Abstract
Background
Sunscreens protect against skin cancer and other harmful effects of solar ultraviolet radiation (UVR). Epidemiologic and public health surveys often rely on self-reported sunscreen use to estimate sun exposure and avoidance, but questions remain about the validity of self-reports. Benzophenone-3 (BP-3), a common sunscreen ingredient, can be detected in the urine. Prior studies suggest that BP-3 concentrations increase after application of sunscreen.
Objectives
The goal of this study was to assess the validity of self-reported frequency of sunscreen use in relation to urinary BP-3 concentrations in a representative sample of the general US population, including in sub-groups defined by age, sex and race/ethnicity.
Methods
To assess the relationship between categorical self-reported sunscreen use and creatinine-corrected urinary BP-3 concentrations, we conducted a linear regression adjusted for age, sex, race/ethnicity, six-month time period, body mass index, education, and sun avoidance behaviors. We tested for effect modification by age, sex, ethnicity and time period of measurement using multiplicative interaction terms and a F test.
Results
BP-3 was positively associated with self-reported frequency of sunscreen use across all ages, sexes, race/ethnicities, and time periods. Crude and multivariate adjusted models were all statistically significant. R-square was relatively low for all models, ranging from 0.15-0.43.
Conclusions
Urinary BP-3 is positively associated with self-reported frequency of sunscreen use in the general US population, even in groups with overall low sunscreen use. These results suggest that self-report is a valid, although weak, way of assessing relative frequencies of sunscreen usage in a population-based study.
Keywords: Sunscreen, benzophenone-3, NHANES
Introduction
Sunscreens protect against harmful effects of sun exposure and solar ultraviolet radiation (UVR), including sunburn, skin aging, and skin cancer, the most common type of cancer in the United States (HHS). Sunscreen use varies considerably by sex and race/ethnicity, with females and non-Hispanic whites using more sunscreen than males and other ethnic groups (Briley et al. 2007; Hall et al. 1997; Pichon et al. 2005).With increasing rates of skin cancer in many countries (Edwards et al. 2014; Staples et al. 2006), public health campaigns around the world promote sunscreen use and reduced solar exposure (CDC; Eide and Weinstock 2006; HHS).
Many epidemiological studies on sun exposure and skin cancer rely on self-report to measure sunscreen use (Kearney et al. 2014; Mortier et al. 2015; Parker et al. 2015). Self-reported sunscreen use is also used as one way of evaluating the efficacy of public health campaigns aimed at increasing sun avoidance behaviors and preventing skin cancer (Buller et al. 2015; Glanz et al. 2015; Youl et al. 2015). It is therefore important to understand the validity of self-reported frequency of sunscreen use.
A limited number of previous studies have sought to evaluate the validity of self-reported sunscreen use. One prior study by Hillhouse et al. used daily and weekly diaries of sun protection behaviors during the summer, and compared them to surveys that summarized several months use that were given at the end of the summer and found good validity between the diary reports and survey results (Hillhouse et al. 2012). The study population in Hillhouse et al. was drawn from a limited geographical area (southeastern United States) and comprised mainly females and whites, the groups most likely to be using sunscreen, so these findings may not be generalizable to males and other ethnicities with lower rates of sunscreen usage (Hillhouse et al. 2012). Another previous study found “fair to good” agreement between self-reported and actual sunscreen use, as measured by swabbing the skin, among children at a swimming pool during the summer, a population in which attention to sun avoidance is increased, and therefore these results may not be generalizable to everyday patterns of sunscreen use or adults (Glanz et al. 2009).
Benzophenone-3 (BP-3) is a common ingredient in sunscreen that absorbs UVR (270-350 nm)(Burnett and Wang 2011). An experimental trial showed that urinary BP-3 concentrations increase following application of sunscreens containing BP-3, regardless of UVR exposure (Gonzalez et al. 2006). These findings are supported by another study by Calafat et al. that found higher BP-3 concentrations in NHANES participants who were more likely to be using sunscreen, namely females and non-Hispanic whites (Calafat et al. 2008). Calafat et al. suggested that the higher levels of BP-3 seen in women and whites could be due to increased usage of sunscreen and personal care products that contain sunscreen, some of which also contain BP-3 as an ingredient (Calafat et al. 2008). Although investigators have confirmed the relationship between sunscreen use and urinary BP-3 concentrations, no study has validated self-reported sunscreen use in a nationally representative US population.
The aim of this study was to use urinary BP-3 concentrations as a biomarker of sunscreen usage to assess the validity of self-reported typical sunscreen usage in adults in a nationally-representative sample of the United States population. To our knowledge, no study in adults has used a biomarker to assess whether self-reported sunscreen use represents actual use. Moreover, prior validation studies of self-reported sunscreen use have not been representative of the general US population, and have been conducted in the summer, when attention to sun protection is increased. In the present study, we sought to assess the validity of self-reported typical year-round sunscreen use in the general adult US population using urinary BP-3 as a biomarker of sunscreen use.
Methods
The National Health and Nutrition Examination Survey (NHANES) is a publically available, representative sample of the civilian, non-institutionalized population of the United States with the goal of assessing the health and nutrition of children and adults in the United States. Sampling is conducted by a non-random, complex, multi-stage sampling design. NHANES data are collected by conducting physical examinations, interviewing participants, and obtaining blood and urine specimens. NHANES has been conducted continuously since 1999, with data reported in 2-year intervals. The survey years 2003-2006 and 2009-2012 were selected for this analysis because they included urine measurements of BP-3, which we used as a biomarker for actual sunscreen use, and self-reported sunscreen use.
BP-3 was measured in the urine of a random one-third subset of NHANES participants aged 6 years and older. Urine specimens were collected and shipped to the Division of Laboratory Sciences, National Center for Environmental Health, at the CDC to be analyzed (CDC 2014). BP-3 was assayed using online solid-phase extraction coupled with high-performance liquid chromatography and tandem mass spectrometry (Calafat et al. 2008; CDC 2014). Details of these analytic procedures are available elsewhere (CDC 2013a). BP-3 was creatinine-corrected and reported in µg/g creatinine, and not per volume of urine, to account for urine dilution. Creatinine was measured at the University of Minnesota in Minneapolis, MN, using a Roche/Hitachi Modular P Chemistry Analyzer. Details on this assay are available elsewhere (CDC 2013b). The coefficients of variation for BP-3 were generally well under 10, with the exception of survey years 2003-2004 where the coefficients were 18.4 and 20.7(CDC 2005, 2009, 2011a, 2013a).
Sunscreen use was assessed by self-report in the interview portion of the examination, which took place in the participants' homes. Participants were asked if they used sunscreen “always,” “most of the time,” “sometimes,” “rarely,” or “never.” Participants were also asked about other sun avoidance behaviors, including how often they stay in the shade and how often they wear a long-sleeved shirt, with the same frequency categories for answers. The dermatology questionnaire, used during the interview to assess sunscreen use and sun avoidance, was only administered to participants aged 20-59 years, so our sample was limited to that age range (n=14,463). Participants were excluded if BP-3 was not measured (n=30,460) or had a BP-3 measurement but were missing urinary creatinine (n=5). Of the participants who had a BP-3 measurement and urinary creatinine, 5,885 were also excluded for missing sunscreen use data, and 1 participant was excluded for reporting “don't know” when asked about frequency of sunscreen use. This resulted in a study population of N=4,412.
Demographic information (age, sex, and race/ethnicity) was also collected during the home interview. Body mass index (BMI) was assessed in the Mobile Examination Center (MEC). BMI was calculated as weight (kilograms) per height (meters squared). Details on these measurements are available elsewhere (CDC 2011b).
Statistical Analyses
To assess the association between self-reported sunscreen use and measured BP-3 concentrations, we used linear regression with sunscreen use as the predictor and log-creatinine-corrected BP-3 as the categorical outcome variable. Survey weights were used to account for the non-random sampling design of NHANES. After combining the data from four survey years, we divided each survey weight by 4 to yield the correct weight for each participant, reflecting the lower weight of each participant in the larger dataset. Our model adjusted for age, sex, ethnicity, BMI, education, sun avoidance behaviors, and six-month time period. These adjustment factors were chosen because they are associated with sunscreen use or BP-3 concentrations (Buck Louis et al. 2014; Calafat et al. 2008). Exam period was confounded by latitude, as the NHANES examination centers are typically located at lower latitudes during the winter months. Sunscreen use was assessed as a categorical variable, and p-trend across the categories was calculated by treating self-reported sunscreen use as an ordinal variable, and reporting the p-value for that model. For each model, we also calculated an R2 value, to determine the percentage of variability in BP-3 explained by the model as a whole. We also calculated a partial R2 value for the percentage of variability explained by self-reported sunscreen use only, which was calculated by subtracting the R2 for a model without sunscreen use from the full model with sunscreen use.
We assessed interaction in several ways. Each model was stratified by either ethnicity, sex, age group (20-29 years, 30-39 years, 40-49 years, 50-59 years), and six-month time period of exam (November-April and May-October) (“exam period”). We chose to stratify by these variables to determine if the association between sunscreen use and BP-3 concentrations differed between strata of groups known to have unequal patterns of sunscreen use. For the interaction test between sunscreen use,race/ethnicity and BP-3, we generated a categorical interaction term, and used an F test as a global test of interaction. For the interaction between sunscreen use and age, we generated a continuous interaction term, treating the categorical sunscreen variable and age both continuous variables. For the interaction between sunscreen use and sex, and sunscreen use and exam period, we generated a multiplicative interaction term for each model that treated sunscreen use as continuous, and sex (male or female) or exam period (November-April or May-October) as categorical. Statistical analyses were conducted using the svy command in Stata (Stata Corp, College Station, TX, USA).
Results
We found higher concentrations of BP-3 in participants reporting higher frequencies of sunscreen use. The geometric mean creatinine-corrected BP-3 concentration is reported in Table 1 for each category of sunscreen use, with higher BP-3 concentrations observed for each increased frequency category. The median BP-3 concentrations are shown in Figure 1.
Table 1. Geometric mean benzophenone-3 (µg/g creatinine) according to frequency of sunscreen use (n=4412).
| Frequency of sunscreen use | Geometric mean BP-3 concentration (µg/g creatinine) | p-value |
|---|---|---|
| Never | 9.3 | ref |
| Rarely | 14.8 | <0.01 |
| Sometimes | 32.3 | <0.01 |
| Most of the time | 74.2 | <0.01 |
| Always | 116.8 | <0.01 |
Figure 1. Median benzophenone-3 (µg/g creatinine) according to frequency of sunscreen use (n=4412).
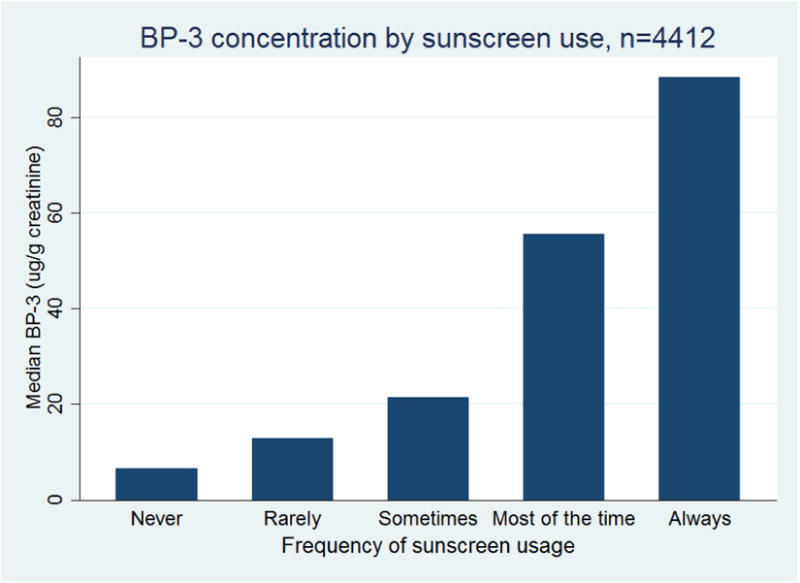
Table 2 reports the number of people, with survey-weighted percentages, reporting each category of sunscreen frequency by race/ethnicity and sex. Across all race/ethnic groups, men were more likely to report “never” using sunscreen, and women were more likely to report using sunscreen “most of the time” or “always” (Table 2). With the exception of non-Hispanic white females, all ethnic groups and sexes were more likely to report “never” using sunscreen than “always” (Table 2). There was a statistically significant trend (p<0.01) between self-reported sunscreen use and BP-3 concentrations in all of the models tested, and within each racial and ethnic group (Table 3), within each sex (Table 4), within each age group (Table 5), and within each exam period (Table 6). All of these associations were positive, indicating that higher frequency of self-reported sunscreen use was associated with higher concentrations of BP-3.
Table 2. Frequency and survey-weighted percentage of self-reported sunscreen use, stratified by race and sex (n=4412).
| Frequency of sunscreen use | ||||||
|---|---|---|---|---|---|---|
| Race | Sex | Never n (%) | Rarely n (%) | Sometimes n (%) | Most of the time n (%) | Always n (%) |
| Non-Hispanic White | Male | 313 (0.29) | 191 (0.21) | 241 (0.28) | 139 (0.16) | 50 (0.06) |
| Female | 204 (0.18) | 131 (0.13) | 236 (0.25) | 222 (0.23) | 200 (0.22) | |
| Non-Hispanic Black | Male | 408 (0.83) | 31 (0.06) | 34 (0.07) | 11 (0.02) | 10 (0.02) |
| Female | 342 (0.70) | 48 (0.09) | 51 (0.11) | 18 (0.03) | 34 (0.07) | |
| Mexican American | Male | 262 (0.68) | 36 (0.11) | 48 (0.13) | 16 (0.04) | 14 (0.03) |
| Female | 162 (0.41) | 49 (0.12) | 74 (0.22) | 33 (0.09) | 54 (0.16) | |
| Other Hispanic | Male | 102 (0.66) | 12 (0.08) | 17 (0.13) | 10 (0.05) | 11 (0.08) |
| Female | 55 (0.31) | 21 (0.11) | 37 (0.21) | 20 (0.14) | 43 (0.22) | |
| Other race/multiracial | Male | 108 (0.49) | 32 (0.18) | 39 (0.16) | 21 (0.11) | 13 (0.07) |
| Female | 64 (0.31) | 22 (0.10) | 40 (0.20) | 41 (0.22) | 44 (0.18) | |
Table 3. Regression coefficients for change in log benzophenone-3 concentrations according to frequency of sunscreen use, stratified by ethnicity (n=4412).
| Frequency of sunscreen use | Ethnicity | ||||
|---|---|---|---|---|---|
| White non-Hispanic | Black non-Hispanic | Mexican American | Other Hispanic | Other race, multi-racial | |
| Never | ref | ref | ref | ref | Ref |
| Rarely | 0.2 (-0.1-0.5) | 0.3 (-0.2-0.9) | 0.5 (0.0-0.9) | 0.2 (-0.2-0.7) | 0.4 (-0.2-1.0) |
| Sometimes | 0.9 (0.6-1.2) | 0.5 (0.1-0.9) | 0.7 (0.4-1.0) | 1.2 (0.7-1.6) | 1.6 (0.9-2.3) |
| Most of the time | 1.5 (1.2-1.9) | 1.4 (0.7-2.1) | 1.8 (1.0-2.6) | 1.6 (0.8-2.5) | 2.2 (1.6-2.8) |
| Always | 2.0 (1.6-2.4) | 1.9 (1.1-2.7) | 1.8 (1.1-2.5) | 2.1 (1.3-2.9) | 1.9 (1.1-2.9) |
| P-trend | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Model R2 | 0.23 | 0.15 | 0.27 | 0.28 | 0.43 |
| Sunscreen R2 | 0.08 | 0.06 | 0.07 | 0.13 | 0.13 |
Adjusted for age, sex, BMI, education, sun avoidance
Table 4. Regression coefficients for change in log benzophenone-3 concentrations according to frequency of sunscreen use, stratified by sex (n=4412).
| Frequency of sunscreen use | Sex | |
|---|---|---|
| Males | Females | |
| Never | ref | ref |
| Rarely | 0.3 (0.0-0.5) | 0.2 (-0.1-0.6) |
| Sometimes | 0.9 (0.7-1.2) | 1.0 (0.6-1.3) |
| Most of the time | 1.4 (1.0-1.7) | 1.8 (1.4-2.1) |
| Always | 2.1 (1.7-2.4) | 1.8 (1.2-2.5) |
| P-trend | <0.01 | <0.01 |
| Model R2 | 0.19 | 0.22 |
| Sunscreen R2 | 0.09 | 0.11 |
Adjusted for age, ethnicity, BMI, education, sun avoidance, and 6-month time period
Table 5. Regression coefficients for change in log benzophenone-3 concentrations according to frequency of sunscreen use, stratified by age (n=4412).
| Frequency of sunscreen use | Age group | |||
|---|---|---|---|---|
| 20-29 years | 30-39 years | 40-49 years | 50-59 years | |
| Never | ref | ref | ref | ref |
| Rarely | 0.0 (-0.3-0.4) | 0.3 (-0.1-0.7) | 0.3 (-0.1-0.8) | 0.3 (-0.2-0.9) |
| Sometimes | 0.8 (0.5-1.2) | 0.8 (0.4-1.2) | 1.2 (0.7-1.6) | 0.8 (0.3-1.3) |
| Most of the time | 1.1 (0.8-1.5) | 1.2 (0.8-1.7) | 1.9 (1.5-2.4) | 1.9 (1.3-2.4) |
| Always | 1.8 (1.2-2.3) | 1.5 (1.0-2.0) | 1.9 (1.2-2.5) | 2.7 (2.0-3.4) |
| P-trend | <0.01 | <0.01 | <0.01 | <0.01 |
| Model R2 | 0.26 | 0.26 | 0.26 | 0.29 |
| Sunscreen R2 | 0.08 | 0.06 | 0.11 | 0.14 |
Adjusted for sex, ethnicity, BMI, education, sun avoidance, and 6-month time period
Table 6. Regression coefficients for change in log benzophenone-3 concentrations according to frequency of sunscreen use, stratified by 6-month time period (n=4412).
| Frequency of sunscreen use | Exam period | |
|---|---|---|
| Nov-April | May-Oct | |
| Never | ref | ref |
| Rarely | 0.3 (0.0-0.6) | 0.2 (0.0-0.5) |
| Sometimes | 0.6 (0.3-0.8) | 1.2 (0.9-1.5) |
| Most of the time | 1.6 (1.3-2.0) | 1.6 (1.3-1.9) |
| Always | 1.9 (1.5-2.3) | 2.1 (1.7-2.4) |
| P-trend | <0.01 | <0.01 |
| Model R2 | 0.25 | 0.23 |
| Sunscreen R2 | 0.08 | 0.11 |
- Urinary benzophenone-3 (BP-3) is a metabolite of a common sunscreen ingredient.
- We modeled urinary BP-3 against self-reported sunscreen usage.
- We observed a positive association between sunscreen use and urinary BP-3 concentrations.
- R2 was low, suggesting self-report is a valid although weak way of assessing frequency of sunscreen use.
When we examined interaction between sunscreen use and various demographic factors, we found no statistically significant interaction by race (F=1.26, p=0.26), nor by sex (β=0.04, p=0.54) or exam time (β=0.02, p=0.67). However, there was an interaction between frequency of sunscreen use and age (β=0.01, p=0.03), with greater association in older participants.
Discussion
In this cross-sectional, representative sample of the US population, we found that self-reported frequency of sunscreen use was significantly associated with urinary BP-3 concentrations in US adults. This association was seen across all age groups, sexes, races/ethnicities, and exam periods.
We also found a statistically significant interaction between sunscreen use and age on BP-3 concentrations, suggesting that the association between self-reported sunscreen use and BP-3 concentrations increases with age; however, the association between sunscreen use and BP-3 was statistically significant across all age strata. While we found strong associations between self-reported sunscreen use and BP-3 across all ages, sexes, ethnicities, and exam periods, the R2 values for all models were relatively small, which may be due to the fact that, among other factors, there are other sources of exposure to BP-3. For example, participants could also have been exposed from BP-3 used in other cosmetics, hair products, shampoo, or food packaging (Calafat et al. 2008; Schlumpf et al. 2001). The relatively low R2 values may also reflect individual differences in metabolizing BP-3, laboratory sources of variation, the psychological difficulties in remembering and summarizing relative frequency of sunscreen use over time, and the use of a single one-time BP-3 measurement. While we treated BP-3 as a biomarker of actual sunscreen use in this study, its use as a biomarker may be limited due to the possibility of exposure from other products, and an emerging popularity of sunscreens not containing BP-3.
Sunscreen has been utilized as an important public health tool for avoiding solar UVR. Skin cancer is very common, and sun avoidance behaviors, including sunscreen use, are promoted as an effective way to reduce risk of sunburn and developing skin cancer (Ghiasvand et al. 2015; Montague et al. 2001). As a result, sunscreen use has increased over time (Ghiasvand et al. 2015). While it is recommended that sunscreen be used in conjunction with other sun avoidance behaviors such as sitting in the shade and wearing long clothing, sunscreen is the most commonly used method to protect against sun exposure (Stanton et al. 2004). A valid assessment of sunscreen use is therefore important in measuring the effectiveness of public health campaigns aimed at reducing UVR exposure and skin cancer risk. The results of this study suggest that frequency of sunscreen use assessed via self-report provides a valid method of relative frequency of sunscreen use. However, given the relatively low R2s for predicting BP-3, self-reported summaries of sunscreen use provide only weak indications of BP-3 concentrations.
While skin cancer is primarily a problem for light-skinned individuals, people of all races and ethnicities are at risk for skin cancer and are advised to practice sun avoidance behaviors (Agbai et al. 2014). The consistent associations we found between sunscreen use and BP-3 concentrations suggests that even among groups with low frequencies of sunscreen usage, self-report is linearly and positively related to BP-3.
An important strength of this study is that the study population is representative of the US population. This is valuable, as sunscreen use varies widely by sex and ethnicity. Among the study limitations are its cross-sectional design. Ideally BP-3 would be measured periodically over the time corresponding to the frequency of sunscreen use. As noted, sunscreen use only accounts for a small percentage of measured BP-3, probably due in part to the fact that sunscreen is not the only source of BP-3. In addition, we have no information on the actual frequency of sunscreen use, only the relative frequencies, represented by the categories presented to the participants. The validity of self-reported sunscreen use could potentially be improved by more precise ways of asking about sunscreen frequency, such as asking individuals how many times per day they apply sunscreen, the volume of sunscreen applied each application, and the SPF rating of the sunscreen.
Conclusions
In this study, we found a statistically significant positive association between self-reported frequency of sunscreen use and urinary BP-3 across all age groups, sexes, and race/ethnicities. Although these results suggest that self-reported use is significantly related to BP-3, our biomarker for actual sunscreen use, further studies will be needed to assess whether there are ways to improve the biomarker for actual use as well as ways to validate self-reporting of sunscreen use with more precise questioning including volume of sunscreen used, number of days per week used, how often sunscreen is reapplied during the day, and typical SPF used.
Acknowledgments
The authors thank Barry Graubard for his helpful comments on the analyses. This work was supported by funding from the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and the U.S. Public Health Service of the Department of Health and Human Services. We also thank the National Center for Health Statistics, Centers for Disease Control and Prevention for administration of NHANES, as well as the Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention for the measurement of BP-3.
Footnotes
The authors have no competing financial interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agbai ON, Buster K, Sanchez M, Hernandez C, Kundu RV, Chiu M, et al. Skin cancer and photoprotection in people of color: A review and recommendations for physicians and the public. Journal of the American Academy of Dermatology. 2014;70:748–762. doi: 10.1016/j.jaad.2013.11.038. [DOI] [PubMed] [Google Scholar]
- Briley JJ, Jr, Lynfield YL, Chavda K. Sunscreen use and usefulness in african-americans. Journal of drugs in dermatology : JDD. 2007;6:19–22. [PubMed] [Google Scholar]
- Buck Louis GM, Kannan K, Sapra KJ, Maisog J, Sundaram R. Urinary concentrations of benzophenone-type ultraviolet radiation filters and couples' fecundity. American journal of epidemiology. 2014;180:1168–1175. doi: 10.1093/aje/kwu285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller DB, Berwick M, Lantz K, Buller MK, Shane J, Kane I, et al. Evaluation of immediate and 12-week effects of a smartphone sun-safety mobile application: A randomized clinical trial. JAMA dermatology. 2015 doi: 10.1001/jamadermatol.2014.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett ME, Wang SQ. Current sunscreen controversies: A critical review. Photodermatology, photoimmunology & photomedicine. 2011;27:58–67. doi: 10.1111/j.1600-0781.2011.00557.x. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Wong LY, Ye X, Reidy JA, Needham LL. Concentrations of the sunscreen agent benzophenone-3 in residents of the united states: National health and nutrition examination survey 2003--2004. Environmental health perspectives. 2008;116:893–897. doi: 10.1289/ehp.11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Sunscreen for your sun day. Available: http://www.cdc.gov/cancer/skin/pdf/sunscreen4sunday.pdf.
- CDC. Laboratory procedure manual. 2005 Available: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l24eph_c_met_phenols.pdf.
- CDC. Laboratory procedure manual. 2009 Available: http://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/eph_d_met_phenols_parabens.pdf.
- CDC. Laboratory procedure manual. 2011a Available: http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/EPH_F_met_phenols_parabens.pdf.
- CDC. Anthropometry procedures manual. 2011b Available: http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/Anthropometry_Procedures_Manual.pdf.
- CDC. Laboratory procedure manual. 2013a Available: http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/EPH_G_met.pdf.
- CDC. Urinary albumin and urinary creatinine. 2013b Available: http://wwwn.cdc.gov/nchs/nhanes/2011-2012/ALB_CR_G.htm.
- CDC. Environmental phenols and parabens. 2014 Available: http://wwwn.cdc.gov/nchs/nhanes/2011-2012/EPH_G.htm.
- Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, et al. Annual report to the nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–1314. doi: 10.1002/cncr.28509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide MJ, Weinstock MA. Public health challenges in sun protection. Dermatologic clinics. 2006;24:119–124. doi: 10.1016/j.det.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Ghiasvand R, Lund E, Edvardsen K, Weiderpass E, Veierod MB. Prevalence and trends of sunscreen use and sunburn among norwegian women. The British journal of dermatology. 2015;172:475–483. doi: 10.1111/bjd.13434. [DOI] [PubMed] [Google Scholar]
- Glanz K, McCarty F, Nehl EJ, O'Riordan DL, Gies P, Bundy L, et al. Validity of self-reported sunscreen use by parents, children, and lifeguards. American journal of preventive medicine. 2009;36:63–69. doi: 10.1016/j.amepre.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanz K, Volpicelli K, Jepson C, Ming ME, Schuchter LM, Armstrong K. Effects of tailored risk communications for skin cancer prevention and detection: The pennscape randomized trial. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24:415–421. doi: 10.1158/1055-9965.EPI-14-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez H, Farbrot A, Larko O, Wennberg AM. Percutaneous absorption of the sunscreen benzophenone-3 after repeated whole-body applications, with and without ultraviolet irradiation. The British journal of dermatology. 2006;154:337–340. doi: 10.1111/j.1365-2133.2005.07007.x. [DOI] [PubMed] [Google Scholar]
- Hall HI, May DS, Lew RA, Koh HK, Nadel M. Sun protection behaviors of the u.S. White population. Preventive medicine. 1997;26:401–407. doi: 10.1006/pmed.1997.0168. [DOI] [PubMed] [Google Scholar]
- HHS. The surgeon general's call to action to prevent skin cancer. Available: http://www.surgeongeneral.gov/library/calls/prevent-skin-cancer/call-to-action-prevent-skin-cancer.pdf. [PubMed]
- Hillhouse J, Turrisi R, Jaccard J, Robinson J. Accuracy of self-reported sun exposure and sun protection behavior. Prevention science : the official journal of the Society for Prevention Research. 2012;13:519–531. doi: 10.1007/s11121-012-0278-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney GD, Phillips C, Allen DL, Hurtado GA, Hsia LL. Sun protection behaviors among latino migrant farmworkers in eastern north carolina. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine. 2014;56:1325–1331. doi: 10.1097/JOM.0000000000000275. [DOI] [PubMed] [Google Scholar]
- Montague M, Borland R, Sinclair C. Slip! Slop! Slap! And sunsmart, 1980-2000: Skin cancer control and 20 years of population-based campaigning. Health education & behavior : the official publication of the Society for Public Health Education. 2001;28:290–305. doi: 10.1177/109019810102800304. [DOI] [PubMed] [Google Scholar]
- Mortier L, Lepesant P, Saiag P, Robert C, Sassolas B, Grange F, et al. Comparison of sun protection modalities in parents and children. Journal of the European Academy of Dermatology and Venereology : JEADV. 2015;29(Suppl 2):16–19. doi: 10.1111/jdv.12897. [DOI] [PubMed] [Google Scholar]
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at travis afb. Military medicine. 2015;180:26–31. doi: 10.7205/MILMED-D-14-00091. [DOI] [PubMed] [Google Scholar]
- Pichon LC, Mayer JA, Slymen DJ, Elder JP, Lewis EC, Galindo GR. Ethnoracial differences among outdoor workers in key sun-safety behaviors. American journal of preventive medicine. 2005;28:374–378. doi: 10.1016/j.amepre.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Schlumpf M, Cotton B, Conscience M, Haller V, Steinmann B, Lichtensteiger W. In vitro and in vivo estrogenicity of uv screens. Environmental health perspectives. 2001;109:239–244. doi: 10.1289/ehp.01109239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton WR, Janda M, Baade PD, Anderson P. Primary prevention of skin cancer: A review of sun protection in australia and internationally. Health promotion international. 2004;19:369–378. doi: 10.1093/heapro/dah310. [DOI] [PubMed] [Google Scholar]
- Staples MP, Elwood M, Burton RC, Williams JL, Marks R, Giles GG. Non-melanoma skin cancer in australia: The 2002 national survey and trends since 1985. The Medical journal of Australia. 2006;184:6–10. doi: 10.5694/j.1326-5377.2006.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Youl PH, Soyer HP, Baade PD, Marshall AL, Finch L, Janda M. Can skin cancer prevention and early detection be improved via mobile phone text messaging? A randomised, attention control trial. Preventive medicine. 2015;71:50–56. doi: 10.1016/j.ypmed.2014.12.009. [DOI] [PubMed] [Google Scholar]


