Abstract
Background
The decreasing effectiveness of antimicrobial agents is a growing global public health concern. Low- and middle-income countries (LMIC) are vulnerable to the loss of antimicrobial efficacy given their high burden of infectious disease and the cost of treating resistant organisms.
Methods
We analyzed data from the World Health Organization’s Antibacterial Resistance Global Surveillance Report. We investigated the importance of out-of-pocket spending and copayment requirements for public sector medications on the level of bacterial resistance among LMIC, adjusting for environmental factors purported to be predictors of resistance, such as sanitation, animal husbandry and poverty as well as other structural components of the health sector.
Findings
Out-of-pocket health expenditures were the only factor demonstrating a statistically significant relationship with antimicrobial resistance. A ten point increase in the percentage of health expenditures that were out-of-pocket was associated with a 3·2 percentage point increase in resistant isolates [95% CI, 1·17 to 5·15, p-value=0·002]. This relationship was driven by countries requiring copayments for medications in the public health sector. Among these countries, moving from the 20th to 80th percentile of out-of-pocket health expenditures was associated with an increase in resistant bacterial isolates from 17·76 [95%CI 12·54 to 22·97] to 36·27 percentage points [95% CI 31·16 to 41·38].
Interpretation
Out-of-pocket health expenditures were strongly correlated with antimicrobial resistance among LMIC. This relationship was driven by countries that require copayments on medications in the public sector.
Our findings suggest cost-sharing of antimicrobials in the public sector may drive demand to the private sector where supply-side incentives to overprescribe are likely heightened and quality assurance less standardized.
Keywords: Antimicrobial Resistance, Out-of-pocket spending, Copayment
Introduction
Antimicrobial resistance is a growing global public health challenge that could undo decades of progress in declining morbidity and mortality from infectious diseases. Common bacterial pathogens have increasingly developed resistance to most of the currently available antibiotics. This phenomenon, coupled with a dry antibiotic pipeline, has led the World Health Organization (WHO) to warn of a “post-antibiotic era, in which common infections and minor injuries can kill.”1
Resistant organisms are more difficult to treat and are associated with higher morbidity and mortality than their susceptible counterparts.2,3 The US Centers for Disease Control and Prevention (CDC) estimates that at least 2 million illnesses and 23000 deaths a year in the USA were caused by antibiotic resistance.4 The economic burden of antimicrobial resistance is difficult to calculate due to insufficient data and the need to account for externalities.5 However, estimates of the impact of antimicrobial resistance on the US economy are exceedingly high, including $20 billion (2008 dollars) in direct health care costs with additional indirect costs as high as $25 billion per year.4
The concern over rising antimicrobial resistance is not limited to the developed world. Okeke et al. document accelerating rates of resistance among enteric, respiratory and sexually transmitted pathogens in developing countries.6
Several factors have been proposed as contributing to the spread of resistance in developing countries. The use of antimicrobial agents for growth promotion in animal husbandry may lead to the spread of antimicrobial resistance when humans consume or are in direct contact with livestock.7–9 Socioeconomic status has also been shown to have an influence on what antibiotic agents are prescribed and posited to be associated with resistance.10–12 Byarugaba points to a direct influence of poverty on antimicrobial use, whereas others suggest that “rising incomes in low-income and middle-income countries” may be an important factor.13,14 Okeke et al. review linkages between poverty and resistance identifying several plausible pathways.15,16 First, those living in developing countries are more exposed to infectious diseases and may be more susceptible due to malnutrition or immunodeficiency, therefore have a greater need for antimicrobial therapy. Second, impoverished individuals may be more at risk to being exposed to sub-inhibitory dosages of antimicrobial agents since poverty may encourage shorter courses of therapy, medication sharing or use of lower quality or expired medications.17 Third, access to appropriate medical care may be more limited in developing countries, thus encouraging individuals to self-medicate or seek care from less tightly regulated, for-profit providers.
Out-of-pocket health expenditures are a major source of health care financing in the developing world. Pharmaceutical purchases (including antimicrobial agents) constitute an estimated 70% of out-of-pocket health expenditures in India and 43% in Pakistan.18–20 In the sample of low and middle income countries (LMIC) used in our main analysis, described below, on average 49% of health expenditures are private. The majority of private health expenditures (76%) are out-of-pocket. Consistent with other reports, the majority of out-of-pocket expenditures in low- and middle-income countries (63%) were for medications.21
Traditionally, cost-sharing in the form of copayments has been viewed as a way to curtail the overuse of medical care. However, in many low- and middle-income countries, copayments in the public sector may have an unintended consequence. Most developing economies have a robust informal private healthcare sector which operates alongside the more traditional public health sector. If the public and private health sectors serve as substitutes for one another to some degree, the prediction from consumer theory is that raising the price (via a higher copayment) in the public health sector for medication will shift more consumers into the private sector, depending upon the elasticity of substitution and transaction costs associated with purchase in the public sector.22 Motivated by this theoretical prediction, we hypothesize that copayments in the public sector promote the development of antibiotic resistance by inducing patients to purchase antibiotic treatment from less well-regulated private providers who have financial incentives to inappropriately prescribe antibiotics, offer truncated courses of treatment, or use lower quality formulations. Even if total consumption of antibiotics were unchanged, the shift of more patients to less-regulated providers could lead to more antibiotic resistance. We develop a mathematical economic model to demonstrate this point and include it as part of the Appendix.
Because of the lack of available data, little empirical work has been done to assess the relative importance of out-of-pocket payments and copayments on antimicrobial resistance in the developing world.14,16 We use a recently published data set collected by the WHO to assess the role of such payments, while adjusting for other key proposed predictors--including poverty, livestock production, sanitation, and institutional features of the country-specific health sector--on the prevalence of antimicrobial resistance across a sample of low- and middle-income countries. While the causes of antimicrobial resistance are complex, our analysis supports an increasing role of the public sector in regulating and subsidizing the distribution of antimicrobial agents.
METHODS
Data Sources
The principal data source for antimicrobial resistance is the WHO Antimicrobial Resistance: Global Report on Surveillance (2014) Annex 2, Reported or Published Resistance Rates in Common Bacterial Pathogens.1 The report was released in April 2014 and represented the “first attempt by WHO to assemble information on national ABR [antibacterial resistance] surveillance and on ABR data for a set of common pathogenic bacteria (p. XIX)”. The WHO sent questionnaires to Member States, 129 responded and 114 provided data. The questionnaire was designed to probe each country on the prevalence of nine bacteria-antimicrobial resistance combinations including: Escherichia coli: Resistance to third-generation cephalosporins, Escherichia coli: Resistance to fluoroquinolones, Klebsiella pneumoniae: Resistance to third-generation cephalosporins, Klebsiella pneumoniae: Resistance to carbapenems, Staphylococcus aureus: Resistance to methicillin (MRSA), Streptococcus pneumoniae: Resistance, or non-susceptibility, to penicillin, Nontyphoidal Salmonella (NTS): Resistance to fluoroquinolones, Shigella species: Resistance to fluoroquinolones, Neisseria gonorrhoeae: Decreased susceptibility to third-generation cephalosporins. If data from national sources were incomplete or unavailable, the WHO accessed data from national and international surveillance networks. If data from these two sources combined were still incomplete (< 30 isolates tested), the WHO sought data from the academic literature by using scientific journal articles published after 2007 to further broaden the sample. For further details on the WHO methodology, see Annex 1 of the Report. Our outcome variable of interest is the percent of bacterial isolates tested that demonstrated resistance to a class of antimicrobial agents. In particular, we compute the average percent of isolates that demonstrate antibiotic resistance for a given bacteria-antibacterial combination in a given country.
Data on poverty (the percent poverty gap at $2 a day (PPP)), sanitation (the percent of population with access to improved sanitation facilities), the percent of health expenditures that are paid out-of-pocket, livestock and hospital bed density are taken from the World Bank Indicators.23 The livestock production index includes meat, dairy products and other derivatives directly from livestock.
We also include data on copayments for medications in the public sector. In sensitivity analyses reported in the Appendix, we include other features of the health sector such as copayments on consultations, physician density and whether private providers can dispense medications. Physician density was taken from the World Bank Indicators. The other variables listed were taken from the WHO Pharmaceutical Sector Country Profile Reports.24 The variables included in the analysis are indicator variables equal to one if a copayment is required or dispensing by private MDs is admissible, and zero otherwise.
Our sample includes 47 countries [23 in Africa, 8 in the Americas, 3 in Europe, 8 in the Middle East, 3 in Southeast Asia, and 2 in the Western Pacific]. A list of countries included in the analysis is presented in Appendix Table A1. A data appendix with sources and precise definitions of all variables is provided in Appendix Table A2.
Statistical Analysis
The data set for the main analysis is a longitudinal panel of bacteria-antimicrobial resistance pairs at the country level. Bacterial-antimicrobial resistance combinations were aggregated within country across data sources using the arithmetic average.
Because the threshold to develop antimicrobial resistance against a given agent varies across pathogens, we modelled these differences using bacteria-antimicrobial combinations and region-specific slopes (holding constant fixed effects, a set of nine indicator variables, one for each of the nine bacteria-antimicrobial combinations and a set of five indicator variables for each of the different regions).25 Statistical testing rejected the hypothesis that these dummy variables were not confounders in understanding the association between environmental and healthcare predictors and resistant bacterial isolates (F-test χ2 =23·46 p<0·001).
It is important to emphasize our main findings are not identified on the basis of comparisons of different bacteria against each other. The inclusion of indicator variables for each bacterial-antibacterial pair in our regression specifications allows us to statistically identify the correlation between the percent of health expenditures that are out-of-pocket and the percent of isolates that are resistant, within (not across) a given bacterial-antibacterial pair.
Country-level linear regression models were estimated with percent of tested isolates that were resistant to a given antimicrobial agent as the outcome. Multivariate regression models added environmental and structural healthcare features thought to influence antimicrobial resistance.
Our basic linear fixed effects statistical model was thus:
where b is bacteria-antibacterial resistance pair, c is country and r represents region. The outcome of interest is the percent of bacterial isolates tested that are classified as resistant. X is a column vector of socioeconomic and environmental variables that vary at the country-level and have been implicated as factors that are accelerating resistance such as sanitation, livestock and poverty. Standard errors are clustered by country to reflect the fact that countries were not sampled independently.
In the Appendix, we include results that weight by population (Table A3) and weight the outcome by number of isolates (Table A4) as well as adding additional healthcare characteristics such as physician density, log of income per capita, and log of total health expenditures (Table A5). We also collapse over bacteria-antimicrobial combinations to the country level (Table A6) Finally, as mentioned above we expand our sample to all countries with data on resistance and the World Development Indicators although they lack data on copayment structure in Table A7. In Table A8 we substitute country for region indicator variables in the regresson above so that we are estimating the relationship between out-of-pocket expenditures and resistance using only within-country variation. Our results are not sensitive to these changes in the main specification.
In addition, we also ran separate regressions with resistance isolates for each bacteria as the dependent variable (rather than the index resistance measure we describe above).
Data were analyzed using Stata version 12·1 (College Park, TX). The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Summary statistics (mean and standard deviation) are displayed in Table 1. The first column represents summary statistics for the entire sample of countries. The second column represents summary statistics for observations in the lowest 50th percentile of antimicrobial resistance, whereas the third column represents summary statistics for those countries in the 50th percentile and above. The median antimicrobial resistance among isolates in our sample of countries is 15·0%.
Table 1.
Summary Statistics Grouped by Median Antimicrobial Resistance.
| All Groups (1) | Resistance<Median (2) | Resistance>=Median (3) | p-value on the difference between cols (3)–(2) (4) | |
|---|---|---|---|---|
| Out-of-pocket Expenditures (%of Total Health Expenditures) | 35·87 | 33·01 | 38·58 | 0·038 |
| (16·79) | (15·88) | (17·24) | ||
| Sanitation (% of Population with Access to Improved Facilities) | 52·45 | 49·20 | 55·53 | 0·116 |
| (28·21) | (28·06) | (28·11) | ||
| Poverty Gap (% <$2 a day) | 44·48 | 47·06 | 42·03 | 0·240 |
| (29·49) | (29·78) | (29·12) | ||
| Livestock Index | 125·67 | 124·68 | 126·61 | 0·574 |
| (18·41) | (21·28) | (15·23) | ||
| Hospital Bed Density | 19·78 | 19·78 | 19·77 | 0·996 |
| (19·30) | (18·78) | (19·85) |
Notes: The mean and standard deviation (in parentheses) are shown. Column (1) demonstrates the summary statistics for the entire sample. Column (2) represents the summary statistics for observations that are below the median for percent of bacterial isolates that are resistant. Column (3) represents the summary statistics for observations that are at or above the median for percent of bacterial isolates that are resistant. Column (4) reports the p-value on the difference between the columns (2) and (3).
Livestock production, hospital bed density and poverty are not statistically different across the two groups. The percent of health expenditures that are out-of-pocket is statistically higher among the upper 50th percentile of antimicrobial resistance. To explore whether these simple differences remain statistically significant conditional on other factors, we use multivariate regression.
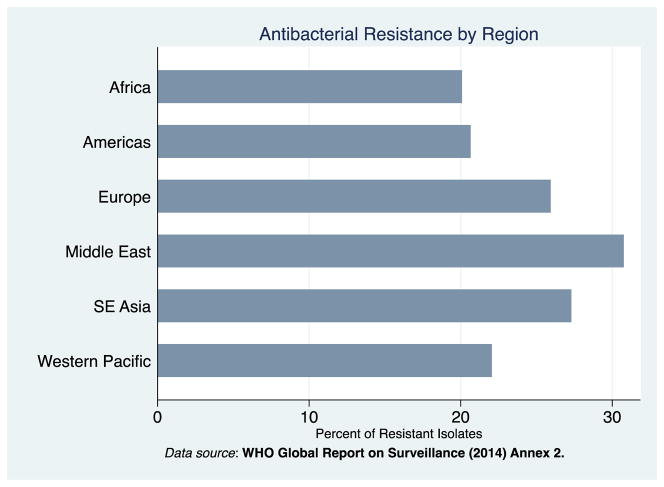
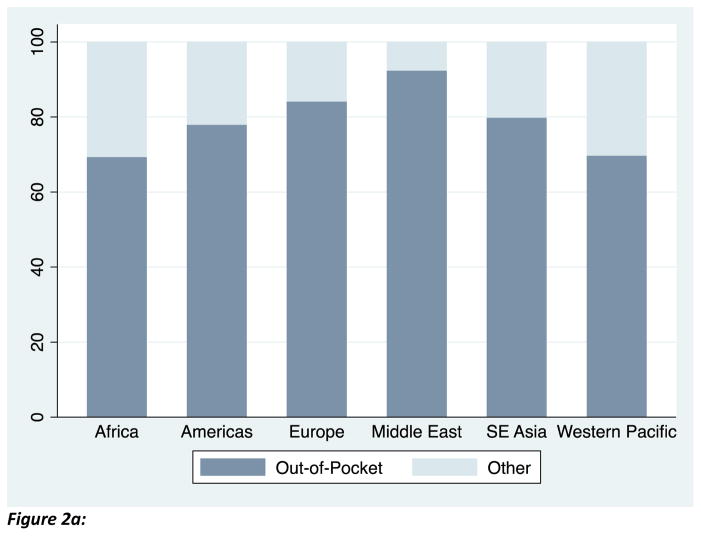
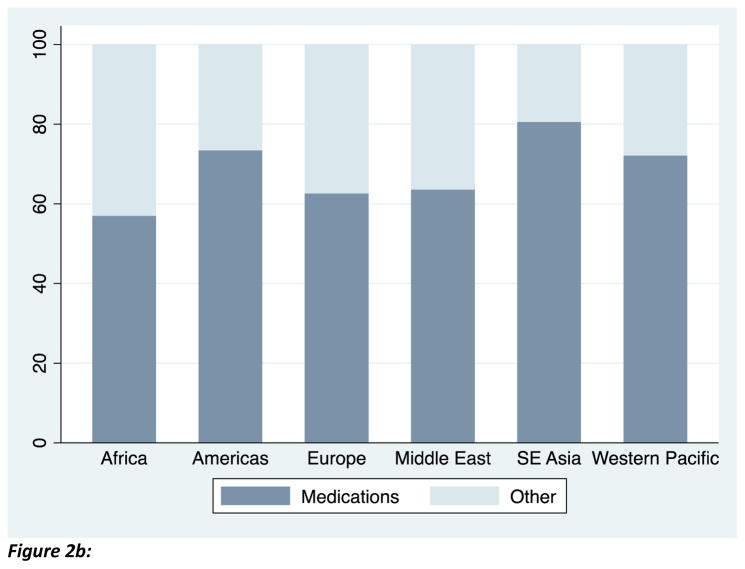
The mean of the outcome variable, percent of bacterial isolates that are resistant, are graphed in Figure 1: the figure demonstrates that resistance exceeds 20 percent in each region. Among our sample of countries, private health expenditures comprise approximately half of total health expenditures on average. Figure 2a demonstrates that the majority of these private health expenditures are out-of-pocket. Consistent with other reports, Figure 2b shows that the majority of out-of-pocket payments in low- and middle-income countries were spent on medications.21
Figure 1. Antimicrobial Resistance by Region.
Antimicrobial resistance is calculated as the mean over data sources, bacterial-antimicrobial combinations, and over low- and middle-income countries within a given region. See appendix for formula. Source: WHO Antimicrobial resistance global report on surveillance, 2014.
Figure 2.
Figure 2a. Out-of-pocket health expenditure (as a percent of private health expenditures) are an important source of health care financing. Source: World Bank, 2012.
Figure 2b: Out-of-pocket spending on medications comprises the majority of out-of-pocket health expenditures. Source: WHO World Health Survey, 2003
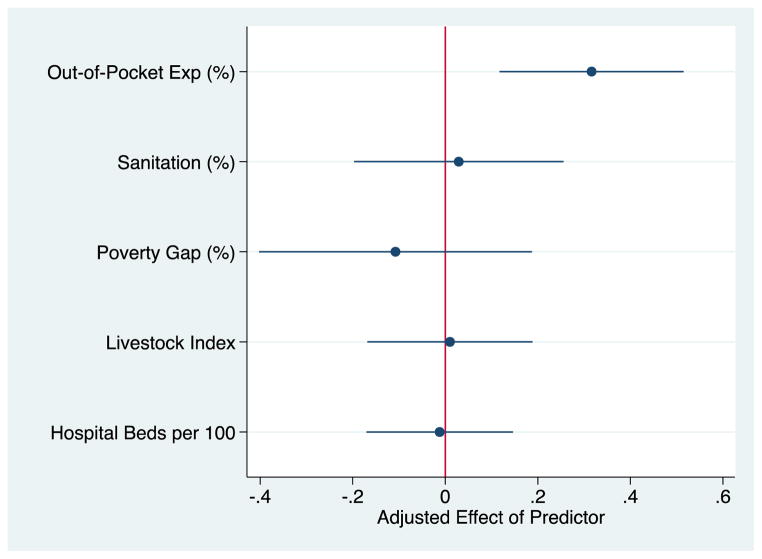
The results of the multivariate analysis are presented in Figures 3 and 4. Figure 3 represents the partial effect of a given predictor variable on anti-bacterial resistance after adjustment for other variables. There is a positive relationship between out-of-pocket spending on healthcare and antimicrobial resistance. A ten point increase in the percentage of health expenditures that were out-of-pocket was associated with a 3·2 percentage point increase in resistant bacteria [95% CI, 1·17 to 5·15, p-value=0·002], representing approximately 15 percent of the sample average of resistance. (This estimate is directly taken from the regression results presented in Figure 3, and is obtained by multiplying the adjusted effect of the percent of health expenditures out-of-pocket on antibiotic resistance and its confidence interval by ten to represent a ten, as opposed to a one, unit increase). None of the other predictor variables (including sanitation) are statistically significant.
Figure 3. Out-of-pocket expenditures (as a % of Total Health Expenditures) predict Antimicrobial Resistance in low- and middle- income countries, 2008–2012.
Coefficients are presented from the regression model specified in equation 1. Model estimated as mentioned in the Methods section, adjusts for poverty, livestock, sanitation, hospital bed density, region and bacteria-antimicrobial combination fixed effects. Error bars are 95% CIs based on robust standard errors clustered by country to reflect non-independence of sampling. Sources: World Bank, 2012 & WHO Antimicrobial resistance global report on surveillance, 2014.
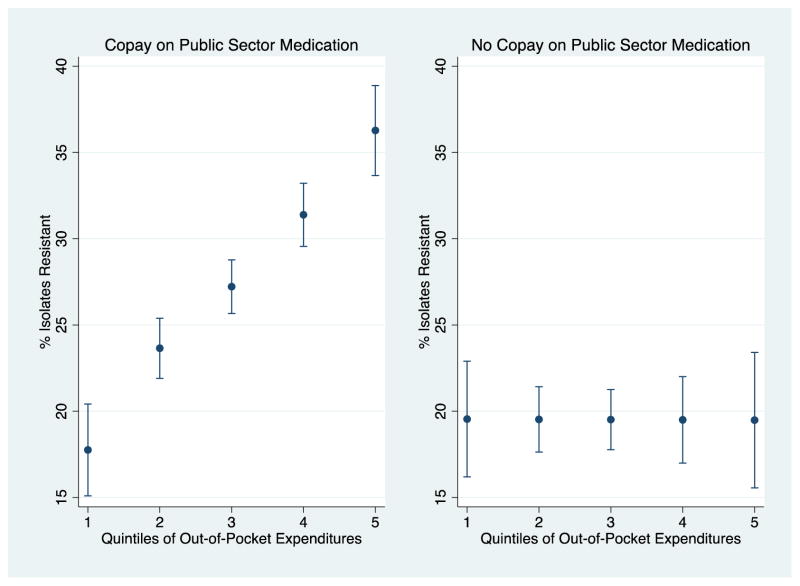
Figure 4. Out-of-pocket Health Expenditures (as a percent of Total Health Expenditure) exhibits a dose-response relationship with antimicrobial resistant isolates only in the presence of public sector copayments on medications.
The figure plots percent of isolates that demonstrate resistance predicted at the following quintiles of out-of-pocket health expenditure [1=6–20%, 2=21–32%, 3=34–41%, 4=41–49%, 5=50–72%]. The left hand side figure is restricted to the sample of countries requiring a copayment on medications in the public sector (N=23). The right hand side figure is restricted to the sample of countries not requiring a copayment on medications in the public sector (N=24). Sources: World Bank, 2012 & WHO Antimicrobial resistance global report on surveillance, 2014.
In Table 2 we report estimates from regressions with percent resistant isolates for each separate organism as the outcome variable. These estimates are imprecise due to small sample sizes. Nevertheless, the marginal effect of the percent of health expenditures that are out-of-pocket was significant and statistically indistinguishable from that provided using the summary resistance index measure presented in Figure 3 in all but one instance. (The p-value for the Wald test for equality rejects the null hypothesis that the point estimates are indistinguishable from the regression specification using the summary measure in 5 of 6 cases and is reported in row 4). The one exception is for the category which has the smallest number of observations (N=21), Gonorrhea resistant to Fluoroquinolones. We believe this provides a strong justification for the summary index and we limit further analyses to the summary measure.
Table 2.
The Correlation of Out-of-Pocket Health Expenditures (as a Percent of Total Health Expenditures) on Resistant Isolates: Analysis by Organism.
| Dependent variable varies across column headings | Methicillin-Resistant Staphyloccus Aureus | Escherichia coli | Klebsiella pneumoniae | Nontyphoidal Salmonella | Shigella species | Gonorrhea-Resistant Flouroquinolones |
|---|---|---|---|---|---|---|
| Out-of-Pocket Exp (%) | 0.309 | 0.608** | 0.107 | 0.016 | 0.168 | −0.0005 |
| 95% CI of Estimate | [−0.156–0.773] | [0.157–1.06] | [−0.385–0.600] | [−0.461–0.492] | [−0.058–0.393] | [−0.004–0.040] |
| No. Observations | 36 | 79 | 58 | 33 | 25 | 21 |
| p-value of Wald Test of Equality with Basline Coefficient Estimate | 0.974 | 0.199 | 0.396 | 0.209 | 0.189 | <0.001 |
Notes: OLS was used to estimate the association between out-of-pocket health expenditures and percent resistant isolates for each separate organism as the outcome (the organisms are given as column headings). All models include the variables in Equation 1 of the main analysis (e.g., livestock, sanitation, poverty, hospital bed density). A Wald test of equality between the separate regression coefficient estimates and the preferred specification fails to reject the null for all but one instance. The results presented above are very similar to the preferred estimates use the summary index. Robust standard errors clustered at the country level are in brackets are reported below the coefficient estimate.
p<0·10
p<0·05
p<0·001.
Figure 4 further probes the relationship between out-of-pocket health expenditures and resistance. In the left panel, the sample of countries that require a copayment for medications in the public sector (N=23) are included. In the right panel, the sample of countries that do not require a copayment for medications in the public sector (N=24) are included. Among countries that require copayments, moving from the 20th to the 80th percentile of out-of-pocket health expenditures was associated with an increase in the bacterial isolates that were resistant from 17·76 [95%CI 12·54 to 22·97] to 36·27 [95% CI 31·16 to 41·38] percentage points. Among countries that had no such requirement, moving from the 20th to the 80th percentile of out-of-pocket health expenditures was not associated with a change in the percentage of bacterial isolates that were resistant: 19·54 [95%CI 12·97 to 26·12] to 19·48 [95% CI 11·78 to 27·18]. Appendix Table A9, an analysis conducted at the country level to investigate factors that vary at that level, demonstrates that copayments for medication strongly positively predict the percent of health expenditures that are out-of-pocket, whereas copayments on consultation do not. This could reflect the fact that medications appear to account for the majority of out-of-pocket payments for health in LMIC (Figure 1); it is difficult to know for certain how much of this out-of-pocket expenditure increase represents quantity versus price.
One threat to the validity of our analysis is that copayments in the public sector are not randomly assigned. It is plausible that the level of public copayments is correlated with other, unobserved determinants of antibiotic resistance thus altering the interpretation of our results, For instance, it could be the case that countries that have low levels of infection control, poor infrastructure, or inadequately trained personnel for healthcare more generally, and thus more antibiotic resistance, require higher copayments in their public sector clinics as a way to supplement their underfunded and overall poor healthcare system. Thus, the omitted variable could be infection control practices or hospital infrastructure or aggregate (public and private) health expenditures.
Although we cannot randomize copayments on medication in the public sector across countries, we checked whether countries with copayments are statistically indistinguishable from those without them. Put differently, we assessed whether countries without public sector copayments were more likely to have poor sanitation, high levels of poverty, large numbers of livestock, a high density of hospitals, etc. (since countries with these latter characteristics are a priori more likely to have high levels of antibiotic resistance).
The results in Appendix Table A10 are not supportive of that view—none of the predictors of antibiotic resistance are correlated with the presence of copayment and there is no difference between the marginal effects between the two groups. These results suggest countries with copayments on medications in the public sector are not observationally different than countries that do not have copayments.
Discussion
Controlling the spread of resistant bacterial pathogens is an urgent global public health priority. Our work highlights an underappreciated policy lever to address this problem – rolling back cost-sharing arrangements for medications in the public sector. In our study, we find that out-of-pocket health expenditures are statistically more important than any other country-level environmental factors – including poverty, livestock production, access to sanitation and other institutional features of the health sector – in predicting patterns of antimicrobial resistance across low-and middle-income countries. Importantly, we find the relationship between out-of-pocket expenditures and resistance is driven by countries that require a copayment for medication in the public sector.
Though no previous research has examined the relationship between out-of-pocket payments and antibiotic resistance in low- and middle-income countries, our findings are consistent with the work of researchers who have found supplier-induced demand is an important determinant for excess use of healthcare.26–28 Data from the European Surveillance of Antimicrobial Consumption Network (ESAC-Net) demonstrate a link between higher outpatient antimicrobial use and antimicrobial resistance.29 Although public use data on antibiotics consumption in LMIC are lacking and coverage by private vendors is incomplete, there exists a positive and significant correlation (correlation coefficient =0.29 and p<0*0001) between out-of-pocket health expenditures and antibiotic consumption among ESAC-Net countries.
To our knowledge, we are the first to emphasize the idea that copayments imposed in the public sector of a health care system lead to overuse of a medication or product in the private sector. Conventional teaching in health economics – which focuses on their effect on the demand for care within a single insurance system – is that copayments tend to discourage use. The most prominent and convincing evidence for this was the RAND health insurance experiment (HIE) conducted in over six US cities on 2000 households, the increase in copayment associated with care led to a significant decline in use of antibiotics (Newhouse, 1993), providing evidence that the demand for health care is not completely inelastic.30
However, when there are two sectors selling the same or similar products, an increase in the price of one does not necessarily reduce overall quantity demanded, and may in fact increase resistance for the same overall consumption level if consumption in the private sector leads to greater resistance. To make this point clear, we have developed a formal model, which we include in the appendix.
In brief, in a spatial differentiation model, patients can substitute between the two sectors according to their own convenience or transaction costs. Heterogeneity among consumers--in terms of willingness and ability to trade off travel, wait time, and uncertainty about quality--lead to some demand in each sector even with different out-of-pocket prices for exactly the same product. An increase in one price may simply shift the marginal consumer from one sector to the other. For example, increasing the co-payment in the public sector, on top of travel and waiting costs, may push consumers to patronize ubiquitous private pharmacies instead.
More interestingly, total resistance may increase even if total consumption stays the same or decreases (up to a point), if the resistance generated per pill is sufficiently higher in the private sector – a plausible assumption, given that truncated courses of treatment are common in the private sector, but drive resistance.
There are important limitations regarding the results presented. First, the data on antimicrobial resistance across developing countries suffers from reporting bias and was most likely derived from hospital settings. Although reporting bias is a problem for all comparative health analyses, it is likely to be particularly problematic for our outcome of interest. We have attempted to mitigate reporting bias by including indicator variables for regions. The multivariate regression analysis therefore compares a set of countries within the same region, and such countries are likely subject to similar constraints on data collection. Accurate surveillance that is standardized across geographical units would be the most direct way to eliminate reporting bias and should be a global health priority. Better data on community-associated resistance may find environmental predictors to be more important than in the current study.
Second, our study has focused on cross-country differences. Several studies have noted important geographical variation within a given country.31,32 Comparing cross-country and within-country predictors of resistance should be an important focus of future research. Finally, what we have presented is a correlation and not definitive evidence of a causal mechanism.
The key insight of our analysis is that cost-sharing on medications in the public sector may shift individuals into a less well-regulated often informal private sector. This is in contrast to the standard health economics view which was developed for a one-sector system whereby cost-sharing tends to decrease demand. To our knowledge, we are the first to point out this important distinction for LMIC. Bolstering the capacity of the public sector to diagnose, prescribe and provide subsidized, high quality antimicrobial agents could deter individuals from seeking medical care from self-employed private providers from whom they may receive lower quality or inappropriate dosage of antibiotics. For instance, research done by Onwujekwe and colleagues in Nigeria found that 78% of low-quality medications came from private facilities compared to public facilities.33 Paruk et al. show that patients receiving antibiotics in the private sector were more likely to receive a truncated course of antimicrobial therapy compared to those in the public sector.34 Since the same individual in the private sector often performs prescribing and dispensing activities and quality controls on medications are often absent, pulling patients into a strengthened public health sector for treatment of communicable disease could reduce the use of inappropriate or low quality antimicrobial agents and thus decrease the spread of resistant organisms. Pharmaceutical companies may have a role, too. For instance, they could work to prevent counterfeit versions of their product by introducing scratch off labels and text messaging for verification as has been done in some parts of the developing world.35
The strategy of reducing cost barriers associated with medications in the public sector is currently being proposed by the Health Ministry of India and has already been implemented in China.36–38 Whether such strategies slow the rate of antimicrobial overuse and resistance will be contingent on the implementation of the program as well as the creation of a surveillance system capable of detecting such an effect.
Supplementary Material
Research in context.
Systematic Review
We searched for evidence on antibiotic resistance in LMIC using the Google scholar search engine and PubMed. Key words included in our searches were “antibiotic resistance” “antibiotic consumption” “out-of-pocket health expenditures” and “low- and middle-income countries.” We did not use a specific language or date filter. We assessed the quality of the evidence based on a number of characteristics such as the statistical analysis employed and the way in which the data were collected.
Interpretation
We find that the percent of health expenditures that are out-of-pocket strongly correlate with antimicrobial resistance among low-and middle-income countries (LMIC). This relationship was driven by countries that require copayments on medications in the public sector.
Our results suggest cost-sharing of antimicrobials in the public sector may shift demand from the public to the private sector where supply-side incentives to overprescribe are likely heightened and quality assurance less standardized.
Our findings have two broader implications: first the standard one-sector model of demand for healthcare is inadequate in LMIC. Second antimicrobial consumption is a key driver of resistance in LMIC, however, attention must also be paid to the quality of healthcare advice and the antimicrobial product.
Acknowledgments
The authors would like to thank Ramanan Laxminarayan, Saskia Nahrgang, Kara Hanson, Jason Andrews and Doug Owens for comments.
Funding/Support: Financial support for this research was provided by the National Institutes of Health (grant P30 150127-5054662-0002)
Footnotes
Conflict of Interest Disclosures: The authors have no conflicts of interest with regard to this study.
Contributors: Marcella Alsan and Lena Schoemaker had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Design and conduct of the study: Marcella Alsan, Lena Schoemaker, Nagamani Kammili and Jay Bhattacharya. Collection, management, analysis, and interpretation of the data: Marcella Alsan, Lena Schoemaker, Nagamani Kammili and Jay Bhattacharya. Preparation, review, or approval of the manuscript: Marcella Alsan, Lena Schoemaker, Nagamani Kammili, Prasanthi Kolli, Jay Bhattacharya and Karen Eggleston.
Conflict of interest statement: We declare that we have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. Antimicrobial resistance: global report on surveillance. 2014 [Google Scholar]
- 2.Wolkewitz M, Frank U, Philips G, Schumacher M, Davey P for the BURDEN Study Group. Mortality associated with in-hospital bacteremia caused by Staphylococcus aureus: a multistate analysis with follow-up beyond hospital discharge. J Antimicrob Chemother. 2011;66:381–386. doi: 10.1093/jac/dkq424. [DOI] [PubMed] [Google Scholar]
- 3.de Kraker ME, Davey PG, Gundmann H for the BURDEN study group. Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: estimating the burden of antibiotic resistance in Europe. PLoS Med. 2011;8:e1001104. doi: 10.1371/journal.pmed.1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Centers for Disease Control and Prevention. [accessed July 24, 2014];Antibiotic resistance threats in the United States 2013. 2013 Apr; http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf.
- 5.Howard DH, Scott DR. The Economic Burden of Drug Resistance. Clinical Infectious Disease. 2005;41:S283–286. doi: 10.1086/430792. [DOI] [PubMed] [Google Scholar]
- 6.Okeke IN, Klugman K, Bhutta Z, Duse AG, Jenkins P, O’Brien T, Pablos-Mendez A, Laxminarayan R. Antimicrobial Resistance in developing countries II: strategies for containment. Lancet Infectious Disease. 2005;5:568–580. doi: 10.1016/S1473-3099(05)70217-6. [DOI] [PubMed] [Google Scholar]
- 7.Mathew AG, Cissell R, Liamthong S. Antibiotic Resistance in Bacteria Associated with Food Animals: A United States Perspective of Livestock Production. Foodborne Pathogens and Disease. 2007;4(2):115–33. doi: 10.1089/fpd.2006.0066. [DOI] [PubMed] [Google Scholar]
- 8.Levy SB, FitzGerald GB, Macone AB. Spread of antibiotic-resistant plasmids from chicken to chicken and from chicken to man. Nature. 1976;260:40–42. doi: 10.1038/260040a0. [DOI] [PubMed] [Google Scholar]
- 9.Webster P. Poultry, politics, and antibiotic resistance. Lancet. 2009;374:773–74. doi: 10.1016/s0140-6736(09)61578-6. [DOI] [PubMed] [Google Scholar]
- 10.Glass SK, Pearl DL, McEwen SA, Finley R. Canadian province-level risk factor analysis of macrolide consumption patterns (2000–2006) Journal of Antimicrobial Chemotherapy. 2010;65:148–55. doi: 10.1093/jac/dkp391. [DOI] [PubMed] [Google Scholar]
- 11.Glass SK, Pearl DL, McEwen SA, Finley R. A province-level risk factor analysis of fluoroquinolone consumption patterns in Canada. Journal of Antimicrobial Chemotherapy. 2010;65:2019–27. doi: 10.1093/jac/dkq225. [DOI] [PubMed] [Google Scholar]
- 12.Reed S, Sullivan S, Laxminarayan R. Socioeconomic issues related to antibiotic use. In: Low DE, editor. Appropriate antibiotic use. London: The Royal Society of Medicine Press; 2001. pp. 41–46. [Google Scholar]
- 13.Byarugaba DK. A view on antimicrobial resistance in developing countries and responsible risk factors. International Journal of Antimicrobial Agents. 2004:105–10. doi: 10.1016/j.ijantimicag.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Laxminarayan R, Duse A, Wattal C, et al. Antibiotic resistance—the need for global solutions. Lancet Infectious Disease. 2013;13(12):1057–98. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 15.Okeke IN. Antimicrobial Resistance in Developing Countries. Springer; New York: 2010. Poverty and Root Causes of Resistance in Developing Countries; pp. 27–35. [Google Scholar]
- 16.Okeke IN, Laxminarayan R, Bhutta ZA, Duse AG, Jenkins P, O’Brien TF, Pablos-Mendez A, Klugman KP. Antimicrobial resistance in developing countries. Part I: recent trends and current status. Lancet Infectious Disease. 2005;5:481–93. doi: 10.1016/S1473-3099(05)70189-4. [DOI] [PubMed] [Google Scholar]
- 17.Okeke IN, Lamikanra A. Quality and Bioavailability of tetracycline capsules in Nigerian semi-urban community. International Journal of Antimicrobial Agents. 1995:245–50. doi: 10.1016/0924-8579(94)00064-2. [DOI] [PubMed] [Google Scholar]
- 18.Garg CG, Karan KK. Reducing out-of-pocket expenditures to reduce poverty: a disaggregated analysis at the rural-urban state level in India. Health Policy and Planning. 2008;24:115–28. doi: 10.1093/heapol/czn046. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz C. Out-of-pocket household health expenditures and their use in National Health Accounts: Evidence from Pakistan. Stanford University Working Paper Series on Health and Demographic Change in the Asia-Pacific. 2009 [Google Scholar]
- 20.Whitehead M, Dahlgren G, Evans T. Equity and health sector reforms: can low-income countries escape the medical poverty trap? Lancet. 2001;358:833–36. doi: 10.1016/S0140-6736(01)05975-X. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. World Health Survey. 2003 [Google Scholar]
- 22.Clotfelter CT. Public services, private substitutes, and the demand for protection against crime. American Economic Review. 1977;67:867–77. [Google Scholar]
- 23.World Bank. World Development Indicators. 1960–2013 [Google Scholar]
- 24.World Health Organization. Development of Country Profiles and monitoring of the pharmaceutical situation in countries: Pharmaceutical Sector Country Profiles Data and Reports. http://www.who.int/medicines/areas/coordination/coordination_assessment/en/index1.html.
- 25.Nikaido H. Multiple antibiotic resistance and efflux. Curr Opin Microbiol. 1998;1(5):516–23. doi: 10.1016/s1369-5274(98)80083-0. [DOI] [PubMed] [Google Scholar]
- 26.Wennberg J, Barnes B, Zubkoff M. Professional uncertainty and the problem of supplier-induced demand. Soc Sci Med. 1982;16(7):811–24. doi: 10.1016/0277-9536(82)90234-9. [DOI] [PubMed] [Google Scholar]
- 27.Clemens J, Gottlieb J. Do physicians’ financial incentives affect medical treatment and patient health? American Economic Review. 2014;104(4):1320–49. doi: 10.1257/aer.104.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong H, Bogg L, Rehnberg C, Diwan V. Association between health insurance and antibiotics prescribing in four counties in rural China. Health Policy. 1999;48:29–45. doi: 10.1016/s0168-8510(99)00026-3. [DOI] [PubMed] [Google Scholar]
- 29.Goossens H, Ferech M, Stichele RV, Elseviers M for the ESAC Project Group. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365:579–87. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 30.Newhouse J. Free for All? Lessons from the RAND Health Insurance Experiment. Harvard University Press. 1993 [Google Scholar]
- 31.Nitzan O, Low M, Lavi I, Hammerman A, Klang S, Raz R. Variability in outpatient antimicrobial consumption in Israel. Infection. 2010;38:12–18. doi: 10.1007/s15010-009-9065-8. [DOI] [PubMed] [Google Scholar]
- 32.Matuz M, Benko R, Doro P, et al. Regional variations in community consumption of antibiotics in Hungary, 1996–2003. Br J Clin Parmacol. 2006;61:96–100. doi: 10.1111/j.1365-2125.2005.02525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onwujekwe O, Kaur H, Dike N, et al. Quality of anti-malarial drugs provided by public and private healthcare providers in south-east Nigeria. Malaria Journal. 2009;8(22):1–9. doi: 10.1186/1475-2875-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paruk F, Richards G, Scribante J, Bhagwanjee S, Mer M, Perrie H. Antibiotic prescription practices and their relationship to outcome in South African intensive care units: Findings of the Prevalence of Infection in South African Intensive Care Units (PISA) Study. The South African Medical Journal. 2012;102(7):613–16. doi: 10.7196/samj.5833. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman J. A Way to Check That Drugs Are Not Counterfeit. [accessed 21 Nov 2014];The New York Times; 2011 Sep 26; Available from: http://www.nytimes.com/2011/09/27/health/27counterfeit.html?_r=2&.
- 36.Bagcchi S. India will make 50 essential drugs available free of charge. BMJ. 2014;349:4321. doi: 10.1136/bmj.g4321. [DOI] [PubMed] [Google Scholar]
- 37.IHS. Indian government revives plans for free essential drugs. [accessed 7 Aug 2014];Economics & Country Risk. 2014 Jun 2; Available from: http://www.ihs.com/products/global-insight/industry-economic-report.aspx?id=1065990151.
- 38.Chen Zhu. Launch of the health-care reform plan in China. Lancet. 2009;373:1322–23. doi: 10.1016/S0140-6736(09)60753-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.