Abstract
Purpose
PD1/PD-L1 signaling promotes tumor growth while inhibiting effector cell-mediated anti-tumor immune responses. Here we assessed the impact of single and dual blockade of PD1/PD-L1, alone or in combination with lenalidomide, on accessory and immune cell function as well as multiple myeloma (MM) cell growth in the BM milieu.
Experimental Design
Surface expression of PD1 on immune effector cells, and PD-L1 expression on CD138+MM cells and myeloid derived suppressor cells (MDSC) were determined on tumor cells from newly diagnosed (ND)-MM and relapsed/refractory (RR)-MM BM versus healthy donor (HD). We defined the impact of single and dual blockade of PD1/PD-L1, alone and with lenalidomide, on autologous anti-MM immune response and tumor cell growth.
Results
Both ND and RR patient MM cells have increased PD-L1 mRNA and surface expression compared to HD. There is also a significant increase in PD1 expression on effector cells in MM. Importantly, PD1/PD-L1-blockade abrogates BM-stroma cell (BMSC)-induced MM growth, and combined blockade of PD1/PD-L1 with lenalidomide further inhibits BMSC-induced tumor growth. These effects are associated with induction of intracellular expression of IFNγ and Granzyme-B in effector cells. Importantly, PD-L1 expression in MM is higher on MDSC than on antigen presenting cells, and PD1/PD-L1-blockade inhibits MDSC-mediated MM growth. Finally, lenalidomide with PD1/PD-L1-blockade inhibits MDSC-mediated immune suppression.
Conclusion
Our data therefore demonstrates that checkpoint signaling plays an important role in providing the tumor-promoting, immune-suppressive microenvironment in MM, and that PD1/PD-L1-blockade induces anti-MM immune response that can be enhanced by lenalidomide, providing the framework for clinical evaluation of combination therapy.
Keywords: PD-1/PD-L1, lenalidomide, MDSC, multiple myeloma, immunotherapy
Introduction
Multiple Myeloma (MM) is a clonal B cell malignancy associated with a monoclonal (M) protein in blood and/or urine, bone lesions, and immunodeficiency. It usually evolves from monoclonal gammopathy of undetermined significance (MGUS), with low levels of plasmacytosis and M protein without osteolytic lesions, anemia, hypercalcemia and renal failure.(1) MM is characterized by genetic signatures including frequent translocations into the immunoglobulin heavy chain switch region (IgH), oncogenes, and abnormal chromosome number.(2, 3) Most patients with translocations have non-hyperdiploid chromosome number (NHMM), while those patients lacking IgH translocations have hyperdiploid chromosome number (HMM) with trisomies of chromosomes 3,5,7,9,11,15,19 and 21. Importantly, patients with hyperdiploid MM have a better outcome with prolonged survival.(4, 5)
Advances in MM biology have established that the bidirectional interaction between MM cells, bone marrow stroma cells (BMSC), extracellular matrix, and accessory cells can induce autocrine and paracrine signaling that regulates tumor development and growth on the one hand, while transforming the bone marrow microenvironment into an immune-suppressive milieu on the other.(6, 7) We and others have extensively studied the impact of the interaction between BMSC and MM cells on pathogenesis and cell adhesion mediated-drug resistance (CAM-DR) in order to identify and validate new targeted therapeutics.(1) Immunomodulatory drugs (IMiDs) thalidomide and lenalidomide, and proteasome inhibitor bortezomib are novel agents which target the tumor cell in its microenvironment and can overcome CAM-DR; they have been rapidly integrated into MM treatment, resulting in at least a 2–3 fold prolongation of median survival.(8–10) Even though these novel drugs have transformed the treatment paradigm and patient outcome, most MM relapses due to minimal residual disease (MRD) and drug resistance.(11) Generation of more effective therapeutic strategies may therefore not only require targeting the tumor and stroma, but also overcoming blockade of anti-tumor immune response. Tumor associated immune suppressor cells such as regulatory T cells (Treg) and myeloid derived suppressor cells (MDSC) can effectively block anti-tumor immune responses, representing an important obstacle for immunotherapy. We have recently assessed the presence, frequency, and functional characteristics of MDSC in patients with newly diagnosed (ND-MM), responsive MM, and relapsed, refractory MM (RR-MM) compared to healthy donor (HD), and identified an increased MDSC population (CD11b+CD14−HLA-DR−/lowCD33+CD15+) with tumor-promoting and immune-suppressive activity in both the peripheral blood (PB) and bone marrow (BM) of MM patients. Moreover, we have shown that lenalidomide does not target MDSC in the BM milieu.(12)
Programmed cell death-1 (PD1, CD279), a member of the CD28 receptor family, and its ligands either PD-L1 (B7-H1, CD274) or PD-L2 (B7-DC, CD273), play a fundamental role in tumor immune escape by inhibiting immune effector functions. PD1 gene is encoded on chromosome 2, and PD-L1 gene is on chromosome 9. PD1 expression is induced on antigen activated T cells and exhausted T cells and B cells; PD-L1 is mainly expressed by antigen presenting cells (APCs) and various non-hematopoietic cells; and PD-L2 is found on hematopoietic cells including dendritic cells and macrophages.(13) Recent studies in solid tumors have demonstrated that expression of PD-L1 is significantly increased and associated with progressive disease in lung cancer, breast cancer, renal cell cancer, colorectal cancer, gastric cancer, esophageal cancer, and pancreatic cancer. (7, 8, 14–21) Most importantly, remarkable responses have been observed to PD1 blockade in malignant melanoma, leading to recent FDA approval of PD1 monoclonal antibody therapies. To date, increased PD-L1 expression has been shown in MM cells compared to HD plasma cells (13, 22–26), and increased PD1 expression has been demonstrated on CD4T cells in MM.(11, 13, 22, 24, 25, 27) Since PD1/PD-L1 signaling promotes tumor growth while inhibiting effector cell-mediated anti-tumor immune response, we here assessed the impact of single and dual blockade of PD1/PD-L1 signaling, alone or in combination with lenalidomide, on accessory (MDSC, BMSC) and immune cell (CD4T cells, CD8T cells, NK cells, NKT cells and Monocytes/Macrophages) function, as well as MM cell growth, in the BM milieu. Our studies provided the framework for targeting PD1 and PD-L1 in combination with lenalidomide to inhibit tumor cell growth and restore immune function in MM.
Materials and Methods
Cell isolation
Heparinized venous blood samples and/or aspirates of BM from patients with ND-MM (n=6) or RR-MM (n=10) and healthy donors (HD, n=10) were obtained after written informed consent per the Declaration of Helsinki and approval by the Institutional Review Board of the Dana-Farber Cancer Institute.
Cell lines
MM1.S, U266 and H929 MM cells were purchased from American Type Culture Collection (ATCC, Manassas, VA); plasma cell leukemia (PCL) cells OPM1 and OPM2 were provided by Dr. Edward Thompson (University of Texas Medical Branch, Galveston, TX). Cell lines have been tested and authenticated by STR DNA fingerprinting analysis (Molecular Diagnostic Laboratory, DFCI), and used within three months after thawing. All cell lines were maintained in RPMI1640 (Bio Whittaker) containing 10% FBS, 100Units/ml penicillin, and 100ug/ml streptomycin (Life Technologies).
Reagents and compounds
Functional grade PD1 and PD-L1 blocking antibodies, anti-human PD1 (clone J116) and anti-human PD-L1 (clone MIH1), were obtained from eBiosiences (San Diego, CA). Immunomodulatory drug lenalidomide (10mM) was dissolved in DMSO and stored at −20 °C. Anti-CD3 and anti-CD28 MAbs (10ug/ml) (Becton Dickinson Biosciences, San Jose, CA) were used to stimulate cells.
Cell phenotyping
Cell surface expression of PD1 (CD279) on CD4+T cells, CD8+T cells, CD56+ NK cells and CD3+CD8+CD56+NKT cells, and PD-L1 (CD274) on CD138+ MM cells, CD14+ monocytes/macrophages, CD11b+CD14+HLA-DR+ antigen presenting cells (APCs), CD11b+CD14−HLA-DR−/lowCD33+CD15+ nMDSC, and CD11b+CD14+HLA-DR−/low mMDSC was determined on PBMCs or BMMCs from MM patients or healthy donors by multiparameter flow cytometry analysis. Cells were stained with CD11b APCCy7, CD14 Pacific blue, HLA-DR PECy7, CD33 PECy5, and CD15 FITC conjugated MAbs (BD Biosciences, San Jose, CA) for MDSC; as well as CD138 APC for MM cells, and CD4 PE-Cy5, CD8 PE-Cy7, CD56 FITC for effector cells (BD Biosciences, San Jose, CA).
Intracellular cytokine analysis
Autologous effector cells and CD138+ MM cells or MDSCs were cocultured in the absence or presence of anti-human PD1 (10ug/ml) and anti-human PD-L1 blocking Ab (10ug/ml) alone or in combination with or without addition of lenalidomide (1uM) for 16 hours to 4 days. 5 ug/ml of brefeldin-A solution (eBiosciences, San Diego, CA) was added during the last 2 hours of incubation. Cells were then fixed in 4% paraformaldehyde-PBS and stained with PE-conjugated interferon-gamma (IFNγ) and FITC-conjugated granzyme-B (Gzm-B) MAbs (Becton Dickinson Biosciences, San Jose, CA) in permeabilization buffer (0.5% saponin-PBS). Intracytoplasmic cytokines in T cells, NK cells, NKT cells and monocytes/macrophages was detected by flow cytometry using BD-LSR Fortessa (Becton Dickinson Biosciences, San Jose, CA) and analyzed using Flowjo software (TreeStar, Ashland, OR).
Cell proliferation assay
CD138+MM cells were isolated by FACSAria IIu sorter, labeled with CFSE, and cultured either alone or with BMSC generated from BM aspirates of MM patients, or autologous MDSC, with or without anti-human PD1 and anti-human PD-L1 Ab alone or in combination for 24 hours to 4 days (2:1 ratio). MM cell growth was measured following propidium iodide addition (PI, 1ug/ml) by CFSE/PI flow cytometry analysis using BD-LSR Fortessa (Becton Dickinson Biosciences, San Jose, CA), and data were analyzed using Flowjo software (TreeStar, Ashland, OR).
MDSC and CD3+T cells were isolated from PB or BM aspirates of MM patients by FACS-sorting, and MDSC were cocultured for 4 days with CFSE labeled autologous T cells (MDSC:T cell ratio 1:4) in the absence or presence of checkpoint blockade antibodies with lenalidomide (1uM).
Cytotoxicity Assay
CD138+MM cells and autologous effector cells (CD3T cells and NK cells) were isolated by FACS-sorting from MM bone marrow. CD138+MM cells were labeled with CFSE and cultured with each effector cell population in the absence or presence of anti-PD1 or anti-PD-L1, alone or in combination, for 4 hours. PI (1ug/ml) was added before analysis. Apoptotic/dead MM cells were identified as CD138+CFSE+PI+ by multi-parameter flow cytometry analysis using BD-LSR Fortessa (Becton Dickinson Biosciences, San Jose, CA), and data were analyzed using Flowjo software (TreeStar, Ashland, OR).
To determine the effect of checkpoint blockade with lenalidomide on anti-MM cytotoxicity in the bone marrow, bone marrow mononuclear cells (BMMC) from patients with RR-MM were labeled with CFSE and cultured in the absence or presence of anti-PD1 (10ug/ml) and anti-PD-L1 (10ug/ml), alone or in combination, or with the addition of lenalidomide (1uM). PI (1ug/ml) was added before analysis. Immune complex with Apoptotic/dead MM cells were identified as CD138+CFSE+PI+ by multi-parameter flow cytometry analysis using BD-LSR Fortessa (Becton Dickinson Biosciences, San Jose, CA), and data were analyzed using Flowjo software (TreeStar, Ashland, OR). Minimum of 10,000 live events per sample were acquired using multi-parameter flow cytometry.
Statistics
All in vitro experiments were performed in triplicate and repeated at least three times. Statistical significance was determined by non-parametric T test, two-tailed distribution, with minimal significance level *p<0.05. Statistical analysis was performed using GraphPad Prism (v6) software.
Results
Increased PD-L1 gene expression in MM
We first assessed expression and frequency of PD-L1 gene in the BM bone marrow CD138+MM cells from patients with MM at diagnosis (ND-MM) (Intergroupe Francophone du Myelome (IFM), n=170) versus normal bone marrow plasma cells (HD, n=6) (Supplementary Figure S1A). Exon array profiling analysis of CD138+ plasma cells demonstrated that there was a significant increase in PD-L1 mRNA expression in MM cells from patients with ND-MM compared to HD (p=0.0064). Furthermore, 45% patients had increased copy number of PD-L1 gene in their tumor clone (Supplementary Figure S1B). Expression and copy number of PD-L1 gene was significantly correlated in clonal MM cells (Pearson's correlation, R2=0.37 and p=5.5e-07) (Supplementary Figure S1C). Since PD-L1 gene is encoded on chromosome 9, we next compared PD-L1 gene expression among normal, hyperdiploid MM (HMM) and non-hyperdiploid MM (NHMM) groups. PD-L1 gene expression was significantly upregulated in NHMM (p=0.03), and even more highly expressed in HMM (p=0.0007), compared to normal plasma cells. In addition, there was a significant difference between HMM and NHMM subgroups (p=1.75e-09) (Supplementary Figure S1D). In contrast, there was no significant association between PD-L1 expression and chromosomal abnormalities in MM including t(4; 14), del(17p), del (1q), t(11; 14), del(13), and t(14; 16) (data not shown).
Increased frequency of PD1 and PD-L1 surface expression in MM bone marrow microenvironment
We next assessed surface expression of PD-L1 by multi-parameter flow cytometry in BM CD138+MM cells from patients with ND-MM (n=6), RR-MM (n=10), and normal plasma cells (n=3); as well as in a panel of MM and plasma cell leukemia (PCL) cell lines (MM1.S, OPM1, OPM2, U266 and H929) (Figure 1). PD-L1 surface expression was significantly increased in MM cells from patients with ND-MM (mean=16.5±6.5) and RR-MM (mean=26.6±8.2) compared to normal plasma cells (mean=4.7±1.8) (p<0.05) (Figure 1A, left graph and lower right panel), and was detectable only in MM1.S, OPM2 and H929 MM cell lines (Figure 1A, top right panel). There was no significant expression of PD-L2 on MM cells (data not shown).
Figure 1. Increased frequency of PD1 and PD-L1 expression in MM bone marrow microenvironment.
Cell surface expression of PD1 (CD279) and PD-L1 (CD274) is shown on CD138+MM cells, immune effector cells, and immune-suppressive MDSC in ND-MM and RR-MM. A. Cell surface expression of PD-L1 is quantitated on patient MM cells obtained from patients with ND-MM (n=6), RR-MM (n=10), and compared to healthy donor plasma cells (HD, n=3). Data represent percentage of PD-L1 expressing CD138+MM cells. Representative histogram plots of PD-L1 expression (red) relative to control (gray) is shown on a gated population of CD138+ plasma cells. Top panel demonstrates PD-L1 expression on MM cell lines (MM1.S, OPM2, H929), and lower panel represents PD-L1 expression on BM CD138+ plasma cells from patients with ND-MM and RR-MM, as well as HD-BM. B. Cell surface expression of PD1 is quantitated on immune effector cells (CD4T cells, CD8T cells, NK cells and NKT cells) from patients with ND-MM (n=6) and RR-MM (n=10) compared to healthy donors (HD, n=10). Data represent percentage of PD1 coexpressing CD4T cells (top left graph), CD8T cells (top right graph), NK cells (lower left graph), and NKT cells (lower right graph) in BM of patients with ND-MM and RR-MM compared to HD-PBMC. Representative histogram plots of PD1 expression (blue) versus control (grey) on BM immune effector cells: CD4T cells, CD8T cells, NK cells, and NKT cells with gating strategy is shown by multi-parameter dot plots (right panel). C. The frequency of PD-L1 cell surface expression is shown in monocytic MDSC (mMDSC) and neutrophilic MDSC (nMDSC) compared to antigen presenting cells (APC) from BM of patients with ND-MM (left graph) and RR-MM (right graph). Representative flow cytometry histogram of PD-L1 expression (red) versus control (grey) on MDSC in healthy donor and RR-MM bone marrow (right panel). With gating strategy for mMDSC and nMDSc is shown by multi-parameter dot plots (right panel).
We next evaluated surface expression of PD1 in immune effector cells in both BM and peripheral blood of patients with active MM using multi-parameter flow cytometry (Figure 1B). Even though CD4T cells from several patients with ND-MM and RR-MM expressed high levels of PD1, there was no significant difference in PD1 expression on CD4T cells in MM BM, either ND-MM (mean=10.8±3.4) or RR-MM (mean=12±2.8), compared to normal CD4T cells (mean=10.2±1.3) (Figure 1B, top left graph). In contrast, there was a significant increase in expression of PD1 on CD8T cells in ND-MM (mean=10.4±1.7) and RR-MM (mean=9.4±2.3) compared to normal CD8T cells (mean=6.1±0.6) (Figure 1B, top right graph). Likewise, PD1 expression was higher in BM NK cells in patients with ND-MM (mean=7.9±1.4) and RR-MM (mean=14.2±3.2) compared to normal NK cells (mean=1.6±0.2) (Figure 1B, lower left graph). In contrast, PD1 expression was significantly lower in BM CD8+CD56+NKT cells of ND-MM (mean=14.3±2.6) and RR-MM (mean=14.1±2.2) than normal NKT cells (mean=24.7±1.1) (Figure 1B, lower right graph). Increased expression of PD1 is demonstrated by histogram of PD1 coexpressing effector cells (CD8T cells and NK) in RR-MM (Figure 1B, right panel). Similar changes in PD1 expression were observed in effector cells in the PBMC of ND-MM and RR-MM (data not shown).
We have recently characterized the neutrophil-like CD11b+CD14−HLA-DRlow/−CD33+CD15+ MDSC population with tumor-promoting and immune-suppressive activity in the MM BM.(12) To determine whether MDSC-induced MM cell growth and immune effector cell suppression is mediated by PD1/PD-L1 pathway, we next assessed cell surface expression and frequency of PD-L1 in the BM MDSC of patients with ND-MM and RR-MM. Since MDSC are either absent or present at a very low numbers in healthy donors, we first evaluated the frequency of PD-L1 expressing within myeloid cell subpopulations in ND-MM and RR-MM (Figure 1C). Myeloid cell subpopulations in each MM patient BM were phenotypically characterized as CD11b+CD14+HLA-DR+ antigen presenting cells (APC), CD11b+CD14+HLA-DRlow/−monocytic myeloid derived suppressor cells (mMDSC), and CD11b+CD14−HLA-DRlow/−CD33+CD15+ neutrophilic myeloid derived suppressor cells (nMDSC). Multi-parameter flow cytometry analysis showed that PD-L1 expression was increased in nMDSC (mean=37.8±18) compared to APCs (mean=21.5±7.9) in the BM of patients with ND-MM (Figure 1C, left graph). Of note, there was a more significant increase in PD-L1 in mMDSC (mean=33.9±10) and nMDSC (mean=40.1±11.5) compared to APC (mean=21.8±6) in the BM of patients with RR-MM. PD-L1 expressing mMDSC increased in RR-MM (mean=33.9±10) versus ND-MM (mean=22±9.4), but there was no significant change in PD-L1 expressing nMDSC with disease progression (Figure 1C right graph). Shown is a representative histogram of PD-L1 expression in mMDSC and nMDSC of RR-MM and HD (Figure 1C, right panel). Thus, PD-L1 in MM cells and MDSC, along with PD1 in immune effector (CD8T cells and NK) cells, were increased in BM of ND-MM and RR-MM.
Checkpoint blockade overcomes bone marrow stroma-mediated MM growth
We next investigated whether PD1/PD-L1 signaling plays a role in BMSC-mediated MM cell growth in cocultures of MM cells and BMSC from patients with RR-MM (Figure 2). BMSC were generated from MM BM and cultured either with MM cell lines or autologous CD138+MM cells with or without anti-PD1 and anti-PD-L1, alone or in combination. MM cell viability/growth was measured by CFSE-flow cytometry analysis or 3H-thymidine cell proliferation assays. We first determined whether BMSC affects expression of PD-L1 on MM cells. BMSC significantly induced PD-L1 expression on H929 MM cells (>2 fold increase), as shown by multi-parameter flow cytometry analysis (Figure 2A). The regulatory role of PD1/PD-L1 in BMSC-mediated MM growth is further evidenced in the cocultures of CFSE-labeled CD138+MM cells from RR-MM with BMSC, with or without single and dual PD1/PD-L1 signaling blockade. BMSC significantly increased CD138+CFSElow MM cell viability/growth (CD138+CFSElow cells:80% of CD138+ cells in BM) compared to MM cells alone (CD138+CFSElow cells:2% of CD138+ cells in BM); and blockade of PD-L1 (CD138+CFSElow cells:69% of CD138+ cells in BM), PD1 (CD138+CFSElow cells:77% of CD138+ cells in BM), or the combination (CD138+CFSElow cells:62% of CD138+ cells in BM) overcame BMSC-mediated MM cell growth (Figure 2B). Therefore PD1/PD-L1 pathway may play an important role in stroma-mediated tumor growth, independent of immune effector cell function.
Figure 2. Checkpoint blockade overcomes bone marrow stroma-mediated MM growth.
PD1/PD-L1 signaling role in the bidirectional interaction between MM cells and bone marrow stroma cells (BMSC) is shown in coculture of BMSC and MM cells. A. BMSC effect on PD-L1 expression in MM cells is demonstrated by multi-parameter flow cytometry analysis of MM cell-BMSC cocultures. Representative histogram plot for PD-L1 expression in CD138+MM cell population (red) versus control (grey) is demonstrated in MM cell line (H929) alone and cultured with BMSC. B. Checkpoint blockade effect on BMSC-mediated MM growth is demonstrated by CFSE-flow analysis in cocultures of CD138+MM cells from patient with RR-MM with BMSC. Shown are multi-parameter-dot plots for MM cells alone and cultured with BMSC with or without single and dual blockade of PD1 and PD-L1. CD138+CFSElow cell population represents live/growth MM cells (large gated box) and CD138+CFSEhigh cell population represents non-dividing/dead MM cells (small gated box).
Checkpoint blockade enhances immune effector cell-mediated anti-MM response in MM BM
We next investigated the immune-suppressive role of PD1/PD-L1 signaling in the MM microenvironment, and determined whether blockade of PD1/PD-L1 signaling can reverse tumor-induced immune suppression in the MM BM microenvironment (Figure 3). Immune effector cell-mediated MM cytotoxicity was measured by CFSE/PI apoptotic/dead cell detection assays in cocultures of effector cells (autologous T cells and NK cells) and target cells (CD138+MM cells) from RR-MM. CD3+T cells, CD56+NK cells and CD138+MM cells were isolated by FACS-sorting from BM of patients with RR-MM. CD138+ target MM cells were then labeled with CFSE and cocultured for four hours with each autologous effector cell population in the absence or presence of anti-PD1 and anti-PD-L1, alone or in combination. Apoptotic/dead CD138+MM cells were characterized as CD138+CFSE+PI+ MM cells using CFSE/PI-flow cytometry analysis. Blockade of PD1 and PD-L1, alone and more significantly in combination, induced effector cell-mediated MM cytotoxicity. Within the effector cell populations, NK cells (4 fold, p<0.05) demonstrated more pronounced anti-MM cytotoxicity than T cells (2 fold increase, p<0.05) with blockade of PD1 (2 fold, p<0.05) and PD-L1 (3 fold, p<0.05) alone, or in combination (4 fold, p<0.05) (Figure 3A).
Figure 3. Checkpoint blockade enhances immune effector cell-mediated anti-MM responses in MM bone marrow.
A. Impact of checkpoint blockade on immune effector cell-mediated anti-MM response is demonstrated by a multi-parameter CFSE/PI flow cytometry analysis. CFSE labeled target CD138+MM cells and autologous immune effector cells (CD3T cells and NK cells) were cultured with or without anti-PD1 and anti-PD-L1, alone or in combination. Effector cell mediated-cytotoxicity was determined by CD138+CFSE+PI+ apoptotic/dead MM cells. Fold change is relative to control (effector cell-mediated MM cytotoxicity without checkpoint blockade). Impact of checkpoint blockade on effector cytokines IFNγ (B) and Gzm-B (C) mediating cytotoxicity against MM is shown in effector cells in cocultures of CD138+MM cells and autologous effector cells.
To define the impact of checkpoint blockade on effector cytokine pattern mediating cytotoxicity, we next evaluated intracellular production of effector cytokines (IFNγ and Gzm-B) in CD4T cells, CD8T cells, and NK cells cocultured with autologous CD138+MM cells from RR-MM BM (Figure 3B and C). Intracellular cytokine analysis by multi-parameter flow cytometry demonstrated that checkpoint blockade induced higher IFNγ production in NK cells (anti-PD-L1:1.3 fold, anti-PD1: 1.7 fold, anti-PD1/PD-L1: 2.2. fold) than T cells during anti-MM cytotoxicity (Figure 3B), along with increased production of Gzm-B (Figure 3C). Even though checkpoint blockade modulates effector cytokine production, there was no associated change in effector cell proliferation (data not shown), indicating that checkpoint molecules regulate effector cell function. Of note, checkpoint blockade-induced cell activation varies in each effector cell subpopulation, indicating a differential anti-tumor response in patients.
Checkpoint blockade partially reverses MDSC-mediated MM growth and immune suppression in MM BM
Since both mMDSC and nMDSC express PD-L1 in RR-MM BM, we next assessed whether blockade of PD1/PD-L1 signaling can reverse MDSC-mediated tumor growth and immune suppression in MM BM (Figure 4). The impact of checkpoint blockade on MDSC-mediated MM growth was analyzed in autologous cocultures of CD138+MM cells and mMDSC and nMDSC from RR-MM BM. CD138+MM cells, CD11b+CD14+HLA-DRlow/neg mMDSC, and CD11b+CD14−HLA-DRlow/negCD33+CD15+ nMDSC were isolated from RR-MM BM by FACS-sorting. CFSE-labeled CD138+MM cells were cultured for two days with autologous mMDSC or nMDSC with or without anti-PD1 and anti-PD-L1, alone or in combination. CD138+CFSElow MM cells were assessed by CFSE-flow analysis. As shown in Figure 4A mMDSC induced CD138+MM cell growth (21% to 34% CD138+CFSElow cells of CD138+MM cells); importantly, dual blockade of PD1 and PD-L1 abrogated MDSC-mediated MM cell growth in RR-MM BM (34% to 22% CD138+CFSElow cells of CD138+MM cells). In contrast, there was no significant change in MDSC-mediated MM growth by either PD1 or PD-L1 single blockade (data not shown).
Figure 4. Checkpoint blockade partially reverses MDSC-mediated MM growth and immune suppression in MM bone marrow.
A. Impact of checkpoint blockade on MDSC-mediated tumor growth is demonstrated in the bone marrow of patients with RR-MM by CFSE-flow cytometry analysis. CFSE labeled CD138+MM cells and autologous MDSC were cultured in the absence or presence of anti-PD1 and anti-PD-L1, alone or in combination. Viability/growth of CD138+MM cells (CD138+CFSElow) is shown by representative histogram plots. B. Effect of checkpoint blockade on MDSC-mediated immune suppression is shown by intracellular effector cytokine analysis in RR-MM BM. Autologous T cells cultured either alone or with mMDSC and nMDSC in the absence or presence of anti-PD1 and anti-PD-L1, alone or in combination. Gated boxes demonstrate percent intracellular IFNγ expression in T cells cultured alone (top panel), with mMDSC (middle panel), and with nMDSC (lower panel) with or without dual PD1 and PD-L1 blockade. C. Impact of checkpoint blockade with lenalidomide on MDSC-mediated immune suppression is shown in RR-MM bone marrow by flow cytometry intracellular cytokine analysis. A representative bar graph shows percent intracellular Gzm-B expression in all effector cells cultured alone or with autologous mMDSC and nMDSC (top graph). A representative bar graph of intracellular Gzm-B expression is shown in each effector cell population from the coculture of effector cells with autologous mMDSC (middle graph) and with autologous nMDSC (lower graph).
The impact of checkpoint blockade on MDSC-mediated immune suppression was next assessed in RR-MM BM. mMDSC, nMDSC, and autologous effector cells were isolated by FACS-sorting from RR-MM BM. MDSC and autologous effector cells (T cells, NK cells, and NKT cells) were cultured with or without anti-PD1 and anti-PD-L1, alone or in combination, and intracellular production of cytokines IFNγ and Gzm-B was determined in effector cells (Figure 4B and C). As shown by representative multi-parameter dot plots of intracellular IFNγ expression in gated CD3T cells, both mMDSC and nMDSC significantly suppressed IFNγ production in T cells, and only combined blockade of PD1 and PD-L1 overcame this suppression (Figure 4B).
We next tested whether targeting PD1/PD-L1 inhibitory signaling using checkpoint blockade antibodies while inducing immune effector cell activity with lenalidomide can reverse MDSC-mediated immune-suppression in RR-MM BM (Figure 4C). Intracellular cytokine analysis in effector cells cultured with either autologous mMDSC or nMDSC demonstrated that both mMDSC and nMDSC induced suppression of intracellular Gzm-B production in all effector cells (Figure 4C, top graph). Specifically, checkpoint blockade in cocultures of mMDSC and autologous effector cells induced Gzm-B production in CD8T cells, NK and NKT cells; and the combination of lenalidomide with checkpoint blockade further enhanced Gzm-B production, particularly in CD8T cells, NK and NKT cells (Figure 4C, middle graph). Similarly, checkpoint blockade in cocultures of nMDSC with autologous effector cells from RR-MM BM significantly increased intracellular Gzm-B production in T cells and NKT cells, but not NK cells (Figure 4C, lower graph). Of note, checkpoint blockade, either alone or with lenalidomide, was not able to enhance effector cell proliferation in the presence of MDSC (data not shown).
Lenalidomide reduces expression of PD1 and PD-L1 in BM cells and enhances checkpoint blockade-induced MM cytotoxicity
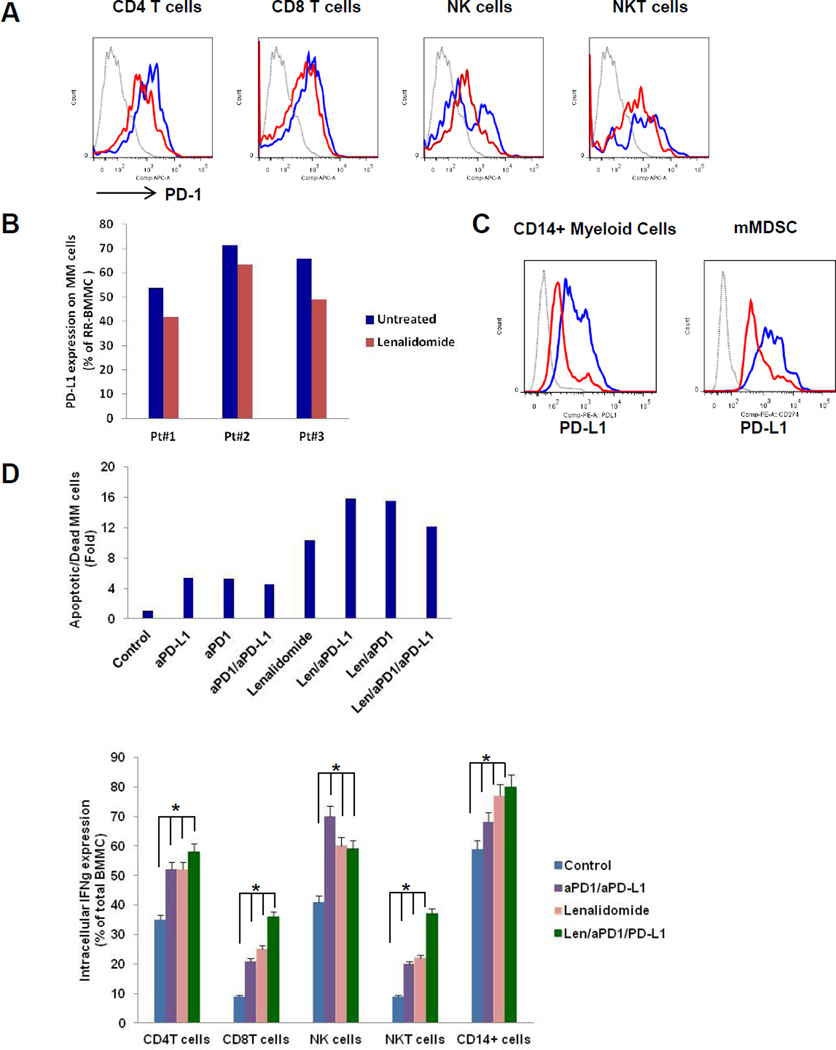
The impact of lenalidomide on surface expression of PD1 and PD-L1 in MM cells and BM accessory cells was next defined in RR-MM BM. BMMC from patients with RR-MM were cultured with lenalidomide (1uM); and cell surface expression of PD1 in effector cells (CD4T cells, CD8T cells, NK cells, and NKT cells), as well as surface expression of PD-L1 in CD138+MM cells, MDSC, and CD14+ monocytes/macrophages, was then determined by multi-parameter flow cytometry analysis (Figure 5). Lenalidomide significantly reduced PD1 surface expression on CD4T cells, CD8T cells, and NK cells in RR-MM BM (Figure 5A). Lenalidomide modestly decreased surface expression of PD-L1 on CD138+MM cells (Figure 5B), and more significantly downregulated PD-L1 expression on monocytes/macrophages and mMDSC in the BM from RR-MM (Figure 5C).
Figure 5. Lenalidomide reduces expression of PD1 and PD-L1 and enhances checkpoint blockade-induced MM cytotoxicity in MM bone marrow.
Impact of lenalidomide on cell surface expression of PD1 on effector cells (A) and PD-L1 on CD138+MM cells (B), as well as CD14+myeloid cells and MDSCs (C) in RR-MM bone marrow is shown by representative histogram plots. Percent PD1 expression on the effector cells and PD-L1 on the CD14+myeloid cells and MDSCs of untreated BM cells (blue) and lenalidomide treated BM cells (red) is shown relative to control (grey). D. Impact of lenalidomide on checkpoint blockade-induced MM cytotoxicity is shown in a representative graph of RR-MM bone marrow. Mononuclear cells from patient with RR-MM bone marrow were labeled with CFSE and cultured in the absence or presence of anti-PD1 and anti-PD-L1, alone or in combination, and with or without addition of lenalidomide. Shown is a percent Apoptotic/Dead (CD138+CFSE+PI+) MM cells in BMMC (top graph). Impact of lenalidomide on checkpoint blockade-induced effector cell activity is shown in RR-MM bone marrow by intracellular effector cytokine analysis. RR-MM bone marrow cells were cultured with anti-PD1 and anti-PD-L1 alone, or in combination, or with the addition of lenalidomide. Representative bar graph shows intracellular expression of IFNγ in each effector cell populations in RR-MM bone marrow (lower graph).
Since lenalidomide downregulates surface expression of checkpoint molecules in MM cells and accessory cells in MM BM, we next investigated anti-MM cytotoxic activity of lenalidomide in combination with checkpoint blockade in RR-MM (Figure 5D). To mimic the BM microenvironment, all BM cells were labeled with CFSE and cultured with anti-PD1 anti-PD-L1, alone or together, and with lenalidomide. Apoptotic/dead CD138+CFSE+PI+ MM cells were identified by multi-parameter CFSE/PI-flow cytometry analysis. There was a significant increase in CFSE+PI+ apoptotic/dead CD138+ MM cells in BM cultured with anti-PD1 and anti-PD-L1; and lenalidomide further enhanced checkpoint blockade-mediated MM cytotoxicity in RR-MM BM (Figure 5D, top graph). We further analyzed the effect of checkpoint blockade, alone or with lenalidomide, on cytokine production in BM effector cells. Bone marrow cells were cultured with anti-PD1, anti-PD-L1, or anti-PD1/PD-L1, alone or with lenalidomide. Intracellular expression of effector cytokines (IFNγ and Gzm-B) was then measured in CD4T cells, CD8T cells, NK cells, NKT cells, and monocytes/macrophages by multi-parameter flow cytometry analysis (Figure 5D, lower graph). Intracellular cytokine analysis of effector cells in RR-MM BM demonstrated that dual blockade of PD1 and PD-L1 significantly induced IFNγ production in all effector cells (Figure 5D, lower graph); as well as Gzm-B production in NK cells and NKT cells (data not shown). Importantly, lenalidomide further enhanced dual checkpoint blockade-induced IFNγ production in all effector cells (Figure 5D, lower graph).
Discussion
The MM microenvironment is transformed in the presence of tumor cells to promote MM development/growth while allowing tumor escape from immune surveillance due to suppression of anti-MM immune effector responses. As a result, infections remain a major cause of death.(28) Recent studies have defined immune checkpoint receptor PD1/PD-L1 signaling as a key pathway regulating the critical balance between immune activation and tolerance.(23, 28–31) Binding of PD1 on effector cells to PD-L1 or PD-L2 on non-hematopoietic cells triggers inhibitory signaling in effector cells, leading to induction and maintenance of tolerance.(32) Recent studies in solid tumors have demonstrated that PD1/PD-L1 signaling allows for escape from immune surveillance, transforming the tumor microenvironment into a tumor-protective, immune-suppressive milieu.(7, 8, 16, 18, 33–36) Specifically, PD-L1 expression on tumor cells inhibits T cell activation and CTL mediated-tumor lysis. Importantly, recent studies have shown increased expression of PD-L1 on lung, skin, renal, gastric, pancreatic, colorectal, breast and ovarian cancers. (8, 15, 16, 20, 35, 37–40) Moreover, blockade of PD1/PD-L1 signaling using clinically-relevant anti-PD1 monoclonal antibodies restored immune responses and achieved remarkable clinical responses in solid tumors including melanoma and lung cancer, providing a very promising novel immunotherapeutic strategy.
Studies in hematologic malignancies have shown increased expression of PD-L1 in B-cell lymphomas, chronic lymphocytic leukemia, acute myeloid leukemia, and multiple myeloma.(24, 25, 33, 41–45) In the present study, we investigated the role of PD1/PD-L1 inhibitory signaling in the bidirectional interaction between tumor, stroma, and immune accessory cells in the MM BM microenvironment. Importantly, we assessed the impact of single and dual blockade of PD1/PD-L1 signaling, alone or in combination with lenalidomide, on the tumor-promoting, immune-suppressive MM microenvironment. Previous studies have demonstrated that PD-L1 is not expressed on normal plasma cells, but is expressed on MM cell lines and primary MM cells.(25–27) Here we showed that both mRNA and cell surface expression of PD-L1 is increased on CD138+ MM cells from ND-MM and further elevated in RR-MM, compared to normal BM plasma cells. However, within a broad panel of MM cell lines constitutive PD-L1 expression is limited to MM1.S, OPM2 and H929 cells, suggesting that PD-L1 expression is induced on MM cells by the bidirectional interaction between tumor and accessory cells. PD-L1 gene is encoded on chromosome 9, which is increased in copy number in HMM. Importantly, patients with HMM have a better prognosis and outcome than patients with NHMM. Analysis of PD-L1 gene expression in tumor cells from patients with ND-MM demonstrated that the expression of PD-L1 gene was significantly correlated with the copy number in most patients’ tumor clones. Although expression of PD-L1 gene was significantly increased in NHMM relative to normal donor plasma cells, it was even higher in HMM. Enhancing anti-MM immune response by targeting checkpoint molecules may therefore improve outcome even in HMM.
PD1 expression is increased on CD4T cells from patients, and returns to levels in normal CD4T cells following autologous transplant.(25) Benson et al.(22) have shown that PD1 is expressed on NK cells from MM patients, but not normal NK cells; that blockade of PD1 signaling by anti-PD1 antibody induces cytolytic activity of NK cells; and that lenalidomide further induces NK-mediated anti-tumor responses. Here we extended these studies to determine the impact not only of PD1 blockade but also of dual blockade of PD1 and PD-L1, alone or with lenalidomide, on the functional sequelae in MM cells, stroma, immune effector cells (CD4T cells, CD8T cells, NK cells, NKT cells and monocytes/macrophages) and immune suppressor MDSCs in the MM bone marrow.
We first determined that PD1 expression is significantly increased on effector immune cells, particularly on CD8T cells and NK cells, whereas PD-L1 is expressed by myeloid effector cells monocytes/macrophages. Previous studies have shown that MDSC express high levels of B7 in murine models of solid tumor.(46–48) Moreover, It has been recently demonstrated that PD1 and PD-L1 are expressed at low levels in MDSC of patients with MM.(49) Here we compared PD-L1 expression on myeloid cell subpopulations including antigen presenting cells, mMDSC and nMDSC in the BM of patients with MM. PD-L1 expression is significantly higher in MDSC in RR-MM than ND-MM. Increased expression of PD1 on immune effector cells, and increased PD-L1 on both MM cells and immune suppressor MDSC, indicates that PD1/PD-L1 inhibitory signaling plays an important role in providing a tumor-promoting, immune-suppressive microenvironment in MM BM.
Extensive studies focusing on the interaction of BMSC with MM cells have demonstrated that BMSC promote MM cell growth and drug resistance. Tamura H et al.(26) have demonstrated that BMSC also upregulates PD-L1 expression on MM cells. To delineate whether PD1/PD-L1 plays a role in BMSC-mediated MM growth, we assessed the impact of single and dual blockade of PD1/PD-L1 signaling in cocultures of tumor cells from patients with RR-MM and BMSC. Importantly, BMSC markedly induced PD-L1 expression in MM cells, and BMSC-mediated MM cell growth was abrogated by blockade of PD1 and PD-L1, suggesting that checkpoint blockade may have a direct effect on BMSC-induced MM growth, independent of its immune accessory cell activity.
Immunomodulatory drug lenalidomide not only targets the MM cell directly, but also induces anti-MM activity of immune effector cells. We have recently shown that lenalidomide does not alter MDSC-mediated tumor growth and immune suppression in MM.(14) However, lenalidomide reduces PD1 expression on NK cells and PD-L1 expression on tumor cells from patients with MM.(22) Here we found that lenalidomide decreased PD1 expression in all effector cells (CD4T cells, CD8T cells, NK cells, and NKT cells), as well as PD-L1 expression in MM cells, MDSC, and Monocytes/Macrophages. We characterized the immunomodulatory effects of PD1/PD-L1 blockade with lenalidomide in autologous cocultures of immune effector cells with MM cells from patients with RR-MM. Even though there was no change in effector cell proliferation, PD1/PD-L1 blockade significantly induced cytotoxic activity of autologous T cells, NK cells, and monocytes/macrophages against MM cells; and lenalidomide further enhanced effector cell-mediated cytotoxicity. PD1/PD-L1 blockade also induced intracellular expression of cytotoxic cytokines IFNγ and Gzm-B in CD4T cells, CD8T cells, NK cells, and monocytes/macrophages in RR-MM. Furthermore, MDSC-mediated MM cell growth was significantly decreased by PD1/PD-L1 blockade. Finally, PD1/PD-L1 blockade induced intracellular expression of IFNγ and Gzm-B in T cells, NK cells, and NKT cells cultured with autologous MDSC; and lenalidomide further enhanced this effector cell activation. Of note, checkpoint blockade induced response in each effector cell population regardless of PD1 expression level.
Our data therefore demonstrate that immune checkpoint signaling plays an important role conferring the tumor-promoting, immune-suppressive microenvironment in MM BM. Importantly, blockade of PD1 or PD-L1, alone and in combination, induces anti-MM immune responses, which can be further enhanced by lenalidomide. Targeting checkpoint signaling using PD1 and PD-L1 blocking antibodies, particularly in combination with lenalidomide, therefore represents a promising novel immune-based therapeutic strategy to inhibit tumor cell growth, restore host immune function in MM, and improve patient outcome in MM.
Supplementary Material
Translational Relevance.
The interaction of tumor cells with their surrounding accessory cells and extracellular matrix provides a tumor-promoting environment, while suppressing immune response. Recent studies in solid tumors have demonstrated that programmed death-1 (PD1) signaling plays an important role in tumor-induced immune-suppression; and conversely, that blockade of PD1/PD-L1 by therapeutic antibodies restores anti-tumor immune response. Remarkable responses have been observed to PD1-blockade in malignant melanoma, leading to recent FDA approval of anti-PD1 antibody therapies. Here we assessed the impact of single and dual blockade of PD1/PD-L1 alone or in combination with lenalidomide, on accessory and immune cell function, as well as on MM cell growth in the BM milieu. Our study demonstrates that PD1/PD-L1-blockade can induce anti-MM immune responses, which are enhanced by lenalidomide. Our studies provide the preclinical rationale for evaluation of combined PD1/PD-L1-blockade with lenalidomide to inhibit tumor cell growth, restore host immune function, and improve patient outcome in MM.
Acknowledgments
Financial Support: This study was supported by NIH/NCI Specialized Program of Research Excellence in Myeloma P50 CA100707 (Anderson KC); NIH/NCI Host-Tumor Cell Interactions in Myeloma: Therapeutic Applications P01 CA78378 (Anderson KC), and NIH/NCI Molecular Sequelae of Myeloma-Bone Marrow Interactions: Therapeutic Applications R01 CA50947 (Anderson KC) grants. KCA is an American Cancer Society Clinical Research Professor.
Footnotes
Conflict-of-interest disclosure: Anderson, KC: Celgene, Millennium, Onyx: Richardson P: Celgene, Millennium, Johnson&Johnson. Munshi N: Celgene, Millennium, Novartis. Hideshima, T: Acetylon. Raje, N: Amgen, Celgene, Novartis, AstraZeneca.
Authorship Contributions: G.G. made the hypothesis, designed and conducted the research, performed experiments; K.B.C. and S.P. provided assistance for flow cytometry analysis, M.K.S. performed statistical analysis, G.B., J.E.A., H.O., A.S., T.H, R.S., S.K. and T.H. assisted the experiments, R.E.W. and Y.T delivered patient samples, F.M., S.M., H.A.L and N.C.M. provided genomic data, and J.P.L., N.R., N.C.M., D.M.D., P.G.R. and K.C.A. provided advice and clinical samples, G.G. analyzed results and made figures; G.G. and K.C.A. wrote and edited the manuscript.
References
- 1.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nature reviews Cancer. 2007;7:585–598. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 2.Chng WJ, Santana-Davila R, Van Wier SA, Ahmann GJ, Jalal SM, Bergsagel PL, et al. Prognostic factors for hyperdiploid-myeloma: effects of chromosome 13 deletions and IgH translocations. Leukemia. 2006;20:807–813. doi: 10.1038/sj.leu.2404172. [DOI] [PubMed] [Google Scholar]
- 3.Van Wier S, Braggio E, Baker A, Ahmann G, Levy J, Carpten JD, et al. Hypodiploid multiple myeloma is characterized by more aggressive molecular markers than non-hyperdiploid multiple myeloma. Haematologica. 2013;98:1586–1592. doi: 10.3324/haematol.2012.081083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smadja NV, Fruchart C, Isnard F, Louvet C, Dutel JL, Cheron N, et al. Chromosomal analysis in multiple myeloma: cytogenetic evidence of two different diseases. Leukemia. 1998;12:960–969. doi: 10.1038/sj.leu.2401041. [DOI] [PubMed] [Google Scholar]
- 5.Fonseca R, Debes-Marun CS, Picken EB, Dewald GW, Bryant SC, Winkler JM, et al. The recurrent IgH translocations are highly associated with nonhyperdiploid variant multiple myeloma. Blood. 2003;102:2562–2567. doi: 10.1182/blood-2003-02-0493. [DOI] [PubMed] [Google Scholar]
- 6.Gorgun G, Calabrese E, Soydan E, Hideshima T, Perrone G, Bandi M, et al. Immunomodulatory effects of lenalidomide and pomalidomide on interaction of tumor and bone marrow accessory cells in multiple myeloma. Blood. 2010;116:3227–3237. doi: 10.1182/blood-2010-04-279893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10:5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 8.Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta histochemica. 2006;108:19–24. doi: 10.1016/j.acthis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Hideshima T, Mitsiades C, Akiyama M, Hayashi T, Chauhan D, Richardson P, et al. Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341. Blood. 2003;101:1530–1534. doi: 10.1182/blood-2002-08-2543. [DOI] [PubMed] [Google Scholar]
- 10.Hideshima T, Chauhan D, Shima Y, Raje N, Davies FE, Tai YT, et al. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96:2943–2950. [PubMed] [Google Scholar]
- 11.Atanackovic D, Luetkens T, Kroger N. Coinhibitory molecule PD-1 as a potential target for the immunotherapy of multiple myeloma. Leukemia. 2014;28:993–1000. doi: 10.1038/leu.2013.310. [DOI] [PubMed] [Google Scholar]
- 12.Gorgun GT, Whitehill G, Anderson JL, Hideshima T, Maguire C, Laubach J, et al. Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. Blood. 2013;121:2975–2987. doi: 10.1182/blood-2012-08-448548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luptakova K, Rosenblatt J, Glotzbecker B, Mills H, Stroopinsky D, Kufe T, et al. Lenalidomide enhances anti-myeloma cellular immunity. Cancer immunology, immunotherapy : CII. 2013;62:39–49. doi: 10.1007/s00262-012-1308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nature medicine. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 15.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–198. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 17.Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011;28:682–688. doi: 10.1007/s12032-010-9515-2. [DOI] [PubMed] [Google Scholar]
- 18.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 19.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer research. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 20.Droeser RA, Hirt C, Viehl CT, Frey DM, Nebiker C, Huber X, et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer. 2013;49:2233–2242. doi: 10.1016/j.ejca.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Huang S, Gong D, Qin Y, Shen Q. Programmed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8+ T lymphocytes in human non-small cell lung cancer. Cellular & molecular immunology. 2010;7:389–395. doi: 10.1038/cmi.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benson DM, Jr, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116:2286–2294. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. The EMBO journal. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kearl TJ, Jing W, Gershan JA, Johnson BD. Programmed death receptor-1/programmed death receptor ligand-1 blockade after transient lymphodepletion to treat myeloma. J Immunol. 2013;190:5620–5628. doi: 10.4049/jimmunol.1202005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenblatt J, Glotzbecker B, Mills H, Vasir B, Tzachanis D, Levine JD, et al. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. J Immunother. 2011;34:409–418. doi: 10.1097/CJI.0b013e31821ca6ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamura H, Ishibashi M, Yamashita T, Tanosaki S, Okuyama N, Kondo A, et al. Marrow stromal cells induce B7-H1 expression on myeloma cells, generating aggressive characteristics in multiple myeloma. Leukemia. 2013;27:464–472. doi: 10.1038/leu.2012.213. [DOI] [PubMed] [Google Scholar]
- 27.Zhang C, Wang W, Qin X, Xu Y, Huang T, Hao Q, et al. B7-H1 protein vaccine induces protective and therapeutic antitumor responses in SP2/0 myeloma-bearing mice. Oncology reports. 2013;30:2442–2448. doi: 10.3892/or.2013.2686. [DOI] [PubMed] [Google Scholar]
- 28.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annual review of immunology. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lafferty KJ, Cunningham AJ. A new analysis of allogeneic interactions. The Australian journal of experimental biology and medical science. 1975;53:27–42. doi: 10.1038/icb.1975.3. [DOI] [PubMed] [Google Scholar]
- 30.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nature medicine. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 31.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. The Journal of experimental medicine. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clinic proceedings. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 33.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer research. 2013;73:6900–6912. doi: 10.1158/0008-5472.CAN-13-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer research. 2013;73:3591–3603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. The New England journal of medicine. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hodi FS. Hematology/Oncology Clinics of North America. Melanoma. Preface. Hematology/oncology clinics of North America. 2014;28:xiii–xiv. doi: 10.1016/j.hoc.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Choueiri TK, Fay AP, Gray KP, Callea M, Ho TH, Albiges L, et al. PD-L1 Expression in Non-clear cell Renal Cell Carcinoma. Annals of oncology. 2014;25:2178–2184. doi: 10.1093/annonc/mdu445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kronig H, Kremmler L, Haller B, Englert C, Peschel C, Andreesen R, et al. Interferon-induced programmed death-ligand 1 (PD-L1/B7-H1) expression increases on human acute myeloid leukemia blast cells during treatment. European journal of haematology. 2014;92:195–203. doi: 10.1111/ejh.12228. [DOI] [PubMed] [Google Scholar]
- 42.Greaves P, Gribben JG. The role of B7 family molecules in hematologic malignancy. Blood. 2013;121:734–744. doi: 10.1182/blood-2012-10-385591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hao Y, Chapuy B, Monti S, Sun HH, Rodig SJ, Shipp MA. Selective JAK2 inhibition specifically decreases Hodgkin lymphoma and mediastinal large B-cell lymphoma growth in vitro and in vivo. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:2674–2683. doi: 10.1158/1078-0432.CCR-13-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossille D, Gressier M, Damotte D, Maucort-Boulch D, Pangault C, Semana G, et al. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. Leukemia. 2014;28:2367–2375. doi: 10.1038/leu.2014.137. [DOI] [PubMed] [Google Scholar]
- 45.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. The New England journal of medicine. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host Cancer research. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 47.Fujimura T, Ring S, Umansky V, Mahnke K, Enk AH. Regulatory T cells stimulate B7-H1 expression in myeloid-derived suppressor cells in ret melanomas. The Journal of investigative dermatology. 2012;132:1239–1246. doi: 10.1038/jid.2011.416. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y, Zeng B, Zhang Z, Zhang Y, Yang R. B7-H1 on myeloid-derived suppressor cells in immune suppression by a mouse model of ovarian cancer. Clin Immunol. 2008;129:471–481. doi: 10.1016/j.clim.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 49.Favaloro J, Liyadipitiya T, Brown R, Yang S, Suen H, Woodland N, et al. Myeloid derived suppressor cells are numerically, functionally and phenotypically different in patients with multiple myeloma. Leukemia & lymphoma. 2014:1–8. doi: 10.3109/10428194.2014.904511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.