Abstract
Aims
Infants with persistent pulmonary hypertension of the newborn (PPHN) have elevated pulmonary vascular resistance that can lead to right ventricular (RV) failure and death. Clinicians must decide which infants will fail conventional therapy and require transfer to extra corporeal membrane oxygenation (ECMO) centres, but accurate echocardiographic predictors have not been identified. We assessed echocardiographic measurements of RV pressure and function in predicting progression to death or ECMO in infants with PPHN.
Methods and results
Echocardiograms for infants ≥35-week gestation with a clinical diagnosis of PPHN were retrospectively reviewed. Traditional and strain echocardiographic measures were compared for those with or without the primary outcome of ECMO/cardiovascular death. Receiver operator curves identified cut points for measures that were significantly different. Of the 86 subjects analysed, 25 (29%) of the patients had the primary outcome of ECMO/death. The ECMO/death group had diminished tricuspid annular plane systolic excursion (TAPSE; P = 0.002) and RV global longitudinal peak strain (GLPS; P = 0.03), a predominant right-to-left shunt across the patent ductus arteriosus (PDA; P = 0.05), and an elevated oxygenation index (OI; P < 0.001). Sensitivity/specificity for TAPSE <4 mm was 56 and 85%, and for GLPS greater than or equal to −9% was 52 and 77%.
Conclusion
TAPSE, GLPS, and right-to-left PDA shunting were associated with progression to death/ECMO. RV free wall strain was not associated with the outcome, suggesting that diminished global strain better reflects clinical outcomes in this group. These thresholds may assist in the decision-making to transfer high-risk infants to ECMO centres.
Keywords: persistent pulmonary hypertension, echocardiogram, mortality, ECMO, strain
Introduction
Persistent pulmonary hypertension of the newborn (PPHN) is a potentially fatal condition in which foetal circulation fails to adapt to the extrauterine environment. Pulmonary pressures remain elevated potentially leading to a persistent right-to-left shunt, hypoxaemia, and right ventricular (RV) failure.1 The incidence is 1.9 per 1000 live births, and the national average for mortality is 11%.2 Patients with more severe disease may require advanced medical care including inhaled nitric oxide (iNO), high-frequency ventilation (HFV), and extracorporeal membranous oxygenation (ECMO).3,4
Echocardiography is a preferred tool for diagnosing and monitoring PPHN, despite a lack of reliable measures.5,6 Tricuspid regurgitant jet peak velocity (TRJV), interventricular septal shape, and direction of Doppler flow across an intra-atrial shunt and patent ductus arteriosus (PDA) are commonly used indices. However, many of these measures demonstrate poor correlation, accuracy, and specificity compared with catheterization.6,7 Furthermore, these measures are limited to pressure and/or volume assessments, while patients with severe PPHN may have decreased cardiac function secondary to persistent RV afterload or prolonged hypoxaemia.8 The American Society of Echocardiography recently released RV guidelines for children that include conventional measures, such as tricuspid annular plane systolic excursion (TAPSE) and fractional area change (FAC).9 Promising non-traditional measures include left ventricular eccentricity index (LVEI), which offers a more objective measure of septal geometry changes, and RV global longitudinal peak strain (GLPS), which provides an assessment of global ventricular function.10,11
This study aimed to identify measurements of RV pressure and systolic function that are associated with progression to death or ECMO in patients with PPHN. We hypothesize that early echocardiographic indices of RV pressure and systolic function are associated with a composite outcome of death or ECMO.
Methods
Cohort
We conducted a retrospective review of patients admitted to the Duke Intensive Care Nursery between January 2010 and October 2013. Patients were identified using a clinical database developed and maintained by the Division of Neonatal-Perinatal Medicine. Inclusion criteria included infants ≥35-week gestation at birth with a clinical diagnosis of PPHN and an ‘early echocardiogram’ within the first 6 days of life. In cases of multiple echocardiograms, the first study was used. Exclusion criteria included known chromosomal abnormality, congenital cardiac disease (aside from a PDA or small atrial level shunt at the fossa ovalis), or the lack of a pre-ECMO echocardiogram. This protocol was reviewed and approved by the Duke Institutional Review Board.
Echocardiographic measurements
Complete echocardiograms were obtained except in cases of cardiovascular instability. Images were acquired using the Phillips IE33 ultrasound scanner with the 8 or 12 MHz transducer and analysed using Xcelera® (Phillips Medical Systems, Koninklijke, Netherlands) after standard data compression to 30 frames per second. An experienced reader blinded to the clinical outcome performed all measurements.
TRJV was obtained from the view that afforded the best alignment with regurgitant flow using continuous wave Doppler.10 The predominant PDA direction was visually determined using the spectral Doppler tracing and color Doppler flow.12 RV/LV ratio was calculated from RV and LV diameters measured between endocardial surfaces in the mid-papillary parasternal short-axis view during mid-systole.13 FAC was calculated by the formula: (RV areadiastole − RV areasystole)/RV areadiastole.10 TAPSE was determined by the difference in the distance from tricuspid lateral annulus to transducer interface along a parallel line in diastole and systole from 2D apical four-chamber views.14 Systolic : diastolic ratio was calculated with systole defined as the duration of TRJV and diastole defined as the interval between successive tricuspid regurgitation jets.15 LVEI was measured in diastole and systole in the mid-papillary parasternal short-axis view by calculating the ratio of the diameter of the left ventricle parallel to the septum and the diameter bisecting and perpendicular to the septum.10 LV fractional shortening (FS) was measured in MMode in parasternal short-axis views.16 Qualitative LV systolic function was made subjectively from multiple views.
Strain echocardiography
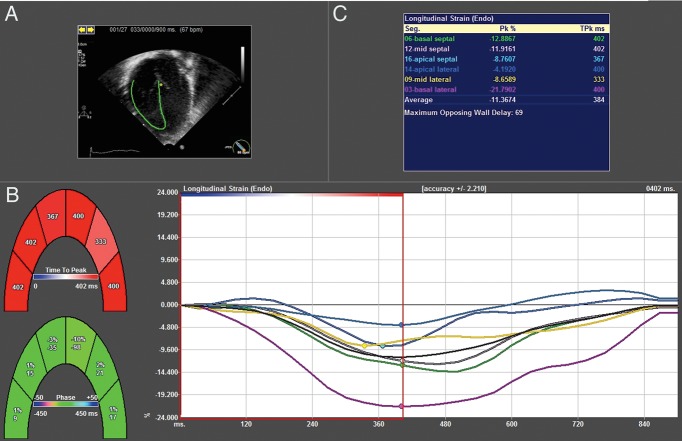
All studies were converted to DICOM format, imported into Tomtec Velocity Vector Imaging (VVI), and analysed with 2D Cardiac Performance Analysis® (Tomtec, Munich, Germany). Strain analysis was performed on a single four-chamber apical image. The cardiac cycle was defined by QRS onset to aortic valve closure, which was determined on spectral Doppler tracing. A single ROI was placed along the endocardial border from the tricuspid valve lateral annulus to medial annulus (Figure 1). Software tracking integrity was automatically detected and visually confirmed. In cases of poor tracking, tracing was readjusted and segments with persistent inadequate tracking were excluded. GLPS was calculated as an average of peak systolic strain in six segments, including basal, mid, and apical segments on the septal and RV walls. Free wall longitudinal peak strain (FWLPS) was calculated as an average of the mid and basal lateral wall segments.
Figure 1.
Example of VVI strain output. (A) Anatomic motion is tracked using RV endocardial border. (B) Time to peak velocity is calculated for each segment. (C) Global longitudinal strain is displayed as the average of the six segments.
Definitions
The primary outcome was cardiovascular death or need for ECMO prior to discharge. Cardiovascular death was defined as non-viable cardiac function, despite maximal medical support resulting in death. The decision to use ECMO was made at the discretion of the clinical care team and typically involved respiratory failure on maximum ventilator settings and/or imminent cardiovascular collapse. Diagnoses associated with PPHN were subclassified as congenital diaphragmatic hernia, acute vasoconstriction (hypoxic ischaemic encephalopathy, meconium aspiration, hypoglycemia, respiratory distress syndrome, sepsis), or idiopathic pulmonary hypertension. Haemodynamics were documented from the time of echocardiogram. OI was calculated retrospectively for all mechanically ventilated patients as previously reported.17 Length of stay among survivors was defined as number of days between first admission and discharge or transfer to lower acuity centre.
Statistics
Continuous variables are reported as median (minimum − maximum). Counts, proportions, and/or percentages were used to summarize categorical variables. Wilcoxon rank-sum or Fishers exact test was used to evaluate differences between patients with the primary outcome (death/ECMO) and those who survived without ECMO. Inter-observer reliability between two readers was determined on random samples of significant findings using Wilcoxon rank-sum and Cohen's kappa tests. A value of P ≤ 0.05 was considered significant.
Receiver operator curves were used to determine cut points for continuous variables. Multivariate regression analysis was used to determine odds ratios for the combination of reported cut points. A Kaplan–Meier curve was created for survival or days of known contact above and below one of the cut points. A subgroup analysis was performed assessing only those subjects who had an echocardiogram within 48 h prior to a primary outcome.
Results
Demographics and outcomes
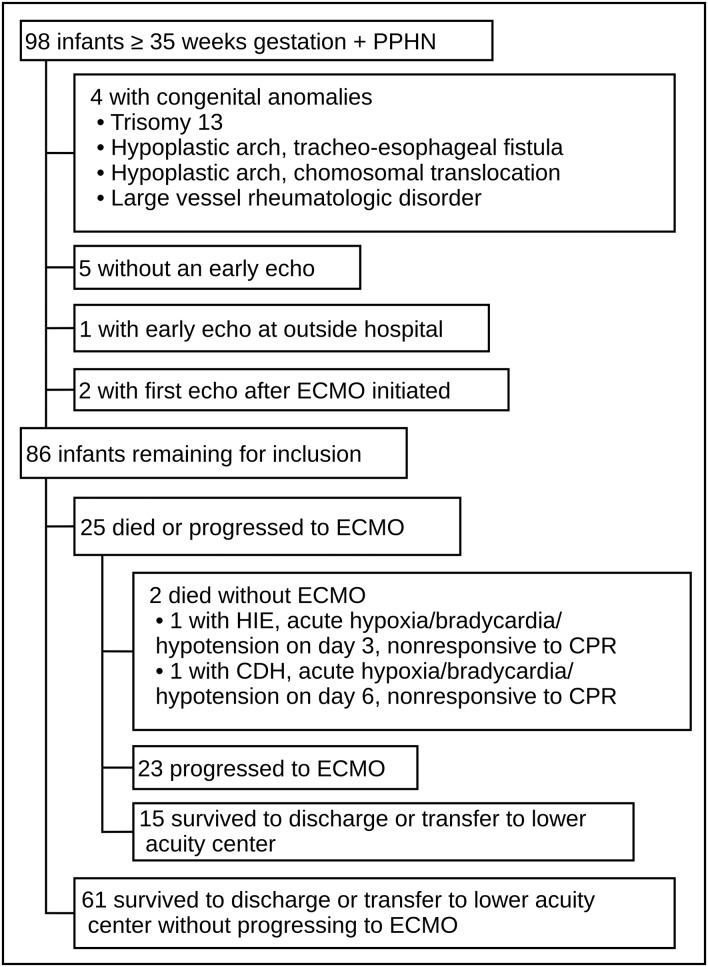
We identified 98 consecutive patients who met our inclusion criteria, and 86 (88%) were included in the study (Figure 2). The primary outcome of cardiovascular death or progression to ECMO was met in 25/86 (29%) of the patients. The median length of time between echocardiogram and death/ECMO was 10 h (range 1–118 h). There were no differences in demographics between groups, but death/ECMO patients required more cardiorespiratory support (Table 1). Overall, cohort survival was 87% (76/86). The median length of stay for all survivors was 31 days (7–229). Length of stay was lower in patients who survived without ECMO compared with ECMO survivors [24 days (7–229) vs. 62 days (29–129), P < 0.001].
Figure 2.
Patient selection.
Table 1.
Demographics and haemodynamics at echocardiogram
| Survived without ECMO | Death/ECMO | P | |
|---|---|---|---|
| N 61 | N 25 | ||
| Demographics | |||
| Gestational age (weeks) | 39 (35–41) | 39 (35–42) | 0.68 |
| Birth weight (g) | 3300 (1940–5650) | 3220 (1900–4500) | 0.30 |
| 5 min Apgar | 7 (0–9) | 7 (0–9) | 0.43 |
| Male | 37 (61) | 16 (64) | 0.81 |
| Race | 0.95 | ||
| White | 27 (44) | 12 (48) | |
| Black | 24 (39) | 10 (40) | |
| Other | 10 (16) | 3 (12) | |
| Caesarean section | 38 (64) | 16 (67) | 0.81 |
| Inborn | 21 (34) | 10 (40) | 0.63 |
| Diagnosis | 0.62 | ||
| CDH | 10 (16) | 6 (24) | |
| Acute vasoconstriction | 35 (57) | 12 (48) | |
| Idiopathic | 16 (26) | 7 (28) | |
| Age at echocardiogram (hours) | 32 (1–130) | 29 (2–135) | 0.94 |
| Haemodynamics | |||
| OI | 11 (2–29) | 29 (9–59) | <0.001 |
| pH | 7.29 (6.95–7.49) | 7.23 (6.88–7.43) | 0.18 |
| Mean airway pressure (mmHg) | 9.6 (6–18.4) | 12.1 (6–18.6) | <0.001 |
| High-frequency ventilation | 28 (46) | 19 (76) | 0.10 |
| Mean arterial pressure (mmHg) | 49 (27–71) | 53 (29–74) | 0.13 |
| Dopamine infusion | 29 (48) | 18 (72) | 0.04 |
| Epinephrine infusion | 9 (15) | 14 (56) | <0.001 |
Continuous variables—median (min − max); categorical variables—n (%).
Acute vasoconstriction = hypoxic ischaemic encephalopathy, asphyxia, respiratory distress syndrome, hypoglycemia, sepsis.
CDH, congenital diaphragmatic hernia; OI, oxygenation index.
Subgroup analysis was performed in 20/86 (23%) in whom death or progression to ECMO occurred within 48 h of the echocardiogram.
Echocardiographic measurements
Infants who died or received ECMO had diminished TAPSE and GLPS, and a predominantly right-to left (pulmonary artery to aorta) PDA shunt (Table 2). TRJV was the most difficult measurement to quantify, while TAPSE and LV functional measures were the most reliably obtained. The four-chamber apical view was adequate for strain analysis in 79/86 (92%) patients. The basal and mid segments tracked more consistently (basal septal 97%; mid septal 94%; basal RV free wall 94%, mid RV free wall 92%) than apical segments (apical septal 55%; apical RV free wall 71%). Overall, the percentage of adequately tracked segments was similar between death/ECMO group (83%) and the control group (84%).
Table 2.
Echocardiographic measurements
| Overall cohort |
Subgroup analysis |
|||||||
|---|---|---|---|---|---|---|---|---|
| Normal range | Survived without ECMO | Death/ECMO | P | Measurement reliability | Survived without ECMO | Death/ECMO | P | |
| N 61 | N 25 | N 86 | N 66 | N 20 | ||||
| RV pressure measures | ||||||||
| TRJV (m/s) | <2.5 | 3.53 (2.20 to 4.56) | 3.73 (2.60 to 5) | 0.40 | 64 (74) | 3.52 (2.2 to 4.56) | 3.77 (2.6 to 5) | 0.19 |
| PDA direction | 78 (91) | |||||||
| Left to right | 8 (13) | 0 (0) | 0.10* | 8 (13) | 0 (0) | 0.19* | ||
| Bidirectional (predominantly left to right) | 26 (40) | 8 (32) | 29 (48) | 5 (28) | ||||
| Predominantly right to left | 21 (33) | 15 (60) | 0.05** | 23 (38) | 13 (72) | 0.02** | ||
| RV/LV ratio | N/A | 1.13 (0.55 to 1.93) | 1.2 (0.49 to 1.92) | 0.52 | 83 (97) | 1.1 (0.49 to 1.93) | 1.2 (0.59 to 1.92) | 0.44 |
| Systolic LVEI | N/A | 1.42 (1 to 2.20) | 1.40 (1.06 to 2.03) | 0.96 | 83 (97) | 1.4 (1 to 2.2) | 1.45 (1.06 to 2.03) | 0.47 |
| Diastolic LVEI | N/A | 1.21 (0.93 to 1.77) | 1.22 (0.93 to 1.55) | 0.99 | 83 (97) | 1.21 (0.93 to 1.77) | 1.23 (1 to 1.55) | 0.88 |
| RV functional measures | ||||||||
| FAC (%) | 0.33 (0.22 to 0.44) | 0.27 (0.05 to 0.56) | 0.19 (0.03 to 0.47) | 0.19 | 80 (93) | 0.27 (0.05 to 0.56) | 0.19 (0.03 to 0.47) | 0.12 |
| TAPSE (mm) | 9.2 (6.4 to 12) | 5.5 (1 to 14) | 3 (1 to 9.7) | 0.002 | 85 (99) | 5 (1 to 14) | 3 (1 to 8.4) | 0.0006 |
| GLPS (%) | >−18 | −11 (−19.7 to −3.2) | −8.8 (−15.7 to −3.1) | 0.03 | 79 (92) | −11 (−19.7 to −3.2) | −8.2 (−14.1 to −3.1) | 0.006 |
| FWLPS (%) | N/A | −12.4 (−27.4 to −2.1) | −13.4 (−27.4 to −2.1) | 0.55 | 79 (92) | −12.4 (29.1 to −2.1) | −13.4 (−23.1 to −3.1) | 0.29 |
| RV combined measure | ||||||||
| Systolic : diastolic ratio | 1.08 (0.56 to 1.6) | 1.31 (0.45 to 2.82) | 1.39 (0.68 to 2.4) | 0.62 | 84 (98) | 1.33 (0.45 to 2.82) | 1.39 (0.68 to 2.4) | 0.76 |
| LV functional measures | ||||||||
| FS (%) | 30–45 | 37 (18.5 to 66.2) | 35 (14.3 to 50) | 0.38 | 86 (100) | 36.6 (18.5 to 66.2) | 35.6 (14.3 to 50) | 0.81 |
| Systolic function | 86 (100) | |||||||
| Hyperdynamic/Normal | 47 (77) | 20 (80) | 1‡ | 52 (79) | 15 (75) | 0.76‡ | ||
| Mild-moderately decreased | 12 (20) | 1 (4) | 12 (18) | 1 (5) | ||||
| Severely decreased | 2 (3) | 4 (16) | 0.06‡‡ | 2 (3) | 4 (20) | 0.02‡‡ | ||
Continuous variables—median (min − max); categorical variables—n (%).
TAPSE, tricuspid annular plane systolic excursion; FAC, fractional area change; TRJV, tricuspid regurgitant jet peak velocity; PDA, patent ductus arteriosus; GLPS, right ventricular global longitudinal peak strain; FWLPS, free wall longitudinal peak strain; RV/LV ratio, left ventricular/right ventricular ratio; LVEI, left ventricular eccentricity index; FS, fractional shortening; N/A, not applicable (not validated in this population).
*Left to right vs. the other two categories (right to left and bidirectional predominantly left to right) combined.
**Right to left vs. the other two categories combined.
‡Hyperdynamic/normal vs. the other two categories combined.
‡‡Severely decreased vs. the other two categories combined.
Inter-observer analysis demonstrated good reliability as evidenced by non-significant differences for TAPSE (P = 0.75), GLPS (P = 0.68), and FWLPS (P = 0.42), and kappa coefficients close to 1 for PDA (κ = 0.91) and LV qualitative function (κ = 0.87).
In the subgroup analysis, death/ECMO patients had more severe qualitative LV dysfunction (Table 2). Otherwise, the results were similar, with increased strength of significance to TAPSE, GLPS, and right-to-left PDA shunt.
Predictive analyses
For the overall cohort, receiver operator curves demonstrated an area under the curve of 0.91 [95% confidence interval (CI) 0.84–0.98] for OI, 0.73 (95% CI 0.61–0.85) for TAPSE, and 0.65 (0.52–0.78) for GLPS. Based on these curves, OI >20, TAPSE <4 mm, and GLPS greater than or equal to −9% (RV global peak strain worse than or equal to −9%) were identified as optimal thresholds (Table 3). Sensitivities were slightly improved in the subgroup analysis. A Kaplan–Meier curve shows lower survival or days of known contact for infants with TAPSE <4 mm (Figure 3).
Table 3.
Receiver operator curves
| Overall cohort |
Subgroup analysis |
|||||||
|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | Sensitivity | Specificity | Positive predictive value | Negative predictive value | |
| OI ≥20 | 76 | 88 | 76 | 88 | 80 | 84 | 64 | 92 |
| TAPSE <4 mm | 56 | 85 | 61 | 82 | 60 | 83 | 52 | 87 |
| GLPS greater than or equal to −9% | 52 | 77 | 48 | 80 | 61 | 77 | 44 | 87 |
| TAPSE <4 mm + R-L PDA | 57 | 91 | 72 | 83 | 61 | 88 | 61 | 88 |
| TAPSE <4 mm + severe LV | 16 | 100 | 100 | 74 | 20 | 100 | 100 | 80 |
| TAPSE <4 mm + OI ≥20 | 44 | 96 | 85 | 77 | 50 | 94 | 77 | 84 |
All values are %.
CI, confidence interval; TAPSE, tricuspid annular plane systolic excursion; GLPS, right ventricular global longitudinal peak strain; R-L PDA, predominantly right-to-left patent ductus arteriosus; Severe LV, qualitative severe LV dysfunction.
Figure 3.
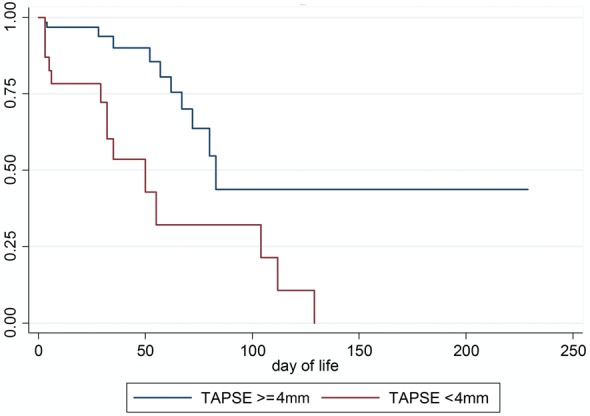
Kaplan–Meier curve for TAPSE 4 mm.
In the entire cohort, 23/86 (27%) had a TAPSE <4 mm, while 14/25 (56%) who met the primary outcome had a TAPSE <4 mm. 18/86 (21%) had the combination of TAPSE <4 mm and a predominantly right-to-left PDA, and 13/18 (72%) of these patients met the primary outcome. 4/6 (67%) patients with qualitative severe LV dysfunction met the primary outcome.
A multivariable logistic regression included OI >20, TAPSE <4 mm, GLPS greater than or equal to −9%, and predominant right-to-left PDA. The odds ratio for OI [30.3 (95% CI 4.75–190)] and TAPSE [29.7 (2.63–335.45)] remained significant when controlling for covariates. The confidence intervals for GLPS and right-to-left PDA were not significant in this analysis (GLPS, 95% CI 0.02–1.71; right-to-left PDA, 0.38–19.43).
Discussion
We found that infants with PPHN who died or required ECMO had elevated OI at the time of echocardiogram, diminished TAPSE and GLPS, and a predominant right-to-left PDA. OI and TAPSE remained significant in a multivariate model. Additionally, we found reasonably predictive characteristics for OI >20, TAPSE <4 mm, and GLPS greater than or equal to −9%. Our subgroup analysis demonstrated that severe LV dysfunction became more prominent in death/ECMO patients. This analysis also demonstrated that the sensitivity and negative predictive value of these measures modestly improved when the echocardiogram occurred within 48 h of decompensation.
PPHN therapeutic options and cohort survival
iNO and HFV have proved to be efficacious in PPHN infants, although a significant minority will still fail conventional ventilation and benefit from ECMO.3,4,17 Cardiorespiratory support was higher in our death/ECMO group, reflecting their worse clinical picture. Survival for infants with idiopathic PPHN treated with ECMO is reported at 81%.18 Survival in our population was lower at 65%, likely due to our inclusion of patients at higher risk for mortality, including those with congenital diaphragmatic hernia and birth asphyxia. Their inclusion did not bias the overall cohort as there was no significant difference in diagnoses between the two groups, and overall cohort survival was similar to the national average.2 The evidence clearly demonstrates the utility of predicting progression to ECMO to facilitate timely management decisions and ensure early transfer to tertiary care centres with access to iNO, HFV, and ECMO.
Echocardiographic measures of RV pressure in PPHN
Evaluations of RV pressure in infants with PPHN have mostly focused on TRJV and PDA direction or velocity. TRJV is elevated in infants with PPHN but does not consistently correlate with clinical outcomes in infants with PPHN.19–22 In our study, TRJV was not significantly different between the two groups. It was measurable in only 73% of patients, as incomplete Doppler envelopes frequently hindered this evaluation. The combination of lack of feasibility and inaccuracy makes TRJV of questionable value in clinical decision-making. A possible substitute for an estimation of pulmonary artery pressure is pulmonary regurgitation, which could be used in future studies.
A predominant right-to-left shunt via the PDA is indicative of suprasystemic pulmonary pressures and pulmonary hypertension.23 Studies that have attempted to correlate PDA direction to outcomes in PPHN infants have found mixed results.12,20,21 We found that a predominantly right-to-left direction was associated with poor outcomes. However, PDA directionality can change dynamically in response to pain/agitation, physical pressure from transducers, or ventilator changes.20 Therefore, a bidirectional shunt with predominant left-to-right direction should not rule out severe disease. Also, the in utero function of the PDA as a pathway to lower resistance may persist after birth, diminishing the right-sided pressure/volume and somewhat ameliorating the severity of PPHN. However, this pop-off effect may not prevent RV failure since placental separation results in a dramatic increase in systemic pressures. Therefore, the RV may have to contend with the higher ex utero systemic vascular resistance while struggling with persistent in utero pulmonary resistance.
Other estimates of RV pressure, including RV/LV ratio and LVEI, have not been well studied in PPHN infants and were not different between groups. Considering the relative RV dominance of foetal life and that RV pressure can remain transiently elevated even in healthy newborns, measurements that rely on the comparative size and geometry of the ventricles may be less reliable indicators of clinical status.
Echocardiographic measures of RV function in PPHN
The evaluation of right heart function in PPHN infants is limited to one study that found FAC was not linked to outcomes.20 RV myocardial performance index, a measure of global cardiac function, may be elevated in infants with PPHN.24 Our echocardiography protocol was not optimized to measure this index retrospectively using tissue Doppler, but future studies should consider its inclusion.
Strain is emerging as a useful tool in adult pulmonary hypertension where RV global longitudinal and free wall strain are diminished.11 Analysis of RV strain in infants is limited to establishing norms and evaluating the post-operative period in congenital heart disease.25,26 In our study, GLPS, but not FWLPS, was significantly diminished in death/ECMO patients. GLPS is more robust vs. FWLPS as it accounts for septal and free wall deformation and likely minimizes regional tracking strain issues. Further study with prospectively optimized images is necessary to evaluate the value of these strain measures in this population.
The predictive characteristics of TAPSE were slightly better than GLPS. Also, TAPSE remained significant in multivariate modelling and demonstrated differences in the Kaplan–Meier curve. TAPSE is a simple and highly robust measure of RV myocardial deformation that is less influenced by imaging artifacts.27 The theoretical benefits of GLPS over TAPSE, the inclusion of both RV free wall and septum, and the freedom from translational and tethering motions are limited to populations with regional heterogeneity. Furthermore, we did not employ strain optimization protocols, and temporal resolution was lower due to the data compression on transfer to the archives. These factors may have led to less accurate strain values that did not affect TAPSE.
Combined echocardiogram measures of function and pressure in PPHN
Systolic : diastolic ratio has been associated with clinical outcomes in older children with pulmonary hypertension,15 but it has not been studied in infants with PPHN. This measure is a complex interplay of RV contractility, preload, afterload, and heart rate. Higher values could be explained by diastolic shortening from cardiac dysfunction and/or systolic lengthening due to elevated afterload. In our study, the ratio was not associated with poor outcomes. The accuracy may vary if an incomplete tricuspid regurgitant jet envelope or inadequate tissue Doppler is obtained, which occurred in 26% of our subjects. The lack of a significant difference in this population also may be reflective of the role that a right-to-left PDA shunt plays in the pathophysiology of PPHN compared with other forms of pulmonary hypertension.
Another approach to a combined function/pressure analysis is to use the pressure and functional markers with the best predictive characteristics. In this study, TAPSE <4 mm and a predominant right-to-left PDA identified 72% of the patients who progressed to ECMO and/or death. While multivariate analysis demonstrated that TAPSE was the only independently associated measure, the addition of PDA direction modestly improved the screening characteristics.
LV function and haemodynamics
We evaluated LV function due to the potential for ventricular interdependence and secondary LV dysfunction leading to poor outcomes.20,21 Death/ECMO patients were more likely to require pressors and have a higher OI, although the inclusion of OI in ECMO guidelines provides a strong selection bias.17 LV qualitative function was inadequate in predicting decompensation as evidenced by no difference in the overall cohort, although this difference was significant in the subgroup analysis. The 100% specificity and positive predictive value of the combination of TAPSE <4 mm and severe LV dysfunction suggests patients with LV dysfunction in the setting of RV dysfunction will likely need increased support, but the sensitivity was poor. Although FS is quantitative, the abnormal septal geometry in PPHN makes it less reliable than qualitative LV systolic function. An alternative is 3D ejection fraction, although high neonatal heart rates and low frame rates could lead to inaccuracies.
Limitations
This study was a retrospective, observational analysis with a limited number of primary outcomes, and was not adequately powered to find small differences. Although the subgroup analysis suggests some benefit to repeat echocardiograms, future studies would need to validate our findings in closer proximity to outcomes. While the addition of OI added useful clinical information, it also presents a selection bias as OI is a guideline for ECMO initiation. Patient agitation and/or pain may have resulted in transient increases in pulmonary pressures thereby altering echocardiographic measurements, but documenting or quantifying this effect was not feasible. Z-scores based on gestational age have been established for TAPSE.27 Although our TAPSE cut-off was below the 95% CI of any age infant in our cohort, even when considering that 2D measurements are ∼1 mm less than M-mode,14 a larger study may be powered to allow for gestational age appropriate Z-scores.
RV strain analysis was performed using a single apical view rather than the newly reported comprehensive RV analysis using three RV apical views.28 Images were not optimized for strain analysis, which disproportionately affected apical segments. Tomtec VVI software has the limitation of analysing studies stored at the lower frame rate of 30 frames per second. Prior studies that analysed strain in infants and children using Tomtec VVI at 30 frames per second found reproducible results that were validated against higher rates.29,30 Although further study is required to completely validate this frame rate, the lower rate should underestimate peak strain, meaning the difference seen in GLPS in this study would by reinforced at higher frame rates.
Conclusions
This study provides evidence that OI >20, TAPSE <4 mm, GLPS greater than or equal to −9%, and a predominantly right-to-left PDA shunt is associated with poor outcomes in infants with PPHN. However, transfer should not be delayed for patients who do not meet these thresholds but are still thought to be at high risk for poor outcome. Repeated echocardiographic assessment with concurrent OI in infants with a persistently concerning clinical picture may identify patients with initially reassuring markers who subsequently meet the cut-offs. A larger prospective trial could validate a model using a combination of RV pressure and functional markers as predictors of poor outcome to assist in the decisions to intensify cardiovascular support, transfer patients to ECMO centres, and ECMO initiation.
Funding
P.B.S. receives salary support for research from the National Institutes of Health and the National Center for Advancing Translational Sciences (HHSN267200700051C, HHSN275201000003I, and UL1TR001117); he also receives research support from industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp).
Acknowledgements
The authors acknowledge the assistance of the staff of the Duke Pediatric Echocardiographic Laboratory and the Intensive Care Nursery in this project.
Conflict of interest: None declared.
References
- 1.Puthiyachirakkal M, Mhanna MJ. Pathophysiology, management, and outcome of persistent pulmonary hypertension of the newborn: a clinical review. Front Pediatr 2013;1:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh-Sukys MC, Tyson JE, Wright LL, Bauer CR, Korones SB, Stevenson DK, et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics 2000;105(1 Pt 1):14–20. [DOI] [PubMed] [Google Scholar]
- 3.Finer NN, Barrington KJ. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst Rev 2006:CD000399. [DOI] [PubMed] [Google Scholar]
- 4.Kennaugh JM, Kinsella JP, Abman SH, Hernandez JA, Moreland SG, Rosenberg AA. Impact of new treatments for neonatal pulmonary hypertension on extracorporeal membrane oxygenation use and outcome. J Perinatol 1997;17:366–9. [PubMed] [Google Scholar]
- 5.Janda S, Shahidi N, Gin K, Swiston J. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart 2011;97:612–22. [DOI] [PubMed] [Google Scholar]
- 6.Mourani PM, Sontag MK, Younoszai A, Ivy DD, Abman SH. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics 2008;121:317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groh GK, Levy PT, Holland MR, Murphy JJ, Sekarski TJ, Myers CL, et al. Doppler echocardiography inaccurately estimates right ventricular pressure in children with elevated right heart pressure. J Am Soc Echocardiogr 2014;27:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinsella JP, McCurnin DC, Clark RH, Lally KP, Null DM., Jr Cardiac performance in ECMO candidates: echocardiographic predictors for ECMO. J Pediatr Surg 1992;27:44–7. [DOI] [PubMed] [Google Scholar]
- 9.Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr 2010;23:465–95; quiz 576–7. [DOI] [PubMed] [Google Scholar]
- 10.Forfia PR, Vachiery JL. Echocardiography in pulmonary arterial hypertension. Am J Cardiol 2012;110(6 Suppl):16S–24S. [DOI] [PubMed] [Google Scholar]
- 11.Rajagopal S, Forsha DE, Risum N, Hornik CP, Poms AD, Fortin TA, et al. Comprehensive assessment of right ventricular function in patients with pulmonary hypertension with global longitudinal peak systolic strain derived from multiple right ventricular views. J Am Soc Echocardiogr 2014;27:657–65 e3. [DOI] [PubMed] [Google Scholar]
- 12.Gotteiner NL, Harper WR, Gidding SS, Berdusis K, Wiley AM, Reynolds M, et al. Echocardiographic prediction of neonatal ECMO outcome. Pediatr Cardiol 1997;18:270–5. [DOI] [PubMed] [Google Scholar]
- 13.Jone PN, Hinzman J, Wagner BD, Ivy DD, Younoszai A. Right ventricular to left ventricular diameter ratio at end-systole in evaluating outcomes in children with pulmonary hypertension. J Am Soc Echocardiogr 2014;27:172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qureshi MY, Eidem BW, Reece CL, O'Leary PW. Two-dimensional measurement of tricuspid annular plane systolic excursion in children: can it substitute for an m-mode assessment? Echocardiography 2015;32:528–34. [DOI] [PubMed] [Google Scholar]
- 15.Alkon J, Humpl T, Manlhiot C, McCrindle BW, Reyes JT, Friedberg MK. Usefulness of the right ventricular systolic to diastolic duration ratio to predict functional capacity and survival in children with pulmonary arterial hypertension. Am J Cardiol 2010;106:430–6. [DOI] [PubMed] [Google Scholar]
- 16.Louie EK, Lin SS, Reynertson SI, Brundage BH, Levitsky S, Rich S. Pressure and volume loading of the right ventricle have opposite effects on left ventricular ejection fraction. Circulation 1995;92:819–24. [DOI] [PubMed] [Google Scholar]
- 17.Elbourne D, Field D, Mugford M. Extracorporeal membrane oxygenation for severe respiratory failure in newborn infants. Cochrane Database Syst Rev 2002:CD001340. [DOI] [PubMed] [Google Scholar]
- 18.Lazar DA, Olutoye OO, Cass DL, Fernandes CJ, Welty SE, Johnson KE, et al. Outcomes of neonates requiring extracorporeal membrane oxygenation for irreversible pulmonary dysplasia: the Extracorporeal Life Support Registry experience. Pediatr Crit Care Med 2012;13:188–90. [DOI] [PubMed] [Google Scholar]
- 19.Skinner JR, Stuart AG, O'Sullivan J, Heads A, Boys RJ, Hunter S. Right heart pressure determination by Doppler in infants with tricuspid regurgitation. Arch Dis Child 1993;69:216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson AL, Deatsman S, Frommelt MA, Mussatto K, Frommelt PC. Correlation of echocardiographic markers and therapy in persistent pulmonary hypertension of the newborn. Pediatr Cardiol 2009;30:160–5. [DOI] [PubMed] [Google Scholar]
- 21.Fraisse A, Geva T, Gaudart J, Wessel DL. Doppler echocardiographic predictors of outcome in newborns with persistent pulmonary hypertension. Cardiol Young 2004;14:277–83. [DOI] [PubMed] [Google Scholar]
- 22.Skinner JR, Hunter S, Hey EN. Haemodynamic features at presentation in persistent pulmonary hypertension of the newborn and outcome. Arch Dis Child Fetal Neonatal Ed 1996;74:F26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musewe NN, Poppe D, Smallhorn JF, Hellman J, Whyte H, Smith B, et al. Doppler echocardiographic measurement of pulmonary artery pressure from ductal Doppler velocities in the newborn. J Am Coll Cardiol 1990;15:446–56. [DOI] [PubMed] [Google Scholar]
- 24.Patel N, Mills JF, Cheung MM. Use of the myocardial performance index to assess right ventricular function in infants with pulmonary hypertension. Pediatr Cardiol 2009;30:133–7. [DOI] [PubMed] [Google Scholar]
- 25.Petko C, Uebing A, Furck A, Rickers C, Scheewe J, Kramer HH. Changes of right ventricular function and longitudinal deformation in children with hypoplastic left heart syndrome before and after the Norwood operation. J Am Soc Echocardiogr 2011;24:1226–32. [DOI] [PubMed] [Google Scholar]
- 26.Pena JL, da Silva MG, Faria SC, Salemi VM, Mady C, Baltabaeva A, et al. Quantification of regional left and right ventricular deformation indices in healthy neonates by using strain rate and strain imaging. J Am Soc Echocardiogr 2009;22:369–75. [DOI] [PubMed] [Google Scholar]
- 27.Koestenberger M, Nagel B, Ravekes W, Urlesberger B, Raith W, Avian A, et al. Systolic right ventricular function in preterm and term neonates: reference values of the tricuspid annular plane systolic excursion (TAPSE) in 258 patients and calculation of Z-score values. Neonatology 2011;100:85–92. [DOI] [PubMed] [Google Scholar]
- 28.Forsha D, Risum N, Kropf PA, Rajagopal S, Smith PB, Kanter RJ, et al. Right ventricular mechanics using a novel comprehensive three-view echocardiographic strain analysis in a normal population. J Am Soc Echocardiogr 2014;27:413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bharucha T, Khan R, Mertens L, Friedberg MK. Right ventricular mechanical dyssynchrony and asymmetric contraction in hypoplastic heart syndrome are associated with tricuspid regurgitation. J Am Soc Echocardiogr 2013;26:1214–20. [DOI] [PubMed] [Google Scholar]
- 30.Koopman LP, Slorach C, Manlhiot C, McCrindle BW, Jaeggi ET, Mertens L, et al. Assessment of myocardial deformation in children using Digital Imaging and Communications in Medicine (DICOM) data and vendor independent speckle tracking software. J Am Soc Echocardiogr 2011;24:37–44. [DOI] [PubMed] [Google Scholar]