Abstract
Aims
Left atrial appendage (LAA) device imaging after endovascular closure is important to assess for device thrombus, residual leak, positioning, surrounding structures, and pericardial effusion. Cardiac CT angiography (CCTA) is well suited to assess these non-invasively.
Methods and results
We report our consecutive series of non-valvular atrial fibrillation patients who underwent CCTA post-LAA closure with Amplatzer Cardiac Plug (ACP), Amulet (second generation ACP), or WATCHMAN devices. Patients underwent CCTA typically 1–6 months post-implantation. Prospective cardiac-gated CCTA was performed with Toshiba 320-detector or Siemens 2nd generation 128-slice dual-source scanners, and images interpreted with VitreaWorkstation™. GFR <30 mL/min/1.73 m2 was an exclusion. We assessed for device thrombus, residual LAA leak, device embolization, position, pericardial effusion, optimal implantation, and device lobe dimensions. Forty-five patients underwent CCTA at median 97 days post-LAA closure (18 ACP, 9 Amulet, 18 WATCHMAN). Average age was 75.5 ± 8.9 years, mean CHADS2 score 3.1 ± 1.3, and CHADS-VASc score 4.9 ± 1.6. All had contraindications to oral anticoagulation. Post-procedure, 41 (91.1%) were discharged on DAPT. There was one device embolization (ACP, successfully retrieved percutaneously) and one thrombus (WATCHMAN, resolved with 3 months of warfarin). There were two pericardial effusions, both pre-existing and not requiring intervention. Residual leak (patency) was seen in 28/44 (63.6%), and the mechanisms of leak were readily identified by CCTA (off-axis device, gaps at orifice, or fabric leak). Mean follow-up was 1.2 ± 1.1year, with no death, stroke, or systemic embolism.
Conclusion
CCTA appears to be a feasible alternative to transoesophageal echocardiography for post-LAA device surveillance to evaluate for device thrombus, residual leak, embolization, position, and pericardial effusion.
Keywords: left atrial appendage closure, cardiac CTA, Amplatzer cardiac plug, WATCHMAN, Amulet
Introduction
Percutaneous endovascular left atrial appendage (LAA) closure is increasingly performed worldwide for patients with atrial fibrillation (AF), especially those with contraindications to long-term oral anticoagulation (OAC). This is supported by guidelines from the European Society of Cardiology, which had implemented a class IIB recommendation for LAA closure in AF patients with high stroke risk and contraindications to long-term OAC.1 The majority of procedures performed in Europe adhere to this guideline as reported by Tzikas of ∼1000 patients who underwent LAA closure with the Amplatzer Cardiac Plug (ACP) (St Jude Medical, Maple Grove, MN, USA) device,2 with 74% performed in patients with major bleeding or at high bleeding risk. Other reported indications were coronary stenting requiring dual antiplatelet therapy (DAPT; 23%), drug interaction (18%), stroke on warfarin (16%), renal or hepatic disease (13%), labile INR (7%), and risk of falls (7%). In Canada, LAA closure may be performed under the Health Canada special access program for patients with CHADS2 ≥1 and contraindications to long-term OAC. In the USA, the WATCHMAN (Boston Scientific Corporation, Natick, MA, USA) device is anticipated to receive FDA approval in early 2015 for patients who are eligible for OAC.
Given the projected more than doubling of the prevalence of AF to 15.9 million by 2050,3 and since ∼50% of patients eligible for OAC are not receiving OAC in the community,4,5 percutaneous LAA closure is expected to increasingly play a dominant role in stroke prevention for AF. Following LAA closure, routine surveillance with transoesophageal echocardiogram (TEE) is usually performed at 1–6 months post-procedure to evaluate for device-associated thrombus and residual leak into LAA (patency). The implications of these findings include the necessity to institute short-term anticoagulation (heparin or OAC) to dissolve device thrombus, and consideration of resumption of OAC or repeat LAA closure with a different device in the setting of large residual leak, respectively.
We have reported our preliminary experience of using cardiac computed tomography angiography (CCTA) as an alternative to TEE for post-procedural surveillance with the ACP device.6 We showed that CCTA provided accurate assessment of the position and function of ACP compared with transthoracic echocardiography (TTE). Moreover, CT linear attenuating coefficient (degree of attenuation, Hounsfield) measurements allowed the detection of residual flow (patency) into the LAA. With the added advantage of non-invasiveness, we proposed that CCTA is a valid alternative to TEE for post-LAA closure surveillance, and this has become the routine imaging modality of choice for this indication at our institution. In this study, we report our experience with our consecutive series of patients who underwent endovascular LAA closure followed by CCTA for surveillance.
Methods
We report our consecutive series of patients who underwent CCTA for post-procedural surveillance after endovascular LAA closure with the ACP, Amulet (2nd generation ACP), or WATCHMAN devices at Vancouver General Hospital. All patients underwent baseline pre-planning imaging, typically with TEE at 4–8 weeks prior to their LAA closure procedure. Since November 2013, we also routinely performed baseline CCTA in addition to baseline TEE prior to LAA closures. GFR <30 mL/min/1.73 m2 was an exclusion for CCTA. Indications for LAA closure were high stroke risk, non-valvular AF patients with contraindications for long-term OAC, with CHADS2 ≥1 and CHADS-VASc ≥2 (in accordance to the Canadian Cardiovascular Society and American College of Cardiology AF guidelines for OAC).3,7
Endovascular LAA closures were performed under TEE guidance and general anaesthesia. Transseptal punctures were performed inferoposteriorly at the fossa ovalis with SL1 (St Jude Medical) transseptal sheath and either BRK-1™ XS (St Jude Medical) or NRG® radiofrequency (Baylis Medical, Burlington, MA, USA) transseptal needles. Volume loading to achieve mean left atrial pressure ∼15 mmHg and intravenous heparin was given to maintain ACT >250 s. A 5F marker pigtail was advanced into the LAA and cineangiograms taken for measurements. For ACP/Amulet, the widest landing zone was measured at ∼10 mm inside the orifice for ACP and ∼12 mm for Amulet. For WATCHMAN, the widest anatomic orifice (from circumflex artery inferiorly to a point 1–2 cm inside the tip of the pulmonary vein ridge superiorly) was recorded. Corresponding measurements on TEE were recorded after volume loading (and also on baseline CCTA if available). Device sizing was based on the widest landing zone dimensions and we typically upsize by 3–5 mm for ACP, 2–4 mm for Amulet, and 9–25% for WATCHMAN. The LAA shape was categorized into four major shapes using baseline CCTA and fluoroscopic images.8
The appropriately sized TorqueVue 45 × 45 delivery sheath or the WATCHMAN double-curve (or single-curve) sheath was advanced into the LAA typically using a pigtail as a rail. Our steps for LAA device deployment were previously described.9 We strive to achieve the recommended five signs of proper deployment for ACP/Amulet: (i) tire-shaped lobe, (ii) separation of the lobe from the disc, (iii) concavity of the disc, (iv) axis of the lobe should be perpendicular to the neck axis at landing zone, and (v) width of the lobe is ≥2/3 within the circumflex artery. For WATCHMAN, the PASS (position, anchor, size, and seal) criteria were achieved prior to release. The presence of residual leak was assessed on TEE and cineangiograms after device release. The presence and degree of peri-device leak were measured on TEE with the Nyquist limit set low (<50 cm/s).
Post-procedure, patients were admitted overnight for observation. Antithrombotic regimen post-LAA closure typically consisted of DAPT (aspirin + clopidogrel) for 1–3 months, followed by aspirin alone indefinitely. Patients were followed clinically at 1–3 months and annually post-procedure. Patients routinely underwent post-procedural TTE the next day and at 1 month. In this cohort, CCTA was typically performed at 1–6 months for surveillance. One patient had CCTA performed the day post-procedure due to concern of device embolization on TTE. A subset of patients also underwent TEE post-procedure for follow-up, typically at 1–6 months.
Prospective systolic-triggered ECG synchronized cardiac-gated CCTA was performed with Aquilion One 320-detector (Toshiba Medical Systems Corporation, Tokyo, Japan) or Siemens 2nd generation 128-slice dual-source CT (Somatom Definition Flash, Siemens Healthcare, Forchheim, Germany) scanners, and the digital images were interpreted with the VitreaWorkstation™ (Vital, Toshiba Medical Systems Group Company, The Netherlands). Our CT protocol for pre-planning and post-procedural LAA closures is described in Table 1. Our approach to digital post-processing reconstructions and interpretations of the pre-planning and post-procedural CCTA images has been previously described.10 The processed images were assessed for atrial-side device thrombus, residual contrast leak (patency) into the LAA, device embolization, device positioning, pericardial effusion, the presence of the five signs of proper implantation, the presence of peri-device gap, and device lobe dimensions. Lobe compression (%) was calculated as: [(manufacturer device diameter − measured diameter)/manufacturer device diameter] × 100%. Residual leak (patency) into the LAA was assessed by measurements of the linear attenuation coefficient (Hounsfield unit, HU) in the LAA distal to the device and comparison of contrast density to surrounding cardiac chambers.
Table 1.
Vancouver General Hospital CT protocol for pre-planning and post-procedure for LAA closures with the Toshiba or Siemens scanners
| Prospective cardiac-gated scans | Values |
|---|---|
| Tube potential | 100 kV for BMI <30 120 kV if BMI >30 |
| Tube current | 300–500 mA with ECG tube current modulation |
| Scan direction | Cranial to caudal |
| Scan volume | Heart to diaphragm (14–16 cm) |
| Size | Images reconstructed to 0.5 and 0.6 mm with 40% overlap, 512 × 512 mm matrix, FOV 25cm |
| Detector collimation | 320 × 0.5 mm Toshiba or 128 × 0.6 mm Siemens |
| Cardiac phase reconstruction | Relative triggering 30–40% of RR interval or absolute triggering 250 ms after R wave |
| Contrast bolus tracking | Sure Start (Toshiba) or Cardiac Definition (Siemens) |
| IV contrast injection (5 cc/s) | 50–80 cc Optiray contrast, followed by 50 cc 30% contrast/70% saline mixture, and final 30 cc saline chaser |
| Heart rate | No restriction |
| Beta-blocker and nitrates | Not required |
BMI, body mass index.
Statistical analysis
Descriptive statistics were used to describe the baseline characteristics of patients. Continuous variables were summarized as mean ± standard deviation, or median and inter-quartile range. Categorical variables were summarized as frequency and percentage. Categorical variables were compared using a χ2 test or Fisher exact test, and continuous variables using the Student t-test or Mann–Whitney test. Statistical analyses were performed with the SPSS software (IBM SPSS version 20, New York).
Results
Forty-five patients with non-valvular AF underwent routine CCTA at a mean of 141.6 ± 130.5 days (median 97 days) post-LAA closure (18 ACP, 9 Amulet, 18 WATCHMAN) for device surveillance at our hospital. Baseline characteristics are described in Table 2 and the indications for LAA closure in Table 3. The average age was 75.5 ± 8.9 years. The mean CHADS2 score was 3.1 ± 1.3, and CHADS-VASc score was 4.9 ± 1.6. The mean radiation dose for these patients with our standard post-LAA CCTA protocol was 5.3 ± 3.7 mSv. All patients had contraindications to long-term OAC: 42 (93.3%) had a history of major bleeding, 1 (2.2%) had oesophageal varices from chronic liver disease, 1 (2.2%) had high risk of falls due to seizures, and 1 (2.2%) had non-healing venous ulcers on warfarin requiring skin grafting. Eleven (24.4%) were on anticoagulation prior to LAA closure, including four who were found to have thrombus in the LAA on baseline pre-planning TEE (requiring OAC with subsequent proven thrombus resolution prior to LAA closure), and two on short-term, low-molecular weight heparins because of systemic embolization and pulmonary embolism prior to LAA closure.
Table 2.
Baseline characteristics
| Baseline characteristics | n = 45 |
|---|---|
| Age (years) | 75.5 ± 8.9 |
| Sex (women) | 20 (44.5%) |
| Height (cm) | 167.8 ± 11.8 |
| Weight (kg) | 75.4 ± 19.3 |
| BMI | 26.6 ± 5.5 |
| AF paroxysmal | 14 (31.1%) |
| AF chronic | 31 (68.9%) |
| History stroke/TIA | 20 (44.4%) |
| CAD | 18 (40.0%) |
| CHF | 21 (47.7%) |
| Diabetes mellitus | 13 (28.9%) |
| Hypertension | 38 (84.4%) |
| Previous bioprosthesis | 3 (6.7%) |
| Liver disease | 6 (13.3%) |
| Renal failure (GFR<60 mL/min/1.73 m2) | 21 (46.7%) |
| LVEF (%) | 52.8 ± 13.4 |
| LVEF ≤40% | 9 (20.0%) |
Table 3.
Indications for LAA closure and baseline antithrombotic agents
| Indications for LAA closure | n = 45 |
|---|---|
| CHADS2 score | 3.1 ± 1.3 |
| CHADS-VASc score | 4.9 ± 1.6 |
| HASBLED score | 4.2 ± 1.0 |
| Previous major bleeding | 42 (93.3%) |
| Intracranial bleed | 17 (37.8%) |
| Intraocular bleed | 2 (4.4%) |
| Gastrointestinal bleed | 21 (46.7%) |
| Retroperitoneal bleed | 3 (6.7%) |
| Genitourinary bleed | 1 (2.2%) |
| Transfusions | 22 (48.9%) |
| High fall risk (seizure) | 1 (2.2%) |
| Other reasons: oesophageal varices, non-healing venous ulcers | 2 (4.4%) |
| Baseline antithrombotic | |
| None | 16 (35.6%) |
| Aspirin | 16 (35.6%) |
| Clopidogrel | 3 (6.7%) |
| Aspirin + clopidogrel | 1 (2.2%) |
| Warfarin | 7 (15.6%) |
| Novel OAC | 2 (4.4%) |
| Heparin | 2 (4.4%) |
Procedural success with device release was 100% (devices implanted are listed in Table 4), and there were no procedural stroke or major bleeding. There was one clinically insignificant transseptal puncture-related pericardial effusion that did not require intervention. Post-procedure, 41 (91.1%) were discharged on DAPT [21 for 1 month (2 stopped aspirin or clopidogrel after a week because of bleeding), and 20 for 3 months]; only 1 patient was discharged on warfarin (concomitant pulmonary embolism requiring short-term OAC), 2 on aspirin alone, and 1 on clopidogrel alone (Table 5).
Table 4.
LAA device and size implanted
| Device implanted | n = 45 |
|---|---|
| Amplatzer Cardiac Plug (ACP) | 18 |
| Mean size | 25.6 ± 3.4 mm |
| Amulet (second generation ACP) | 9 |
| Mean size | 22.7 ± 3.2 mm |
| WATCHMAN | 18 |
| Mean size | 27.5 ± 4.3 mm |
Table 5.
Antithrombotic regimen post-LAA closure
| Antithrombotic regimen post-LAA | n = 45 |
|---|---|
| ASA + clopidogrel: 1 month | 21 (46.7%)a |
| ASA + clopidogrel: 3 months | 20 (44.4%) |
| ASA + clopidogrel: 6 months | 1 (2.2%) |
| Warfarin | 1 (2.2%) |
| ASA alone | 2 (4.4%) |
| Clopidogrel alone | 1 (2.2%) |
aOne stopped clopidogrel and one stopped aspirin after 1 week due to bleeding.
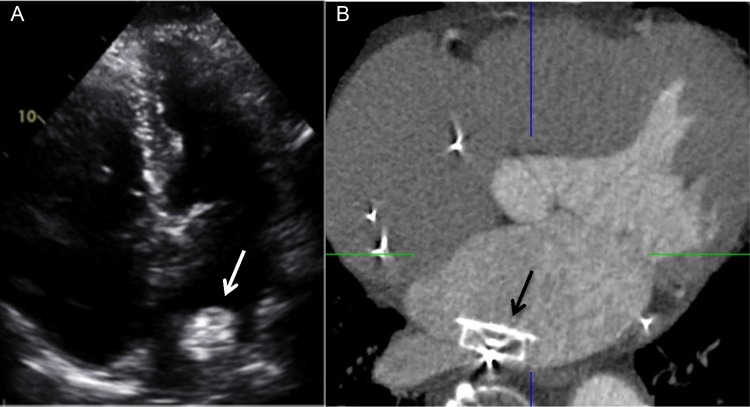
There was one device-associated thrombus (2.2%) with WATCHMAN (Figure 1) found on routine CCTA follow-up at 78 days post-implant while on DAPT (planned DAPT for 3 months post-LAA closure) that was successfully treated with 3 months of warfarin without clinical sequelae. There was no residual LAA leak in this patient. There was one device embolization (2.2%) with ACP demonstrated on CCTA done 1 day post-implant, as the post-TTE showed device in a high posterior position (Figure 2); this was successfully retrieved percutaneously without consequence. Two patients had pericardial effusion (4.4%) on post-CCTA, which were both present pre-LAA closure that did not require intervention.
Figure 1.
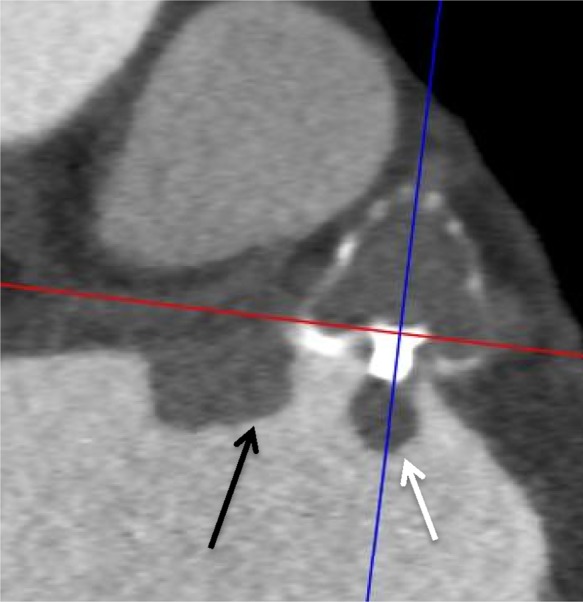
Contrast-enhanced CT images revealed an atrial-side device thrombus on a WATCHMAN device at the fabric insert (white arrow) and adjacent to the device (black arrow).
Figure 2.
Embolized ACP device in the left atrium that was lodged posteriorly, seen on (A) TTE (white arrow) and confirmed on (B) contrast-enhanced four-chamber CT image (black arrow).
Residual leak (patency) was seen in 28/44 (63.6%) of the follow-up CCTA and was not significantly different with the various devices: ACP 12/17 (70.6%), Amulet 6/9 (66.7%), and WATCHMAN 10/18 (55.6%) (P = NS). Of note, only 6/44 (13.6%) had peri-device leak into the LAA on TEE at the end of the procedure. Of 23 patients who had both TEE and CCTA for post-procedural follow-up, CCTA patency was present in 12/23 (52.2%, of which 7/12 had TEE peri-device leak), and any TEE peri-device leak was present in 8/23 (34.8%). The radiodensity of the LAA was evaluated for contrast patency. Patent LAA (residual leak) had mean 352.2 ± 136.4 Hounsfield unit (HU) compared with mean 65.2 ± 17.4 HU in occluded LAA (P < 0.0001). In fact, all occluded LAA had radiodensity <100 HU, and contrast opacification <25% of the left atrium (Figure 3).
Figure 3.
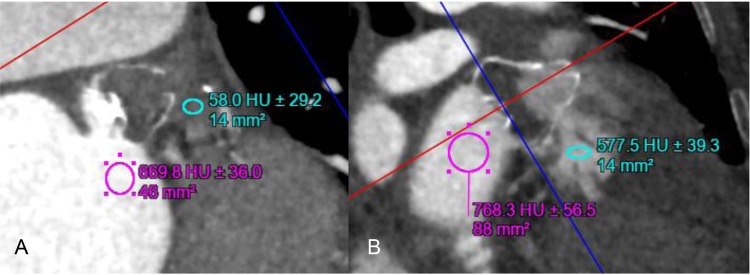
Linear attenuation coefficient measured in Hounsfield units on contrast-enhanced CT images of the LAA after WATCHMAN closures showing (A) complete occlusion of the LAA (no residual contrast patency, 58.0 HU) and (B) patent LAA (residual contrast patency, 577.5 HU).
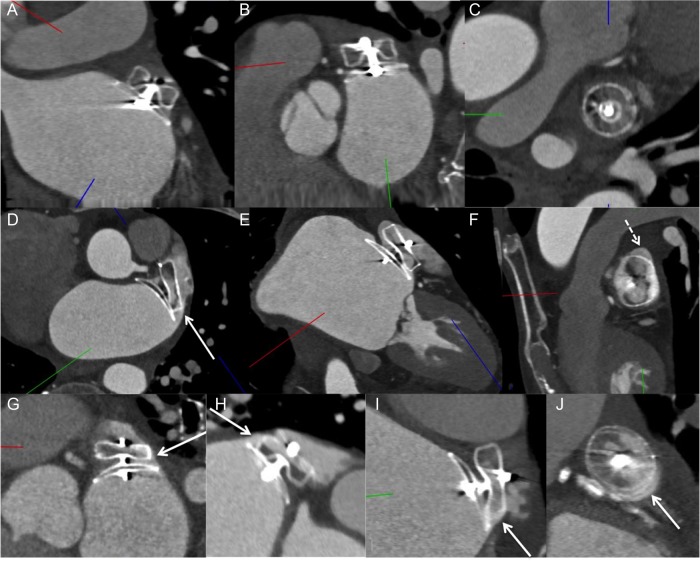
We evaluated the five signs of proper deployment with the ACP and Amulet devices on follow-up CCTA. The device lobe was tire shaped, and there was separation of the lobe and disk in all 26/26 of cases. The disc was concave in 22/26 (84.6%) of cases. In 24/26 (92.3%) cases, the lobe was at least 2/3 within the circumflex artery. However, only 12/26 (46.2%) of cases had good alignment of the lobes (Figure 4). Of the 14/26 (53.8%) of ACP/Amulet cases where the lobes were off-axis at the landing zone on CCTA, only 1/14 appeared to be off-axis on procedural TEE.
Figure 4.
Contrast-enhanced CT images: (A–C) Amulet case with good alignment of lobe perpendicular to the neck of the LAA, with complete occlusion of the LAA. (D–F) ACP case with off-axis lobe (not perpendicular to the LAA neck) (white arrow in D) and superior gap (dashed arrow in F) causing residual contrast patency of LAA. (G) Example of ACP case with off-axis on the right side (arrow) with contrast leak through that edge, whereas there was no lobe leak on the left side. (H) Example of ACP case with off-axis lobe and contrast leak through the superior edge (arrow) (bottom half of lobe with no contrast opacification). (I) ACP lobe off-axis inferiorly (arrow) with contrast leaking through the edge and gap (arrow in J).
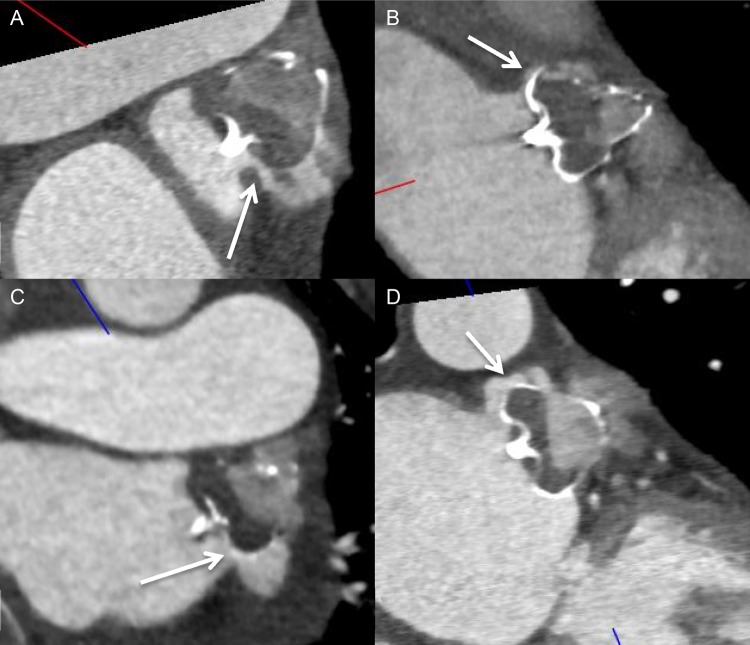
We evaluated potential contributing causes of residual leak into the LAA on CCTA. For ACP/Amulet, patent LAA had significantly higher proportion of off-axis lobes (13/18, 72.2%) compared with occluded LAA (1/8, 12.5%), P = 0.007. Furthermore, the mean maximum lobe compression on CCTA was 9.2 ± 6.8% in the patent LAA group and 16.1 ± 7.6% in the occluded LAA group (P = 0.011). The mean minimum lobe compression was not different in the patent LAA and occluded LAA groups (5.4 vs. 6.5%, P = NS). For WATCHMAN, the mean maximum and minimum lobe compressions on CCTA were not different between the patent LAA and occluded LAA groups, 14.8 vs. 14.2% (P = NS) and 9.2 vs. 8.9% (P = NS), respectively. However, all WATCHMAN devices with patent LAA on CCTA had demonstrable ostial peri-device gaps that led to contrast opacification of the LAA (Figure 5). Furthermore, one patient also had fabric leak through the WATCHMAN device at 90 days post-implant (Figure 6). Residual leaks were observed in all four major LAA shapes and were not statistically different [chicken-wing 11/18 (61.1%), windsock 9/14 (64.3%), cactus 5/7 (71.4%), cauliflower 3/6 (50.0%)].
Figure 5.
Contrast-enhanced CT images of four different examples of WATCHMAN implanted devices with ostial peri-device gaps with residual contrast patencies of the LAA.
Figure 6.
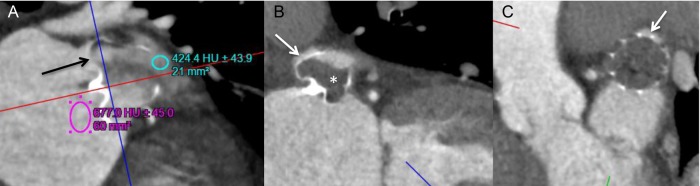
Contrast-enhanced CT images: (A) example of fabric leak through an incompletely endothelialized WATCHMAN device, with contrast seeping through the fabric at the proximal shoulder of the device (black arrow). (B and C) In comparison, this WATCHMAN device had no contrast opacification of the proximal shoulder of the device (*), but instead contrast into the LAA came through the superior gap (white arrows in B and C).
A second repeat post-CCTA was performed in eight patients (ACP or Amulet) with LAA patency at a mean of 327.1 ± 170.4 days post-implant. Six (75.0%) had persistent leak, all had off-axis lobes and ostial gaps (all <5 mm). Two repeat CCTA showed subsequent occluded LAA, both initial LAA patency were presumed predominantly related to fabric leak as the first CCTAs were performed at 37 days (slight off-axis lobe) and 57 days (good lobe alignment) post-implant in these cases.
The mean duration of follow-up was 1.2 ± 1.1 years in this cohort. There was no occurrence of death, stroke, or systemic embolism. One patient had a transient ischaemic attack with transient speech disturbance, and investigations did not show new cerebral infarction, device thrombus, or leak. As mentioned, one patient had device-associated thrombus, which resolved with warfarin without clinical sequelae. Follow-up bleeding (non-procedural) occurred in 4/45 (8.9%) of patients; 1 minor bleed while on warfarin, 2 occurred 1 week post-procedure while on DAPT (lower GI bleeds requiring discontinuation of one antiplatelet agent), and 1 required transfusion for unexplained haemoglobin drop to 76 mg/dL while on DAPT (planned 3-month therapy).
Discussion
Our study showed that CCTA is a useful, non-invasive post-procedural surveillance test for endovascular LAA closure devices in patients with contraindications for OAC, with safe long-term clinical outcomes. We were able to evaluate for atrial-side device thrombus, residual leak (patency) into the LAA, device embolization, device position, pericardial effusion, the presence of the five signs of proper deployment, and device lobe and compression dimensions.
We found that endovascular LAA devices were well visualized on CCTA in the LAA, with high spatial resolution that enabled assessment of device angles and positions. The presence of pericardial effusion and atrial-side device thrombus can be readily identified on CCTA. Embolized devices in the heart chambers can also be clearly visualized on CCTA, as in our case of ACP embolization into the left atrium that was successfully retrieved percutaneously. CCTA is also useful to visualize embolized devices in the left ventricle and assess if they are trapped by papillary muscles and trabeculations, which may be relevant when considering percutaneous retrieval.
The linear attenuation coefficient within the LAA can also be readily measured in Hounsfield units and ascertained for residual LAA patency from contrast leak. In fact we found that all occluded LAA have an attenuation of <100 HU and <25% of the contrast opacification of the left atrium. These thresholds have become our criteria to evaluate for LAA patency after percutaneous closure.
Using CCTA, we found that a large proportion of patients (63.6%) had residual LAA patency after ACP, Amulet, and WATCHMAN closures. This is contrary to reports based upon TEE follow-up that showed much lower incidence of residual leak with ACP, up to 11.9% in the report by Tzikas.2 Other series have shown that leak >5 mm has not been documented with ACP; leak of 3–5 mm was reported in 0–1% of cases,11 and leak <3 mm was reported in 0–16%.12–14 The clinical significance of residual leak with the ACP device has not been explored. With the WATCHMAN device, residual leak was shown to occur in a significant proportion of patients on TEE follow-up, with some degree of leak seen in 32% of cases in the PROTECT-AF study at 12-month follow-up (36.8% >3 mm and 63.2% ≤3 mm). In this study, residual leak was not associated with increased risk of subsequent thromboembolism;15 however, the event-rate was low and these findings were considered hypothesis generating. CCTA appears to be a much more sensitive modality to detect residual leak compared with TEE, and the clinical significance of CCTA-detected residual leak into LAA after closure with the ACP/Amulet or WATCHMAN devices is unknown and needs further study.
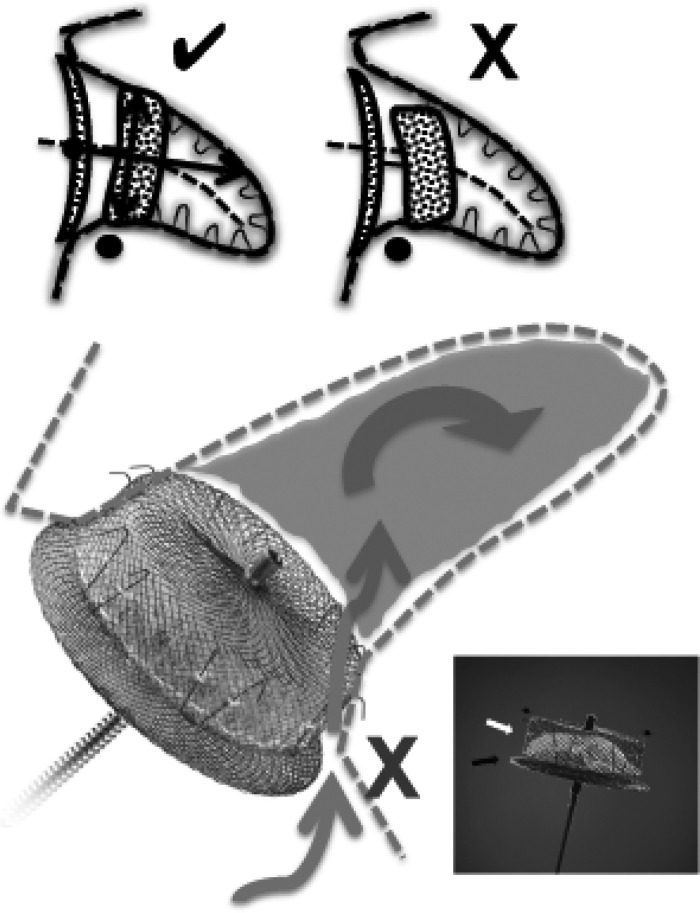
With the superior resolution of CCTA, we were able to clearly evaluate the five signs of proper deployment as defined by the instructions for use for the ACP/Amulet devices on CCTA. Interestingly, we identified a large proportion of cases where the alignment of the device lobe was suboptimal as assessed on CCTA, even though they were deemed to be well aligned based upon the final procedural TEE images. This highlights the challenges with TEE in depicting the three-dimensional LAA structure; alternatively, the devices may have shifted in position since implantation. In addition, a larger proportion of patients with residual patent LAA had suboptimal alignment (off-axis at the landing zone) compared with patients with occluded LAA. A schematic explaining the mechanism of lobe off-axis leak with this device is depicted in Figure 7. Given that the polyester fabric sewn into the lobe is thin and proximally located within the lobe, contrast can readily diffuse through the edges of the lobe when malapposed at the landing zone. Thus, achieving proper alignment is not only important to minimize device embolization, but also promote complete LAA occlusion with this device. The use of CT co-registration during percutaneous LAA closure to improve ACP/Amulet device alignment at the landing zone may be useful.
Figure 7.
Schematic showing how an off-axis ACP/Amulet lobe results in contrast diffusing through the side edge of the lobe (not covered by polyester fabric, which is a thin layer sewn to the proximal part of the lobe). Proper alignment of the lobe entails perpendicular apposition of the lobe against the wall of the LAA at the neck (✓); poor alignment with the lobe is off-axis at the LAA neck (X).
For the ACP/Amulet device, we found that patients with residual LAA patency had lower mean maximum lobe compression (9.2%) compared with patients with occluded LAA (16.1%) on CCTA. Moreover, those with residual leak were also more likely to have suboptimal alignment (off-axis lobe) (72.2%) compared with those without leak (12.5%). Thus, achieving adequate lobe compression and optimal lobe alignment appear to reduce residual leak. This was similarly described by Jaguszewski et al.16 in their smaller 24-patient ACP series, where they found residual contrast patency in 62%, and also found that occluded LAA had greater lobe compression (>10%) and perpendicular lobe axis.
For the WATCHMAN device, when deployment was optimal according to the instruction for use PASS criteria, we found that device compression measurements were not different among patients with occluded or patent LAA on CCTA. On the other hand, all WATCHMAN patients with residual LAA patency had peri-device gaps at the LAA ostia. This highlights the importance of minimizing peri-device leaks during the procedure, which will presumably correspond to lower incidence of contrast patency on CCTA. In addition, one patient had fabric leak at 90 days post-implantation, which contrasts with other WATCHMAN patients without fabric leak, highlighting the variability in timing of complete endothelialization in humans. In canine models, complete endothelialization was shown to occur within 28–45 days; however, the endothelialization process appears to be accelerated in dogs.17,18
In summary, from our experience, it appears that there are three underlying important mechanisms of residual LAA contrast patency after endovascular closure: (i) off-axis lobe with the ACP/Amulet device, enabling contrast diffusion across non-fabric covered sides of the lobe; (ii) ostial peri-device LAA gaps, especially important with the WATCHMAN device as it is a single-lobe occluder without a disc that covers the echocardiographic orifice; (iii) fabric leak through non-endothelialized portion of the devices. Since two of our ACP patients with fabric leak on their initial CCTA (performed <60 days post-implant) were subsequently found to have occluded LAA on repeat CCTA, it would be preferable to scan patients at least 3 months post-LAA closure with endovascular devices to minimize demonstrating leaks due to incomplete endothelialization.
The mean radiation dose with our standard post-LAA CCTA protocol was only 5.3 mSv, which is considered low and equivalent to annual background radiation dose of 3–5 mSv. To put in context, the potential risk of fatal cancer with 10 mSv exposure is only 1 : 2000. In addition, the 2011 position statement from the Radiologic Society of North America and the American Association of Physicists in Medicine stated that the risks of medical imaging at effective doses <50 mSv for single procedures are too low to be detectable and may be non-existent. Thus, the radiation dose incurred by our post-surveillance CCTA protocol is low and acceptable.
Limitations
Our study is limited by being a small retrospective series of patients who underwent CCTA after endovascular LAA closure, with low clinical events. Only a subset of patients had both TEE and CCTA post-procedure. Furthermore, the clinical significance of CCTA residual LAA contrast patency is unknown.
Conclusions
CCTA is a feasible, non-invasive post-procedural surveillance imaging modality after endovascular LAA closure to evaluate for atrial-side device thrombus, residual leak (and mechanisms of leak), device embolization, device position, and pericardial effusion. Thus, CCTA may a suitable alternative to TEE for post-LAA closure surveillance; however, the clinical significance of residual LAA patency on CCTA should be further explored in larger studies.
Conflict of interest: None declared.
Funding
There was no funding or support for work performed in this manuscript. However, J.S. has received unrestricted research grant supports (from the Canadian Institutes of Health Research, University of British Columbia Division of Cardiology, AstraZeneca, Abbott Vascular, St Jude Medical, Boston Scientific, and Servier), speaker honoraria (AstraZeneca, St Jude Medical, Boston Scientific, Bayer, and Sunovion), consultancy and advisory board honoraria (AstraZeneca, St Jude Medical, Boston Scientific, and Abbott Vascular), and proctorship honoraria (St Jude Medical and Boston Scientific).
References
- 1.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719–47. [DOI] [PubMed] [Google Scholar]
- 2.Tzikas A, Shakir S, Gafoor S, Omran H, Berti S, Santoro G, et al. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the AMPLATZER Cardiac Plug. EuroIntervention 2015;10. [DOI] [PubMed] [Google Scholar]
- 3.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006;114:119–25. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Hylek EM, Borowsky LH, Phillips KA, Selby JV, Singer DE. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med 1999;131:927–34. [DOI] [PubMed] [Google Scholar]
- 5.Kakkar AK, Mueller I, Bassand JP, Fitzmaurice DA, Goldhaber SZ, Goto S, et al. Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the international, observational, prospective GARFIELD registry. PLoS ONE 2013;8:e63479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poulter RS, Tang J, Jue J, Ibrahim R, Nicolaou S, Mayo J, et al. Cardiac computed tomography follow-up of left atrial appendage exclusion using the Amplatzer Cardiac Plug device. Can J Cardiol 2012;28:119 e1–3. [DOI] [PubMed] [Google Scholar]
- 7.Skanes AC, Healey JS, Cairns JA, Dorian P, Gillis AM, McMurtry MS, et al. Focused 2012 update of the Canadian cardiovascular society atrial fibrillation guidelines: recommendations for stroke prevention and rate/rhythm control. Can J Cardiol 2012;28:125–36. [DOI] [PubMed] [Google Scholar]
- 8.Di Biase L, Santangeli P, Anselmino M, Mohanty P, Salvetti I, Gili S, et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol 2012;60:531–8. [DOI] [PubMed] [Google Scholar]
- 9.Saw J, Lempereur M. Percutaneous left atrial appendage closure: procedural techniques and outcomes. JACC Cardiovasc Interv 2014;7:1205–20. [DOI] [PubMed] [Google Scholar]
- 10.Saw J, Lopes JP, Reisman M, Bezerra H. CT imaging for percutaneous LAA closure. In: Saw J, Kar S, Price MJ, eds. Left Atrial Appendage Closure: Mechanical Approaches to Stroke Prevention in Atrial Fibrillation. 1st ed New York, USA: Springer; 2015. [Google Scholar]
- 11.Park JW, Sievert H, Schillinger W, Lickfett L, Lopez-Minguez JR, Omran H, et al. Results of the amplatzer cardiac plug European multicenter prospective observational study. J Am Coll Cardiol 2012;60:B27. [Google Scholar]
- 12.Lam YY, Yip GW, Yu CM, Chan WW, Cheng BC, Yan BP, et al. Left atrial appendage closure with AMPLATZER cardiac plug for stroke prevention in atrial fibrillation: initial Asia-Pacific experience. Catheter Cardiovasc Interv 2012;79:794–800. [DOI] [PubMed] [Google Scholar]
- 13.Urena M, Rodes-Cabau J, Freixa X, Saw J, Webb JG, Freeman M, et al. Percutaneous left atrial appendage closure with the AMPLATZER cardiac plug device in patients with nonvalvular atrial fibrillation and contraindications to anticoagulation therapy. J Am Coll Cardiol 2013;62:96–102. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Minguez JR, Eldoayen-Gragera J, Gonzalez-Fernandez R, Fernandez-Vegas C, Fuentes-Canamero ME, Millan-Nunez V, et al. Immediate and one-year results in 35 consecutive patients after closure of left atrial appendage wthe amplatzer cardiac plug. Rev Esp Cardiol 2013;66:90–7. [DOI] [PubMed] [Google Scholar]
- 15.Viles-Gonzalez JF, Kar S, Douglas P, Dukkipati S, Feldman T, Horton R, et al. The clinical impact of incomplete left atrial appendage closure with the watchman device in patients with atrial fibrillation: a PROTECT AF (percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation) substudy. J Am Coll Cardiol 2012;59:923–9. [DOI] [PubMed] [Google Scholar]
- 16.Jaguszewski M, Manes C, Puippe G, Salzberg S, Muller M, Falk V, et al. Cardiac CT and echocardiographic evaluation of peri-device flow after percutaneous left atrial appendage closure using the AMPLATZER cardiac plug device. Catheter Cardiovasc Interv 2015;85:306–12. [DOI] [PubMed] [Google Scholar]
- 17.Kar S, Hou D, Jones R, Werner D, Swanson L, Tischler B, et al. Impact of watchman and amplatzer devices on left atrial appendage adjacent structures and healing response in a canine model. JACC Cardiovasc Interv 2014;7:801–9. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz RS, Holmes DR, Van Tassel RA, Hauser R, Henry TD, Mooney M, et al. Left atrial appendage obliteration: mechanisms of healing and intracardiac integration. JACC Cardiovasc Interv 2010;3:870–7. [DOI] [PubMed] [Google Scholar]