Abstract
Objectives
Cocaine intoxication leads to over 500,000 emergency department visits annually in the USA and ethanol co-intoxication occurs in 34% of those cases. Cardiotoxicity is an ominous complication of cocaine and cocaethylene overdose for which no specific antidote exists. Because infusion of lipid emulsion (Intralipid) can treat lipophilic local anesthetic toxicity and cocaine is an amphipathic local anesthetic, the authors tested whether lipid emulsion could attenuate cocaine cardiotoxicity in vivo. The effects of lipid emulsion were compared with the metabolically inert sulfobutylether-β-cyclodextrin (Captisol) in an isolated heart model of cocaine and cocaethylene toxicity to determine if capture alone could exert similar benefit as lipid emulsion which exhibits multi-modal effects. The authors then tested if cocaine and cocaethylene, like bupivacaine, inhibit lipid-based metabolism in isolated cardiac mitochondria.
Methods
For whole animal experiments, Sprague-Dawley rats were anesthetized, instrumented, and pretreated with lipid emulsion followed by a continuous infusion of cocaine to assess time of onset of cocaine toxicity. For ex vivo experiments, rat hearts were placed onto a non-recirculating Langendorff system perfused with Krebs-Henseleit solution. Heart rate, left ventricle maximum developed pressure (LVdevP), left ventricle diastolic pressure (LVDP), maximum rate of contraction (+dP/dtmax), maximum rate of relaxation (−dP/dtmax), rate-pressure product (RPP = heart rate*LVdevP), and line pressure were monitored continuously during the experiment. A dose-response to cocaine (10, 30, 50, and 100 μM) and cocaethylene (10, 30, 50 μM) was generated in the absence or presence of either 0.25% lipid emulsion, or sulfobutyl-β-cyclodextrin. Substrate-specific rates of oxygen consumption were measured in interfibrillar cardiac mitochondria in the presence of cocaine, cocaethylene, ecgonine, and benzoylecgonine.
Results
Treatment with lipid emulsion delayed onset of hypotension (140 seconds vs. 279 seconds p = 0.008) and asystole (369 seconds vs. 607 seconds; p = 0.02) in whole animals. Cocaine and cocaethylene induced dose-dependent decreases in RPP, +dP/dtmax, and −dP/dtmaxabs (p < 0.0001) in Langendorff hearts; line pressure was increased by cocaine and cocaethylene infusion, but not altered by treatment. Lipid emulsion attenuated cocaine- and cocaethylene-induced cardiac depression. Sulfobutyl-β-cyclodetrin alone evoked a mild cardio-depressant effect (p < 0.0001) but attenuated further cocaine- and cocaethylene-induced decrements in cardiac contractility at high concentrations of drug (100 μM; p < 0.001). Finally, both cocaine and cocaethylene, but not ecgonine and benzoylecgonine, inhibited lipid-dependent mitochondrial respiration by blocking carnitine exchange (p < 0.05).
Conclusions
A commercially available lipid emulsion was able to delay progression of cocaine cardiac toxicity in vivo. Further, it improved acute cocaine- and cocaethylene-induced cardiac toxicity in rat isolated heart while sulfobutyl-β-cyclodetrin was effective only at the highest cocaine concentration. Further, both cocaine and cocaethylene inhibited lipid-dependent mitochondrial respiration. Collectively, this suggests that scavenging-independent effects of lipid emulsion may contribute to reversal of acute cocaine and cocaethylene cardiotoxicity, and the beneficial effects may involve mitochondrial lipid processing.
INTRODUCTION
Cocaine is a commonly abused psychostimulant that acts on the central nervous system. Recreational use of cocaine contributes to more than half-million emergency department (ED) visits annually in the United States, the most for any illicit drug of abuse.1 Cardiotoxicity is an ominous complication of cocaine overdose.2,3 Sodium bicarbonate is used to overcome sodium channel blockade and reduce arrhythmias,4 along with benzodiazepines and α-adrenergic antagonists to reduce the sympathomimetic effects of cocaine.5 However, no specific antidote exists to address cocaine toxicity. Further, in 34% of ED visits, cocaine toxicity presents with concurrent alcohol intoxication.1 Co-intoxication slows clearance of cocaine,6,7 produces the cardio-toxic metabolite cocaethylene,8,9 and increases the likelihood of hospitalization.10
The complexity of molecular targets and cardiovascular effects of cocaine2,11 makes treatment of overdose difficult; no single, site-specific antagonist can address all of the associated clinical complications. Case reports have identified the potential efficacy of intravenous (IV) lipid emulsion (ILE) as a treatment for cocaine overdose in humans12,13 and in animal models5,14 based on ILE’s ability to reverse local anesthetic toxicity15 via a number of mechanisms, including partitioning of toxic drugs16–20 and improved cardiac output.20–22 We wanted to assess how these effects might combine to combat cocaine-induced cardiac depression. To this end, we tested the efficacy of ILE in preventing progression of cocaine toxicity to cardiac collapse in vivo. We further investigated mechanistic contributions from two perspectives. First, we tested the potential contribution of non-partitioning effects by comparing ILE to the metabolically inert sulfobutyl-β-cyclodextrin (SBE-β-CD; Captisol, Ligand Tech, La Jolla, CA) in a isolated rat heart model. β-cyclodextrins are a class of macromolecular structures designed to bind lipophilic moieties and have been proposed as detoxification agents for drug overdose,23 and for cocaine in particular.24,25 They have the ability to scavenge drugs, but no metabolic activity. Second, because cocaine and other local anesthetics impair lipid-based respiration,26,27 we specifically tested the hypothesis that cocaine and its metabolites impair mitochondrial carnitine exchange to contribute to the cardiac depression that ILE may directly combat.
METHODS
Study Design
This was a laboratory study of ILE vs. SBE-β-CD for cocaine toxicity in a murine model. All experimental protocols were approved by the Chicago Veterans Affairs Health Care System Animal Care and Use Subcommittee. Rats were housed as pairs in the Veterinary Medical Unit at the Jesse Brown Veterans Affairs Medical Center (JBVAMC). Protocols were approved by the Institutional Animal Care and Utilization Committee of the JBVAMC.
Animal Handling and Preparation
We carried out lipid emulsion infusion experiments as previously described.28 Following induction in a Bell jar, we intubated animals (n = 12 total), then mechanically ventilated and anesthetized them with 2% isoflurane in oxygen. We measured blood pressure via a femoral artery cannula and electrocardiogram (ECG) by needle electrodes. Animals received infusions through separate femoral vein catheters, after 20-minute equilibration of vital signs and arterial blood gas readings. We first randomized each animal to receive an infusion of either saline (n = 6) or 20% lipid emulsion (Intralipid, Baxter Pharmaceuticals, Deerfield, IL, n = 6) at 3 ml/kg/min for five minutes. Subsequently we delivered a continuous infusion of 10 mg/kg/min cocaine, until asystole was achieved. We assessed for cocaine toxicity based on both a drop in systolic blood pressure (sBP) of below 40 mmHg and asystole of 5 seconds or more. We chose a blood pressure of < 40 mmHg to assess for profound hypotension that was indicative of poor perfusion and risk of ischemic sequelae.29,30
Study Protocol
Isolated heart experiments
We performed isolated heart experiments as previously described.21 In brief, Sprague-Dawley rats (n = 20) weighing between 350 g and 430 g were heparinized, anesthetized with pentobarbital, and their hearts removed and placed onto a Langendorff system perfused at a constant rate of 16 mL/min with Krebs-Henseleit solution warmed to 37 °C; pH adjusted to 7.40; and containing 100 mM sodium chloride (NaCl), 4.74 mM potassium chloride (KCl), 1.18 mM monopotassium phosphate (KH2PO4), 1.18 mM magnesium sulfate (MgSO4), 1.00 mM calcium chloride (CaCl2), 25.00 mM sodium bicarbonate (NaHCO3), 11.50 mM glucose, 4.92 mM pyruvate, and 5.39 mM fumarate. Krebs solution was also equilibrated with a mixture of oxygen (95%) and carbon dioxide (5%) by passing through a membrane oxygenator. We measured pressure with a latex balloon in the left ventricle and recorded as in the intact animal experiments using LabChart 5.2 (ADInstruments, Colorado Springs, CO). We recorded heart rate, left ventricle maximum developed pressure (LVdevP), left ventricle diastolic pressure (LVDP), maximum rate of contraction (+dP/dtmax), maximum rate of relaxation (−dP/dtmax), rate-pressure-product (RPP = heart rate*LVdevP), and line pressure continuously during the experiment. RPP is a measure of cardiac work or cardiac output that takes into consideration both the maximum pressure generated and rate of contractions. It is important because it provides a good quantification of energy demand of the heart and its ability to perfuse tissues. We assessed the dose-response of perfused hearts to cocaine (10, 30, 50, and 100 μM, Sigma-Aldrich, St. Louis MO, n = 5) and cocaethylene (10, 30, 50 μM, Sigma-Aldrich, St. Louis MO, n = 5), in the absence or presence of either 0.25% lipid emulsion (n = 5) or 1.2 μmol/min SBE-β-CD (n = 5). Cocaethylene was made as a 5 mM stock solution in 50% ethanol, with final concentrations of 0.1%, 0.3%, 0.5% ethanol for 10, 30, and 50 μM cocaethylene, respectively. A cocaethylene-free ethanol control was run for the 0.1, 0.3, and 0.5% ethanol conditions.
Mitochondrial preps
We isolated interfibrillar cardiac mitochondria as previously described27,31 using differential centrifugation. Following calibration with a Clark oxygen electrode, the addition of 0.1 mM adenosine diphosphate (ADP) depleted the endogenous substrates. Subsequently we added cocaine, cocaethylene, benzoylecgonine, or ecgonine and assessed mitochondrial respiration supported by non-lipid substrate (pyruvate) and by carnitine-acylcarnitine translocase (CACT) dependent activity. We measured concentration-dependent effects of cocaine (0 to 1 mM), cocaethylene, and cocaine metabolites (benzoylecgonine, ecgonine at 1 mM for both), for state III (ADP-stimulated) respiration supported by pyruvate (10 mM). Further, we assessed mitochondrial CACT activity by adding malonate, pyruvate, and carnitine to respiring mitochondria and determining the rate of carnitine-stimulated pyruvate oxidation. Malonate inhibits the tricarboxylic acid cycle, so acetyl-coenzyme A (acetyl-CoA) accumulates in mitochondria and respiration is suppressed by feedback inhibition of pyruvate dehydrogenase, which catalyzes the reaction converting pyruvate to acetyl-CoA, generating reduced nicotinamide adenine dinucleotide (NADH) in the process. The addition of carnitine to this system leads to the conversion of acetyl-CoA to the acetylcarnitine, which is shuttled out of mitochondria by CACT, thereby relieving the inhibition of pyruvate dehydrogenase by acetyl-CoA and allowing generation of NADH, which supports respiration by providing reducing equivalents to the hydrogen electron transport system. Under these conditions, activation of respiration by added carnitine is directly correlated to CACT activity. Kinetic analysis comprised measuring oxidative rates (nanogram atom of oxygen per minute per milligram of protein: ngatomolO * min−1 * mol−1 protein) at different respiratory substrate and inhibitor concentrations. We assembled Dixon plots for cocaine and cocaethylene by graphing reciprocal respiratory rate (1/V) against cocaine and cocaethylene concentrations (millimolar). Subsequently, we calculated inhibitory constants (Ki) based on intersection of fit curves for multiple acetyl-carnitine and cocaine/cocaethylene concentrations as previously described.32,33
Sample size calculations
Sample size calculations were conducted based on previous experience and data for the same systems using bupivacaine as the local anesthetic including in vivo,28 ex vivo,21,34 and mitochondrial experiments.27 For the in vivo experiments we assumed an effect size of 2, with α = 0.05, and power = 0.8 resulting in a total sample size of 12 (six in each group.) Power calculations for isolated heart experiments were computed based on post-test criterion, assuming four post-tests (10, 30, 50, 100 μM cocaine), splitting the alpha equally among them (α = 0.0125 per test), and checking for an effect at the highest concentration, assuming an effect size of three (30% difference in contractility with a maximal standard deviation of 10%). This yields a total sample size of 10 (five per group). For mitochondrial experiments we assumed complete inhibition (> 60%) of carnitine-stimulated pyruvate oxidation by cocaine and alpha split between the four post-test comparisons producing a sample size of three per group.
Data Analysis
We analyzed the toxicity data using survival analysis and quantified significance using the log-rank (Mantel-Cox) test. We conducted analysis of physiological data in Prism 6.0 (GraphPad, San Diego, CA) by two-way analysis of variance (ANOVA), taking into account both drug dose (0 to 100 μM) and treatment group (null vs. ILE, or null vs. SBE-β-CD) with parameters normalized to baseline. Cocaine and cocaethylene dose-effects are reported as well as treatment effects, significance values from post-hoc Sidak tests controlling for multiple comparisons for cocaine data and post-hoc Tukey tests for multiple comparisons for cocaethylene and ethanol data.
For mitochondrial data we used a Mann-Whitney U-test to compare baseline respiration levels with respiration levels after administration of accompanying drug (cocaine, cocaethylene, ecgonine, benzoylecgonine.)
RESULTS
Lipid emulsion pretreatment for cocaine toxicity in intact animals
We previously found that pretreating rats with an ILE increased the dose of IV bupivacaine (a lipophilic, cardiotoxic, local anesthetic) required to produce asystole.28 We studied whether this salutary effect could similarly attenuate the toxicity of cocaine. Blood pressure in controls (n = 6) dropped below 40 mmHg after a median of 140 seconds (95% CI = 103 to 240) of cocaine infusion (p = 0.008), while the blood pressure of those pretreated with lipid emulsion (n = 6) reached 40 mmHg after median 279 seconds (95% CI = 184 to 411) (Figure 1-A). Saline-treated controls developed asystole at 369 seconds (95% CI = 281 to 474) compared to 607 seconds (95% CI = 397 to 705) (Figure 1-B) among those pretreated with ILE (p = 0.0195). Thus, animals pretreated with lipid required roughly double the cocaine dose to reduce blood pressure to 40 mmHg systolic, and 50% more cocaine than controls to develop asystole.
Figure 1. Lipid pretreatment increases time to <40 mmHg and asystole in whole animal in vivo preparation.
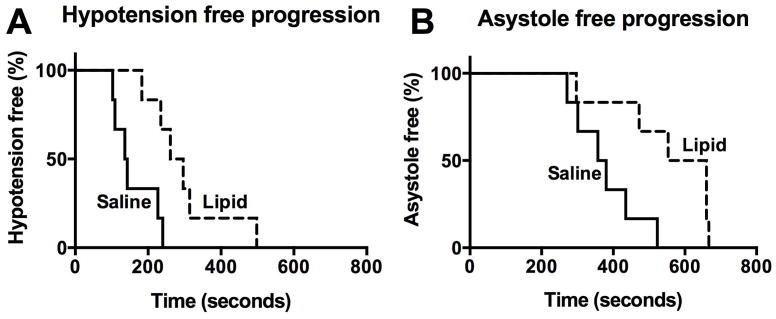
(A) Hypotension free progression, with a systolic blood pressure (sBP) >40 mmHg following an infusion of 10 mg/kg/mL of cocaine with either a pretreatment of 3 mL/kg/min of saline over 5 minutes (control), or 3 mL/kg/min of 20% lipid emulsion (Intralipid) over 5 minutes (Lipid). Significantly prolonged median survival of 279 seconds in lipid treated vs. 140 seconds in saline treated (p = 0.008, log-rank test).
(B) Asystole free progression. Significantly prolonged median survival to 607 seconds in lipid treated versus 369 seconds in saline treated (p = 0.0195, log-rank test)
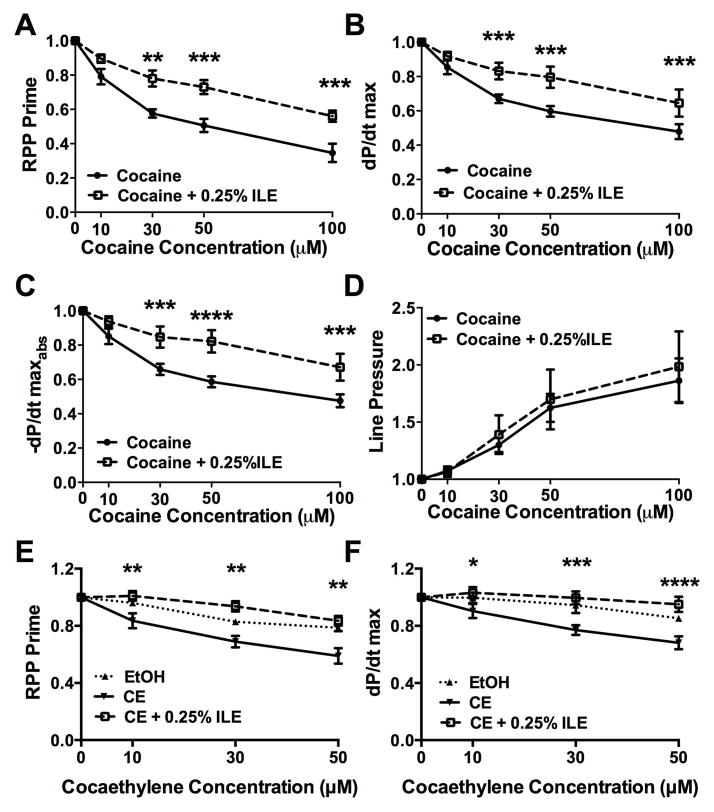
Lipid emulsion, cocaine, and cocaethylene in the isolated heart (Figure 2, Table 1)
Figure 2. Lipid attenuates cocaine & cocaethylene induced cardiovascular depression in the isolated heart.
(A) Rate-pressure product (RPP) following treatment with increasing concentrations of cocaine and concurrent 0.25% infusion of lipid emulsion (cocaine + 0.25% ILE) normalized to pre-ILE baseline. Treatment with ILE improved cardiovascular parameters relative to cocaine alone (** p < 0.01, *** p < 0.001).
(B) Maximal contraction force (dP/dt max, *** p < 0.001).
(C) Maximal relaxation force (−dP/dt maxabs, *** p < 0.001, **** p < 0.0001).
(D) Line pressure
(E) RPP following infusions of different concentrations of cocaethylene (CE), ethanol control at 0.1%, 0.3%, and 0.5% final infusion (EtOH) or cocaethylene and concurrent 0.25% infusion of lipid emulsion (CE + 0.25% ILE) normalized to post-ILE baseline. ILE improved cardiovascular parameters relative to CE alone (** p < 0.01 for CE vs. CE+0.25% ILE). EtOH was elevated relative to CE and indistinguishable from CE+0.25% ILE.
(F) dP/dt max during cocaethylene and lipid infusion (* p < 0.05, *** p < 0.001, **** p < 0.0001 for CE vs. CE+0.25% ILE). EtOH was elevated relative to CE and indistinguishable from CE+0.25%ILE. All values plotted as mean ±SEM
Table 1.
Hemodynamic parameters following cocaine and cocaethylene with treatment from ILE
| Cocaine
|
Cocaethylene
|
||||||
|---|---|---|---|---|---|---|---|
| RPP | dp/dt | −dp/dt | Line-P | RPP | dp/dt | ||
| 10μM | 79 ±5 | 85 ±4 | 85 ±5 | 107 ±3 | 10μM | 84 ±5 | 90 ±5 |
| +ILE | 90 ±2 | 92 ±3 | 93 ±3 | 106 ±5 | 0.1% EtOH | 96 ±2* | 99 ±4 |
| 30μM | 58 ±2 | 67 ±3 | 66 ±3 | 130 ±6 | +ILE | 101 ±3§ | 103 ±4* |
| +ILE | 78 ±5 § | 83 ±5¶ | 85 ±6¶ | 139 ±17 | 30μM | 69 ±4 | 77 ±3 |
| 50μM | 51 ±4 | 59 ±3 | 59 ±3 | 162 ±12 | 0.3% EtOH | 82 ±2* | 94 ±5§ |
| +ILE | 73 ±4¶ | 80 ±6¶ | 82 ±7† | 170 ±26 | +ILE | 94 ±4 | 100 ±5¶ |
| 100μM | 35 ±5 | 48 ±4 | 47 ±4 | 186 ±20 | 50μM | 59 ±5 | 68 ±4 |
| +ILE | 56 ±3¶ | 64 ±8¶ | 67 ±8¶ | 198 ±31 | 0.5% EtOH | 79 ±2¶ | 85 ±1¶ |
| +ILE | 84 ±3§ | 95 ±5† | |||||
Values plotted as mean ± standard error (SEM)
RPP = rate pressure product; Line-P = line pressure; ILE = 0.25% infusion of lipid emulsion; EtOH = ethanol; dp/dt =
Difference from analogous treatment condition with cocaine or cocaethylene alone italicized
<0.05
<0.01
<0.001
<0.0001
We studied the effects of cocaine in isolated rat hearts and further explored whether it exerted salutary effects on cocaine- or cocaethylene-induced cardiac depression in a Langendorff, isovolumic, non-recirculating, constant rate perfusion system. Cocaine induced dose-dependent decreases in RPP (Figure 2-A, p < 0.0001), dP/dtmax (Figure 2-B, p < 0.0001), and −dP/dtmax absolute value (−dP/dtmaxabs; Figure 2-C, p < 0.0001); and an increase in line pressure at a constant rate of coronary perfusion (Figure 2-D, p = 0.004). Hearts co-treated with ILE exhibited less depression of cardiovascular parameters (RPP p = 0.01, dP/dtmax p = 0.0033, −dP/dtmaxabs p = 0.0046) at cocaine concentrations >10 μM, but with no effect on line pressure. Cocaethylene also induced dose-dependent decreases in RPP (Figure 2-E, p < 0.0001) and dP/dtmax (Figure 2-F, p < 0.0001), which were alleviated by co-treatment with ILE (p = 0.001, 0.0058 respectively). No differences were observed between ethanol-treated controls and cocaethylene plus ILE groups. No interaction effects were noted in any condition.
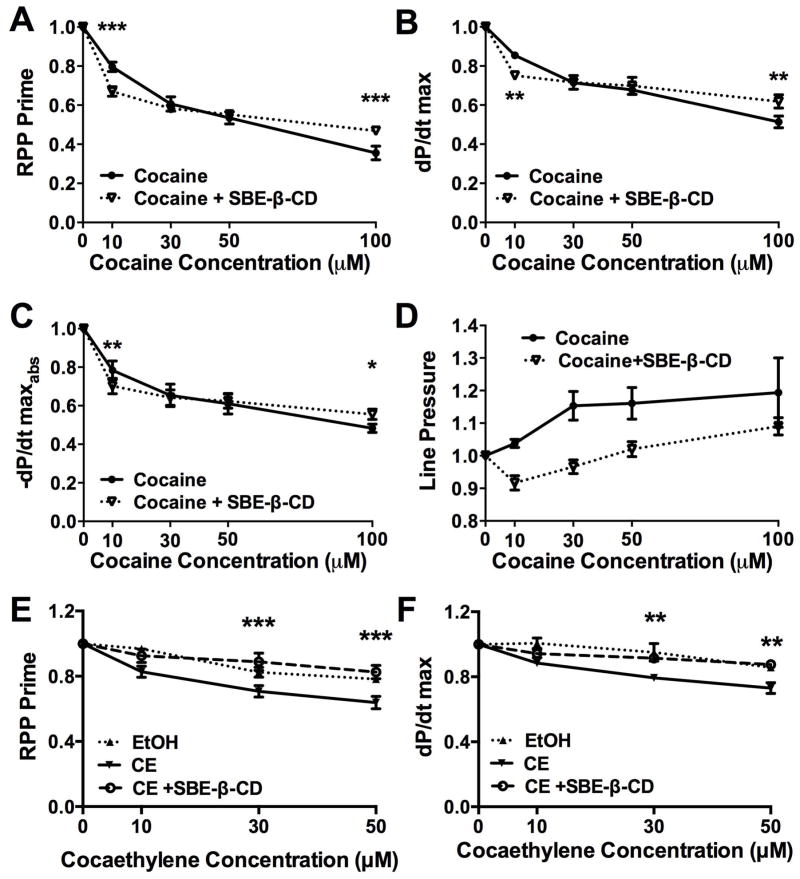
Cyclodextrin, cocaine, and cocaethylene in isolated heart (Figure 3, Table 2)
Figure 3. Sulfobutylether-β-cyclodetrin induces mild cardiac depression but blocks further cardiac depression at high cocaine and cocaethylene concentrations.
(A) Rate-pressure product (RPP) following treatment with increasing concentrations of cocaine and concurrent sulfobutylether-β-cyclodextrin (cocaine + SBE-β-CD) normalized to pre- SBE-β-CD baseline. SBE-β-CD diminished cardiovascular parameters at low cocaine concentrations but blocked additional cardiodepression at high cocaine concentrations (*** p < 0.001).
(B) Maximal contraction (dP/dt max, ** p < 0.01).
(C) Maximal relaxation (−dP/dt maxabs, *p < 0.05, ** p < 0.01).
(D) Line pressure
(E) RPP following infusions of different concentrations of cocaethylene (CE), ethanol control at 0.1, 0.3 and 0.5% final infusion (EtOH) and concurrent SBE-β-CD normalized to post- SBE-β-CD baseline (*** p < 0.001 for CE vs. CE+SBE-β-CD). EtOH was elevated relative to CE and indistinguishable from CE+SBE-β-CD.
(F) dP/dt max following cocaethylene and SBE-β-CD (** p < 0.01 for CE vs. CE+SBE-β-CD). EtOH was elevated relative to CE and indistinguishable from CE+ SBE-β-CD.
All values plotted as mean ±SEM
Table 2.
Hemodynamic parameters following cocaine and cocaethylene with treatment from sulfobutylether-β-cyclodextrin
| Cocaine
|
Cocaethylene
|
||||||
|---|---|---|---|---|---|---|---|
| RPP | dp/dt | −dp/dt | Line-P | RPP | dp/dt | ||
| 10mM | 80±2 | 85±1 | 78±5 | 104±1 | 10mM | 82±3 | 88±2 |
| +SBE-β-CD | 67±2¶ | 75±2§ | 70±4§ | 91±2 | 0.1% EtOH | 97±1§ | 100±3§ |
| 30mM | 61±4 | 71±2 | 65±7 | 115±4 | +SBE-β-CD | 93±4 | 94±2 |
| +SBE-β-CD | 58±2 | 71±3 | 64±4 | 96±2 | 30mM | 70±3 | 73±3 |
| 50mM | 53±3 | 68±2 | 61±5 | 116±5 | 0.3% EtOH | 82±3* | 95±5¶ |
| +SBE-β-CD | 55±2 | 70±4 | 62±4 | 102±2 | +SBE-β-CD | 89±5¶ | 91±1§ |
| 100mM | 35±3 | 51±3 | 48±2 | 120±10 | 50mM | 64±4 | 73±3 |
| +SBE-β-CD | 47±2¶ | 62±3§ | 56±3* | 109±3 | 0.5% EtOH | 78±2§ | 86±1§ |
| +SBE-β-CD | 83±4¶ | 88±2§ | |||||
Values plotted as mean ± standard error
RPP = rate pressure product; Line-P = line pressure; SBE-β-CD = Sulfobutylether-β-cyclodextrin; EtOH = ethanol control (vehicle) without cocaethylene.
Difference from analogous treatment condition with cocaine or cocaethylene alone italicized
p <0.05
p <0.01
p <0.001
SBE-β-CD alone evoked a mild cardio-depressant effect independent of cocaine treatment (p = 0.0001 compared to baseline) but attenuated further cocaine-induced decreases in RPP (Figure 3-A), dP/dtmax (Figure 3-B), and −dP/dtmaxabs (Figure 3-C) at high cocaine concentrations. No significant effect was seen on line pressure (Figure 3-D). At the highest cocaine concentration tested (100 μM) SBE-β-CD alleviated the most pronounced cardiac depression for RPP, dP/dtmax, and −dP/dtmaxabs. This differential effect of SBE-β-CD at low and high concentrations produced an interaction effect in the two-way ANOVA for RPP (p < 0.0001), dP/dtmax (p = 0.0001), and −dP/dtmaxabs (p = 0.0015), and prevented any overall treatment effect. Infusing SBE-β-CD also attenuated the cardiac depression associated with cocaethylene toxicity for RPP (Figure 3-E) and dP/dtmax (Figure 3-F). No differences were observed between ethanol-treated controls and cocaethylene plus SBE-β-CD groups.
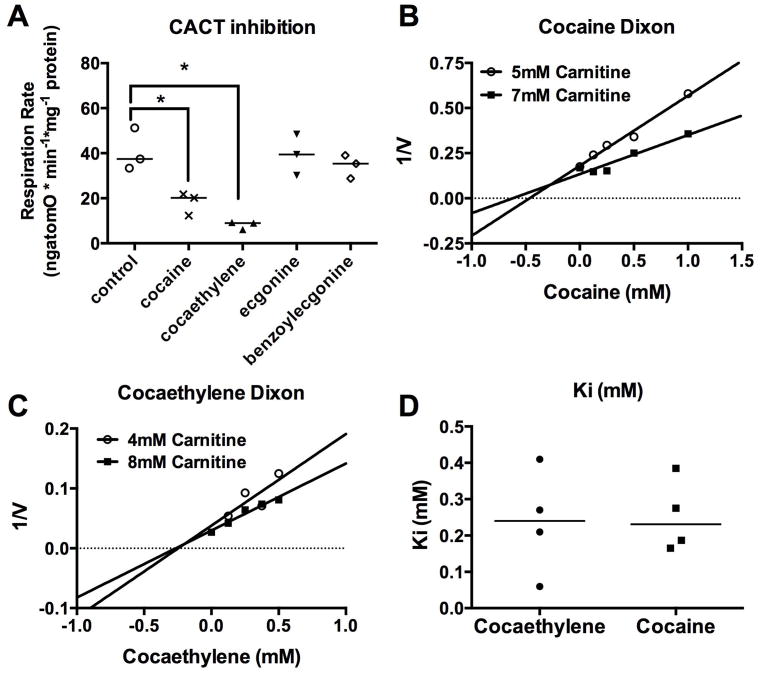
Effect of cocaine and metabolites on mitrochondrial carnitine exchange
Based on the alleviation of cocaine- and cocaethylene-induced cardiac depression, we further tested if cocaine, cocaethylene, and the less cardiotoxic cocaine metabolites (benzoylecgonine and ecgonine) exhibited different effects on carbohydrate- versus lipid-based mitochondrial respiration. Cocaine and cocaethylene (up to 1 mM) did not inhibit respiration supported by the non-lipid substrate pyruvate (data not shown). However, when carnitine is added to pyruvate and malonate, both cocaine (1 mM) and cocaethylene (1 mM) inhibited respiration down to 20 (min:max, 12:21) ngatomO*min−1*mg−1 (p < 0.05) and 9 (min:max, 6–9) ngatomO*min−1*mg−1 (p < 0.05), respectively from a baseline of 37 (min:max, 33:51) ngatomO*min−1*mg−1; neither benzoylecgonine (1 mM) nor ecgonine (1 mM) inhibited CACT-dependent respiration (Figure 4-A). Using different concentrations of carnitine, we used the Dixon method32 to calculate the inhibitory constants (Ki) of both cocaine (Figure 4-B), and cocaethylene (Figure 4-C) for carnitine-stimulated pyruvate oxidation – a measure of mitochondrial CACT activity and carnitine exchange. Both cocaine and cocaethylene had similar Ki (Figure 4-D), with means of 253 (SEM ± 50 μM) for cocaine (n = 4) and 237 (SEM ± 72 μM) for cocaethylene (n = 4).
Figure 4. Both cocaine and cocaethylene preferentially inhibit carnitine exchange in isolated mitochondria.
(A) Carnitine-acylcarnitine translocase (CACT)-dependent respiration in rat, interfibrillar cardiac mitochondria at baseline and in the presence of cocaine, cocaethylene, and the cocaine metabolites ecgonine and benzoylecgonine (all 1 mM concentration). Both cocaine and cocaethylene significantly depress respiration (* p < 0.05). All data points plotted with median line.
(B) Representative Dixon plot of cocaine-based inhibition of carnitine-dependent respiration. Cocaine produced a non-competitive inhibition of carnitine-dependent respiration.
(C) Representative Dixon plot of cocaethylene based inhibition of carnitine dependent respiration. Cocaethylene produced a non-competitive inhibition of carnitine dependent respiration.
(D) Inhibitory constants (Ki) of CACT for both cocaine and cocaethylene. All data points plotted with median line.
DISCUSSION
Our results demonstrate that lipid emulsion is effective at relieving cardiac depression caused by either cocaine or cocaethylene. We further found that rescue of cardiac contractility by ILE is more effective than that provided by SBE-β-CD, a metabolically inert scavenging agent. This indicates the importance of cardiotonic21,22 or direct effects17,18 of the lipid on the heart during recovery. Like bupivacaine, both cocaine and cocaethylene potently inhibited mitochondrial carnitine exchange, while the less cardio-toxic metabolites35 ecgonine and benzoylecgonine did not. These parallels, shared with bupivacaine toxicity and the commonality of reversal by ILE, suggest that ILE-based improvements in lipid metabolism during cocaine intoxication might underlie the cardiovascular recovery. Our results comport with prior clinical reports of the efficacy of ILE in combating cocaine cardiac toxicity,12,13 and with previous animal models14 that demonstrate that ILE can assist in recovery from toxicity. We have extended the results to cocaethylene, which is known to have significant detrimental effects on cardiovascular function,36,37 and is found in a significant proportion of patients with cocaine-related visits to the ED.1 Further, we identify CACT inhibition as a common mechanism of toxicity.
Cocaine is a pharmacodynamically complex drug that binds and interacts with many different targets, including the serotonin-norepinephrine-dopamine-transporter, in addition to the sodium, potassium, and calcium ion channels, leading to alterations in cardiac contractility, excitability, conduction, and repolarization.2,11 The sodium channel-blocking capacity of cocaine underlies its historical use as the first local anesthetic. However, this beneficial effect of cocaine is offset by its potential as a severe cardiotoxin, first identified more than a century ago. Given that overdose caused by the toxic local anesthetic bupivacaine responds to ILE,15 it is reasonable to hypothesize that cocaine toxicity would respond similarly. Bupivacaine, like cocaine, exerts cardiotoxic effects at a variety of targets.38 The shared effect of bupivacaine, cocaine, and cocaethylene of inhibiting mitochondrial fatty acid metabolism points to a more general mechanism of local anesthetic induced cardiac toxicity. In particular, ATP supplementation to isolated myocardial strips can reverse bupivacaine-induced contractile depression39; this suggests that interference with mitochondrial energy generation is a key element of toxicity. Our results, coupled with prior work,27 could provide a common mechanism for the beneficial metabolic effect of lipid emulsion and a way to test what cardiac drug toxicities would derive benefit from lipid emulsion therapy.
Scavenging of drug away from sites of toxicity15 is a putative mechanism underlying the efficacy of both ILE and SBE-β-CD. The drug delivery community is increasingly interested in developing improved drug uptake methods,40,41 as sequestration by liposomes,42–44 microemulsions,45 macromolecular carrier systems,23 and other agents46,47 all hold significant promise for the treatment of toxicity. However, in isolation, scavenging may not be enough. In whole animal models of bupivacaine toxicity, partitioning alone (the ‘lipid sink’) cannot account for the rapidity of recovery that ILE produces,48 and recent reports demonstrate that liposomes do not rescue cardiovascular parameters as effectively as lipid emulsions during acute toxicity.49 Recovery in these models requires an additional cardiotonic effect22 as observed in the absence of toxicity.21 In the case of overdose by drugs like cocaine and cocaethylene, toxicity is caused by altering a number of different targets,2,50 and an effective treatment would need to address toxicity at many of these targets. A synergistic therapy including both indirect (e.g., drug-scavenging) and direct effects (e.g., improvement in cellular energetics) would most likely be needed to achieve optimal recovery. While both the SBE-β-CD and ILE can partition drug, only ILE contributes a cardiotonic effect.20 Interestingly, β-cyclodetrins including SBE-β-CD and hydroxypropyl-β-cyclodextrin can improve recovery from toxicity from organophosphates,51 calcium channel blockers,52 and bacterial toxins,53 indicating the potential of scavenging agents. However, in more intelligently designed agents, molecular capture systems will likely complement agents that directly improve metabolism or physiologic performance in the same way that lipid emulsion provides benefit beyond just a partitioning effect.
LIMITATIONS
In the whole animal experiments, our study is limited by the choice of pre-treatment and potential clearance of lipid. We used a large dose (15 mL/kg total) based on prior experience in animal models14,54,55 and allometric scaling of human-to-rat doses.56 Assuming a half-life of lipid (about 15 minutes), we expect a severely lipaemic plasma, but the lack of continuous infusion could mean that lipid is cleared. Further, we restricted our model to pretreatment, while in clinical settings post-treatment is the required method of therapy. However, based on previous successful translation of bupivacaine pretreatment28 to clinical post-treatment,57 and the proposed mechanisms of action,20 we believe that evidence of efficacy in delaying cocaine toxicity indicates lipid will also be effective as a post-treatment for that indication. Finally, we did not blind the in vivo experiments, so experimenter effects may bias our results. Our isolated heart experiments are limited in terms of the choice of ‘pure capture’ agent. SBE-β-CD interfered slightly with recovery at lower cocaine doses, possibly due to binding of extracellular calcium or uptake of cholesterol from cardiac membranes.58,59 Moreover, SBE-β-CD might not be the most effective scavenging agent for cocaine or cocaethylene.60 Sugammadex, a γ-cyclodextrin, was developed as a capture agent for rocuronim,61 and is highly effective for that purpose. Presumably, a similar cocaine-specific capture molecule could reverse cocaine-induced cardiac toxicity with similar efficacy. Finally, we calculated our sample sizes to look for effects of lipid at the highest concentration of cocaine and cocaethylene. We might therefore miss beneficial effects or trends at the lowest cocaine and cocaethylene doses, but this is of less translational relevance as findings for highly toxic doses.
CONCLUSIONS
We found that a commercially available lipid emulsion attenuates cocaine toxicity in the whole animal, and improved acute cocaine- and cocaethylene-induced cardiac toxicity in isolated rat heart. In contrast, a metabolically inert sulfobutyl-β-cyclodextrin (Captisol) was effective in reversing contractile depression in isolated heart only at the highest cocaine concentration. Cocaine and cocaethylene both impaired carnitine exchange, like bupivacaine, suggesting a shared metabolic target for causing cardio-toxicity. Collectively, these data suggest that effects other than drug scavenging contribute to lipid emulsion-induced reversal of cocaine cardiotoxicity. This work provides evidence in support of others,5,12–14 that lipid emulsion could provide an effective antidote to cocaine overdose. Future studies should focus on the development of more targeted therapeutics and optimal treatment of this potentially fatal overdose.
Acknowledgments
Funding: United States Veterans Administration (Washington DC, USA) Merit Review (Weinberg and Rubinstein), NIH CounterACT grant 1U01NS083457-01 (Weinberg and Rubinstein), Department of Anesthesiology, University of Illinois College of Medicine (Chicago, IL, USA) & American Heart Association (Dallas, TX, USA) Predoctoral Fellowship 13PRE16810063 (Fettiplace)
Footnotes
Presentations: American Thoracic Society International Conference, San Diego, California, May 2014 and the 2000 American Society of Anesthesiologists, New Orleans, Louisiana, October 2000.
Disclosures: Guy Weinberg holds a US patent related to lipid resuscitation. Guy Weinberg & Israel Rubinstein are co-founders of ResQ Pharma, LLC.
References
- 1.U.S. Department of Health and Human Services. [Accessed Feb 8, 2015];Drug Abuse Warning Network, 2011: national estimates of drug-related emergency department visits. Available at: http://www.samhsa.gov/data/sites/default/files/DAWN2k11ED/DAWN2k11ED/DAWN2k11ED.pdf.
- 2.Maraj S, Figueredo VM, Lynn Morris D. Cocaine and the heart. Clin Cardiol. 2010;33:264–9. doi: 10.1002/clc.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kloner RA, Hale S, Alker K, Rezkalla S. The effects of acute and chronic cocaine use on the heart. Circulation. 1992;85:407–19. doi: 10.1161/01.cir.85.2.407. [DOI] [PubMed] [Google Scholar]
- 4.Wood DM, Dargan PI, Hoffman RS. Management of cocaine-induced cardiac arrhythmias due to cardiac ion channel dysfunction. Clin Toxicol. 2009;47:14–23. doi: 10.1080/15563650802339373. [DOI] [PubMed] [Google Scholar]
- 5.Connors N, Hoffman R. Experimental treatments for cocaine toxicity: a difficult transition to the bedside. J Pharm Exp Ther. 2013;347:251–7. doi: 10.1124/jpet.113.206383. [DOI] [PubMed] [Google Scholar]
- 6.Harris DS, Everhart ET, Mendelson J, Jones RT. The pharmacology of cocaethylene in humans following cocaine and ethanol administration. Drug Alcohol Depen. 2003;72:169–82. doi: 10.1016/s0376-8716(03)00200-x. [DOI] [PubMed] [Google Scholar]
- 7.Pan WJ, Hedaya M. Cocaine and alcohol interactions in the rat: effect of cocaine and alcohol pretreatments on cocaine pharmacokinetics and pharmacodynamics. J Pharm Sci. 1999;88:1266–74. doi: 10.1021/js990184j. [DOI] [PubMed] [Google Scholar]
- 8.Hearn WL, Flynn DD, Hime GW, et al. Cocaethylene: a unique cocaine metabolite displays high affinity for the dopamine transporter. J Neurochem. 1991;56:698–701. doi: 10.1111/j.1471-4159.1991.tb08205.x. [DOI] [PubMed] [Google Scholar]
- 9.Jatlow P, Elsworth JD, Bradberry CW, et al. Cocaethylene: a neuropharmacologically active metabolite associated with concurrent cocaine-ethanol ingestion. Life Sci. 1991;48:1787–94. doi: 10.1016/0024-3205(91)90217-y. [DOI] [PubMed] [Google Scholar]
- 10.Wiener SE, Sutijono D, Moon CH, et al. Patients with detectable cocaethylene are more likely to require intensive care unit admission after trauma. Am J Emerg Med. 2010;28:1051–5. doi: 10.1016/j.ajem.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 11.O’Leary ME, Hancox JC. Role of voltage-gated sodium, potassium and calcium channels in the development of cocaine-associated cardiac arrhythmias. Brit J Clin Pharmacol. 2010;69:427–42. doi: 10.1111/j.1365-2125.2010.03629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jakkala-Saibaba R, Morgan PG, Morton GL. Treatment of cocaine overdose with lipid emulsion. Anaesthesia. 2011;66:1168–70. doi: 10.1111/j.1365-2044.2011.06895.x. [DOI] [PubMed] [Google Scholar]
- 13.Arora NP, Berk WA, Aaron CK, Williams KA. Usefulness of intravenous lipid emulsion for cardiac toxicity from cocaine overdose. Am J Cardiol. 2013;111:445–7. doi: 10.1016/j.amjcard.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 14.Carreiro S, Blum J, Hack JB. Pretreatment with intravenous lipid emulsion reduces mortality from cocaine toxicity in a rat model. Ann Emerg Med. 2014;64:32–7. doi: 10.1016/j.annemergmed.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Weinberg G. Lipid emulsion infusion resuscitation for local anesthetic and other drug overdose. Anesthesiology. 2012;117:180–7. doi: 10.1097/ALN.0b013e31825ad8de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinberg G, Lin B, Zheng S, et al. Partitioning effect in lipid resuscitation: further evidence for the lipid sink. Crit Care Med. 2010;38:2268–9. doi: 10.1097/CCM.0b013e3181f17d85. [DOI] [PubMed] [Google Scholar]
- 17.Nadrowitz F, Stoetzer C, Foadi N, et al. The distinct effects of lipid emulsions used for “lipid resuscitation” on gating and bupivacaine-induced inhibition of the cardiac sodium channel Nav1. 5. Anesth Analg. 2013;117:1101–8. doi: 10.1213/ANE.0b013e3182a1af78. [DOI] [PubMed] [Google Scholar]
- 18.Wagner M, Zausig Y, Ruf S, et al. Lipid rescue reverses the bupivacaine-induced block of the fast Na+ current (INa) in cardiomyocytes of the rat left ventricle. Anesthesiology. 2014;120:724–36. doi: 10.1097/ALN.0b013e3182a66d4d. [DOI] [PubMed] [Google Scholar]
- 19.Hori K, Matsuura T, Mori T, Kuno M, Sawada M, Nishikawa K. The effect of lipid emulsion on intracellular bupivacaine as a mechanism of lipid resuscitation: an electrophysiological study using voltage-gated proton channels. Anesth Analg. 2013;117:1293–301. doi: 10.1213/ANE.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 20.Fettiplace MR, Lis K, Ripper R, et al. Multi-modal contributions to detoxification of acute pharmacotoxicity by a triglyceride micro-emulsion. J Control Release. 2015;198:62–70. doi: 10.1016/j.jconrel.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fettiplace MR, Ripper R, Lis K, et al. Rapid cardiotonic effects of lipid emulsion infusion. Crit Care Med. 2013;41:e156–62. doi: 10.1097/CCM.0b013e318287f874. [DOI] [PubMed] [Google Scholar]
- 22.Fettiplace MR, Akpa B, Ripper R, et al. Resuscitation with lipid emulsion: dose-dependent recovery from cardiac pharmacotoxicity requires a cardiotonic effect. Anesthesiology. 2014;120:915–25. doi: 10.1097/ALN.0000000000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertrand N, Gauthier MA, Bouvet C, et al. New pharmaceutical applications for macromolecular binders. J Control Release. 2011;155:200–10. doi: 10.1016/j.jconrel.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 24.Yu J, Zhao Y, Holterman M, Venton D. Cocaine detoxification by combinatorially substituted β-Cyclodextrin libraries. Bioorg Med Chem. 2002;10:3291–9. doi: 10.1016/s0968-0896(02)00205-5. [DOI] [PubMed] [Google Scholar]
- 25.Nesnas N, Lou J, Breslow R. The binding of cocaine to cyclodextrins. Bioorg Med Chem Lett. 2000;10:1931–3. doi: 10.1016/s0960-894x(00)00371-1. [DOI] [PubMed] [Google Scholar]
- 26.Weinberg G, Ripper R, Bern S, et al. Pioglitazone attenuates acute cocaine toxicity in rat isolated heart: potential protection by metabolic modulation. Anesthesiology. 2011;114:1389–95. doi: 10.1097/ALN.0b013e318218a7f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinberg GL, Palmer JW, VadeBoncouer TR, Zuechner MB, Edelman G, Hoppel CL. Bupivacaine inhibits acylcarnitine exchange in cardiac mitochondria. Anesthesiology. 2000;92:523–8. doi: 10.1097/00000542-200002000-00036. [DOI] [PubMed] [Google Scholar]
- 28.Weinberg G, VadeBoncouer T, Ramaraju G, Garcia-Amaro M, Cwik M. Pretreatment or resuscitation with a lipid infusion shifts the dose-response to bupivacaine-induced asystole in rats. Anesthesiology. 1998;88:1071–5. doi: 10.1097/00000542-199804000-00028. [DOI] [PubMed] [Google Scholar]
- 29.Ferrari M, Wilson D. Effects of graded hypotension on cerebral blood flow, blood volume, and mean transit time in dogs. Am J Physiol. 1992;262:H1908–14. doi: 10.1152/ajpheart.1992.262.6.H1908. [DOI] [PubMed] [Google Scholar]
- 30.Taira Y, Marsala M, Rosenblum WI. Effect of proximal arterial perfusion pressure on function, spinal cord blood flow, and histopathologic changes after increasing intervals of aortic occlusion in the rat. Stroke. 1996;27:1850–8. doi: 10.1161/01.str.27.10.1850. [DOI] [PubMed] [Google Scholar]
- 31.Palmer J, Tandler B, Hoppel C. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem. 1977;252:8731–9. [PubMed] [Google Scholar]
- 32.Dixon M. The determination of enzyme inhibitor constants. Biochem J. 1953;55:170–1. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burlingham B, Widlanski T. An intuitive look at the relationship of Ki and IC50: a more general use for the Dixon plot. J Chem Educ. 2003;80:214–8. [Google Scholar]
- 34.Weinberg GL, Ripper R, Murphy P, et al. Lipid infusion accelerates removal of bupivacaine and recovery from bupivacaine toxicity in the isolated rat heart. Reg Anesth Pain Med. 2006;31:296–303. doi: 10.1016/j.rapm.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Morishima H, Whittington R, Iso A, Cooper T. The comparative toxicity of cocaine and its metabolites in conscious rats. Anesthesiology. 1999;90:1684–90. doi: 10.1097/00000542-199906000-00025. [DOI] [PubMed] [Google Scholar]
- 36.Wilson LD, Malik M, Willson H. Cocaine and ethanol: combined effects on coronary artery blood flow and myocardial function in dogs. Acad Emerg Med. 2009;16:646–55. doi: 10.1111/j.1553-2712.2009.00443.x. [DOI] [PubMed] [Google Scholar]
- 37.Wilson L, Jeromin J, Garvey L, Dorbandt A. Cocaine, ethanol, and cocaethylene cardiotoxity in an animal model of cocaine and ethanol abuse. Acad Emerg Med. 2001;8:211–22. doi: 10.1111/j.1553-2712.2001.tb01296.x. [DOI] [PubMed] [Google Scholar]
- 38.Albright G. Cardiac arrest following regional anesthesia with etidocaine or bupivacaine. Anesthesiology. 1979;51:285–7. doi: 10.1097/00000542-197910000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Eledjam JJ, de La Coussaye JE, Brugada J, et al. In vitro study on mechanisms of bupivacaine-induced depression of myocardial contractility. Anesth Analg. 1989;69:732–5. [PubMed] [Google Scholar]
- 40.Leroux J-C. Injectable nanocarriers for biodetoxification. Nat Nanotechnol. 2007;2:679–84. doi: 10.1038/nnano.2007.339. [DOI] [PubMed] [Google Scholar]
- 41.Graham L, Nguyen T, Lee S. Nanodetoxification: emerging role of nanomaterials in drug intoxification treatment. Nanomedicine (London) 2011;6:921–8. doi: 10.2217/nnm.11.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 43.Fallon MS, Chauhan A. Sequestration of amitriptyline by liposomes. J Colloid Interface Sci. 2006;300:7–19. doi: 10.1016/j.jcis.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 44.Forster V, Luciani P, Leroux J-C. Treatment of calcium channel blocker-induced cardiovascular toxicity with drug scavenging liposomes. Biomaterials. 2012;33:3578–85. doi: 10.1016/j.biomaterials.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 45.Minton N, Goode A, Henry J. The effect of a lipid suspension on amitriptyline disposition. Arch Toxicol. 1987;60:467–9. doi: 10.1007/BF00302392. [DOI] [PubMed] [Google Scholar]
- 46.Hu C-MJ, Fang RH, Copp J, Luk BT, Zhang L. A biomimetic nanosponge that absorbs pore-forming toxins. Nat Nanotechnol. 2013;8:336–40. doi: 10.1038/nnano.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoshino Y, Koide H, Furuya K, et al. The rational design of a synthetic polymer nanoparticle that neutralizes a toxic peptide in vivo. Proc Natl Acad Sci. 2012;109:33–8. doi: 10.1073/pnas.1112828109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuo I, Akpa BS. Validity of the lipid sink as a mechanism for the reversal of local anesthetic systemic toxicity: a physiologically based pharmacokinetic model study. Anesthesiology. 2013;118:1350–61. doi: 10.1097/ALN.0b013e31828ce74d. [DOI] [PubMed] [Google Scholar]
- 49.Cave G, Harvey M, Shaw T, Damitz R, Chauhan A. Comparison of intravenous lipid emulsion, bicarbonate, and tailored liposomes in rabbit clomipramine toxicity. Acad Emerg Med. 2013;20:1076–9. doi: 10.1111/acem.12224. [DOI] [PubMed] [Google Scholar]
- 50.Koner R, Rezkalla S. Cocaine and the heart. New Engl J Med. 2003;348:487–8. doi: 10.1056/NEJMp020174. [DOI] [PubMed] [Google Scholar]
- 51.Verster RS, Botha CJ. Evaluation of hydroxypropyl-β-cyclodextrin in the treatment of aldicarb poisoning in rats. J S Afr Vet Assoc. 2004;75:182–5. doi: 10.4102/jsava.v75i4.480. [DOI] [PubMed] [Google Scholar]
- 52.Mottram A, Bryant S, Aks S. Dose-dependent response to cyclodextrin infusion in a rat model of verapamil toxicity. West J Emerg Med. 2012;13:63–7. doi: 10.5811/westjem.2011.3.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.May C, Stewart PL. Development of a toxin-binding agent as a treatment for tunicaminyluracil toxicity: protection against tunicamycin poisoning of sheep. Aust Vet J. 1998;76:752–6. doi: 10.1111/j.1751-0813.1998.tb12307.x. [DOI] [PubMed] [Google Scholar]
- 54.Harvey M, Cave G. Intralipid outperforms sodium bicarbonate in a rabbit model of clomipramine toxicity. Ann Emerg Med. 2007;49:178–85. doi: 10.1016/j.annemergmed.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 55.Fettiplace MR, Ripper R, Lis K, Feinstein DL, Rubinstein I, Weinberg G. Intraosseous lipid emulsion: an effective alternative to IV delivery in emergency situations. Crit Care Med. 2014;42:e157–60. doi: 10.1097/01.ccm.0000435677.76058.15. [DOI] [PubMed] [Google Scholar]
- 56.US Department of Health and Human Services: Food and Drug Administration Center for Drug Evaluation and Research. [Accessed Feb 8, 2015];Guidance for Industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Available at: http://www.fda.gov/ohrms/dockets/98fr/02d-0492-gdl0002.pdf.
- 57.Weinberg G, Ripper R, Feinstein DL, Hoffman W. Lipid emulsion infusion rescues dogs from bupivacaine-induced cardiac toxicity. Reg Anesth Pain Med. 2003;28:198–202. doi: 10.1053/rapm.2003.50041. [DOI] [PubMed] [Google Scholar]
- 58.Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim Biophys Acta. 2007;1768:1311–24. doi: 10.1016/j.bbamem.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stella V, Rajewski R. Cyclodextrins: their future in drug formulation and delivery. Pharm Res. 1997;14:556–67. doi: 10.1023/a:1012136608249. [DOI] [PubMed] [Google Scholar]
- 60.Kurkov SV, Madden DE, Carr D, Loftsson T. The effect of parenterally administered cyclodextrins on the pharmacokinetics of coadministered drugs. J Pharm Sci. 2012;101:4402–8. doi: 10.1002/jps.23329. [DOI] [PubMed] [Google Scholar]
- 61.Sorgenfrei IF, Norrild K, Larsen PB, et al. Reversal of rocuronium-induced neuromuscular block by the selective relaxant binding agent sugammadex: a dose-finding and safety study. Anesthesiology. 2006;104:667–74. doi: 10.1097/00000542-200604000-00009. [DOI] [PubMed] [Google Scholar]