Abstract
Background
The development of robust plasma-based biomarkers in Parkinson disease (PD) could lead to new approaches for identifying those at risk for PD and developing novel therapies. Here we validate plasma Apolipoprotein A1 (ApoA1) as a correlate of age at onset and motor severity in PD.
Methods
Plasma ApoA1 and high-density lipoprotein at baseline, six months, and 12 months were measured in 254 research volunteers [154 patients with PD and 100 normal controls] enrolled in the Parkinson's Progression Markers Initiative (PPMI) study.
Results
Lower baseline plasma ApoA1 levels associate with an earlier age at PD onset in early-stage, drug-naïve PPMI PD patients (p=0.023). Moreover, lower baseline ApoA1 levels trend towards association with worse motor severity in PPMI PD patients (p=0.080). Over 12 months of follow-up, plasma ApoA1 levels do not predict motor decline in the PPMI PD cohort. Finally, a meta-analysis of five PD cohorts encompassing >1000 patients confirms significant association of lower plasma ApoA1 with earlier age at PD onset (p<0.001) and greater motor severity (p<0.001).
Conclusions
Our results confirm the previously-reported association of lower plasma ApoA1 levels with two clinical features suggesting poorer dopaminergic system integrity – earlier age at PD onset and greater motor severity – in early-stage, drug-naïve PD patients. This is the first report of a plasma-based biomarker evaluated in the PPMI study. Future investigations are warranted evaluating plasma ApoA1 as a longitudinal correlate of disease progression, and investigating the potential of ApoA1 as a therapeutic target in PD.
Keywords: Apolipoprotein A1, Biomarker, Parkinson disease
INTRODUCTION
The development of biomarkers, objective proxy indicators of physiological or pathophysiological states, is a major research priority in the field of neurodegenerative diseases. The need for biomarkers arises because the organ involved in these diseases, the brain, is difficult to assess in an objective manner and impractical to sample directly.1 Moreover, neurodegenerative diseases are common, and, currently, none can be treated with disease-modifying therapies.2,3
Parkinson disease (PD) is the second most prevalent neurodegenerative disease after Alzheimer disease (AD), with an estimated five million individuals affected with PD worldwide.4 Characterized by the loss of dopaminergic neurons of the substantia nigra, PD presents clinically with bradykinesia, rigidity, tremor, and postural instability.5 By the time patients are symptomatic, however, it is estimated that 40-60% of neurons may be lost.6
We previously identified plasma Apolipoprotein A1 (ApoA1) as a candidate biomarker associating with PD risk. Emerging from an unbiased screen of ~100 proteins, plasma ApoA1 correlated with measures of dopaminergic integrity. Specifically, in a cross-sectional study, lower levels of plasma ApoA1 – the major component of high-density lipoprotein (HDL)7 – associated with an earlier age at PD onset and poorer performance on the motor section of the Unified Parkinson Disease Rating Scale (UPDRS-III).8 Moreover, in an asymptomatic at-risk cohort for PD, lower ApoA1 levels associated with decreased putaminal dopamine transporter binding.8 Finally, we have recently demonstrated that plasma ApoA1 levels are significantly lower in PD patients compared to neurologically normal controls (NC).9
The Parkinson's Progression Markers Initiative (PPMI) is a longitudinal observational study of early PD patients and controls recruited from 32 clinical sites in the United States, Europe, and Australia.10 With the goal of facilitating PD biomarker development, the PPMI cohort is extensively characterized, and biofluid sampling from the PPMI cohort occurs under standardized protocols. In the present study, we evaluated plasma ApoA1 levels in the PPMI cohort, with our primary goal being to validate and confirm our prior findings in an international, drug-naive group of PD patients.
METHODS
For additional details, please see Supplemental Methods.
Participants
The parent cohort for our current study is the Parkinson's Progression Markers Initiative, a five-year multicenter international cohort with sites in the United States, Europe, and Australia.
The PPMI study protocol was approved by the institutional review board at all participating clinical sites, and written informed consent was obtained from all participants prior to their inclusion in the study.
For our current analysis, we included all PPMI subjects for whom plasma samples were available from the baseline visit, six-month follow-up visit (PPMI visit 2), and 12-month follow-up visit (PPMI visit 4) through August, 2013 (Table 1, Supplementary Table 1). Exclusion criteria are detailed in the Supplemental Methods. In brief, subjects were only excluded if there was no evidence of dopaminergic deficit by DATscan, or if samples failed specific quality control criteria.
Table 1.
Comparison of Clinical and Demographic Information between normal controls (NC) and patients with Parkinson disease (PD).
| Clinical/Demographic Features | NC (n=100) | PD (n=154) | P–value |
|---|---|---|---|
| Baseline Age at plasma Mean (SD) [95% CI] | 63 (9) [62-65] | 63 (8) [61-64] | 0.95a |
| Female/Male No. (% Male) | 39/61 (61.0) | 48/106 (68.8) | 0.20b |
| Age at diagnosis Mean (SD) [95% CI] | - | 63 (9) [61-64] | - |
| Disease Duration Median in years (range) | - | 0.6 (0.0-3.0) | - |
| UPDRS Part III Motor Score Mean (SD) | 1.16 (1.9) | 20.4 (7.9) | <0.0001a |
| Hoehn and Yahr stage Mean (SD) | 0.01 (0.1) | 1.54 (0.50) | <0.0001a |
| MoCA Mean (SD) | 28.31 (1.01) | 27.05 (2.12) | <0.001a |
Abbreviations: NC, Normal Controls; MoCA, Montreal Cognitive Assessment; PD, Parkinson disease; UPDRS, Unified Parkinson's Disease Rating Scale.
Based on Mann Whitney Test.
Based on χ2 test.
Plasma Sample Collection and Handling
Plasma collection was performed at baseline, six months, and 12 months at each clinical site as described in the PPMI biologics manual (http://www.ppmi-info.org).
Measurement of Plasma Analytes
Plasma ApoA1 and a basic lipid panel (HDL, low density lipoprotein (LDL), total cholesterol, and triglycerides) were measured using a Roche Cobas c501 bioanalyzer (Tina-quant assay, catalog number 03032566-122), using standard manufacturer protocols.
Statistical Analyses of PPMI Data
In our primary analysis, multivariate linear regression models adjusted for age at plasma sampling and sex were used to evaluate the association between baseline plasma ApoA1 levels and two main outcome measures (age at PD onset, motor score on the UPDRS (UPDRS-III)).
In secondary baseline ApoA1 analyses, multivariate linear regression models adjusted for additional covariates were also evaluated as indicated in the text, and additional outcomes (e.g. Montreal Cognitive Assessment, or MoCA) were also evaluated as indicated in the text. Moreover, baseline plasma HDL was assessed as a correlate of age at PD onset and UPDRS-III in multivariate linear regression models otherwise identical to those used to evaluate plasma ApoA1.
Plasma ApoA1 longitudinal analyses were also performed. We used linear mixed-effects models11 to evaluate whether baseline plasma ApoA1 levels predicted rates of disease progression and to ascertain whether changes in ApoA1 correlated with disease progression (UPDRS-III) over time. A random intercept was included in the mixed-effects models. For these longitudinal analyses, we excluded subjects who started symptomatic therapy for PD motor symptoms, since this could affect UPDRS-III scores; 81/154 PD patients remained after excluding those who initiated therapy within 12 months of follow-up.
Logistic regression and Cox proportional hazards analyses were performed to investigate whether baseline ApoA1 or HDL plasma levels predicted the initiation of symptomatic therapy over the 12-month follow-up period. These analyses adjusted for age at plasma sampling and sex.
Analyses for sources of pre-analytical variability are described in Supplemental Methods.
Meta-analysis
To meta-analyze the effect of ApoA1 on age at PD onset and PD motor severity, raw baseline ApoA1 values from four prior PD cohorts8,9,12 along with baseline ApoA1 values from the present study, were standardized for differences in measurement platform. Specifically, within each cohort, the raw ApoA1 value was subtracted from the mean ApoA1 value, dividing by the standard deviation.
After standardization of ApoA1 values, the combined dataset was analyzed using linear regression models as in the primary analyses above. In addition, Cox proportional hazards models were used to estimate hazard ratios for tertiles of plasma ApoA1 values, with respect to age at PD onset and UPDRS III motor severity, adjusting for age at plasma sampling and sex.
Two tailed p-values are reported throughout the text. Values with P < 0.05 were regarded as statistically significant. All statistical analyses were performed using the open source statistical software R (http://www.r-project.org) or GraphPad Prism (San Diego, CA). All R-scripts are available upon request.
RESULTS
Demographic and clinical characteristics of study subjects
154 PD patients and 100 neurologically normal controls (NC) from 19 PPMI clinical sites were included in this study. Their demographic and clinical characteristics are summarized in Table 1. Mean age at plasma sampling and sex did not differ between PD and NC. For PD patients, the median disease duration was 0.6 years and the mean Hoehn and Yahr stage was 1.5 at baseline (Table 1). As expected, PD patients performed significantly worse on tests of motor function (UPDRS-III, Hoehn and Yahr), as well as the Montreal Cognitive Assessment (MoCA), compared to NC (Table 1).
Potential sources of pre-analytical variability in PPMI ApoA1 measures
We evaluated three different potential sources of pre-analytical variability in the measures obtained for plasma ApoA1 from the PPMI cohort: differences by clinical site, differences by time period of biofluid sampling, and differences by biorepository. In each case, we considered PD and NC separately.
Comparing ApoA1 plasma levels across clinical sites of origin, one site, with few samples (Clinical site 327, n=6, all PD), demonstrated an unusually large variation in ApoA1 values (Fig. 1a, 1b). Comparing seven 6-month time periods starting with July-December 2010 and ending in July-December 2013, we found the earliest time period of plasma sampling exhibited greater ApoA1 variation in the NC only; again, the high-variation time period consisted of very few samples (n=11 NC, Fig. 1c, 1d). Comparing across biorepositories, neither demonstrated an unusually large variation in ApoA1 plasma measures (Fig. 1e, 1f). Thus, clinical site of origin and time period of plasma sampling demonstrated unusually large variation in some subgroups, suggesting that these two factors could be sources of pre-analytical variability in ApoA1 measures. However, in each case, the subgroup exhibiting an unusually large amount of variation was comprised of very few samples.
Figure 1. Pre-analytical variability in PPMI plasma ApoA1.
Across clinical sites, site 327 (arrow) had higher-than-expected ApoA1 variability compared to other sites and to values expected based on prior ApoA1 measures at our site (A, B). Across time periods of plasma collection, the earliest period (arrow) showed higher-than-expected ApoA1 variability in neurologically normal controls only (C, D). Mean +/- SEM are indicated for each clinical site, time period, and biorepository.
Based on these findings, we performed our primary analyses without adjustment for clinical site, time period of biofluid sampling, or biorepository. However, for any significant associations, we performed secondary analyses adjusting for clinical site and for time period of biofluid sampling to ensure that this did not change our results.
Lower plasma ApoA1 correlates with earlier age at PD onset in the PPMI cohort
We previously demonstrated significant associations between lower levels of plasma ApoA1 and (1) an earlier age at PD onset, and (2) more severe PD assessed by the motor subscale of the UPDRS (UPDRS-III). These associations were first demonstrated in a cohort of mid-stage PD patients.8 We sought to extend these relationships to the early-stage, drug-naive PPMI cohort.
As shown in Table 2, lower levels of plasma ApoA1 were significantly associated with earlier age at PD onset in the PPMI PD patients (β=0.41, SE=0.18, two-tailed p=0.023). Lower levels of plasma ApoA1 also showed a trend towards association with greater motor impairment (higher UPDRS-III score) in PPMI PD patients (β=-3.54, SE=2.01, two-tailed p=0.080). As both sex and age have been reported to affect ApoA1 levels, with female sex in particular noted to correlate with higher ApoA1 levels,13 models adjusted for age at plasma sampling and sex. Of note, in this cohort, only sex affected ApoA1 levels significantly (Supplementary Fig. 1, Supplementary Fig. 2).
Table 2.
Associations between clinical outcomes and baseline plasma ApoA1 or HDL levels in PPMI cohort Parkinson disease patients.
| Outcome | Predictor(s) | P-value | Direction |
|---|---|---|---|
| Age at PD Onset | Plasma ApoAl | p=0.023 | + |
| Plasma HDL | p=0.020 | + | |
| UPDRS Part III | Plasma ApoAl | p=0.080 | - |
| Plasma HDL | p=0.155 | - | |
| MoCA | Plasma ApoAl | p=0.280 | + |
| Plasma HDL | p=0.545 | + |
Abbreviations: ApoA1=Apolipoprotein A1; HDL=High-Density Lipoprotein; MoCA=Montreal Cognitive Assessment; UPDRS Part III=Unified Parkinson's Disease Rating Scale Part III (motor section, higher scores denote worse performance). All linear regression models adjusted for age at plasma and sex, and all reported p-values are two-tailed.
In addition, due to the potential pre-analytical variability introduced by time period of plasma sampling or clinical site, we repeated our analyses adjusting for these variables. Adjusting for both time period of plasma sampling and clinical site of origin did not affect the association of plasma ApoA1 with age at PD onset (two-tailed p=0.032) and strengthened the association with UPDRS-III score (two-tailed p=0.019).
In contrast to observed associations with motor severity, plasma ApoA1 levels were not correlated with cognitive performance (MoCA, Table 2).
Lower plasma HDL correlates with earlier age at PD onset in the PPMI cohort
ApoA1 is the primary component of HDL, and we observed a strong correlation between plasma ApoA1 and HDL measures (Pearson R2=0.80, Fig. 2a). We therefore considered plasma HDL as an alternative predictor of age at PD onset and motor severity.
Figure 2. Plasma ApoA1 and HDL increase over time.
Plasma HDL levels correlate with ApoA1 values (A; R2 = 0.80). While no baseline differences were observed in plasma ApoA1 (B) or HDL (C) between neurologically normal controls (NC) and Parkinson disease (PD) patients, a trend towards less increase in ApoA1 levels over time was observed in PD patients (p=0.065).
Lower plasma HDL measures were significantly associated with earlier age at PD onset in linear regression models adjusted for age at plasma sampling and sex (β=0.76, SE=0.32, two-tailed p=0.020, Table 2). This result persisted in further models additionally adjusting for time period of plasma sampling and clinical site of origin (two-tailed p=0.041). In contrast, HDL measures were not significantly correlated with PD motor severity as measured by the UPDRS-III score, nor with cognitive performance as measured by the MoCA (Table 2). No significant associations between LDL or total cholesterol with age at onset or UPDRS-III score were found.
Plasma ApoA1 does not correlate with PD motor progression over a 12-month period of longitudinal follow-up
The PPMI participants selected for inclusion in the present study had clinical visits and plasma sampling at baseline, six months, and 12 months. As expected, UPDRS-III scores increased in PD patients over time (two-tailed p<0.001, Supplementary Fig. 3), with an average increase of 20% indicating worse clinical status over time. We next performed longitudinal analyses of plasma ApoA1 and HDL levels as correlates of PD motor progression. Using linear mixed-effects models, we found no significant association between baseline ApoA1 or HDL plasma levels and rate of motor decline, as reflected in the UPDRS-III score over 12 months of follow-up. The difference in annual change in UPDRS-III score between the highest tertile and lowest tertile of plasma ApoA1 was 0.19 (95% CI: -3.75, 4.11). In addition, longitudinal measures of plasma ApoA1 and HDL were not significantly associated with UPDRS-III score, using linear mixed-effects models.
Surprisingly, however, we observed a significant increase in both ApoA1 and HDL measures over time (two-tailed p<0.001 for ApoA1, Fig. 2b; two-tailed p<0.001 for HDL, Fig. 2c). In PD subjects, ApoA1 and HDL measures appeared to initially increase in the first six months, but then remained relatively constant from 6-12 months. In contrast, in NC subjects, ApoA1 and HDL plasma levels continued to increase from 6-12 months. Indeed, the difference in change in ApoA1 values over time, comparing PD and NC subjects, trended towards significance (two-tailed p=0.065). In addition, in contrast to our previous finding of baseline higher ApoA1 plasma levels in NC compared to PD in a cohort of mid-stage PD subjects, we find here no significant differences in ApoA1 levels comparing NC vs. PD at baseline (Supplementary Fig. 4), 6 months, or 12 months, although at 12 months, the two groups are starting to separate.
Given the surprising nature of this apparent increase in ApoA1 levels, we investigated the possibility that ApoA1 values may vary according to time a given sample spends in storage. Since the onset of our ApoA1 studies, we have always included a reference sample with each ApoA1 assay. Because all aliquots of this reference sample were created from a single pool of plasma at the same time, but measured at different timepoints that spanned 3-21 months spent in storage, any differences in values obtained would reflect the effects of assay noise or time spent in storage. As shown in Supplementary Fig. 5, we found no trend with increasing time in storage.
Finally, 73/154 PD patients initiated therapy for motor symptoms over the course of the 12-month follow-up. Initiating symptomatic therapy can be perceived as a marker of motor disease severity. Baseline ApoA1 or HDL values, however, did not predict initiation of therapy in logistic regression or Cox proportional hazards models, adjusting for age at plasma sampling and sex.
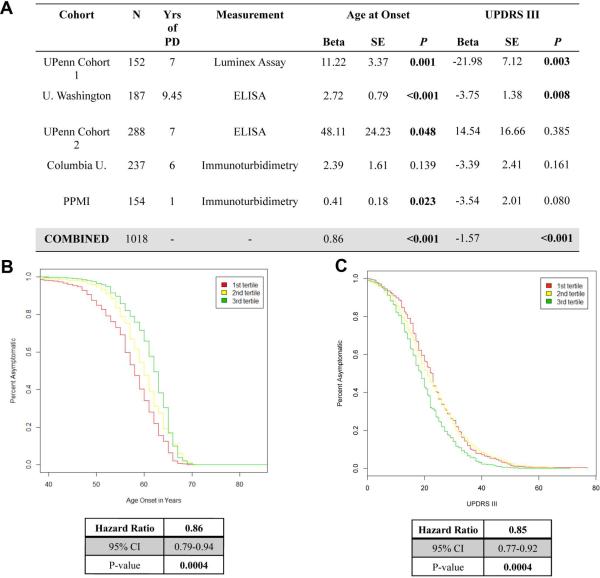
Meta-analysis of five PD cohorts validates association of lower plasma ApoA1 levels with earlier age at PD onset and greater motor severity
Starting with our initial report nominating plasma ApoA1 as a PD biomarker from an unbiased screen of ~100 proteins, we have evaluated plasma ApoA1 levels in 1018 PD patients from five different, extensively characterized cohorts.8,9,12 Moreover, across these studies, plasma ApoA1 has been measured using three different technical platforms.
We meta-analyzed the association of lower plasma ApoA1 levels with (1) earlier age at PD onset, and (2) higher UPDRS-III score in this combined dataset. Because two earlier technical platforms yielded relative ApoA1 values rather than absolute ApoA1 values, we standardized these values within each cohort, and then used the standardized ApoA1 values in our analyses.
In this combined dataset, we found a significant correlation between plasma ApoA1 levels and age at PD onset, with lower plasma ApoA1 associated with earlier age at onset (Fig. 3a, two-tailed p<0.001 for linear regression model, adjusting for age at plasma sampling and sex). Moreover, we found a significant association between plasma ApoA1 levels and UPDRS-III score, with lower levels of plasma ApoA1 associated with poorer motor performance (Fig. 3c, two-tailed p<0.001, adjusting for age at plasma sampling and sex).
Figure 3. Meta-analysis of >1000 PD patients confirms association of plasma ApoA1 with age at PD onset and motor severity.
Plasma ApoA1 significantly associates with age at onset and motor severity (UPDRS III motor score) in a meta-analysis of five cohorts of >1000 PD patients, using linear models adjusted for age at plasma sampling and sex (A). Cox proportional hazard (CPH) models confirm the association of low plasma ApoA1 levels (red) with earlier age at onset (B) and worse (higher) UPDRS-III score (C), compared with medium (yellow) or high (green) levels of plasma ApoA1. Hazard ratio is always expressed in terms of the protective effect of increased ApoA1 expression for one tertile compared to the next. UPDRS=Unified Parkinson's disease Rating Scale.
We further explored this combined dataset using Cox proportional hazard (CPH) models, comparing tertiles of standardized ApoA1 values, adjusted for age at plasma sampling and sex. As shown in Fig. 3b, lower levels of plasma ApoA1 were again associated with earlier age at PD onset (two-tailed p<0.001; HR 0.86, 95% CI 0.79-0.94 for each tertile increase in ApoA1 plasma levels). Additionally, lower levels of plasma ApoA1 were associated with worse motor performance (higher score on the UPDRS-III) as well (Fig. 3d; two-tailed p<0.001; HR 0.85, 95% CI 0.77-0.92 for each tertile change in ApoA1 plasma levels).
DISCUSSION
In the present study, we report that (1) lower baseline plasma ApoA1 levels associate with an earlier age at PD onset in early-stage, drug-naïve PD patients, (2) lower baseline plasma ApoA1 levels trend towards association with higher UPDRS-III score in early-stage, drug-naïve PD patients, (3) meta-analysis of >1000 PD patients demonstrates a highly significant association of plasma ApoA1 with age at PD onset and motor severity, and (4) over a 12-month period, plasma ApoA1 levels do not predict disease course longitudinally.
Our primary goal in the present study was to understand the robustness of our previously-reported results to various sources of clinical noise. Thus, we first replicated our findings from an unbiased screen of ~100 plasma proteins in a cohort of mid-stage PD patients,8 reporting that lower plasma ApoA1 levels associate with earlier age at PD onset and trend towards association with more severe PD in the PPMI cohort. As the PPMI cohort is an international cohort and the current study included 19 clinical sites, our finding suggests that the association of plasma ApoA1 levels with various indicators of dopaminergic system integrity is robust to many potential sources of clinical noise. Moreover, because the PPMI cohort is untreated at baseline, the possibility that the previously-observed signal was due to effects of dopaminergic therapy can be excluded. We note that although we have chosen to report two-tailed p-values throughout this study, one-tailed p-values may be just as appropriate in a replication study. If we were to use one-tailed p-values, the association between lower plasma ApoA1 levels and greater motor severity (higher UPDRS-III score) would achieve strict statistical significance.
Further adding to our confidence in the robustness of the association between lower plasma ApoA1 levels and earlier age at PD onset and higher UPDRS-III scores is the meta-analysis reported here. Across >1000 PD patients in five different cohorts, at different stages of disease, assayed on three different technical platforms, the ApoA1 association persists and in fact strengthens. While it is possible, and indeed likely, that there are additional sources of pre-analytical variability beyond the ones formally considered in this study, it is highly unlikely that in multiple cohorts, across multiple testing platforms, the directions of confounding remain the same, and could thus result in a false positive result. Rather, it is more likely that the many sources of potential noise may be obscuring the true magnitude of the association between ApoA1 levels and age at PD onset and motor severity.
Most PD biomarker candidates have yet to be robustly characterized across multiple testing platforms and independent clinical cohorts, with three important exceptions. First, levels of total alpha-synuclein (ASyn) in the cerebrospinal fluid (CSF) have been evaluated by multiple groups, on multiple testing platforms.14-17 Unfortunately, results are conflicting with respect to whether CSF ASyn levels differ in PD vs. controls,15,16,18-20 and even in cases where PD patients are reported to differ in CSF ASyn levels, there is a great degree of overlap with controls.21,22 Second, CSF beta-amyloid 1-42 (Aβ42) and CSF tau have been evaluated in PPMI PD patients and controls, where lower levels of CSF Aβ42 and CSF phosphorylated tau were found to associate with PD diagnosis.15 Finally, lower levels of serum or plasma uric acid and urate have been demonstrated across many different cohorts to associate with increased risk of PD.23-28 The current study thus places plasma ApoA1 among just a handful of potential PD biomarkers successfully replicated in many different contexts.
Having demonstrated the robustness of the association between plasma ApoA1 levels and age at PD onset and PD severity, several scenarios are compatible with our data. First, higher ApoA1 levels may be protective against more rapid dopaminergic neurodegeneration, thus delaying clinical onset and mitigating disease severity. Second, dopaminergic neurodegeneration itself may affect plasma ApoA1 levels though a mechanism that remains to be determined. Third, both decreases in plasma ApoA1 and dopaminergic neurodegeneration may be driven by an external factor. To definitively discriminate among these possibilities, experiments manipulating ApoA1 levels may be needed.
In addition to the cross-sectional results discussed above, the PPMI cohort offered for the first time a chance to longitudinally evaluate the association of plasma ApoA1 levels with PD severity. Over a 12-month follow-up period, no significant association was observed. This could reflect underlying biology – examples of risk biomarkers or diagnostic biomarkers that do not correlate with or predict disease progression certainly exist. However, the longest period of follow-up in this study was 12 months, and the PPMI subjects have early PD, so it is also possible that longer follow-up is needed to observe a longitudinal signal. Indeed, the overall difference in UPDRS-III score over the course of 12 months was only 20% (~4 points), and the wide confidence interval comparing rate of change in UPDRS-III in high-ApoA1 vs. low-ApoA1 PPMI PD patients suggests that our signal-to-noise ratio is too low in this cohort at this time.
In summary, we provide here further evidence that lower plasma ApoA1 levels associate with two measures of poorer dopaminergic system integrity – earlier age at PD onset and greater motor severity. This signal replicates across many different clinical cohorts and many technical platforms; we note additionally that this is the first report evaluating a plasma-based biomarker in the PPMI cohort. It will be critical to continue following this cohort to further evaluate the relationship between plasma ApoA1 levels and clinical status as PD continues to progress in these subjects. Indeed, while the overlap between ApoA1 levels in PD vs. control individuals suggests that ApoA1 will not be a good diagnostic biomarker for PD, the significance of the observed association stems from the fact that ApoA1 levels are modifiable, with existing, safe drugs. Thus, further investigation into the mechanism of the ApoA1 effect, and evaluation of whether the pharmacological elevation of ApoA1 levels might hold therapeutic promise in PD, is warranted.
Supplementary Material
ACKNOWLEDGEMENTS
Data used in the preparation of this article were obtained from the Parkinson's Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org. PPMI-a public-private partnership-is funded by the Michael J. Fox Foundation for Parkinson's Research and funding partners, including Abbvie, Avid Radiopharmaceuticals, Biogen Idec, Bristol-Meyers Squibb, Covance, GE Healthcare, Genentech, GlaxoSmithKline, Eli Lilly and Company, Lundbeck, Merck, Meso Scale Discovery, Pfizer Inc., Piramal Imaging, Roche CNS group, and UCB. Dr. Chen-Plotkin receives research support from the NIH (P50 NS053488, U01 NS082134, R01 NS082265), the Doris Duke Charitable Foundation, the Burroughs Wellcome Fund, and the Benaroya Fund. Dr. Swanson is supported by the Brody Family Trust Fund. Dr. Alcalay is supported by the NIH (NINDS: K02 NS080915), the Parkinson's Disease Foundation, the Michael J. Fox Foundation and the Smart Foundation. He received travel support from Sanofi/Genzyme. Source of CUMC funding: NIH (NINDS: K02 NS080915; and UL1 TR000040, formerly the National Center for Research Resources, Grant Number UL1 RR024156) and the Parkinson's Disease Foundation. We thank Travis L. Unger and Theodore Mifflin for technical assistance.
Funding Agencies: Data used in the preparation of this article were obtained from the Parkinson's Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org. PPMI, a public-private partnership, is funded by the Michael J. Fox Foundation for Parkinson's Research and funding partners, including Abbvie, Avid Radiopharmaceuticals, Biogen Idec, Bristol-Meyers Squibb, Covance, GE Healthcare, Genentech, GlaxoSmithKline, Eli Lilly and Company, Lundbeck, Merck, Meso Scale Discovery, Pfizer Inc., Piramal Imaging, Roche CNS group, and UCB. Dr. Chen-Plotkin receives research support from the NIH (P50 NS053488, U01 NS082134, R01 NS082265), the Doris Duke Charitable Foundation, the Burroughs Wellcome Fund, and the Benaroya Fund. Dr. Swanson is supported by the Brody Family Trust Fund. Dr. Alcalay is supported by the NIH (NINDS: K02 NS080915), the Parkinson's Disease Foundation, the Michael J. Fox Foundation and the Smart Foundation. He received travel support from Sanofi/Genzyme. Source of CUMC funding: NIH (NINDS: K02 NS080915; and UL1 TR000040, formerly the National Center for Research Resources, Grant Number UL1 RR024156) and the Parkinson's Disease Foundation.
Author Roles: CRS and ACP had full access to the data in this study and take responsibility for the integrity of the data and accuracy of the data analysis. Study concept and design: CRS and ACP
Acquisition, analysis, and interpretation of the data: CRS and YB performed experiments. CRS, YB, SX, and ACP analyzed data. LC and RA recruited patients, performed neurological examinations, collected and recorded clinical data, and assisted in interpretation of PPMI data.
Statistical analysis: CRS, SX, ACP
Drafting of manuscript: CRS and ACP drafted the manuscript
Critical Revision of manuscript for important intellectual content: YB, SX, LC, RA; All authors reviewed and edited the manuscript for accuracy and content.
Study Supervision: ACP
Footnotes
Relevant conflicts of interest/financial disclosures: Nothing to disclose
Conflict of Interest Disclosures: No disclosures were reported relevant to the research in this study.
References
- 1.Sherer T. Biomarkers for Parkinson's Disease. Sci Transl Med. 2011;3:79ps14. doi: 10.1126/scitranslmed.3002488. [DOI] [PubMed] [Google Scholar]
- 2.Olanow CW, Kieburtz K, Schapira AH. Why have we failed to achieve neuroprotection in Parkinson's disease? Ann Neurol. 2008;64(Suppl 2):S101–10. doi: 10.1002/ana.21461. [DOI] [PubMed] [Google Scholar]
- 3.Rascol O, Lozano A, Stern M, Poewe W. Milestones in Parkinson's disease therapeutics. Mov Disord. 2011;26:1072–1082. doi: 10.1002/mds.23714. [DOI] [PubMed] [Google Scholar]
- 4.Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 5.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 7.Schonfeld G, Pfleger B. The structure of human high density lipoprotein and the levels of apolipoprotein A-I in plasma as determined by radioimmunoassay. J Clin Invest. 1974;54:236–246. doi: 10.1172/JCI107758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiang JK, Wong YC, Siderowf A, et al. Plasma apolipoprotein A1 as a biomarker for parkinson's disease. Ann Neurol. 2013 doi: 10.1002/ana.23872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swanson CR, Li K, Unger TL, et al. Lower plasma ApoA1 levels are found in Parkinson's disease and associate with APOA1 genotype. Mov Disord. doi: 10.1002/mds.26022. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parkinson Progression Marker Initiative The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol. 2011;95:629–635. doi: 10.1016/j.pneurobio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 12.Berlyand Y, Alcalay RN, Swanson CR, et al. Statin Use, Apolipoprotein A1, and Parkinson's Disease [abstract]. Neurology. 2014;82(Suppl P2):055. [Google Scholar]
- 13.Jungner I, Marcovina SM, Walldius G, Holme I, Kolar W, Steiner E. Apolipoprotein B and A-I values in 147576 Swedish males and females, standardized according to the World Health Organization-International Federation of Clinical Chemistry First International Reference Materials. Clin Chem. 1998;44:1641–1649. [PubMed] [Google Scholar]
- 14.Tokuda T, Salem SA, Allsop D, et al. Decreased alpha-synuclein in cerebrospinal fluid of aged individuals and subjects with Parkinson's disease. Biochem Biophys Res Commun. 2006;349:162–166. doi: 10.1016/j.bbrc.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 15.Kang JH, Irwin DJ, Chen-Plotkin AS, et al. Association of cerebrospinal fluid beta-amyloid 1-42, T-tau, P-tau181, and alpha-synuclein levels with clinical features of drug-naive patients with early Parkinson disease. JAMA Neurol. 2013;70:1277–1287. doi: 10.1001/jamaneurol.2013.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mollenhauer B, Cullen V, Kahn I, et al. Direct quantification of CSF alpha-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp Neurol. 2008;213:315–325. doi: 10.1016/j.expneurol.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Hong Z, Shi M, Chung KA, et al. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson's disease. Brain. 2010;133:713–726. doi: 10.1093/brain/awq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foulds PG, Yokota O, Thurston A, et al. Post mortem cerebrospinal fluid alpha-synuclein levels are raised in multiple system atrophy and distinguish this from the other alpha-synucleinopathies, Parkinson's disease and Dementia with Lewy bodies. Neurobiol Dis. 2012;45:188–195. doi: 10.1016/j.nbd.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park MJ, Cheon SM, Bae HR, Kim SH, Kim JW. Elevated levels of alpha-synuclein oligomer in the cerebrospinal fluid of drug-naive patients with Parkinson's disease. J Clin Neurol. 2011;7:215–222. doi: 10.3988/jcn.2011.7.4.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Dijk KD, Bidinosti M, Weiss A, Raijmakers P, Berendse HW, van de Berg WD. Reduced alpha-synuclein levels in cerebrospinal fluid in Parkinson's disease are unrelated to clinical and imaging measures of disease severity. Eur J Neurol. 2014;21:388–394. doi: 10.1111/ene.12176. [DOI] [PubMed] [Google Scholar]
- 21.Mollenhauer B, Locascio JJ, Schulz-Schaeffer W, Sixel-Doring F, Trenkwalder C, Schlossmacher MG. alpha-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. Lancet Neurol. 2011;10:230–240. doi: 10.1016/S1474-4422(11)70014-X. [DOI] [PubMed] [Google Scholar]
- 22.Shi M, Bradner J, Hancock AM, et al. Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann Neurol. 2011;69:570–580. doi: 10.1002/ana.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis JW, Grandinetti A, Waslien CI, Ross GW, White LR, Morens DM. Observations on serum uric acid levels and the risk of idiopathic Parkinson's disease. Am J Epidemiol. 1996;144:480–484. doi: 10.1093/oxfordjournals.aje.a008954. [DOI] [PubMed] [Google Scholar]
- 24.Weisskopf MG, O'Reilly E, Chen H, Schwarzschild MA, Ascherio A. Plasma urate and risk of Parkinson's disease. Am J Epidemiol. 2007;166:561–567. doi: 10.1093/aje/kwm127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwarzschild MA, Schwid SR, Marek K, et al. Serum urate as a predictor of clinical and radiographic progression in Parkinson disease. Arch Neurol. 2008;65:716–723. doi: 10.1001/archneur.2008.65.6.nct70003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum uric acid levels and the risk of Parkinson disease. Ann Neurol. 2005;58:797–800. doi: 10.1002/ana.20663. [DOI] [PubMed] [Google Scholar]
- 27.Shen L, Ji HF. Low uric acid levels in patients with Parkinson's disease: evidence from meta-analysis. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2013-003620. e003620-2013-003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winquist A, Steenland K, Shankar A. Higher serum uric acid associated with decreased Parkinson's disease prevalence in a large community-based survey. Mov Disord. 2010;25:932–936. doi: 10.1002/mds.23070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.