Abstract
Background:
Biosurfactants constitute a structurally diverse group of surface-active compounds derived from microorganisms. They are widely used industrially in various industrial applications such as pharmaceutical and environmental sectors. Major limiting factor in biosurfactant production is their production cost.
Objectives:
The aim of this study was to investigate biosurfactant production under laboratory conditions with potato peels as the sole source of carbon source.
Materials and Methods:
A biosurfactant-producing bacterial strain (Bacillus pumilus DSVP18, NCBI GenBank accession no. GQ865643) was isolated from motor oil contaminated soil samples. Biochemical characteristics of the purified biosurfactant were determined and its chemical structure was analyzed. Stability studies were performed and biological activity of the biosurfactant was also evaluated.
Results:
The strain, when grown on modified minimal salt media supplemented with 2% potato peels as the sole carbon source, showed the ability to reduce Surface Tension (ST) value of the medium from 72 to 28.7 mN/m. The isolated biosurfactant (3.2 ± 0.32 g/L) was stable over a wide range of temperatures (20 - 120 ºC), pH (2-12) and salt concentrations (2 - 12%). When characterized using high-performance liquid chromatography (HPLC) and Fourier transform infrared spectroscopy, it was found to be a lipopeptide in nature, which was further confirmed by Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (mass peak 1044.60) and nuclear magnetic resonance (NMR) studies. Data showed that the isolated biosurfactant at the concentration range of 30 - 35 µg/ml had strong antimicrobial activity when tested against standard strains of Bacillus cereus, Escherichia coli, Salmonella enteritidis, Staphylococcus aureus and Paenibacillus larvae.
Conclusions:
Potato peels were proved to be potentially useful substrates for biosurfactant production by B. pumilus DSVP18. The strain possessed a unique property to reduce surface tension of the media from 72 to 28.7 mN/m. In addition, it showed a stable surface activity over a wide range of temperatures, pH, and saline conditions and had strong antimicrobial activity. This potential of the identified biosurfactant can be exploited by pharmaceutical industries for its commercial usage.
Keywords: Surface Active Agents, Surface Tension, Lipopeptides, Anti-Microbial Agents, Paenibacillus
1. Background
Biosurfactants are a unique class of structurally diverse compounds, which are amphiphilic in nature and have pronounced surface active properties with a wide variety of applications related to environmental protection, enhanced oil recovery, cosmetic, food processing and pharmaceutical industries (1-6). These compounds have been shown to have several advantages including high biodegradability, biocompatibility, low toxicity and low irritancy over their synthetic counterparts (7). However, a major concern behind the restricted commercial application of these microbially produced biosurfactants is their high production costs (6, 8, 9). This cost can be reduced by utilizing alternative substrates, such as agricultural based industrial wastes, for economical biosurfactant production, and the choice of microorganism, which plays an important role in biosurfactant production. A wide variety of alternative raw materials, namely various agricultural and industrial byproducts, have been evaluated for biosurfactant production (8-11). Keeping the above factors into account, modified minimal salt medium supplemented with potato peels was selected and used as the sole carbon source for production of biosurfactant. India being the third largest producer of potato in the world (10) generates large quantities of potato peels, which are generally attained by steam, abrasive or lye peeling by potato processing industries.
2. Objectives
In the present investigation the ability of a bacterial isolate to utilize potato peels, as a cost effective substrate for biosurfactant production, was examined. Extraction, purification and characterization of the isolated biosurfactant were done to ensure the role of the isolated biosurfactant as a potent antimicrobial agent.
3. Materials and Methods
3.1. Screening and Identification of Biosurfactant-Producing Strains
A biosurfactant-producing microbial strain was isolated from motor oil contaminated soil samples, obtained from a warehouse (Roorkee, India), based on surface active measurements (12). Briefly, surface tension of the cell-free broth (50 mL) of the culture supernatant, obtained at different time intervals, was measured by a tensiometer (Sigma 703 KSV instruments Ltd., Finland), using the Wilhelmy plate measurement technique. All the measurements were done in triplicates. The selected bacterial strain (DSVP18) from the above evaluation tests was identified by microscopic and conventional biochemical tests, in accordance with Bergey’s Manual of Systematic Bacteriology (13).
The strain was identified as Bacillus pumilus, DSVP18 (NCBI GenBank accession no. GQ865643), using morphological, biochemical and 16S rRNA sequence analysis. Furthermore, identification of the selected bacterial isolate DSVP18 was performed using standard 16S rRNA-specific universal primers (Genescript, India), with a forward primer (5′-AGAGTTTGATCCTGGCTCAG-3′) and reverse primer (5′-AAGGAGGTGATCCAGCCGCA-3′). A reaction mixture containing approximately 50 ng of template DNA, Polymerase Chain Reaction (PCR) buffer (10 mM Tris-HCl, pH 8.3; 50 mM KCl; 2.5 mM MgCl2; and 0.001% gelatin), a 0.2 mM concentration of each PCR primer, 0.2 mM concentration of each dNTP, and 2.5 U of Taq DNA polymerase in a total volume of 50 μL was prepared. Polymerase Chain Reaction conditions were as follows; initial denaturation at 95°C for two minutes, followed by 30 cycles of denaturation at 95°C for one minute, annealing for one minute at 58°C, and extension at 72°C for one minute. At completion of the PCR reaction, 5 μL of the PCR product was run on a 1% agarose gel containing 1 mM ethidium bromide for visualization of DNA in the amplified bands on a UV illuminator (12). The partial sequences were subjected to BLAST analysis available at www.ncbi.nlm.nih.gov/BLAST. The taxonomic position and relationship of the isolated strain, B. pumilus DSVP18, was determined using MEGA 6.0 by constructing a phylogenetic tree (14).
3.2. Medium and Growth Conditions
Biosurfactant production by Bacillus pumilus DSVP18 (OD600nm = 0.8) was carried out using modified minimal salt medium (MSM) supplemented with potato peels (2%) as the sole carbon source under optimized growth conditions at 37ºC for 10 - 12 hours. The composition of the used mineral salt medium (MSM) (g/L) was follows: (NH4)2SO4 (3.0), NaNO3 (2.0), K2HPO4 (4.5), KH2PO4 (2.0), NaCl (0.1), MgSO4 H2O (0.01), CaCl2 (0.01) and FeSO4 7H2O (0.01) with pH adjusted to 7.0 (15). All experiments were carried out in triplicates.
3.3. Substrate Preparation and Compositional Analysis
Potato peels were obtained as waste material from a potato chips factory located in Haridwar, India. They were dried in an oven at 60ºC and ground in a hammer mill. The composition of potato peels, before use for experimental work, in terms of percentage protein (16), carbohydrate (17), sugar (18) and ash contents (19) are mentioned in Table 1.
Table 1. Chemical Composition of Potato Peels.
| Composition | Content, % |
|---|---|
| Total Dry Matter | 18.6 |
| Carbohydrate | 58.2 |
| Crude Protein | 13.6 |
| Fat/lipid content | 2.2 |
| Ash content | 7.4 |
| pH | 5.9 |
3.4. Dry Biomass Estimation
Dry biomass of bacterial growth was estimated at different time intervals (0, 24, 48, 72 and 120 hours) by centrifuging the samples (250 mL) at 16300x g for 25 minutes. The cell pellet obtained after centrifugation was dried overnight at 105ºC and weighed (20).
3.5. Extraction and Purification of the Biosurfactant
The biosurfactant was extracted from the cell free broth using acid precipitation techniques, as described by Yakimov et al. (21). Briefly, biosurfactant precipitates were formed when cell free broth samples, obtained at different time intervals, were adjusted to pH 2 using 6N HCl and kept overnight at 4ºC. Cell free broth was then centrifuged at 10 000x g for 15 minutes, and the obtained precipitate was collected and suspended in a minimal amount of distilled water, which was further neutralized to pH 7.0 using 1 N NaOH. The solution was then lyophilized, weighed and stored at -20ºC (21).
3.6. Determination of Protein and Lipid Content
The total protein content of the biosurfactant was determined according to the methods described by Lowry et al. (16), using bovine serum albumin as the standard. Total lipid content was determined as described by Arutchelvi et al. (22).
3.7. Chromatographic Analysis
Thin Layer Chromatography (TLC) analysis of the isolated biosurfactant was done on silica gel (Si 60 F254, 0.25 mm, Merck) using chloroform-methanol-water 65:25:4 (v/v/v) as the solvent system. Spots were revealed by spraying with distilled water and heating at 110ºC for five minutes, for detection of hydrophilic compounds. Surfactin (Sigma-Aldrich, USA) was used as the standard. HPLC of purified biosurfactant was performed using a 30 cm C18 µ-Bondapak column (Waters) at 25°C and separated using reverse phase HPLC. The used mobile phase, constituted of 80% acetonitrile with 0.1% trifluoroacetic acid and a flow rate of 1 mL/minute. The eluted peaks were detected at 210 nm (21).
3.8. Spectroscopic Analysis
3.8.1. Fourier Transformation Infra-Red (FTIR) Analysis
Characterization of purified biosurfactant was performed using a Fourier Transformation Infra-Red (FTIR) spectrophotometer (Thermo-Nicolet, USA) equipped with OMNIC software for data analysis. A pinch of isolated biosurfactant was ground with 100 mg of KBr in pestle and mortar for 30 seconds to obtain translucent pellets. The FTIR spectra was obtained at a frequency range of 4000 - 500 cm-1 (23).
3.8.2. Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Analysis
Furthermore, 0.5 μL of purified biosurfactant was spotted onto the anchor chip position on a Matrix Assisted Laser Desorption/Ionization (MALDI) plate, and 0.5 μL of the matrix was added to the sample spot. The matrix was a saturated solution of 2, 5-dihydroxybenzoic acid in water and 0.3 mg/ml α-cyano-4-hydroxycinnamic acid in acetone/ethanol (2:1 v/v). A peptide standard was also spotted for external calibration. The spots were left at room temperature to dry and analyzed on Applied Biosystems Voyager System 4402 Mass Spectrometer with the delayed mode and an acceleration voltage of 20 kV.
3.8.3. Nuclear Magnetic Resonance Spectroscopy Analysis
One-dimensional 1 H nuclear magnetic spectra were recorded on 298 K on a 500MHz NMR spectrophotometer (Bruker, Germany). The samples were prepared as solutions in 100% CDCl3, using approximately 1-3 mg of the biosurfactant (12).
3.9. Properties of the Biosurfactant
3.9.1. Effect of pH, NaCl and Temperature on Biosurfactant Stability
Temperature, pH and the effect of NaCl (%) stability on Surface Tension (ST) values of crude biosurfactant were determined (24, 25). Briefly, ST was measured by a tensiometer (Sigma 703 KSV instruments Ltd., Finland) using the Wilhelmy plate measurement technique, and for evaluating the critical micelle dilutions, the cell free broth was diluted ten-fold (CMD-1) and 100-fold (CMD-2) with distilled water. The ST of these solutions was then plotted against time. To check thermal stability, 50 mL of the crude biosurfactant aq. solution (0.1 %) was incubated for five hours at different temperature ranges from 20 - 120ºC, followed by determination of surface activities (ST, CMD-1 and CMD-2). The pH stability studies of crude biosurfactant aq. solution (0.1 %) were done by adjusting the pH range from 2 - 12 and keeping it for 24 hours before the measurement of surface activities. The effect of salt concentration on surface active values was determined by dissolving 2 - 12% (w/v) NaCl in the crude biosurfactant aq. solution (0.1 %).
3.9.2. Antimicrobial Assay of Purified Biosurfactant and Determination of Minimum Inhibitory Concentration (MIC)
Antimicrobial tests of the purified biosurfactant (50 µg/mL) were performed against standard strains, such as Bacillus cereus (MTCC 430), Escherichia coli (MTCC 1687), Salmonella enteritidis (MTCC 3219), and Staphylococcus aureus (MTCC 5021), procured from the Microbial Type Culture Collection (MTCC, IMTECH, Chandigarh, India) and Paenibacillus larvae (ATCC 9545), respectively. The antibacterial activity of the purified biosurfactant was determined using a growth inhibition zone assay (23). Briefly, overnight cultured bacterial strains with a standardized inoculum size of 1.5 × 108 CFU/mL were inoculated on surface of nutrient agar plates, separately. Purified biosurfactant at varying concentrations (20 - 50 µg/mL) was loaded into wells of these nutrient agar seeded plates with respective bacterial inoculum. The plates were incubated for 24 hours at 37ºC. The MIC value was defined as the lowest concentration of biosurfactant that inhibited the visible growth of microorganisms after overnight incubation. The MIC values, of purified biosurfactant showing maximum zone of inhibition when tested on the above-mentioned microbial strains, were recorded. A negative control was prepared using the same solvent as employed to obtain the extract. Ofloxacin (5 µg, Sigma-Aldrich, USA) and cefoperazone-sulbactam (5 µg, Sigma-Aldrich, USA) were used for Gram-positive and Gram-negative bacteria, respectively. All the above-mentioned experiments were conducted in triplicates.
4. Results
4.1. Identification of Biosurfactant-Producing Strain
Among 78 bacterial isolates obtained from oil contaminated soil samples, strain DSVP18 was chosen based on its ability to reduce ST of the medium from 72 to 28.7 mN/m. This bacterial isolate (DSVP18) was then subjected to biochemical tests using Bergey’s manual of systematic bacteriology, and it was found to be a Gram-positive, motile, rod-shaped and endospore-forming bacterium. This isolate also showed positive results for urease, citrate utilization and for IMViC test. Genomic DNA was isolated and an amplified product of 1.5 Kb was obtained (Figure 1). Sequence alignment of the fragment using BLAST revealed 100% identity to B. pumilus JS-45 16S rRNA gene partial sequence.
Figure 1. Polymerase Chain Reaction Amplified a Product of 1.5 kb Corresponding to 16S rRNA Gene.
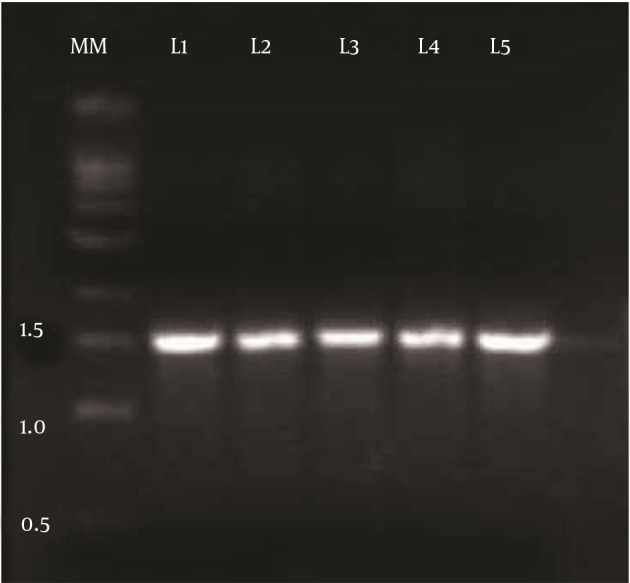
MM, Molecular Marker (1 kb DNA ladder); L1, DSVP13; L2, DSVP14; L3, DSVP18; L4, DSVP25; L5, DSVP26.
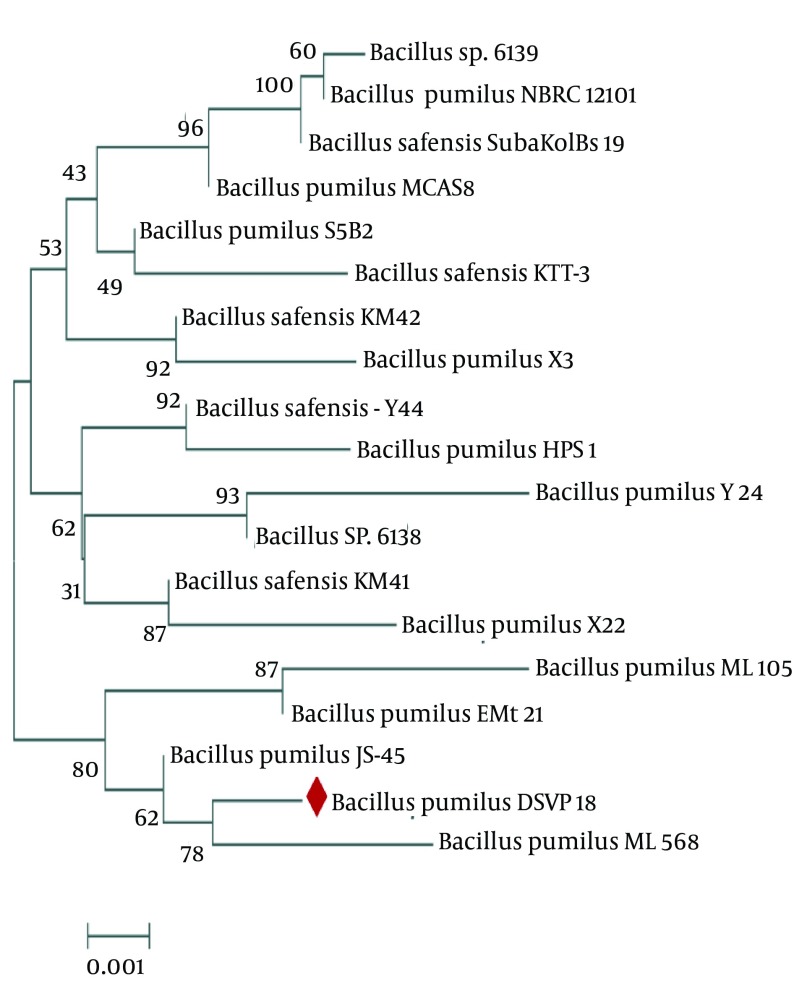
The physiological and biochemical characteristics, and the 16S rRNA sequence analysis confirmed that the strain was DSVP18 belonging to Bacillus pumilus (NCBI Gen Bank accession no. GQ865643). The isolate’s evolutionary history was inferred using the Neighbor-Joining method. The optimal tree, with the sum of branch length equal to 0.02602863, was constructed (Figure 2). The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) has been shown next to the branches (14). The tree was drawn to scale, with branch lengths in the units of the number of base substitutions per site. The evolutionary distances were computed for 19 nucleotide sequences using the Kimura 2-parameter method. There were a total of 1536 positions in the final dataset. Evolutionary analyses were conducted using the MEGA6 software (14).
Figure 2. The Phylogenetic Tree of Bacillus pumilus DSVP18 Constructed Using the Neighbor-Joining Method (MEGA 6 Software).
4.2. Biosurfactant Extraction
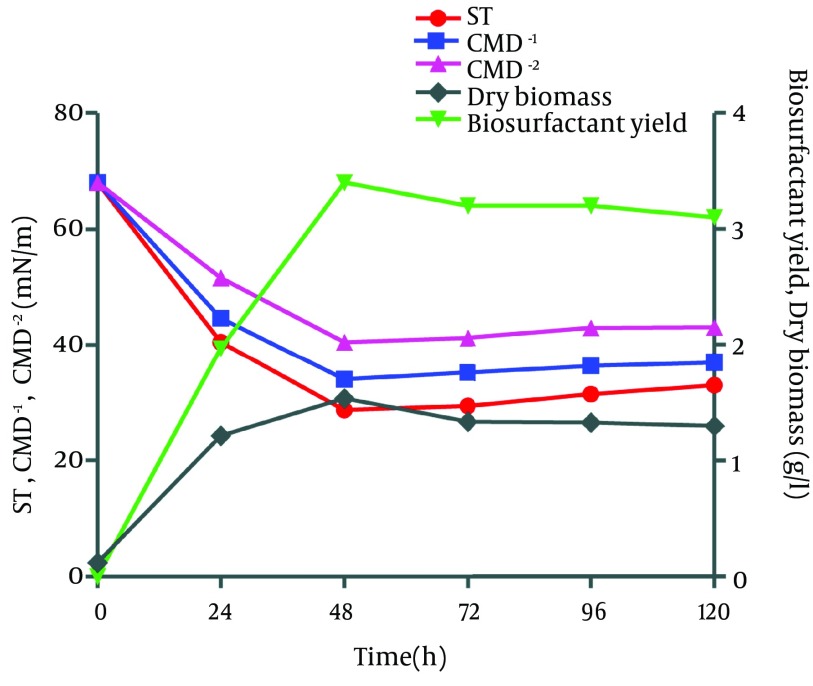
Biosurfactant was extracted from Bacillus pumilus DSVP18 using the acid precipitation technique. Data showed maximum biosurfactant level of 3.2 g/L obtained after 48 hours of culture incubation at 37ºC with maximum dry biomass (1.3 g/L). The extracted biosurfactant was also capable of reducing the ST of modified MSM medium from 70 to 28.7 mN/m when 2% potato peels were used as the sole carbon source while critical micelle dilutions (CMD-1 and CMD-2) were recorded to be 36.4 and 33.0 mN/m, respectively (Figure 3).
Figure 3. Surface Tension (ST), Critical Micelle Dilutions (CMD-1, CMD-2), Dry Biomass and Biosurfactant (BS) Production by B. pumilus DSVP18 at Different Time Intervals.
4.3. Characterization of Biosurfactant
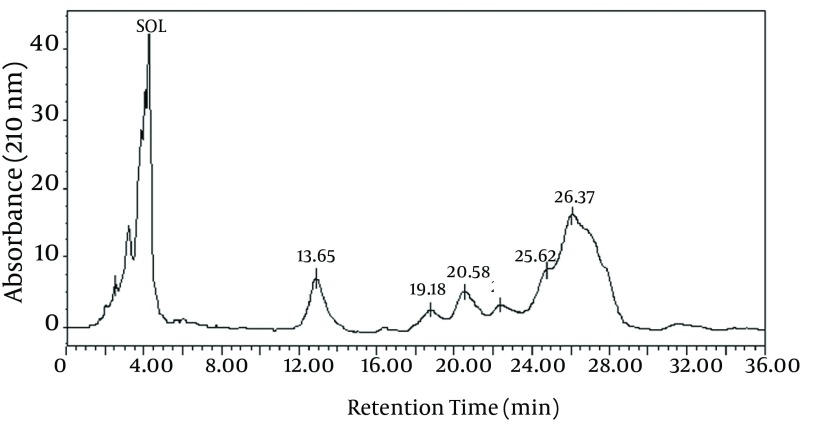
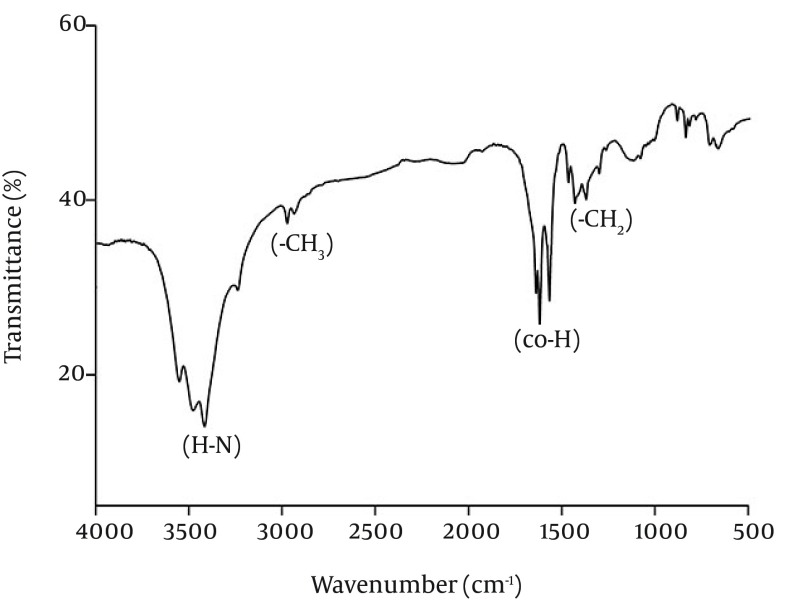
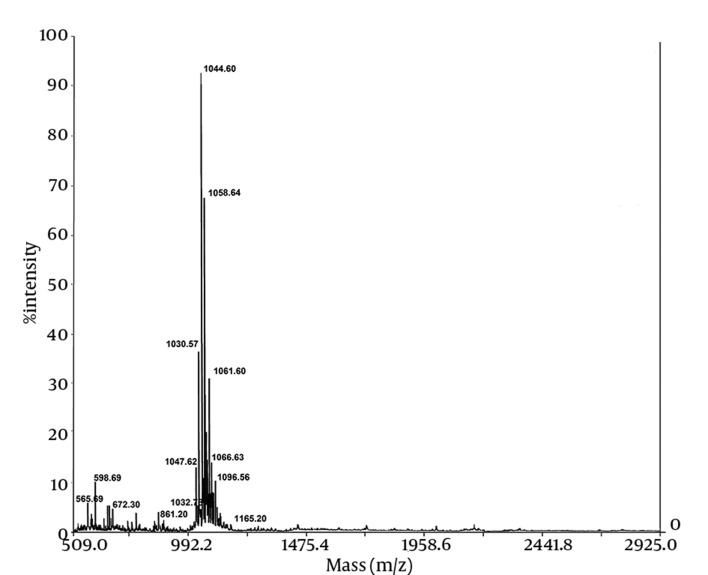
The chemical composition of the biosurfactant showed that it was composed of 14.7% protein and 18% lipid. The proportion of the surfactant ratio of protein and lipid remained constant in the acid precipitate at different periods of harvesting. Bacillus pumilus produced a lipopeptide, which was stable when checked for surface activities (ST, CMD-1 and CMD-2) at a wide range of pH (2.0 to 12.0), temperatures (20 - 120ºC) and 2% to 12% salt conditions (Table 2). HPLC analysis of the partially purified biosurfactant showed six major peaks at retention times: 13.65, 19.18, 20.58, 22.61, 25.62, and 26.37, respectively (Figure 4). Fourier Transformation Infra-Red spectrum of the biosurfactant from Bacillus pumilus DSVP18 (Figure 5) showed strong absorption bands of peptides at 3405 cm-1, 1604 cm-1 and 1541 cm-1 resulting from N-H stretching, C = O stretching and combined C-N stretching modes. The predominant adsorption bands were 1423 cm-1 and 2964 cm-1, which indicated the presence of aliphatic chains (CH2, CH3) in the sample. The intense band at 1604 cm-1 corresponds to the CO-NH-R group. The absorption region at 1730 cm-1 was due to an ester carbonyl band. Data obtained from MALDI-TOF-MS of purified biosurfactant showed well-resolved groups of peaks at m/z values 1000 - 1096 (Figure 6). The mass peaks at m/z 1047.6, 1030.5, 1044.6 and 1058.6 indicated a lipopeptide with mixture of structural analogs. Mass spectrometry confirmed isomers of surfactin and iturin in purified fractions. The mass peak at 1044.60 was attributed to a surfactin isoform containing a β-hydroxy fatty acid with a chain of 14 carbon atoms. Figure 6 shows the assignments of peaks obtained from MALDI-TOF-MS.
Table 2. Surface Activities of Isolated Biosurfactant Aqueous Solution (0.1%) at Different pH (2 - 12), Temperature (2 - 120 ºC) and NaCl Concentrations (2 - 12%).
| Parameters | Surface Tension, mN/m | CMD-1, mN/m | CMD-2, mN/m |
|---|---|---|---|
| pH | |||
| 4 | 34.5 | 38.2 | 46.3 |
| 6 | 32.4 | 36.7 | 43.8 |
| 8 | 34.6 | 38.8 | 46.5 |
| 10 | 36.2 | 39.4 | 48.8 |
| 12 | 36.4 | 39.4 | 49.2 |
| Temperature, ºC | |||
| 20 | 34.7 | 36.5 | 41.8 |
| 40 | 30.5 | 32.5 | 40.5 |
| 60 | 36.2 | 36.2 | 42.0 |
| 80 | 38.0 | 43.1 | 51.5 |
| 100 | 42.8 | 45.0 | 54.0 |
| 120 | 42.8 | 43.4 | 54.0 |
| NaCl, % | |||
| 2 | 36.0 | 42.1 | 49.8 |
| 4 | 36.2 | 42.0 | 53.0 |
| 6 | 38.8 | 48.0 | 56.0 |
| 8 | 45.6 | 53.4 | 59.8 |
| 10 | 48.1 | 55.4 | 60.2 |
| 12 | 48.4 | 56.6 | 60.8 |
Figure 4. High-Performance Liquid Chromatography (HPLC) Chromatogram of Biosurfactant Obtained From Bacillus pumilus DSVP18.
Figure 5. Fourier Transformation Infra-Red Spectrum of Purified Biosurfactant Obtained from Bacillus pumilus DSVP18.
Figure 6. Matrix-Assisted Laser Desorption Ionization–Time of Flight (MALDI-TOF) Mass Spectrometry Spectra of Purified Biosurfactant Obtained From Bacillus pumilus DSVP18.
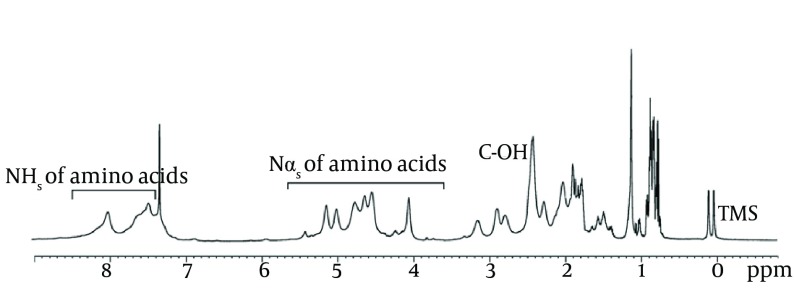
Results obtained with 500 MHz for 1 H NMR (Figure 7), clearly indicated that the molecule being studied was a lipopeptide. The spectrum confirmed the presence of a long aliphatic chain (CH2 at 1.5 - 1.2 ppm) and that the peptide backbone-amide-NH groups were at the region from 7.95 to 7.2 ppm. The doublet signal obtained at 0.859 ppm corresponded to the (CH3)2-CH group; specifying terminal branching in the fatty acid component. The spectrum indicated the resonance of the following amino acids: valine with peptide CH at 4.6, CH2 at 1.7, and 2 CH3 at 0.9 ppm; leucine with peptide CH2 at 1.7, CH at 1.3 and 2 CH at 0.9 ppm; isoleucine with peptide CH at 4.2, CH at 2.2, CH2 at 1.9 and 2 CH3 at 0.9 ppm; aspartic acid or asparagines with peptide CH at 4.7 and CH2 at 2.8 ppm; and glutamic acid or glutamine peptide CH at 4.3, internal CH2 at 1.9, and CH2 α to the carbonyl at 2.1 ppm.
Figure 7. Nuclear Magnetic Resonance Spectra of Purified Biosurfactant Obtained from Bacillus pumilus DSVP18.
4.4. Antimicrobial Action of Lipopeptide
Lipopeptide produced by B. pumilus DSVP18 showed antimicrobial activity against E. coli, B. cereus, S. enteritidis, S. aureus and P. larvae at different concentrations using the well diffusion method. The MIC value of the lipopeptide varied from 30 to 35 µg/mL. The maximum zone of inhibition recorded with respect to lipopeptide concentration was 30 µg/mL against E. coli (23 ± 0.3 mm) followed by B. cereus (19 ± 0.5 mm), S. enteritidis (18.6 ± 0.3 mm) and S. aureus (18 ± 0.7 mm) while for P. larvae (15.3 ± 0.8 mm) this was noted at 35 µg/mL lipopeptide concentration.
5. Discussion
Potato peels hold a major portion of processing waste and cause severe disposal problems for the potato industry (10). In the present study, these waste products of the potato industry were used for biosurfactant production, as they are a rich source of carbohydrate and protein, which eventually can be used as a potential energy source, thereby alleviating the waste disposal problem (26). Previous researches have made numerous attempts to use potato peels and mushrooms to obtain products, such as enzymes, organic acids, ethanol and polysaccharides with little success (26).
The production of compounds from such agricultural waste requires the selection of microorganisms with relevant enzymatic activities. Most Bacillus sp. have a wide range of hydrolytic enzyme systems and are often capable of utilizing organic matter consisting of complex mixtures (27). In our study, soil samples contaminated with motor oil were collected for the isolation of biosurfactant-producing bacterial strains capable of utilizing potato peels as a cheap carbon source. Amongst various known screening tests for biosurfactant production, such blood agar lysis, drop collapse assay and emulsification activity, ST reduction assay was chosen for the screening of potential microorganisms that produce biosurfactant (28). The B. pumilus DSVP18 isolate was selected for further studies, as this microorganism possessed efficient ST reduction ability and fulfilled the above criteria thus suggesting that it could be an efficient biosurfactant producer. Physiological and biochemical tests and 16S rRNA sequencing allowed the identification of the potential biosurfactant producer, Bacillus pumilus DSVP18, at the species level with 100% identity to Bacillus pumilus JS-45 from the GenBank database (29).
The selected strain, Bacillus pumilus DSVP18, was capable of utilizing potato peels as the sole carbon source. In our study biosurfactant production by Bacillus pumilus DSVP18 was growth-associated because a good correlation was observed between the maximum yield of biosurfactant and biomass at 48 hours of incubation (at late log phase) and the surface-active compound was stable over time, which is in good agreement with earlier findings (30). Maximum biosurfactant production (3.2 ± 0.32 g/L) was detected during late log growth phase with the least ST value of 28.7 mN/m. These results suggest that expressions of the gene responsible for biosurfactant production are involved in reducing ST values (31). Previous studies have also reported the maximum yield of surfactin to be approximately 110 mg/l by the Bacillus subtilis S 499 strain (32). Similarly, biosurfactant production of 1.74 g/L was observed when the microbial consortium of Enterobacter cloacae and Pseudomonas sp. (ERCPPI-2) was grown on minimal salt medium supplemented with olive oil (33).
The lipopeptide produced by B. pumilus was quantified using HPLC (34), for which six major peaks were attained at the retention time (13.65 - 26.37). The HPLC data strongly suggest that the biosurfactant obtained in this study had a lipopeptide structure, which is in support of previous findings (35). The molecular mass of the purified lipopeptide biosurfactants was attained using MALDI-TOF-MS. The groups of obtained mass spectra resembled that of surfactins and iturins, which represent well-known biosurfactant families produced by Bacillus sp. (36). The nature of the biosurfactant was evaluated comparing the FTIR spectra of this fraction to that of pure surfactin. Analysis by HNMR and FTIR of isolated biosurfactant led us to suggest their structural relatedness to lipopeptides. Production of different biosurfactant isoforms by Bacillus sp. can be attributed to a set of conserved genes responsible for these enzymes by non-ribosomal synthesis. These changes in structure confer an advantage to the microorganism to survive under drastic environments. Considering the importance of bioremediation of oil-contaminated sites (oil spills), it is necessary to isolate and screen potent biosurfactant producers, which can survive in harsh conditions.
The high stability of the biosurfactant in a wide pH (2 - 12) range, temperature (20 - 120 ºC) and salinity conditions (2 - 12 %), makes it very suitable for these extreme conditions as depicted by Nitschke et al. using cassava wastewater (2). The pH and thermal stability and stability over high saline conditions of the biosurfactants increase the scope of its application in microbial enhanced oil recovery (MEOR) processes. Several lipopeptide biosurfactants produced by Bacillus sp. have been noted for their antibacterial activity (23). Lipopeptide biosurfactants are most prominently known as surfactin, produced mostly by the Bacillus sp. despite similar global structures; surfactins, iturins and fengycin differ in some aspects regarding their biological activities. The antimicrobial potential of DSVP18 biosurfactant against several standard test pathogens, including S. enteritidis, S. aureus, B. cereus, E. coli and P. larvae was evaluated in this study. The obtained zone of inhibition clearly represents the potential of DSVP18 biosurfactant as an antimicrobial agent. The response of these microorganisms to biosurfactants may be attributed to their different membrane structures and their relative permeability. Sensitivity towards biosurfactants was in the following order as per their zone of inhibition E. coli > B. cereus > S. aureus > S. enteritidis > P. larvae. This application potential of the biosurfactant from B. pumilus DSVP18 is of great importance as in the future its medicinal use may further be assessed to protect against gastrointestinal pathogenic infections.
The antimicrobial activity increased with increasing concentration of biosurfactant. Our results are in accordance with data for biosurfactants obtained from B. subtilis natto (37), B. circulans (38) and B. licheniformis (39). The biosurfactant from B. pumilus DSVP18 showed good inhibitory action against Gram-negative bacteria. This is in contrast to previous reports by Singh and Cameotra (40), where they found the lipopeptide biosurfactant to be active mostly against Gram-positive bacteria having little or no effect on Gram-negative bacteria. A Bacillus subtilis isolate showing antagonistic activity against food-borne pathogens was also reported recently (41). In their study, Moore et al. (41) reported three B. subtilis strains with proficient biosurfactant activity against test pathogens, i.e. Salmonella and Staphylococcus cultures, also known as clinical multi-resistant strains. Sabate et al. (42), reported on the antibacterial action of surfactin against P. larvae. In their studies they stated that vegetative cells of P. larvae were affected as soon as they came in contact with the surfactin.
The current work proposes for the first time the use of potato peels as the sole carbon source for biosurfactant production. Our results are of great importance, since the uniqueness of the compound and the possibility of cost effective production and stability of biosurfactant makes it a potential candidate for use in environmental applications. The biosurfactant produced by the B. pumilus DSVP18 isolate was characterized as a lipopeptide with bioremediation activities under extreme conditions. Purification and study of the mode of action of this low mass compound could lead to the development of an innovative antimicrobial compound in the medical sector. With an alarming increase in drug resistant microorganisms, such new biomolecules can provide an alternative to the existing antimicrobial agents. The potential of this microorganism utilizing other agricultural-based cheap raw materials, for cheaper and large-scale production of biosurfactant, can also be evaluated.Biosurfactant-producing bacterial strain Bacillus pumilus DSVP18 (Gene Bank Accession No. GQ865643) screened from oil contaminated soil was identified by 16S rRNA gene sequencing. The strain showed unique ability to utilize potato peels as a carbon source for biosurfactant production and was able to reduce surface tension of the media from 72 to 28.7 mN/m. Structural characterization of the stable (pH, temperature and salt concentration) biosurfactant using HPLC, FTIR, NMR, GC-MS and MALDI-TOF/MAS analysis showed that it was lipopeptide in nature, which possess strong antimicrobial activity. This potential of biosurfactant can be exploited by pharmaceutical industries for its commercial usage.
Footnotes
Authors’ Contributions:All authors supervised and managed the research project. Deepak Sharma, Mohammad Javed Ansari and Sonam Gupta conducted the research. Ahmad Al Ghamdi, Parul Pruthi and Vikas Pruthi helped in writing the manuscript. Deepak Sharma and Mohammad Javed Ansari contributed equally to this work.
Funding/Support:The authors are grateful to deanship of scientific research, college of food and agriculture science research, King Saud university Riyadh for providing research support.
References
- 1.Padmapriya B, Suganthi S. Antimicrobial and anti adhesive activity of purified biosurfactants produced by Candida species. Middle East J Sci Res. 2013;14(10):1359–69. [Google Scholar]
- 2.Nitschke M, Pastore GM. Production and properties of a surfactant obtained from Bacillus subtilis grown on cassava wastewater. Bioresour Technol. 2006;97(2):336–41. doi: 10.1016/j.biortech.2005.02.044. [DOI] [PubMed] [Google Scholar]
- 3.Abdul HA, Fooladi T, Yusoff WMW, Shafiei. Surface Active Components: Review. Curr Res J Biol Sci. 2014;6(2):89–95. [Google Scholar]
- 4.Geys R, Soetaert W, Van Bogaert I. Biotechnological opportunities in biosurfactant production. Curr Opin Biotechnol. 2014;30:66–72. doi: 10.1016/j.copbio.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Rebello S, Asok AK, Mundayoor S, Jisha MS. Surfactants: toxicity, remediation and green surfactants. Environ Chem Lett. 2014;12(2):275–87. doi: 10.1007/s10311-014-0466-2. [DOI] [Google Scholar]
- 6.Gudina EJ, Rodrigues AI, Alves E, Domingues MR, Teixeira JA, Rodrigues LR. Bioconversion of agro-industrial by-products in rhamnolipids toward applications in enhanced oil recovery and bioremediation. Bioresour Technol. 2015;177:87–93. doi: 10.1016/j.biortech.2014.11.069. [DOI] [PubMed] [Google Scholar]
- 7.Rahman PKSM, Gakpe E. Production, Characterisation and Applications of Biosurfactants-Review. Biotechnol (Faisalabad). 2008;7(2):360–70. doi: 10.3923/biotech.2008.360.370. [DOI] [Google Scholar]
- 8.Lopes Ferreira N. [Industrial exploitation of renewable resources: from ethanol production to bioproducts development]. J Soc Biol. 2008;202(3):191–9. doi: 10.1051/jbio:2008021. [DOI] [PubMed] [Google Scholar]
- 9.Al-Bahry SN, Al-Wahaibi YM, Elshafie AE, Al-Bemani AS, Joshi SJ, Al-Makhmari HS, et al. Biosurfactant production by Bacillus subtilis B20 using date molasses and its possible application in enhanced oil recovery. Int Biodeterior Biodegradation. 2013;81:141–6. doi: 10.1016/j.ibiod.2012.01.006. [DOI] [Google Scholar]
- 10.Schieber A, Saldana MA. Potato peels: a source of nutritionally and pharmacologically interesting compounds-a review. Food. 2009;3(2):23–9. [Google Scholar]
- 11.de Oliveira DW, Franca IW, Felix AK, Martins JJ, Giro ME, Melo VM, et al. Kinetic study of biosurfactant production by Bacillus subtilis LAMI005 grown in clarified cashew apple juice. Colloids Surf B Biointerfaces. 2013;101:34–43. doi: 10.1016/j.colsurfb.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Pemmaraju SC, Sharma D, Singh N, Panwar R, Cameotra SS, Pruthi V. Production of microbial surfactants from oily sludge-contaminated soil by Bacillus subtilis DSVP23. Appl Biochem Biotechnol. 2012;167(5):1119–31. doi: 10.1007/s12010-012-9613-z. [DOI] [PubMed] [Google Scholar]
- 13.Sneath PHA, Mair NS, Sharpe ME. Bergey's manual of systematic bacteriology. Baltimore: Williams & Wilkins; 1986. [Google Scholar]
- 14.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–9. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pruthi V, Singh Cameotra S. Rapid method for monitoring maximum biosurfactant production obtained by acetone precipitation. Biotechnol Tech. 1995;9(4):271–6. doi: 10.1007/bf00151574. [DOI] [Google Scholar]
- 16.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–75. [PubMed] [Google Scholar]
- 17.DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric Method for Determination of Sugars and Related Substances. Anal Chem. 1956;28(3):350–6. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 18.Miller GL. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal Chem. 1959;31(3):426–8. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 19.Gumul D, Ziobro R, Noga M, Sabat R. Characterisation of five potato cultivars according to their nutritional and pro-health components. Acta Sci Pol Technol Aliment. 2011;10(1):2. [PubMed] [Google Scholar]
- 20.Pruthi V, Cameotra SS. Production of a biosurfactant exhibiting excellent emulsification and surface active properties bySerratia marcescens. World J Microbiol Biotechnol. 1997;13(1):133–5. doi: 10.1007/bf02770821. [DOI] [Google Scholar]
- 21.Yakimov MM, Timmis KN, Wray V, Fredrickson HL. Characterization of a new lipopeptide surfactant produced by thermotolerant and halotolerant subsurface Bacillus licheniformis BAS50. Appl Environ Microbiol. 1995;61(5):1706–13. doi: 10.1128/aem.61.5.1706-1713.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arutchelvi J, Doble M, Bhaduri S, Uppara PV. Production and Characterization of Biosurfactant from Bacillus subtilis YB7. J Appl Sci. 2009;9(17):3151–5. doi: 10.3923/jas.2009.3151.3155. [DOI] [Google Scholar]
- 23.Das P, Mukherjee S, Sen R. Antimicrobial potential of a lipopeptide biosurfactant derived from a marine Bacillus circulans. J Appl Microbiol. 2008;104(6):1675–84. doi: 10.1111/j.1365-2672.2007.03701.x. [DOI] [PubMed] [Google Scholar]
- 24.Roongsawang N, Thaniyavarn J, Thaniyavarn S, Kameyama T, Haruki M, Imanaka T, et al. Isolation and characterization of a halotolerant Bacillus subtilis BBK-1 which produces three kinds of lipopeptides: bacillomycin L, plipastatin, and surfactin. Extremophiles. 2002;6(6):499–506. doi: 10.1007/s00792-002-0287-2. [DOI] [PubMed] [Google Scholar]
- 25.Das K, Mukherjee AK. Crude petroleum-oil biodegradation efficiency of Bacillus subtilis and Pseudomonas aeruginosa strains isolated from a petroleum-oil contaminated soil from North-East India. Bioresour Technol. 2007;98(7):1339–45. doi: 10.1016/j.biortech.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 26.Bacha U, Nasir M, Khalique A, Anjum AA, Jabbar MA. Comparative assessment of various agro-industrial wastes for Saccharomyces cerevisiae biomass production and its quality Evaluation as single cell protein. J Anim Plant Sci. 2011;21(4):844–9. [Google Scholar]
- 27.Mahmood AU, Greenman J, Scragg AH. Orange and potato peel extracts: Analysis and use as Bacillus substrates for the production of extracellular enzymes in continuous culture. Enzyme Microb Technol. 1998;22(2):130–7. doi: 10.1016/s0141-0229(97)00150-6. [DOI] [Google Scholar]
- 28.Youssef NH, Duncan KE, Nagle DP, Savage KN, Knapp RM, McInerney MJ. Comparison of methods to detect biosurfactant production by diverse microorganisms. J Microbiol Methods. 2004;56(3):339–47. doi: 10.1016/j.mimet.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Ouoba LI, Thorsen L, Varnam AH. Enterotoxins and emetic toxins production by Bacillus cereus and other species of Bacillus isolated from Soumbala and Bikalga, African alkaline fermented food condiments. Int J Food Microbiol. 2008;124(3):224–30. doi: 10.1016/j.ijfoodmicro.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 30.Vater J, Kablitz B, Wilde C, Franke P, Mehta N, Cameotra SS. Matrix-assisted laser desorption ionization--time of flight mass spectrometry of lipopeptide biosurfactants in whole cells and culture filtrates of Bacillus subtilis C-1 isolated from petroleum sludge. Appl Environ Microbiol. 2002;68(12):6210–9. doi: 10.1128/AEM.68.12.6210-6219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sekhon K, Khanna S, Cameotra SS. Enhanced biosurfactant production through cloning of three genes and role of esterase in biosurfactant release. Microb Cell Fact. 2011;10(1):49. doi: 10.1186/1475-2859-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandrin C, Peypoux F, Michel G. Coproduction of surfactin and iturin A, lipopeptides with surfactant and antifungal properties, by Bacillus subtilis. Biotechnol Appl Biochem. 1990;12(4):370–5. [PubMed] [Google Scholar]
- 33.Darvishi P, Ayatollahi S, Mowla D, Niazi A. Biosurfactant production under extreme environmental conditions by an efficient microbial consortium, ERCPPI-2. Colloids Surf B Biointerfaces. 2011;84(2):292–300. doi: 10.1016/j.colsurfb.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Aguilar ML. Methods in Molecular biology. HPLC of Peptides and Proteins. Totowa New Jersey: Humana Press; 2004. [Google Scholar]
- 35.Pecci Y, Rivardo F, Martinotti MG, Allegrone G. LC/ESI-MS/MS characterisation of lipopeptide biosurfactants produced by the Bacillus licheniformis V9T14 strain. J Mass Spectrom. 2010;45(7):772–8. doi: 10.1002/jms.1767. [DOI] [PubMed] [Google Scholar]
- 36.Kim H, Yoon B, Lee C, Suh H, Oh H, Katsuragi T, et al. Production and properties of a lipopeptide biosurfactant from Bacillus subtilis C9. Journal of Fermentation and Bioengineering. 1997;84(1):41–6. doi: 10.1016/s0922-338x(97)82784-5. [DOI] [Google Scholar]
- 37.Cao XH, Liao ZY, Wang CL, Yang WY, Lu MF. Evaluation of a lipopeptide biosurfactant from Bacillus natto TK-1 as a potential source of anti-adhesive, antimicrobial and antitumor activities. Braz J Microbiol. 2009;40(2):373–9. doi: 10.1590/s1517-83822009000200030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukherjee S, Das P, Sivapathasekaran C, Sen R. Antimicrobial biosurfactants from marine Bacillus circulans: extracellular synthesis and purification. Lett Appl Microbiol. 2009;48(3):281–8. doi: 10.1111/j.1472-765X.2008.02485.x. [DOI] [PubMed] [Google Scholar]
- 39.Thaniyavarn J, Roongsawang N, Kameyama T, Haruki M, Imanaka T, Morikawa M, et al. Production and characterization of biosurfactants from Bacillus licheniformis F2.2. Biosci Biotechnol Biochem. 2003;67(6):1239–44. doi: 10.1271/bbb.67.1239. [DOI] [PubMed] [Google Scholar]
- 40.Singh P, Cameotra SS. Potential applications of microbial surfactants in biomedical sciences. Trends Biotechnol. 2004;22(3):142–6. doi: 10.1016/j.tibtech.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Moore T. Antagonistic Activity of Bacillus Bacteria against Food-Borne Pathogens. Journal of Probiotics & Health. 2013;01(03):2. doi: 10.4172/2329-8901.1000110. [DOI] [Google Scholar]
- 42.Sabate DC, Carrillo L, Audisio MC. Inhibition of Paenibacillus larvae and Ascosphaera apis by Bacillus subtilis isolated from honeybee gut and honey samples. Res Microbiol. 2009;160(3):193–9. doi: 10.1016/j.resmic.2009.03.002. [DOI] [PubMed] [Google Scholar]