Abstract
Different authors have modelled myofascial tissue connectivity over a distance using cadaveric models, but in vivo models are scarce. The aim of this study was to evaluate the relationship between pelvic motion and deep fascia displacement in the medial gastrocnemius (MG). Deep fascia displacement of the MG was evaluated through automatic tracking with an ultrasound. Angular variation of the pelvis was determined by 2D kinematic analysis. The average maximum fascia displacement and pelvic motion were 1.501 ± 0.78 mm and 6.55 ± 2.47 °, respectively. The result of a simple linear regression between fascia displacement and pelvic motion for three task executions by 17 individuals was r = 0.791 (P < 0.001). Moreover, hamstring flexibility was related to a lower anterior tilt of the pelvis (r = 0.544, P < 0.024) and a lower deep fascia displacement of the MG (r = 0.449, P < 0.042). These results support the concept of myofascial tissue connectivity over a distance in an in vivo model, reinforce the functional concept of force transmission through synergistic muscle groups, and grant new perspectives for the role of fasciae in restricting movement in remote zones.
Keywords: fascia, musculoskeletal, tracking motion, ultrasound
Introduction
Fascia is a term derived from Latin meaning band or bandage (Benjamin, 2009). Within the field of anatomy, no area creates more confusion in terminology than the fasciae systems. For some authors, ‘fascia’ only includes densely banded connective tissues, where fibres have more than one dominant direction (Benetazzo et al. 2011). Other authors include within ‘fascia’ sheets of soft, transparent tissue, such as the hypodermis or subcutaneous tissue (Schleip et al. 2012). Importantly, Stecco (2015) defined the aponeurotic fascia as the deep fascia. Anatomically, two types of fasciae are generally considered: the superficial fascia, which is part of the subcutaneous tissue; and the deep fascia, which considers the connective tissue surrounding muscular groups (Benjamin, 2009). The term aponeurosis is used to describe flattened tendon fibres, whether intra- or extramuscular (Dauber, 2007). Aponeurosis is generally found within muscular tissue, such as a developing tendon. Moreover, in some wide muscles, such as the oblique abdominal muscles, the aponeurosis extends beyond the muscular tissue, thus making it visible upon dissection. For the purpose of this study, fascia was defined as the connective tissue constituted by irregularly arranged collagen fibres, as represented by a superficial fascia, present within the subcutaneous tissue, and a deep fascia, distinguished as the connective tissue surrounding and splitting the muscle groups. Moreover, fasciae are distinct from the parallel arrangement of collagen fibres present in structures, such as ligaments, tendons or aponeurosis (Standring, 2008).
A myofascial bond involves a close link between the deep fascia and the actual muscular tissue, and these structures cannot separate in terms of movement. The myofascial complex helps to modulate the transmission of force by muscles (Stecco, 2015). Moreover, several anchoring points are of interest, such as the juncture between the sacrotuberous ligament and the myofascial complex of the hamstring muscles. Recent studies using magnetic resonance have confirmed the continuity that exists between the sacrotuberous ligament and the tendon group of the semitendinosus and biceps femoris muscles, therefore establishing a clear point of force transmission between these anatomical elements (Bierry et al. 2014).
Additionally, the gastrocnemius muscle presents multiple fascial insertions and connections that connect with neighbouring structures, such as the hamstring and soleus. It is important to consider that the triceps surae muscle complex is formed by the two heads of the gastrocnemius and the soleus, varying in arrangement of their respective aponeuroses. The aponeurosis of the soleus is located on the superficial face, whereas the aponeurosis of the medial gastrocnemius (MG) is located deep within the complex. Independent of this, these aponeuroses do not bond until reaching the inferior myotendinous junction, a point that is continued by the Achilles tendon. The deep fascia of the popliteal region extends between the borders of the popliteal fossa and the superior hamstring muscles and the inferior gastrocnemius, thus creating a connection between both muscles (Latarjet & Ruiz-Liard, 2004).
In an in vivo context, muscle is not an isolated entity, as myofascial tissues mechanically connect neighbouring muscular and non-muscular structures (Yucesoy, 2010). Indeed, the tension produced by the muscle is transmitted not only by the tendon, but also inside and around the muscle by the endomysium, perimysium and epimysium and by extramuscular connective tissues, such as the deep fasciae and the neurovascular tract (Huijing, 2009; Maas & Sandercock, 2010; Purslow, 2010). This degree of connectivity is complemented by the intermuscular septa, fascial compartments and tendon sheaths, as well as by the bone through the periosteum (Stecco et al. 2006).
Several studies support the concept that connective tissue participates in the transmission of myofascial force (Huijing & Baan, 2008; Yucesoy et al. 2010). For example, the thoracolumbar fascia responds to the mechanical traction induced by muscular activity in order to effectively transfer the load between the spine, pelvis, legs and arms (Vleeming et al. 1995). This connectivity of fascial tissue has been observed in cadavers, suggesting fascial continuity at different regions between the pelvis and feet (Gerlach & Lierse, 1990). Furthermore, fasciae play an important role not only in the transmission of force but also in proprioception, as supported by the encapsulated receptors observed in fasciae, specifically the Ruffini and Pacini corpuscles (Schleip, 2003; Stecco et al. 2007).
Although the force transmission and proprioceptive functions of fasciae are known, biomechanical models seem to fail to account for the former, and this failure to account for the force transmission could mean misinterpreting the true extent of biomechanical complexity (Carvalhais et al. 2013). Therefore, studying the deep fascia has become a focal point for scientists and manual therapists (Grimm, 2007). However, in vivo models demonstrating myofascial tissue connectivity over a distance are scarce (Carvalhais et al. 2013). Indeed, some authors have questioned the existence of myofascial force transmission in response to the physiological motion of joints (Herbert et al. 2008; Maas & Sandercock, 2008). Thus, an integrated comprehension of the myofascial connectivity paradigm and its respective therapeutic bias would help to support the theory of fascial connectivity over a distance, as previously observed in cadaveric dissections (Vleeming et al. 1995; Myers, 2001; Stecco et al. 2009).
The aim of the present study was to demonstrate the relationship between pelvic motion when seated with the knees extended with the displacement of the deep fascia of the MG of the dominant limb during pelvic anteversion.
Materials and methods
Subjects
With the approval of the local Health Service, 17 asymptomatic, sedentary young male individuals were recruited. Individuals were excluded if they presented any current or former neuromusculoskeletal dysfunction, any pathological condition of the spine, pre-existing systemic rheumatological condition, or any current or chronic respiratory disease. Ethical approval was obtained from the Northern Metropolitan Health Service of Santiago, Chile, and informed consent from each participant was required.
Equipment
A 5–10-MHz linear transducer (SonoSite Titan; Sonosite, Bothell, WA, USA) was used. Ultrasound videos were recorded at a depth of 39 mm and a sampling rate of 30 frames per second (fps) with a video camera (Pinacle Dazzle DVD Recorder HD).
For 2D video-photogrammetric analysis of pelvic motion, a GoPro Hero 3 (GoPro, San Mateo, CA, USA) camera was used at a recording rate of 60 fps and resolution of 1440 × 1440 pixels. Synchronization between pelvis kinematics and the detection of deep fascia displacement of the MG was performed offline with pressure sensors.
Procedure and task execution
For each individual, the range of joint motion (ROM) of the dominant limb was evaluated with a universal goniometer. Hamstring elasticity was determined using the active knee extension test, which has excellent reliability for both intra- and inter-raters (Hamid et al. 2013). To determine the ROM, 180 ° was subtracted from the range obtained in the test. The elasticity of the MG was evaluated by weight bearing. This measurement provides a reliable evaluation with low measurement error, even by inexperienced raters (Konor et al. 2012).
Pelvic motion during the exercise was determined according to established 2D marking protocols (Kuo et al. 2009). The accurate application of this protocol must follow certain recommendations to diminish the soft tissue artefact (STA), especially on the anterior superior iliac spine. For instance, this measurement must be taken in young subjects (Kuo et al. 2008) with a healthy weight (Vanneuville et al. 1996). Furthermore, studies recommend not exceeding 90 ° of hip flexion because this can produce a range of error of −2 ± 1 ° (Hara et al. 2014).
The ultrasound equipment transducer was positioned on the muscle belly of the MG on the dominant limb using a fixing device that was composed of a thermoplastic polymer and two elastic bands with Velcro (Fig.1).
Fig 1.

Placement of the ultrasound transducer.
The task was rehearsed for 5 min prior to measurements. Subsequently, the individual rested for 10 min. Each individual was asked to perform the exercise three times. Each task consisted of moving from maximum retroversion of the pelvis, with the hips at 80 °, knees fully extended and ankles at 0 °, to a position of pelvic anteversion. To ensure that movement only occurred at the pelvis, the knee was fixed at full extension by a strap across the patella, which was adjusted as tightly as possible without causing pain or irritation for the subject. The task was considered successful if movement was only recorded for the pelvis and, if this condition was not met, then the test was nulled. Task execution was standardized with a metronome, and the individual had to move from pelvic retroversion to anteversion within 4 s (Fig.2).
Fig 2.

Retroversion–anteversion cycle.
2D tracking of the pelvis and deep fascia displacement of the MG tracking
Measurement was performed by a three-stage automatic method: (i) tracking of the pelvis; (ii) deep fascia displacement of the MG tracking; and (iii) manual method comparison.
To track the markers of the pelvis, Lucas–Kanade affine template tracking (Kroon, 2009) was used based on automatic range of interest (ROI) tracking. Finally, the pelvis angular variation curve during the retroversion–anteversion cycle was measured with a low-pass 6-Hz filter (Winter et al. 1974).
To determine deep fascia displacement of the MG, a Lucas–Kanade tracker with pyramid and iteration (Wiggin, 2009) was used. This method has also been used to evaluate deep fascia displacement of the MG near the myotendinous junction (Magnusson et al. 2003; Bojsen-Møller et al. 2004, 2010; Stenroth et al. 2012; Peltonen et al. 2013). This algorithm is accurate in regards to a known distance (10.0 mm) to 0.2 mm. This indicates an acceptable degree of precision and a repeatability of 98% for the measurement of the deep fascia of the MG (Magnusson et al. 2003) during isometric contractions.
In this study, the code implemented for follow-up of the fascia consisted of determining a ROI, in which a line was traced above the deep fascia of the MG. The width of the ROI was 1.42 mm. Within the ROI, 9 pixels to greater represent the sample were automatically detected with the points detect SURF Features Matlab® software function. Lucas–Kanade pyramidal tracking was implemented in such a manner that if the standard deviation of pixel displacement was greater than 1, this pixel would be removed, leaving at least 6 pixels for analysis in order to average the final displacement.
To establish the reliability of the displacement of the deep fascia during MG tracking using tracking of pelvic markers, the maximum value obtained by automated tracking vs. manual tracking was offset using the Bland–Altman plot method in 20 videos.
Data analysis
All of the data were analysed with spss software (v. 22.00 for Windows, IL, USA). A P-value of < 0.05 was considered statistically significant. To evaluate normal data distribution, a Shapiro–Wilk test was used. All data had a normal distribution except for the hamstring elasticity of the dominant limb variable. To establish the reliability of the proposed model, intrasubject reliability for deep fascia displacement of the MG and maximum anteversion ROM was determined by using the intraclass correlation coefficient.
To analyse the correlation of averages between different variables for the three tests, the Spearman and Pearson correlations were used according to the normality test. The levels of correlation were categorized as low (r > 0.3), mid (r ≤ 0.6) and high (r > 0.6) correlations (Portney & Watkins, 2009).
Finally, a simple linear regression was performed between the ROM variables of the pelvis and displacement of the fascia during task execution.
Results
All participants were male, right-hand dominant in the lower limb, aged 22.76 ± 1.8 years, and 1.74 ± 0.5 m tall. The participants' body mass index (BMI) was 23.95 ± 1.33 kg∙m−2.
Intrasubject reliability for the fascia displacement model was 0.903 [confidence interval (CI) 95%; 0.782–0.962], with P < 0.001 and a variation coefficient of 30 ± 15%. The intrasubject reliability for the pelvis ROM was 0.781 (CI 95%; 0.509–0.914), with P < 0.001 and a variation coefficient of 30 ± 16%.
Reliability between manual tracking and tracking with the Lucas–Kanade pyramidal algorithm was 0.973 (CI 95%; 0.993–0.989; P < 0.001). Using the Bland–Altman plot, an average difference of 0.034 mm was observed between the two methods, with an according percentage of < 0.95%. Lucas–Kanade template tracking reliability for determining the maximum inclination of the pelvis during task execution was 0.967 (CI 95%; 0.917–0.987; P < 0.001). An average difference of 0.879 ° was observed with the Bland–Altman plot between both methods, with an according percentage of < 0.95%.
The maximum average displacement of all of the individuals was 1.501 ± 0.78 mm, and the average maximum range reached by the pelvis during the retroversion–anteversion cycle was 6.55 ± 2.47 ° (Fig.3).
Fig 3.
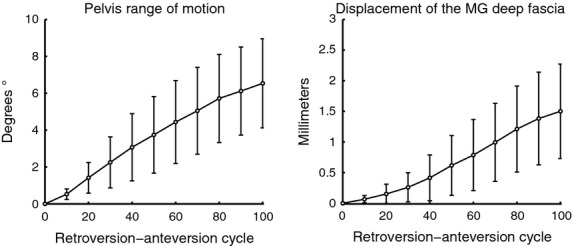
Average pelvis range of motion and displacement of the MG deep fascia during the retroversion–anteversion cycle.
The result of normalized simple linear regression for each 10% of the total cycle between fascia displacement and the pelvis ROM for the three task executions of the 17 individuals was r = 0.791 (P < 0.001). The relationship between the maximum range of anteversion and the maximum pelvis displacement in the individuals was r = 0.620 (P > 0.001; Table1).
Table 1.
Correlations among variables
| RP | DF | HE | MGE | BMI | |
|---|---|---|---|---|---|
| RP | 1 | r = 0.620* (P = 0.008) | r = 0.544* (P = 0.024) | r = 0.112 (P = 0.668) | r = −0.560* (P = 0.019) |
| DF | 1 | r = 0.499* (P = 0.042) | r = −0.191 (P = 0.464) | r = −0.394 (P = 0.117) | |
| HE | 1 | r = −0.113 (P = 0.333) | r = −0.533* (P = 0.028) | ||
| MGE | 1 | r = −0.054 (P = 0.838) | |||
| BMI | 1 |
BMI, body mass index; DF, displacement of the fascia; HE, hamstring elasticity; MGE, medial gastrocnemius elasticity; RP, range of motion of the pelvis.
P < 0.05.
Discussion
An important step towards understanding distant myofascial tissue connectivity is to validate measurement methodology. The present model of myofascial connectivity over a distance between the pelvis and leg showed good reliability. To the authors' current knowledge, this study is the first to suggest a high correlation between pelvis motion and the displacement of the MG fascia during anteversion. Although other studies have confirmed an in vivo transmission of myofascial force, for example between the soleus and gastrocnemius (Bojsen-Møller et al. 2010; Huijing et al. 2011), studies that show the degree of myofascial tissue connectivity over a distance are scarce (Carvalhais et al. 2013). While some authors have unsuccessfully attempted to demonstrate connectivity between pelvic motion and the position of the ankle (Mitchell et al. 2008), these studies have not directly evaluated fascia motion.
Key anatomical points involved in fascial connectivity
The gastrocnemius is surrounded by the deep fascia on the superficial surface, but this fascia presents few important insertions. This is contrary to what occurs with the fascia covering the deep face, which is intimately connected with the aponeurosis of the muscle (Saraph et al. 2000; Blitz & Eliot, 2007; Fig.4).
Fig 4.
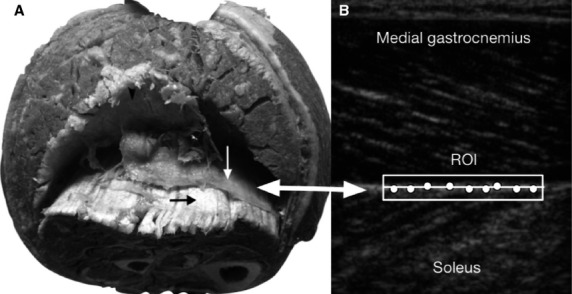
(A) Transverse slice of the leg, showing the partition of the deep fascia (white arrow) between the gastrocnemius and soleus. The aponeuroses of the soleus (black arrow) and gastrocnemius (black arrowhead) are also shown. (B) Sagittal slice. Ultrasound image with the ROI and the points automatically selected for posterior tracking of the deep fascia.
Moreover, there are anatomical anchoring points key to understanding the present study, including: (i) the juncture between the sacrotuberous ligament and the myofascial complex of the hamstring muscles (Fig.5); (ii) the juncture between the hamstring and gastrocnemius through the deep fascia in the popliteal region (Latarjet & Ruiz-Liard, 2004); and (iii) the juncture between the gastrocnemius and soleus through multiple fascial insertions and connections. This final point represents the closest bond within the inferior myotendon, a point at which both aponeuroses join and continue with the Achilles tendon (Fig.6). These three anchoring points provide an anatomical background for explaining the deep fascia displacement of the MG during the pelvis anteversion observed in the present study. This observation contributes to comprehending the anatomical mechanisms involved in force transmission between the pelvis and the legs.
Fig 5.
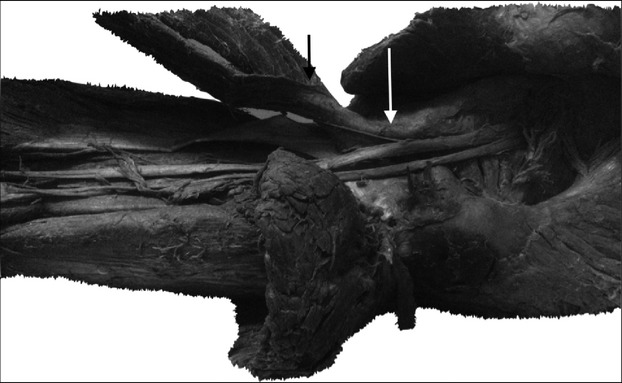
Arrangement of the sacrotuberous ligament at its distal insertion on the level of ischial tuberosity and its continuity with the semitendinosus and biceps femoris muscles. Sacrotuberous ligament (white arrow) and hamstring muscles (black arrow).
Fig 6.
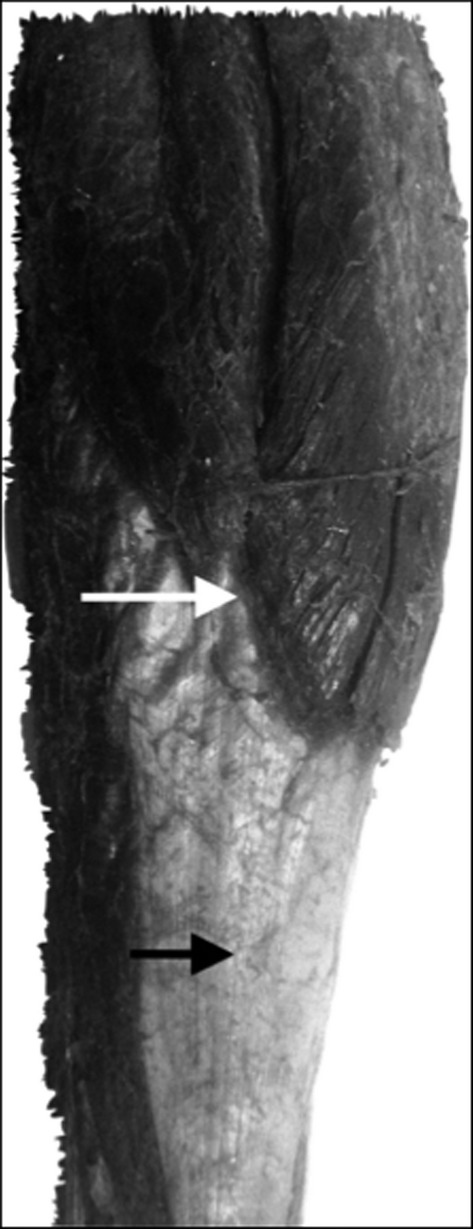
Image showing the myotendinous junction between the gastrocnemius and its aponeurosis (white arrow). Below this is the formation of the Achilles tendon (black arrow).
Functional implications
A high correlation between individuals with a higher pelvis ROM and deep fascia displacement of the MG was found. This correlation is confirmed by Barker & Briggs (1999), who produced a model suggesting that fasciae in the thoracolumbar region facilitate a charge transfer between the legs and the trunk. A long portion of the biceps femoris, which continues with the sacrotuberous ligament and thoracolumbar fascia, would provide for this transmission of force (Vleeming et al. 1995). Likewise, this concept can be complemented by observations made by Carvalhais et al. (2013), who empirically demonstrated the transmission of myofascial force over a distance, for example, between the latissimus dorsi and contralateral gluteus maximus.
The current observations showed that hamstring flexibility is related to a lower anterior tilt of the pelvis (r = 0.544, P < 0.024) and lower deep fascia displacement of the MG (r = 0.449, P < 0.042). This result is consistent with previous studies in which hamstring muscle shortening is related to decreased motion amplitude in pelvic anteversion and lumbar mobility (Gajdosik et al. 1994; Congdon et al. 2005; López-Miñarro & Alacid, 2010), and it reinforces the concept of force transmission through synergistic muscle groups and grants new perspectives for the role of fasciae in restricting movement in remote zones.
From a functional point of view, fascial tissue connectivity over a distance would play an important role due to the association between deep fasciae and muscle, which uses both fasciae and tendon expansion for force transmission (Stecco et al. 2008; Benjamin, 2009). Moreover, from a physiological point of view, this mechanism most likely serves as a mechanosensitive signalling system, as an analogous integration function to the nervous system (Langevin et al. 2004, 2006).
Nevertheless, to confirm this physiological mechanism of force transmission in the musculoskeletal system, future studies should include similar study models in other muscular groups and consider the pathological conditions affecting the viscoelastic behaviour of the fasciae.
Methodological aspects of in vivo fascia displacement measurements
The ROI selected with the ultrasound indicated the deep fascia partition between the soleus and the gastrocnemius (Fig.4). In turn, the hyperechogenic zone selected within the ROI indicated the connective tissue. This zone would automatically include the deep fascia partition and the aponeurosis. This final point is important to mention, as it was difficult to differentiate between the fascia and aponeurosis in the automatic ultrasound tracking analysis. While defining the hyperechogenic zone as fascia or aponeurosis is currently practical and accessible, the interpretation of the present study is that it is the fascia involved in the detected displacement.
On the other hand, different studies have registered displacement of the fascia using the same algorithm as in this study (Bojsen-Møller et al. 2004, 2010; Stenroth et al. 2012; Peltonen et al. 2013). Specifically, Bojsen-Møller et al. (2004) observed an average proximal displacement of 12.6 ± 1.7 mm of the MG deep fascia during maximum isometric contraction with an extended knee. The present study found a distal movement of the MG deep fascia of 1.501 ± 0.78 mm, corresponding to 11% of the total deep fascia displacement of the MG during maximum, voluntary isometric contraction.
The current study used 2D uniaxial tracking to monitor the passive stretching of the MG deep fascia during pelvic anteversion. This method was based on a recent study by Azizi & Roberts (2009), who observed biaxial deformation of the aponeurosis in wild turkeys using high-speed biplanar fluoroscopy to track the deformation of the lateral gastrocnemius aponeurosis. They observed that as a passive force was produced, the aponeurosis only deformed in a longitudinal direction. This is consistent with uniaxial deformation; however, during active force, the aponeurosis stretches both longitudinally and transversely.
To the authors' current understanding, pelvic anteversion through fascial connections would only generate passive longitudinal movement in the deep fascia of the MG. This supposition is in line with recent findings by Andrade et al. (2015) who, using a model similar to that of the present study, found that while hip flexion decreased the range of movement in the ankles, it did not increase the electrical activity of the gastrocnemius and the soleus, suggesting that the restriction could be due to the fascial and neural tissues. Along these same lines, other studies have observed that hamstring stretching in cycling loads, as well as increased tension of the sciatic nerve, did not increase electrical activity in the hamstrings (Nordez et al. 2008; McHugh et al. 2012). Considering the prior, it is probable that the movement recorded for the deep fascia of the MG in the present study is due to passive movement and not due to reflex mechanisms as a result of muscular stretching. However, it is important to stress that these findings were for asymptomatic subjects without central nervous system damage, a condition that would exacerbate the myotatic reflex (Powers et al. 1988; Sterling et al. 2001).
Within the limitations of this study, it is important to mention that fascia displacement was measured only at the level of the MG. However, incorporating more than one ultrasound transducer allowed for simultaneously determining the fascia displacement of the hamstring and gastrocnemius. However, this only modelled the movement of the fascia over a distance, and further investigation is needed to evaluate the displacement of other tissues with this proposed model, such as in neural tissue. With regards to the marking protocol used in the present study, it is important to highlight that while certain criteria were used to diminish the STA, the final range of anteversion could have been greater than that observed when considering that reported by Hara et al. (2014). In addition to this, it is worth mentioning that these results are replicable only in young subjects with a healthy weight because under other conditions the STA could generate a greater range of error than that managed by this study.
Conclusions
The findings of this study validate a simple and reliable model for assessing deep fascia displacement through pelvis anteversion. The results reaffirm and enforce the concepts of myofascial tissue connectivity. This reinforces the functional concept of force transmission through synergistic muscle groups and grants new perspectives for the role of the fasciae in restricting movement in remote zones.
Acknowledgments
The authors would like to thank the Programa de Anatomía y Biología del Desarrollo, Faculty of Medicine, University of Chile, Santiago, Chile. MC is funded by grants FONDECYT 3140447, ICM P09-015-F and the Latin American Cancer Research Network (US-LACRN).
Author contributions
Carlos Cruz-Montecinos: acquisition of data, data analysis, interpretation, contributions to concept and design, drafting of the manuscript, critical revision of the manuscript, and approval of the article; Alberto González Blanche, David López Sánchez and Mauricio Cerda: data interpretation, contributions to concept and design, critical revision of the manuscript, and approval of the article; Rodolfo Sanzana-Cuche: contributions to concept and design, critical revision of the manuscript, and approval of the article; and Antonio Cuesta-Vargas: data interpretation, contributions to concept and design, drafting of the manuscript, critical revision of the manuscript, and approval of the article.
References
- Andrade RJ, Lacourpaille L, Freitas SR, et al. Effects of hip and head position on ankle range of motion, ankle passive torque, and passive gastrocnemius tensión. Scand J Med Sci Sports. 2015 doi: 10.1111/sms.12406. doi: 10.1111/sms.12406. [DOI] [PubMed] [Google Scholar]
- Azizi E, Roberts TJ. Biaxial strain and variable stiffness in aponeuroses. J Physiol. 2009;587:4309–4318. doi: 10.1113/jphysiol.2009.173690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker PJ, Briggs CA. Attachments of the posterior layer of lumbar fascia. Spine. 1999;24:1757–1764. doi: 10.1097/00007632-199909010-00002. [DOI] [PubMed] [Google Scholar]
- Benetazzo L, Bizzego A, De Caro R, et al. 3D reconstruction of the crural and thoracolumbar fasciae. Surg Radiol Anat. 2011;33:855–862. doi: 10.1007/s00276-010-0757-7. [DOI] [PubMed] [Google Scholar]
- Benjamin M. The fascia of the limbs and back – a review. J Anat. 2009;214:1–18. doi: 10.1111/j.1469-7580.2008.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierry G, Simeone FJ, Borg-Stein JP, et al. Sacrotuberous ligament: relationship to normal, torn, and retracted hamstring tendons on MR images. Radiology. 2014;271:162–171. doi: 10.1148/radiol.13130702. [DOI] [PubMed] [Google Scholar]
- Blitz NM, Eliot DJ. Anatomical aspects of the gastrocnemius aponeurosis and its insertion: a cadaveric study. J Foot Ankle Surg. 2007;46:101–108. doi: 10.1053/j.jfas.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Bojsen-Møller J, Hansen P, Aagaard P, et al. Differential displacement of the human soleus and medial gastrocnemius aponeuroses during isometric plantar flexor contractions in vivo. J Appl Physiol. 2004;97:1908–1914. doi: 10.1152/japplphysiol.00084.2004. [DOI] [PubMed] [Google Scholar]
- Bojsen-Møller J, Schwartz S, Kalliokoski KK, et al. Intermuscular force transmission between human plantarflexor muscles in vivo. J Appl Physiol. 2010;109:1608–1618. doi: 10.1152/japplphysiol.01381.2009. [DOI] [PubMed] [Google Scholar]
- Carvalhais VO, Ocarino Jde M, Araújo VL, et al. Myofascial force transmission between the latissimus dorsi and gluteus maximus muscles: an in vivo experiment. J Biomech. 2013;46:1003–1007. doi: 10.1016/j.jbiomech.2012.11.044. [DOI] [PubMed] [Google Scholar]
- Congdon R, Bohannon R, Tiberio D. Intrinsic and imposed hamstring length influence posterior pelvic rotation during hip flexion. Clin Biomech. 2005;20:947–951. doi: 10.1016/j.clinbiomech.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Dauber W. Pocket Atlas of Human Anatomy by Feneis. 4th edn. New York: Thieme Verlag; 2007. [Google Scholar]
- Gajdosik RL, Albert CR, Mitman JJ. Influence of hamstring length on the standing position and flexion range of motion of the pelvic angle, lumbar angle, and thoracic angle. J Orthop Sports Phys Ther. 1994;20:213–219. doi: 10.2519/jospt.1994.20.4.213. [DOI] [PubMed] [Google Scholar]
- Gerlach UJ, Lierse W. Functional construction of the superficial and deep fascia system of the lower limb in man. Acta Anat. 1990;139:11–25. doi: 10.1159/000146973. [DOI] [PubMed] [Google Scholar]
- Grimm D. Biomedical research. Cell biology meets rolfing. Science. 2007;318:1234–1235. doi: 10.1126/science.318.5854.1234. [DOI] [PubMed] [Google Scholar]
- Hamid MS, Ali MR, Yusof A. Interrater and intrarater reliability of the active knee extension (AKE) test among healthy adults. J Phys Ther Sci. 2013;25:957–961. doi: 10.1589/jpts.25.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara R, Sangeux M, Baker R, et al. Quantification of pelvic soft tissue artifact in multiple static positions. Gait Posture. 2014;39:712–717. doi: 10.1016/j.gaitpost.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Herbert RD, Hoang PD, Gandevia SC. Are muscles mechanically independent? J Appl Physiol. 2008;104:1549–1550. doi: 10.1152/japplphysiol.90511.2008. [DOI] [PubMed] [Google Scholar]
- Huijing PA. Epimuscular myofascial force transmission: a historical review and implications for new research. International Society of Biomechanics Muybridge Award Lecture, Taipei, 2007. J Biomech. 2009;42:9–21. doi: 10.1016/j.jbiomech.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Huijing PA, Baan GC. Myofascial force transmission via extramuscular pathways occurs between antagonistic muscles. Cells Tissues Organs. 2008;188:400–414. doi: 10.1159/000118097. [DOI] [PubMed] [Google Scholar]
- Huijing PA, Yaman A, Ozturk C, et al. Effects of knee joint angle on global and local strains within human triceps surae muscle: MRI analysis indicating in vivo myofascial force transmission between synergistic muscles. Surg Radiol Anat. 2011;33:869–879. doi: 10.1007/s00276-011-0863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konor MM, Morton S, Eckerson JM, et al. Reliability of three measures of ankle dorsiflexion range of motion. Int J Sports Phys Ther. 2012;7:279–287. [PMC free article] [PubMed] [Google Scholar]
- Kroon D-J. 2009. OpenCV. Available at www.mathworks.com (accessed August 2014)
- Kuo YL, Tully EA, Galea MP. Skin movement errors in measurement of sagittal lumbar and hip angles in young and elderly subjects. Gait Posture. 2008;27:264–270. doi: 10.1016/j.gaitpost.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Kuo YL, Tully EA, Galea MP. Video analysis of sagittal spinal posture in healthy young and older adults. J Manipulative Physiol Ther. 2009;32:210–215. doi: 10.1016/j.jmpt.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Langevin HM, Cornbrooks CJ, Taatjes DJ. Fibroblasts form a body-wide cellular network. Histochem Cell Biol. 2004;122:7–15. doi: 10.1007/s00418-004-0667-z. [DOI] [PubMed] [Google Scholar]
- Langevin HM, Storch KN, Cipolla MJ, et al. Fibroblast spreading induced by connective tissue stretch involves intracellular redistribution of alpha- and beta-actin. Histochem Cell Biol. 2006;125:487–495. doi: 10.1007/s00418-005-0138-1. [DOI] [PubMed] [Google Scholar]
- Latarjet M, Ruiz-Liard A. Anatomía Humana, Ed. Médica Panamericana. 4th edn. Panamericana: Buenos Aires; 2004. [Google Scholar]
- López-Miñarro PA, Alacid F. Influence of hamstring muscle extensibility on spinal curvatures in young athletes. Sci Sports. 2010;25:188–193. [Google Scholar]
- Maas H, Sandercock TG. Are skeletal muscles independent actuators? Force transmission from soleus muscle in the cat. J Appl Physiol. 2008;104:1557–1567. doi: 10.1152/japplphysiol.01208.2007. [DOI] [PubMed] [Google Scholar]
- Maas H, Sandercock TG. Force transmission between synergistic skeletal muscles through connective tissue linkages. J Biomed Biotechnol. 2010;2010:575672. doi: 10.1155/2010/575672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson SP, Hansen P, Aagaard P. Differential strain patterns of the human gastrocnemius aponeurosis and free tendon, in vivo. Acta Physiol Scand. 2003;177:185–195. doi: 10.1046/j.1365-201X.2003.01048.x. [DOI] [PubMed] [Google Scholar]
- McHugh MP, Johnson CD, Morrison RH. The role of neural tension in hamstring flexibility. Scand J Med Sci Sports. 2012;22:164–169. doi: 10.1111/j.1600-0838.2010.01180.x. [DOI] [PubMed] [Google Scholar]
- Mitchell B, Bressel E, McNair PJ, et al. Effect of pelvic, hip, and knee position on ankle joint range of motion. Phys Ther Sport. 2008;9:202–208. doi: 10.1016/j.ptsp.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Myers TW. Anatomy Trains. Oxford: Churchill Livingstone; 2001. [Google Scholar]
- Nordez A, McNair P, Casari P, et al. Acute changes in hamstrings musculo-articular dissipative properties induced by cyclic and static stretching. Int J Sports Med. 2008;29:414–418. doi: 10.1055/s-2007-964980. [DOI] [PubMed] [Google Scholar]
- Peltonen J, Cronin NJ, Stenroth L, et al. Viscoelastic properties of the Achilles tendon in vivo. Springerplus. 2013;2:212. doi: 10.1186/2193-1801-2-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. 3rd edn. Upper Saddle River, NJ: Pearson Prentice Hall; 2009. [Google Scholar]
- Powers RK, Marder-Meyer J, Rymer WZ. Quantitative relations between hypertonia and stretch reflex threshold in spastic hemiparesis. Ann Neurol. 1988;23:115–124. doi: 10.1002/ana.410230203. [DOI] [PubMed] [Google Scholar]
- Purslow PP. Muscle fascia and force transmission. J Bodyw Mov Ther. 2010;14:411–417. doi: 10.1016/j.jbmt.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Saraph V, Zwick EB, Uitz C, et al. The Baumann procedure for fixed contracture of the gastrosoleus in cerebral palsy. Evaluation of function of the ankle after multilevel surgery. J Bone Joint Surg Br. 2000;82:535–540. doi: 10.1302/0301-620x.82b4.9850. [DOI] [PubMed] [Google Scholar]
- Schleip R. Fascial plasticity – a new neurobiological explanation: part. J Bodyw Mov Ther. 2003;7:11–19. [Google Scholar]
- Schleip R, Jäger H, Klingler W. What is “fascia”? A review of different nomenclatures. J Bodyw Mov Ther. 2012;16:496–502. doi: 10.1016/j.jbmt.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Standring S. Gray's Anatomy, the Anatomical Basis of Clinical Practice. 40th edn. Edinburgh: Elsevier Churchill Livingstone; 2008. [Google Scholar]
- Stecco C. Functional Atlas of the Human Fascial System. Edinburgh: Elsevier Health Sciences; 2015. pp. 51–102. [Google Scholar]
- Stecco C, Porzionato A, Macchi V, et al. Histological characteristics of the deep fascia of the upper limb. Ital J Anat Embryol. 2006;111:105–110. [PubMed] [Google Scholar]
- Stecco C, Gagey O, Belloni A, et al. Anatomy of the deep fascia of the upper limb. Second part: study of innervation. Morphologie. 2007;91:38–43. doi: 10.1016/j.morpho.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Stecco C, Porzionato A, Macchi V, et al. The expansions of the pectoral girdle muscles onto the brachial fascia: morphological aspects and spatial disposition. Cells Tissues Organs. 2008;188:320–329. doi: 10.1159/000121433. [DOI] [PubMed] [Google Scholar]
- Stecco A, Macchi V, Stecco C, et al. Anatomical study of myofascial continuity in the anterior region of the upper limb. J Bodyw Mov Ther. 2009;13:53–62. doi: 10.1016/j.jbmt.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Stenroth L, Peltonen J, Cronin NJ, et al. Age-related differences in Achilles tendon properties and triceps surae muscle architecture in vivo. J Appl Physiol. 2012;10:1537–1544. doi: 10.1152/japplphysiol.00782.2012. [DOI] [PubMed] [Google Scholar]
- Sterling M, Jull G, Wright A. The effect of musculoskeletal pain on motor activity and control. J Pain. 2001;2:135–145. doi: 10.1054/jpai.2001.19951. [DOI] [PubMed] [Google Scholar]
- Vanneuville G, Monnet JP, Vacheron JJ, et al. Determination of the position of the pelvic girdle by the method of external markers in the sagittal plane: a preliminary feasibility study. Surg Radiol Anat. 1996;18:245–247. [PubMed] [Google Scholar]
- Vleeming A, Pool-Goudzwaard AL, Stoeckart R, et al. The posterior layer of the thoracolumbar fascia. Its function in load transfer from spine to legs. Spine. 1995;20:753–758. [PubMed] [Google Scholar]
- Wiggin E. 2009. OpenCV. Available at www.mathworks.com (accessed August 2014)
- Winter DA, Sidwall HG, Hobson DA. Measurement and reduction of noise in kinematics of locomotion. J Biomech. 1974;7:157–159. doi: 10.1016/0021-9290(74)90056-6. [DOI] [PubMed] [Google Scholar]
- Yucesoy CA. Epimuscular myofascial force transmission implies novel principles for muscular mechanics. Exerc Sport Sci Rev. 2010;38:128–134. doi: 10.1097/JES.0b013e3181e372ef. [DOI] [PubMed] [Google Scholar]
- Yucesoy CA, Baan G, Huijing PA. Epimuscular myofascial force transmission occurs in the rat between the deep flexor muscles and their antagonistic muscles. J Electromyogr Kinesiol. 2010;20:118–126. doi: 10.1016/j.jelekin.2008.09.012. [DOI] [PubMed] [Google Scholar]


