Abstract
The effects of ex vivo preservation techniques on the quality of diffusion tensor magnetic resonance imaging in hearts are poorly understood, and the optimal handling procedure prior to investigation remains to be determined. Therefore, 24 porcine hearts were examined in six groups treated with different preservation techniques, including chemical fixation and freezing. Diffusion properties of each heart were assessed with diffusion tensor imaging in terms of fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (Da) and radial diffusivity (Dr). Tractography was performed to visualize the course of the cardiomyocytes, assuming greater diffusivity in the longitudinal than the transverse axis of individual cardiomyocytes. Significant differences in MD, Da and Dr were found, as well as in FA between groups (P < 0.001). Freezing of specimens resulted in the lowest mean FA of 0.21 (0.06) and highest Dr of 8.92 (1.5) mm2 s−1. The highest mean FA was found to be 0.43 (0.11) in hearts perfusion-fixed with formalin. Calculated tractographies were indistinguishable among groups except in frozen specimens, where no fibres could be tracked. Perfusion fixation with formalin provided the best tractography, but immersion fixation yielded diffusion data most similar to fresh hearts. These findings suggest that parameters derived from diffusion tensor imaging in ex vivo hearts are sensitive to fixation and storage methods. In particular, freezing of specimens should be avoided prior to diffusion tensor imaging investigation due to significant changes in diffusion parameters and subsequent image deteriorations.
Keywords: ex vivo DTMRI, fibre tracking, myocardium, preservation methods, tractography
Introduction
Diffusion tensor magnetic resonance imaging (DTMRI) was initially used to describe the neural axons of white matter and their neural connectivity. The method has recently shown potential for the investigation of the spinal cord and peripheral nerves, kidney, blood vessels, skeletal muscles and the heart (Smerup et al. 2009; Gaudiano et al. 2011; Flamini et al. 2013; Jirjis et al. 2013; Naraghi et al. 2013; Scheel et al. 2013). In the heart a direct correlation has been shown between myocyte angulations measured by histology and tractography produced from DTMRI (Holmes et al. 2000), allowing for novel representations of the complex 3D cardiomyocyte network (Smerup et al. 2009). Unfortunately, in vivo applications of DTMRI in the heart or abdomen are very difficult as DTMRI sequences are intrinsically sensitive to movement. However, post mortem DTMRI can be performed on excised specimens with high spatial resolution.
Autolysis processes occur rapidly when specimens are removed from the body. The autolytic effects on diffusion-weighted imaging have been shown in different organs, i.e. the brain (D'Arceuil & de Crespigny, 2007) as well as the heart, as assessed by Eggen and colleagues (Eggen et al. 2012). Eggen showed that tissue preservation is important in order to preserve the diffusion properties of the tissue. Preserving soft tissue morphology prior to histopathological examination is performed by rapid chemical fixation or, less frequently, by freezing of the specimen. The most common fixative for soft tissue is formalin (Holmes et al. 2000; Helm et al. 2006; Nielsen et al. 2009; Smerup et al. 2009), but glutaraldehyde or combinations of the two are also commonly used (Kung et al. 2011). Both formalin and glutaraldehyde act by establishing covalent binding of proteins, the chemical and physical mechanisms of which are well documented (Kiernan, 2000). However, there is currently no consensus regarding handling of soft tissue prior to DTMRI, be it fresh, frozen, fixed, or frozen and fixed tissue. Moreover, the optimal method of fixation (immersion or perfusion fixation) has not been resolved (Holmes et al. 2000; Nielsen et al. 2009; Smerup et al. 2009). Bearing in mind that many other methods of tissue fixation exist, this study aimed at comparing DTMRI-derived parameters in hearts exposed to the most common methods of tissue preservation in cardiac DTMRI being immersion or perfusion fixation in either formalin or glutaraldehyde, and storage at either room temperature or frozen. Because human heart specimens are difficult to obtain, the benefits of porcine hearts were used. Access to such hearts is easy, and they are similar both anatomically and physiologically to human hearts.
DTMRI-measured parameters in the myocardium
Holmes et al. evaluated the consequences of formalin fixation on results of DTMRI, and concluded that measurements of so-called cardiac muscle fibre orientations in fresh hearts were comparable with those obtained in formalin-fixed hearts (Holmes et al. 2000). Based on his and subsequent studies of other groups, it is now widely accepted that the diffusion patterns as measured with DTMRI follow the direction of the cardiomyocytes, being elongated cells forming aggregates with a preferred direction. Estimating myocyte orientation using DTMRI tractography does not reveal the microstructural properties of a tissue, but the diffusion tensor in itself is a widely used and sensitive measure for characterizing tissue microstructure (Alexander et al. 2007). Typical DTMRI measurements include: the fractional anisotropy (FA); the mean diffusivity (MD), the axial diffusivity (Da), that is the magnitudes of the diffusion along the primary eigenvector of the diffusion tensor; and the radial diffusivity (Dr), being the average magnitude of diffusion perpendicular to Da, i.e. the average of the magnitudes of the secondary and tertiary eigenvectors.
Materials and methods
Animal procedures and specimen handling
Twenty-four hearts from healthy female Danish Landrace pigs (50–60 kg) obtained from standard Danish farming facilities were used. The animals were sedated intramuscularly with Midazolam, intravenous access was established for supplemental sedatives (Midazolam) and analgesics (Fentanyl), and intubation was performed for ventilatory control. Anaesthesia was maintained using inhalational Sevoflurane aiming at a minimum alveolar concentration (MAC value) of 1. Heparin was administered to prevent blood clotting. Following median sternotomy, the animals were killed by exsanguination when the hearts were excised and flushed with 1 litre of cold cardioplegic solution (Cardioplex®) evenly distributed between the coronary vessels. After excision, the hearts were randomized into six groups (four hearts in each group). Group 1: scanned after excision with no further handling; Group 2: frozen for 24 h at −18 °C; Group 3: frozen for 24 h at −18 °C, subsequently thawed and perfusion-fixed with formalin (4%); Group 4: immersion-fixed with formalin (4%); Group 5: perfusion-fixed with formalin (4%); and Group 6: perfusion-fixed with glutaraldehyde (4%). During all perfusion procedures, 300 mL of fixation fluid was injected directly into the left coronary artery, while 150 mL was administered into the right coronary artery. The concentrations of fixatives are chosen based on what is commonly used in literature in ex vivo cardiac DTMRI.
Fresh hearts (Group 1)
Hearts in this group were scanned < 2 h after excision and rigor had ceased. They were kept in phosphate-buffered solution (PBS) at room temperature between excision and scanning.
Freezing and thawing (Groups 2 and 3)
Freezing was performed at −18 °C for at least 24 h, and the specimens were subsequently allowed to thaw out for 18 h at 5 °C. The hearts were allowed to reach room temperature before scanning.
Fixation (Groups 3–6)
Hearts in groups 3–5: the specimens were fixed with formalin (Lillies Fluid). Group 3 was fixed after thawing. Hearts in Group 4 were submerged into formalin immediately after excision. Group 5 also was fixed immediately after excision, by perfusion of formalin through the coronary vessels using a 5-mm coronary cannula. A volume of 300 mL was administered through the left coronary artery and 150 mL through the right. All formalin-fixed hearts were stored in formalin in a closed container for a minimum of 24 h at room temperature to allow complete tissue penetration of fixative. Hearts in Group six were perfusion-fixed with glutaraldehyde in the same manner as Group 5. They were subsequently stored in glutaraldehyde at room temperature.
DTMRI
Aldehyde-based fixatives are known to have an impact on MRI properties of nerve tissue (Shepherd et al. 2009). Therefore, the fixed hearts were removed from fixative solution and flushed through the coronary vessels with PBS at room temperature removing excess fixative. During scanning all hearts were removed from the storage solution avoiding motion artefacts due to floatation. The hearts were transferred to an airtight plastic bag to avoid evaporation and drying during DTMRI, and they were then placed inside a surface RF-coil folded into a tube in a soft cradle of towels. Within this cradle non-fixed specimens were allowed to sag while fixed specimens retained their shape because of rigidity induced by fixation. This difference in shape was considered irrelevant because only local tissue diffusional parameters and not overall anatomical parameters were compared. MRI examinations were performed with a Philips Achieva 1.5 T clinical system (Philips Medical Systems, Best, Netherlands), equipped with Nova Dual Gradients and Software Release 2.1.3. Room temperature was maintained constant at 22.0 ± 1.5 °C and humidity of 50 ± 10%. A DTMRI sequence was employed with a multi-slice 2D spin-echo sequence with an isotropic voxel resolution of 1.3 × 1.3 × 1.3 mm3; slice gap: 0 mm; repetition time: 3900 ms; echo time: 72 ms. The number of slices and field-of-view varied according to the size of the hearts. With one average (NSA = 1), diffusion sensitive images were acquired with 32 diffusion directions isotropic distributed with diffusion sensitivity b-values of 1271 s mm−2 and one image without diffusion-weighting. This scheme was used previously by the authors (Smerup et al. 2009). The b-value is selected based on the authors' experiences as being optimal for ex vivo cardiac DTMRI and are very similar to what is found optimal by other groups (Wu et al. 2013). Total scan times ranged between 12 and 15 h depending on the number of slices necessary to cover the entire heart.
Data analysis and statistics
DTMRI data were visualized in a three-dimensional space using custom-made software (Smerup et al. 2009). In order to obtain a representative amount of data, four regions of interest were chosen in the left ventricle, equally distributed in the equatorial plane at the level of the papillary muscles (Fig.1). Within each region, FA, MD, Da and Dr values were extracted from 80–100 voxels, depending on the wall thickness, and evenly distributed from endocardium to epicardium. FA is the most widely used measure of anisotropy in tissues; it reflects the shape of the diffusion tensor and the degree of diffusive anisotropy in the tissue. However, the FA alone is inadequate to describe the diffusion tensor as different combinations and magnitudes of eigenvalues (diffusivities) can result in the same FA. Therefore, several different measures of diffusivity are needed to provide a more complete interpretation of the DTMRI data. MD will increase and FA will decrease in case of increased extracellular fluid, such as in oedema and inflammation (Alexander et al. 2007). Neurological studies showed that Dr is dependent on the amount of myelin and that Da is sensitive to axonal degeneration (Song et al. 2002). Investigating myocardial infarction using DTMRI, Wu et al. found increased MD in the infarcted area while FA decreased (Wu et al. 2006). However, no general interpretations of the DTMRI-derived parameters in cardiac tissue exist in literature, and no direct clinical implications can therefore be deduced from the current results.
Fig 1.
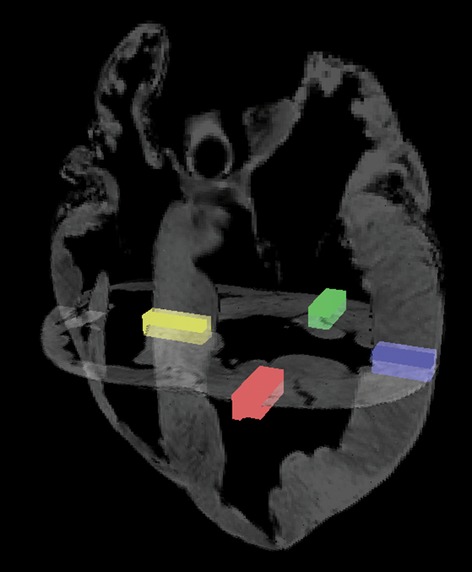
The regions of interest. Four transmural selections at papillary muscle level all at equatorial level. Red: superior wall. Green: inferior wall. Yellow: interventricular septum. Blue: free wall. Papillary muscles were not included in the data sets.
The DTMRI data of the hearts were evaluated qualitatively by tractography. In order to reduce the visual complexity of the images, the analysis software was set to use a maximum track-length threshold of 100 mm. The software was allowed to track through primary eigenvectors with a FA threshold of maximum 0.15 and an inner product threshold of minimum 0.75. These thresholds are commonly used in literature (Wakana et al. 2004; Rollins et al. 2005). Because this study did not focus on regional differences within the heart, data from all regions-of-interests were pooled into a unified representation of the entire heart.
After confirming normality using histograms and Q-Q plots, FA values were compared between groups using anova statistics. Subsequently, post hoc testing was performed with Bonferroni correction. Statistical tests were performed with a significance level of 5%; standard deviations are shown in parentheses. All statistical analyses were performed using stata® 11.
Ethical considerations
This study was approved by the Danish Inspectorate of Animal Experimentation. Likewise, the investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). Any sign of pain or stress during surgery was dealt with immediately.
Results
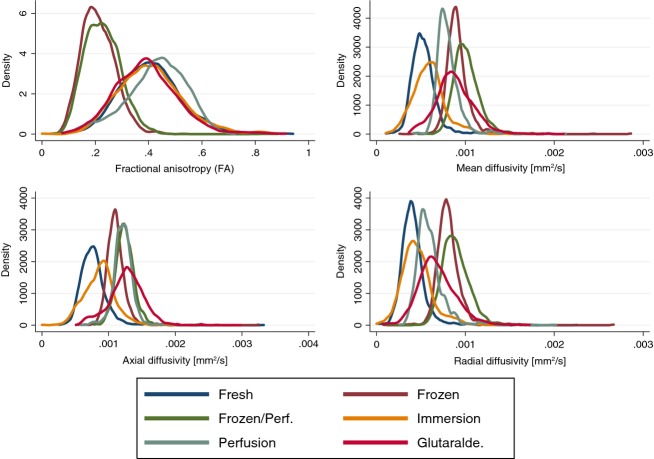
Results of FA, MD, Da and Dr measurements are shown in Table1, and plotted as density histograms in Fig.2. Overall differences between groups were found statistically significant in all variables using anova and Kruskal–Wallis rank test, P < 0.0001. Post hoc analyses found statistically significant differences between all groups. The highest mean FA of 0.43 (0.11) was found in Group 5 with perfusion-fixed hearts. This was higher than all other groups (P < 0.0001). The mean FA in the perfusion-fixed hearts was twice the value of frozen hearts that constituted the group with the lowest mean FA of 0.21 (0.06). Subjecting frozen hearts to perfusion fixation (Group 3) increased the mean FA significantly (P < 0.001). Treatment of fresh hearts with formalin (Groups 4 and 5) also increased mean FA significantly (P < 0.001).
Table 1.
Diffusion properties
| Fresh | Frozen | Frozen/perf | Immersion | Perfusion | Glutaraldehyde | P-value | |
|---|---|---|---|---|---|---|---|
| FA | 0.41 (0.12) | 0.21 (0.06) | 0.23 (0.07) | 0.41 (0.12) | 0.43 (0.11) | 0.39 (0.12) | < 0.001 |
| MD (mm2 s−1) (× 10−4) | 5.36 (1.7) | 9.14 (1.5) | 10.1 (1.5) | 5.93 (1.9) | 8.06 (1.5) | 8.80 (2.1) | < 0.001 |
| Da (mm2 s−1) (× 10−4) | 7.75 (2.1) | 11.15 (1.7) | 12.5 (1.5) | 8.65 (2.4) | 12.17 (1.6) | 12.69 (2.6) | < 0.001 |
| Dr (mm2 s−1) (× 10−4) | 4.16 (1.6) | 8.14 (1.5) | 8.92 (1.5) | 4.57 (1.8) | 6.01 (1.8) | 6.84 (2.1) | < 0.001 |
Da, axial diffusivity; Dr, radial diffusivity; FA, fractional anisotropy; MD, mean diffusivity.
Results reported as mean (SD).
Fig 2.
Histogram density plots of fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (Da) and radial diffusivity (Dr) in each group.
Diffusion tensor magnetic resonance imaging of hearts perfused with glutaraldehyde yielded a mean FA of 0.39 (0.12), which was comparable to FA values in fresh hearts, and significantly lower than in those perfused with formalin 0.43 (0.11; P < 0.001).
The MD, Da and Dr increased in all groups compared with fresh hearts. MD was highest in the frozen perfused hearts [10.1 (1.5) × 10−4 mm2 s−1] with 94% increase, followed by the frozen hearts [9.14 (1.5) × 10−4 mm2 s−1] with 74% increase.
The mean Da was highest in hearts perfused with glutaraldehyde [12.17 (1.6) × 10−4 mm2 s−1]; a 69% increase compared with fresh hearts. In frozen perfused hearts the mean Da increased by 11.15 (1.7) × 10−4 mm2 s−1, i.e. 65%.
The highest mean Dr values were found in the frozen [8.14 (1.5) × 10−4 mm2 s−1] and frozen perfused hearts [8.92 (1.5) × 10−4 mm2 s−1]; both being more than 100% increased compared with fresh hearts.
In general, the smallest change in diffusion parameters compared with fresh hearts was found in immersion-fixed hearts.
In fresh, immersion- and perfusion-fixed hearts, tractography images were visually indistinguishable from one another. Tractography of hearts frozen prior to DTMRI, being subsequently perfusion-fixed or not, was visually very different from all others. In fact, tractography of frozen hearts was practically impossible with the settings chosen in this study (Fig.3).
Fig 3.
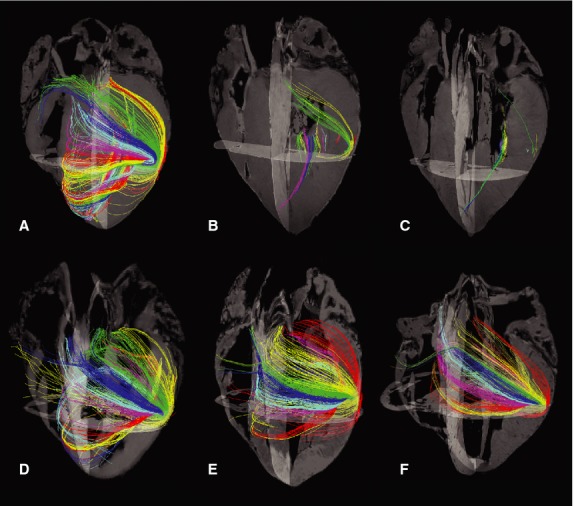
Tractography of the six groups. (A) Fresh. (B) Frozen. (C) Frozen followed by perfusion fixation. (D) Immersion fixation. (E) Perfusion fixation. (F) Glutaraldehyde perfusion fixation. Changing of tractography colours through the myocardium serve only as a visual aid enabling the reader to distinguish between the course of different tracks.
Discussion
This DTMRI study shows that several diffusion-derived parameters in myocardial tissue were affected by the treatment of the specimen in terms of preservation and fixation method. It was found that freezing had a significant negative impact on the measured FA, MD, Da and Dr regardless of subsequent formalin fixation. Tractography was predicated on the directionality of these parameters. If no preferred diffusion directionality prevails after preservation the algorithm cannot produce the suggested tracts. This was reflected when attempting to perform tractography in specimens subjected to sub-zero temperatures (Fig.3). Moreover, it was found that fixation in itself had a marked effect on the diffusion signals; the largest effect was found in hearts fixed with glutaraldehyde by perfusion and the least in hearts fixed with formalin by immersion.
While these results advocate an appropriate handling procedure to be used in excised tissue or organs, the underlying explanations of these findings remain speculative, such as why the diffusion properties of tissues are affected by fixation or temporary freezing? It is known that tissues subjected to sub-zero temperatures are susceptible to cryoinjuries depending on cooling rate and temperature. According to Mazur (1963) and Lovelock (1957), cooling of tissues at a slow rate, as in household freezers, will result in osmotic dehydration and affect the lipid–protein complexes in cell membranes, leading to electrolyte leakage, cellular swelling and potentially membrane rupture with a consequent loss of mechanical integrity. This theory fits well with the current results as the lowest FA values and the highest MD and Dr values can be seen in the groups of frozen/thawed specimens (Groups 2 and 3). An intact cell membrane is of paramount importance when establishing the relationship between FA and the fibrous properties of tissues as it is the hydrophobic cell membrane that maintains the parallel directionality of the water diffusion. Thus, permeable cell membranes, particularly in a frozen and thawed specimen, will lead to increased Dr, which is incompatible with maintaining a FA sufficient for the use of DTMRI tractography. Flamini and colleagues found similar results in 2013 in their study of DTMRI in fresh vs. frozen aortae (Flamini et al. 2013). In spite of FA values not being directly shown, FA images were darker pointing towards a lower mean FA in frozen vessels. Furthermore, tractography of the frozen aorta was far more scattered than in the fresh, all in line with the current results in the heart.
Subjecting frozen/thawed hearts to perfusion fixation (Group 3) significantly increased the mean FA, however, within a magnitude that can hardly be given any clinical or practical significance as emphasized by the tractography in this group being comparable to that of frozen and subsequently thawed hearts not perfused. Thus, it can be concluded that fixation cannot rescue the presumed damage done by freezing and thawing.
Commonly available fixatives were employed, and it was found that all investigated parameters were dependent on the choice of fixative and fixation method. Hearts fixed with formalin by immersion showed a diffusion profile most equal to that of fresh hearts, implying that the process of perfusion also inflicts some sort of damage to the tissue as seen by increased MD, Da and Dr, however, with no relevant consequences on the measured FA or calculated tractography. It can be speculated that the perfusion pressure, which was not measured in this study, might increase the fluid contents of the tissue by rupturing of capillaries and expansion of extracellular space, thereby increasing the MD, but without changing the FA. However, it is beyond the scope of this study to test this.
It would be of significant relevance to investigate further the microstructure of the tissue in the different groups especially by the use of histology as the gold standard in tissue evaluation. However, the preparation of tissue for histological examination in itself introduces the use of fixation agents. Hence, investigating the difference between fresh and fixed tissues in this way is far from straightforward. Therefore, in the current study chose not to proceed with histology, albeit would seem logical, and focus on the main purpose of demonstrating the difference between groups when utilizing DTMRI. It might be speculated that the length of the time period from fixation to scanning might influence the results due to differences in tissue penetration. In the field of cardiac DTMRI, little if any attention at all is given to this notion and it is the authors' experience that as long as the tissue is completely fixed the storage time is of minor relevance. Thus, the time from fixation to scanning was not assessed in this study, and the similar variances between groups are indicative of the current assumption being correct. However, to the authors' knowledge this has never been put to a test in cardiac DTMRI. Furthermore, it might be suspected that the fresh hearts might change their diffusional properties during the course of the scanning due to autolysis or rigor. Schmid and co-workers investigated the effect of tissue autolysis over the course of 11 days and found no changes in their DTMRI results on fresh hearts embedded in agarose and stored at 6 °C (Schmid et al. 2007). The slow contraction induced by rigor could potentially cause motion artefacts because of the long scan time, but as long as rigor has been allowed to cease completely before the onset of scanning, motion artefacts do not occur. Thus, artefacts due to tissue decay and motion were considered unlikely to have influenced the current results.
This is the first study to evaluate the effect of fixation on the molecular diffusion properties of water in hearts. Previous studies compared the angulation of myofibres using histology in fresh hearts with DTMRI in formalin-fixed hearts (Scollan et al. 1998; Helm et al. 2006). They concluded that water diffusion in fixed tissue was comparable to those in fresh tissue. These findings were derived from the main direction of diffusion, the so-called primary eigenvector, and not on measures of diffusivity and FA as in the current study. Nevertheless, their results were in accordance with the current study, in which the difference in mean FA between fresh and formalin-fixed hearts was found, although being statistically significant, to be below 5%. This difference is too small to affect the tractography at the resolution chosen in this study (Fig.3). While the tractography images of fresh and fixed hearts were virtually indistinguishable, it was also found that overall tissue diffusivity changed due to perfusion fixation, but without consequences on the directionality of the diffusion as measured by FA. The results of this study translate directly to human specimens.
In conclusion, the importance of meticulousness when selecting methods, and awareness in specimen handling and preservation prior to DTMRI is emphasized. Because specimens are often shipped between researchers, care should be taken when packing specimens especially for airplane shipment to avoid freezing damage in tissues. Furthermore, the current findings suggest that freezing of the hearts should be avoided prior to DTMRI due to significant deterioration of the accompanied tractography and the highly significant decrease in FA and overall diffusivity parameters. It can be speculated that molecular cryoprotectants may prevent the observed cryoinjuries allowing cryopreservation without destroying the diffusion properties in the tissue. However, this is a topic for future investigations. In theory, the current results indicate that immersion fixation should be preferred over perfusion fixation due to better preservation of overall diffusion properties. However, it is known that the diffusion properties of the myocardium become less restricted as time passes after death due to autolytic processes (Eggen et al. 2012), hence immersion fixation is not an optimal treatment of large specimens, due to slow tissue penetration of formalin. Finally, it should be noted that the mean FA was increased in perfusion-fixed hearts, resulting in optimal conditions for tractography and myocardial angle measurements, in accordance with findings of earlier studies (Scollan et al. 1998; Holmes et al. 2000).
Acknowledgments
The authors would like to thank Robert S. Stephenson, Liverpool John Moores University, UK for valuable linguistic editing and scientific comments. This work was supported by Arvid Nilsson's Foundation.
Author contributions
PA designed the study. PA and MP acquired the data. TL and PA analysed the data. JF made the analysis software. PA drafted and finalized the manuscript. MS and MP revised the manuscript. All authors approved the final article.
References
- Alexander AL, Lee JE, Lazar M, et al. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Arceuil H, de Crespigny A. The effects of brain tissue decomposition on diffusion tensor imaging and tractography. NeuroImage. 2007;36:64–68. doi: 10.1016/j.neuroimage.2007.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggen MD, Swingen CM, Iaizzo PA. Ex vivo diffusion tensor MRI of human hearts: relative effects of specimen decomposition. Magn Reson Med. 2012;67:1703–1709. doi: 10.1002/mrm.23194. [DOI] [PubMed] [Google Scholar]
- Flamini V, Kerskens C, Simms C, et al. Fibre orientation of fresh and frozen porcine aorta determined non-invasively using diffusion tensor imaging. Med Eng Phys. 2013;35:765–776. doi: 10.1016/j.medengphy.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Gaudiano C, Clementi V, Busato F, et al. Renal diffusion tensor imaging: is it possible to define the tubular pathway? A case report. Magn Reson Imaging. 2011;29:1030–1033. doi: 10.1016/j.mri.2011.02.032. [DOI] [PubMed] [Google Scholar]
- Helm PA, Younes L, Beg MF, et al. Evidence of structural remodeling in the dyssynchronous failing heart. Circ Res. 2006;98:125–132. doi: 10.1161/01.RES.0000199396.30688.eb. [DOI] [PubMed] [Google Scholar]
- Holmes AA, Scollan DF, Winslow RL. Direct histological validation of diffusion tensor MRI in formaldehyde-fixed myocardium. Magn Reson Med. 2000;44:157–161. doi: 10.1002/1522-2594(200007)44:1<157::aid-mrm22>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Jirjis MB, Kurpad SN, Schmit BD. Ex vivo diffusion tensor imaging of spinal cord injury in rats of varying degrees of severity. J Neurotrauma. 2013;30:1577–1586. doi: 10.1089/neu.2013.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan JA. Formaldehyde, formalin, paraformaldehyde and glutaraldehyde: what they are and what they do. Micros Today. 2000;00–1:8–12. [Google Scholar]
- Kung GL, Nguyen TC, Itoh A, et al. The presence of two local myocardial sheet populations confirmed by diffusion tensor MRI and histological validation. J Magn Reson Imaging. 2011;34:1080–1091. doi: 10.1002/jmri.22725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovelock JE. The denaturation of lipid-protein complexes as a cause of damage by freezing. Proc R Soc Lond B Biol Sci. 1957;147:427–433. doi: 10.1098/rspb.1957.0062. [DOI] [PubMed] [Google Scholar]
- Mazur P. Kinetics of water loss from cells at subzero temperatures and the likelihood of intracellular freezing. J Gen Physiol. 1963;47:347–369. doi: 10.1085/jgp.47.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naraghi A, da Gama LL, Menezes R, et al. Diffusion tensor imaging of the median nerve before and after carpal tunnel release in patients with carpal tunnel syndrome: feasibility study. Skeletal Radiol. 2013;42:1403–1412. doi: 10.1007/s00256-013-1670-z. [DOI] [PubMed] [Google Scholar]
- Nielsen E, Smerup M, Agger P, et al. Normal right ventricular three-dimensional architecture, as assessed with diffusion tensor magnetic resonance imaging, is preserved during experimentally induced right ventricular hypertrophy. Anat Rec (Hoboken) 2009;292:640–651. doi: 10.1002/ar.20873. [DOI] [PubMed] [Google Scholar]
- Rollins N, Reyes T, Chia J. Diffusion tensor imaging in lissencephaly. Am J Neuroradiol. 2005;26:1583–1586. [PMC free article] [PubMed] [Google Scholar]
- Scheel M, von Roth P, Winkler T, et al. Fiber type characterization in skeletal muscle by diffusion tensor imaging. NMR Biomed. 2013;26:1220–1224. doi: 10.1002/nbm.2938. [DOI] [PubMed] [Google Scholar]
- Schmid P, Lunkenheimer PP, Redmann K, et al. Statistical analysis of the angle of intrusion of porcine ventricular myocytes from epicardium to endocardium using diffusion tensor magnetic resonance imaging. Anat Rec (Hoboken) 2007;290:1413–1423. doi: 10.1002/ar.20604. [DOI] [PubMed] [Google Scholar]
- Scollan DF, Holmes A, Winslow R, et al. Histological validation of myocardial microstructure obtained from diffusion tensor magnetic resonance imaging. Am J Physiol. 1998;275:H2308–H2318. doi: 10.1152/ajpheart.1998.275.6.H2308. [DOI] [PubMed] [Google Scholar]
- Shepherd TM, Thelwall PE, Stanisz GJ, et al. Aldehyde fixative solutions alter the water relaxation and diffusion properties of nervous tissue. Magn Reson Med. 2009;62:26–34. doi: 10.1002/mrm.21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smerup M, Nielsen E, Agger P, et al. The three-dimensional arrangement of the myocytes aggregated together within the mammalian ventricular myocardium. Anat Rec (Hoboken) 2009;292:1–11. doi: 10.1002/ar.20798. [DOI] [PubMed] [Google Scholar]
- Song S-K, Sun S-W, Ramsbottom MJ, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, et al. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wu M-T, Tseng W-YI, Su M-YM, et al. Diffusion tensor magnetic resonance imaging mapping the fiber architecture remodeling in human myocardium after infarction: correlation with viability and wall motion. Circulation. 2006;114:1036–1045. doi: 10.1161/CIRCULATIONAHA.105.545863. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zou C, Liu W, et al. Effect of b-value in revealing postinfarct myocardial microstructural remodeling using MR diffusion tensor imaging. Magn Reson Imaging. 2013;31:847–856. doi: 10.1016/j.mri.2013.02.010. [DOI] [PubMed] [Google Scholar]