Introduction
It seems implausible that an injection of a simple, off-the-shelf, intravenous nutritional solution could be acutely life-saving for a patient with severe drug overdose. Yet, dozens of published case reports support this observation, first made more than a decade ago in a rodent model of bupivacaine toxicity. It is even more surprising that such a simple formulation can rapidly reverse severe clinical toxicity from a variety of vastly disparate medications with distinct pharmacodynamics and mechanisms of action. This review will focus on the clinical application of lipid emulsion therapy in resuscitation from drug-related toxicity and provide an introduction to the development of the method, guidelines for its use and insights into potential controversies and future applications.
Background
Weinberg et al1 first showed in 1998 that an infusion of soy-bean oil emulsion, total parenteral nutrition solution, could prevent (by pretreatment) or improve efficacy of resuscitation from cardiovascular collapse caused by severe bupivacaine overdose in the intact, anesthetized rat. Subsequent studies from the same laboratory confirmed these findings in the rat isolated heart2 and the anesthetized dog3. Under the latter experimental model, return of spontaneous circulation after a bupivacaine challenge occurred in all animals receiving lipid, but in none of the saline controls3. This study was accompanied by an editorial asking whether lipid might be the long-sought ‘silver bullet’ for local anesthetic systemic toxicity (LAST). Since then, the effectiveness of lipid emulsion infusion in reversing LAST has been confirmed in other laboratories and by systematic analysis4 in the clinical setting as well.
Instructive Case Reports: Conclusions and Contradictions
The level of evidence from case reports is less rigorous than that provided by prospective, randomized, controlled clinical trials; however, such study designs are unethical and unsuited to clinical investigations of local anesthetic toxicity. Nonetheless, careful evaluation of even a single case can provide useful information about a particular toxic syndrome and its treatment. Taken together, dozens of such reports can provide valuable clinical insights, as a clear picture emerges of the typical course of a particular overdose and its response to lipid therapy.
LAST
Rosenblatt et al5 reported the first clinical application of lipid emulsion therapy in treating LAST. A middle aged man developed cardiac arrest shortly after a peripheral nerve block combining mepivacaine and bupivacaine. The patient failed to respond to standard resuscitative efforts for approximately 20 minutes but achieved normal vital signs shortly after receiving a 100 mL bolus of lipid emulsion. He subsequently recovered completely with no neurological deficit or cardiovascular sequelae.
This case is now recognized as typical of many lipid resuscitation cases and exemplifies key features repeated in virtually every subsequent report of reversal by lipid infusion of LAST- related cardiac arrest: 1) the event was witnessed (meaning, little to no associated asphyxia or delay in treatment); 2) the patient failed to recover with epinephrine, vasopressin and anti-arrhythmic medications; and 3) spontaneous circulation was re-established shortly after lipid infusion. An additional feature common to many of the early case reports of lipid therapy for severe LAST was the presence of underlying heart disease, suggesting that coronary ischemia, baseline conduction defects or cardiomyopathy could lower the threshold for LAST, thereby defining one subgroup of vulnerable patients.
McCutchen and Gerancher6 reported that in a patient with seizures and ventricular tachycardia following a combined femoral catheter (ropivacaine) and sciatic (bupivacaine) block, the use of lipid emulsion early in the sequence of rapidly worsening toxicity appeared to attenuate or prevent progression of local anesthetic cardiac toxicity. This observation suggests that early lipid infusion might provide an advantage, presumably by interrupting the vicious cycle of low-output, tissue acidosis and worsening toxicity, thereby preventing progression to a low-output state or frank cardiac arrest. Several other recent cases seem to support this notion. These reports contribute directly to the controversy surrounding optimal timing of the lipid emulsion infusion that is addressed below.
Lipid infusion can also reverse neurological signs and symptoms of LAST, including seizures and altered mental status, suggesting that the benefit is not limited to the cardiovascular system7,8. This similarly contributes to the debate regarding the mechanisms underlying lipid resuscitation, since the metabolic hypothesis would not hold in the case of neurotoxicity because the central nervous system does not normally depend on lipid substrates (see the section on ‘Mechanisms’). Successful treatment of severe cardiac toxicity has been reported in children, including two neonates, including a two day old9; the oldest patient reported in a successful lipid resuscitation was a 92 year old woman in asystole following an infraclavicular block with ropivavcaine. Though Intralipid (Fresenius Kabi, Uppsala, Sweden) has been the predominant lipid emulsion brand used in instances of lipid resuscitation reported in the medical literature, successful treatment of severe toxicity has also been reported with other formulations including Liposyn III (Hospira, Lake Forest, IL) and Medialipid (B. Braun, Melsungen, Germany) a mixture of long and medium chain fatty acid triglycerides.
More cases of lipid resuscitation from local anesthetic toxicity can be found in recent reviews of the topic4,10. Additional cases, along with descriptions of the use of lipid in treating many other types of drug toxicity, are posted at the educational website*. This website serves as a forum for discussing lipid therapy and related topics and contains useful links to relevant literature. Practitioners are invited to post cases here and at a sister site†. Together, these two websites comprise an open source registry for reporting the clinical experience with lipid-based resuscitation. Though presumably only a small fraction of all cases is reported, the hope is that over time methods will be identified to increase the rate of case capture and reporting.
Other Toxicological Emergencies
Following the reports of laboratory success in resuscitation from bupivacaine toxicity, lipid emulsion infusion was studied in animal models of a variety of other overdoses, typically those expected to be seen in the emergency room. These include models showing a benefit of lipid in treating overdose of tricyclic antidepressants, beta-blockers, and calcium channel blockers. However, the first use of lipid emulsion therapy in treating non-local anesthetic drug toxicity was described by Sirianni et al11 who reported on the remarkable rescue of an adolescent near-suicide who suffered a witnessed cardiac arrest hours after hospitalization for a massive overdose of bupropion and lamotrigine. After 90 min of ventricular tachycardia/fibrillation and narrow complex pulseless electrical activity, which were unresponsive to maximal medical therapy, including high dose pressors and multiple countershocks, an anesthesiologist recommended using lipid. Within one min of a single bolus of lipid emulsion, the patient recovered normal vital signs and ultimately left the hospital with no major neurologic deficit.
This publication, along with the above-mentioned animal studies, opened the door to more widespread use of lipid emulsion for emergency treatment of toxicity due to a range of lipophilic drugs. Notably, published examples now include toxicities related to verapamil, diltiazem, amlodipine, quetiapine and sertraline, haldoperidol, lamotrigine, olanzapine, propranolol, atenolol, nevibolol, doxepin, dosulepin, imipramine, amitriptyline, glyosphate herbicide, flecainide, venlafaxine, moxidectin and others. It is arguable whether lipid infusion was the proximate cause of toxic reversal in all these singlet case reports. However, on balance it appears lipid might be generally effective in cases where the agent(s) are lipophilic, despite possessing disparate pharmacologic profile infusions. This could apply to treating cases of multi-drug overdose such as that reported by Harvey et al12. Others have recently found that treatment of such mixed drug overdose with lipid emulsion resulted in a reduced incidence of intubation and shorter duration of stay in the intensive care unit than matched controls not receiving lipid (personal communication, Dr. David Uncles, Worthing Hospital, England). Thus the salutary clinical effects of lipid infusion might extend to cost savings as well.
Interestingly, the reversal of haldol-induced Torsades occurred in the same hospital where a year earlier the case report of rescue from bupropion overdose occurred. The treating physician was aware of the previous case and therefore ordered the infusion of lipid emulsion after fifteen min of failed standard resuscitation. Similarly, Smith et al13 reported an apparent save of a patient in cardiac arrest following a bupivacaine-based peripheral nerve block when physicians who had recently attended a simulator course that included a lipid scenario administered the emulsion. These cases emphasize the positive effect that education and awareness of the method can have on patient outcome.
Caveats in Case Reports
It is essential to consider the overall limitations inherent in the interpretation and clinical extrapolation from of such cases. Several types of reporting bias are at play. First is the bias to report positive outcomes and the correlative, presumed underreporting of failed lipid resuscitations. This could impart an overly optimistic account of the efficacy of this therapy. On the obverse, failures of lipid resuscitation should not be automatically ascribed to the use of emulsion since many other patient or treatment factors can contribute to a bad outcome. Severe co-morbidities, overwhelming overdose, delayed intervention, inadequate support of the airway, poor quality Basic Life Support, use of the wrong dose or formulation of lipid could each lead to a failed resuscitation. Another reporting failure occurs as use of lipid becomes more commonplace and therefore fewer physicians are likely to report these cases and editors are less inclined to publish them. Unfortunately, potentially important information is lost in this manner. Such under-reporting could ‘balance’ the effect of positive reporting bias, but the relative importance of these opposing factors is unknown. Since prospective, randomized clinical studies are not feasible, a clinical registry of all resuscitations incorporating lipid could provide a more realistic assessment of therapeutic efficacy, and provide insight into the key factors that improve or reduce the odds of recovery. This underscores the need for establishing a robust method of tracking and evaluating use of lipid emulsion for this purpose.
Safety of Lipid Emulsion Infusion
Previous reports of pulmonary complications following lipid emulsion infusion have involved large volumes of high concentrations for parenteral nutrition particularly in neonates. Fortunately, no such events have been reported in lipid-based resuscitations and no serious clinical complications have been reported following use of lipid emulsion for treating drug-induced toxicity. One notable case of massive overdose reported by West et al14 involved a 72 year old patient with severe amlodipine overdose; two liters of 20% lipid emulsion was infused because of confusion about the treatment protocol. The patient suffered no direct cardiopulmonary complications from the massive overdose. However, the extreme lipemia interfered with various laboratory studies and such instances serve to emphasize the importance of implementing clinical systems and guidelines focused on patient safety in preventing drug errors and iatrogenic harm. In this case, a protocol to prevent inadvertent continuous infusion beyond 30 min would have prevented the lipid and volume overload.
Marwick et al15 reported one patient who developed chemical hyperamylasemia without symptoms of pancreatitis following successful lipid rescue from cardiac arrest due to bupivacaine. Therefore, there is at least a theoretical concern for hyperlipidemia-induced pancreatitis and it is reasonable to assess patients for this after acute lipid infusion. Another instructive twist in this case is that cardiovascular instability recurred 45 min after stopping the lipid infusion and no additional lipid was available. Fortunately the patient responded to pressors and ultimately recovered. This occurrence suggests that patients need careful monitoring for several hours after lipid-based resuscitation and doctors should be prepared to re-institute lipid therapy if needed. Furthermore, in this case and other reports, it has been noted that the extreme lipemia following lipid emulsion infusion can interfere with standard laboratory tests. Given the short (∼15 min) half life of the resulting hypertriglyceridemia, it is expected that this effect should completely dissipate after a few hours.
It is important to ask, ‘What is the acceptable upper limit of lipid infusion’? There is no clear cut answer at this point. Hiller et al16 used a Dixon up-down method to arrive at a first approximation for an LD50 of 20% lipid infusion in anesthetized rats. Using death within 48 hours as the endpoint, they found the maximum likelihood estimate as LD50 = 67 +/- 11 (SEM) mL/kg. The LD 50 is not the ideal metric for identifying the highest safe dose. However, given that the average dose used in the first dozen case reports of lipid resuscitation was 3.7 mL/kg, even scaling the dose to correct for size differences between rats and humans, there appears to be a reasonable margin of safety in limiting the total dose to approximately 10-12 mL/kg over thirty min.
Mechanism
Understanding the mechanism(s) that underlie the effects of lipid emulsion infusion could lead to improved treatment of drug toxicity and possibly extend the use of lipid resuscitation to other clinical scenarios. The typical solutions used for parenteral nutrition are very complex mixtures of natural (e.g. soy) products and this complexity might explain the wide spectrum of resulting physiologic and pharmacologic effects. Two effects in particular, partitioning and enhanced metabolism were initially posited to explain the benefit of lipid infusion during bupivacaine toxicity. However, evidence has recently accumulated indicating several other important potential sites of action (see Figure 1).
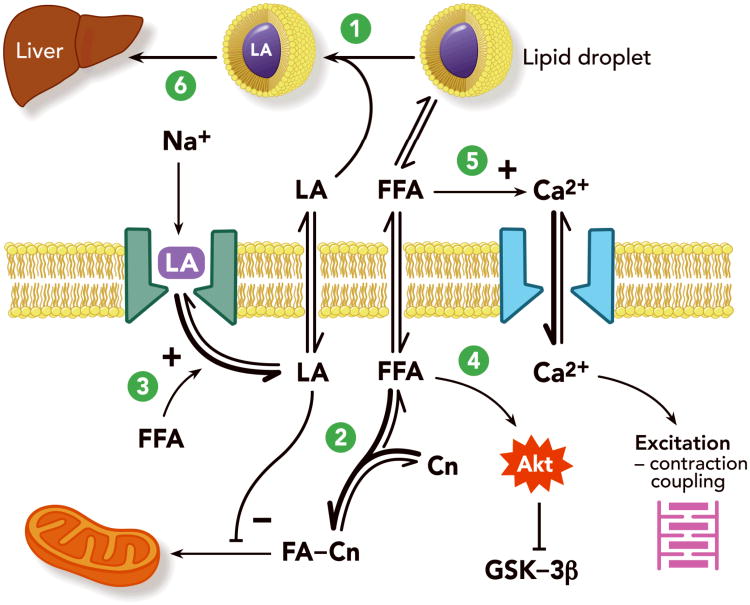
Figure 1.
Proposed mechanisms of lipid resuscitation. After infusion, the lipid emulsion exists in the blood as emulsified oil droplets or multi-lamellar vesicles which for the sake of simplicity are represented as micelles. 1. Capture of LA (lipid sink); 2. Increased fatty acid uptake by mitochondria (metabolic effect); 3. Inteference with LA binding of sodium channels (membrane effect); 4. Activation of Akt cascade leading to inhibition of GSK-3beta (cytoprotection); 5. Promotion of calcium entry via voltage-dependent calcium channels (ionotropic/inotropic; can also involve mitochondrial calcium dynamics); 6. Accelerated shunting (pharmacokinetic effects). FFA: free fatty acids; LA: local anesthetic; Cn: carnitine; FA-Cn: fatty acyl carnitine; Na: sodium ion; Ca: calcium ion; Akt: a serine/threonine protein kinase important in cell survival, proliferation and migration (also called Protein Kinase B); GSK-3beta: glycogen synthase kinase (phophorylates and thereby inhibits glycogen synthase; inhibition of GSK-3beta has been implicated in preventing myocardial ischemia-reperfusion injury).
Partitioning
The early weight of evidence, both direct and indirect, favored an especially important role for the so-called ‘lipid sink’ in reversal of local anesthetic overdose. Simply stated, the infused intravascular lipid mass binds the offending toxin in sufficient quantity to pull drug from the target tissue, thereby reversing the toxicity. Indirect evidence in support of this theory includes the fact that lipid can reverse both neurologic and cardiac toxicity, though the brain does not metabolize fatty acids as an energy source to an appreciable degree. Furthermore, as noted above, lipid infusion is reported to reverse toxicity caused by an array of drugs lacking a common mechanism, site of action, chemical structure or clinical effect (e.g., calcium channel blockers, beta blockers, typical and atypical antipsychotics, tricyclic and other antidepressants, local anesthetics and parasiticides among others). The only shared factor among these drugs is high lipid solubility, indicated by a log P (octanol: water partition coefficient) greater than 2. Weinberg et al2 also reported that after a bolus injection of radiolabeled bupivacaine into the buffer perfusing isolated rat hearts, a subsequent lipid infusion results in more rapid decline of myocardial bupivacaine content than observed in controls – an effect predicted by the sink mechanism. More direct evidence in support of the lipid sink model is provided by studies from Mazoit et al17 showing that lipid emulsion solutions bind very large amounts of lipid-soluble local anesthetic. Supplementing this in vitro experiment, Niiya et al18 found that pretreating pigs with lipid, protected against amiodarone-induced hypotension. Furthermore, ultracentrifuging the plasma to allow separation of the lipid-bound drug indicated that amiodarone was preferentially partitioned into the newly formed lipid phase. Direct evidence in support of a lipid sink effect was then provided by the observation that the resulting lipid-free aqueous phase had a lower concentration of amiodarone than found in that of the pigs given saline instead of lipid. Similarly, Weinberg et al19 analyzed stored blood samples taken at the end of an experimental series comparing lipid versus vasopressin treatment of rats resuscitated from bupivacaine overdose. They found the aqueous (lipid-free) phase bupivacaine concentration was substantially greater in the vasopressin group than in the lipid treated subjects. Among the lipid treated animals (n=5), the ratio of bupivacaine concentrations in the lipid versus aqueous phases following centrifugation was 18.7. Moreover, myocardial bupivacaine content showed a strong positive correlation with the aqueous plasma concentration and a strong negative correlation with rate-pressure product. Therefore, lipid treatment resulted in lower aqueous bupivacaine concentration, lower myocardial content and better cardiac performance. Samuels et al20 assessed the efficacy of partitioning by measuring the production of methemoglobin in blood by drugs of varying lipid solubility. Adding lipid emulsion substantially reduced methemoglobin production caused by the most lipid soluble drug, but did not suppress methemoglobin production caused by less lipid soluble drugs. This supports the relevance of a lipid sink in changing an adverse, drug-related physiologic endpoint. Finally, French et al21 examined in vitro reduction of aqueous drug concentration following addition of lipid emulsion to serum. They found that both the partition coefficient (75% of variation) and volume of distribution (13% of variation) were correlated with the amount of decrease in drug concentration. Combining these factors the authors were able to predict with great accuracy (r2=0.88), the measured decrease of in vitro serum concentration caused by adding 2% lipid emulsion to solutions of eleven drugs previously reported in cases of successful lipid resuscitation. Taken together, these studies provide support for the role of partitioning in lipid resuscitation in treating bupivacaine toxicity and other lipophilic drug overdoses.
However, other studies do not support a substantial contribution of partitioning to lipid resuscitation. For instance, Litonius et al22 measured bupivacaine concentrations in the blood of volunteers given small doses of bupivacaine then treated with lipid or control infusion. There was no difference in free (non-lipid or non-protein bound) bupivacaine concentration indicating a lack of a lipid sink effect. However, lipid infusion shortened the context-sensitive half-life of total plasma bupivacaine concentration by more than 40%, suggesting a positive effect on distribution of bupivacaine to peripheral tissues.
Metabolism
Lipids comprise the hearts preferred energy substrate under normal aerobic conditions and it was reasoned that flux through this pathway could directly affect cardiac function. Therefore, an early theory was that a large lipid load could offset the potent inhibition of fatty acid metabolism caused by bupivacaine. Evidence in support of this theory was first published by Stehr et al23 who showed that in isolated rat heart, concentrations of lipid too low to substantially reduce bupivacaine content in the perfusate were able to reverse bupivacaine-induced reduction in cardiac function. More recently, Partownavid et al24 confirmed a metabolic effect by showing that inhibition of fatty acid oxidation prevents lipid reversal of bupivacaine-induced cardiac toxicity. The improved metabolism was associated with additional cytoprotective effects that suppressed the mitochondrial permeability transition, a key step in programmed cell death. Aside from the reversal of bupivacaine toxicity, we have observed that lipid infusion can exert a direct cardiotonic effect in both intact rats and rat isolated hearts. Although the precise mechanism of this phenomenon is unknown it occurs very rapidly and might contribute to lipid resuscitation.
Other Mechanisms
Mottram et al25 showed in a heterologous tissue culture expression system that free fatty acids reduced bupivacaine inhibition of sodium channel currents. They suggest that modulation of cardiac sodium channels could contribute to reversal of bupivacaine toxicity. Taking a much different approach, Rahman et al26 showed that lipid infusion attenuates cardiac ischemia reperfusion injury. They found that post ischemic infusion of lipid in rodents, as observed in the experiments with metabolic inhibitors, reduced the likelihood of mitochondrial permeability transition and apoptosis. It is very possible that such activation of cytoprotective pathways contributes to the clinically observed benefit of lipid-based resuscitation – a much more complex clinical phenomenon than was initially appreciated. We can now consider separating the results of lipid infusion into intracellular (metabolic, signaling), intravascular (partitioning, sink) and membrane (channel) effects. Future scientific investigation will hopefully identify all the underlying consequences of lipid emulsion infusion and determine their relative contributions to reversing drug overdose.
Controversies in Lipid Emulsion Resuscitation
There is ample evidence that hypoxia and acidosis exacerbate local anesthetic toxicity and potentially inhibit lipid resuscitation. Therefore, the clinician must approach local anesthetic toxicity by first ensuring optimal ventilation, oxygenation and, if necessary circulation and organ perfusion (read, high quality Basic Life Support). Thereafter, the relevant question becomes, ‘How should lipid be positioned in the context of conventional resuscitation, i.e., Advanced Cardiac Life Support’? This is a complex issue and we are still waiting for the best answer given that the available data are not completely consistent.
Experiments comparing lipid to standard resuscitation methods for local anesthetic toxicity, found that lipid was superior to either epinephrine27, vasopressin28 or a combination of the two in treating bupivacaine overdose in the intact rat. Both studies used rate-pressure product, a surrogate of cardiac work, as the key measure of recovery. Metabolic metrics including arterial pH and central venous mixed oxygen saturation were also much better after lipid than in any of the pressor-treated groups. However, work by Mayr et al29 and Hicks et al30, both using pig models of bupivacaine overdose, has contradicted these findings. Mayr et al showed that the combination of high dose epinephrine and vasopressin, was superior to lipid in treating bupivacaine overdose. Hicks et al found no benefit in treating with lipid compared with saline control. Several factors are likely to account for these discrepant findings.
The model reported by Mayr et al29 included a challenge of several minutes of apnea following the bupivacaine injection. Furthermore, they used systolic pressure as the key metric of recovery – a parameter for which pressors will specifically show a positive effect. Moreover, it has been shown that asphyxia31 and acidosis may reduce the efficacy of lipid infusion. Hicks et al30 administered high doses of both vasopressin and epinephrine for resuscitation before treatment with either lipid or saline. Notably, Hiller et al32 showed in a rat model of bupivacaine overdose that a single dose of epinephrine at 10 μg/kg or greater significantly impairs the efficacy of lipid resuscitation from bupivacaine overdose. All epinephrine-treated animals recovered quickly and exhibited high rate-pressure product early in the resuscitation. However, animals receiving the larger doses of epinephrine developed pulmonary edema, and exhibited poor hemodynamic and metabolic metrics after 15 min despite the initially robust blood pressure and heart rate measurements. The mechanisms underlying this delayed decline is not entirely clear but might explain the negative findings of Hicks et al30 since the very large doses of epinephrine would be expected to defeat any benefit from lipid infusion. Finally, it should be noted that Niiya et al18 and others have reported that pigs develop generalized mottling of the skin after lipid infusion. This is very consistent with the well-known and studied phenomenon of complement activation related pseudoallergy (CARPA) seen when pigs receive liposomal preparations. It is not clear whether these are precisely comparable phenomena; however, the observation does call into question the validity of a porcine model for lipid resuscitation.
The optimal formulation of lipid emulsion has recently been questioned by Ruan et al33. Standard long chain triglyceride (LCT) and mixtures of long and medium chain triglyceride (LCT/MCT) emulsions have both been reported to reverse local anesthetic toxicity. However, Ruan et al showed in an experiment using human serum that an added LCT/MCT (50:50) mixture extracted bupivacaine, ropivacaine and mepivacaine to a greater extent than did adding the LCT emulsion. The authors suggest on the basis of this in vitro study that recommendations for the standard LCT formulations be called into question. However, Li et al34 showed in an intact rat model that LCT and LCT/MCT were equally effective in initial reversal of severe bupivacaine overdose but that 8/23 rats given LCT/MCT subsequently developed intractable cardiac arrest while only 2/24 animals in the LCT group did. Moreover, survival times were longer, blood pressure higher and cardiac and plasma bupivacaine concentrations were lower in the LCT group. Optimizing the emulsion will require further comparison of these and other formulations.
Current Recommendations and Practical Considerations
Guidelines for the use of lipid emulsion in resuscitation are available through the American Society of Regional Anesthesia (downloadable from the website‡), and the Association of Anaesthetists of Great Britain and Ireland§. The American Heart Association has also included lipid emulsion infusion in their recommendations for resuscitation in special situations specifically for local anesthetic overdose. The Helsinki Declaration on Patient Safety in Anaesthesiology requires that every department of anesthesiology in Europe has protocols for a range of specific issues, including management of LAST. These national protocols are available online**. Though minor differences exist among these versions, there is a generally accepted approach establishing airway management as the first priority in order to assure optimal oxygenation and ventilation; then seizure suppression, preferably with a benzodiazepine; then lipid emulsion infusion to reverse signs and symptoms of toxicity. Basic life support including chest compressions must be used when clinically indicated in order to assure tissue perfusion and circulation of resuscitation drugs including lipid.
When lipid infusion is used, it requires a large initial intravenous bolus of 20% lipid emulsion (approximately 1.5mL/kg of lean body mass) that is followed immediately by a continuous infusion (approximately 0.25-0.5mL/kg/min) for roughly 10 min following recovery of vital signs. The bolus injection is key to rapid clinical improvement since a large mass of lipid is apparently necessary to achieve the desired effect. A single bolus has been used in most case reports; however, this should be repeated or the infusion increased for failure of return of spontaneous circulation or declining blood pressure (respectively). Given that the lipid infusion must circulate to the coronary vascular bed, high quality basic life support is a necessary element of lipid resuscitation in the setting of a low output state. Specifically, this entails rapid, deep compressions (100 per min of at least 2 inches or 5 cm), allowing complete recoil of the chest wall after each compression and minimizing interruptions (e.g., for delivering a shock or checking for a pulse). It is important to recognize that cardiovascular collapse from LAST is different from other more common causes of cardiac arrest such as ischemia. In toxic cardiomyopathy, raising peripheral vascular resistance with potent vasopressors can impair cardiac output and impede resuscitation. Therefore, vasopressin is not considered useful in this setting and epinephrine should be used in small doses (e.g., <1 μ/kg). Moreover, other agents that reduce contractility (e.g., beta blockers or calcium channel blockers) should be avoided when there is evidence of cardiovascular instability. Given the current gaps in our understanding of the scientific basis of lipid resuscitation, these guidelines will likely be subject to frequent modification based on the evolving experimental literature and continued clinical experience. For instance, Neal et al35 recently showed that performance in simulated management of LAST was dramatically improved with use of a checklist. This important advance is reflected in the revised American Society of Regional Anesthesia and Pain Medicine's practice advisory for managing LAST.
The Future
It is important that the use and methods of lipid emulsion therapy be guided by laboratory evidence and clinical experience. One important advance in achieving this goal will be the implementation of a global registry to allow collation and analysis of a comprehensive database of lipid resuscitation cases. A first attempt to achieving this concept is found at the website††. These data will hopefully inform practitioners regarding the factors in treatment that improve or impede patient survival. Improvements may also come from modifications in lipid formulation or refinements in the regimen of administration, (e.g., by tying it to specific clinical metrics). Finally, it is possible that novel, potential applications in resuscitation or other clinical scenarios will be identified, such as attenuating myocardial reperfusion injury26 or pulmonary hypertension36. In the meantime, we must do our best to educate and encourage colleagues in all specialties practice adopt the accepted, effective guidelines for treating LAST. Together, we can improve patient safety and save lives.
Acknowledgments
Supported by: National Institute of Drug Abuse/National Institutes of Health 1R21DA17892-01. Bethesda, Maryland USA. Veterans Administration Merit Award (Weinberg); Washington, DC, USA.
Footnotes
www.lipidrescue.org (last accessed 4.4.12)
www.lipidregistry.org (last accessed 4.4.12)
http://www.euroanesthesia.org/sitecore/Content/Publications/Helsinki%20Declaration/Protocols.aspx (last accessed 4.6.12)
www.lipidregistry.org (last accessed 4.4.12)
Summary statement: Rapid infusion of lipid emulsion can reverse toxicity caused by lipophilic drugs including local anesthetics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weinberg GL, VadeBoncouer T, Ramaraju GA, Garcia-Amaro MF, Cwik MJ. Pretreatment or resuscitation with a lipid infusion shifts the dose-response to bupivacaine-induced asystole in rats. Anesthesiology. 1998;88:1071–5. doi: 10.1097/00000542-199804000-00028. [DOI] [PubMed] [Google Scholar]
- 2.Weinberg GL, Ripper R, Murphy P, Edelman LB, Hoffman W, Strichartz G, Feinstein DL. Lipid infusion accelerates removal of bupivacaine and recovery from bupivacaine toxicity in the isolated rat heart. Reg Anesth Pain Med. 2006;31:296–303. doi: 10.1016/j.rapm.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Weinberg G, Ripper R, Feinstein DL, Hoffman W. Lipid emulsion infusion rescues dogs from bupivacaine-induced cardiac toxicity. Reg Anesth Pain Med. 2003;28:198–202. doi: 10.1053/rapm.2003.50041. [DOI] [PubMed] [Google Scholar]
- 4.Jamaty C, Bailey B, Larocque A, Notebaert E, Sanogo K, Chauny JM. Lipid emulsions in the treatment of acute poisoning: A systematic review of human and animal studies. Clin Toxicol (Phila) 2010;48:1–27. doi: 10.3109/15563650903544124. [DOI] [PubMed] [Google Scholar]
- 5.Rosenblatt MA, Abel M, Fischer GW, Itzkovich CJ, Eisenkraft JB. Successful use of a 20% lipid emulsion to resuscitate a patient after a presumed bupivacaine-related cardiac arrest. Anesthesiology. 2006;105:217–8. doi: 10.1097/00000542-200607000-00033. [DOI] [PubMed] [Google Scholar]
- 6.McCutchen T, Gerancher JC. Early Intralipid therapy may have prevented bupivacaine-associated cardiac arrest. Reg Anesth Pain Med. 2008;33:178–80. doi: 10.1016/j.rapm.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Foxall G, McCahon R, Lamb J, Hardman JG, Bedforth NM. Levobupivacaine-induced seizures and cardiovascular collapse treated with Intralipid. Anaesthesia. 2007;62:516–8. doi: 10.1111/j.1365-2044.2007.05065.x. [DOI] [PubMed] [Google Scholar]
- 8.Spence AG. Lipid reversal of central nervous system symptoms of bupivacaine toxicity. Anesthesiology. 2007;107:516–7. doi: 10.1097/01.anes.0000278864.75082.72. [DOI] [PubMed] [Google Scholar]
- 9.Shah S, Gopalakrishnan S, Apuya J, Martin T. Use of Intralipid in an infant with impending cardiovascular collapse due to local anesthetic toxicity. J Anesth. 2009;23:439–41. doi: 10.1007/s00540-009-0754-3. [DOI] [PubMed] [Google Scholar]
- 10.Rothschild L, Bern S, Oswald S, Weinberg G. Intravenous lipid emulsion in clinical toxicology. Scand J Trauma Resusc Emerg Med. 2010;18:51. doi: 10.1186/1757-7241-18-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sirianni AJ, Osterhoudt KC, Calello DP, Muller AA, Waterhouse MR, Goodkin MB, Weinberg GL, Henretig FM. Use of lipid emulsion in the resuscitation of a patient with prolonged cardiovascular collapse after overdose of bupropion and lamotrigine. Ann Emerg Med. 2008;51:412–5. doi: 10.1016/j.annemergmed.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Harvey M, Cave G. Case report: successful lipid resuscitation in multi-drug overdose with predominant tricyclic antidepressant toxidrome. Int J Emerg Med. 2012;5:8. doi: 10.1186/1865-1380-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith HM, Jacob AK, Segura LG, Dilger JA, Torsher LC. Simulation education in anesthesia training: A case report of successful resuscitation of bupivacaine-induced cardiac arrest linked to recent simulation training. Anesth Analg. 2008;106:1581–4. doi: 10.1213/ane.0b013e31816b9478. [DOI] [PubMed] [Google Scholar]
- 14.West PL, McKeown NJ, Hendrickson RG. Iatrogenic lipid emulsion overdose in a case of amlodipine poisoning. Clin Toxicol (Phila) 2010;48:393–6. doi: 10.3109/15563651003670843. [DOI] [PubMed] [Google Scholar]
- 15.Marwick PC, Levin AI, Coetzee AR. Recurrence of cardiotoxicity after lipid rescue from bupivacaine-induced cardiac arrest. Anesth Analg. 2009;108:1344–6. doi: 10.1213/ane.0b013e3181979e17. [DOI] [PubMed] [Google Scholar]
- 16.Hiller DB, Di Gregorio G, Kelly K, Ripper R, Edelman L, Boumendjel R, Drasner K, Weinberg GL. Safety of high volume lipid emulsion infusion: A first approximation of LD50 in rats. Reg Anesth Pain Med. 2010;35:140–4. doi: 10.1097/aap.0b013e3181c6f5aa. [DOI] [PubMed] [Google Scholar]
- 17.Mazoit JX, Le Guen R, Beloeil H, Benhamou D. Binding of long-lasting local anesthetics to lipid emulsions. Anesthesiology. 2009;110:380–6. doi: 10.1097/ALN.0b013e318194b252. [DOI] [PubMed] [Google Scholar]
- 18.Niiya T, Litonius E, Petaja L, Neuvonen PJ, Rosenberg PH. Intravenous lipid emulsion sequesters amiodarone in plasma and eliminates its hypotensive action in pigs. Ann Emerg Med. 2010;56:402–8. doi: 10.1016/j.annemergmed.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Weinberg G, Lin B, Zheng S, Di Gregorio G, Hiller D, Ripper R, Edelman L, Kelly K, Feinstein D. Partitioning effect in lipid resuscitation: Further evidence for the lipid sink. Crit Care Med. 2010;38:2268–9. doi: 10.1097/CCM.0b013e3181f17d85. [DOI] [PubMed] [Google Scholar]
- 20.Samuels TL, Willers JW, Uncles DR, Monteiro R, Halloran C, Dai H. In vitro suppression of drug-induced methaemoglobin formation by Intralipid((R)) in whole human blood: Observations relevant to the ‘lipid sink theory’. Anaesthesia. 2012;67:23–32. doi: 10.1111/j.1365-2044.2011.06914.x. [DOI] [PubMed] [Google Scholar]
- 21.French D, Smollin C, Ruan W, Wong A, Drasner K, Wu AH. Partition constant and volume of distribution as predictors of clinical efficacy of lipid rescue for toxicological emergencies. Clinical toxicology. 2011;49:801–9. doi: 10.3109/15563650.2011.617308. [DOI] [PubMed] [Google Scholar]
- 22.Litonius E, Tarkkila P, Neuvonen PJ, Rosenberg PH. Effect of intravenous lipid emulsion on bupivacaine plasma concentration in humans. Anaesthesia. 2012 doi: 10.1111/j.1365-2044.2012.07056.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Stehr SN, Ziegeler JC, Pexa A, Oertel R, Deussen A, Koch T, Hubler M. The effects of lipid infusion on myocardial function and bioenergetics in l-bupivacaine toxicity in the isolated rat heart. Anesth Analg. 2007;104:186–92. doi: 10.1213/01.ane.0000248220.01320.58. [DOI] [PubMed] [Google Scholar]
- 24.Partownavid P, Umar S, Li J, Rahman S, Eghbali M. Fatty acid oxidation and calcium homeostasis are involved in the rescue of bupivacaine induced cardiotoxicity by lipid emulsion in rats. Crit Care Med. 2012 doi: 10.1097/CCM.0b013e3182544f48. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mottram AR, Valdivia CR, Makielski JC. Fatty acids antagonize bupivacaine-induced I(Na) blockade. Clinical toxicology. 2011;49:729–33. doi: 10.3109/15563650.2011.613399. [DOI] [PubMed] [Google Scholar]
- 26.Rahman S, Li J, Bopassa JC, Umar S, Iorga A, Partownavid P, Eghbali M. Phosphorylation of GSK-3beta mediates intralipid-induced cardioprotection against ischemia/reperfusion injury. Anesthesiology. 2011;115:242–53. doi: 10.1097/ALN.0b013e318223b8b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinberg GL, Di Gregorio G, Ripper R, Kelly K, Massad M, Edelman L, Schwartz D, Shah N, Zheng S, Feinstein DL. Resuscitation with lipid versus epinephrine in a rat model of bupivacaine overdose. Anesthesiology. 2008;108:907–13. doi: 10.1097/ALN.0b013e31816d91d2. [DOI] [PubMed] [Google Scholar]
- 28.Di Gregorio G, Schwartz D, Ripper R, Kelly K, Feinstein DL, Minshall RD, Massad M, Ori C, Weinberg GL. Lipid emulsion is superior to vasopressin in a rodent model of resuscitation from toxin-induced cardiac arrest. Crit Care Med. 2009;37:993–9. doi: 10.1097/CCM.0b013e3181961a12. [DOI] [PubMed] [Google Scholar]
- 29.Mayr VD, Mitterschiffthaler L, Neurauter A, Gritsch C, Muller T, Luckner G, Lindner KH, Strohmenger HU. A comparison of the combination of epinephrine and vasopressin with lipid emulsion in a porcine model of asphyxial cardiac arrest after intravenous injection of bupivacaine. Anesthesia and Analgesia. 2008;106:1566–71. doi: 10.1213/01.ane.0000278866.01963.79. [DOI] [PubMed] [Google Scholar]
- 30.Hicks SD, Salcido DD, Logue ES, Suffoletto BP, Empey PE, Poloyac SM, Miller DR, Callaway CW, Menegazzi JJ. Lipid emulsion combined with epinephrine and vasopressin does not improve survival in a swine model of bupivacaine-induced cardiac arrest. Anesthesiology. 2009;111:138–46. doi: 10.1097/ALN.0b013e3181a4c6d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harvey M, Cave G, Kazemi A. Intralipid infusion diminishes return of spontaneous circulation after hypoxic cardiac arrest in rabbits. Anesth Analg. 2009;108:1163–8. doi: 10.1213/ane.0b013e31819367ba. [DOI] [PubMed] [Google Scholar]
- 32.Hiller DB, Gregorio GD, Ripper R, Kelly K, Massad M, Edelman L, Edelman G, Feinstein DL, Weinberg GL. Epinephrine impairs lipid resuscitation from bupivacaine overdose: a threshold effect. Anesthesiology. 2009;111:498–505. doi: 10.1097/ALN.0b013e3181afde0a. [DOI] [PubMed] [Google Scholar]
- 33.Ruan W, French D, Wong A, Drasner K, Wu AH. A mixed (long- and medium-chain) triglyceride lipid emulsion extracts local anesthetic from human serum in vitro more effectively than a long-chain emulsion. Anesthesiology. 2012;116:334–9. doi: 10.1097/ALN.0b013e318242a5f1. [DOI] [PubMed] [Google Scholar]
- 34.Li Z, Xia Y, Dong X, Chen H, Xia F, Wang X, Dong H, Jin Z, Ding X, Papadimos TJ, Xu X. Lipid resuscitation of bupivacaine toxicity: Long-chain triglyceride emulsion provides benefits over long- and medium-chain triglyceride emulsion. Anesthesiology. 2011;115:1219–28. doi: 10.1097/ALN.0b013e318238be73. [DOI] [PubMed] [Google Scholar]
- 35.Neal JM, Mulroy MF, Weinberg GL. American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity: 2012 version. Reg Anesth Pain Med. 2012;37:16–8. doi: 10.1097/AAP.0b013e31822e0d8a. [DOI] [PubMed] [Google Scholar]
- 36.Umar S, Nadadur RD, Li J, Maltese F, Partownavid P, van der Laarse A. Eghbali M: Intralipid prevents and rescues fatal pulmonary arterial hypertension and right ventricular failure in rats. Hypertension. 2011;58:512–8. doi: 10.1161/HYPERTENSIONAHA.110.168781. [DOI] [PMC free article] [PubMed] [Google Scholar]