Abstract
Prenatal organophosphate (OP) pesticide exposure has been reported to be associated with adverse birth outcomes and neurodevelopment. However, the mechanisms of toxicity of OP pesticides on human fetal development have not yet been elucidated. Our pilot study birth cohort, the Study of Asian Women and Offspring’s Development and Environmental Exposures (SAWASDEE cohort) aimed to evaluate environmental chemical exposures and their relation to birth outcomes and infant neurodevelopment in 52 pregnant farmworkers in Fang district, Chiang Mai province, Thailand. A large array of data was collected multiple times during pregnancy including approximately monthly urine samples for evaluation of pesticide exposure, three blood samples for pesticide-related enzyme measurements and questionnaire data. This study investigated the changes in maternal acetylcholinesterase (AChE) and paraoxonase 1 (PON1) activities and their relation to urinary diakylphosphates (DAPs), class-related metabolites of OP pesticides, during pregnancy. Maternal AChE, butyrylcholinesterase (BChE) and PON1 activities were measured three times during pregnancy and urinary DAP concentrations were measured, on average, 8 times from enrollment during pregnancy until delivery. Among the individuals in the group with low maternal PON1 activity (n = 23), newborn head circumference was negatively correlated with log10 maternal ΣDEAP and ΣDAP at enrollment (gestational age=12±3 weeks; β = −1.0 cm, p = 0.03 and β = −1.8 cm, p <0.01, respectively) and at 32 weeks pregnancy (β = −1.1 cm, p = 0.04 and β = −2.6 cm, p = 0.01, respectively). Furthermore, among these mothers, newborn birthweight was also negatively associated with log10 maternal ΣDEAP and ΣDAP at enrollment (β = −219.7 g, p = 0.05 and β = −371.3 g, p = 0.02, respectively). Associations between maternal DAP levels and newborn outcomes were not observed in the group of participants with high maternal PON1 activity. Our results support previous findings from US birth cohort studies. This is the first study to report the associations between prenatal OP pesticide exposure and birth outcomes in Thailand.
Keywords: Paraoxonase 1 (PON1), organophosphate pesticides, birth outcomes, birth cohort, exposure assessment
1. Introduction
Organophosphate (OP) pesticides are among the most widely used pesticides in many areas of the world (Janine and Knight, 2011). In Thailand, following the decision to ban organochlorine pesticides for agricultural use in 1988, OP pesticides have gained popularity for effective control of multiple insects during the cultivation of various tropical fruits, vegetable, rice, legumes, etc. More than a million kilograms of OP pesticides (accounting for about 15% of the total amounts of imported insecticides) are imported into the country annually. The most highly used OP pesticides include chlorpyrifos, profenophos, dimethoate, and ethion (OAE, 2013). Because OP pesticides are widely used, in multiple crop types and likely in all cultivating seasons, their contaminations of produce largely consumed by local populations has been reported (Harnpicharnchai et al. 2013; Prapamontol et al. 2010; Sapbamrer and Hongsibsong 2014).
Prenatal or in utero exposure to OP pesticides has also been linked to more subtle effects such as fetal neurodevelopment and adverse birth outcomes. Studies of rodents found that prenatal exposure to some OP pesticides was associated with decreased fetal growth in offspring (Chanda and Pope 1996; Srivastava and Raizada 1996). In human epidemiological studies, adverse birth outcomes and neurodevelopmental deficits in children prenatally exposed to pesticides were observed consistently at several ages. For instance, prenatal OP pesticide exposures, as measured by the levels of maternal parent OP pesticides in blood or their urinary dialkylphosphate (DAP) metabolites, were linked to decreases in gestational duration (Eskenazi et al. 2004), birth weight, birth length (Whyatt et al. 2004), and head circumference (Berkowitz et al. 2004). Similarly, prenatal OP pesticide exposures have been associated with neurodevelopmental deficits (Bouchard et al. 2011; Engel et al. 2007; Marks et al. 2010; Rauh et al. 2006; Young et al. 2005; Zhang et al. 2014).
The primary acute mechanism of OP toxicity is the inhibition of acetylcholinesterase (AChE) enzyme activity in the nervous systems (Gupta 2006). AChE is responsible for breaking down the neurotransmitter, acetylcholine, at the skeletal neuromuscular junction and synapses. The mechanism of inhibition involves the oxon form of the OP pesticide binding to the serine hydroxyl group on the AChE active site, thus preventing acetylcholine binding leading to cholinergic hyperactivity (Gupta 2006). The hydrolysis of some active OP pesticides into their metabolites is mediated by the paraoxonase 1 (PON1) enzyme. Generally, PON1 polymorphisms contribute to differential susceptibility of humans to OP toxicity (Costa et al. 2013; Eckerson et al. 1983; Holland et al. 2006; Li et al. 2000). Several human studies reported the mediation of birth outcomes and neurodevelopment deficits associated with prenatal OP pesticide exposure by maternal PON1 polymorphism characteristics (Berkowitz et al. 2004; Engel et al. 2007; Engel et al. 2011; Rauch et al. 2012).
We were particularly interested in investigating the birth outcomes of a population with high risk of OP pesticide exposures in Thailand. Unlike any other previous U.S. cohort studies, we focused on pregnant women who actively engaged in agricultural activities without proper training in pesticide applications, lived and worked (Lorenz et al. 2012) where different OP pesticides were used with high volume and frequency (Prapamontol et al. 2010) compared to the U.S. population. This population could enable us to investigate different adverse health outcomes related to exposure to different OP pesticides. Therefore, we investigated the relationship between prenatal OP exposure and birth outcomes in our pilot cohort in the Study of Asian Women and OffSpring’s Development and Environmental Exposures (SAWASDEE). The SAWASDEE study was established in Fang district, an important agricultural area in Chiang Mai province, Thailand. Birth outcomes (birth weight, birth length, and head circumference) and maternal biomarkers including red blood cell AChE activity, plasma butyrylcholinesterase (BChE) activity, PON1 activity, and urinary DAP levels across pregnancy were the primary variables in this study.
2. Materials and methods
2.1 Study site
Collectively, agricultural areas in Fang district of Chiang Mai province cover 239 square kilometres comprising about a quarter of its total area. Fang district is the major producer of oranges that include mandarin oranges (Citrus reticulata Blanco), and, to a lesser extent, tangerines (Citrus tangerina). Production of citrus fruits in this area requires intensive use of pesticides during the pre- and post-harvest periods. Owing to a suitable climate and altitude, up to three harvests can be obtained per year per farm, potentially resulting in constant exposures to pesticides among farmworkers.
We previously established strong evidences of OP pesticide contamination in food and the environment in this area resulting from agricultural pesticide use (Prapamontol et al. 2009; Prapamontol et al. 2010). Furthermore, we previously reported that a group of pregnant farmworkers in this area had inadequate knowledge and unsafe pesticides practices (Lorenz et al. 2012), suggesting they might be prone to high pesticide exposures and any resulting adverse effects.
2.2 Study participants
The SAWASDEE study, a collaborative effort of Chiang Mai University’s Research Institute for Health Sciences, Thailand, and Emory University’s Rollins School of Public Health, was a pilot prospective birth cohort study conducted in Fang Hospital (Ministry of Public Health), Fang district, Chiang Mai province, Thailand with enrollment in early pregnancy, temporally-resolved pesticide exposure assessment during pregnancy and adverse neurodevelopmental outcomes measures in neonates shortly after birth.
Fifty-nine pregnant women were recruited from the antenatal care unit at Fang Hospital from March 2011 to February 2012. The inclusion criteria included: (1) aged 18–35 years old; (2) was pregnant with gestation at enrollment in first or early second trimester (≤16 weeks of pregnancy); (3) were farmworkers; (4) had no serious medical problems; and (5) planned to live in Fang district longer than 6 months. All participants volunteered to participate and signed an informed consent form before data collection began. This study protocol was approved by: 1) Office of Research Ethics, Research Institute for Health Sciences, Chiang Mai University; 2) Ethical Review Committee for Research in Human Participants, Ministry of Public Health of Thailand; and 3) Emory University’s Institutional Review Board.
2.3 Questionnaire data collection
Structured questionnaires were employed to collect demographics, personal habits and behaviors, medical and pregnancy history, occupational information and pesticide use. Data were collected three times: at enrollment, 24 weeks gestation, and shortly after delivery. All questionnaires were administered by trained staff in a face-to-face setting.
2.4 Maternal blood collection
Maternal blood was collected three times during pregnancy: at enrollment (M1); 32 weeks of pregnancy (M2); and, at delivery (M3). Maternal venous blood was collected in two 10-mL heparinized Vacutainer® blood collection tubes (BD, Franklin Lakes, NJ, U.S.A.). Whole blood was centrifuged at 1,500×g for 20 minutes, 4 °C. Heparinized plasma was aspirated using a disposable pasture pipette. The buffy coat was transferred to a new micro-centrifuge tube for further study. The packed red blood cells were washed twice with phosphate buffer (pH 7.4). After washing, the red blood cells were mixed and aliquoted (2 × 1 mL) into a 1.5-mL polystyrene tube using a disposable pasture pipette. All maternal plasma and red blood cells samples were stored in −20 °C until analysis.
2.5 Cord blood collection
The umbilical cord blood sample (C), 2 × 10 mL, was immediately collected from the umbilical cord vein when the umbilical cord was cut. Cord blood was processed as was the maternal blood. Samples were also stored in −20 °C until analysis.
2.6 Enzyme assay
2.6.1 Measurements of cholinesterase enzyme activities
Red blood cells and plasma from maternal venous and cord blood were assayed for AChE and BChE enzyme activities using the Ellman’s spectrophotometric method (Ellman et al. 1961) with a slight modification. For the AChE assay, 20 μL of red blood cells in phosphate buffer (dilution: 1:200) and 200 μL of 0.52 mM 5, 5′-dithiobis-2-nitrobenzoic acid (DTNB) in 5 mM phosphate buffer (pH 8) were added in each well of a 96-well polystyrene plate. A 20 μL of 5.5 mM acetylthiocholine substrate was added. The rate of formation of TNB was monitored for 10 min at 405 nm (25 °C). For the BChE assay, 20 μL of plasma in phosphate buffer (dilution: 1:100) was tested using butyrylthiocholine as the substrate. The absorbance at 405 nm was used to calculate the concentration of 5-thio-2-nitrobenzoic acid (TNB) using Lambert–Beer’s law (ɛTNB = 13,600 M−1 cm−1).
2.6.2 Measurements of paraoxonase (PONase) and arylesterase (AREase) activities of PON1
Plasma from maternal venous and cord blood were assayed for PONase and AREase activities using Huen’s method (Huen et al. 2009). For PONase assay, 20 μL of plasma (dilution: 1:10) was added to 200 μL of 1.2 mM paraoxon (0.1 M Tris-HCl, pH 8.5, 2 mM CaCl2, 2 M NaCl). The concentration of ρ-nitrophenol as the paraoxon hydrolytic product was determined at 405 nm every 15 s over 2 min. For AREase assay, 20 μL of plasma (dilution: 1:80) was added to 200 μL of 3.26 mM phenyl acetate solution (9 mM Tris–HCl pH 8.0 with 0.9 mM CaCl2) in each well of a 96-well UV transparent plate. The concentration of phenol as the hydrolytic product of phenyl acetate was determined at 270 nm every 15 s over 2 min. The concentrations of ρ-nitrophenol and phenol were calculated using Lambert–Beer’s law (ɛρ-nitrophenol = 18,000 M−1 cm−1 and ɛphenol =1,310 M−1cm−1, respectively).
All assays were performed in triplicate. Internal quality control aliquots using pooled samples were used for every assay. The inter-assay relative standard deviations (RSDs) of AChE, BChE, PONase and AREase assays were 5.3%, 5.2%, 4.2% and 1.6%, respectively.
2.7 Paraoxonase 1 phenotyping
An individual’s PON1 phenotype was determined by using the ratio of salt-stimulated PONase activity to AREase activity according to Eckerson’s method (Eckerson et al. 1983). Intra-person activities of each enzyme from three collections were averaged before calculating the ratio. The cumulative distribution and the histogram of the ratio were plotted. The obvious gaps from the plot were used to separate into three PON1 phenotypes of low (AA), middle (AB) and high (BB) activity which are related to QQ, QR and RR genotypes, respectively, at codon192 of PON1 (Humbert et al. 1993). One of the PON1 polymorphisms is the Q192R polymorphism. The change of codon in exon 6 of PON1 gene results in substitution of amino acid glutamine with arginine at the position 192. The activities of these two alloenzymes towards different OP pesticides were different because the amino acid at the position 192 is an important residue of PON1 active site (Harel et al. 3004). From In vivo study, R192 alloenzyme provides more protection against chlorpyrifos oxon (Li et al., 2000).
2.8 Urinary DAPs analysis
Fifty mL of spot urine were collected from the participants at every visit in the hospital, on average 8 samples per participant. Urine samples were aliquot (5 × 10 mL) into 12-mL polystyrene tubes and stored at −20 °C until analysis. Six DAPs including dimethylphosphate (DMP), dimethylthiophosphate (DMTP), dimethydithiophosphate (DMDTP), diethylphosphate (DEP), diethylthiophosphate (DETP), and diethydithiophosphate (DEDTP) were measured in urine samples using GC-FPD (Prapamontol et al. 2014) which was cross-validated with GC-MS. Details of the analysis and data characteristics are described elsewhere (Ryan et al. submitted).
2.9 Birth outcome measurements
Body weight and length, and head circumference of newborns were abstracted from medical records.
2.10 Statistical analysis
All statistical tests were performed using SPSS, version 17 (SPSS Inc., Thailand) and considered significant where p-values were less than 0.05. Descriptive statistics were calculated for general characteristics of study participants and all measurements.
All enzyme activity data were tested for normal distribution using Kolmogorov-Smirnov test. Since intra-person AChE data across pesticide-applying seasons from all participants was not available in this study, the differences of within-person data across pregnancy was calculated for AChE activity inhibition. Percent inhibition of maternal AChE activity was calculated as the % change in activity in blood collected at enrollment and the activity at 3rd trimester (32 wks of pregnancy) and delivery. Participants worked extensively in agriculture at enrollment and they stopped working during the 3rd trimester until delivery. Maternal AChE activities at 32 wks of pregnancy and delivery were averaged and used as a person’s baseline level. AChE activity inhibition fell into 3 distinct categories: less than 0%, > 0–20% and > 20%. Many publications use >20% inhibition of AChE activity as the determinant of biological significance (Hackathorn et al. 1983; Stefanidou et al. 2003). These categories from maternal activities were used for comparing newborn birth outcomes.
The distributions of concentrations of all six maternal urinary DAPs were skewed. Therefore, ΣDMAP (molar sum of DMP, DMTP and DMTDP), ΣDEAP (molar sum of DEP, DETP and DETDP) and ΣDAP (molar sum of all six DAPs) were calculated and also transformed into log10 values for statistical analysis. Detailed calculation can be seen elsewhere (Panuwet et al. 2008).
The differences between variables such as enzyme activities from three collection times were tested by paired-sample t-test while the differences among categorical variables such as enzymes activities and birth outcomes among the different group of AChE activity inhibition and PON1 phenotypes were tested by one-way ANOVA. The paired correlations between continuous variables were analysed by Pearson’s correlation analysis.
Linear regression was used to examine the association of maternal log10 DAP levels and birth outcomes in each group, either classified by AChE inhibition or PON1 phenotype. For PON1 phenotype stratification, AA and AB phenotype (n = 6 and 17, respectively) were pooled together (n = 23) to increase the number before performing regression analysis. Covariates used in the regression model were selected from variables which are known to be potential confounders such as maternal age, pre-pregnancy BMI, weight gain during pregnancy and gestational age. Smoking and alcohol consumption were not used in the models because none of participants reported smoking and consuming drugs and alcohol.
3. Results
3.1 Characteristics of study population
Characteristics of the study population are shown in Table 1. At enrollment, 59 participants were recruited. Three participants were excluded during follow-up period; one participant moved from the study area and two participants miscarried. Four participants were removed from analysis because of incomplete blood sample collection. A total of 52 participants were included in statistical analyses.
Table 1.
Characteristics of study population
| Characteristics (n = 52) | ||
|---|---|---|
| Mean ± SD | Min-Max | |
| Age (years) | 26 ± 4 | 18–35 |
| Gestational age at enrollment (wk) | 12 ± 3 N (%) |
7–16 |
| Ethnicity | ||
| Thai | 11 (21.1%) | |
| Others (Tai Yai, Palong Chinese Yunnan, Lahu and Akha) | 41 (78.9%) | |
| Marital status | ||
| married | 5 (9.6%) | |
| living as married | 47 (90.4%) | |
| Income (poverty level = 2,400 Baht/month) | ||
| below poverty | 31 (59.6%) | |
| above poverty | 18 (34.6%) | |
| not known | 3 (5.8%) | |
| Level of education | ||
| no education | 33 (63.5%) | |
| primary school | 9 (17.3%) | |
| secondary school | 2 (3.8%) | |
| high school | 7 (13.5%) | |
| College/University | 1 (1.9%) | |
| Worked during pregnancy | ||
| 1st trimester | 50 (96.2%) | |
| 2nd trimester | 37 (71.1%) | |
| 3rd trimester | 9 (17.3%) | |
| Sprayed pesticides by themselves during pregnancy | ||
| 1st trimester | 14 (26.9%) | |
| 2nd trimester | 7 (13.5%) | |
| 3rd trimester | 0 (0%) | |
| Helped another person spraying pesticide during pregnancy (such as holding spray hose) | ||
| 1st trimester | 44 (84.6%) | |
| 2nd trimester | 35 (67.3%) | |
| 3rd trimester | 9(17.3%) | |
| Delivery | 51 (98.2%) | |
| full-term | 1 (1.8%) | |
| pre-term | ||
| Mean ± SD | Min-Max | |
| Newborn body weight (g) | 2,857 ± 428 | 1,560–3,750 |
| Newborn body length (cm) | 51.4 ± 2.8 | 41.0–56.0 |
| Newborn head circumference (cm) | 32.7 ± 1.6 | 28.0–37.0 |
Mean±SD gestational age at enrollment was 12 ± 3 wks which was between the late 1st trimester and the early 2nd trimester of gestation. Most participants (71.4%) still worked until the 2nd trimester of gestation and some of them (12.5%) applied pesticides in their work. Most of participants (78.9%) were migrant farmworkers with work-permitting card. About half of participants worked in a tangerine orchard. Out of 59 participants, 55 of the babies (98.2%) were full-term gestation (> 36 wks). Eight participants (15%) delivered the babies with low birth weight (< 2,500 g).
3.2 Enzyme activities
Activities of red blood cell AChE, plasma BChE, PONase and AREase in maternal blood during pregnancy and cord blood are presented in Table 2. Mean maternal red blood cell AChE activity in samples collected at enrollment (M1) (5.5 ± 1.0 U/mL) were significantly lower than those in samples collected at 32 wks of pregnancy (M2) and delivery (M3) (6.1±0.9 and 5.9±1.1 U/mL, respectively); however, the mean activities at 32 wks of pregnancy (M2) and delivery (M3) were not significantly different. Mean AREase activities in all three maternal plasma sample collections were significantly different (enrolment <32 weeks < delivery). No significant differences were seen in BuChE activities across pregnancy.
Table 2.
Activities of red blood cell AChE, plasma BChE, PONase and AREase in maternal blood during pregnancy and cord blood.
| Enzymes | Activities (mean ± SD)
|
|||
|---|---|---|---|---|
| Maternal blood (n = 52)
|
Cord blood (n = 52) | |||
| M1 | M2 | M3 | ||
| AChE (U/mL) | 5.5±1.0#†δ | 6.1±0.9*δ | 5.9 ±1.1*δ | 3.1 ± 0.7*#† |
| BChE (U/mL) | 2.6±0.5 | 2.4±0.6 | 2.5±0.6 | 2.5 ± 0.8 |
| PONase (U/L) | 541.3± 214.2#†δ | 631.0 ± 228.4*δ | 637.6 ± 257.3*δ | 169.0 ± 101.1*#† |
| AREase (U/mL) | 94.4± 30.7#†δ | 121.5±40.7*†δ | 133.7±38.8*#δ | 39.6±24.1*#† |
M1 = maternal blood from 1st collection at enrollment, M2 = maternal blood from 2nd collection at 32nd wk of pregnancy and M3 = maternal blood from 3rd collection at delivery.
*, #, † and δ represent significant activity difference from M1, M2, M3 and cord blood, respectively (p-value < 0.05) using paired-sample t-test.
AChE, plasma PONase and AREase activities in cord blood were significantly lower than in maternal blood from all collection time points,, while BChE activities in maternal and cord plasma were similar. In addition, AChE, POnase and AREase activities were significantly correlated between maternal blood collected at delivery and cord blood (Pearson’s correlations were 0.413, 0.348 and 0.415, respectively (all p<0.05)). Despite being statistically significant, these values suggest modest associations.
Newborn body weight, length and head circumference were not statistically associated with AChE inhibition.
3.3 Paraoxonase1 phenotyping
The distribution histogram of the ratio between PONase and AREase activities had 3 distinct modes, divided at the ratio of 2.92 and 5.86 (Fig. 2). Using Eckerson’s method, the low, middle and high ratios were identified as AA, AB and BB phenotypes, respectively (Eckerson et al. 1983). There were 6, 17 and 29 participants in AA, AB and BB phenotype groups, respectively. Cord blood PON1 phenotyping could not be performed. Because, in the cumulative plot and distribution histogram of the ratio between PONase and AREase activities in cord blood, no group separation was observed (data not shown).
Figure 2.
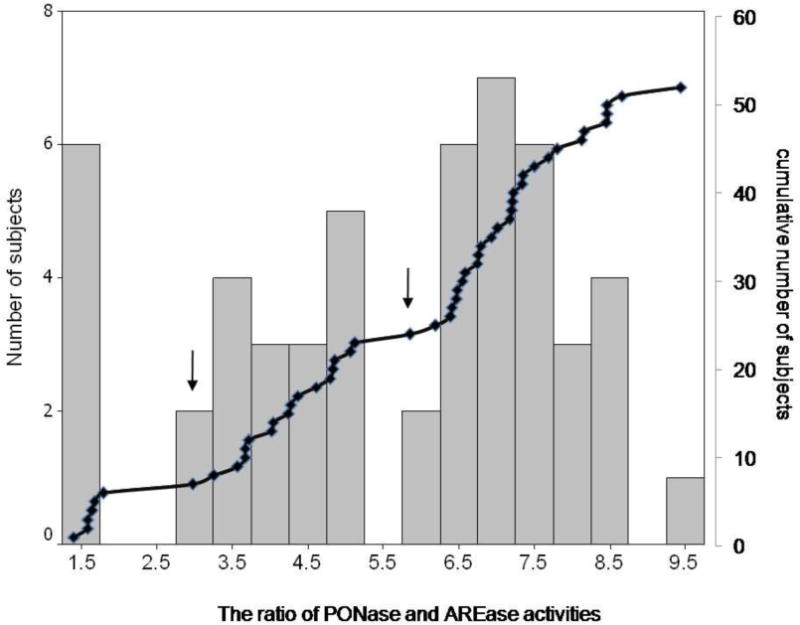
Distribution histogram and cumulative curve of the ratio of maternal paraoxonase and arylesterase activities from 52 participants. Arrows show points of the ratio that divided this population into three distinct groups according to their PON1 phenotypes.
3.4 Paraoxonase1 phenotypes and their relationship with maternal enzymatic activities and newborn outcomes
There were no differences between maternal and cord AChE and BChE activities across maternal AA, AB and BB phenotypes. Newborn outcomes according to the maternal PON1 phenotype are shown in Table 3. Newborn body weight, length and head circumferences were not significantly different among three maternal phenotypes.
Table 3.
Newborn birth outcomes according to maternal PON1 phenotypes
| Newborn outcomes | Maternal phenotype (N=52)
|
||
|---|---|---|---|
| AA (n = 6, 11%) | AB (n = 17, 33%) | BB (n = 29, 56%) | |
| Body weight (grams) | 2980.0 ± 495.1 | 2937.6 ± 318.0 | 2783.8 ± 467.5 |
| Body height (cm) | 53.0±1.4 | 51.9 ± 1.8 | 50.9 ± 3.3 |
| Head circumference (cm) | 33.0±1.1 | 32.9 ± 1.6 | 32.5 ± 1.8 |
3.5 Relationships among maternal acetylcholinesterase inhibition, paraoxonase1 phenotype, OP pesticide exposure, and newborn outcomes
When evaluating all samples collectively (n = 52), no significant associations were observed between birth outcomes and any of the following variables: maternal enzyme activities, AChE inhibition, and urinary DAPs. Table 4 summarizes the maternal urinary DAP concentrations measured at different time across pregnancy and stratified by maternal PON1 phenotypes. Following the stratification of PON1 phenotypes, no significant difference was detected for OP pesticides exposure levels at different time points, except for ΣDAP and ΣDEAP at delivery.
Table 4.
Urinary DAP concentrations in maternal urine collected at enrollment, 32-wk gestation and delivery, stratified by maternal PON1 phenotype.
| Phenotype | Maternal Urine Concentration (μmol/mol cr)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| At enrollment | At 32 -wk gestation | At delivery | ||||||||
|
| ||||||||||
| ΣDAP | ΣDMAP | ΣDEAP | ΣDAP | ΣDMAP | ΣDEAP | ΣDAP | ΣDMAP | ΣDEAP | ||
| AA (n=6) | Mean | 15.3 | 5.82 | 9.51 | 11.1 | 2.90 | 8.22 | 21.9 | 9.31 | 12.6 |
| SD | 12.8 | 8.64 | 5.76 | 5.06 | 1.71 | 5.53 | 24.9 | 12.3 | 13.9 | |
| Median | 10.8 | 2.32 | 8.52 | 10.8 | 2.00 | 7.93 | 12.6 | 3.94 | 10.2 | |
| Minimum | 3.78 | 1.11 | 1.70 | 5.92 | 1.60 | 1.20 | 4.42 | 1.37 | 0.60 | |
| Maximum | 39.5 | 23.4 | 16.2 | 17.8 | 5.58 | 15.8 | 68.2 | 33.8 | 34.4 | |
|
| ||||||||||
| AB (n=17) | Mean | 21.2 | 5.97 | 15.3 | 18.8 | 5.75 | 13.50 | 33.0 | 10.5 | 22.6 |
| SD | 20.4 | 6.24 | 20.6 | 19.7 | 5.27 | 20.60 | 33.9 | 20.2 | 28.0 | |
| Median | 18.5 | 4.07 | 6.62 | 9.93 | 3.21 | 5.06 | 16.7 | 2.59 | 10.9 | |
| Minimum | 1.16 | 0.98 | 0.18 | 5.30 | 1.66 | 0.94 | 4.95 | 1.17 | 1.08 | |
| Maximum | 68.5 | 26.6 | 66.8 | 70.5 | 20.8 | 68.0 | 100 | 65.8 | 84.9 | |
|
| ||||||||||
| BB (n=29) | Mean | 22.9 | 5.45 | 17.4 | 13.1 | 4.44 | 8.62 | 13.2 | 4.69 | 8.49 |
| SD | 41.0 | 4.68 | 40.4 | 15.2 | 2.71 | 14.6 | 16.6 | 2.93 | 15.7 | |
| Median | 10.2 | 4.05 | 3.96 | 7.37 | 3.74 | 1.93 | 7.80 | 4.16 | 2.07 | |
| Minimum | 2.27 | 0.74 | 0.43 | 2.37 | 1.33 | 0.38 | 2.50 | 1.14 | 0.30 | |
| Maximum | 194 | 25.6 | 187 | 63.3 | 12.7 | 58.2 | 73.7 | 13.6 | 66.9 | |
|
| ||||||||||
| Total (n=52) | Mean | 21.5 | 5.66 | 15. 8 | 14.7 | 4.69 | 10.1 | 20.7 | 7.11 | 13.6 |
| SD | 32.8 | 5.62 | 32.2 | 16.1 | 3.72 | 15.9 | 25.6 | 12.4 | 21.0 | |
| Median | 10.6 | 3.91 | 5.02 | 8.38 | 3.41 | 3.98 | 9.93 | 3.90 | 4.48 | |
| Minimum | 1.16 | 0.74 | 0.18 | 2.37 | 1.33 | 0.38 | 2.50 | 1.14 | 0.30 | |
| Maximum | 194 | 26.6 | 187 | 70.5 | 20.8 | 68.0 | 100 | 65.8 | 84.9 | |
|
| ||||||||||
| p-value * | 0.892 | 0.736 | 0.750 | 0.202 | 0.266 | 0.209 | >0.05 | 0.771 | >0.05 | |
p-value for ANOVA test of log10-transformed urinary DAP concentrations among three PON1 phenotypes
Six DAPs were associated with OP pesticide usage in Fang District. The OP pesticides currently in use are chlorpyrifos, diazinon, dimethoate ethion, malathion. pirimiphos ethyl, prothiophos, profenophos and triazophos. Among these, dimethoate and malathion are metabolized to DMAPs while the rest are metabolized to DEAPs.
There was found that diet was associated with OP exposure. Sources of water used, behaviors related to vegetable and fruit consumption as well as work activity during pregnancy were significantly associated with maternal ΣDMAP and ΣDEAP. However, there was no association between exposure levels and proximity to agricultural field. This is not a surprising phenomenon because farmworkers in Thailand usually live on or very close to their farms. Their houses are typically surrounded by agricultural fields (their own fields or those belonging to their neighbors) and are situated without any buffer zones.
When PON1 phenotypes were stratified, we observed significant associations between log10 ΣDMAP, ΣDEAP and ΣDAP in maternal urine and birth outcomes (Table 5). Within a group of combined maternal AA and AB phenotypes (n = 23), log10 ΣDEAP and ΣDAP at enrollment and 32 wks pregnancy were significantly associated with body weight and head circumference of newborns. Head circumference was negatively associated with log10 ΣDEAP and ΣDAP at enrollment (β = −1.0 cm, p = 0.03 and β = −1.8 cm, p <0.01, respectively) and at 32 wks of pregnancy (β = −1.1 cm, p = 0.04 and β = −2.6 cm, p = 0.01, respectively). Newborn body weight was negatively associated with log10 ΣDEAP and ΣDAP at enrollment (β = −219.7 g, p = 0.05 and β = −371.3 g, p = 0.02). Newborn body length was not associated with any exposure status whether stratified by PON1 phenotype or not. Further, no associations between maternal DAP levels and newborn outcomes we observed in the BB phenotype group.
Table 5.
Associations between log10 ΣDMAP, ΣDEAP, and ΣDAP in maternal urine collected at enrollment, 32-wk of pregnancy and delivery and birth outcomes, stratified by maternal PON1 phenotype
| PON1 phenotype | Birth lengtha (cm) | Birth weighta (g) | Head circumferencea (cm) | Gestation ageb (wk) | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| log10 ΣDMAP at enrollment | ||||||||
| AA, AB (n=23) | −0.3 (−2.6, 2.0) | 0.81 | −90.65 (−480.4, 299.2) | 0.63 | −1.6 (−3.0, −0.1) | 0.04* | 0.5 (−0.6, 1.5) | 0.36 |
| BB (n=29) | 1.9 (−1.5, 5.4) | 0.26 | 490.9 (−76.7, 920.4) | 0.02* | 0.4 (−1.9, 2.7) | 0.71 | 1.0 (−2.8, 0.9) | 0.29 |
| log10 ΣDEAP at enrollment | ||||||||
| AA, AB (n=23) | −0.4 (−1.8, 1.1) | 0.61 | −219.7 (−441.8, 2.34) | 0.05* | −1.0 (−2.0, −0.1) | 0.03* | −0.2 (−0.9, 0.5) | 0.54 |
| BB (n=29) | 0.2 (−1.6, 2.1) | 0.80 | 37.5 (−194.2, 269.3) | 0.74 | 0.3 (−0.8, 1.4) | 0.53 | −0.2 (−1.2, 0.8) | 0.69 |
| log10 ΣDAP at enrollment | ||||||||
| AA, AB (n=23) | −1.1 (−2.8, 0.6) | 0.19 | −371.3 (−674.7, −67.93) | 0.02* | −1.8 (−3.0, −0.6) | <0.01* | −0.2 (−1.2, 0.8) | 0.68 |
| BB (n=29) | 1.0 (−1.4, 3.4) | 0.40 | 165.2 (−136.2, 466.6) | 0.27 | 0.1 (−1.4, 1.6) | 0.85 | −0.3 (−1.6, 1.1) | 0.68 |
| log10 ΣDMAP at 32-wk of pregnancy | ||||||||
| AA, AB (n=23) | 1.4 (−1.5, 4.4) | 0.33 | 164.2 (−319.0, 647.3) | 0.49 | −0.3 (−2.4, 1.8) | 0.74 | −0.3 (−1.6, 1.1) | 0.68 |
| BB (n=29) | 2.2 (−2.2, 6.7) | 0.31 | 28.9 (−280.3, 842.2) | 0.31 | −1.0 (−3.7, 1.7) | 0.46 | −0.1 (−2.6, 2.3) | 0.90 |
| log10 ΣDEAP at 32-wk of pregnancy | ||||||||
| AA, AB (n=23) | −0.7 (−2.2, 0.8) | 0.35 | −178.2 (−509.5, 153.0) | 0.27 | −1.1 (−2.1, −0.1) | 0.04* | 0.7 (−0.1, −1.4) | 0.06 |
| BB (n=29) | 0.2 (−1.7, 2.2) | 0.80 | 78.6 (−168.1, 325.2) | 0.52 | −0.6 (−1.8, 0.5) | 0.31 | −0.3 (−1.3, 2.3) | 0.51 |
| log10 ΣDAP at 32-wk of pregnancy | ||||||||
| AA, AB (n=23) | −0.2 (−3.0, 2.6) | 0.90 | −138.7 (−682.6, 405.1) | 0.60 | −2.6 (−4.4, −0.8) | 0.01* | 1.1 (−0.1, 2.4) | 0.08 |
| BB (n=29) | 0.9 (−2.1, 3.9) | 0.55 | 171.1 (−203.8, 546.1) | 0.36 | −1.1 (−2.8, 0.7) | 0.49 | −0.5 (−2.0, 1.0) | 0.49 |
| log10 ΣDMAP at delivery | ||||||||
| AA, AB (n=23) | −0.9 (−0.7, 2.4) | 0.25 | 76.9 (−214.0, 367.7) | 0.59 | 2 2.0E10 (−1.3, 1.4) | 0.98 | 0.1 (−0.7, 1.0) | 0.72 |
| BB (n=29) | −0.8 (−4.0, 2.5) | 0.63 | −94.3 (−500.4, 311.4) | 0.63 | −1.3 (−3.1, 0.4) | 0.12 | 0.4 (−1.3, 2.1) | 0.63 |
| log10 ΣDEAP at delivery | ||||||||
| AA, AB (n=23) | −0.2 (−1.6, 1.1) | 0.69 | −95.9(−341.8, 150.0) | 0.42 | −0.2 (−1.2, 0.8) | 0.66 | 0.6 (0.1, 1.2) | 0.04* |
| BB (n=29) | −0.4 (−2.3, 1.5) | 0.66 | 10.3 (−232.6, 253.2) | 0.93 | −0.4 (−1.6, 0.7) | 0.42 | 0.3 (−0.8, 1.3) | 0.58 |
| log10 ΣDAP at delivery | ||||||||
| AA, AB (n=23) | 0.4 (−1.4, 2.2) | 0.62 | −56.8 (−394.0, 280.6) | 0.73 | −0.3 (−1.7, 1.0) | 0.59 | 0.7 (−0.1, 1.5) | 0.07 |
| BB (n=29) | −0.6 (−5.3, 4.0) | 0.78 | −188.4 (−769.9, 393.0) | 0.51 | −1.1 (−3.8, 1.7) | 0.42 | 0.1 (−2.4, 2.7) | 0.93 |
p <0.05 shows the significant difference.
Models adjusted for maternal age, preprenancy BMI, weight gain and gestational age.
Models adjusted for maternal age, preprenancy BMI and weight gain.
4. Discussion
4.1 Enzyme activities of maternal and cord blood
AChE, PONase and AREase activities in the blood collected at enrollment in our study were significantly lower than in the blood collected at 32 wks and delivery, likely a result of exposure during pregnancy. However, other studies have noted that AChE activity was remarkably increased during pregnancy in non-farmworker mothers with normal labor and vaginal deliveries (de Peyster et al. 1994; Venkataraman et al. 1990; Vlachos et al. 2008). As well as AChE activities, PONase and AREase enzyme activities at delivery were slightly higher during pregnancy among non-farmworker mothers living in an agricultural community (Holland et al. 2006). Researchers have hypothesized that the increasing activities of these enzymes during pregnancy may be due to enzyme expression depending on pregnancy status which leads to altered hemodynamics and other inter-related changes occurring in pregnancy (Venkataraman et al. 1990; Huen et al. 2010). In our study, the lower levels are most likely a result of farmworker activities that resulted in higher OP pesticide exposures in our participants than observed in other studies.
The work activity during each blood collection time is also crucial to explain low AChE activity at the early and middle periods of pregnancy. Our questionnaire data indicated that most of our participants still worked through the early 2nd trimester of pregnancy. They were exposed to anti-AChE OP pesticides during this time as indicated by their urinary DAP levels, thus likely resulting in lower AChE activities. Questionnaire data also demonstrated that some participants worked until delivery. Although only a few of the participants directly handled the pesticides, most were likely exposed in their workplaces and from farm-related tasks like thinning and reaping tangerines. However, enzymes activities from samples collected from working and not working participants in each collection time were not significantly different (data not shown).
4.2 Paraoxonase1 activity and phenotype
Maternal PONase and AREase activities increased with gestational age. These results are similar to a previous study in which these enzyme activities were slightly elevated during pregnancy (Holland et al. 2006). Therefore, PON1 phenotyping should not be affected by the increase of PONase and AREase activities over time). In human studies, the R allele showed higher activity of chlorpyrifos oxon hydrolysis than the Q allele which more actively hydrolyzed diazinon oxon (Ginsberg et al. 2009). Further, the Q allele have been associated with early pregnancy failure (Toy et al. 2009) and high BChE inhibition (Hofmann et al. 2009). However, the R allele has been associated with risk of low birth weight in newborns (Andersen et al. 2012). Clearly, PON1 phenotypes/genotypes provide different degrees of protection against different OP pesticides which may, in turn, lead to different degrees of adverse effects.
4.3 Association among maternal cholinesterase activities, paraoxonase1 phenotyping, pesticide exposure and newborn outcomes
We found no correlation between maternal AChE inhibition and urinary DAP level. This is consistent with the reported insensitivity of AChE activity to exposures to OP pesticides that are far below lethal levels. A study in workers involved in the manufacturing of chlorpyrifos found that the decreased AChE activity was not associated with low PON1 status (Albers et al. 2010). Similarly, a study in agricultural workers observed inhibition of AChE and BChE after chlorpyrifos application but an association of PON1 activity with inhibition was not found (Ellison et al. 2012). Conversely, low PON1 catalytic efficiency and low plasma PON1 activity have been associated with increased plasma BChE inhibition (over baseline levels) in pesticide handlers exposed to various OPs and carbamates (Hofmann et al. 2009).
In this study, a direct correlation between maternal biomarkers and birth outcomes was only observed after stratification by PON1 phenotype. In the low maternal PON1 activity group, a negative association between birth outcomes and prenatal pesticide exposure (as measured by urinary DAP metabolites) was found, although an association between enzyme activities and birth outcome was not observed. Specifically, the association between decreased birth weight and head circumference and maternal urinary ΣDEAP and ΣDAP levels measured at early and late pregnancy was observed among newborns of mothers with the low activity PON1 phenotype. This finding was similar to that previously reported in which low maternal PON1 activity in conjunction with maternal chlorpyrifos exposures was reportedly associated with reduced newborn head circumference (Berkowitz et al., 2004). In addition, another study also reported a negative association between birth weight/length and chlorpyrifos levels in maternal blood (Whyatt et al., 2004). The studies on the association of maternal exposure to OP pesticides with birth outcomes are shown in Table 6.
Table 6.
Comparison of studies of maternal exposure to OP pesticides and birth outcomes
| Cohort | Studies | Populations | Prenatal biomarkers of exposure to OP pesticides | Outcomes | Interaction with PON1 |
|---|---|---|---|---|---|
|
| |||||
| SAWASDEE (Agricultural worker cohort) | The present study | Farmworkers in Thailand (N=52) | ΣDEAP and ΣDAP in urine | ↓ head circumference | Among mothers with low PON1 activity |
| ΣDEAP and ΣDAP in urine | ↓ birth weight | Among mothers with low PON1 activity | |||
|
| |||||
| Columbia (urban Dominican and African American) | Whyatt et al., 2004 | New York (N=314) | Chlorpyrifos in blood | ↓ birth weight | – |
| ↓ birth length | – | ||||
|
| |||||
| Mt. Sinai (urban NYC) | Berkowitz et al. 2004 | New York (N=404) | TCPy in urine | ↓ head circumference | Among mothers with low PON1 activity |
|
| |||||
| Wolff et al. 2007 | New York (N=404) | ΣDEAP in urine | ↓ birth weight | Among mothers with QQ PON1 phenotype | |
| ΣDMAP in urine | ↓ birth length | Among mothers with low AREase activity | |||
|
| |||||
| CHAMACOS (Latina Agricultural Cohort | Eskenazi et al. 2004 | Mexican-American in California (N=488) | ΣDMAP in urine | ↓ gestational age | – |
|
| |||||
| Harley et al. 2011 | Mexican-American in California (N=470) | ΣDMP and ΣDAP in urine | ↓ head circumference | Among newborns with QQ PON1 phenotype | |
|
| |||||
| HOME (suburban Cincinnati, OH) | Rauch et al., 2012 | Cincinnati, OH (N=306) | ΣDAP in urine | ↓ birth weight in black children | Strongest relationship in QR PON1 phenotypes |
Since the major OP pesticide in Thailand is chlorpyrifos with the possible metabolites DEP and DETP. DEAP concentrations drove the overall DAP concentrations in our study and thus the association of DAP levels with birth outcomes was observed in this study. Unlike populations in United States where both chlorpyrifos and chlorpyrifos methyl were used among many other dimethyl-substituted OP pesticides, DMAPs drove the overall DAP levels and thus some birth outcome associations were primarily based upon DMAPs (Eskenazi et al. 2004; Wolff et al. 2007; Harley et al. 2011). It has to be noted that maternal urinary ΣDEAP and ΣDAP during pregnancy were obviously higher than other agricultural-community cohorts such as CHAMACOS (Bradman et al. 2005; Mark et al. 2010) and another birth cohort from central and northeast of Thailand (Kongthip et al. 2013) and Netherland (Ye et al. 2008) but China (Zhang et al. 2014).
Newborn PON1 genotype and activities were reported to be associated with birth outcomes (Huen et al. 2009; Harley et al. 2011; Eskenazi et al. 2004) and neurodevelopment deficits in children (Eskenazi et al. 2014). The shorter gestation period and smaller head circumference that were observed among newborns in the CHAMACOS study may be a direct result of low PON1 activity and the susceptible genotype of newborns. Collectively, these studies suggest that low maternal and newborn PON1 activity may contribute to greater susceptibility of newborns to prenatal exposures.
We would like to emphasize that about 15% of newborns in this study had low birth weight (LBW) which is higher than the overall prevalence in Thailand (8%) and twice the target percentage (7%) of Thailand’s goal to reduce LBW according to the National Economic and Social Development in Thailand (The National Economic and Social Development Board, 2002). In low birthweight newborns in our study, maternal ΣDEAP and ΣDAP at enrollment were slightly higher than in mothers of normal birth weight newborns, however, the differences were not statistically significant. The higher prevalence of LBW in our cohort is something that needs to be further studied and intervention measures should be undertaken.
This study highlights the finding of the negative association of prenatal OP exposure and birth outcomes especially in low PON1 activity mothers. These current data have important implications for public health. Education policy should be adapted to include research translation because it is important to raise the awareness of the population at risk regarding health outcomes of low level OP exposures and genetic influences. Health promotion and preventive measure recommendation such as using proper personal protective equipment (PPE) must be frequently updated and tailored to suit different groups of population. In addition, this issue should be raised as a necessary program for prenatal care in Thailand health policy.
5. Conclusion
This is the first report on the association between OP exposure and birth outcomes in the Thai population. In summary, this study suggested the decrease birth weight and head circumference in newborns born from mothers with low PON1 activity who were exposed to OP pesticides. Although the sample size is small, our results support the increasing body of evidence linking prenatal pesticide exposure to adverse birth outcomes.
Figure 1.
Map of Fang District, Chiang Mai Province, Thailand
Highlights.
We studied the relation of maternal OP pesticide exposure, PON1 and birth outcomes.
Prenatal OP exposure was associated with low birth weight and head circumference.
Adverse birth outcomes were observed in babies whose mothers had low PON1 activity.
We are first to report associations of OP exposure and birth outcomes in Thailand.
Acknowledgments
This study was funded by the Royal Golden jubilee PhD program (RGJ 12), the Thailand Research Fund (reference number PHD/0020/2009) and US National Institute of Environmental Health Sciences (R21 ES015465-01A2 and P30 ES019776). All laboratory facilities were supported by Research Institute for Health Sciences, Chiang Mai University. Finally, a special thanks to the SAWASDEE cohort participants, and medical and nurses team at Fang Hospital.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albers JW, Garabrant DH, Berent S, Richardson RJ. Paraoxonase status and plasma butyrylcholinesterase activity in chlorpyrifos manufacturing workers. J Expo Sci Environ Epidemiol. 2010;20(1):79–89. doi: 10.1038/jes.2009.9. [DOI] [PubMed] [Google Scholar]
- Andersen HR, Wohlfahrt-Veje C, Dalgard C, Christiansen L, Main KM, Nellemann C, Murata K, Jensen TK, Skakkebæk NE, Grandjean P. Paraoxonase 1 polymorphism and prenatal pesticide exposure associated with adverse cardiovascular risk profiles at school age. PloSone. 2012;7(5):15. doi: 10.1371/journal.pone.0036830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behra M, Cousin X, Bertrand C, Vonesch JL, Biellmann D, Chatonnet A, Strähle U. Acetylcholinesterase is required for neuronal and muscular development in the zebrafish embryo. Nat Neurosci. 2002;5(2):111–118. doi: 10.1038/nn788. [DOI] [PubMed] [Google Scholar]
- Berkowitz GS, Wetmur JG, Birman-Deych E, Obel J, Lapinski RH, Godbold JH, Holzman IR, Wolff MS. In utero pesticide exposure, maternal paraoxonase activity, and head circumference. Environ Health Perspect. 2004;112(3):388–391. doi: 10.1289/ehp.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A, Eskenazi B, Barr DB, Bravo R, Castorina R, Chevrier J, Kogut K, Harnly ME, McKonel TE. Environ Health Perspect. 2005;113(12):1802–1807. doi: 10.1289/ehp.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, Trujillo C, Johnson C, Bradman A, Barr DB, Eskenazi B. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ Health Perspect. 2011;119(8):1189–1195. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryk B, BenMoyal-Segal L, Podoly E, Livnah O, Eisenkraft A, Luria S, Cohen A, Yehezkelli Y, Hourvitz A, Soreq H. Inherited and acquired interactions between ACHE and PON1 polymorphisms modulate plasma acetylcholinesterase and paraoxonase activities. J Neurochem. 2005;92(5):1216–1227. doi: 10.1111/j.1471-4159.2004.02959.x. [DOI] [PubMed] [Google Scholar]
- Chanda SM, Pope CN. Neurochemical and neurobehavioral effects of repeated gestational exposure to chlorpyrifos in maternal and developing rats. Pharmacol Biochem Behav. 1996;53(4):771–776. doi: 10.1016/0091-3057(95)02105-1. [DOI] [PubMed] [Google Scholar]
- Costa LG, Giordano G, Cole TB, Marsillach J, Furlong CE. Paraoxonase 1 (PON1) as a genetic determinant of susceptibility to organophosphate toxicity. Toxicology. 2013;307:115–122. doi: 10.1016/j.tox.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das KP, Barone S., Jr Neuronal differentiation in PC12 cells is inhibited by chlorpyrifos and its metabolites: is acetylcholinesterase inhibition the site of action? Toxicol Appl Pharmacol. 1999;160(3):217–230. doi: 10.1006/taap.1999.8767. [DOI] [PubMed] [Google Scholar]
- de Peyster A, Willis WO, Liebhaber M. Cholinesterase activity in pregnant women and newborns. J Toxicol Clin Toxicol. 1994;32(6):683–696. doi: 10.3109/15563659409017975. [DOI] [PubMed] [Google Scholar]
- Eckerson HW, Wyte CM, La Du BN. The human serum paraoxonase/arylesterase polymorphism. Am J Hum Genet. 1983;35(6):1126–1138. [PMC free article] [PubMed] [Google Scholar]
- El-Demerdash FM. Lipid peroxidation, oxidative stress and acetylcholinesterase in rat brain exposed to organophosphate and pyrethroid insecticides. Food Chem Toxicol. 2011;49(6):1346–1352. doi: 10.1016/j.fct.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Ellison CA, Crane AL, Bonner MR, Knaak JB, Browne RW, Lein PJ, Olson JR. PON1 status does not influence cholinesterase activity in Egyptian agricultural workers exposed to chlorpyrifos. Toxicol Appl Pharmacol Toxicology. 2012;265(3):308–315. doi: 10.1016/j.taap.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Engel SM, Berkowitz GS, Barr DB, Teitelbaum SL, Siskind J, Meisel SJ, Wetmur JG, Wolff MS. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. AmJ Epidemiol. 2007;165(12):1397–1404. doi: 10.1093/aje/kwm029. [DOI] [PubMed] [Google Scholar]
- Engel SM, Wetmur J, Chen J, Zhu C, Barr DB, Canfield RL, Wolff MS. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ Health Perspect. 2011;119(8):1182–1188. doi: 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Harley K, Bradman A, Weltzien E, Jewell NP, Barr DB, Eskenazi B. Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ Health Perspect. 2004;112(10):1116–1124. doi: 10.1289/ehp.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Kogut K, Huen K, Harley KG, Bouchard M, Bradman A, Boyd-Barr D, Johnson C, Holland N. Organophosphate pesticide exposure, PON1, and neurodevelopment in school-age children from the CHAMACOS study. Environ Res. 2014;134:149–57. doi: 10.1016/j.envres.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahat FM, Ellison CA, Bonner MR, McGarrigle BP, Crane AL, Fenske RA, Lasarev MR, Rohlman DS, Anger WK, Lein PJ, Olson JR. Biomarkers of chlorpyrifos exposure and effect in Egyptian cotton field workers. Environ Health Perspect. 2011;119(6):801–806. doi: 10.1289/ehp.1002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg G, Neafsey P, Hattis D, Guyton KZ, Johns DO, Sonawane B. Genetic polymorphism in paraoxonase 1 (PON1): Population distribution of PON1 activity. J Toxicol Environ Health B Crit Rev. 2009;12(5–6):473–507. doi: 10.1080/10937400903158409. [DOI] [PubMed] [Google Scholar]
- Giordano G, Afsharinejad Z, Guizzetti M, Vitalone A, Kavanagh TJ, Costa LG. Organophosphorus insecticides chlorpyrifos and diazinon and oxidative stress in neuronal cells in a genetic model of glutathione deficiency. Toxicol Appl Pharmacol. 2007;219(2–3):181–189. doi: 10.1016/j.taap.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Gonzalez V, Huen K, Venkat S, Pratt K, Xiang P, Harley KG, Kogut K, Trujillo CM, Bradman A, Eskenazi B, Holland NT. Cholinesterase and paraoxonase (PON1) enzyme activities in Mexican-American mothers and children from an agricultural community. J Expo Sci Environ Epidemiol. 2012;22(6):641–648. doi: 10.1038/jes.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Garcia B, Olave ME, Ramos-Martinez E, Gonzalez-Horta C, Levario-Carrillo M, Sanchez-Ramirez B. Decrease of muscarinic cholinergic receptors expression in placenta from rats exposed to methyl parathion. Hum Exp Toxicol. 2008;27(3):241–246. doi: 10.1177/0960327108091863. [DOI] [PubMed] [Google Scholar]
- Gupta RC. Toxicology of organophosphate and carbamate compounds. Elsevier Academic Press; New York: 2006. [Google Scholar]
- Guven M, Bayram F, Unluhizarci K, Kelestimur F. Endocrine changes in patients with acute organophosphate poisoning. Hum Exp Toxicol. 1999;18(10):598–601. doi: 10.1191/096032799678839419. [DOI] [PubMed] [Google Scholar]
- Hackathorn DR, Brinkman WJ, Hathaway TR, Talbott TD, Thompson LR. Validation of a whole blood method for cholinesterase monitoring. Am Ind Hyg Assoc J. 1983;44(7):547–551. doi: 10.1080/15298668391405283. [DOI] [PubMed] [Google Scholar]
- Hanneman E, Westerfield M. Early expression of acetylcholinesterase activity in functionally distinct neurons of the zebrafish. J Comp Neurol. 1989;284(3):350–361. doi: 10.1002/cne.902840303. [DOI] [PubMed] [Google Scholar]
- Harnpicharnchai K, Chaiear N, Charerntanyarak L. Residues of organophosphate pesticides used in vegetable cultivation in ambient air, surface water and soil in Bueng Niam Subdistrict, Khon Kaen, Thailand. Southeast Asian J Trop Med Public Health. 2013;44(6):1088–97. [PubMed] [Google Scholar]
- Harley KG, Huen K, Aguilar Schall R, Holland NT, Bradman A, Barr DB, Eskenazi B. Association of Organophosphate Pesticide Exposure and Paraoxonase with Birth Outcome in Mexican-American Women. PLoS ONE. 2011;6(8):e23923. doi: 10.1371/journal.pone.0023923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel M, Aharoni A, Gaidukov L, Brumshtein B, Khersonsky O, Meged R, Dvir H, Ravelli RB, McCarthy A, Toker L, Silman I, Sussman JL, Tawfik DS. Structure and evolution of the serum paraoxonase family of detoxifying and anti-atherosclerotic enzymes. Nat Struct Mol Biol. 2004;11:412–9. doi: 10.1038/nsmb767. [DOI] [PubMed] [Google Scholar]
- Haviland JA, Butz DE, Porter WP. Long-term sex selective hormonal and behavior alterations in mice exposed to low doses of chlorpyrifos in utero. Reprod Toxicol. 2010;29(1):74–79. doi: 10.1016/j.reprotox.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Hofmann JN, Keifer MC, Furlong CE, De Roos AJ, Farin FM, Fenske RA, van Belle G, Checkoway H. Serum cholinesterase inhibition in relation to paraoxonase-1 (PON1) status among organophosphate-exposed agricultural pesticide handlers. Environ Health Perspect. 2009;117(9):1402–1408. doi: 10.1289/ehp.0900682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland N, Furlong C, Bastaki M, Richter R, Bradman A, Huen K, Beckman K, Eskenazi B. Paraoxonase polymorphisms, haplotypes, and enzyme activity in Latino mothers and newborns. Environ Health Perspect. 2006;114(7):985–991. doi: 10.1289/ehp.8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen K, Harley K, Bradman A, Eskenazi B, Holland N. Longitudinal changes in PON1 enzymatic activities in Mexican-American mothers and children with different genotypes and haplotypes. Toxicol Appl Pharmacol. 2010;244(2):181–189. doi: 10.1016/j.taap.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen K, Harley K, Brooks J, Hubbard A, Bradman A, Eskenazi B, Holland N. Developmental changes in PON1 enzyme activity in young children and effects of PON1 polymorphisms. Environ Health Perspect. 2009;117(10):1632–1638. doi: 10.1289/ehp.0900870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert R, Adler DA, Disteche CM, Hassett C, Omiecinski CJ, Furlong CE. The molecular basis of the human serum paraoxonase activity polymorphism. Nat Genet. 1993;3(1):73–76. doi: 10.1038/ng0193-73. [DOI] [PubMed] [Google Scholar]
- Jin QH, He HY, Shi YF, Lu H, Zhang XJ. Overexpression of acetylcholinesterase inhibited cell proliferation and promoted apoptosis in NRK cells. Acta pharmacologica Sinica. 2004;25(8):1013–1021. [PubMed] [Google Scholar]
- Keller M, Robitzki A, Layer PG. Heterologous expression of acetylcholinesterase affects proliferation and glial cytoskeleton of adherent chicken retinal cells. Cell Tissue Res. 2001;306(2):187–198. doi: 10.1007/s004410100444. [DOI] [PubMed] [Google Scholar]
- Kongtip P, Nankongnab N, Woskie S, Phamonphon A, Tharnpoophasiam P, Wilaiwan K, Srasom P. Organophosphate Urinary Metabolite Levels during Pregnancy, Delivery and Postpartum in Women Living in Agricultural Areas in Thailand. J Occup Health. 2013;55(5):367–375. doi: 10.1539/joh.13-0040-oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layer PG, Klaczinski J, Salfelder A, Sperling LE, Thangaraj G, Tuschl C, Vogel-Höpker A. Cholinesterases in development: AChE as a firewall to inhibit cell proliferation and support differentiation. Chem Biol Interact. 2013;203(1):269–276. doi: 10.1016/j.cbi.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Lev-Lehman E, Ginzberg D, Hornreich G, Ehrlich G, Meshorer A, Eckstein F, Soreq H, Zakut H. Antisense inhibition of acetylcholinesterase gene expression causes transient hematopoietic alterations in vivo. Gene Ther. 1994;1(2):127–135. [PubMed] [Google Scholar]
- Li WF, Costa LG, Richter RJ, Hagen T, Shih DM, Tward A, Lusis AJ, Furlong CE. Catalytic efficiency determines the in-vivo efficacy of PON1 for detoxifying organophosphorus compounds. Pharmacogenet. 2000;10(9):767–779. doi: 10.1097/00008571-200012000-00002. [DOI] [PubMed] [Google Scholar]
- London L, Beseler C, Bouchard MF, Bellinger DC, Colosio C, Grandjean P, Harari R, Kootbodien T, Kromhout H, Little F, Meijster T, Moretto A, Rohlman DS, Stallones L. Neurobehavioral and neurodevelopmental effects of pesticide exposures. Neurotoxicol. 2012;33(4):887–896. doi: 10.1016/j.neuro.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz AN, Prapamontol T, Narksen W, Srinual N, Barr DB, Riederer AM. Pilot study of pesticide knowledge, attitudes, and practices among pregnant women in northern Thailand. Int J Environ Res Publ Health. 2012;9(9):3365–3383. doi: 10.3390/ijerph9093365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, Calderon N, Eskenazi B. Organophosphate pesticide exposure and attention in young Mexican-American children: the CHAMACOS study. Environ Health Perspect. 2010;118(12):1768–1774. doi: 10.1289/ehp.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Economic and Social Development Board. The 9th the National Economic and Social Development Plan (2002–2006) Bangkok, Thailand: 2002. [Google Scholar]
- Prapamontol T, Hongsibsong S, Pakvilai N, Polyiem W, Kawichai S, Santasup C. Pesticide residues in tangerines (Citrus reticulata Blanco) cultivated in different types from Chiang Mai Province, northern Thailand. Toxicol Lett 196, Supplement(0) 2010:S334–S335. [Google Scholar]
- Prapamontol T, Kerdnoi T, Hongsibsong S, Prommuangyong K, Pakvilai N, Polyiem W, Chaimongkol U, Mangklabruks A. Expansion of the research outcome for safety of vegetable and fruit consumption in upper northern Thailand Final report 2009 [Google Scholar]
- Prapamontol T, Sutan K, Laoyang S, Hongsibsong S, Lee G, Yano Y, Hunter RE, Ryan PB, Barr DB, Panuwet P. Cross validation of gas chromatography-flame photometric detection and gas chromatography-mass spectrometry methods for measuring dialkylphosphate metabolites of organophosphate pesticides in human urine. Int J Hyg Environ Health. 2014;217(4–5):554–556. doi: 10.1016/j.ijheh.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Rauch SA, Braun JM, Barr DB, Calafat AM, Khoury J, Montesano AM, Yolton K, Lanphear BP. Associations of prenatal exposure to organophosphate pesticide metabolites with gestational age and birth weight. Environ Health Perspect. 2012;120(7):1055–1060. doi: 10.1289/ehp.1104615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, Whitehead R, Tang D, Whyatt RW. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among innercity children. Pediatrics. 2006;118(6):e1845–1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recio R, Ocampo-Gomez G, Moran-Martinez J, Borja-Aburto V, López-Cervante M, Uribe M, Torres-Sánchez L, Cebrián ME. Pesticide exposure alters follicle-stimulating hormone levels in Mexican agricultural workers. Environ Health Perspect. 2005;113(9):1160–1163. doi: 10.1289/ehp.7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PB, Prapamontol T, Panuwet P, Hongsibsong S, Loayang S, Naksen W, Srinual N, Riederer AM, Barr DB. Maternal urinary Diakylphosphate metabolites of organophosphorus insecticides in a pilot birth cohort in Thailand: the SAWASDEE study Submitted. [Google Scholar]
- Sapbamrer R, Hongsibsong S. Organophosphorus pesticide residues in vegetables from farms, markets, and a supermarket around Kwan Phayao Lake of Northern Thailand. Arch Environ Contam Toxicol. 2014;67(1):60–7. doi: 10.1007/s00244-014-0014-x. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Ryde IT, Levin ED, Seidler FJ. Organophosphate insecticides target the serotonergic system in developing rat brain regions: disparate effects of diazinon and parathion at doses spanning the threshold for cholinesterase inhibition. Environ Health Perspect. 2006;114(10):1542–1546. doi: 10.1289/ehp.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Violin JD, Seidler FJ, Slotkin TA. Modeling the developmental neurotoxicity of chlorpyrifos in vitro: macromolecule synthesis in PC12 cells. Toxicol Appl Pharmacol. 1998;151(1):182–191. doi: 10.1006/taap.1998.8424. [DOI] [PubMed] [Google Scholar]
- Srisomboon N. Office of disease prevention and control 10. Thailand: 2013. Current Situation of Pesticide poisoning during January–April 2013. (in Thai) [Google Scholar]
- Srivastava MK, Raizada RB. Development effect of technical dimethoate in rats: maternal and fetal toxicity evaluation. Indian J Exp Biol. 1996;34(4):329–333. [PubMed] [Google Scholar]
- Stefanidou M, Athanaselis S, Velonakis M, Pappas F, Koutselinis A. Occupational exposure to cholinesterase inhibiting pesticides: a Greek case. Int J Environ Health Res. 2003;13(1):23–29. doi: 10.1080/0960312021000063287. [DOI] [PubMed] [Google Scholar]
- Toy H, Camuzcuoglu H, Celik H, Erel O, Aksoy N. Assessment of serum paraoxonase and arylesterase activities in early pregnancy failure. Swiss Med Wkly. 2009;139(5–6):76–81. doi: 10.4414/smw.2009.12495. [DOI] [PubMed] [Google Scholar]
- Venkataraman BV, Iyer GY, Narayanan R, Joseph T. Erythrocyte and plasma cholinesterase activity in normal pregnancy. Indian J Physiol Pharmacol. 1990;34(1):26–28. [PubMed] [Google Scholar]
- Vlachos DG, Schulpis KH, Parthimos T, Mesogitis S, Vlachos GD, Partsinevelos GA, Antsaklis A, Tsakiris S. The effect of the mode of delivery on the maternal-neonatal erythrocyte membrane acetylcholinesterase activity. Clin Biochem. 2008;41(10–11):818–823. doi: 10.1016/j.clinbiochem.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Rauh V, Barr DB, Camann DE, Andrews HF, Garfinkel R, Hoepner LA, Diaz D, Dietrich J, Reyes A, Tang D, Kinney PL, Perera FP. Prenatal insecticide exposures and birth weight and length among an urban minority cohort. Environl Health Perspect. 2004;112(10):1125–1132. doi: 10.1289/ehp.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Pierik FH, Hauser R, Duty S, Angerer J, Park MM, Burdorf A, Hofman A, Jaddoe V, Mackenbach JP, Steegers E, Tiemeier H, Longnecker MP. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: The Generation R Study. Environmental Research. 2008;108(2):260–267. doi: 10.1016/j.envres.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JG, Eskenazi B, Gladstone EA, Bradman A, Pedersen L, Johnson C, Barr DB, Furlong CE, Holland NT. Association between in utero organophosphate pesticide exposure and abnormal reflexes in neonates. Neurotoxicology. 2005;26(2):199–209. doi: 10.1016/j.neuro.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Han S, Liang D, Shi X, Wang F, Liu W, Zhang L, Chen L, Gu Y, Tian Y. Prenatal exposure to organophosphate pesticides and neurobehavioral development of neonates: a birth cohort study in Shenyang, China. PloS one. 2014;9(2):e88491. doi: 10.1371/journal.pone.0088491. [DOI] [PMC free article] [PubMed] [Google Scholar]


