Abstract
Background and Purpose
CLR1404 is a phospholipid ether that exhibits selective uptake and retention in malignant tissues. Radiolabeled CLR1404 enables tumor-specific positron-emission tomography (PET) imaging (124I) and targeted delivery of ionizing radiation (131I). Here we describe the first preclinical studies of this diapeutic molecule in head and neck cancer (HNC) models.
Material and Methods
Tumor-selective distribution of 124I-CLR1404 and therapeutic efficacy of 131I-CLR1404 was tested in HNC cell lines and patient-derived xenograft tumor models. Monte Carlo dose calculations and 124I-CLR1404 PET/CT imaging were used to examine 131I-CLR1404 dosimetry in preclinical HNC tumor models.
Results
HNC tumor xenograft studies including patient-derived xenografts demonstrate tumor-selective uptake and retention of 124I-CLR1404 resulting in a model of highly conformal dose distribution for 131I-CLR1404. We observe dose-dependent response to 131I-CLR1404 with respect to HNC tumor xenograft growth inhibition and this effect is maintained together with external beam radiation.
Conclusions
We confirm the utility of CLR1404 for tumor imaging and treatment of HNC. This promising agent warrants further investigation in a developing phase I trial combining 131I-CLR1404 with reduced-dose external beam radiation in patients with loco-regionally recurrent HNC.
Keywords: Head and neck cancer, CLR1404, diapeutic, radiation, radionuclide
Introduction
Approximately two-thirds of head and neck cancer (HNC) patients receive radiation in the definitive, adjuvant or palliative setting. Although significant technical advances have been made in delivery and dose shaping of radiation with intensity modulated radiation therapy (IMRT), normal tissue toxicity remains dose limiting. This challenge is amplified among HNC patients who manifest loco-regional disease recurrence following initial treatment with radiation [1-3]. Although a proportion of these patients remain potentially curable with additional local treatment approaches (surgery, radiation, chemoradiation), retreatment is challenging because of the risk it confers for irreversible damage to normal tissues [4-6]. Consequently there is a compelling need to identify improved treatment approaches for patients with loco-regional HNC recurrence.
It has long been recognized that many tumors cells contain much higher concentrations of naturally occurring ether lipids than normal tissues, perhaps reflecting a reduced capacity of tumor cells to metabolize these lipids [7, 8]. In order to exploit the differences in phospholipid ether metabolism between normal and tumor cells we have synthesized and evaluated a series of over 30 radio-iodinated phospholipid ether analogs as potential tumor selective imaging agents [9]. These phospholipid ether analogs are designed to incorporate aromatic radioiodine to stabilize the molecule from in vivo de-iodination. Tumor-specific uptake of these agents is theorized to result from selective insertion into sphingolipid- and cholesterol-rich microdomains of the plasma membrane known as “lipid rafts”. In varied contexts a 6-10 fold increase in lipid rafts has been identified in the plasma membrane of tumor cells as compared to normal cells [9-12].
CLR1404 (18-(p-iodophenyl)octadecyl phosphocholine) is an alkyl phosphocholine (Figure 1A) that accumulates in lipid rafts and is taken up in cells [13-15]. The greater abundance of lipid rafts in many cancer cells compared to normal cells provides a semi-selective mechanism for CLR1404 uptake [16]. When radiolabeled with 131I, the selective accumulation of CLR1404 inside cancer cells affords precise delivery of radiation. In complementary fashion, positron emission from 124I radiolabeled CLR1404 enables anatomically precise modeling of this in vivo distribution using PET [15]. As a result radiolabeled CLR1404 confers a diapeutic capacity to molecularly target radiation to tumor cells and quantitatively model the resulting radiation dose to tumor and normal tissues. CLR1404 has entered early phase clinical investigation as both a diagnostic (124I-CLR1404) and therapeutic (131 I-CLR1404) agent with early results confirming safety, potent tumor uptake, and acceptable total body and normal tissue dosimetry [15, 17].
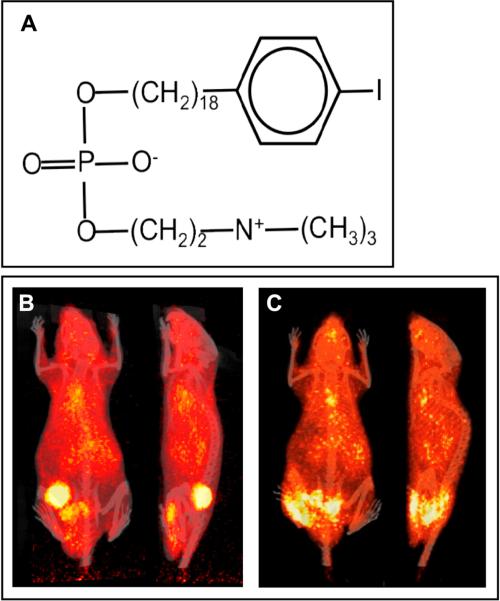
Figure 1. Bio-distribution of 124I-CLR1404 in HNC xenografts.
(A) Distinct isotopes of iodine can be coupled to the CLR1404 backbone. 124I is particularly valuable for PET imaging applications and 131I offers a powerful tool for cancer therapy. (B) Human tumor xenograft SCC22B. (C) Human patient-derived xenograft UW-SCC1P. Once tumors reach ~200 mm3,100 μCi 124I-CLR1404 is given IV and PET-CT performed at 4d confirming profound CLR1404 uptake and retention.
We hypothesize that CLR1404 may be selectively taken up in HNC and thereby enable precise delivery of radiation to sites of gross disease. Here we demonstrate selective in vivo distribution of CLR1404 and confirm the therapeutic potential of radiolabeled CLR1404 alone and together with moderate dose external beam radiation in murine models of HNC. Using PET/CT imaging of HNC tumor models treated with 124I-CLR1404, we present dosimetric proof-of-principle for the capacity of radiolabeled CLR1404 to enable both normal tissue-sparing and tumor dose escalation. It is anticipated that further investigation combining this diapeutic agent with reduced dose external beam radiation will provide opportunities to facilitate local disease control and improved quality of life in the context of re-irradiation for loco-regionally recurrent HNC.
Materials and Methods
Reagents
Synthesis of CLR1404 [9] as well as radio-iodination and purification (>99% radiochemical purity) have been previously described [15]. Pharmaceutical grade 124I-CLR1404 and 131I-CLR1404 with pre-determined activity and purity were provided for all preclinical and clinical studies by Cellectar Biosciences (Madison, WI) (formerly Novelos Therapeutics).
Cell culture
All cells were cultured at 37°C and 5% CO2. The human head and neck squamous cell carcinoma UM-SCC-22B (SCC-22B) cell line was provided by Dr. Thomas E. Carey (University of Michigan, Ann Arbor, MI) and the SCC1483 cell line was provided by Dr. Jennifer Grandis (University of Pittsburgh, Pittsburgh, PA). The authenticity of these cell lines was regularly verified on the basis of cell morphology and genomic short tandem repeat (STR) profile. Cells were cultured in Dulbeccos’ Modified Eagle's Medium (DMEM) cell culture media supplemented with 10% FBS, MEM non-essential amino acids solution, 1 μg/mL hydrocortisone, 100 U/mL penicillin, and 100 μg/mL streptomycin. Patient-derived xenograft establishment was performed as previously described [18, 19]. All cell culture reagents were purchased from Life Technologies Inc.
Tumor xenograft studies
Athymic nude (Hsd:Athymic Nude-Foxn1nu) male mice aged 4-6 weeks were obtained from Harlan Laboratories (Indianapolis, IN). Care and treatment of experimental animals was conducted in accordance with the institutional guidelines of the Animal Care and Use Committee of the University of Wisconsin. Tumors were engrafted on the flank of animals by subcutaneous injection of 2 × 106 cells/site. Tumor volumes were monitored twice weekly by measuring the small and large diameters of each tumor using digital calipers and extrapolating tumor volumes with the formula volume = (π)/6x (largest diameter) × (perpendicular diameter)2. Once average tumor volumes reached 150 – 200 mm3 mice were randomly assigned to treatment groups. Mice were treated as indicated with non-radiolabeled CLR1404 or 131I-CLR1404 via tail vein intravenous (IV) injection. Non-radioactive animals were housed in groups of three to four in cages separated from the radioactive animal cages. Radioactive animals were housed individually with lead shielding between cages. For external beam radiotherapy animals were treated using an X-RAD 320 biological irradiator (Precision X-ray, N. Branford, CT) using custom-designed lead jigs to immobilize animals and minimize radiation exposure to normal tissues away from the tumor-bearing flank.
Patient-derived tumor xenografts
A patient-derived tumor xenograft was generated as previously described [18]. Briefly, patients consenting to participate in this IRB-approved protocol completed a brief questionnaire collecting information regarding tobacco use, alcohol use, prior malignancy, sex, age, and prior treatment received. At the time of surgery or staging biopsy, a section of tumor was collected for research. This tumor section was transported to the laboratory in ice-cold Dulbecco's Modified Eagle Medium (DMEM) with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, and 25 μg/mL amphotericin and minced to less than 1 mm3 pieces under sterile conditions. Minced tumor pieces were mixed 1:1 with reduced growth factor Matrigel (BD Biosciences, Inc) and injected subcutaneously into NOD SCID gamma (NSG, Jackson Laboratories) mice with an 18 gauge needle. Subsequent passages were made in a similar fashion into either NSG or athymic nude mice.
In vivo micro PET/CT imaging
PET/CT imaging with 124I-CLR1404 in mice was performed as previously described [15] using a Siemens Inveon micro-PET/CT scanner (Siemens Preclinical Systems). CT scans were acquired with x-ray energy of 70 kVp at 1000 mA for 200 ms with 220° of rotation and 220 back-projections. CT images were reconstructed with filtered back-projection using system software with a Shepp-Logan filter to voxel sizes of 0.2 × 0.2 × 0.2 mm3. PET scans were acquired for 10 to 30 min (40 to 100 million counts) and reconstructed with either 2DOSEM (2D ordered–subset expectation–maximization) with 16 subsets and 4 EM (expectation maximization) iterations or 3D-OSEM with 16 subsets and 18 iterations to voxel size of 0.8 × 0.8 × 0.8 mm3. Corrections of normalization, dead time, scatter, and attenuation were applied using the system software and the co-registered CT scan. PET data were quantified using a calibration specific to 124I. Image analysis, including region of interest quantification and co-registration of multiple time points, was performed with the Inveon Research Workplace (Siemens Preclinical Systems) and Amira (Visage Imaging Inc.). To permit dosimetric calculations PET/CT images for mice were obtained 4, 24, 48 and 72 hours post-124I-CLR1404 injection.
Radiation dosimetry
Dosimetry calculations were performed using computational architecture of Geant4, which is a well-benchmarked Monte Carlo code that has been used for high-energy physics, medical and space applications [20]. The Monte Carlo-based platform is capable of processing PET/CT scans taken at multiple time points in order to account for important pharmacokinetic changes that occur in the small animals over the observation period, including changes in the activity distribution in tissues adjacent to the tumor. Computations were performed using a 16-core 64 CPU high throughput cluster specifically for Monte Carlo dose calculations.
Statistical methods
For animal experiments statistical power calculations were performed for proposed sample sizes and confidence interval construction based on Hotelling's T2-statistic. One-way repeated measurement ANOVA was performed to analyze tumor growth in control and treatment groups. Two-tailed t-test was used to compare between individual groups when ANOVA values reached significance. P-values less than 0.05 were considered statistically significant. All analyses were performed using JMP and SAS statistical software (SAS Institute, Cary, NC).
Results
Radionuclide-labeled CLR1404 distribution and dosimetry in murine HNC models
To evaluate the tumor-selective in vivo distribution of CLR1404 in HNC we utilized xenograft tumor models in which nude mice were injected subcutaneously with the SCC-22B human HNC squamous cell carcinoma cell line. Mice bearing macroscopic tumors (150-200 mm3) were given a single tail vein injection of 100 μCi 124I-CLR1404. Four days later these animals were imaged using PET/CT (Figure 1B). These imaging studies demonstrated a 6-fold ratio of tumor/normal tissue uptake, confirming tumor-selective in vivo distribution of CLR1404 in this preclinical mouse model of human HNC. These findings mirror those previously reported in a variety of other tumor cell lines [15] and we have observed similar specificity in xenograft tumors with a second human HNC squamous cell carcinoma line, SCC-1483 (not shown). The absence of thyroid radioactivity in these imaging studies confirms the in vivo stability of radio-iodinated CLR1404 in these models and this has been previously demonstrated [15].
To explore the generalizability of these findings and their relevance to human HNC we evaluated the tumor-selective distribution of CLR1404 using a patient-derived human papilloma virus (HPV)-positive xenograft tumor model. Such direct-from-patient tumor models have been reported to more closely reflect the biology of human cancer compared to immortalized cell lines [18]. Following subcutaneous engraftment, mice bearing macroscopic patient-derived xenografts were given 100 μCi 124I-CLR1404 by IV injection and imaged by PET/CT four days later (Figure 1C). Again we observed highly selective tumor-specific uptake and retention of CLR1404.
The novel diapeutic capacity of radiolabeled CLR1404 affords the possibility of precise modeling of radiation dosimetry with this compound. For this purpose 124I-CLR1404 PET/CT images are utilized to anatomically assess the pharmacokinetic behavior of CLR1404 in a given subject and this information is used to model and predict the absorbed doses to tumor volumes and healthy tissues/organs with 131I-CLR1404 administration. Our group and others have previously demonstrated the ability of these methods to predict radiation dosimetry for radionuclide therapy in animal models and patients [21]. Here, we attained PET/CT images taken 4, 24, 48, and 72 hours post-injection of 100 μCi 124I-CLR1404 in mice bearing SCC-22B human HNC tumors. Using these images to define the distribution of source activity over time we modeled the cumulative absorbed dose per injected unit of activity of 131I-CLR1404 with a Monte Carlo computational platform. This method accounts for all aspect of 131I beta particle interactions within 3D heterogeneous media such as the human body [22, 23] and small animals [24]. As a result we generated a model to predict absorbed dose to tumor and critical structures (Figure 2A) and a color wash display of cumulative absorbed dose distribution normalized per injected activity of 131I-CLR1404 (Figure 2B). These models predict an HNC tumor-selective dose distribution following 131I-CLR1404 administration with a 2.7 fold or greater increase in mean dose rate (Gy/MBq) to tumor compared to other normal tissue organs (Figure 2B).
Figure 2. Tumor and normal tissue dosimetry in mice.
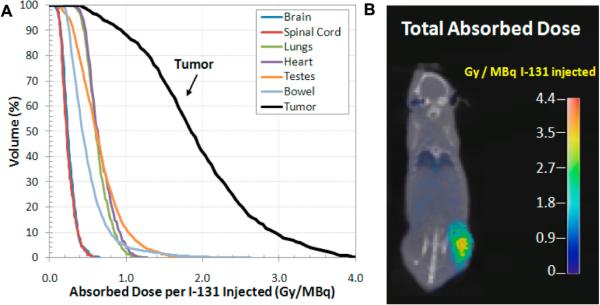
(A) Dose volume histograms calculated for the tumor and several important normal structures in an individual mouse carrying SCC-22B xenografts using micro-PET/CT images (4, 24, 48 and 72 hours post injection). (B) Color wash of cumulative absorbed dose calculated by our Monte Carlo framework per injected activity of 131I.
Therapeutic efficacy of 131I-CLR1404 in murine xenograft models of human HNC
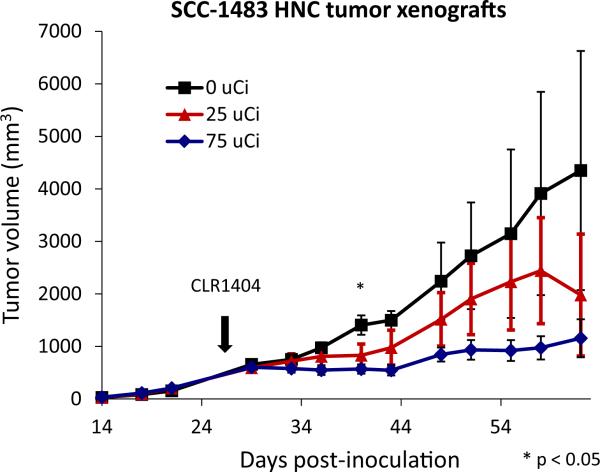
Given the tumor-specific distribution of CLR1404 in xenograft HNC models we next evaluated the potential for 131I-CLR1404 to elicit a therapeutic effect in this context. For this we engrafted nude mice subcutaneously with the human HNC squamous cell carcinoma cell line SCC1483 and treated mice bearing macroscopic tumors with a single IV injection of 25 or 75 μCi of 131I-CLR1404 or non-radiolabeled CLR1404 control (Figure 3). These studies identified a dose-dependent effect of 131I-CLR1404 on tumor growth. This observation is consistent with a therapeutic anti-tumor effect resulting from the selective distribution of radiolabeled CLR1404 in HNC.
Figure 3. Dose-dependent effect of 131I-CLR1404 on HNC xenograft tumor growth inhibition.
Dose response profile for 131I-labeled CLR1404 (single administration) in SCC-1483 HNC tumor xenograft model confirms dose-dependent tumor growth inhibition. Differences in mean tumor size between groups reach significance by one way ANOVA on day 40 (p < 0.05) (indicated by *) but not thereafter due to loss of several animals and variance within groups.
Although we have modeled the dosimetry of 131I-CLR1404 in the context of macroscopic disease, the micro-dosimetry of molecular targeted radionuclides remains challenging to define at the cellular level. As a result it is difficult to predict the in vivo dosimetry that may be achieved using targeted radionuclides for treatment of microscopic disease. Yet in the clinical context, even in the retreatment setting, it is generally feasible to utilize external beam treatments to deliver sufficient radiation dose to control microscopic disease. Nonetheless, it remains a challenge to deliver potentially curative doses of radiation to recurrent sites of gross disease in HNC. In this context the use of molecular targeted radionuclides may facilitate a tumor-selective boost of radiation dose at sites of gross disease. To test the capacity of 131I-CLR1404 to augment external beam radiation, we utilized human HNC xenograft tumor models. Following subcutaneous engraftment with SCC-1483 or SCC-22B cells, macroscopic tumors were treated with a fractionated course of external beam radiation and a single IV injection of either 131I-CLR1404 or non-radiolabeled CLR1404 (Figure 4A and B). In these HNC xenograft tumor model experiments, the molecular targeted delivery of a tumor-directed radiation boost with 131I-CLR1404 resulted in at least a clear trend toward improved tumor control.
Figure 4. 131I-CLR1404 antagonizes tumor growth when administered together with fractionated external beam radiation.

Combination therapy with 131I-CLR1404 and external beam radiation therapy (EBRT) showed greater potency for tumor growth inhibition than external beam radiation alone in (A) SCC-22B and a non-significant trend toward this effect in (B) SCC-1483 HNC tumor xenograft models. For the SCC-22B tumor model one-way ANOVA indicates significance (p < 0.05) for the comparison between all groups from day 12 onward. Two-tailed t-test comparison between the 6 Gy × 4 and 6 Gy × 4 + 200 μCi 131I-CLR1404 groups was significant (p< 0.05) from day 58 onward (indicated by *).
Discussion
The current study demonstrates the capacity of radiolabeled CLR1404 to selectively target xenograft tumors arising from either human HNC cell lines or direct-from-patient HNC tumor cells. We illustrate that tumor-selective delivery of radionuclides enables conformal radiation dosimetry resulting in anti-tumor activity against human HNC xenograft tumors. Importantly, we also present PET/CT imaging and the results of associated dosimetric modeling computations to support the feasibility of using radiolabeled CLR1404 to deliver tumor-selective radiation in HNC tumor models.
We recently completed a phase I trial evaluating the tumor-selective uptake of 124I-CLR1404 in advanced malignancy (NCT01662284). This trial included patients with HNC and we anticipate that 124I-CLR1404 PET/CT imaging and Monte Carlo dosimetric modeling from these patients will confirm that CLR1404 can indeed selectively target to loco-regionally advanced sites of HNC. On the basis of results presented herein, we are now preparing a phase I clinical trial protocol that combines CLR1404 with reduced-dose external beam radiation in patients with locoregional recurrence following prior HNC radiation. This trial will examine feasibility, toxicity, tumor response, salivary and swallowing function, and specific quality of life metrics. This will be the first clinical study to examine combined external beam radiation plus tumor-directed radionuclide treatment using CLR1404 in HNC patients. As part of this study we will utilize 124I-CLR1404 PET/CT imaging and Monte Carlo dosimetric modeling to guide the personalized prescription of 131I-CLR1404. All HNC patients will receive a dosimetry test dose of 124I-CLR1404 to establish drug uptake and enable Monte Carlo dose estimation based on CLR1404 PET/CT imaging evaluation. A unique aspect of this trial is that patients will have their external beam radiation dose prescribed based on dose estimation from their individual 124I-CLR1404 PET/CT.
Clinical trials employing external beam radiation in HNC during the last several decades demonstrate the importance of patient-specific dosimetry in order to determine accurate dose-response relationships. The value of this data reflects our ability to predict 3D dose distributions in patient-specific geometries. Here we illustrate the feasibility of patient-specific Monte Carlo-based dosimetry capable of providing compatible dose metrics for preclinical HNC tumor models (Figure 1) that involve the combination of external beam radiation therapy and radionuclide therapy [25, 26]. This platform is capable of calculating doses to any organ in the body. Accounting for the dosimetric impact of radionuclide therapy to critical organs will make the combined treatment of radionuclide and external beam radiation therapy safer and potentially more effective.
The uptake of CLR1404 by human tumors may reflect specific biological parameters that can be studied in the human xenograft model systems. The two established biomarkers that demonstrate robust correlation with HNC patient survival outcome at present include EGFR expression and HPV status [27, 28]. EGFR is known to associate with lipid rafts in tumor cells and we hypothesize that tumors rich in EGFR expression will demonstrate increased uptake of CLR1404 compared with tumors showing low expression of EGFR. As for HPV, it is known that HPV+ tumors of the HNC show a lower frequency of mutations than HPV- tumors [29]. HPV+ patients also demonstrate lower mean age and reduced tobacco use history than HPV- patients [30]. It is plausible that less mutated host cells from HPV+ tumors may exhibit a distinct susceptibility to CLR1404 uptake compared to tumor cells from HPV- hosts. Such possibilities are intriguing but speculative and the number of tumor samples evaluated in this initial report does not allow meaningful conclusions to be drawn in this regard. In future patient-derived xenograft studies as well as a submitted phase I clinical trial we will specifically examine these biomarkers for their association with CLR1404 uptake in HNC.
Conclusions
A substantial number of HNC patients (30-50%) manifest loco-regional disease recurrences following their initial therapy. When loco-regional recurrence manifests without evidence of distant metastatic disease, long term disease free survival and even cure can be obtained in as many as 10-25% of patients with retreatment [4-6]. However, the risks associated with re-irradiation are high, and novel approaches to deliver effective retreatment while limiting collateral normal tissue damage are needed. Should the paradigm of combining a tumor-specific radiolabeled molecule prove successful in this setting when combined with external beam radiation, this would provide strong rationale to investigate CLR1404 in other HNC settings including definitive treatment, perhaps with de-intensified external beam radiation for the purpose of reducing normal tissue toxicities.
Acknowledgements
We thank the University of Wisconsin head and neck cancer research team for their valuable discussions and contributions to this work. P. M. Harari acknowledges a laboratory research agreement with Cellectar Biosciences. R. J. Kimple acknowledges partial funding support from CA160639.
Role of the Funding Source
This work was funded in part by Cellectar Biosciences however this study sponsor had no direct involvement in experimental study design; collection, analysis and interpretation of data; nor in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Dr. Jamey Weichert declares ownership and employment interest in Cellectar Biosciences (Founder and Chief Scientific Officer). Dr. Paul Harari and the University of Wisconsin held a laboratory research agreement with Cellectar Biosciences from 2007-2010 to perform preclinical studies of CLR1404 in murine tumor model systems.
References
- 1.Cognetti DM, Weber RS, Lai SY. Head and neck cancer: an evolving treatment paradigm. Cancer. 2008;113(7 Suppl):1911–32. doi: 10.1002/cncr.23654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forastiere A, Weber R, Ang K. Treatment of head and neck cancer. The New England journal of medicine. 2008;358(10):1076. doi: 10.1056/NEJMc073274. author reply 1077-8. [DOI] [PubMed] [Google Scholar]
- 3.Peters LJ, et al. Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer: results from TROG 02.02. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(18):2996–3001. doi: 10.1200/JCO.2009.27.4498. [DOI] [PubMed] [Google Scholar]
- 4.Chen AM, Phillips TL, Lee NY. Practical considerations in the re-irradiation of recurrent and second primary head-and-neck cancer: who, why, how, and how much? International journal of radiation oncology, biology, physics. 2011;81(5):1211–9. doi: 10.1016/j.ijrobp.2011.06.1998. [DOI] [PubMed] [Google Scholar]
- 5.Mendenhall WM, et al. Re-irradiation of head and neck carcinoma. American journal of clinical oncology. 2008;31(4):393–8. doi: 10.1097/COC.0b013e3181637398. [DOI] [PubMed] [Google Scholar]
- 6.Hoebers F, et al. Reirradiation for head-and-neck cancer: delicate balance between effectiveness and toxicity. Int J Radiat Oncol Biol Phys. 2011;81(3):e111–8. doi: 10.1016/j.ijrobp.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Snyder F, Wood R. Alkyl and alk-1-enyl ethers of glycerol in lipids from normal and neoplastic human tissues. Cancer Res. 1969;29(1):251–7. [PubMed] [Google Scholar]
- 8.Snyder F, Blank ML, Morris HP. Occurrence and nature of O-alkyl and O-alk-I-enyl moieties of glycerol in lipids of Morris transplanted hepatomas and normal rat liver. Biochim Biophys Acta. 1969;176(3):502–10. doi: 10.1016/0005-2760(69)90217-3. [DOI] [PubMed] [Google Scholar]
- 9.Pinchuk AN, et al. Synthesis and structure-activity relationship effects on the tumor avidity of radioiodinated phospholipid ether analogues. J Med Chem. 2006;49(7):2155–65. doi: 10.1021/jm050252g. [DOI] [PubMed] [Google Scholar]
- 10.Carrasco MP, et al. Disruption of cellular cholesterol transport and homeostasis as a novel mechanism of action of membrane-targeted alkylphospholipid analogues. Br J Pharmacol. 2010;160(2):355–66. doi: 10.1111/j.1476-5381.2010.00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li YC, et al. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol. 2006;168(4):1107–18. doi: 10.2353/ajpath.2006.050959. quiz 1404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patra SK. Dissecting lipid raft facilitated cell signaling pathways in cancer. Biochim Biophys Acta. 2008;1785(2):182–206. doi: 10.1016/j.bbcan.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Mollinedo F. Antitumour ether lipids: proapoptotic agents with multiple therapeutic indications. Expert Opin. Ther. Patents. 2007;17:385–405. [Google Scholar]
- 14.van Blitterswijk WJ, Verheij M. Anticancer alkylphospholipids: mechanisms of action, cellular sensitivity and resistance, and clinical prospects. Current pharmaceutical design. 2008;14(21):2061–74. doi: 10.2174/138161208785294636. [DOI] [PubMed] [Google Scholar]
- 15.Weichert JP, et al. Alkylphosphocholine analogs for broad-spectrum cancer imaging and therapy. Sci Transl Med. 2014;6(240):240ra75. doi: 10.1126/scitranslmed.3007646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li YC, et al. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. The American journal of pathology. 2006;168(4):1107–18. doi: 10.2353/ajpath.2006.050959. quiz 1404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grudzinski JJ, et al. A phase 1 study of 131I-CLR1404 in patients with relapsed or refractory advanced solid tumors: dosimetry, biodistribution, pharmacokinetics, and safety. PLoS One. 2014;9(11):e111652. doi: 10.1371/journal.pone.0111652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimple RJ, et al. Development and characterization of HPV-positive and HPV-negative head and neck squamous cell carcinoma tumorgrafts. Clin Cancer Res. 2013;19(4):855–64. doi: 10.1158/1078-0432.CCR-12-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein AP, et al. Influence of handling conditions on the establishment and propagation of head and neck cancer patient derived xenografts. PLoS One. 2014;9(6):e100995. doi: 10.1371/journal.pone.0100995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agostinelli S. GEANT4—a simulation toolkit. Nucl. Instrum. Methods Phys. 2003;A 506:250–303. [Google Scholar]
- 21.Bednarz B, et al. Towards multiscale personalized dosimetry for diapeutic radiopharmaceuticals. 8th International Conference on Isotopes; Chicago, IL. 2014. [Google Scholar]
- 22.Bednarz B, Hancox C, Xu XG. Calculated organ doses from selected prostate treatment plans using Monte Carlo simulations and an anatomically realistic computational phantom. Physics in medicine and biology. 2009;54(17):5271–86. doi: 10.1088/0031-9155/54/17/013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bednarz B, Xu XG. A feasibility study to calculate unshielded fetal doses to pregnant patients in 6-MV photon treatments using Monte Carlo methods and anatomically realistic phantoms. Medical physics. 2008;35(7):3054–61. doi: 10.1118/1.2938519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taschereau R, Chatziioannou AF. Monte Carlo simulations of absorbed dose in a mouse phantom from 18-fluorine compounds. Medical physics. 2007;34(3):1026–36. doi: 10.1118/1.2558115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bednarz B, Besemer A, Y. Y A Monte Carlo-Based Small Animal Dosimetry Platform for Pre-Clinical Trials: Proof of Concept Med. Phys. 2012;39:3899. [Google Scholar]
- 26.Besemer A, et al. Towards Personalized Dosimetry Using Diapeutic Radiopharmaceuticals. Med. Phys. 2013;40(6):382. [Google Scholar]
- 27.Ang KK, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. The New England journal of medicine. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh B, Pfister DG. Individualized treatment selection in patients with head and neck cancer: do molecular markers meet the challenge? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(19):3114–6. doi: 10.1200/JCO.2007.14.7298. [DOI] [PubMed] [Google Scholar]
- 29.Lassen P. The role of Human papillomavirus in head and neck cancer and the impact on radiotherapy outcome. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2010;95(3):371–80. doi: 10.1016/j.radonc.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 30.Gillison ML. HPV and prognosis for patients with oropharynx cancer. European journal of cancer. 2009;45(Suppl 1):383–5. doi: 10.1016/S0959-8049(09)70058-9. [DOI] [PubMed] [Google Scholar]