Abstract
Purpose
Elevated cartilage stress has been identified as a potential mechanism for retropatellar pain; however, there are limited data in the literature to support this mechanism. Females are more likely to develop patellofemoral pain than males, yet the causes of this dimorphism are unclear. We used experimental data and computational modeling to determine whether patients with patellofemoral pain had elevated cartilage stress compared to pain-free controls and test the hypothesis that females exhibit greater cartilage stress than males.
Methods
We created finite element models of 24 patients with patellofemoral pain (11 males; 13 females) and 16 pain-free controls (8 males; 8 females) to estimate peak patellar cartilage stress (strain energy density) during a stair climb activity. Simulations took into account cartilage morphology from MRI, joint posture from weight-bearing MRI, and muscle forces from an EMG-driven model.
Results
We found no difference in peak patellar strain energy density between patellofemoral pain (1.9 ± 1.23 J/m3) and control subjects (1.66 ± 0.75 J/m3, p=0.52). Females exhibited greater cartilage stress compared to males (2.2 vs 1.3 J/m3, respectively, p=0.0075), with large quadriceps muscle forces (3.7BW females vs 3.3BW males) and 23% smaller joint contact area (females: 467 ± 59 mm2 vs males: 608 ± 95mm2).
Conclusion
Patellofemoral pain patients did not display significantly greater patellar cartilage stress compared to pain-free controls; however, there was a great deal of subject variation. Females exhibited greater peak cartilage stress compared to males, which might explain the greater prevalence of patellofemoral pain in females compared to males but other mechanical and biological factors are clearly involved in this complex pathway to pain.
Keywords: patellofemoral pain, joint stress, finite element model, stair climb
Introduction
Patellofemoral pain is one of the most common disorders of the knee (33,35), yet the etiology of dull, aching, retro-patellar pain remains poorly understood. Pain is often aggravated by activities that place large loads on the joint, such as stair climbing, squatting, and running, suggesting that mechanical loads play an important role. Although cartilage is aneural, the subchondral bone is richly innervated, and elevated cartilage/bone mechanical stress has long been proposed to be a possible cause of pain (28). Immunohistochemistry and confocal microscopy studies confirm the sensory and sympathetic innervation of bone and its potential role in skeletal pain (26) and our recent study using F-NaF positron emission tomography showed that chronic patellofemoral pain patients display elevated bone metabolic activity (15). However, testing a hypothesis that relates joint pain to mechanical stress remains difficult due to an inability to measure stress in vivo and the subjective assessment of pain.
Females are two-times more likely to develop patellofemoral pain than males (8) yet we do not understand why. Several mechanical risk factors have been identified with this dimorphism (1), including: increased Q-angle (12), impaired muscular control of the hip (30), and altered muscle forces across the knee (5,12). However, if stress is the driving mechanical factor related to patellofemoral pain, then it is important to understand what factors contribute to the distribution of stress and how these factors differ between males and females. When normalized by the anterior-posterior knee dimension, females have narrower medial-lateral knee width compared to males (10), reducing the contact area of the patellofemoral joint after adjusting for body size and weight (as shown by surface area measurements of Otterness and Eckstein (27)), and reducing the moment arms of muscles crossing the joint (38). Our previous work estimating weight bearing patellofemoral joint contact area showed that males have 34% greater contact area compared to females (4). This sex difference was not significant when contact area was normalized by height × width dimensions of the patella (4), suggesting that the patellofemoral joint contact areas scale with body size, independent of sex. However, if females require greater muscle forces to produce knee extension moments, by virtue of smaller muscle moment arms, they may have greater cartilage stress compared to males. Using an EMG-driven musculoskeletal model that takes into account individual muscle activation patterns, we estimated muscle forces during walking and running and found that females indeed produce greater muscle forces compared to males, when normalized to body weight (5). A comprehensive modeling framework that takes into account subject-specific articulating geometry, muscle and joint contact forces due to individual muscle activation patterns, and joint posture is necessary to determine how these individual factors influence cartilage stress and whether stress is indeed related to joint pain.
Using finite element (FE) models of the patellofemoral joint, Farrokhi et al. (17) showed that females with patellofemoral pain had greater cartilage stress than pain-free controls, supporting the role of mechanical stress in patellofemoral pain. Elevated cartilage stress might also explain why females are more likely to develop patellofemoral pain compared to males, although there are no patellofemoral cartilage stress data for males in the literature to support this idea. We have developed a computational framework to estimate in vivo cartilage stress (6), taking into account subject-specific cartilage morphology, joint posture from weight-bearing magnetic resonance imaging (4), and individual muscle forces from an EMG-driven musculoskeletal model (25). Using this framework to estimate patellofemoral joint cartilage stress, the purpose of this study was to; a) determine whether patients with patellofemoral pain exhibit elevated cartilage stress compared to pain-free controls, and b) test the hypothesis that females experience greater peak patellar cartilage stress than males. We estimated peak patellar cartilage stress during stair climbing with the knee at 60° of flexion, a task often associated with joint pain and involving large loads at the patellofemoral joint (11,34).
Methods
Subjects
Experimental data were collected from 24 patients with patellofemoral pain (11 males; 13 females) and 16 pain-free controls (8 males; 8 females). There were no differences in age, mass, or height between sex-matched patellofemoral pain and pain-free controls (Table 1). Patients were diagnosed with patellofemoral pain by a single physician and were accepted into the study if they had pain originating from the patellar region that was reproducible during at least two of the following functional activities; stair ascent or descent, squatting, kneeling, prolonged sitting, or isometric quadriceps contraction (9). Since this study was concerned with patients who experience dull, achy retropatellar pain with no obvious signs of structural joint damage, subjects were excluded if they had visible cartilage damage on magnetic resonance images. Subjects were also excluded if they, showed signs of patella tendonitis, or if they reported having a previous history of knee surgery, history of traumatic patellar dislocation, or any neurological involvement that would influence gait. Patellofemoral pain subjects completed an Anterior Knee Pain questionnaire (22) to evaluate symptoms and functional limitations related to their patellofemoral pain (Table 1). Prior to participation, subjects were informed of all experimental procedures and gave their written consent in accordance with the Institutional Review Board (IRB) of Stanford University.
Table 1.
Subject demographics displaying mean ± one standard deviation. A score of 100 on the Anterior Knee Pain Scale (20) means no pain or dysfunction.
| Subject Group | Age [yr] | Mass [kg] | Height [m] | Anterior Knee Pain Score |
|---|---|---|---|---|
| Control [n=16] | ||||
| Male [n=8] | 27 ± 3 | 73.5 ± 4.3 | 1.79 ± 0.07 | 100 |
| Female [n=8] | 29 ± 5 | 58.3 ± 4.6 | 1.66 ± 0.05 | 100 |
| Patellofemoral Pain [n=24] | ||||
| Male [n=11] | 30 ± 4 | 72.4 ± 12.5 | 1.78 ± 0.09 | 70 ± 11 |
| Female [n=13] | 29 ± 5 | 60.4 ± 9.1 | 1.65 ± 0.06 | 74 ± 13 |
Motion Capture Experiments
Subjects performed a stair climb task up three steps in a motion analysis laboratory to obtain lower limb kinematics, kinetics, and electromyographic (EMG) data necessary to estimate muscle forces (25). Retro-reflective markers were placed on lower limb landmarks (21) and three-dimensional marker trajectories measured at 60Hz using a 6-camera motion capture system (Motion Analysis Corporation, Santa Rosa, CA). Ground reaction forces and EMG signals were simultaneously recorded at 2400Hz using a force plate (Bertec Corporation, Columbus, OH) and 16-channel EMG system (MotionLab Systems, Baton Rouge, LA), respectively. Surface EMG electrodes were placed on seven muscles crossing the knee in accordance with the placements of Perotto et al (29); vastus medialis, vastus lateralis, rectus femoris, semimembranosus, biceps femoris (long head), medial gastrocnemius and lateral gastrocnemius. For patellofemoral pain patients with bilateral pain, EMG recordings were taken from the more painful leg. For the control subjects, the selected knee for EMG data was randomly chosen. Subjects performed four sets of maximum isometric muscle contractions with the knee near 90 deg flexion to elicit maximum activation of knee extensors and knee flexors. Subjects then performed four sets of calf raises against resistance to elicit maximum activation of the ankle plantar flexors. Prior to electrode placement, the skin was shaved and cleaned with alcohol. EMG signals were recorded with preamplified single differential, rectangular Ag electrodes with 10 mm inter-electrode distance (DE-2.1, DelSys, Inc, Boston, MA, USA). Signals were band-pass filtered (30 500 Hz, 4th order, Butterworth), before being passed to the EMG-Driven model for further processing (see Computational Modeling of Muscle Forces below). Marker trajectories and force plate data were low-pass filtered using a zero-lag fourth-order Butterworth filter with a cut-off frequency of 15 Hz. Standard Newton-Euler inverse dynamics calculations were performed using custom-written Matlab code (Mathworks, Natick, MA) to calculate lower limb joint kinematics and kinetics. Net internal knee moments are presented.
Magnetic Resonance Imaging
To segment the geometry of the bones and cartilage of the patellofemoral joint, magnetic resonance (MR) images of each subject’s knee were acquired with a 1.5T GE Signa MR scanner (GE Healthcare, Milwaukee, WI) using a fat-suppressed spoiled gradient echo sequence (repetition time: 60ms, echo time: 5ms, flip angle: 40°, matrix: 256×256, field of view: 12×12cm, slice thickness: 1.5mm, scan time: 10:25 min). Each subject was also scanned in an open-configuration MR scanner (0.5T SP/i MR, GE Healthcare, Milwaukee, WI) in an upright, weight-bearing posture with the knees at 60° of knee flexion. This posture was chosen because this is the position at which peak knee extension moments are produced during stair climbing. Weight-bearing scans enabled accurate registration of the finite element model (described below) and measurement of patellofemoral joint contact areas to validate model predictions for each subject (6). A 3D fast spoiled gradient echo sequence was used to obtain contiguous sagittal plane MR images of the patellofemoral joint (repetition time: 33ms, echo time: 9ms, flip angle: 45°, matrix: 256×160, field of view: 20×20cm, slice thickness: 2mm, scan time: 2:10 min).
Computational Modeling of Muscle Forces
Lower limb muscle forces were calculated for each subject during the stair climb activity using an EMG-driven musculoskeletal model of the knee (25). Quadriceps muscle forces when the knee reached 60° of flexion were used as input into a finite element model of the patellofemoral joint. The anatomical model used for the muscle force predictions treated the vastus medialis and vastus medialis oblique muscles as a single muscle group. Similarly, the representation of the vastus lateralis in the EMG-driven model did not differentiate the oblique fibers of this muscle. In the finite element model, a percentage of muscle force in the vastus medialis and vastus lateralis muscles were redistributed to oblique fibers of these muscles. These distributions were based on the work of Farahmand et al. (16), who showed that the vastus medialis oblique fibers accounted for ~40% of the total muscle physiological cross sectional area of the vastus medialis, and that the vastus lateralis oblique muscles accounted for ~25% of the total cross sectional area of the vastus lateralis.
Computational Modeling of Cartilage Stress
High resolution MR images were manually segmented and triangulated surface meshes were fit to point cloud data and smoothed using Geomagic Studio (Geomagic, NC). Triangulated surface meshes of the bones of the femur, tibia, and patella and hexahedral volume elements (edge length ~1mm) of the anterior femoral cartilage and patellar cartilage were created using Hypermesh (Hyperworks, MI). The bones of the femur, tibia, and patella were treated as rigid bodies to reduce the computational complexity of the simulation. Since subchondral bone is at least two orders of magnitude stiffer than cartilage (3), treating the subchondral bone as rigid is a reasonable approximation without influencing the stress distribution in the cartilage (13,23). The femoral and patellar cartilage was modeled as a linear elastic solid with an elastic modulus of 12 MPa and a Poisson ratio of 0.47. The interaction between the patellar and femoral surfaces was resolved using surface-to-surface contact with a friction coefficient of 0.01 (2).
The patellar tendon and quadriceps muscles were modeled as tension-only connector elements. The patellar tendon was modeled using ten connector elements, evenly distributed across the attachment area of the tibia and inferior patellar pole with a total stiffness of 2000 N/mm (31). The weight-bearing MR images were used to align the patellar tendon and quadriceps connector elements. Functional components of the quadriceps were actuated independently, including: vastus intermedius (2 elements), rectus femoris (3 elements), vastus lateralis (3 elements), vastus lateralis oblique (3 elements), vastus medialis (3 elements), and vastus medialis oblique (3 elements).
Quasi-static, finite-sliding simulations were performed using a non-linear FE solver (ABAQUS Explicit, SIMULIA, Providence, RI) to compute patellar cartilage stress with the knee at 60° of flexion. For each simulation the femur and tibia were fixed and the patella was unconstrained in six degrees of freedom. Quadriceps muscle forces from the EMG-driven model were applied to the quadriceps connector elements, causing the patella to settle into the trochlear groove of the femur until reaching static equilibrium. The initial position of the femur and tibia were determined by registering the FE meshes of the femur and tibia to the weight-bearing MR data set (see (7) for details). The initial orientation of the patella was also determined by registration to the weight-bearing images. However, to ensure no ‘overlap’ between the cartilage of the patella and femur at the start of the simulation, the patella was displaced in an anterior direction until a gap was present between the femoral and patellar cartilage. The final position of the patella was determined when the applied muscle forces and resulting patellar tendon and joint contact forces achieved a state of static equilibrium.
Cartilage strain energy density (SED) was calculated to represent the mechanical ‘stress’ that might excite nociceptors in the subchondral bone. Strain energy density is a scalar quantity that incorporates both hydrostatic pressure and octahedral shear stress (measures commonly used to quantify cartilage stress (6),(7),(17)). Peak strain energy density was calculated as the mean of the top 10% within the layer of patellar cartilage elements closest to the bone-cartilage interface.
Statistical Analysis
We performed a two-way ANOVA (sex × pain condition) to make comparisons of peak cartilage strain energy density between females and males with and without patellofemoral pain. Scheffé post-hoc tests were performed to investigate significant interactions. We performed the same comparisons for the net joint moment measured from inverse dynamics during the stair climb activity (normalized to body mass * height), the absolute and body weight normalized quadriceps forces predicted by the EMG-driven model, and the knee flexion-extension moment arms for the quadriceps muscles. Statistical analyses were performed using DataDesk (Data Description Inc., Ithaca, NY) using a p-value of <0.05 to indicate significance.
Results
The peak patellar cartilage strain energy density estimated during stair climbing was not statistically significant between patellofemoral pain (1.9 ± 1.23 J/m3) and control subjects (1.66 ± 0.75 J/m3, p=0.52; females and males combined; Fig. 1). However, females exhibited 70% greater peak patellar cartilage strain energy density compared to males (Fig. 2, p=0.0075; pain and control subjects combined). Females with patellofemoral pain had greater cartilage strain energy density than males with patellofemoral pain (Fig. 3, p=0.02). The trend for female control subjects was the same, although peak cartilage strain energy density was not statistically different to male control subjects (Fig. 3, p=0.10). Females with patellofemoral pain experienced similar peak SED to females in the control group (Fig. 3; SED: 2.35 ± 1.42 J/m3 vs 2.07 ± 0.83 J/m3), respectively.
Figure 1.
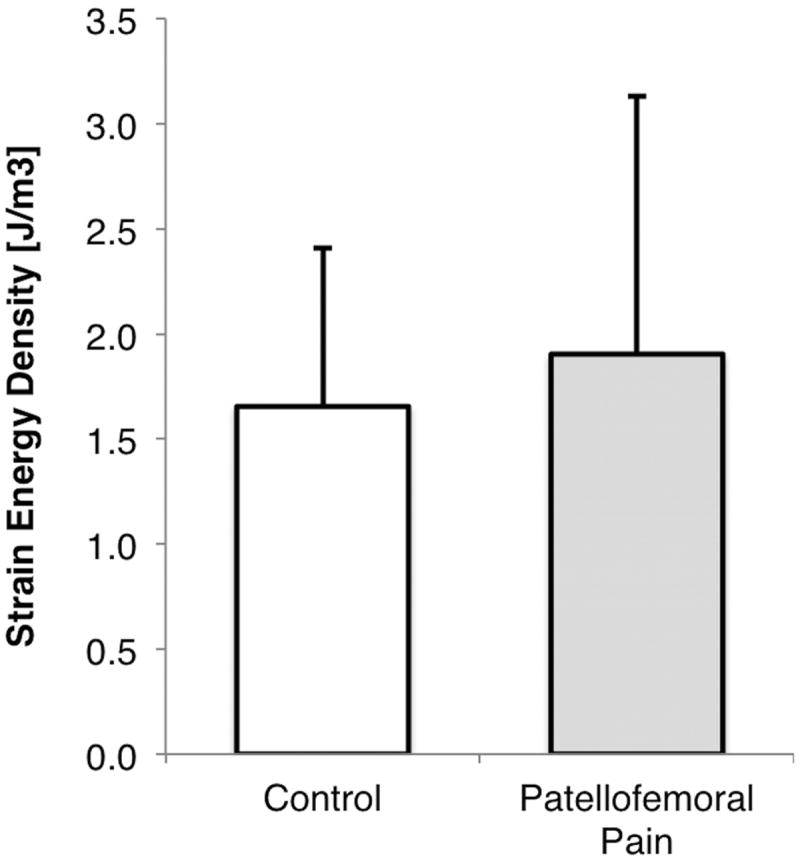
Peak strain energy density in the patellar cartilage of pain-free control subjects and patellofemoral pain patients during a stair climb at 60 degrees of knee flexion.
Figure 2.
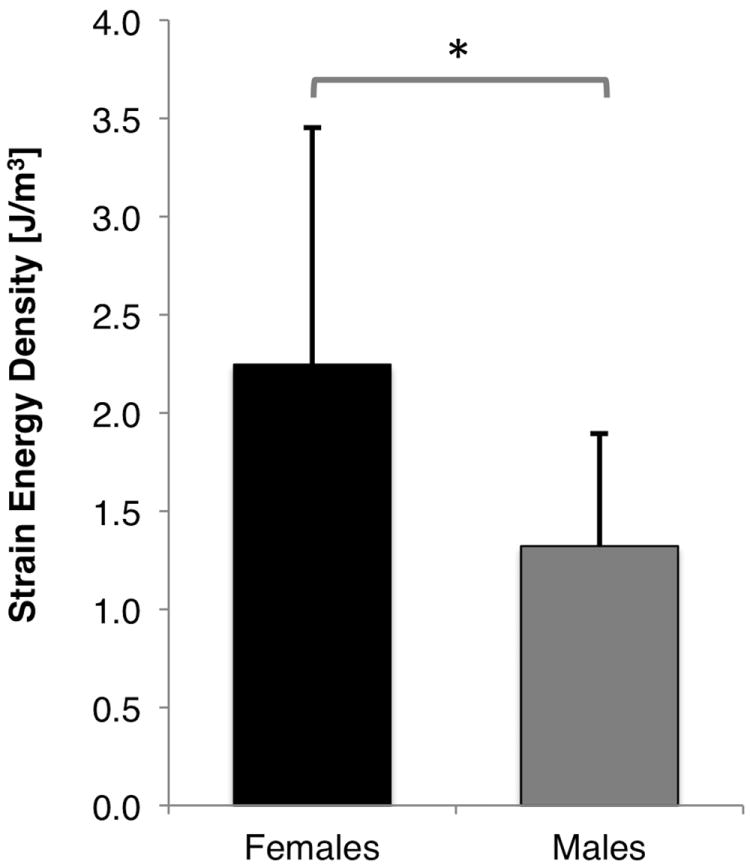
Peak strain energy density in the patellar cartilage of females and males during a stair climb at 60 degrees of knee flexion. * p = 0.0075.
Figure 3.
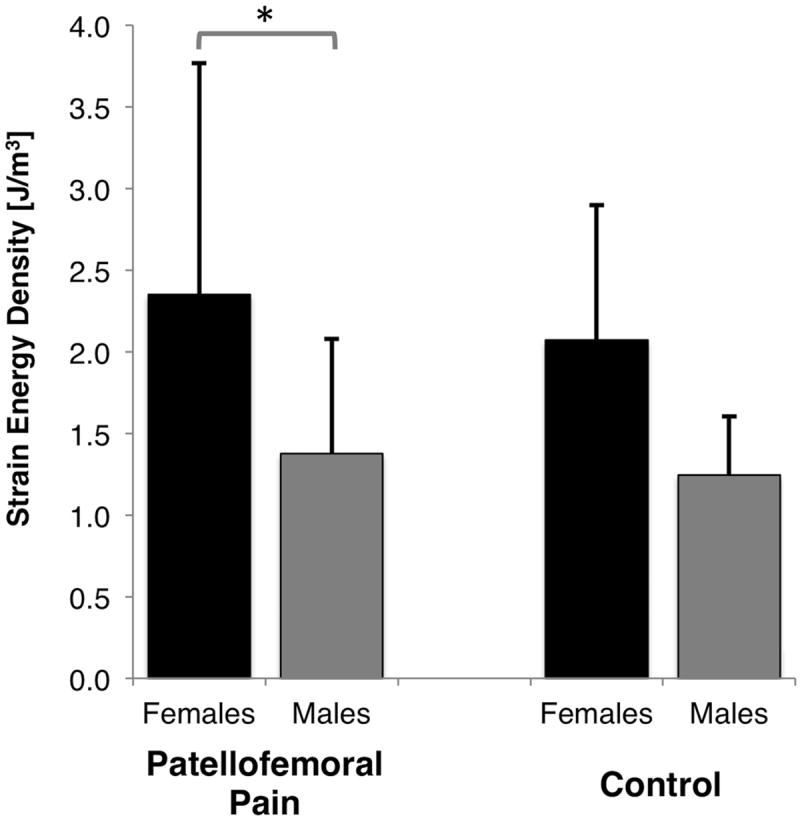
Peak strain energy density in the patellar cartilage of females and males with and without patellofemoral pain during a stair climb at 60 degrees of knee flexion. * p = 0.02.
The normalized net internal knee extension moment estimated from inverse dynamics was similar between females and males for both control and patellofemoral pain subjects (Fig. 4A). The normalized quadriceps force was also similar between females and males, although there was a trend for females to have higher normalized quadriceps force compared to males (average 3.7BW for females and 3.3BW for males, pain and controls combined; p=0.12; Fig. 4B). Table 2 provides the predicted absolute and normalized quadriceps muscle forces. The extension moment arm of the quadriceps muscles at 60° of knee flexion (not shown; taken from the scaled musculoskeletal model) was smaller in the female subjects compared to the males (3.39cm vs 3.67cm, respectively, p=0.0012).
Figure 4.
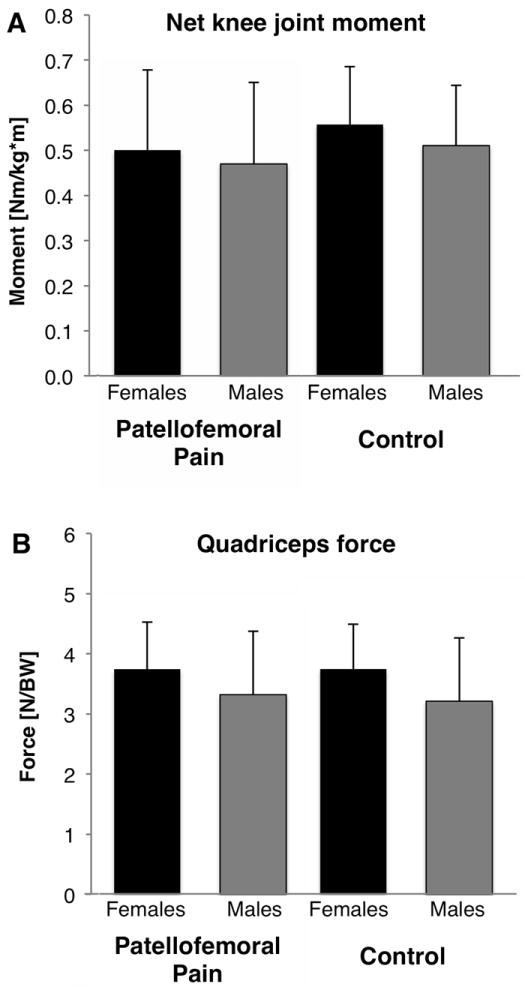
A) Net joint knee extension moment from inverse dynamics, and B) normalized quadriceps muscle forces predicted from the EMG-driven musculoskeletal model when the knee was at 60° of flexion during a stair climb activity (mean ± 1 standard deviation). BW = body weight.
Table 2.
Absolute (a) and normalized (b) quadriceps muscle forces predicted by the EMG-driven musculoskeletal model
| a | ||||
|---|---|---|---|---|
| Patellofemoral Pain | Control | |||
| Muscle | Females | Males | Females | Males |
| Vastus Medialis | 354 ± 110 | 374 ± 162 | 350 ± 116 | 385 ± 191 |
|
| ||||
| Vastus Medialis Oblique | 236 ± 73 | 250 ± 108 | 233 ± 77 | 257 ± 128 |
|
| ||||
| Vastus Lateralis | 693 ± 135 | 764 ± 311 | 663 ± 131 | 717 ± 224 |
|
| ||||
| Vastus Lateralis Oblique | 231 ± 45 | 255 ± 104 | 221 ± 44 | 239 ± 75 |
|
| ||||
| Vastus Intermedius | 651 ± 138 | 707 ± 282 | 637 ± 152 | 693 ± 266 |
|
| ||||
| Rectus Femoris | 22 ± 38 | 52 ± 55 | 45 ± 85 | 50 ± 57 |
|
| ||||
| Total Quadriceps Force [N] | 2187 | 2401 | 2148 | 2341 |
|
| ||||
| b | ||||
| Patellofemoral Pain | Control | |||
| Muscle | Females | Males | Females | Males |
|
| ||||
| Vastus Medialis | 0.60 ± 0.17 | 0.52 ± 0.22 | 0.61 ± 0.18 | 0.53 ± 0.24 |
|
| ||||
| Vastus Medialis Oblique | 0.40 ± 0.11 | 0.35 ± 0.15 | 0.40 ± 0.12 | 0.35 ± 0.16 |
|
| ||||
| Vastus Lateralis | 1.20 ± 0.27 | 1.05 ± 0.33 | 1.16 ± 0.24 | 0.99 ± 0.27 |
|
| ||||
| Vastus Lateralis Oblique | 0.40 ± 0.09 | 0.35 ± 0.11 | 0.39 ± 0.08 | 0.33 ± 0.09 |
|
| ||||
| Vastus Intermedius | 1.12 ± 0.24 | 0.98 ± 0.33 | 1.11 ± 0.23 | 0.95 ± 0.33 |
|
| ||||
| Rectus Femoris | 0.04 ± 0.06 | 0.07 ± 0.07 | 0.07 ± 0.14 | 0.07 ± 0.08 |
|
| ||||
| Total Quadriceps Force [BW] | 3.74 | 3.32 | 3.75 | 3.21 |
Contact areas predicted by the finite element model were within 8% of those measured from the weight bearing MR images (predicted: 537 ± 102mm2 vs measured: 580 ± 154mm2). Males had predicted contact areas that were, on average, 33% larger than females. Predicted contact areas were as follows: female patellofemoral pain 487 ± 52 mm2; female control = 447 ± 66 mm2; male patellofemoral pain = 603 ± 93 mm2; male control = 614 ± 97 mm2.
Discussion
The purpose of this study was two-fold. Firstly, to determine whether patients with patellofemoral pain exhibit greater cartilage stress than pain-free controls, since stress is often cited as a potential mechanism for dull, achy retropatellar pain. Our findings suggest that the peak patellar cartilage stress in patellofemoral pain patients is no different to pain-free controls when performing a stair climb activity. These data seem to contradict the findings of Farrokhi et al. (17), who reported 30-60% greater mean and peak cartilage stress in females with patellofemoral pain (n=10), compared to a pain-free control group (n=10). However, it is important to note that Farrokhi and colleagues reported significant differences in peak stresses at 15° of knee flexion but found no differences at 45° of knee flexion (17). As the knee flexes, the patella becomes engaged within the trochlear groove and contact area increases (4), potentially mitigating differences in cartilage area (and therefore stress) between patellofemoral pain and pain-free controls. Although Farrokhi et al. did not report contact areas or muscle forces, reduced contact area at 15 degrees of knee flexion seems a likely cause of the large stress differences they reported. In our study we did not see differences in contact area between patellofemoral pain and pain-free control subjects. Direct comparison between our results and those of Farrokhi et al. is difficult due to differences in muscle force estimates (optimization vs EMG-driven), imaging protocol (supine vs weight-bearing), and model boundary conditions (constrained patella vs unconstrained patella).
It is interesting to highlight the large range of cartilage stress across the patellofemoral pain group, as indicated by the large standard deviations in Fig. 1. Within this cohort there were some patients who experienced high peak cartilage stress; for these patients stress might be an important mechanical contributor to pain. However, there were other patients within the pain cohort who experienced relatively low cartilage stress, suggesting that some other factor is related to their sensation of pain. Cause and effect is difficult to determine in this cross-sectional study as we do not know to what extent patients adjusted their movement patterns in response to pain when performing the stair climb activity in the lab. Integrating stress measures with an estimate of loading cycles might yield a difference in total ‘load exposure’ in these patients and provide useful data for clinicians monitoring work load of patients during rehabilitation. Using a patient-specific model to classify patients based on their peak cartilage stress and/or load exposure could be useful to identify patients who might respond to load-altering interventions and warrants further investigation.
The second purpose of this paper was to test the hypothesis that females exhibit greater peak patellar cartilage stress than males during a stair climb activity. Our data showed a sex-difference in the peak cartilage stress with females experiencing 70% greater stress compared to males. Again, we cannot prove cause and effect, but elevated patellar cartilage stress might explain why females, in general, are more susceptible to patellofemoral pain compared to males.
What contributed to elevated cartilage stress in our female subjects? Although the normalized net joint moments at the knee were similar between females and males, females produced this extension moment with quadriceps forces that were comparable to males. These higher muscle forces are consistent with our previous estimates of muscle forces during walking and running, which showed females produced higher normalized forces compared to males (5). One reason for this larger force is due to the smaller extension moment arms of the quadriceps muscles of females compared to males. Morphological sex differences have previously been identified as playing a role in the development of patellofemoral pain (1), but not in the context of smaller muscle moment arms, which would result in greater muscle and joint contact forces. Females have narrower femoral intercondylar distance (10,18) and smaller patella width and thickness compared to males (19), reducing the moment arms of knee muscles when producing knee moments.
Cartilage stress is also influenced by joint orientation, the morphology of the articulating surfaces, and cartilage thickness, so it is possible that some of these factors also play a role in females having greater patellar cartilage stress compared to males. Csintalan et al. (12) investigated sex differences using cadaveric patellofemoral joints and, compared to males, females had reduced contact area and increased contact pressures, which is consistent with the current findings. Our previous in vivo measurements of patellofemoral joint contact area using weight-bearing MR imaging showed similar sex differences (4). Csintalan et al. (12) also showed that the difference in contact area was greater than the difference in bone dimensions between male and female specimens, suggesting that there might be sex differences in articulating geometry. Varadarajan et al. (36) found that females have a medially oriented proximal trochlear groove compared to males, although the functional relevance of this remains unclear. It is worth noting that contact area is highly dependent on load (4), in particular, the quadriceps muscle force distribution which influences patellar tracking (24). From the few studies that have investigated three-dimensional patellofemoral joint kinematics, there does not appear to be any significant sex difference (32,37). Lastly, cartilage thickness can alter the magnitude and distribution of patellar cartilage stress. We previously showed that females have thinner load-bearing patellar cartilage compared to males (14), which might result in greater cartilage stress. These data are consistent with previous research showing females to have significantly less knee cartilage than males (20).
Although we have taken care to accurately model the physiological loading conditions, limitations of our model should be considered. Firstly, we used a simple linear elastic constitutive model to represent the mechanical behaviour of cartilage, which was constant for all subjects. Modeling cartilage as a linear elastic solid is a reasonable assumption for a dynamic activity, such as the stair climb task modeled here, although we appreciate that this does not capture the complex time-varying and inhomogenous properties of the tissue. It is reasonable to assume that the differences in material properties amongst subjects might account for the differences seen in measured vs. predicted contact area. Secondly, this study was limited to quasi-static simulations of the patellofemoral joint with the knee at 60° of flexion, as we were interested in the peak cartilage stress under maximal knee joint extension loads. A dynamic model would enable us to estimate stress through the entire range of motion with time-varying muscle forces, which might result in different stresses than those presented here.
In summary, this paper showed that, on average, patients with patellofemoral pain do not exhibit greater peak cartilage stress compared to pain-free controls during a stair climb task at 60 degrees of knee flexion; however, there is a great deal of subject variation. Females exhibit greater peak cartilage stress compared to males, which might explain the greater prevalence of patellofemoral pain in females compared to males, but other mechanical and biological factors are involved in this complex pathway to pain. Patient-specific computational models can be used to classify patellofemoral pain patients based on mechanical factors, such as cartilage stress, providing a useful clinical tool to identify targeted treatment options.
Acknowledgments
We thank Parastou Eslami for help with creating the finite element meshes.
The authors have no additional professional or financial affiliations related to the subject of this study. The results of the present study do not constitute endorsement by ACSM.
Source of Funding: This work was funded by the National Institutes of Health (EB0002524, EB005790) and the Department of Veterans Affairs (A2592R).
Footnotes
Conflicts of Interest: None of the authors have any conflicts of interest to declare.
Publisher's Disclaimer: Medicine & Science in Sports Exercise® Published ahead of Print contains articles in unedited manuscript form that have been peer reviewed and accepted for publication. This manuscript will undergo copyediting, page composition, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered that could affect the content.
References
- 1.Arendt EA. Dimporphism and Patellofemoral Disorders. Orthop Clin N Am. 2006;37:593–9. doi: 10.1016/j.ocl.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Ateshian GA, Hung CT. Patellofemoral joint biomechanics and tissue engineering. Clinical Orthopaedics and Related Research. 2005;81:81–90. doi: 10.1097/01.blo.0000171542.53342.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beaupré GS, Stevens SS, Carter DR. Mechanobiology in the development, maintenance, and degeneration of articular cartilage. J Rehabil Res Dev. 2000;37(2):145–51. [PubMed] [Google Scholar]
- 4.Besier TF, Draper CE, Gold GE, Beaupre GS, Delp SL. Patellofemoral joint contact area increases with knee flexion and weight-bearing. Journal of Orthopaedic Research. 2005;23(2):345–50. doi: 10.1016/j.orthres.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Besier TF, Fredericson M, Gold GE, Beaupre GS, Delp SL. Knee muscle forces during walking and running in patellofemoral pain patients and pain-free controls. J Biomech. 2009;42(7):898–905. doi: 10.1016/j.jbiomech.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Besier TF, Gold GE, Beaupre GS, Delp SL. A modeling framework to estimate patellofemoral joint cartilage stress in vivo. Medicine and Science in Sports and Exercise. 2005;37(11):1924–30. doi: 10.1249/01.mss.0000176686.18683.64. [DOI] [PubMed] [Google Scholar]
- 7.Besier TF, Gold GE, Delp SL, Fredericson M, Beaupre GS. The influence of femoral internal and external rotation on cartilage stresses within the patellofemoral joint. Journal of Orthopaedic Research. 2008;26(12):1627–35. doi: 10.1002/jor.20663. [DOI] [PubMed] [Google Scholar]
- 8.Boling M, Padua D, Marshall S, Guskiewicz K, Pyne S, Beutler A. Gender differences in the incidence and prevalence of patellofemoral pain syndrome. Scand J Med Sci Sports. 2010;20(5):725–30. doi: 10.1111/j.1600-0838.2009.00996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brechter JH, Powers CM. Patellofemoral joint stress during stair ascent and descent in persons with and without patellofemoral pain. Gait Posture. 2002;16(2):115–23. doi: 10.1016/s0966-6362(02)00090-5. [DOI] [PubMed] [Google Scholar]
- 10.Conley S, Rosenberg A, Crowninshield R. The female knee: anatomic variations. J Am Acad Orthop Surg. 2007;15(Suppl 1):31–6. doi: 10.5435/00124635-200700001-00009. [DOI] [PubMed] [Google Scholar]
- 11.Costigan PA, Deluzio KJ, Wyss UP. Knee and hip kinetics during normal stair climbing. Gait Posture. 2002;16(1):31–7. doi: 10.1016/s0966-6362(01)00201-6. [DOI] [PubMed] [Google Scholar]
- 12.Csintalan RP, Schulz MM, Woo J, McMahon PJ, Lee TQ. Gender differences in patellofemoral joint biomechanics. Clin Orthop Relat Res. 2002;402:260–9. doi: 10.1097/00003086-200209000-00026. [DOI] [PubMed] [Google Scholar]
- 13.Donahue TL, Hull ML, Rashid MM, Jacobs CR. A finite element model of the human knee joint for the study of tibio-femoral contact. J Biomech Eng. 2002;124:273–80. doi: 10.1115/1.1470171. [DOI] [PubMed] [Google Scholar]
- 14.Draper CE, Besier TF, Gold GE, et al. Is cartilage thickness different in young subjects with and without patellofemoral pain? Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2006;14(9):931–7. doi: 10.1016/j.joca.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Draper CE, Quon A, Fredericson M, et al. Comparison of MRI and (1)(8)F-NaF PET/CT in patients with patellofemoral pain. Journal of magnetic resonance imaging : JMRI. 2012;36(4):928–32. doi: 10.1002/jmri.23682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farahmand F, Senavongse W, Amis AA. Quantitative study of the quadriceps muscles and trochlear groove geometry related to instability of the patellofemoral joint. Journal of Orthopaedic Research. 1998;16(1):136–43. doi: 10.1002/jor.1100160123. [DOI] [PubMed] [Google Scholar]
- 17.Farrokhi S, Keyak JH, Powers CM. Individuals with patellofemoral pain exhibit greater patellofemoral joint stress: a finite element analysis study. Osteoarthritis and Cartilage. 2011;19(3):287–94. doi: 10.1016/j.joca.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hislop-Jambrich J. A 3D radiological study of age-related quantitative and morphological differences in the human femur: Clinical and anthropological applications. Melbourne, Australia: The University of Melbourne; 2010. p. 296. [Google Scholar]
- 19.Introna F, Di Vella G, Campobasso CP. Sex determination by discriminant analysis of patella measurements. Forensic science international. 1998;95(1):39–45. doi: 10.1016/s0379-0738(98)00080-2. [DOI] [PubMed] [Google Scholar]
- 20.Jones G, Glisson M, Hynes K, Cicuttini F. Sex and site differences in cartilage development: a possible explanation for variations in knee osteoarthritis in later life. Arthritis and rheumatism. 2000;43(11):2543–9. doi: 10.1002/1529-0131(200011)43:11<2543::AID-ANR23>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 21.Kadaba MP, Ramakrishnan HK, Wootten ME. Measurement of lower extremity kinematics during level walking. Journal of Orthopaedic Research. 1990;8(3):383–92. doi: 10.1002/jor.1100080310. [DOI] [PubMed] [Google Scholar]
- 22.Kujala UM, Jaakkola LH, Koskinen SK, Taimela S, Hurme M, Nelimarkka O. Scoring of patellofemoral disorders. Arthroscopy. 1993;9(2):159–63. doi: 10.1016/s0749-8063(05)80366-4. [DOI] [PubMed] [Google Scholar]
- 23.Li G, Lopez O, Rubash H. Variability of a three-dimensional finite element model constructed using magnetic resonance images of a knee for joint contact stress analysis. J Biomech Eng. 2001;123:341–6. doi: 10.1115/1.1385841. [DOI] [PubMed] [Google Scholar]
- 24.Lin F, Wilson NA, Makhsous M, et al. In vivo patellar tracking induced by individual quadriceps components in individuals with patellofemoral pain. J Biomech. 2010;43(2):235–41. doi: 10.1016/j.jbiomech.2009.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lloyd DG, Besier TF. An EMG-driven musculoskeletal model to estimate muscle forces and knee joint moments in vivo. J Biomech. 2003;36(6):765–76. doi: 10.1016/s0021-9290(03)00010-1. [DOI] [PubMed] [Google Scholar]
- 26.Mach DB, Rogers SD, Sabino MC, et al. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113(1):155–66. doi: 10.1016/s0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 27.Otterness IG, Eckstein F. Women have thinner cartilage and smaller joint surfaces than men after adjustment for body height and weight. Osteoarthritis and Cartilage. 2007;15(6):666–72. doi: 10.1016/j.joca.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Outerbridge RE, Dunlop JA. The problem of chondromalacia patellae. Clin Orthop Relat Res. 1975;110:177–96. doi: 10.1097/00003086-197507000-00024. [DOI] [PubMed] [Google Scholar]
- 29.Perotto A, Delagi EF, Iazzetti J, Morrison D. Anatomical Guide for the Electromyographer. IL: Springfield; 2005. [Google Scholar]
- 30.Powers CM. The influence of abnormal hip mechanics on knee injury: a biomechanical perspective. J Orthop Sports Phys Ther. 2010;40(2):42–51. doi: 10.2519/jospt.2010.3337. [DOI] [PubMed] [Google Scholar]
- 31.Reeves ND, Maganaris CN, Narici MV. Effect of strength training on human patella tendon mechanical properties of older individuals. The Journal of Physiology. 2003;548(Pt 3):971–81. doi: 10.1113/jphysiol.2002.035576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seisler AR, Sheehan FT. Normative three-dimensional patellofemoral and tibiofemoral kinematics: a dynamic, in vivo study. IEEE transactions on bio-medical engineering. 2007;54(7):1333–41. doi: 10.1109/TBME.2007.890735. [DOI] [PubMed] [Google Scholar]
- 33.Taunton JE, Ryan MB, Clement DB, McKenzie DC, Lloyd-Smith DR, Zumbo BD. A retrospective case-control analysis of 2002 running injuries. Br J Sports Med. 2002;36(2):95–101. doi: 10.1136/bjsm.36.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor WR, Heller MO, Bergmann G, Duda GN. Tibio-femoral loading during human gait and stair climbing. Journal of Orthopaedic Research. 2004;22(3):625–32. doi: 10.1016/j.orthres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 35.van Middelkoop M, van Linschoten R, Berger MY, Koes BW, Bierma-Zeinstra SM. Knee complaints seen in general practice: active sport participants versus non-sport participants. BMC Musculoskelet Disord. 2008;9:36. doi: 10.1186/1471-2474-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varadarajan KM, Gill TJ, Freiberg AA, Rubash HE, Li G. Gender differences in trochlear groove orientation and rotational kinematics of human knees. J Orthop Res. 2009;27(7):871–8. doi: 10.1002/jor.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varadarajan KM, Gill TJ, Freiberg AA, Rubash HE, Li G. Patellar tendon orientation and patellar tracking in male and female knees. J Orthop Res. 2010;28(3):322–8. doi: 10.1002/jor.20977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wretenberg P, Nemeth G, Lamontagne M, Lundin B. Passive knee muscle moment arms measured in vivo with MRI. Clin Biomech (Bristol, Avon) 1996;11(8):439–46. doi: 10.1016/s0268-0033(96)00030-7. [DOI] [PubMed] [Google Scholar]


