Abstract
Objective
Examine influences of diabetes-specific social support (D-SS) and depressive symptoms on glycemic control over time, among adults randomized to a diabetes self-management education and support (DSME/S) intervention or usual care.
Methods
Data were from 108 African-American and Latino participants in a six-month intervention trial. Multivariable linear regression models assessed associations between baseline D-SS from family and friends and depressive symptoms with changes in HbA1c. We then examined whether baseline D-SS or depression moderated intervention-associated effects on HbA1c.
Results
Higher baseline D-SS was associated with larger improvements in HbA1c (adjusted ΔHbA1c -0.39% for each +1-point D-SS, p=0.02), independent of intervention-associated HbA1c decreases. Baseline depressive symptoms had no significant association with subsequent HbA1c change. Neither D-SS nor depression moderated intervention-associated effects on HbA1c.
Conclusions and Practice Implications
Diabetes self-management education and support programs have potential to improve glycemic control for participants starting with varying levels of social support and depressive symptoms. Participants starting with more support for diabetes management from family and friends improved HbA1c significantly more over six months than those with less support, independent of additional significant DSME/S intervention-associated HbA1c improvements. Social support from family and friends may improve glycemic control in ways additive to DSME/S.
1. Introduction
Improving glycemic control among adults with diabetes is important to decreasing mortality and morbidity from this increasingly common condition. Improvement of glycemic control is especially important among urban, low-income Latino and African American adults with diabetes who, on average, have worse glycemic control than non-Latino white adults with diabetes.1 Efforts to improve glycemic control through diabetes self-management education and support (DSME/S) are widespread and often successful.2,3 However, the effectiveness of DSME/S interventions varies widely across studies and, within studies, across individual participants.4–6
To date, little is known about patient-level factors that influence the effectiveness of diabetes interventions in improving glycemic control. Several studies have shown that participants starting with higher levels of HbA1c are more likely to benefit from DSME/S;7–10 and one review found a stronger effect with higher patient age.11 However two recent systematic reviews of self-management interventions concluded that there is very little information available about which subgroups of patients (e.g. age, gender, or race/ethnicity) will benefit more from these interventions.12,13 In particular, psychosocial factors known to affect diabetes management in observational studies have rarely been examined as moderating factors in DSME/S effectiveness, even though they have often been theorized to affect the potential to benefit from diabetes treatment.14 The exception is patient health literacy, which was found not to moderate DSME/S effectiveness in one study,15 but in another study patients with low health literacy benefitted from a DSME/S intervention more than those with high health literacy.16
Two factors that observational studies suggest may be especially important in influencing whether participants fully benefit from DSME/S interventions are: 1) the level of social support participants receive from family and friends; and 2) whether participants are experiencing depressive symptoms. In semi-structured interviews, adults with diabetes named diabetes-specific social support from family and friends as a critical factor when initiating or sustaining changes in self-management routines.17–21 Social support itself is defined as “an exchange of resources between at least two persons, aimed at increasing the well-being of the receiver”.22 Diabetes-specific social support (D-SS) refers to social support for diabetes care tasks, and is frequently studied in the context of laypeople such as family members, friends, or peers with diabetes. In observational studies, higher levels of social support are associated with better diabetes self-management, including better medication adherence, consistent blood glucose monitoring, healthier eating, and more physical activity.23,24 However, a direct association between D-SS and subsequent glycemic control has not been conclusively established, and most previous studies of the association between social support and glycemic control have been limited by cross-sectional design.25–30 In addition, no known previous studies have examined whether people starting a comprehensive diabetes management intervention with low social support have more improvement in their glycemic control from the intervention.
Higher levels of depressive symptoms have been more conclusively linked with worse glycemic control among people with diabetes, particularly through worse self-management behaviors.31–33 It should be noted that depressive symptoms, defined as a count of the number or severity of symptoms of major depressive disorder not associated with a particular life circumstance, is a distinct entity from diabetes distress, defined as emotional distress linked specifically to diabetes and its management. Key studies have demonstrated that patients’ depressive symptoms affect diabetes management differently than diabetes distress.34,35 Improving depressive symptoms concurrently with diabetes self-management has been attempted in a few specialized DSME/S interventions, while improving diabetes distress is often a core goal of general DSME/S. No known previous studies have examined whether pre-existing depressive symptoms moderate the effectiveness of a general DSME/S intervention.
We sought to address these gaps in knowledge by examining the influences of baseline D-SS and depressive symptoms, separately and together, on changes in glycemic control over a six month period among adults who were randomized to receive either a comprehensive DSME/S intervention or usual care over that time period. We hypothesized that higher baseline levels of D-SS and lower baseline levels of depressive symptoms would be associated with larger subsequent improvements in HbA1c among participants in both groups. We were uncertain, however, whether baseline D-SS or depressive symptoms would moderate the ability of those in the intervention group to improve their glycemic control, beyond any improvements made by the control group. It is possible that a diabetes intervention would be more beneficial to those with depressive symptoms or without other sources of support for diabetes management. On the other hand, because DSME/S interventions most often focus on individuals and not their support systems or mental health, it is possible that those with better pre-existing social support and mental health would be better able to engage in the intervention to make greater improvements in glycemic control.
2. Methods
2.1 Intervention Content, Participants, and Overall Effectiveness
We analyzed data from a randomized-controlled trial of a culturally tailored, empowerment-based,36 community health worker (CHW)-led intervention that aimed to improve diabetes self-management and glycemic control. The intervention design, implementation, and outcomes have been described in detail elsewhere.37–40 The intervention was developed, conducted, and evaluated by a collaborative of community, health system, and academic partners (the REACH Detroit Partnership) using community-based participatory research (CBPR) principles.41 The study was approved by the University of Michigan and Henry Ford Health System Institutional Review Boards.
Participants were African-American or Latino adults with type 2 diabetes, identified from medical records at a federally-qualified community health center and an urban health care system. Eligible participants lived in pre-specified zip-codes in either eastside Detroit, which is predominantly African-American (80%) and had a median household income of $25,020, or southwest Detroit, whose residents are predominantly Latino (70%) and had an annual median household income of $11,500 (U.S. Census Bureau 2007). Individuals found to have serious diabetes-related complications, such as kidney failure (defined as being on dialysis), on a screening survey were excluded. The six-month intervention included CHW-delivered group diabetes management classes, home visits to help participants set and follow up on diabetes management goals, and accompaniment to physician appointments to model activated participation. Neither increasing diabetes social support from family and friends, nor reducing depressive symptoms were goals of the intervention. Participants were individually randomized between September 2004 and July 2006 to either receive the six-month intervention or usual care. Due to concern about certain participants’ need for prompt assistance with severe medical conditions, the participating health centers requested that five participants originally randomized to the control group be switched to the intervention group. In as-randomized, intention-to-treat analyses, intervention participants had an -0.8% additional decrease in HbA1c over the six-month study period as compared to control participants.37
2.2 Independent Variable Measures
Independent variables were measured through survey instruments with validated scales. Surveys were conducted in participants’ homes at enrollment, 6 months, and 12 months after enrollment, in either English or Spanish. Diabetes social support was measured with the Diabetes Care Profile42,43 on a 0–4 scale, with higher scores indicating more D-SS (Cronbach alpha in our sample 0.95). The PHQ-944 was used to measure depressive symptoms, raw scores ranged from 0–27; for this analysis raw scores were multiplied by 4/27 to create a 0–4 scale to facilitate comparisons with D-SS (Cronbach alpha in our sample 0.83). Other variables measured with survey data included sex, age, race/ethnicity, education level, year of diabetes diagnosis, marital/partner status, diabetes medication regimen, and self-rated health status with the SF-145.
2.3 Primary Outcome Measure
Our main outcome measure for this study was change in HbA1c, from baseline to six-months. Participants were asked at the time of their baseline and six-month interviews to go to the laboratory at their health care site within 1 week to give samples for HbA1c analysis. All recruitment sites sent lab samples to the central laboratory of the urban healthcare system for analysis. We did not obtain same week lab samples from a handful of participants (9 at baseline and 4 at 6-months), so for these participants an HbA1c level was obtained from chart review, always within 3 months of the baseline interview and within 2 months of the 6-month interview. The time difference between baseline and 6-month HbA1c measurements was always greater than three months.
2.4 Analyses
For this study of potential influences on intervention-associated effects, we examined participants in their ‘as-treated’ groups. Changes in HbA1c, diabetes social support, and depressive symptoms over time were described and statistical significance of changes were assessed using paired t-tests.
The impact of pre-intervention D-SS on changes in HbA1c was assessed using multivariable linear regression models adjusting for baseline HbA1c. This analysis method will produce point estimates similar to repeated measures mixed models when used in a pretest-posttest (two time point) design, provided that missing data is missing at random.46 In this dataset, the baseline HbA1c mean did not differ by whether the participants’ 6-month HbA1c data was missing, and 6-month HbA1c means did not differ by whether the participants’ baseline HbA1c data was missing. All models had the outcome of change in HbA1c from baseline to 6 months. The initial model (Model A) included baseline HbA1c, our principal independent variables (baseline D-SS and baseline depressive symptoms), socio-demographic characteristics (age, gender, education level, race/ethnicity, and marital/partner status), study design elements (participant’s health care site, randomization group), and measures of health status (self-rated health status and diabetes medication regimen). Models included any participant characteristics that differed significantly between groups at baseline. Alternate models A1 and A2 examine baseline social support and depressive symptoms in separate models.
Model B assessed possible moderator effects of baseline social support and depressive symptoms. Interaction terms for baseline social support*randomization group and baseline depressive symptoms*randomization group were added to Model A to determine if baseline values had more of an association with HbA1c changes among those receiving the intervention during this time period. Alternate models B1 and B2 examine the social support and depressive symptom interaction terms in separate models.
Finally, because social support may lessen depressive symptoms, or buffer the health effects of depressive symptoms,47–50 in Model C we examined whether baseline depressive symptoms moderated associations of baseline social support with change in HbA1c, by adding an interaction term baseline social support*baseline depressive symptoms to Model A.
Power calculations based on the Likelihood Ratio test, N=108 participants, and the 10 predictors in our models, showed over 80% power to detect an increase of 0.05 in model R-squared by adding an additional predictor of interest51. Regression diagnostics revealed one participant outlier with baseline triglycerides above 1200 mg/dl. We excluded this participant from our reported results, but alternate analyses with this participant included had similar results. SAS version 9.3 (SAS Institute, 2011) was used in all analyses.
3. Results
3.1 Participants
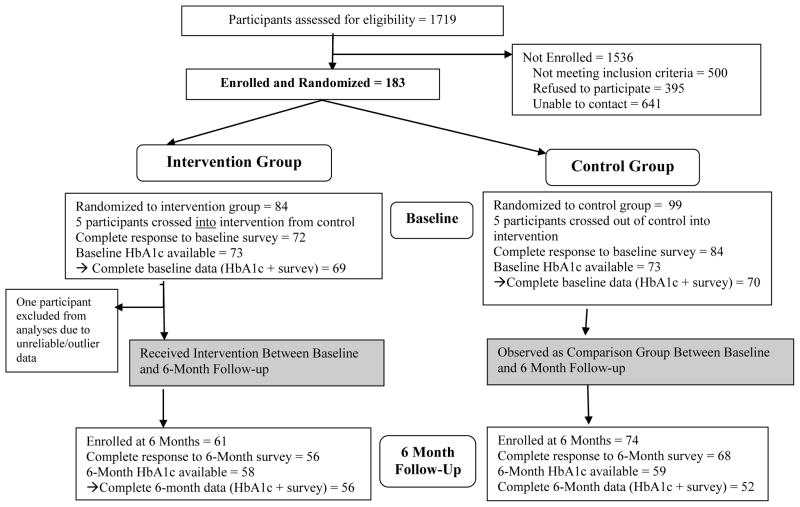
Complete data for the multivariable models were available for 108 out of 163 enrolled participants (Figure 1). African-Americans were more likely than Latinos to drop out of the study, and therefore have missing HbA1c data. The characteristics of participants included in study analyses are shown in Table 1. Participants in the intervention group were slightly younger on average (mean 50 years old) compared to control group participants (mean 56 years old). The percent of participants using insulin and mean time since diabetes diagnosis did not significantly differ between the two groups. Baseline social support and baseline depressive symptoms were not significantly correlated (correlation coefficient 0.09, p=0.37, not reported in table). There were no significant differences in baseline social support or depressive symptoms between African-American and Latino participants (data not shown in table).
Figure 1.
Study Participant Flow Chart
Table 1.
Characteristics of Study Participants
| All Participants (n = 108) | Intervention Group (n = 56) | Control Group (n = 52) | p-value for Intervention Group vs. Control Group* | |
|---|---|---|---|---|
| Participant Characteristic | N (%) or Mean (SD) | |||
| Female | 77 (71.3%) | 42 (75.0%) | 35 (67.3%) | 0.38a |
| Age (years) | 53.2 (11.6) | 50.2 (10.2) | 56.4 (12.3) | <0.01b |
| High School or GED Graduate | 57 (52.8%) | 31 (55.4%) | 26 (50.0%) | 0.58a |
| Race/Ethnicity | 0.45a | |||
| African-American | 52 (48.1%) | 25 (44.6%) | 27 (51.9%) | |
| Latino/a | 56 (51.9%) | 31 (55.4%) | 25 (48.1%) | |
| Healthcare Site | 0.02a | |||
| Community health center | 68 (63.0%) | 41 (73.2%) | 27 (51.9%) | |
| Urban health system | 40 (37.0%) | 15 (26.8%) | 25 (48.1%) | |
| Married or Partnered | 80 (74.1%) | 43 (76.8%) | 37 (71.2%) | 0.50a |
| Self-Rated Health Status (range 1–5) | 3.6 (0.9) | 3.5 (0.9) | 3.6 (0.9) | 0.58b |
| Diabetes medication regimen | 0.62c | |||
| No medications | 9 (8.3%) | 6 (10.7%) | 3 (5.8%) | |
| Oral medications only | 71 (65.7%) | 37 (66.1%) | 34 (65.4%) | |
| Insulin +/− oral medications | 28 (25.9%) | 13 (23.2%) | 15 (28.8%) | |
| Time since diabetes diagnosis, years | 8.8 (8.1) | 7.8 (6.9) | 9.9 (9.1) | 0.13d |
| Baseline Diabetes Social Support from Family and Friends (range 0–4) | 2.6 (1.0) | 2.8 (1.0) | 2.5 (0.9) | 0.08b |
| Baseline Depression Symptom Severity (range 0–4) | 0.7 (0.7) | 0.8 (0.9) | 0.7 (0.6) | 0.43e |
| Baseline HbA1c% | 8.7 (2.2) | 8.7 (2.3) | 8.6 (2.1) | 0.95e |
| Baseline Diabetes Self-Management Behavior (range 0–3) | 2.0 (0.8) | 2.0 (0.8) | 2.0 (0.8) | 0.97e |
P value for statistical differences between Intervention vs. Control groups determined by:
Pearson chi-square test,
Student t-test,
Fisher’s exact test,
Log-rank test,
Wilcoxon test.
3.2 Descriptive Changes in Key Variables
As shown in Table 2, participants in the intervention group significantly improved HbA1c values over the 6 month intervention period (mean change -1.0%, p=<0.01), while, over the same six months, the control group had no significant change in HbA1c (mean change 0.0%, p = 0.85). Laboratory HbA1c measurements were obtained an average of 5 days after the baseline interview and 23 days after the six-month interview (See Supplemental Table 1 for details). The HbA1c values for 13 people were >90 days from their corresponding interview date. The time interval between baseline and six-month HbA1c measurement averaged 6.8 months, with no interval less than three months. T-tests indicated no significant differences between the treatment groups in any of these HbA1c time parameters. Participants’ average diabetes social support did not significantly change from baseline (enrollment) to 12 months (mean change among all participants D-SS -0.1 points, SD 0.9, p = 0.19); this was not measured at 6 months. Similarly, overall depressive symptoms did not significantly change while participants received the six-month intervention.
Table 2.
Changes in glycemic control, social support, and depressive symptoms by as-treated group, N=108a
| Baseline | Endpointb | Difference | P-value (T-Test) | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| HbA1c% Intervention group | 8.7 (2.3) | 7.7 (1.7) | -1.0 (1.9) | <0.01c |
| HbA1c% Control group | 8.6 (2.1) | 8.6 (2.4) | 0.0 (1.5) | 0.85 |
| HbA1c% All participants combined | 8.7 (2.2) | 8.1 (2.1) | -0.5 (1.8) | <0.01c |
| Diabetes Social Support Intervention group | 2.8 (1.0) | 2.6 (0.9) | -0.2 (1.0) | 0.15 |
| Diabetes Social Support Control group | 2.5 (0.9) | 2.3 (1.0) | 0.0 (0.9) | 0.80 |
| Diabetes Social Support All participants combined | 2.6 (1.0) | 2.5 (1.0) | -0.1 (1.0) | 0.19 |
| Depressive symptoms Intervention group | 5.2 (6.0) | 4.6 (4.5) | -0.4 (5.3) | 0.58 |
| Depressive symptoms Control group | 4.8 (3.8) | 5.2 (4.9) | 0.7 (4.8) | 0.29 |
| Depressive symptoms All participants combined | 5.0 (5.0) | 4.9 (4.7) | 0.1 (5.1) | 0.78 |
All participants whose data was included in Table 3 models were included in these analyses
Endpoint for all variables was measured at 6 months after baseline except for diabetes social support which was measured at 12 months after baseline.
p <0.05
3.3 Impact of Baseline Diabetes Social Support and Depressive Symptoms on Subsequent Changes in HbA1c From Baseline to 6 Months
In Model A (Table 3), greater baseline diabetes social support was associated with greater improvements in HbA1c over six months, both among participants receiving, and those not receiving, the DSME/S intervention. For each 1-point increase in the 5-point diabetes social support scale, the adjusted decrease in HbA1c was 0.39% larger (p = 0.02). Notably, the positive association of baseline social support on HbA1c change was independent of the positive effect of intervention assignment itself on HbA1c change (adjusted intervention group decrease in HbA1C was 0.89% larger than in the control group, p=0.004). Baseline depressive symptoms were not associated with 6-month change in HbA1c (p=0.16). In alternate Models A1 and A2 (Supplemental Table 2), results were not significantly different when social support and depressive symptoms were analyzed in separate models.
Table 3.
Effect of Baseline Diabetes Social Support and Baseline Depressive Symptoms on Change in HbA1c from Baseline to Month 6: Multiple Regression Results
| Model Aa N = 108 |
Model Ba N=108 |
Model C N=108 |
|
|---|---|---|---|
| Independent Variables | Coefficient (Std Err), p-value | ||
| Baseline Diabetes Social Support from Family and Friendsc | -0.39 (0.16), p=0.02b | -0.38 (0.23), p=0.11 | -0.39 (0.16), p=0.02b |
| Baseline Depressive Symptomsd | 0.31 (0.22), p=0.16 | 0.52 (0.40), p=0.20 | 0.32 (0.23), p=0.16 |
| Intervention Group (Ref = Control Group) | -0.89 (0.30), p=0.004b | -0.89 (0.30), p=0.004b | -0.88 (0.30), p=0.005b |
| Interaction: Baseline Diabetes Social Support * Intervention Group | xx | -0.02 (0.31), p=0.94 | xx |
| Interaction: Baseline Depressive Symptoms * Intervention Group | xx | -0.28 (0.45), p=0.54 | xx |
| Interaction: Baseline Diabetes Social Support * Baseline Depressive Symptoms | xx | xx | -0.04 (0.18), p=0.82 |
| Male (Ref = Female) | -0.31 (0.34), p=0.37 | -0.29 (0.35), p=0.40 | -0.30 (0.34), p=0.38 |
| Age (Years) | -0.02 (0.01), p=0.11 | -0.02 (0.02), p=0.16 | -0.02 (0.02), p=0.14 |
| African-American (Ref=Latino/a) | 0.16 (0.50), p=0.75 | 0.17 (0.50), p=0.73 | 0.17 (0.50), p=0.74 |
| Patient at urban health system (Ref=Community health center) | 0.42 (0.48), p=0.38 | 0.40 (0.48), p=0.42 | 0.42 (0.48), p=0.39 |
| Married or Partnered (Ref = Single) | 0.35 (0.36), p=0.33 | 0.36 (0.36), p=0.33 | 0.38 (0.38), p=0.32 |
| High-School Graduate (Ref = Not high school graduate) | 0.28 (0.38), p=0.47 | 0.29 (0.39), p=0.45 | 0.29 (0.39), p=0.46 |
| Self-Rated Health Status (5 point scale) | 0.07 (0.18), p=0.72 | 0.06 (0.18), p=0.73 | 0.07 (0.18), p=0.70 |
| Use Insulin (Ref = do not use insulin) | 0.25 (0.35), p=0.48 | 0.24 (0.36), p=0.51 | 0.24 (0.36), p=0.50 |
| Baseline HbA1c% | -0.44 (0.07), p<.0001b | -0.45 (0.07), p<.0001b | -0.44 (0.07), p<.0001b |
| Model R2 | 0.41 | 0.41 | 0.41 |
See Supplemental Tables 2 and 3 for alternate analyses of Models A and B examining diabetes social support and depressive symptoms separately. These alternate analyses did not show significantly different results than the models the above table
p value <0.05
Continuous measure of Diabetes Social Support on 0–4 point scale
Continuous measure of Depressive Symptoms on 0–4 point scale
3.4 Moderating Effects of Diabetes Social Support or Depressive Symptoms on Intervention Effect on HbA1c
In Model B (Table 3), neither interaction term added to Model A was statistically significant, indicating that neither baseline D-SS nor baseline depressive symptoms had more association with change in HbA1c among the intervention group as compared to the control group. In alternate Models B1 and B2 (Supplemental Table 3), results were not significantly different when independent variables related to social support were analyzed separately from those related to depressive symptoms.
3.5 Moderating Effect of Depressive Symptoms on Diabetes Social Support Effect on HbA1c
Given Model A results that baseline D-SS was associated with change in HbA1c, Model C was used to analyze whether baseline depressive symptoms moderated this association. However the interaction term depressive symptoms * social support was not statistically significant, while baseline social support itself remained a significant predictor of change in HbA1c (coefficient -0.39, p=0.02).
3.6 Additional Alternate Models
Alternate models containing a covariate of ‘time from baseline to six-month HbA1c measurement’ and an interaction term for ‘HbA1c interval time’ x study group did not show these covariates were significantly associated with model outcomes. Additional analyses excluding the 13 participants with HbA1c values >90 days from the corresponding interview date did not give significantly different results than reported models (data not shown in tables).
4. Discussion and Conclusion
4.1 Discussion
In this sample of low-income urban African American and Latino adults with type 2 diabetes, participants reporting higher levels of baseline diabetes social support from their family and friends had significantly greater improvements in HbA1c in the following six months than those with less support, independent of additional significant DSME/S intervention-associated HbA1c improvements (Model A). The positive impact of diabetes social support on improvement in glycemic control was independent of any influence of depressive symptoms (Model C). Baseline depressive symptoms were not associated with 6-month change in HbA1c (Model A). Neither social support nor depressive symptoms moderated the effect of being assigned to the diabetes intervention on improvement in HbA1c (Model B).
The key implication of these findings for the design of DSME/S interventions is that participants at all baseline levels of social support or depressive symptoms benefitted equally from the intervention. It is important to note that the intervention we examined did not aim to increase family or friend social support or decrease depressive symptoms among participants. In fact, participants’ mean perceived levels of diabetes-specific social support and depressive symptoms did not change from baseline to 6 and 12-month follow-up.
This is the first study we are aware of to examine pre-intervention social support from family and friends as a potential moderator of the effectiveness of a general and multi-faceted diabetes intervention. In contrast, a study by Barrera and colleagues examined whether a diabetes intervention that aimed to increase participants’ social support was more successful among those whose perceived support changed during the intervention.52 They found that increases in social connection to family and friends, but also professionals and community members, during the program were correlated with intervention effectiveness. In contrast to this [Barrera] study, this DSME/S intervention did not aim to change participants’ social support.
This longitudinal study also contributes to evidence that social support is associated with subsequent improvements in glycemic control, an association that previous cross-sectional studies had not conclusively established. Social support may be linked to improvements in glycemic control through improvements in diabetes self-management behaviors such as healthy eating, physical activity, or medication adherence.24,53,54 However, although we adjusted for many characteristics known to be associated with level of received social support (age, gender, health status, and depressive symptoms), social support may also be associated with an unexamined participant characteristic (including possible unmeasured differences in socioeconomic resources) that was in turn predictive of change in HbA1c.
Notably, the positive impact of diabetes social support was independent of baseline level of depressive symptoms, which was not associated with change in HbA1c and did not moderate intervention effects on HbA1c. In contrast to these results, other studies have found that higher depressive symptoms predict increases in HbA1c and more difficulty with self-management over time.31,55 However one previous study examining the effect of pre-intervention depressive symptoms for people with diabetes in a lifestyle-focused weight loss intervention showed that participants at all levels of baseline depressive symptoms lost similar amounts of weight during the intervention.56 Two similar studies of participants in DSME/S interventions showed that changes in depressive symptoms over the course of an intervention were not correlated with changes in HbA1c over the course of the intervention.57,58 Previous studies have rarely examined the associations of social support and depression on subsequent health outcomes simultaneously. However one such study with patients with heart failure found that social support and depression were both independently associated with patient mortality.59
Our findings must be interpreted in the face of several important limitations. First, study participants were low-income, racial/ethnic minority adults, potentially limiting generalizability beyond these groups. In particular, African-American participants were more likely than Latinos to be missing complete study data, due to increased difficulty in completing follow-up contacts as well as two unexpected deaths among African-American participants. This may limit generalizability particularly among African-Americans with diabetes. Second, as is the case with other interventions targeting high-risk, low-income communities, there was significant participant drop out between study enrollment and post-intervention follow-up. However, we included correlates of missing data in our regression models. Third, while most participants’ HbA1c values were close to their interview dates, we allowed a notable variation in HbA1c timing for a subset of participants. In this CBPR study, this method was used to accommodate community partners who were concerned that some participants would not want to give samples to the study directly. Alternate analyses adjusting for timing of HbA1c measurement did not give disparate results. As community and academic partners developed their working relationship and trust grew, HbA1c samples were obtained directly from participants for a subsequent diabetes management study. Finally, our study assessed the effects of only one type of diabetes social support – positive support from family and friends – and not the effects of pre-existing support from other sources or the effects of ‘negative support’. Negative support, or barriers to diabetes management, from family and friends can have a particularly deleterious effect on diabetes management success60–64 and could be assessed concurrently with positive support in future studies.
4.2 Practice Implications
Our findings that pre-existing social support from family and friends may add to the effects of a diabetes behavioral intervention can contribute to the design and evaluation of interventions in two principal ways. First, baseline diabetes social support will be important to measure as a potentially independent predictor of changes in glycemic control over a DSME/S intervention study period. Second, diabetes programs could encourage participants with high diabetes social support to involve supporters in change efforts, in order to capitalize on this pre-existing, and potentially additive, resource. In addition, diabetes programs could attempt to directly involve supportive family members and friends in ways that maximize the effectiveness of their support.65 Factorial trials that compare DSME/S effects on patients enrolled alone versus enrolled with a supporter could help define what incremental effects could be obtained through this type of additional programmatic focus on patients’ supporters.
Diabetes social support may be particularly important for glycemic control in socially disadvantaged groups, such as the low income, racial/ethnic minority participants in this study. Observational studies among African-Americans and Latinos with diabetes show associations between social support and self-management behaviors,66–70 with some evidence that social support is more important for these groups than for non-Hispanic whites with diabetes.37,38 In the Diabetes Attitudes Wishes and Needs (DAWN) 2 study, African-Americans and Latinos reported larger support networks than non-Hispanic Whites, however they also reported more diabetes-related family arguments.71 Qualitative studies among racial/ethnic minority adults with diabetes further support the important, culturally influenced, and potentially unique, roles that family and friend support play in these adults’ diabetes self-management success.66,72 Furthermore, while African-Americans and Latinos have disproportionate rates of diabetes and continue to suffer worse diabetes outcomes, diabetes management programs focused on racial/ethnic minority groups are often less successful.73 Diabetes programs could test whether efforts to incorporate diabetes social support and minimize negative diabetes support from friends and family in culturally-relevant ways can increase diabetes intervention success among these groups.7
4.3 Conclusion
Within this population of low SES African American and Latino adults with type 2 diabetes, individuals with higher baseline diabetes social support from family and friends had subsequently larger improvements in glycemic control that were independent of additional improvements in glycemic control related to assignment to a general DSME/S intervention. However effects of the DSME/S intervention on glycemic control were not moderated by baseline depressive symptoms or social support. Diabetes self-management and education programs have potential to be successful for participants starting with varying levels of social support and depressive symptoms. Participants with higher diabetes social support from family and friends may be more likely to improve glycemic control over time in ways that complement gains made in general diabetes self-management programs.
Supplementary Material
Highlights.
We examined how diabetes social support and depressive symptoms affected change in glycemic control over six months.
Participants were randomized to a diabetes self-management intervention or usual care
Higher social support at baseline predicted larger improvements in glycemic control
These improvements were independent of intervention improvements in glycemic control
Baseline social support and depression levels did not change how effective the intervention was
Acknowledgments
We thank the CHASS/REACH Detroit Partnership staff, the REACH Detroit Partnership Steering Committee, and the REACH Detroit Partnership intervention participants for their contributions and dedication to the work of REACH Detroit (www.reachdetroit.org). The REACH Detroit Partnership is affiliated with the Detroit Community-Academic Urban Research Center (www.sph.umich.edu/URC/). We also thank Deborah Levine and John Piette for helpful comments on earlier versions of this manuscript, and Alison VonAchen for preliminary work on analyses.
Funding
Support for this study was provided by the National Institute of Health Grant R18-DK078558, the Michigan Center for Diabetes Translational Research (P30DK092926, National Institute of Diabetes and Digestive and Kidney Diseases R18DK078558), the Centers for Disease Control and Prevention Cooperative Agreement No. U50/CCU417409, and the Robert Wood Johnson Foundation Clinical Scholars Program. Ann-Marie Rosland is a VA HSR&D Career Development Awardee. None of the funders had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Author Contributions
AR developed the analysis plan, analyzed data and wrote the manuscript; EK contributed to data analysis and reviewed/edited the manuscript; MS conducted the clinical trial, contributed to data analysis, and reviewed/edited the manuscript; BS analyzed data and reviewed/edited the manuscript; GP conducted the clinical trial and reviewed/edited the manuscript; MV reviewed/edited the manuscript; EN reviewed/edited the manuscript; MH developed the analysis plan and reviewed/edited the manuscript.
No authors have relevant conflicts of interest.
Registered as Clinical Trial NCT00800410.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fan T, Koro CE, Fedder DO, Bowlin SJ. Ethnic disparities and trends in glycemic control among adults with type 2 diabetes in the U.S. from 1988 to 2002. Diabetes Care. 2006;29:1924–1925. doi: 10.2337/dc05-2238. [DOI] [PubMed] [Google Scholar]
- 2.Pimouguet C, Goff ML, Thiébaut R, Dartigues JF, Helmer C. Effectiveness of disease-management programs for improving diabetes care: a meta-analysis. Can Med Assoc J. 2011;183:E115–E127. doi: 10.1503/cmaj.091786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark M. Diabetes self-management education: A review of published studies. Prim Care Diabetes. 2008;2:113–120. doi: 10.1016/j.pcd.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25:1159–71. doi: 10.2337/diacare.25.7.1159. [DOI] [PubMed] [Google Scholar]
- 5.Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. Jama. 2002;288:2469–75. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- 6.Loveman E, Frampton GK, Clegg AJ. The clinical effectiveness of diabetes education models for Type 2 diabetes: a systematic review. Health Technol Assess. 2008;12:1–136. doi: 10.3310/hta12090. [DOI] [PubMed] [Google Scholar]
- 7.Sarkisian CA, et al. A systematic review of diabetes self-care interventions for older, African American, or Latino adults. Diabetes Educ. 2003;29:467–79. doi: 10.1177/014572170302900311. [DOI] [PubMed] [Google Scholar]
- 8.Hörnsten A, Stenlund H, Lundman B, Sandström H. Improvements in HbA1c remain after 5 years–a follow up of an educational intervention focusing on patients’ personal understandings of type 2 diabetes. Diabetes Res Clin Pract. 2008;81:50–55. doi: 10.1016/j.diabres.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Lorig K, et al. Online diabetes self-management program: a randomized study. Diabetes Care. 2010;33:1275–1281. doi: 10.2337/dc09-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welch G, Garb J, Zagarins S, Lendel I, Gabbay RA. Nurse diabetes case management interventions and blood glucose control: results of a meta-analysis. Diabetes Res Clin Pract. 2010;88:1–6. doi: 10.1016/j.diabres.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 11.Tshiananga JKT, et al. The effect of nurse-led diabetes self-management education on glycosylated hemoglobin and cardiovascular risk factors: a meta-analysis. Diabetes Educ. 2011 doi: 10.1177/0145721711423978. 0145721711423978. [DOI] [PubMed] [Google Scholar]
- 12.Coster S, Norman I. Cochrane reviews of educational and self-management interventions to guide nursing practice: a review. Int J Nurs Stud. 2009;46:508–528. doi: 10.1016/j.ijnurstu.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Minet L, Møller S, Vach W, Wagner L, Henriksen JE. Mediating the effect of self-care management intervention in type 2 diabetes: A meta-analysis of 47 randomised controlled trials. Patient Educ Couns. 2010;80:29–41. doi: 10.1016/j.pec.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan SH, Billimek J, Sorkin DH, Ngo-Metzger Q, Greenfield S. Who Can Respond to Treatment?: Identifying Patient Characteristics Related to Heterogeneity of Treatment Effects. Med Care. 2010;48:S9–S16. doi: 10.1097/MLR.0b013e3181d99161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glasgow RE, et al. Twelve-month outcomes of an Internet-based diabetes self-management support program. Patient Educ Couns. 2012;87:81–92. doi: 10.1016/j.pec.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothman RL, et al. Influence of patient literacy on the effectiveness of a primary care–based diabetes disease management program. Jama. 2004;292:1711–1716. doi: 10.1001/jama.292.14.1711. [DOI] [PubMed] [Google Scholar]
- 17.Gallant M. Help or hindrance? How family and friends influence chronic illness self-management among older adults. Res Aging. 2007;29:375–409. [Google Scholar]
- 18.Schlundt DG, Rea MR, Kline SS, Pichert JW. Situational obstacles to dietary adherence for adults with diabetes. J Am Diet Assoc. 1994;94:874–876. 879. doi: 10.1016/0002-8223(94)92367-1. quiz 877–878. [DOI] [PubMed] [Google Scholar]
- 19.El-Kebbi IM, et al. Diabetes in urban African Americans. V Use of discussion groups to identify barriers to dietary therapy among low-income individuals with non-insulin-dependent diabetes mellitus. Diabetes Educ. 1996;22:488–92. doi: 10.1177/014572179602200508. [DOI] [PubMed] [Google Scholar]
- 20.Trief PM, et al. Describing support: a qualitative study of couples living with diabetes. Fam Syst Health. 2003;21:57–67. [Google Scholar]
- 21.Gomersall T, Madill A, Summers LKM. A Metasynthesis of the Self-Management of Type 2 Diabetes. Qual Health Res. 2011;21:853–871. doi: 10.1177/1049732311402096. [DOI] [PubMed] [Google Scholar]
- 22.Shumaker SA, Brownell A. Toward a theory of social support: Closing conceptual gaps. J Soc Issues. 1984;40:11–36. [Google Scholar]
- 23.Gallant M. The influence of social support on chronic illness self-management: a review and directions for research. Health Educ Behav. 2003;30:170–195. doi: 10.1177/1090198102251030. [DOI] [PubMed] [Google Scholar]
- 24.Rosland AM, et al. Social support and lifestyle vs. medical diabetes self-management in the Diabetes Study of Northern California (DISTANCE) Ann Behav Med. 2014 doi: 10.1007/s12160-014-9623-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trief PM, Grant W, Elbert K, Weinstock RS. Family environment, glycemic control, and the psychosocial adaptation of adults with diabetes. Diabetes Care. 1998;21:241–5. doi: 10.2337/diacare.21.2.241. [DOI] [PubMed] [Google Scholar]
- 26.Griffith LS, Field BJ, Lustman PJ. Life stress and social support in diabetes: association with glycemic control. Int J Psychiatry Med. 1990;20:365–72. doi: 10.2190/APH4-YMBG-NVRL-VLWD. [DOI] [PubMed] [Google Scholar]
- 27.Brody GH, Kogan SM, Murry VM, Chen Y, Brown AC. Psychological functioning, support for self-management, and glycemic control among rural African American adults with diabetes mellitus type 2. Health Psychol. 2008;27:S83–90. doi: 10.1037/0278-6133.27.1.S83. [DOI] [PubMed] [Google Scholar]
- 28.Nakahara R, et al. Prospective study on influence of psychosocial factors on glycemic control in Japanese patients with type 2 diabetes. Psychosomatics. 2006;47:240–6. doi: 10.1176/appi.psy.47.3.240. [DOI] [PubMed] [Google Scholar]
- 29.Okura T, Heisler M, Langa KM. Association between cognitive function and social support with glycemic control in adults with diabetes mellitus. J Am Geriatr Soc. 2009;57:1816–24. doi: 10.1111/j.1532-5415.2009.02431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fortmann AL, et al. Glycemic control among US Hispanics/Latinos with diabetes from the HCHS/SOL Sociocultural Ancillary Study: Do structural and functional social support play a role? J Behav Med. 2014;38:153–159. doi: 10.1007/s10865-014-9587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lustman P, et al. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000;23:934–42. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 32.Aikens J. Prospective associations between emotional distress and poor outcomes in type 2 diabetes. Diabetes Care. 2012;35:2472–8. doi: 10.2337/dc12-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiu C, Wray L, Beverly E, Dominic O. The role of health behaviors in mediating the relationship between depressive symptoms and glycemic control in type 2 diabetes: a structural equation modeling approach. Soc Psychiatry Psychiatr Epidemiol. 2010;45:67–76. doi: 10.1007/s00127-009-0043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher L, et al. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33:23–28. doi: 10.2337/dc09-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisher L, Glasgow RE, Strycker LA. The Relationship Between Diabetes Distress and Clinical Depression With Glycemic Control Among Patients With Type 2 Diabetes. Diabetes Care. 2010;33:1034–1036. doi: 10.2337/dc09-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Funnell MM, Anderson RM. Empowerment and self-management of diabetes. Clin Diabetes. 2004;22:123–127. [Google Scholar]
- 37.Spencer MS, et al. Effectiveness of a community health worker intervention among African American and Latino adults with type 2 diabetes: A randomized controlled trial. Am J Public Health. 2011;101:2253–2260. doi: 10.2105/AJPH.2010.300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kieffer EC, et al. Reducing disparities in diabetes among African-American and Latino residents of Detroit: the essential role of community planning focus groups. Ethn Dis. 2004;14:S27–37. [PubMed] [Google Scholar]
- 39.Two Feathers J, et al. Racial and Ethnic Approaches to Community Health (REACH) Detroit partnership: improving diabetes-related outcomes among African American and Latino adults. Am J Public Health. 2005;95:1552–60. doi: 10.2105/AJPH.2005.066134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Two Feathers J, et al. The development, implementation, and process evaluation of the REACH Detroit Partnership’s Diabetes Lifestyle Intervention. Diabetes Educ. 2007;33:509–520. doi: 10.1177/0145721707301371. [DOI] [PubMed] [Google Scholar]
- 41.Wallerstein NB, Duran B. Using community-based participatory research to address health disparities. Health Promot Pract. 2006;7:312–313. doi: 10.1177/1524839906289376. [DOI] [PubMed] [Google Scholar]
- 42.Fitzgerald J, et al. The reliability of the diabetes care profile for African Americans. Eval Health Prof. 1998;21:52–65. doi: 10.1177/016327879802100103. [DOI] [PubMed] [Google Scholar]
- 43.Fitzgerald JT, et al. Development and validation of the Diabetes Care Profile. Eval Health Prof. 1996;19:208–30. doi: 10.1177/016327879601900205. [DOI] [PubMed] [Google Scholar]
- 44.Kroenke K, Spitzer R, Williams J. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav. 1997;38:21–37. [PubMed] [Google Scholar]
- 46.Little RJA. Statistical Analysis with Missing Data. 2. Wiley-Interscience; 2002. [Google Scholar]
- 47.Sherman AM. Social relations and depressive symptoms in older adults with knee osteoarthritis. Soc Sci Med. 2003;56:247–57. doi: 10.1016/s0277-9536(02)00023-0. [DOI] [PubMed] [Google Scholar]
- 48.Schulz AJ, et al. Psychosocial stress and social support as mediators of relationships between income, length of residence and depressive symptoms among African American women on Detroit’s eastside. Soc Sci Med. 2006;62:510–22. doi: 10.1016/j.socscimed.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 49.Bisschop MI, Kriegsman DM, Beekman AT, Deeg DJ. Chronic diseases and depression: the modifying role of psychosocial resources. Soc Sci Med. 2004;59:721–33. doi: 10.1016/j.socscimed.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 50.Osborn CY, Egede LE. The relationship between depressive symptoms and medication nonadherence in type 2 diabetes: the role of social support. Gen Hosp Psychiatry. 2012;34:249–253. doi: 10.1016/j.genhosppsych.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maxwell S. Sample size and multiple regression analysis. Psychol Methods. 2000;5:434–58. doi: 10.1037/1082-989x.5.4.434. [DOI] [PubMed] [Google Scholar]
- 52.Barrera MJ, Toobert DJ, Angell KL, Glasgow RE, Mackinnon DP. Social support and social-ecological resources as mediators of lifestyle intervention effects for type 2 diabetes. J Health Psychol. 2006;11:483–95. doi: 10.1177/1359105306063321. [DOI] [PubMed] [Google Scholar]
- 53.Cohen S. Psychosocial models of the role of social support in the etiology of physical disease. Health Psychol. 1988;7:269–97. doi: 10.1037//0278-6133.7.3.269. [DOI] [PubMed] [Google Scholar]
- 54.Uchino BN. Social support and health: a review of physiological processes potentially underlying links to disease outcomes. J Behav Med. 2006;29:377–87. doi: 10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]
- 55.Dzida G, et al. Depressive symptoms prior to and following insulin initiation in patients with type 2 diabetes mellitus: Prevalence, risk factors and effect on physician resource utilisation. Prim Care Diabetes. 2015 doi: 10.1016/j.pcd.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Faulconbridge LF, et al. Changes in depression and quality of life in obese individuals with binge eating disorder: bariatric surgery versus lifestyle modification. Surg Obes Relat Dis. 2013;9:790–796. doi: 10.1016/j.soard.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leyva B, Zagarins SE, Allen NA, Welch G. The relative impact of diabetes distress vs depression on glycemic control in hispanic patients following a diabetes self-management education intervention. Ethn Dis. 2011;21:322–327. [PubMed] [Google Scholar]
- 58.Zagarins SE, Allen NA, Garb JL, Welch G. Improvement in glycemic control following a diabetes education intervention is associated with change in diabetes distress but not change in depressive symptoms. J Behav Med. 2012;35:299–304. doi: 10.1007/s10865-011-9359-z. [DOI] [PubMed] [Google Scholar]
- 59.Friedmann E, et al. Relationship of depression, anxiety, and social isolation to chronic heart failure outpatient mortality. Am Heart J. 2006;152:940.e1–8. doi: 10.1016/j.ahj.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 60.Stephens MAP, et al. Spouses’ Attempts to Regulate Day-to-Day Dietary Adherence Among Patients With Type 2 Diabetes. Health Psychol. 2012 doi: 10.1037/a0030018. [DOI] [PubMed] [Google Scholar]
- 61.Mayberry LS, Osborn CY. Family Support, Medication Adherence, and Glycemic Control Among Adults With Type 2 Diabetes. Diabetes Care. 2012;35:1239–1245. doi: 10.2337/dc11-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mayberry LS, Rothman RL, Osborn CY. Family Members’ Obstructive Behaviors Appear to Be More Harmful Among Adults With Type 2 Diabetes and Limited Health Literacy. J Health Commun. 2014;19:132–143. doi: 10.1080/10810730.2014.938840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mayberry LS, Osborn CY. Family involvement is helpful and harmful to patients’ self-care and glycemic control. Patient Educ Couns. 2014;97:418–425. doi: 10.1016/j.pec.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosland AM, Heisler M, Choi HJ, Silveira MJ, Piette JD. Family influences on self-management among functionally independent adults with diabetes or heart failure: do family members hinder as much as they help? Chronic Illn. 2010;6:22–33. doi: 10.1177/1742395309354608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rosland AM, Heisler M, Piette JD. The impact of family behaviors and communication patterns on chronic illness outcomes: a systematic review. J Behav Med. 2012;35:221–239. doi: 10.1007/s10865-011-9354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gallant MP, Spitze G, Grove JG. Chronic illness self-care and the family lives of older adults: a synthetic review across four ethnic groups. J Cross-Cult Gerontol. 2010;25:21–43. doi: 10.1007/s10823-010-9112-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ford ME, Tilley BC, McDonald PE. Social support among African-American adults with diabetes, Part 2: A review. J Natl Med Assoc. 1998;90:425–32. [PMC free article] [PubMed] [Google Scholar]
- 68.Rosland AM, et al. When is social support important? The association of family support and professional support with specific diabetes self-management behaviors. J Gen Intern Med. 2008;23:1992–1999. doi: 10.1007/s11606-008-0814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bailey BJ, Lherisson-Cedeno D. Diabetes outcomes and practices: comparison of African Americans and Caucasians. J Natl Black Nurses Assoc. 1997;9:66–75. [PubMed] [Google Scholar]
- 70.Rees CA, Karter AJ, Young BA. Race/ethnicity, social support, and associations with diabetes self-care and clinical outcomes in NHANES. Diabetes Educ. 2010;3:435–445. doi: 10.1177/0145721710364419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peyrot M, et al. Ethnic differences in psychological outcomes among people with diabetes: USA results from the second Diabetes Attitudes, Wishes, and Needs (DAWN2) study. Curr Med Res Opin. 2014;30:2241–2254. doi: 10.1185/03007995.2014.947023. [DOI] [PubMed] [Google Scholar]
- 72.Becker G, Beyene Y, Newsom EM, Rodgers DV. Knowledge and care of chronic illness in three ethnic minority groups. Fam Med. 1998;30:173–180. [PubMed] [Google Scholar]
- 73.Peek ME, Cargill A, Huang ES. Diabetes health disparities: a systematic review of health care interventions. Med Care Res Rev. 2007;64:101S–56S. doi: 10.1177/1077558707305409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.