Abstract
Background
This paper reports on the findings of the effect of two structured exercise interventions on secondary cognitive outcomes which were gathered as part of the Progressive Resistance Exercise Training in Parkinson’s disease randomized controlled trial.
Methods
This study was a prospective, parallel-group, single-center trial. Fifty-one non-demented patients with mild-to-moderate Parkinson’s disease were randomly assigned either to modified Fitness Counts or to Progressive Resistance Exercise, and were followed for 24 months. Cognitive outcomes were the Digit Span, Stroop, and Brief Test of Attention.
Results
Eighteen patients in modified Fitness Counts and 20 patients in Progressive Resistance Exercise completed the trial. At 12 and at 24 months no differences between groups were observed. At 12 months, relative to baseline, modified Fitness Counts improved on the Digit Span (estimated change, 0.3; Inter-Quartile Range, 0, 0.7; p=0.04) and Stroop (0.3; 0, 0.6; p=0.04), and Progressive Resistance Exercise improved only on the Digit Span (0.7; 0.3, 1; p<0.01). At 24 months, relative to baseline, modified Fitness Counts improved on the Digit Span (0.7; 0.3, 1.7; p<0.01) and Stroop (0.3; 0.1, 0.5; p=0.03), while Progressive Resistance Exercise improved on the Digit Span (0.5; 0.2, 0.8; p<0.01), Stroop (0.2; −0.1, 0.6; p=0.048), and Brief Test of Attention (0.3; 0, 0.8; p=0.048). No neurologic or cognitive adverse events were seen.
Conclusions
This study provides Class IV level of evidence that 24 months of Progressive Resistance Exercise or modified Fitness Counts may improve attention and working memory in non-demented patients with mild-to-moderate Parkinson’s disease.
Keywords: Progressive resistance exercise, Parkison’s disease, Randomized controlled trial, Attention, Memory
INTRODUCTION
Cognitive impairment1–4 including attention and working memory deficits5 are frequently observed in Parkinson disease (PD). It is estimated that 78% of patients with PD will develop dementia.6 Treating cognitive impairment is a challenge as it is often unresponsive to dopaminergic therapies.7 Recent work from the 90+ study suggests that poor overall physical performance is a strong predictor of cognitive decline.8 The beneficial effects of exercise training on cognitive performance are well recognized in aging,9 as well as in individuals with dementia.10 In PD, preliminary research on the effects of exercise on cognition is promising,11 but long-term randomized controlled trials are needed.12
Recently, the Progressive Resistance Exercise Training in PD (PRET-PD)13 trial reported, that compared to modified Fitness Counts (mFC),14 Progressive Resistance Exercise Training (PRET) reduced the off-medication motor section of the Unified Parkinson’s Disease Rating Scale (UPDRS-III) at the study end-point of 24 months by 7.3 points. More recently, we also reported our findings associated with physical function, a secondary outcome.15 Here, we examine the cognitive domain and report the effect of mFC and PRET on cognitive outcomes related to attention and working memory. This paper addresses the following questions at the 12- and at the 24-month time point: relative to baseline, does exercise improve cognitive function, and is one type of exercise modality better at improving cognitive function in mild-to-moderate PD? Because of the known benefits of exercise on cognitive performance,9–11 we hypothesized that exercise would improve cognitive function in mild-to-moderate PD.
METHODS
Study Design and Participants
The PRET-PD trial was a prospective, parallel-group, single-center, randomized controlled trial between September 2007 and July 2011.13 Patients with idiopathic PD, confirmed by a Movement Disorders specialist,16 were self-referred or recruited from Rush University Medical Center. Patients were eligible if they were 50 to 67 years (the Physical Activity Readiness Questionnaire,17 a screening tool used for exclusion was only validated for use with persons younger than 69, the maximum age at study completion); on stable dopaminergic therapy; and able to walk for six minutes. Patients were ineligible if they had a neurological history other than PD; significant arthritis; failed the Physical Activity Readiness Questionnaire;17 had a Mini-Mental State Examination score<2318; were already exercising; or had deep brain stimulation surgery for PD. Patients were followed every 6 months for 24 months or until they withdrew from the study. Each participant provided written informed consent approved by the local Institutional Review Boards.
Interventions
Details of the exercise intervention used in the PRET-PD trial have been published previously.13 In brief, the mFC was an exercise program recommended by the National Parkinson Foundation and focused on stretches, balance exercises, breathing, and non-progressive strengthening (manual chapters two and three).14 PRET was a weight-lifting program where the load against which the muscle worked was systematically and progressively increased. The PRET program consisted of strengthening exercises that were directed at all the major muscle groups.19 The programs were identical in all aspects (duration of exercise, number of exercise sessions, and time with the personal trainer) except for the specific exercises. Patients participated in their respective interventions twice a week19 for 24 months. One-on-one exercise with a certified personal trainer was provided for both weekly exercise sessions during the first six months; the trainer-assisted sessions were reduced to once per week after six months. Patients in the mFC and PRET groups were instructed not to engage in additional exercise.
Study Procedures
All cognitive assessments were performed after 12-hour overnight withdrawal of dopaminergic medication20 by individuals trained in administering standardized cognitive assessments and blinded to group assignments at the Clinical Motor Control Laboratory at the University of Illinois at Chicago. Off-medication assessment was completed in the morning. The cognitive outcomes were one of many outcome domains tested; they were clinical status, the primary outcome, and bradykinesia and strength, tremor, physical function15 and gait, quality of life, and cognition, which were secondary outcomes. The order of testing was pseudo-randomized between outcome domains and between cognitive outcomes. The off medication testing session lasted for approximately 3 hours. This was followed by ingestion of anti-Parkinsonian medication, a break for lunch, confirming a clinical response to the medication, and then repeating the entire testing procedure while on anti-Parkinsonian medication. In this paper we will report findings related to the cognitive outcomes only while off medication for the following reasons: first, the effects of anti-Parkinsonian medication on cognitive outcomes are not well understood;21 second, anti-Parkinsonian medication could possibly mask the effects of exercise; and third, because on-medication testing was always conducted in the afternoon following off-medication testing in the morning, fatigue was a potential confound with respect to cognitive performance.
Cognitive Outcomes
The cognitive outcomes were: the Digit Span Forwards and Backwards to assess working memory,22 which had a sum score that ranged from 0–30; the Stroop Color-Word Interference to measure selective attention and conflict resolution,23 which had a T-score, a standardized normative metric, with a mean of 50 and a standard deviation of 10; and the Brief Test of Attention to assess selective attention,24 which had a sum score that ranged from 0–20.
Follow-up
Patients in the mFC and PRET groups returned to the laboratory at 6, 12, 18, and 24 months for follow-up. The entire baseline assessment procedure was repeated at each follow-up visit.
Randomization and Blinding
The statistician matched the enrolled patients in pairs by sex and off-medication UPDRS-III scores,25 and randomly assigned one member of each pair to PRET and the other member to mFC using a random-length permuted block design.26 Randomization resulted in a parallel group design with a 1:1 allocation ratio. Patients started exercising within a month of randomization. Research personnel involved in data collection were blinded to group assignment. The patients knew their treatment assignment but were unaware of the study hypothesis and were explicitly instructed not to discuss their exercise program with the raters.
Statistical Analysis
All raw scores were converted to z-scores. For the Digit Span22 and the Stroop23 published norms were used to calculate z-scores. For the Brief Test of Attention, the pooled baseline mean and standard deviation was used to calculate z-scores. To reduce Type I errors, we used only the 12 and 24 month change from baseline z-scores. The distributional assumptions for parametric testing were not met; therefore non-parametric methods were used. The Wilcoxon rank-sum test was used to analyze the differences between the mFC and PRET groups at 12 and 24 months. The Wilcoxon signed-rank test was used to analyze change from baseline at 12 and 24 months. We also calculated correlations of changes in cognitive outcomes with changes in clinical and functional outcomes published previously. In addition, a sensitivity analysis using the last available observation carried forward (LOCF) was performed to evaluate the impact of missing data. Since these outcomes were not used to power the original randomized controlled trial, we did not adjust the significance level for multiple outcomes. All statistical tests were 2-tailed, with P value<0.05 for statistical significance.
RESULTS
Of the 51 patients enrolled, 46 patients (mFC, n=23; PRET, n=23) completed the 12-month follow-up and 38 patients (mFC, n=18; PRET, n=20) completed the 24-month follow-up. At baseline, the mFC and PRET groups did not differ on demographic, clinical, and cognitive outcomes (Table 1).
Table 1.
Characteristics of Patients at Baseline1
| Treatment groups | ||||
|---|---|---|---|---|
| mFC | PRET | PRET vs. mFC (95% CI) |
p-value2 | |
| Demographic | ||||
| Age in years | 58·6 ± 5·6 | 59·0 ± 4·6 | −0·4 (−2·6 to 3·4) | 0·783 |
| Education in years | 15·9 ± 2·7 | 16·8 ± 3·5 | 0.9 (−1.0 to 2.7) | 0.373 |
| Sex - no. (%) | 14 | |||
| Male | 14 (58·3) | 14 (58·3) | ||
| Female | 10 (41·7) | 10 (41·7) | ||
| Ethnicity - no. (%) | 0.194 | |||
| Hispanic or Latino | 5 (20·8) | 1 (4·2) | ||
| Not Hispanic or Latino | 19 (79·2) | 23 (95·8) | ||
| Race - no. (%) | 0.494 | |||
| African American | 0 (0) | 2 (8·3) | ||
| Native American | 0 (0) | 0 (0) | ||
| White | 24 (100) | 22 (91·7) | ||
| Handedness - no. (%) | 14 | |||
| Right | 22 (91·7) | 23 (95·8) | ||
| Left | 2 (8·3) | 1 (4·2) | ||
| Clinical | ||||
| Years since diagnosis | 6·5 ± 47 | 6·5 ± 4·1 | 0·0 (−2·5 to 2·6) | 0·973 |
| Mini-Mental State Examination | 29·1 ± 1·4 | 29·3 ± 1·1 | −0·2 (−0·9 to 0·5) | 0·63 |
| Most affected side - no. (%) | 0·5 (0·2 to 1·6) | 0·374 | ||
| Right | 17 (70·8) | 13 (54·2) | ||
| Left | 7 (29·2) | 11 (45·8) | ||
| Motor Status | ||||
| Unified Parkinson Disease Rating Scale, part III, motor subscale score (range, 0–108) (off medication) | 34·7 ± 11·5 | 34·5 ± 11·9 | −0·2 (−7·0 to 6·6) | 0·953 |
| Hoehn and Yahr Staging Scale (disability; range, 0–5; off medication) | 2 (2, 2.5) | 2 (2, 2.5) | 0 (0 to 0)5 | 0.856 |
| Medication – n (%) | ||||
| Dopamine Precursors | 19 (79) | 17 (71) | 0.514 | |
| Dopamine Agonists | 16 (67) | 17 (71) | 0.764 | |
| Adjuncts | 12 (50) | 10 (42) | 0.564 | |
| Cognitive Outcomes – median (IQR) | ||||
| Digit Span Forwards and Backwards Sum Score (range, 0–30; off medication) | 17 (14.5, 19) | 17 (15, 19.5) | 0 (−2 to 2)5 | 0.846 |
| Stroop Color-Word Interference T-Score (mean, 50; SD, 10; off medication) | 47.5 (41, 53) | 41 (33, 51) | −3 (−11 to 4)5 | 0.416 |
| Brief Test of Attention Sum Score (range, 0–20; off medication) | 16 (13, 18.5) | 17 (13.5, 18) | 0 (−2 to 2)5 | 0.756 |
mFC, Modified Fitness Counts; PRET, Progressive Resistance Exercise Training; CI, Confidence Interval; scPD, Standard of Care Parkinson Disease; IQR, Inter-Quartile Range
Plus-minus values are mean ± 1SD
Representing significance of a test that was performed to determine if values were the same across the mFC and PRET groups
P values calculated using t-test
P values calculated using Chi-Square test (expected cell count ≥ 5) or Fisher’s Exact test (expected cell count <5)
Hodges-Lehman estimate of location shift
P values calculated using Wilcoxon Rank-Sum test
Difference between mFC and PRET groups at 12 and 24 months
There were no differences between the mFC and PRET groups at 12 and 24 months on any of the cognitive outcomes measured in this study (Table 2).
Table 2.
Difference between groups in change from baseline in cognitive outcomes at 12 and 24 months
| PRET vs. mFC (95% CI)1 | |
|---|---|
| Digit Span Forwards and Backwards (z-score) | |
| 12 months | −0.3 (−0.7 to 0.3) p = 0.32 |
| 24 months | 0.3 (−0.3 to 1) p = 0.27 |
| Stroop Color-Word Interference (z-score) | |
| 12 months | 0.1 (−0.5 to 0.5) p = 0.81 |
| 24 months | 0 (−0.4 to 0.5) p = 0.77 |
| Brief Test of Attention (z-score) | |
| 12 months | 0 (−0.5 to 0.5) p = 0.77 |
| 24 months | 0 (−0.8 to 0.5) p = 0.83 |
PRET, Progressive Resistance Exercise Training; mFC, Modified Fitness Counts; CI, Confidence Interval
Hodges-Lehman estimate of location shift and p-values calculated using Wilcoxon Rank-Sum test
Change from baseline: The effect in each intervention arm at 12 and 24 months
Digit Span Forward and Backwards Sum Score
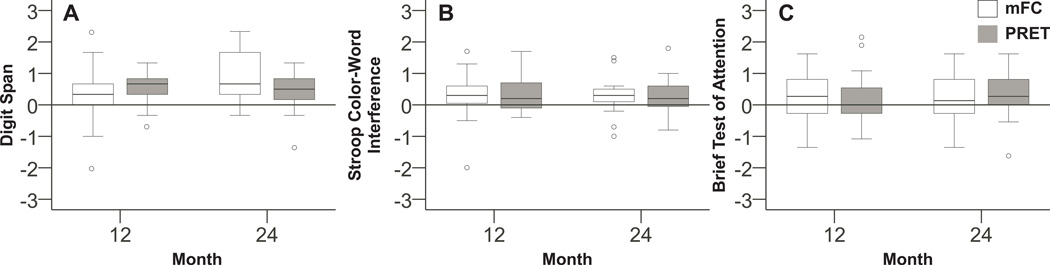
The mFC and PRET groups improved their Digit Span scores at 12 (mFC: estimated difference, 0.3; Inter-quartile range, 0, 0.7; p=0.04; PRET: 0.7; 0.3, 1; p<0.01; Table 3 and Figure 1A) and at 24 months (mFC: 0.7; 0.3, 1.7; p<0.01; PRET: 0.5; 0.2, 0.8; p<0.01; Table 3 and Figure 1A).
Table 3.
Cognitive outcomes at baseline, 12, and 24 months, and change from baseline at 12 and 24 months
| Z-Score at visit: Median (IQR) |
Change from baseline: Median (IQR), p value1 |
|||
|---|---|---|---|---|
| mFC | PRET | mFC | PRET | |
| Digit Span Forwards and Backwards2 | ||||
| Baseline | 0 (−0.3, 0.6) | 0.3 (−0.3, 0.8) | ||
| 12 months | 0.3 (−0.3, 1.3) | 1 (0, 1.7) | 0.3 (0, 0.7) p = 0.04 |
0.7 (0.3, 1) p < 0.01 |
| 24 months | 1 (0, 2) | 1 (0.2, 1.3) | 0.7 (0.3, 1.7) p < 0.01 |
0.5 (0.2, 0.8) p < 0.01 |
| Stroop Color-Word Interference2 | ||||
| Baseline | −0.3 (−0.9, 0.3) | −0.9 (−1.7, 0.1) | ||
| 12 months | −0.1 (−0.8, 0.8) | −0.2 (−1.2, 0.4) | 0.3 (0, 0.6) p = 0.04 |
0.2 (−0.1, 0.8) p = 0.08 |
| 24 months | −0.1 (−0.8, 0.8) | −0.1 (−1.7, 0.4) | 0.3 (0.1, 0.5) p = 0.03 |
0.2 (−0.1, 0.6) p = 0.048 |
| Brief Test of Attention2 | ||||
| Baseline | 0.1 (−0.7, 0.8) | 0.4 (−0.5, 0.7) | ||
| 12 months | 0.4 (−0.4, 1.2) | 0.4 (−0.4, 0.9) | 0.3 (−0.3, 1) p = 0.07 |
0 (−0.3, 0.5) p = 0.11 |
| 24 months | −0.1 (−0.4, 0.9) | 0.4 (0.1, 1.2) | 0.1 (−0.3, 0.8) p = 0.50 |
0.3 (0, 0.8) p = 0.048 |
IQR, Inter-Quartile Range; mFC, Modified Fitness Counts; PRET, Progressive Resistance Exercise Training
P values calculated using the Wilcoxon Signed Rank test
Positive change score from baseline is indicative of improvement
Figure 1.
These box plots illustrate the off-medication change from baseline z-scores in the modified Fitness Counts (mFC) and Progressive Resistance Exercise Training (PRET) for (A) the Digit Span Forwards and Backwards, (B) the Stroop Color-Word Interference, and (C) the Brief Test of Attention at 12, and 24 months. Positive z-scores indicate improvement in the Digit Span Forwards and Backwards sum score, the Stroop Color-Word Interference T-score, and the Brief Test of Attention sum score.
Stroop Color-Word Interference T-Score
The mFC group improved performance on the Stroop at 12 months (mFC: 0.3; 0, 0.6; p=0.04; Table 3 and Figure 1B), while the PRET group presented with no change (0.2; −0.1, 0.8; p=0.08; Table 3 and Figure 1B). At 24 months, the mFC and PRET groups improved their Stroop scores (mFC: 0.3; 0.1, 0.5; p=0.03; PRET: 0.2; −0.1, 0.6; p=0.048; Table 3 and Figure 1B).
Brief Test of Attention Sum Score
At 12 months, the mFC (0.3; −0.3, 1; p=0.07; Table 3 and Figure 1C) and the PRET (0; −0.3, 0.5; p=0.11; Table 3 and Figure 1C) groups did not change on the BTA. At 24 months, mFC remained unchanged, while PRET improved on the BTA (mFC: 0.1; −0.3, 0.8; p=0.50; PRET: 0.3; 0, 0.8; p=0.048; Table 3 and Figure 1C).
Associations of Change in UPDRS-III, Function, and Cognition
There were no significant relationships between change in the cognitive outcomes and the UPDRS-III. With respect to the relationships between change in cognitive outcomes and change in physical function outcomes, only the relationship between the digit span and walking speed was significant (rho=0.46, p=0.004).
In summary, there are 2 findings of importance at the study end-point. First, there are no differences between mFC and PRET for all cognitive outcomes measured in this study. Second, PRET and mFC presented with substantial within group improvement in cognitive performance, i.e., at 24 months, relative to baseline, the PRET group improved on all three of the cognitive outcomes (Digit Span, Stroop, and BTA) measured in this study, while the mFC group improved on two of the three cognitive outcomes (Digit Span and Stroop) measured in this study. These results did not change with LOCF sensitivity analyses (supplementary Table e-1 and e-2).
Adverse events
Adverse events related to this trial have been previously published.13 Of note, there were no neurological or cognitive adverse events reported.
DISCUSSION
This clinical trial demonstrated that 24 months of exercise, twice a week, may be effective in improving attention and working memory in non-demented patients with mild-to-moderate PD when evaluated off medication. Relative to baseline, mFC and PRET improved performance on the Digit Span and Stroop, while PRET also improved performance on the BTA. This is the first randomized clinical trial to examine the effects of 24 months of exercise on cognitive functions in patients with mild-to-moderate PD. The findings from this clinical trial extend the previously known cognitive benefits of exercise in Alzheimer’s disease and in normal aging to PD. In addition, improvements in digit span scores were associated with improvements in walking speed. Given the recently published finding that physical activity reduces major mobility disability in older adults at risk for disability,27 taken together with our recently published findings that exercise improves motor signs of PD13 and physical function,15 and our current finding that exercise might improve cognitive function, the evidence for the benefits of exercise across domains is accumulating.
This clinical trial demonstrates the likely beneficial effects of 24 months of structured exercise on attention and working memory, which are cognitive domains that are frequently impaired in patients with PD. This is especially important for the Stroop because impaired performance on the Stroop has been shown to be associated with greater risk of subsequent dementia in non-demented patients with PD.28,29 Consequently, the finding that structured exercise might improve Stroop scores might indicate that exercise may reduce the risk of subsequent dementia in patients with PD. This possibility is clinically relevant because the cumulative incidence estimates from longitudinal studies indicate that up to 78% of patients with PD will develop dementia.6
PRET was significantly better than mFC at improving motor signs of PD,13 and PRET and mFC were efficacious at improving physical function15 and cognitive outcomes in PD with neither being better than the other. One possible explanation is that because the mFC protocol included non-progressive resistance exercises, it could be considered as a ‘low’ dose resistance exercise regimen, while the PRET protocol could be considered as a ‘high’ dose resistance exercise regimen. It has been shown that both ‘low’ and ‘high’ dose PRET have similar beneficial effects on cognitive outcomes.30 It could be that there is a threshold for a treatment effect on cognitive function. Our findings suggest that PRET and mFC exceeded a threshold for an effect on cognitive function. Further research is needed to understand the mechanisms by which exercise improves cognition and whether different doses of exercise and different types of exercise have the same or different effects on cognition.
In general, repeated administrations of cognitive assessments in healthy individuals are known to have practice effects, and these practice effects are affected by factors such as age, task difficulty,31,32 and IQ.31 We recognize that the improvements observed in this study could have been driven by practice effects. However, there are two arguments that favor the idea that the improvements in cognitive outcomes observed in this study were at least partially attributable to the effect of exercise and not simply an effect of practice. First, cognitive function has been shown to decline with increasing age and the magnitude of this decline is approximated by the improvement in cognitive function due to repeated testing.33 Second, in PD, the neurodegenerative disease process augments the cognitive decline that accompanies the aging process. In fact, prior work has shown that when cognitive tests are administered approximately 36 months apart, more cognitive decline occurs in patients with PD when compared to age-matched controls.34 Consequently, combining the effects of aging and the neurodegenerative disease process one would expect cognitive function to decline or at best remain unchanged over time in patients with PD. However, we observed improvements in performance in the mFC and PRET groups in the cognitive outcomes at 12 and 24 months. This might be suggestive of an effect of exercise beyond that of practice alone.
Another factor that is a potential confound is the increased social and cognitive engagement by virtue of mere participation in the PRET-PD study. The very fact that these patients went out of their houses to the gyms provides for greater opportunities for social and cognitive engagement with others including their family, personal trainers, and the study personnel. The extent to which this increased social and cognitive engagement confounds our findings is unknown. This is a limitation of this study. In addition, due to a relatively younger group of patients, mild-to-moderate disease, and an above average level of education, the lack of generalizability is a limitation. Given these limitations and the fact that we did not adjust our α-level for multiple outcomes, our findings should be interpreted with caution. Despite these limitations, the PRET-PD trial is similar to a phase 2 study that supports the concept of exercise as an adjunct therapy for PD and demonstrates feasibility,35 thereby providing a rationale for larger-scale multi-center trials, that could extend our findings across geographic locations, clinical practices, and a wider group of patients with PD.
In conclusion, this clinical trial found that 24 months of PRET or mFC exercise may improve cognitive outcomes in mild-to-moderate PD while off medication. Our findings suggest that non-demented patients with mild-to-moderate PD might be able to improve cognitive performance by engaging in either mFC or PRET for two 60 to 90 minute sessions a week.
Supplementary Material
Acknowledgments
Full Financial Disclosures: FJD, JAR, SEL and CP received grant support from NIH. DEV received grant support from NIH, Michael J. Fox, and consults for projects at UT Southwestern Medical Center and Great Lakes NeuroTechnologies. WMK receives grant support from the NIH and DoD and consulting fees from the NIH. CLC is or has received research support from Allergan Inc., Merz Pharmaceuticals, Ipsen Limited, NIH, and Parkinson Disease Foundation and consulting fees from Neupathe, Allergan Inc., Merz Pharmaceuticals, Ipsen Limited and Medtronic Corporation. JGG has received grant/research support from NIH, Michael J. Fox Foundation, Rush University, Teva (site-PI) and consulting fees from Merz Pharmaceuticals and Pfizer. DMC received grant support from NIH and Michael J. Fox, and receives lecture and reviewer fees from NIH.
Funding Source: Supported by NIH (R01-NS28127-12 to 16, R01 NS52318, R01 NS75012). The sponsors were not involved in the design, conduct, collection, management, analysis, and/or interpretation of the study results and preparation, review, or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of NIH.
Footnotes
Clinical Trial Registration: clinicaltrials.gov, NCT00591344.
Author Roles: FJD was responsible for acquisition of data, analysis and interpretation of the data, statistical analysis, drafting the manuscript, and administrative, technical, or material support. JAR was responsible for study concept and design, obtaining funding, acquisition of data, analysis and interpretation of the data, critical revision of the manuscript for important intellectual content, and administrative, technical, or material support. CP was responsible for acquisition of data, analysis and interpretation of the data, critical revision of the manuscript for important intellectual content, and administrative, technical, or material support. SEL participated in study concept and design, obtaining funding, interpretation of the data, statistical analysis, and critical revision of the manuscript for important intellectual content. DEV was responsible for study concept and design, obtaining funding, interpretation of the data, critical revision of the manuscript for important intellectual content, study supervision, and administrative, technical, or material support. WMK was responsible for study concept and design, obtaining funding, interpretation of the data, critical revision of the manuscript for important intellectual content, study supervision, and administrative, technical, or material support. CLC was responsible for study concept and design, interpretation of the data, critical revision of the manuscript for important intellectual content, study supervision, and administrative, technical, or material support. JGG was responsible for interpretation of the data, critical revision of the manuscript for important intellectual content, and technical, or material support. DMC was responsible for study concept and design, obtaining funding, interpretation of the data, critical revision of the manuscript for important intellectual content, study supervision, and administrative, technical, or material support.
REFERENCES
- 1.Brown RG, Marsden CD. Cognitive function in parkinsons-disease - from description to theory. Trends Neurosci. 1990;13(1):21–29. doi: 10.1016/0166-2236(90)90058-i. [DOI] [PubMed] [Google Scholar]
- 2.Cooper JA, Sagar HJ, Jordan N, Harvey NS, Sullivan EV. Cognitive impairment in early, untreated parkinsons-disease and its relationship to motor disability. Brain. 1991;114:2095–2122. doi: 10.1093/brain/114.5.2095. [DOI] [PubMed] [Google Scholar]
- 3.Owen AM. Cognitive dysfunction in parkinson disease: The role of frontostriatal circuitry. Neuroscientist. 2004;10(6):525–537. doi: 10.1177/1073858404266776. ER. [DOI] [PubMed] [Google Scholar]
- 4.Kudlicka A, Clare L, Hindle JV. Executive functions in parkinson's disease: Systematic review and meta-analysis. Mov Disord. 2011;26(13):2305–2315. doi: 10.1002/mds.23868. [doi]. [DOI] [PubMed] [Google Scholar]
- 5.Owen AM, James M, Leigh PN, et al. Fronto-striatal cognitive deficits at different stages of parkinsons-disease. Brain. 1992;115:1727–1751. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- 6.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in parkinson disease - an 8-year prospective study. Arch Neurol. 2003;60(3):387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 7.Goldman JG, Litvan I. Mild cognitive impairment in parkinson's disease. Minerva Med. 2011;102(6):441–459. [PMC free article] [PubMed] [Google Scholar]
- 8.Bullain SS, Corrada MM, Shah BA, Mozaffar FH, Panzenboeck M, Kawas CH. Poor physical performance and dementia in the oldest old: The 90+ study. JAMA Neurol. 2013;70(1):107–113. doi: 10.1001/jamaneurol.2013.583. [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 10.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis. Arch Phys Med Rehabil. 2004;85(10):1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Uc EY, Doerschug KC, Magnotta V, et al. Phase I/II randomized trial of aerobic exercise in parkinson disease in a community setting. Neurology. 2014;83(5):413–425. doi: 10.1212/WNL.0000000000000644. [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hindle JV, Petrelli A, Clare L, Kalbe E. Nonpharmacological enhancement of cognitive function in parkinson's disease: A systematic review. Mov Disord. 2013;28(8):1034–1049. doi: 10.1002/mds.25377. doi:10.1002/mds.25377;10.1002/mds.25377. [DOI] [PubMed] [Google Scholar]
- 13.Corcos DM, Robichaud JA, David FJ, et al. A two-year randomized controlled trial of progressive resistance exercise for parkinson's disease. Mov Disord. 2013 doi: 10.1002/mds.25380. doi:10.1002/mds.25380;10.1002/mds.25380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cianci H. Parkinson disease: Fitness counts. 3rd ed. Miami, FL: National Parkinson Foundation; 2006. [Google Scholar]
- 15.Prodoehl J, Rafferty MR, David FJ, et al. Two-year exercise program improves physical function in parkinson's disease: The PRET-PD randomized clinical trial. Neurorehabil Neural Repair. 2014 doi: 10.1177/1545968314539732. [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical-diagnosis of idiopathic parkinsons-disease - a clinicopathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canadian Society for Exercise Physiology. Physical activity readiness questionnaire - PAR -Q (revised 2002) [Accessed 04 May 2013];2013 http://uwfitness.uwaterloo.ca/PDF/par-q.pdf. Updated 2002. [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Feigenbaum MS, Pollock ML. Prescription of resistance training for health and disease. Med Sci Sports Exerc. 1999;31(1):38–45. doi: 10.1097/00005768-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Langston JW, Widner H, Goetz CG, et al. Core assessment program for intracerebral transplantations (capit) Mov Disord. 1992;7(1):2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- 21.Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in parkinson's disease as a function of dopaminergic medication and task demands. Cereb Cortex. 2001;11(12):1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- 22.Wechsler D. Wechsler adult intelligence scale: Administration and scoring Manual <br/>. Third ed. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 23.Golden CJ, Freshwater SM. The stroop color and word test: A manual for clinical and experimental uses. Chicago, IL: Stoelting; 2002. [Google Scholar]
- 24.Schretlen D. Brief test of attention: Professional manual. Lutz, FL: Psychological Assessment Resources, Inc.; 1997. [Google Scholar]
- 25.Fahn S, Elton RL. UPDRS Program Members. Unified parkinson's disease rating scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent developments in parkinsons disease. Vol. 2. Florham Park, NJ: Macmillan Healthcare Information; 1987. pp. 153–163. [Google Scholar]
- 26.Friedman LM, Furberg CD, DeMets DL. Fundamentals of clinical trials. New York: Springer-Verlag; 1998. [Google Scholar]
- 27.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: The LIFE study randomized clinical trial. JAMA. 2014;311(23):2387–2396. doi: 10.1001/jama.2014.5616. [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahieux F, Fenelon G, Flahault A, Manifacier MJ, Michelet D, Boller F. Neuropsychological prediction of dementia in parkinson's disease. J Neurol Neurosurg Psychiatry. 1998;64(2):178–183. doi: 10.1136/jnnp.64.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janvin CC, Aarsland D, Larsen JP. Cognitive predictors of dementia in parkinson's disease: A community-based, 4-year longitudinal study. J Geriatr Psychiatry Neurol. 2005;18(3):149–154. doi: 10.1177/0891988705277540. [DOI] [PubMed] [Google Scholar]
- 30.Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions A 12-month randomized controlled trial. Arch Intern Med. 2010;170(2):170–178. doi: 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabbitt P. Crystal quest: An examination of the concepts of 'fluid' and 'crystallised' intelligence as explanations for cognitive changes in old age. In: Baddeley AD, Weiskrantz LS, editors. Attention, selection, awareness and control. Oxford: Oxford University Press; 1993. pp. 197–231. [Google Scholar]
- 32.Lowe C, Rabbitt P. Test/re-test reliability of the CANTAB and ISPOCD neuropsychological batteries: Theoretical and practical issues. cambridge neuropsychological test automated battery. international study of post-operative cognitive dysfunction. Neuropsychologia. 1998;36(9):915–923. doi: 10.1016/s0028-3932(98)00036-0. [DOI] [PubMed] [Google Scholar]
- 33.Rabbitt P, Diggle P, Smith D, Holland F, Mc Innes L. Identifying and separating the effects of practice and of cognitive ageing during a large longitudinal study of elderly community residents. Neuropsychologia. 2001;39(5):532–543. doi: 10.1016/s0028-3932(00)00099-3. [DOI] [PubMed] [Google Scholar]
- 34.Muslimovic D, Post B, Speelman JD, De Haan RJ, Schmand B. Cognitive decline in parkinson's disease: A prospective longitudinal study. J Int Neuropsychol Soc. 2009;15(3):426–437. doi: 10.1017/S1355617709090614. [DOI] [PubMed] [Google Scholar]
- 35.Rascol O. Physical exercise in parkinson disease: Moving toward more robust evidence? Mov Disord. 2013;28(9):1173–1175. doi: 10.1002/mds.25453. [doi]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.