Abstract
Insulin inhibits eating after its intracerebroventricular (ICV) administration in multiple species and under a variety of conditions. Nevertheless, the results across reports are inconsistent in that ICV insulin does not always reduce food intake. The reasons for this variability are largely unknown. Using mice as a model, we performed several crossover trials with insulin vs. vehicle when infused into the third cerebral ventricle (i3vt) to test the hypothesis that recent experience with the i3vt procedure contributes to the variability in the effect of ICV insulin on food intake. Using a cross-over design with two days between injections, we found that insulin (0.4 µU/mouse) significantly reduced food intake relative to vehicle in mice that received vehicle on the first and insulin on the second trial, whereas this effect was absent in mice that received insulin on the first and vehicle on the second trial. Higher doses (i3vt 4.0 and 40.0 µU/mouse) had no effect on food intake in this paradigm. When injections were spaced 7 days apart, insulin reduced food intake with no crossover effect. Mice that did not reduce food intake in response to higher doses of i3vt insulin did so in response to i3vt infusion of the melanocortin receptor agonist melanotan-II (MT-II), indicating that the function of the hypothalamic melanocortin system, which mediates the effect of insulin on eating, was not impaired by whatever interfered with the insulin effect, and that this interference occurred upstream of the melanocortin receptors. Overall, our findings suggest that associative effects based on previous experience with the experimental situation can compromise the eating inhibition elicited by i3vt administered insulin.
Keywords: adiposity signals, eating, experimental design, peptides
Introduction
When acutely administered intracerebroventricularly (ICV), the pancreatic hormone insulin reduces food intake dose-dependently; and when administered chronically, ICV insulin reduces body weight as well [1–4]. Because of insulin’s important role in all aspects of energy homeostasis regulation, its central effect on food intake has been widely studied, including in several species, under different experimental conditions, and using a wide range of doses [e.g. [1–6]]. Species reducing food intake when administered insulin ICV include mice [2], rats [4], marmots [7], chickens [8] and baboons [6], and humans reduce their food intake when administered insulin intranasally [9]. With regard to potency, insulin doses below 1 µU produced a significant reduction of food intake after administration into the third cerebral ventricle (i3vt) in some reports in mice [2, 3, 10]. Higher insulin doses also reduced food intake and produced different physiological effects in other studies [3, 11, 12]. Even higher insulin doses (1, 2, 4, 8 mU i3vt) elicit an eating-inhibitory effect in rats [1]. Diet has also been varied; most studies were performed in animals maintained on regular lab chow, with relatively low contents of fat and mono- or disaccharides. Air et al. [1] assessed the acute effects of i3vt insulin on a 15% sucrose test meal as well as on 24-h food intake in rats and found that i3vt insulin decreased both sucrose and chow intake in a dose-dependent manner. This implies that insulin can also inhibit the consumption of palatable food, at least when the rats are maintained on chow. In contrast, analogous to the development of peripheral insulin resistance, maintenance on a high-fat diet also decreases sensitivity to i3vt insulin [13].
Collectively, these reports support the general observation that centrally administered insulin can inhibit food intake in numerous species and under different experimental conditions. Nonetheless, while these phenomena are well documented, the hypophagic response to ICV insulin is not always reliable [14], with some investigators failing to observe it at all [15, 16]. That is, despite differences in the insulin doses, the mode of administration (acute and chronic) and the magnitude of the observed insulin effects, most studies report a reliable eating-inhibitory effect of ICV insulin in a more or less dose-dependent manner. Yet, other published studies using rigorous paradigms failed to find a decrease in food intake in rats when insulin was chronically or acutely administered [15, 16], and there are likely unpublished findings with similar outcomes [see [14]]. In the present experiments using mice as the model, we tested the hypothesis that when counterbalanced designs are used such that some subjects get i3vt insulin on Trial 1 and vehicle on Trial 2, whereas others get the two injections in the reverse order, the food intake response on Trial 2 may be altered because of the presence of cues that were present on Trial 1; i.e., recent experience with the procedure (e.g., based on associative learning) may contribute to some of the variability in the effect of i3vt insulin on food intake. We also varied the time between Trial 1 and Trial 2 in order to rule out any carryover effects of the first injection.
Materials and Methods
Animals and housing
Male C57BL/6J mice (13–14 wk old) bred locally were used for the study. Breeding pairs were originally purchased from Jackson Laboratories (Charles River Inc., Germany). The mice were single housed in Makrolon® type III cages (Indulab, Gems, Switzerland) on pine wood chip bedding (Lignocel hygienic animal bedding, IRS, Rosenberg, Germany). The animal room had a 12-h light/12-h dark cycle (lights off at 1400 h) and was kept at 22.5°C with 40–60% humidity and 10/h air exchanges. Prior to surgery the mice were adapted to the handling by a technique developed by G. Pacheco-Lopez (see supplementary video) and food restriction procedures. At the age of 14–16 wk (mean body weight 28 g) the mice underwent 3rd-ventricular (i3vt) cannulation surgery, afterwards the handling procedure was maintained (see supplementary video). All procedures were approved by the Veterinary Office of the Canton of Zurich.
Diet and food intake measurements
Mice were maintained on a low-fat diet (12% energy from fat; extruded KLIBA 3436, Provimi Kliba SA, Switzerland) with a caloric density of 3.1 Kcal/g. Food pellets were always manipulated using forceps to avoid odor contamination. For food intake measurements, fresh, pre-weighed food (3 pellets ≈ 9 g) was offered at a given time point (see details below) and intake was calculated by weighing the remaining food to the nearest 0.1 g using individual food containers (50 ml plastic tubes; Becton Dickinson AG, Switzerland) and a digital balance (PM 460 Delta Range, Mettler, Switzerland) at several time points (see below). Food spillage was neglected because pilot assessments indicate no mayor lost (data not shown). Water was continuously available ad libitum.
I3vt cannulation
Mice received SC injections of antibiotics (20 mg/kg, 4µl/g, Borgal 24%, Intervet, Switzerland) and analgesics (5 mg/kg, 4µl/g, Rimadyl, Pfizer, Switzerland) 30 min prior to the onset of inhalation anesthesia (Isoflurane; induction = 5.0% / 300 cc/min O2; maintenance = 1.5–2.5% / 200 cc/min O2). A digital stereotaxic frame (940 Kopf Instruments, USA) was used to determine the coordinates for the implantation of the guide cannula and the supporting screw. The guide cannula (C315GS-4-SPC; Plastic one Inc., USA) was implanted at coordinates: A-P: −0.83, M-L: 0.0, D-V: −4.80. The sagittal sinus was displaced laterally prior to lowering the guide cannula. Dental acrylic and cyanoacrylate glue were used to secure the cannula to the skull.
Cannula placement and patency verification
When mice had regained their pre-surgical weight (≈ 7 d), cannula placement was verified by i3vt administration of the orexigenic peptide, neuropeptide-Y (NPY). NPY potently increases food intake when administered into the third cerebral ventricle, such that an increase of eating following its injection is indicative that the tip of the cannula is appropriately positioned. During the light phase, food was removed for 2 h, NPY (Bachem AG, Switzerland, 1 µg/1 µl) or saline (1 µl, NaCl 0.9%, B. Braun, Switzerland) was administered, pre-weighed food was returned immediately after the injection and food intake was recorded after 2 and 22 h. Treatments were given in counterbalanced order with one day between treatments. On average, NPY (1 µg/µl) elicited a strong orexigenic effect (+525%) (2 h post injection: NPY 1.26 ± 0.10 g / Sal 0.24 ± 0.02g) (t (15) = 9.603, p ≤ 0.05). This effect was still present at 22 h (NPY 4.40 ± 0.17 g / Sal 3.68 ± 0.11g (t (15) = 4.826, p ≤ 0.05). The inclusion criterion was a minimum food intake of 0.5 g at 2 h after i3vt NPY injection (5).
Experiment 1
Fifteen mice received i3vt infusions of insulin (Actrapid®, NovoNordisk Pharma AG, Switzerland, 0.4 µU / 1 µl) or an equivalent volume of vehicle (saline; NaCl 0.9%) at 10:00 h (4 h prior to dark onset). The infusion took about 30 s, and the injector was kept in the cannula for an additional 30 s following the administration to allow time for the drug to diffuse (see Supplementary video). Pre-weighed food was offered after 4 h of food deprivation, at dark onset, and cumulative food intake was recorded after 2, 4 and 20 h. Every animal received both treatments in a counterbalanced design (insulin then vehicle n= 7 / vehicle then insulin n=8), with two intervening days. Mice were adapted to the experimental procedure for two days prior to the experiments and on the intervening days.
Experiment 2
Five mice received i3vt infusions of 0.0 (vehicle), 0.2 or 0.4 µU insulin as described for Experiment 1, except that the order of treatments was randomized and there were 7 intervening days between infusions in order to minimize any lingering effects of i3vt insulin from prior administrations.
Experiment 3
Because a wide range of insulin doses has been reported to be effective in mice in the literature [2, 10, 15], we asked whether increasing the insulin dose might produce a clearer effect of insulin on food intake that might override any effect due to order or recent experience. Seven mice received 4.0 or 40.0 µU insulin or vehicle in a within-subjects design with one day between trials.
Experiment 4
To assess whether mice that were non-responsive to i3vt insulin would react to a different anorexic agent, the same six mice used in Experiment 3 received i3vt infusions of MTII (H-3902, Bachem AG, Switzerland, 1 µg / 1 µl), an agonist of the melanocortin receptors (MC3-R and MC4-R), or artificial cerebrospinal fluid (aCSF, 1 µl) in a within-subject design with one intervening day between injections.
Other controls
After the experiments, other mice were administered the same batch of insulin (1 mU/g BW) subcutaneously and blood glucose assessed after 15, 30, 45, 60 and 90 min to verify that the insulin was biologically active [17]. In every instance there was a substantial and significant hypoglycemia elicited by the insulin.
Statistical analyses
SPSS for Windows (ver. 17.0) was used for all analyses. Data were tested for normality using the Shapiro-Wilk test. Means were compared using a paired t-test or repeated measures ANOVA, as required by the test design. If data were not normally distributed, we used the Wilcoxon Matched-Pairs test to analyze the data. P-values ≤ 0.05 were considered significant.
Results
Experiment 1
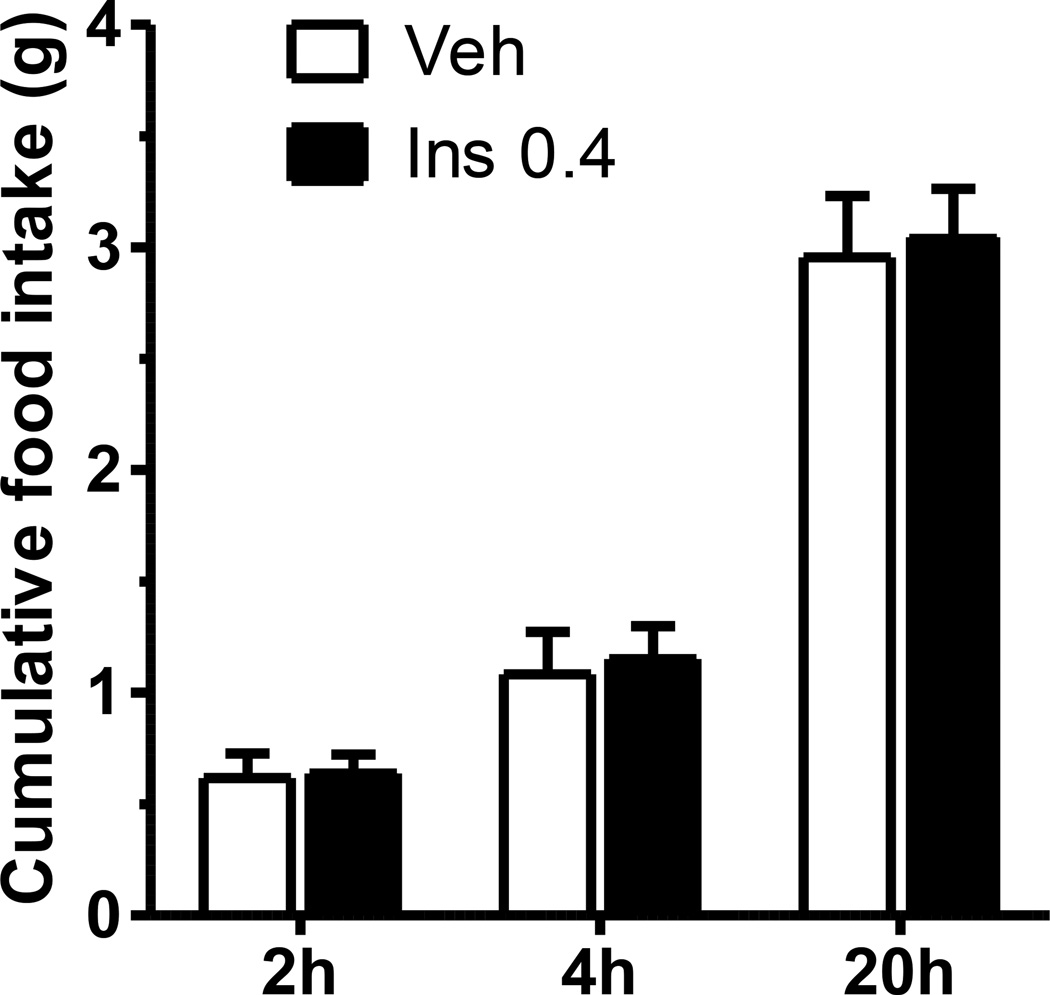
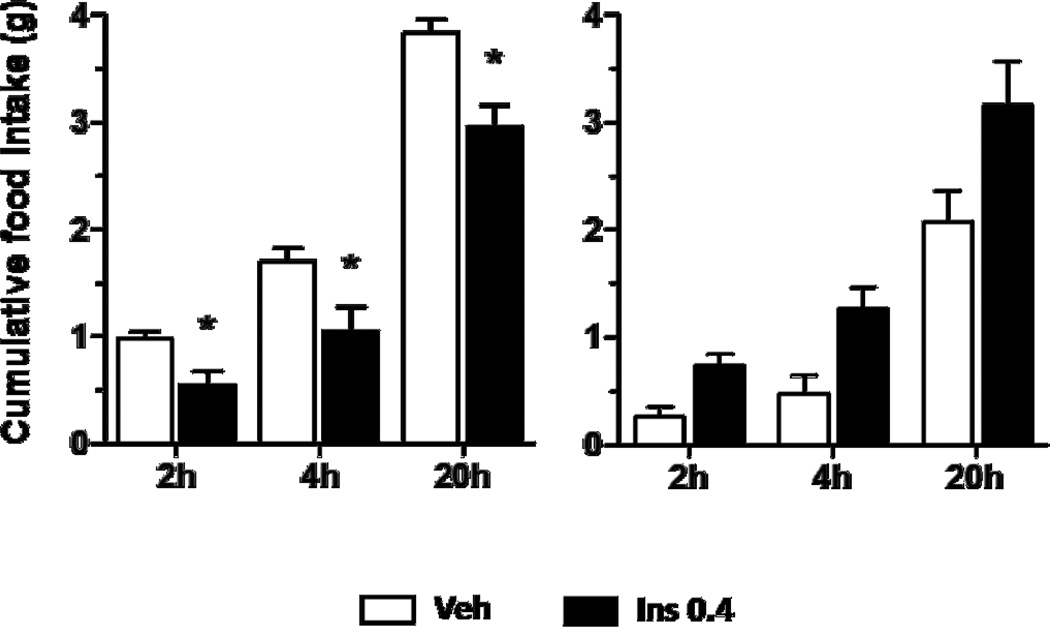
Analysis of food intake combining all data on both days of the cross-over design did not reveal a significant effect of insulin on cumulative food intake at 2, 4, or 20 h (Fig. 1). However, by design, there were two sub-groups, mice receiving insulin on Trial 1 and the saline vehicle on Trial 2 and mice receiving the vehicle on Trial 1 and insulin on Trial 2. Insulin significantly reduced food intake relative to the saline vehicle in those mice that received vehicle on the first trial and insulin on the second trial (Fig. 2, left); i.e., utilizing a within-subject analysis, paired t-test, food intake was reduced at 2 h (44% reduction), t (7) = 3.523, p ≤ 0.05, 4 h (38% reduction), t (7) = 3.029, p ≤ 0.05, and 20 h 23% reduction), t (7) = 3.921, p ≤ 0.05. In contrast, this effect was not evident in mice that received insulin on the first and vehicle on the second trial (n=7): 2 h, t (6) = −2.069, p = 0.084, 4 h, t (6) = −2.240, p = 0.066, and 20 h, t (6) = −1.771, p = 0.127 (Fig. 2, right). In fact, the intake on the saline vehicle day for these mice was considerably lower than intake following saline in the other group; i.e., the lack of a reduction of insulin in this group could be attributed, at least in part, to below-normal intake following the saline vehicle. A between-subjects comparison, unpaired t-test, of the data on Trial 1 revealed a significant effect of insulin at 2 and 4 h when compared to the food intakes of mice that received vehicle. Therefore, while the between-subjects analysis, unpaired t-test, revealed a significant reduction of food intake by i3vt insulin on Trial 1, the effect was smaller than that observed in the within-subject’s analysis of mice receiving the saline vehicle followed by insulin. Thus, there was a substantial effect of order.
Figure 1.
I3vt insulin infusion (0.4 µU/mouse) did not alter food intake in mice in a within-subjects crossover design with two intervening days between trials. Data are means ± SEM (n = 15).
Figure 2.
I3vt insulin infusion (0.4 µU/mouse) reduced 2, 4 and 20 h food intake in animals that received vehicle first (left graph), but had no reliable effect in mice that received insulin first (right graph). Data are means ± SEM (n = 7 and 8, respectively).
Experiment 2
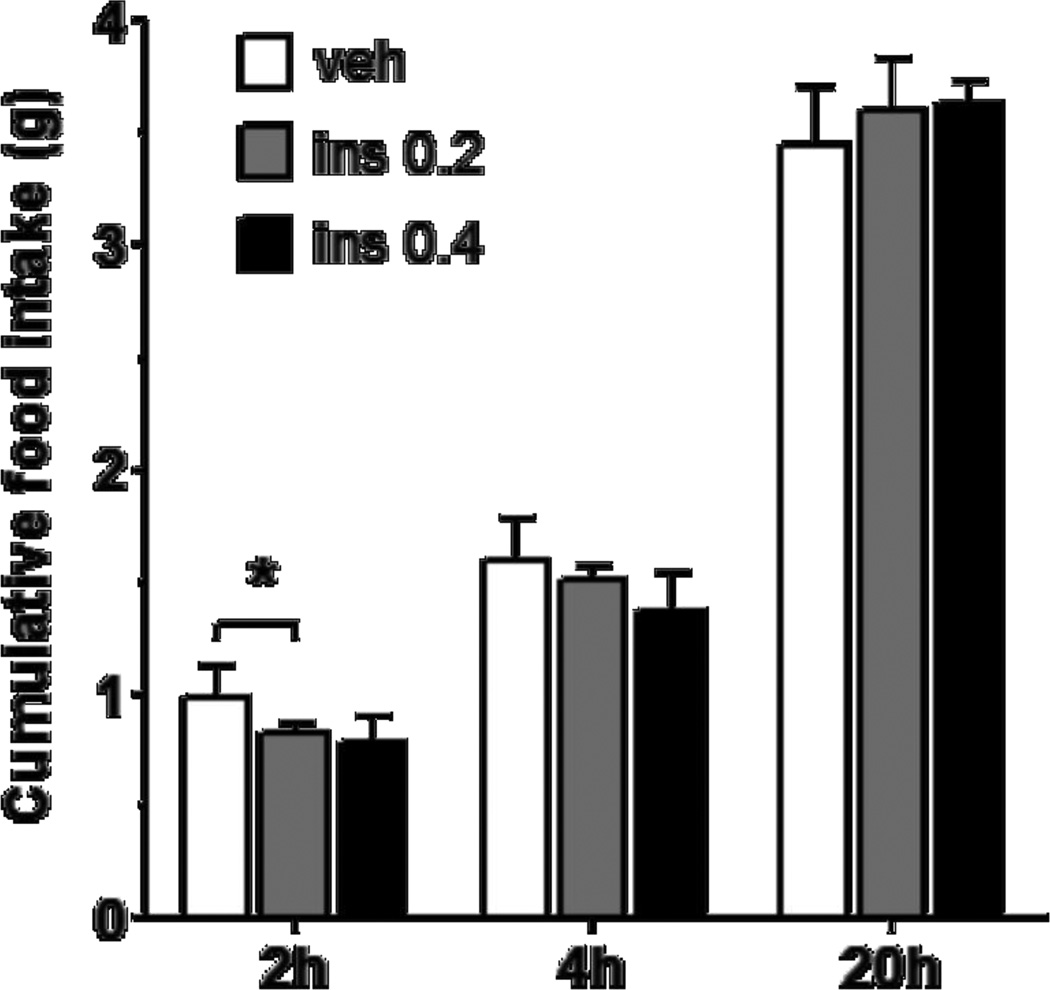
When mice received 0.0 (vehicle), 0.2 or 0.4 µU insulin i3vt in randomized order, but with 7 d between trials, analysis of variance (ANOVA) indicates a significant main effect F (2, 8) = 4.828, p ≤ 0.05 for 2-h cumulative food intake (6 h post infusion) using repeated-measures ANOVA (results are reported only for the 5 mice that completed all 3 trials; data from 2 mice had to be excluded because they developed cannula patency problems and did not receive all three treatments). Subsequent planned contrasts revealed a significant reduction of food intake by insulin at 0.2 µU (F (1, 4) = 12.689, p ≤ 0.05 when compared to vehicle, a 24% reduction; Fig. 3). Reduction by the higher dose of insulin (0.4 µU) at 2 h approached significance (p = 0.073). Repeated measures ANOVA indicated no effect of either dose at 4 h (p = 0.158) or 20 h (p = 0.650), and there was no apparent order effect although the n was small.
Figure 3.
I3vt insulin infusion (0.2 µU/mouse) reduced 2-h food intake whereas the 0.4 µU/mouse dose did not. Data are means ± SEM (n = 5).
Experiment 3
Higher doses of i3vt insulin (4.0 and 40.0 µU) did not produce a reliable reduction of 2 or 20 h food intake in mice that were comparable in all other ways to those in Experiments 1 and 2. Wilcoxon nonparametric analysis reveals that 4 µU insulin/mouse did not reduce food intake at 2 h (Mdn = 1.01 g; z = − 1.60, r = − 0.92, P = 0.11), 4 h (Mdn = 1.77 g; z = − 1.07, r = − 0.61, P = 0.28), or 20 h (Mdn = 3.58 g; z = − 0.45, r = − 0.26, P = 0.65) compared to vehicle (Mdn = 0.84 g at 2h, 1.52 g at 4h, 3.04 g at 20h). Neither did the 40 µU insulin/mouse at 2 h (Mdn = 0.90 g; z = − 0.54, r = −0.31, P = 0.59), 4 h (Mdn = 1.75 g; z = − 0.54, r = − 0.31, P = 0.59), or 20 h (Mdn = 3.87 g; z = − 1.60, r = − 0.92, P = 0.11) compared to vehicle (Mdn = 1.12 g at 2h, 1.68 g at 4h or 2.64 g at 20h).
Experiment 4
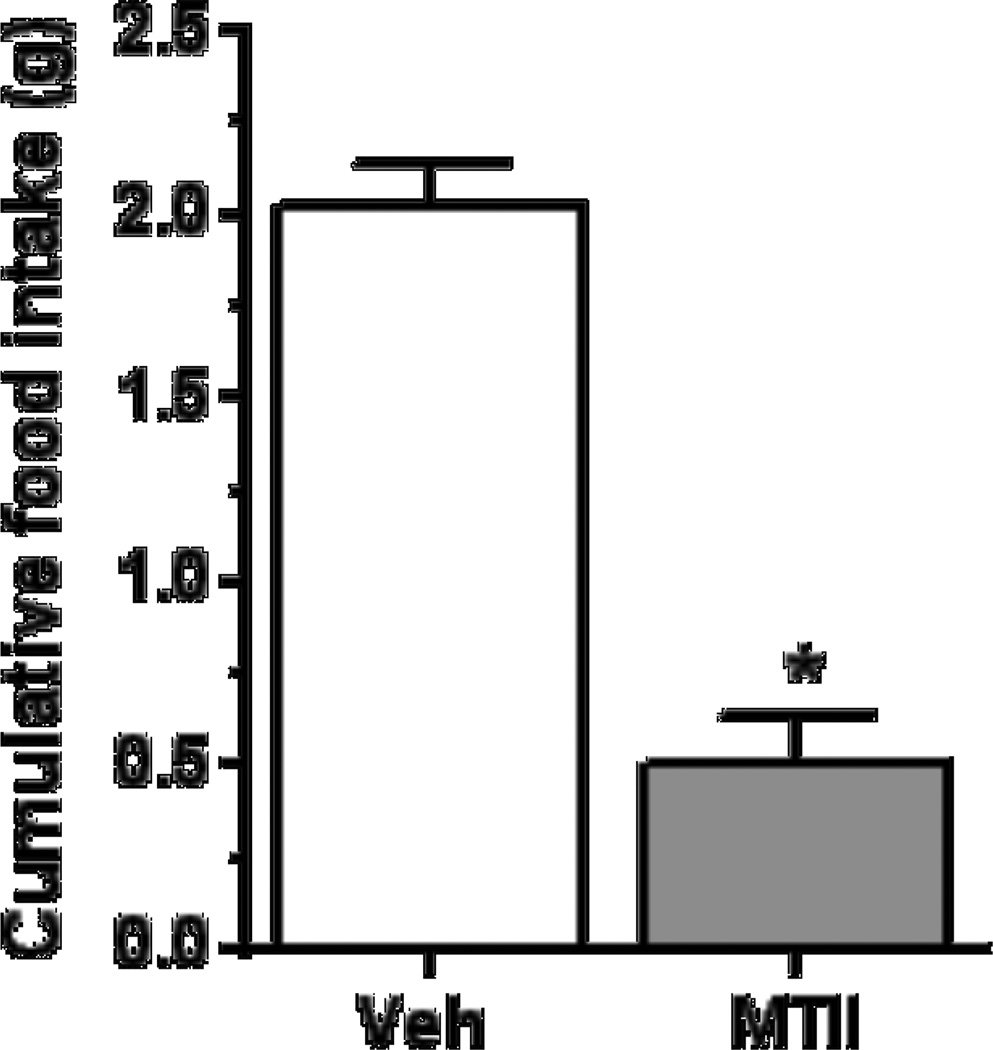
The paired t-test indicates that I3vt MT-II significantly inhibited food intake when assessed at 4 h after injection in mice that had not decreased their food intake to insulin in Experiment 3 (Figure 4, M ± SEM; n = 6, t (5) = 8.23, p < 0.05, r = 0.96). Thus, the ability of central melanocortin to reduce food intake was intact in the mice.
Figure 4.
I3vt MTII infusion (1 µg/mouse) reduced 4-h food intake. Data are means ± SEM (n = 6).
Discussion
Over the last decades, significant progress has been made in understanding insulin’s role in energy homeostasis. While the results with respect to the effects of administration of exogenous insulin into the central nervous system are for the most part consistent, with insulin eliciting an acute catabolic action, not all reports are confirmatory. Several generalizations can be made based on the prior literature. First, insulin’s central catabolic action is consistent across a range of species. Second, the effect is observed following acute administration into fasted animals, during slow constant infusion into free-feeding animals, and during intermittent administration to free-feeding animals. Third, the effect is greater in males than in females, having been demonstrated in rats [18] and humans [19]. Finally, the efficacy of centrally administered insulin to reduce food intake is compromised in genetic or high-fat diet-induced obesity, and in animals maintained on a high-fat diet independent of obesity. That said, and as we have reviewed elsewhere [14], the reduction of food intake in response to insulin administered into the brain is not observed universally. In the present experiments we asked whether the order in which insulin vs. vehicle is administered might be important.
In Experiment 1, insulin reduced food intake the first time it was administered. This was manifest as a mild but significant reduction using a between-subjects analysis on Trial 1, and a more robust reduction in terms of both magnitude and duration was apparent when the mice had had a prior administration of vehicle and utilizing a within-subjects analysis. This suggests that prior experience with the exact protocol, but without insulin, somehow facilitated the subsequent hypophagic response. When the insulin was administered on Trial 1 and saline on Trial 2 two days later, the food intake after the saline infusion was in fact lower than occurred in mice receiving saline on Trial 1, perhaps negating any effect of insulin. One possible explanation could be a carryover effect [20]; i.e., the insulin might have had a long-lasting effect and thus still was able to reduce food intake after vehicle administration two days later. This seems unlikely, however, because we used Actrapid® HM insulin, which supposedly acts only for about 8 hours according to the manufacturer’s description (NovoNordisk Pharma AG, Switzerland), although that refers to systemic as opposed to possible central effects.
In Experiment 1 we mimicked as closely as possible the paradigm of Brown and colleagues where i3vt insulin significantly and dose-dependently reduced food intake in mice [2], except that we allowed only 2 as opposed to 7 days of intervening time between trials. Brown and colleagues reported a substantial decrease in food intake in response to i3vt insulin at doses above 0.05 µU / mouse [2]. When we used 7 intervening days in Experiment 2, insulin did reduce food intake, but the response was not especially robust and there was no order effect.
An alternative explanation for the apparent order effect observed in Experiment 1 is associative learning (i.e. classical conditioning), and several factors, alone or together, might have contributed. While the mice had been adapted to the i3vt infusions by prior vehicle administrations, the injections had not been in association with 4-h food deprivation; i.e., the combination of food deprivation coupled with subsequent access to food was novel on the first test trial and might have served as a conditioned stimulus. When the same stimulus was presented again two days later, the eating-inhibitory effect of insulin that had been elicited on the first trial might thus have become associated with the combination of 4-h deprivation, an i3vt injection, etc., causing the animals to eat less after the control infusion on the second trial. That said, mild deprivation seems unlikely to be a conditioned stimulus in this situation because it also occurred for all mice when they were assessed for cannula placement with NPY. Another novel stimulus on Trial 1 was the odor of m-cresol (3-methylfenol), the aromatic organic compound commercially used as solvent (preservative and stabilizer) of the insulin. Human diabetic patients report detecting the smell of m-cresol in their insulin [21]. The novel odor was present in the animal-housing room for the first time on Trial 1 and presumably detectable by mice whether getting insulin or saline injections. Thus, mice that received insulin might have associated the smell of m-cresol with its eating-inhibitory effect, and this may have prompted those mice to eat less after the subsequent vehicle infusion, resulting in lower food intake than normally seen after saline in Trial 2. Consistent with this, in an experiment assessing conditioned effects of intranasal insulin in humans, Stockhorst et al. [22] included a control group receiving the insulin vehicle with added m-cresol with or without insulin and observed conditioned changes of peripheral insulin secretion in response to vehicle plus m-cresol on a subsequent test trial when no insulin was present. In another study, the same group observed that intravenous insulin triggered an increase in parasympathetic tone and, hence, heart rate changes at maximum hyperinsulinemia, and that this response could also be conditioned [23]. Other previous studies [24, 25] described the conditioned secretion of endogenous insulin and consequent hypoglycemia in response to cues such as odors that had been associated with the prior administration of insulin. These authors reported that after pairing pharmacological doses of exogenous insulin with unique cues, these same cues elicited hypoglycemia in rats in the absence of exogenous insulin. Further, and consistent with the present findings, in those experiments a conditioned response was observed after as few as two insulin trials when an explicit olfactory cue was used [26]. Perhaps, the low food intake in response to saline infusion on the second day of Experiment 1 was caused by a conditioned secretion of endogenous insulin. Considering these possibilities, we used a longer intervening period in Experiment 2, consistent with the paradigm of Brown [2]. Indeed, there was no indication of an order effect, and there was a significant overall effect of i3vt insulin to reduce food intake, although it was relatively small. Future research in this area would benefit from including groups of mice that received saline on both Trials 1 and 2, or that received insulin on both Trials 1 and 2.
To ascertain whether a higher dose of insulin might yield clearer results, in Experiment 3 we administered 10–100 times higher doses of insulin, but none were effective. While a greater hypophagic effect of i3vt insulin has been observed in rats when larger doses are administered [27, 28], the range of administered doses was limited. When much higher doses of insulin are administered into the cerebral ventricles, there is actually a vagally-mediated reflexive increase of pancreatic insulin secretion and consequent hypoglycemia [29–31], an effect that would likely counter any hypophagic action of central insulin. The fact that i3vt insulin initiates several responses is important for understanding the present experiments. These effects include, in addition to hypophagia, countering the NPY-melanocortin system, improving cognitive functioning, increasing pancreatic insulin secretion and lowering blood glucose. Which of these might become conditioned on any trial, or rather which combination of them, is not easily ascertained. If hypophagia were the only unconditioned effect of i3vt insulin, hypophagia should also be the conditioned response (e.g. [32]), but that cannot explain the findings of Experiment 1 where the opposite seems to have occurred. Parsing out these possibilities must be the aim of future experiments.
I3vt MT-II significantly reduced food intake in mice that were non-responsive to i3vt insulin. MT-II is a synthetic analog of α-melanocyte-stimulating hormone (α-MSH). Insulin acts on neurons in the hypothalamic arcuate nucleus to stimulate the expression of its precursor, pro-opiomelanocortin (POMC), and this had been found to mediate the catabolic action of central insulin [33]. The observation that the mice reduced food intake in response to MT-II, but not in response to insulin, indicates that the function of the hypothalamic melanocortin system was not impaired by whatever interfered with the insulin effect. It also implies that the impediment to insulin action took place either at the insulin receptor on POMC neurons or else upstream of the MC4 receptors activated by exogenous MT-II. The significant stimulation of food intake after the i3vt administration of NPY that was used to verify cannula placement also indicated that the energy homeostasis regulating hypothalamic neuropeptide systems, and in particular the anabolic pathway downstream of the pertinent NPY receptors, was functioning properly in our animals. Moreover, it confirmed that the mice were capable to increase food intake under our housing conditions.
It should be noted that the probabilistic nature of the eating-inhibitory effect is, however, not specific for insulin. Rather, it is a well-recognized phenomenon that peptide effects on eating can be variable, often without an easily discernable reason [14]. Such variability has also been reported for cholecystokinin, leptin, peptide tyrosine tyrosine and other peptides [34, 35], but no convincing explanation has so far been found for this phenomenon. Although that remains the case, the present findings, while confirming the capricious nature of the phenomenon, do suggest that associative effects may contribute. Such a phenomenon may well account for some of the negative results with respect to the effects of exogenous insulin on eating.
The present results are significant in identifying one source of variance in the hypophagic response to centrally-administered insulin. Given that associative effects related to environmental cues (e.g., fasting, i3vt injections, odors, and likely others) can alter the magnitude and in fact the actual manifestation of the behavioral response, it is important that future experiments be designed to account for this, and for the precise methods used to be made clear.
Supplementary Material
Highlights.
The present results identify one source of variance in the hypophagic response to centrally-administered insulin.
Associative effects related to environmental cues (e.g., fasting, i3vt injections, odors, and likely others) can alter the magnitude and in fact the actual manifestation of the behavioral response.
It is important that future experiments be designed to account for this, and for the precise methods used to be made clear.
Acknowledgement
Supported by NIDDK R01 DK078201 (SCW and WL), and DK017844 (SCW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Air EL, Benoit SC, Coolen LM, Strauss R, Jackman A, Seeley RJ, et al. Melanocortins mediate insulin-induced hypophagia. Diabetes. 2002;51:A40–A41. [Google Scholar]
- 2.Brown LM, Clegg DJ, Benoit SC, Woods SC. Intraventricular insulin and leptin reduce food intake and body weight in C57BL/6J mice. Physiol Behav. 2006;89:687–691. doi: 10.1016/j.physbeh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Jaillard T, Roger M, Galinier A, Guillou P, Benani A, Leloup C, et al. Hypothalamic reactive oxygen species are required for insulin-induced food intake inhibition: an NADPH oxidase-dependent mechanism. Diabetes. 2009;58:1544–1549. doi: 10.2337/db08-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Air EL, Benoit SC, Blake Smith KA, Clegg DJ, Woods SC. Acute third ventricular administration of insulin decreases food intake in two paradigms. Pharmacology, biochemistry, and behavior. 2002;72:423–429. doi: 10.1016/s0091-3057(01)00780-8. [DOI] [PubMed] [Google Scholar]
- 5.Plata-Salaman CR, Oomura Y, Shimizu N. Dependence of food intake on acute and chronic ventricular administration of insulin. Physiol Behav. 1986;37:717–734. doi: 10.1016/0031-9384(86)90177-0. [DOI] [PubMed] [Google Scholar]
- 6.Woods SC, Lotter EC, McKay LD, Porte D., Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–505. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- 7.Florant GL, Singer L, Scheurink AJW, Park CR, Richardson RD, Woods SC. Intraventricular Insulin Reduces Food-Intake and Body-Weight of Marmots during the Summer Feeding Period. Physiol Behav. 1991;49:335–338. doi: 10.1016/0031-9384(91)90053-q. [DOI] [PubMed] [Google Scholar]
- 8.Honda K, Karnisoyama H, Saneyasu T, Sugahara K, Hasegawa S. Central administration of insulin suppresses food intake in chicks. Neurosci Lett. 2007;423:153–157. doi: 10.1016/j.neulet.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Hallschmidt M, Benedict C, Born J, Kern W. Targeting metabolic and cognitive pathway of the CNS by intranasal insulin administration. Expert Opin Drug Del. 2007;4:319–322. doi: 10.1517/17425247.4.4.319. [DOI] [PubMed] [Google Scholar]
- 10.Won JC, Jang PG, Namkoong C, Koh EH, Kim SK, Park JY, et al. Central administration of an endoplasmic reticulum stress inducer inhibits the anorexigenic effects of leptin and insulin. Obesity. 2009;17:1861–1865. doi: 10.1038/oby.2009.194. [DOI] [PubMed] [Google Scholar]
- 11.Morgan DA, Rahmouni K. Differential effects of insulin on sympathetic nerve activity in agouti obese mice. Journal of hypertension. 2010;28:1913–1919. doi: 10.1097/HJH.0b013e32833c2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller AP, Gnoatto J, Moreira JD, Zimmer ER, Haas CB, Lulhier F, et al. Exercise increases insulin signaling in the hippocampus: physiological effects and pharmacological impact of intracerebroventricular insulin administration in mice. Hippocampus. 2011;21:1082–1092. doi: 10.1002/hipo.20822. [DOI] [PubMed] [Google Scholar]
- 13.Clegg DJ, Gotoh K, Kemp C, Wortman MD, Benoit SC, Brown LM, et al. Consumption of a high-fat diet induces central insulin resistance independent of adiposity. Physiol Behav. 2011;103:10–16. doi: 10.1016/j.physbeh.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woods SC, Langhans W. Inconsistencies in the assessment of food intake. American journal of physiology. Endocrinology and metabolism. 2012;303:E1408–E1418. doi: 10.1152/ajpendo.00415.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jessen L, Clegg DJ, Bouman SD. Evaluation of the lack of anorectic effect of intracerebroventricular insulin in rats. American journal of physiology. Regulatory, integrative and comparative physiology. 2010;298:R43–R50. doi: 10.1152/ajpregu.90736.2008. [DOI] [PubMed] [Google Scholar]
- 16.Nagaraja TN, Patel P, Gorski M, Gorevic PD, Patlak CS, Fenstermacher JD. In normal rat, intraventricularly administered insulin-like growth factor-1 is rapidly cleared from CSF with limited distribution into brain. Cerebrospinal Fluid Res. 2005;2:5. doi: 10.1186/1743-8454-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heikkinen S, Argmann CA, Champy MF, Auwerx J. Evaluation of glucose homeostasis. In: Ausubel Frederick M, et al., editors. Current protocols in molecular biology. 29B. Vol. 29. 2007. p. 3. [DOI] [PubMed] [Google Scholar]
- 18.Clegg DJ, Riedy CA, Smith KA, Benoit SC, Woods SC. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003;52:682–687. doi: 10.2337/diabetes.52.3.682. [DOI] [PubMed] [Google Scholar]
- 19.Hallschmid M, Benedict C, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin reduces body fat in men but not in women. Diabetes. 2004;53:3024–3029. doi: 10.2337/diabetes.53.11.3024. [DOI] [PubMed] [Google Scholar]
- 20.Diaz-Uriarte R. Incorrect analysis of crossover trials in animal behaviour research. Animal Behaviour. 2002;63:815–822. [Google Scholar]
- 21.Rajpar SF, Foulds IS, Abdullah A, Maheshwari M. Severe adverse cutaneous reaction to insulin due to cresol sensitivity. Contact Dermatitis. 2006;55:119–120. doi: 10.1111/j.0105-1873.2006.0866g.x. [DOI] [PubMed] [Google Scholar]
- 22.Stockhorst U, de Fries D, Steingrueber HJ, Scherbaum WA. Unconditioned and conditioned effects of intranasally administered insulin vs placebo in healthy men: a randomised controlled trial. Diabetologia. 2011;54:1502–1506. doi: 10.1007/s00125-011-2111-y. [DOI] [PubMed] [Google Scholar]
- 23.Stockhorst U, Huenig A, Ziegler D, Scherbaum WA. Unconditioned and conditioned effects of intravenous insulin and glucose on heart rate variability in healthy men. Physiol Behav. 2011;103:31–38. doi: 10.1016/j.physbeh.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Reiss WJ. Conditioning of a hyper-insulin type of behavior in the white rat. Journal of comparative and physiological psychology. 1958;51:301–303. doi: 10.1037/h0040308. [DOI] [PubMed] [Google Scholar]
- 25.Woods S. Insulin and the brain: a mutual dependency. Progress in Psychobiology and Physiological Psychology. 1996:53–81. [Google Scholar]
- 26.Woods SC, Makous W, Hutton RA. Temporal parameters of conditioned hypoglycemia. Journal of comparative and physiological psychology. 1969;69:301–307. doi: 10.1037/h0028186. [DOI] [PubMed] [Google Scholar]
- 27.Riedy CA, Chavez M, Figlewicz DP, Woods SC. Central insulin enhances sensitivity to cholecystokinin. Physiol Behav. 1995;58:755–760. doi: 10.1016/0031-9384(95)00108-u. [DOI] [PubMed] [Google Scholar]
- 28.Air EL, Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin combine additively to reduce food intake and body weight in rats. Endocrinology. 2002;143:2449–2452. doi: 10.1210/endo.143.6.8948. [DOI] [PubMed] [Google Scholar]
- 29.Chowers I, Lavy S, Halpern L. Effect of insulin administered intracisternally on the glucose level of the blood and the cerebrospinal fluid in vagotomized dogs. Experimental neurology. 1966;14:383–389. doi: 10.1016/0014-4886(66)90122-1. [DOI] [PubMed] [Google Scholar]
- 30.Woods SC, Porte D., Jr Effect of intracisternal insulin on plasma glucose and insulin in the dog. Diabetes. 1975;24:905–909. doi: 10.2337/diab.24.10.905. [DOI] [PubMed] [Google Scholar]
- 31.Chen M, Woods SC, Porte D., Jr Effect of cerebral intraventricular insulin on pancreatic insulin secretion in the dog. Diabetes. 1975;24:910–914. doi: 10.2337/diab.24.10.910. [DOI] [PubMed] [Google Scholar]
- 32.Ramsay DS, Woods SC. Biological consequences of drug administration: implications for acute and chronic tolerance. Psychological review. 1997;104:170–193. doi: 10.1037/0033-295x.104.1.170. [DOI] [PubMed] [Google Scholar]
- 33.Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ, et al. The catabolic action of insulin in the brain is mediated by melanocortins. Journal of Neuroscience. 2002;22:9048–9052. doi: 10.1523/JNEUROSCI.22-20-09048.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tschop M, Castaneda TR, Joost HG, Thone-Reineke C, Ortmann S, Klaus S, et al. Physiology: does gut hormone PYY3-36 decrease food intake in rodents? Nature. 2004:430–431. doi: 10.1038/nature02665. p following 165; discussion 2 p following. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.