Abstract
Background
Depletion of reduced glutathione is associated with Parkinson’s disease (PD) and glutathione augmentation has been proposed as a disease-modifying strategy.
Objectives
The purpose of this study was to determine the safety and tolerability of intranasal reduced glutathione in individuals with PD.
Methods
30 individuals with PD were randomized to either placebo (saline), 300 mg/day or 600 mg/day intranasal glutathione in 3 divided daily doses. Follow-up visits included side effect screening of PD symptoms and cognition, blood chemistry, sinus irritation, and hyposmia. Tolerability was measured by frequency and severity of reported adverse events, compliance and withdrawals from the study.
Results
After 3 months, there were no substantial differences between groups in the number of adverse events reported or observed among all safety measures assessed. All groups met tolerability criteria.
Conclusions
These data support the safety and tolerability of intranasal glutathione in this population. Pharmacokinetic and dose-finding studies are warranted.
Keywords: glutathione, antioxidant, nutrition, neuroprotection, deficiency
Introduction
Glutathione is a well-established antioxidant, hydrogen peroxide reducing agent, essential for cellular detoxification, as a neuropeptide, and as a reservoir for cysteine, glycine, and glutamic acid. 1, 3 The loss of reduced glutathione (GSH) is the most consistently reported alteration in the antioxidant defense system in PD.7–10 Where most individuals can synthesize enough GSH to maintain redox equilibrium, this is not the case in PD and other neurodegenerative disorders which have consistently been shown to be associated with GSH depletion,10 defining GSH as a conditionally essential nutrient in PD.11
The value of exogenously administered GSH to patients with PD has been formally studied twice using intravenous GSH, (iv)GSH, based on the understanding that oral GSH is poorly absorbed.14 Both studies concluded that further research was warranted on GSH as a neuroprotective agent in PD. 15, 16 (iv)GSH is limited by invasiveness, expense, and necessary clinic visits, which restrict therapeutic utility.
(in)GSH has been used as a potential route of central nervous system (CNS) glutathione augmentation since 2004, based on an acceptable safety and tolerability profile, 17, 18 biological plausibility that small, polar molecules may bypass the blood-brain barrier (BBB) via intranasal administration, and anecdotal case reports of improvement.17, 19
Methods
This study was designed to evaluate the safety and tolerability of (in)GSH in a double-blind, placebo-controlled fashion in a cohort of individuals with PD. The study was approved by the Bastyr University IRB and conducted in accordance with The Code of Ethics of the World Medical Association for experiments involving humans. The FDA granted Investigational New Drug status. All clinical evaluations were conducted at Bastyr University Clinical Research Center, Kenmore, WA, USA. Only the data monitoring committee, the database manager and the compounding pharmacy were unblinded. The study was registered on ClinicalTrials.gov (#NCT01398748).
All participants were English-speaking residents of the Pacific Northwest, USA who reported having been diagnosed with idiopathic PD by a clinical neurologist within the previous 10 years, had a modified Hoehn & Yahr stage ≤ 3, were ≥ 21 years of age, and had been stable on medications, supplements, diet, and exercise for 30 days prior to study entry. Individuals were excluded if they had abnormal liver enzymes or kidney function, cognitive impairment (Montreal Cognitive Assessment (MoCA) score < 25), epilepsy, a history of stroke, a history of brain surgery, structural brain disease, diseases with features common to PD (e.g. essential tremor), chronic sinusitis, or a history of intranasal telangiectasia. All individuals agreed to try to maintain stability of medications, diet, lifestyle, and alternative therapies throughout the study trial, although deviation from baseline routine throughout the trial did not disqualify them from continued participation.
Key Pharmacy (Kent, WA, USA) compounded the study medication for each participant enrolled according to a randomized schedule generated by the study statistician. Purity and potency of glutathione, both in powdered and compounded liquid form, was independently validated by Eagle Analytical (Houston, TX, USA) at the beginning and throughout the study. Liquid glutathione was assessed for potency and purity from both unissued medication and from medication returned by subjects after 30 days of storage. Mucosal Atomization Device (MAD) tips, used to turn the liquid glutathione into a mist for easier administration, were supplied by Wolfe-Tory Medical (Teleflex) and replaced monthly.
Study medication was dispensed as sterile, capped 1-ml syringes in a light-impermeable plastic bag shipped on ice and stored in the refrigerator. GSH has a sulfur smell; to limit risk of unblinding, study clinicians did not participate in dispensation, collection, counting, or disposal of study medication. Participants were instructed to store the study medication in the refrigerator and to rinse MAD tips with warm water and let air dry after each use.
The maximum dose, 4200 mg/ week, was chosen to match the dose used in a 2009 pilot study of intravenous GSH, 1400 mg three times weekly.16 Subjects who passed screening were randomized into one of four groups: 600mg (in)GSH/day, 300mg (in)GSH/day, placebo (sterile saline) or watchful waiting using simple random allocation with uneven distribution (n= 10,10,10, and 4, respectively). In order to evaluate the impact of the saline spray on nasal symptoms, the study sponsor requested four additional individuals be enrolled to a watchful waiting arm, to provide a point of comparison for nasal irritation that could be caused by either the saline placebo or the active glutathione. Because these individuals did not receive placebo, they are excluded from all analyses other than those evaluating nasal irritation. Subjects randomized to intervention arms were instructed to spray one 1 ml syringe full of study medication three times daily for 3 months total. The medication was dispensed one month at a time, with instructions to return both used and unused syringes at the end of each month. Self-reported doses taken were confirmed through counts of returned syringes. Along with the medication, subjects were given a daily log and told to report medication use and any changes in symptoms and well-being.
Subjects returned at weeks 2, 4, 8, 12, and 16 for assessments of complete blood count (CBC), alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), creatinine, and a urinalysis. Monitoring of Side Effects Scale (MOSES) is a standardized questionnaire designed to assess 83 potential symptoms across 8 body systems, and was used to screen for side effects. The SNOT-20, a validated measure of rhinosinusitis 20, was employed in this study because sinus irritation was anticipated. For our purposes, questions 1–10, specific to sinusitis (e.g. runny nose, sneezing, cough), were used to screen for sinus-specific AEs. The UPDRS was used to monitor PD symptoms, and the Sensonics Smell ID Test was used to test olfactory function. AEs were predefined to reflect clinically relevant worsening or occurrence of all outcomes evaluated. According to protocol, study clinicians were blinded and required to have successfully completed the MDS UPDRS Training Program. Each participant was asked to select a time of day when they were most likely to be ‘on’; once that time was selected, all subsequent evaluations were scheduled at the same time to minimize the impact of circadian fluctuations of PD symptoms.
All comparisons presented are between the active arms of the study and placebo; data from the no-intervention arm (n=4) was eliminated from all analyses except sinus irritation, which was anticipated in all arms. Individuals who did not make the three month study visit were dropped from the analysis. Descriptive statistics were the primary outcome measure, and thresholds for reporting were determined a priori. Clinical side events were defined as a 2-point change on the MOSES or a rating of 3 or 4 (severe) on the 0–4 MOSES scale; Laboratory adverse events were predefined as a deviation from accepted reference ranges, e.g. ALT > 50 IU/L. Tolerability was defined as 80% of the group taking 80% of the prescribed dose of study medication.
Results
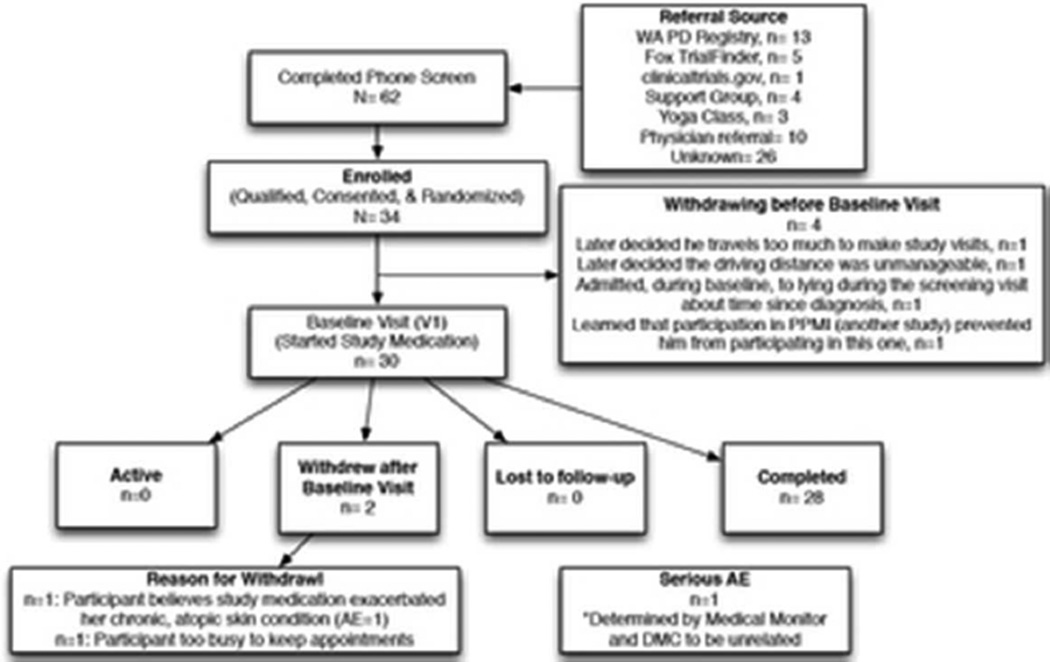
Of the 30 participants assigned to a treatment arm, 28 completed the study intervention; one participant withdrew due to schedule conflicts and the other withdrew due to an adverse event (AE) attributed to the study medication. The AE necessitating withdrawal from the study was a “ringing in her head” following the first use of study medication exacerbation of chronic pruritus that had been several months quiescent prior to the screening visit. The participant reported the ringing sensation resolved over 4–6 hours and the dermal inflammation resolved within two weeks. Across study arms, the predominately Caucasian (96%) participants were evenly distributed for gender (50%/50% male/female) and HY (median 2).
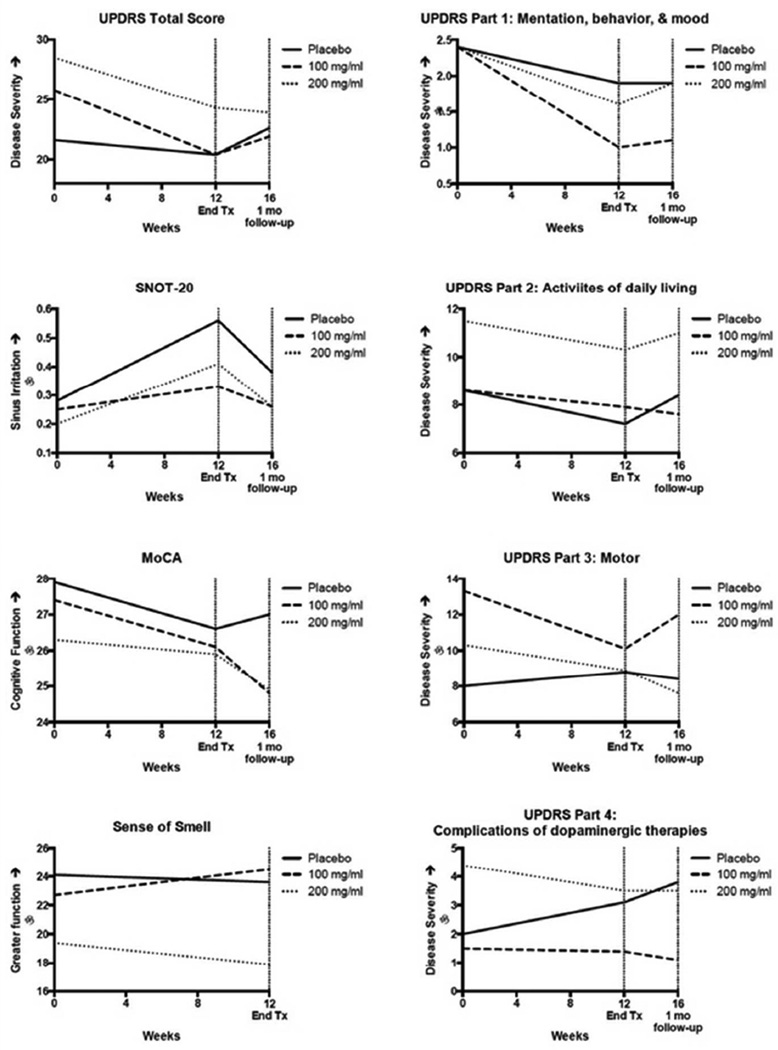
Subject compliance with study medication use met criteria for tolerability in all cohorts. GSH retained 89% of its potency after over 30 days of home storage. As expected, individuals in all intervention arms reported an increase in sinus symptoms, and this was approximately equivalent across arms. There were no statistically significant differences in the frequency of laboratory events as defined by CBC, WBC with differential, ALT, AST, creatinine, blood urea nitrogen, uric acid or urinalysis. UPDRS scores, included as a safety measure, improved in both treatment arms over placebo. In post hoc analysis, UPDRS trends remained consistent after excluding all individuals (n=10) who changed medications throughout the study. Side effects, deviations from laboratory reference ranges, and change from baseline clinical scores are listed in Table 1.
Table 1.
Side effects by cohort. The table reports the number of individuals meeting criteria for adverse events and includes only those symptoms reported by two or more participants in any cohort. Sinusitis and UPDRS reported as mean change in absolute score from baseline, by cohort.
| Table of Side Effects | |||
|---|---|---|---|
| Placebo |
300 mg/d | 600 mg/d | |
| (n=9) | (n=8) | (n=8) | |
| Number of individuals reporting symptom: | |||
| Negative Side Effects | |||
| Labored breathing | 0 | 0 | 2 |
| Sore throat/redness | 0 | 0 | 2 |
| Flatulence | 2 | 0 | 1 |
| Increased thirst | 0 | 0 | 2 |
| Contortions/neck-back arching | 0 | 2 | 0 |
| Chills | 2 | 0 | 0 |
| Positive Side Effects | |||
| Improved blink rate | 1 | 5 | 0 |
| Improved arm swing | 1 | 1 | 2 |
| Fewer muscle pains or aches | 2 | 1 | 2 |
| Reduced edema | 0 | 0 | 2 |
| Improved incontinence/Nocturnal enuresis | 0 | 2 | 0 |
| Reduced urinary frequency | 3 | 0 | 0 |
| Reduced agitation | 0 | 3 | 0 |
| Improved drowsiness/lethargy/sedation | 2 | 1 | 2 |
| Improved insomnia | 1 | 0 | 2 |
| Less crying/feelings of sadness | 2 | 0 | 0 |
| Deviation from laboratory normal reference ranges | |||
| Hemoglobin | 0 | 0 | 2 |
| Hematocrit | 0 | 0 | 2 |
| Creatine | 1 | 1 | 2 |
| Uric acid | 0 | 0 | 2 |
| Change from baseline, Mean (SD): | |||
| Sinusitis (SNOT-20 Score 0–1) | 0.275 | 0.185 | 0.213 |
| Change in Parkinson's Symptoms | |||
| UPDRS total (0–199) | −1.1 (4.1) | −5.3 (4.8) | −4.3 (7.5) |
| UPDRS Part 1: Mentation, behavior, & mood | −0.6(1.2) | −1.4(2.0) | −0.8(1.7) |
| UPDRS Part 2: Activities of daily living | −1.3(3.5) | −0.8 (2.3) | −1.3(3.5) |
| UPDRS Part 3: Motor score | 0.8 (3.7) | −3.1 (2.9) | −1.4(3.7) |
| UPRDS Part 4: Complications of dopaminergic therapy | 1.0(1.5) | −0.1 (1.0) | −0.9 (2.4) |
To evaluate whether individuals were unblinded by the smell, participant feedback was evaluated. Qualitative interviews generated 189 total comments; two comments referenced the salty taste, one mentioned the smell of sulfur in nose and stool. Of the six participants who expressed confidence in knowing their group assignment, two were correct.
Discussion
In this phase I/IIa clinical trial, (in)GSH was well-tolerated. A naturally occurring molecule, exogenously administered GSH has an excellent record of safety. The few studies that have evaluated exogenous administration of GSH to humans with PD have been reassuring. 16, 18
Mild clinical improvement in UPDRS symptoms came as a bit of a surprise for this non-dopaminergic therapy, although exogenous GSH has been shown to increase dopamine transporters.22 The benefit measured may be explained by regression toward the mean, although anecdotal reports suggest at least some individuals do experience an acute improvement in clinical symptoms following administration of exogenously supplied gluathione.19 While the study was double blind with a placebo control, GSH has a distinct smell that unblinded at least one participant.
The clinical response, while fortunate for patients, suggests delayed-start trial (or similar) design should be utilized when attempting to determine the neuroprotective capacity of (in)GSH over time. Symptomatic improvement with (in)GSH should be verified in a larger study powered for detecting differences between groups.
Overall, this study supports the safety and tolerability of (in)GSH in a sample of patients who are within 10 years of PD diagnosis. The identification of a non-dopaminergic strategy capable of improving UPDRS scores may herald a new generation of therapeutics. GSH perturbations have been documented in numerous other disorders of the CNS, such as schizophrenia, dementia, Huntington’s disease, and autism and thus the therapeutic potential of (in)GSH may not be limited to PD.
Outcomes associated with study arms. The mean change, by treatment arm, in clinical outcomes assessed over the course of the three-month study intervention and after a one-month wash out period. UPDRS: Unified Parkinson’s Disease Rating Scale; SNOT-20: SinoNasal Outcomes Test; MoCA: Montreal Cognitive Assessment; Sense of Smell was determined by Sensonics Smell Identification Test.
Enrollment algorithm according to CONSORT guidelines.
Acknowledgements
We gratefully acknowledge the participation of all individuals in this study. We thank Key Pharmacy for collaborating with the research team and their willingness to provide additional product purity and potency data throughout the study. We wish to acknowledge the donation of Mucosal Atomization Device tips by Wolfe-Tory Medical (Teleflex). The SNOT-20 and MoCA were used with permission from J. Piccirillo and Z. Nasreddine, respectively.
Financial Disclosures
LK Mischley: Research funding from NIH NCCAM, Charles and Barbara Wright, Bastyr University Research Institute, honoraria from Kadlec Neurological Resource Center, Union Hospital, Indiana State University, Northwest Parkinson’s Disease Foundation; A Samii: Honoraria from Teva Pharmaceuticals, UCB, and US WorldMeds; LJ Standish: NIH NCCAM, The John and Lotte Hecht Memorial Foundation, Bastyr University School of Naturopathic Medicine; NL Polissar: Fee-for service statistical consulting. JB Leverenz: Consulting Boehringer-Ingelheim, Navidea Biopharmaceuticals, Piramal Healthcare;
Funding: NIH NCCAM K01 AT04404, Bernard Osher Foundation, Veterans Affairs P50 NS062684
Footnotes
Conflict of Interest: None of the authors have any conflicts of interest to disclose.
Author Roles:
Laurie Mischley: Research project conception and execution, manuscript preparation; James Leverenz: Research project organization, review and critique of final manuscript; Richard Lau: Research project organization and execution, execution of statistical analysis plan, manuscript preparation; Nayak Polissar and Moni Neradilek: Statistical analysis design, review, and critique, and manuscript review; Ali Sami: Research project conception, organization; Leanna Standish: Research project conception and organization, manuscript review and critique.
No clients have financial interest in this study; RC Lau and MB Neradilek: None.
References
- 1.Guo N, McIntosh C, Shaw C. Glutathione: new candidate neuropeptide in the central nervous system. Neuroscience. 1992;51(4):835–842. doi: 10.1016/0306-4522(92)90524-6. [DOI] [PubMed] [Google Scholar]
- 2.Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A. The current and projected economic burden of Parkinson's disease in the United States. Mov Disord. 2013;28(3):311–318. doi: 10.1002/mds.25292. [DOI] [PubMed] [Google Scholar]
- 3.Aquilano K, Baldelli S, Ciriolo MR. Glutathione: new roles in redox signaling for an old antioxidant. Frontiers in pharmacology. 2014;5:196. doi: 10.3389/fphar.2014.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lizama-Manibusan B, McLaughlin B. Redox modification of proteins as essential mediators of CNS autophagy and mitophagy. FEBS Lett. 2013;587(15):2291–2298. doi: 10.1016/j.febslet.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Effects of tocopherol and deprenyl on the progression of disability in early Parkinson's disease. N Engl J Med. 1993;328(3):176–183. doi: 10.1056/NEJM199301213280305. [DOI] [PubMed] [Google Scholar]
- 6.NIH. Statement on the Termination of QE3 Study: Natational Institute of Neurological Disorders and Stroke (NINDS) 2011. [Google Scholar]
- 7.Bains JS, Shaw CA. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res Brain Res Rev. 1997;25(3):335–358. doi: 10.1016/s0165-0173(97)00045-3. [DOI] [PubMed] [Google Scholar]
- 8.Lee M, Cho T, Jantaratnotai N, Wang YT, McGeer E, McGeer PL. Depletion of GSH in glial cells induces neurotoxicity: relevance to aging and degenerative neurological diseases. Faseb J. 2010;24(7):2533–2545. doi: 10.1096/fj.09-149997. [DOI] [PubMed] [Google Scholar]
- 9.Pearce RK, Owen A, Daniel S, Jenner P, Marsden CD. Alterations in the distribution of glutathione in the substantia nigra in Parkinson's disease. J Neural Transm. 1997;104(6–7):661–677. doi: 10.1007/BF01291884. [DOI] [PubMed] [Google Scholar]
- 10.Cacciatore I, Baldassarre L, Fornasari E, Mollica A, Pinnen F. Recent advances in the treatment of neurodegenerative diseases based on GSH delivery systems. Oxid Med Cell Longev. 2012;2012:240146. doi: 10.1155/2012/240146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shils MEOJ, Shike Moshe. Modern Nutrition in Health and Disease. Philadelphia: Lippincott Williams & Wilkins; 2006. Evolution of Knowldege of Essential Nutrients: Conditional Essentiality. [Google Scholar]
- 12.DelleDonne A, Klos KJ, Fujishiro H, et al. Incidental Lewy body disease and preclinical Parkinson disease. Archives of neurology. 2008;65(8):1074–1080. doi: 10.1001/archneur.65.8.1074. [DOI] [PubMed] [Google Scholar]
- 13.Sian J, Dexter DT, Lees AJ, et al. Alterations in glutathione levels in Parkinson's disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol. 1994;36(3):348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- 14.Witschi A, Reddy S, Stofer B, Lauterburg BH. The systemic availability of oral glutathione. Eur J Clin Pharmacol. 1992;43(6):667–669. doi: 10.1007/BF02284971. [DOI] [PubMed] [Google Scholar]
- 15.Sechi G, Deledda MG, Bua G, et al. Reduced intravenous glutathione in the treatment of early Parkinson's disease. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20(7):1159–1170. doi: 10.1016/s0278-5846(96)00103-0. [DOI] [PubMed] [Google Scholar]
- 16.Hauser RA, Lyons KE, McClain T, Carter S, Perlmutter D. Randomized, double-blind, pilot evaluation of intravenous glutathione in Parkinson's disease. Mov Disord. 2009;24(7):979–983. doi: 10.1002/mds.22401. [DOI] [PubMed] [Google Scholar]
- 17.Seymour J. In: Use of compunded gluathione by CAM practitioners in the Pacific Northwest. Mischley L, editor. Las Vegas, NV: 2007. [Google Scholar]
- 18.Mischley LK, Vespignani MF, Finnell JS. Safety survey of intranasal glutathione. J Altern Complement Med. 2013;19(5):459–463. doi: 10.1089/acm.2011.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mischley LK. Glutathione Deficiency in Parkinson's Disease: Intranasal Administration as a Method of Augmentation. Journal of Orthomolecular Medicine. 2011;26(1):32–36. [Google Scholar]
- 20.Piccirillo JF, Merritt MG, Jr, Richards ML. Psychometric and clinimetric validity of the 20-Item Sino-Nasal Outcome Test (SNOT-20) Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2002;126(1):41–47. doi: 10.1067/mhn.2002.121022. [DOI] [PubMed] [Google Scholar]
- 21.Hauser RA, Auinger P. Determination of minimal clinically important change in early and advanced Parkinson's disease. Mov Disord. 2011;26(5):813–818. doi: 10.1002/mds.23638. [DOI] [PubMed] [Google Scholar]
- 22.Sechi GNS, Agnetti V, et al. Influence of parenteral GSH on striatal dopamine transporter in PD. Movment Disorders. 2006;21(Suppl 15):S579. [Google Scholar]
- 23.United States National Library of Medicine . Entacapone. In: AM (EST), editor. LiverTox: Clinical and Research Informtion on Drug-Induced Liver Injury. 2014-07-2 10:45:25. National Institute of Diabetes and Digestive and Kidney Diseases, US Department of Health & Human Services; 2014. [Google Scholar]
- 24.Kobrinsky NL, Hartfield D, Horner H, et al. Treatment of advanced malignancies with high-dose acetaminophen and N-acetylcysteine rescue. Cancer Invest. 1996;14(3):202–210. doi: 10.3109/07357909609012140. [DOI] [PubMed] [Google Scholar]
- 25.Wang N, Shi XF, Guo SH, Zhang DZ, Ren H. A clinical study of N-acetylcysteine treatment in chronic hepatitis B patients. Zhonghua Gan Zang Bing Za Zhi. 2008;16(7):487–489. [PubMed] [Google Scholar]
- 26.Pinhel MA, Sado CL, Longo Gdos S, et al. Nullity of GSTT1/GSTM1 related to pesticides is associated with Parkinson's disease. Arquivos de neuro-psiquiatria. 2013;71(8):527–532. doi: 10.1590/0004-282X20130076. [DOI] [PubMed] [Google Scholar]