Abstract
The self-renewal and differentiation of tissue stem cells must be tightly controlled. Unrestrained self-renewal leads to over-proliferation of stem cells, which may cause tumor formation, while uncontrolled differentiation leads to depletion of the stem cell pool. In this issue of The EMBO Journal, Demitrack et al (2015) show that the Notch pathway is a key regulator of Lgr5 antral stem cell self-renewal and differentiation. Notch signaling controls the proliferation and differentiation of stem cells as well as gastric tissue growth, while uncontrolled Notch activity in stem cells leads to polyp formation.
See also: ES Demitrack et al (October 2015)
Stem cells exhibit an extraordinary ability to generate multiple cell types in the body. Besides embryonic stem cells, tissue-specific stem cells serve a critical role during development as well as in homeostasis and injury repair in the adult. Stem cells renew themselves through proliferation as well as generate tissue-specific cell types through differentiation. The characteristics of different stem cells vary from tissue to tissue, and are determined by their intrinsic genetic and epigenetic status. However, the balance between self-renewal and differentiation of different stem cells is all stringently controlled. Uncontrolled self-renewal leads to overgrowth of stem cells and possibly tumor formation, while uncontrolled differentiation may exhaust the stem cell pool, leading to an impaired ability to sustain tissue homeostasis. Thus, stem cells continuously sense their environment and appropriately respond with proliferation, differentiation, or apoptosis. Remarkably, tissue stem cells from different tissues share a limited number of signaling pathways for the regulation of their self-renewal and differentiation, albeit in a very context-dependent manner. One of these pathways is the Notch pathway.
The Notch pathway represents an evolutionarily conserved signaling pathway that possesses a simple but unique mode of action (Fig 1A). The core Notch pathway contains only a small number of components. The canonical Notch pathway is activated through the binding of Notch ligand on the surface of signal-sending cells to the Notch receptor on neighbor signal-receiving cells. This event initiates a cascade of proteolytic cleavages of the Notch receptor, including γ-secretase-mediated release of the Notch intracellular domain (NICD). NICD fragment then enters the nucleus to induce target gene transcription. Under most circumstances, the canonical Notch pathway requires physical contact between neighboring cells; thus, it links the fate of one cell to that of an immediate neighbor, providing a sophisticated way to control the self-renewal and differentiation of stem cells. The Notch pathway has been shown to regulate many types of stem cells, including embryonic stem cells, neural stem cells, and hematopoietic stem cells as well as Lgr5 epithelial stem cells (VanDussen et al, 2012; Koch et al, 2013).
Figure 1. Notch pathway regulates Lgr5 stem cell self-renewal and differentiation.
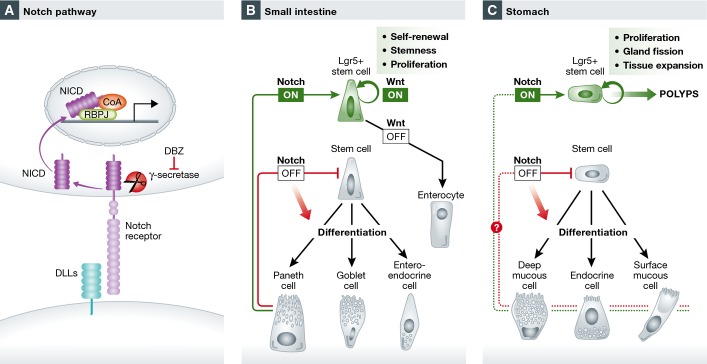
(A) The Notch signaling pathway. (B, C) Notch regulates the self-renewal and the differentiation of Lgr5 stem cells in (B) the small intestine and (C) the stomach as shown in Demitrack et al (2015).
Recently, Lgr5 has been identified in the small intestine and colon as a stem cell marker, where a small population of Lgr5+ crypt base columnar cells actively proliferate and generate all cell types in the epithelium (Barker et al, 2007). Lgr5 stem cells have since been identified in many other tissues, including kidney, hair follicle, and stomach (Haegebarth & Clevers, 2009; Barker et al, 2010). In the small intestine, the Notch pathway has been shown to control the proliferation and fate determination of Lgr5 stem cells (VanDussen et al, 2012) (Fig 1B). The immediate neighbor of Lgr5 stem cells—Paneth cells—provides Notch ligands to the stem cells to maintain their self-renewal (Sato et al, 2011). At the progenitor cell level, Notch activity determines the binary differentiation direction: activated Notch signaling promotes differentiation to cells with absorptive function, while its inactivation promotes differentiation to cells with secretory function (Crosnier et al, 2006). However, the role of the Notch pathway in gastric stem cell is unknown.
In this issue of The EMBO Journal, Demitrack et al (2015) performed a comprehensive analysis of the role of the Notch pathway in the self-renewal and differentiation of Lgr5 gastric stem cells. The authors first used an elegant Notch reporter system (NIP1::CreERT2; ROSAEYFP) to trace the activity of Notch active cells. In this system, Cre is linked to the intracellular domain of the Notch receptor. With the activation of Notch signaling, Cre is cleaved, but only in the presence of Tamoxifen can Cre enter the nucleus to activate the expression of EYFP to permanently mark the Notch activating cells and their progeny. Using this system, the authors showed Notch activating cells located at the base of antral glands, which later can generate all cells in the antral gland.
To test the role of Notch signaling in gastric antral stem cell homeostasis, the authors performed loss- and gain-of-function studies. Inhibiting the Notch pathway using a γ-secretase inhibitor or genetic deletion of Notch reduced overall epithelial cell as well as Lgr5 antral stem cell proliferation, while forced activation of Notch in Lgr5+ stem cells significantly increased epithelial cell and stem cell proliferation. These experiments clearly demonstrated the importance of the Notch pathway in regulating antral epithelial cell proliferation. Organoid formulation from stem cells is direct functional evidence for stem cell activity. Using this strategy, Demitrack et al (2015) further directly demonstrated Notch activity affected the function of Lgr5 stem cells. Increased or decreased Notch activity correspondingly increases or decreases organoid formation and growth. This is consistent with the role of Notch signaling in the intestine.
While in the intestine Notch activity determines fate decisions, a key question is if this also true in the stomach? Demitrack et al (2015) further tested the differentiation of Lgr5 antral stem cell following Notch manipulation. Unlike the intestine, in the stomach, Notch singling appears to uniformly affect all cell lineages via regulation of stem cell and progenitor cell proliferation while blocking their differentiation toward all lineages (Fig1C). This is likely due to the lack of absorptive cell types in the stomach. Thus, it is not surprising that the authors later found that constitutive Notch activation caused polyp formation in the stomach, a phenotype that was not observed in the small intestine. This suggests the possibility that Notch plays a more profound role in cell proliferation in the stomach than in the small intestine, as in the small intestine, simply activating Notch could lead to stem cell proliferation, or enterocyte differentiation, depending on the status of Wnt activity in the cells (Yin et al, 2014) (Fig 1B).
Given the finding that Notch activation promotes Lgr5 antral stem cell proliferation, it is tempting to speculate that continuous Notch activation will also promote stomach tissue growth, as it is postulated that postnatal stomach tissue growth occurs via gland fission in response to increased stem cell number. Indeed, Demitrack et al (2015) further observed an increased rate of gland fission by forced Notch activation in Lgr5 stem cells. Furthermore, using multi-color Lgr5, ROSAconfetti reporter mice, in which daughter cells of different Lgr5 stem cells are marked by different colors, the authors found Notch activation markedly increased the rate of monoclonal gland conversion, suggesting stem cells with constitutive Notch activation possess competitive advantage, which quickly outperform other stem cells and take over available niche spaces.
Finally, Demitrack et al (2015) demonstrate that Notch regulates stem cell proliferation potentially via regulating the mTOR pathway. Notch activation increases mTOR activity, while its inhibition decreases mTOR activity. Furthermore, the mTOR inhibitor rapamycin restores Notch activation-induced cell proliferation to baseline level. These data, although preliminary, offer an intriguing signaling link that may be relevant beyond Lgr5 antral stem cells.
The activity of the Notch pathway in Lgr5 antral stem cells provides a great example of stem cell regulation by extracellular cues. The behavior of stem cells is a function of coordinated interactions between cell autonomous programs and microenvironmental cues (the stem cell niche). The niche instructs the self-renewal of stem cells, while the stem cells compete for available niche real estate for their maintenance (Snippert et al, 2010). In the small intestine, Paneth cells provide Wnt and Notch ligand to stem cells to maintain their stemness, and daughter stem cells that lose contact with Paneth cells leave the niche and differentiate (Snippert et al, 2010; Sato et al, 2011). It will be interesting to explore this in the stomach (Fig 1C). Are there other niche signals for stomach stem cells and how do they cooperate with Notch to regulate stem cell behavior? The answers to these questions will have important implications for stomach-related diseases.
References
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- Demitrack ES, Gifford GB, Keeley TM, Carulli AJ, VanDussen KL, Thomas D, Giordano TJ, Liu Z, Kopan R, Samuelson LC. Notch signaling regulates gastric antral LGR5 stem cell function. EMBO J. 2015;34:2522–2536. doi: 10.15252/embj.201490583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegebarth A, Clevers H. Wnt signaling, lgr5, and stem cells in the intestine and skin. Am J Pathol. 2009;174:715–721. doi: 10.2353/ajpath.2009.080758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch U, Lehal R, Radtke F. Stem cells living with a Notch. Development. 2013;140:689–704. doi: 10.1242/dev.080614. [DOI] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, Clevers H. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- VanDussen KL, Carulli AJ, Keeley TM, Patel SR, Puthoff BJ, Magness ST, Tran IT, Maillard I, Siebel C, Kolterud A, Grosse AS, Gumucio DL, Ernst SA, Tsai YH, Dempsey PJ, Samuelson LC. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development. 2012;139:488–497. doi: 10.1242/dev.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Farin HF, van Es JH, Clevers H, Langer R, Karp JM. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat Methods. 2014;11:106–112. doi: 10.1038/nmeth.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]


