Abstract
Background
Atopic dermatitis (AD) is a heterogenous and highly complex disease characterized by an increased microbial colonization. For unknown reasons, a subgroup of AD patients develops Eczema herpeticum (EH), a severe viral complication due to spreading of herpes simplex virus (HSV). Indoleamine 2,3-dioxygenase (IDO1) is a tryptophan (Trp) catabolizing enzyme which is assumed to be instrumental in the anti-bacterial and anti-viral defence mechanisms.
Methods
Comparative investigation of the IDO1 expression and activity in freshly isolated monocytes, plasmacytoid DC (pDC) and in vitro generated Langerhans cells (LC) obtained from AD patients with HSV-infections and EH and non-atopic controls.
Results
We demonstrate an increase in Trp degradation in the serum of patients during acute EH episodes. Circulating pDC from patients with history of EH display an increased IDO1 expression. An increased Trp degradation is detected in the supernatants of circulating monocytes from AD patients with acute EH. Mature LC from AD patients with history of EH and with acute EH display an increased IDO1 expression and activity respectively. In LC from patients with history of EH, viral signals induce an exaggerated IDO1 expression and activity.
Conclusion
IDO1 expression and activity in LC seems involved in the pathophysiology of EH in AD and could represent a predictive biomarker for patients with risk to develop EH and other viral complications.
Keywords: Atopic dermatitis; Eczema herpeticum; Herpes simplex virus; Indoleamine 2,3- dioxygenase; Langerhans cells; Biomarker
Introduction
Atopic dermatitis (AD) is a complex and heterogeneous disease characterized by chronic eczematous skin lesions with a large clinical phenotype requesting adapted therapeutic approaches (1–5). AD is frequently aggravated by superinfections (1, 2). Eczema herpeticum (EH) is an uncommon but severe viral complication due to herpes simplex virus (HSV) type 1 or 2 which can be observed in a subset of AD patients (6, 7). Recently, a number of risk factors have been identified which may predispose AD patients to develop EH (8–11). Plasmacytoid dendritic cells (pDC) have been identified as important components of the antiviral defence (12, 13) but they seem underrepresented in lesional skin of AD (14). Subsets of myeloid dendritic cells (DCs) including epidermal Langerhans cells (LC) are postulated to be involved in the resistance towards but also in the spreading of viruses (15–17).
An interesting and multifaceted fashion of how DC contribute to immune homeostasis is the expression of the enzyme indoleamine 2,3-dioxygenase (IDO) (18–20) which metabolizes the essential amino acid tryptophan (Trp) as the first step in the so-called kynurenine (Kyn) pathway. The degradation of Trp to Kyn can be reached by three enzymes, i.e. (i) the initially reported IDO1, (ii) its more recently identified analogous enzyme IDO2 and (iii) the tryptophan 2,3-dioxygenase (TDO). Each Trp degrading enzyme seems to have distinct tissue localization and may be induced by distinct ways (18): While TDO regulates the systemic Trp level, IDO1 and IDO2 act more locally in the lymph nodes and the tissues. TDO is constitutively expressed in the liver and can be induced by distinct stimuli such as e.g. glucocorticosteroids. IDO2 is expressed in the tissue of liver, kidney, brain and in antigen-presenting cells. IDO1 is variably expressed in myeloid DC, while pDC can express both IDO1 and IDO2. IDO activity has been reported in a number of antigen-presenting cells e.g. different DC subsets like CD8α positive DCs, CD103 positive DCs, CD19+/B220-/CD11c+ DCs and pDCs (21, 22), but also in epidermal LC (23). IDO activity degrades Trp locally thereby lowering its concentration to an amount critical for T cells (19, 24) or pathogens (25). T cells are either halted in their proliferation or transformed into regulatory T cells (Tregs)(19).There is growing evidence for a role of the IDO1 induced Trp degradation as a broad anti-microbial effector (26, 27) in parasitic infections such as Toxoplasma gondii and bacterial infections such as Listeria monocytogenes (28) or Staphyloccocus aureus (29, 30). Most importantly, IDO1 activity has been shown to be crucial in anti-viral defense including HSV (27, 31, 32). In contrast, some viruses such as HIV, cytomegalovirus or EBV are also able to impair or exploit IDO1 activity (33, 34). Recently, IDO1 has been shown to be of importance in atopic disorders (35–37). This could be linked to the catalytic activity of IDO1 but also to a signalling but non-enzymatic capability of IDO1 relevant for long term tolerance (38).
In the present study, we were interested to gain more insight into the regulation of IDO1 in anti-viral defense on the background of HSV-1 infection in AD patients. We hypothesized that IDO1 expression and/or function in myeloid DC may be critical for the control of HSV-1 infections in AD patients at risk for recurrent episodes of EH. We therefore investigated the expression and function of IDO1 in circulating pDC and monocytes as well as in LC-like DC obtained from healthy non-atopic controls and AD patients with and without recurrent HSV-1 infections and from AD patients with history of EH as well as patients presenting with an acute episode of EH.
Material and methods
Recruitment of patients
Fifty five healthy non-atopic controls and 104 AD patients were recruited and subdivided into the following groups: healthy non-atopic controls without clinical HSV-1 infections (NA; n=33); healthy non-atopic controls with recurrent HSV-1 infections (NA/HSV; n=22)(recurrent HSV-1 infections has been defined as two or more episodes per year); AD patients without recurrent HSV-1 infections (AD; n=34); AD patients with recurrent HSV-1 infections but without EH (AD/HSV; n=37); AD patients with recurrent episodes of HSV-1 infections and with a history of EH (AD/HSV/EH history; n=19); AD patients with recurrent episodes of HSV-1 infections and presenting with an episode of acute EH (AD/HSV/EH acute; n=14). Demographics in our collectives are shown on Table I. The study was approved by the local ethics committee and written informed consent was obtained from all participants of the study.
Table I.
Demographics of the enrolled controls and patients. Results are given as mean ± SD
| Groups | Age | Sex- ratio ♀/♂ |
Frequency HSV-1 infections |
SCORAD | Total IgE | HSV IgG titer |
|---|---|---|---|---|---|---|
| NA (n=33) | 30.4 ± 10.6 | 21/12 | - | - | - | 1:5,929 ± 9,794 |
| NA/HSV (n=22) | 32.1 ± 13 | 13/9 | 2.3 ± 1.3 | - | - | 1:16,959 ± 11,607 |
| AD (n=34) | 29.5 ± 8.7 | 20/14 | - | 34.6 ± 9.7 | 1,607 ± 3,244 | 1:7,644 ± 11,918 |
| AD/HSV (n=37) | 34.3 ± 11.4 | 21/16 | 2,4 ± 1.6 | 31.2 ± 11 | 1,627 ± 3,528 | 1:17,860 ± 8,345 |
| AD/HSV/ EH history (n=19) | 35.3 ± 9.9 | 4/15 | 1.7 ± 2.5 | 39.3 ± 11 | 4,391 ± 6,667 | 14,457 ± 11,743 |
| AD/HSV/EH acute (n=14) | 29.2 ± 8.2 | 4/10 | 1.6 ± 2.9 | 47.2 ± 12.2 | 6,245 ± 9,022 | 14,392 ± 13,708 |
Reagents
The following monoclonal antibodies (mAb) were used for flow cytometric analysis. Phycoerythrin (PE)-labelled monoclonal antibody (mAb) against CD14 (M5E2) (IgG2a κ), the PE-labeled mAb against CD123 (9F5) (IgG1 κ) and the isotype matched controls were purchased from Becton Dickinson (Mountain View, CA, USA). PE-labelled mAb against Langerin (DCGM4) (IgG1) was from Coulter-Immunotech (Marseille, France) and allophycocyanin (APC)-labelled mAb against CD1a (HI149) (IgG1) from BD Biosciences. Appropriate isotype controls were used. APC- labelled mAb against BDCA2 (AC144) (IgG1) from Miltenyi Biotec (Bergisch Gladbach, Germany). 7-amino-actinomycin (7-AAD) was from Sigma (Deisenhofen, Germany). The mAb against IDO1 was a kind gift of Dr. Takikawa (Aichi, Japan). FITC-labelled F(ab’)2 of goat-anti-mouse Ab (GaM/FITC) and mouse serum were from Dianova (Hamburg, Germany). For the generation of LC-like DC, recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) was purchased from Novartis Pharma AG, recombinant human interleukin (IL)-4 from Strathman Biotech GmbH (Hannover, Germany) and recombinant human transforming growth factor beta1 (hTGF-β1) was from R&D Systems.
Maturation of LC was induced with a cytokine cocktail consisting of tumor-necrosis factor-alpha (TNF-α, Sigma-Aldrich, Munich, Germany), IL-1 beta and IL-6 (R&D Systems, Wiesbaden-Nordenstadt, Germany) as reported previously (39). Stimulation was performed with the TLR3 agonist polyriboinosinic-polyribocytidilic acid (Poly I:C), Sigma (Munich, Germany) and the TLR4 ligand lipopolysaccharide (LPS) (Sigma, Germany). Both have been shown to induce IDO1 in myeloid DC (39).
Isolation of cells from peripheral blood
Monocytes were isolated from peripheral blood with a density gradient protocol using Nycoprep™ (Axis-Shield, Oslo, Norway) as reported in details elsewhere (40). CD14 expression was assessed by flow cytometry (FACSCanto™ flow cytometer and FACS Diva software (BD) and was >90%. PBMC were isolated by using density gradient centrifugation with Lymphoprep™ (Axis-Shield) from heparin blood (30min at 1000 × g)(39). pDCs were identified by flow cytometry using mAb against CD123 and BDCA2.
Generation of Langerhans cell-like dendritic cells
Langerhans cell-like dendritic cells (LC-like DCs) were generated as reported in details elsewhere (41). Briefly, monocytes were cultured for 7 days in VLE-RPMI medium containing 500 IU/ml human recombinant granulocyte-macrophage colony stimulating factor (GM-CSF) (10ng/ml; 500 IU/mL) human recombinant IL-4 (500U/ml) and natural hTGF-β1 (10 ng/ml; 25U/ml). Medium was changed every second day adding half of the cytokine dose on day 2 and full cytokine dose on day 4 of culture. For maturation, LC-like DCs were cultured for further 24–48h with a cocktail of pro-inflammatory cytokines containing 10ng/ml TNF-α, 10ng/ml IL-1β and 5 ng/ml IL-6 (39, 42). For stimulation, the immature cells were cultured for 24–48h with Poly I:C (25µg/ml) or 100ng/ml LPS.
Flow cytometric analysis
The viable pDC, CD14+ monocytes or LC-like DCs populations were analyzed for IDO1 expression by intra-cellular staining and analysis on a FACSCanto™ flow cytometer (BD) as reported in details elsewhere (39).
Assessment of IDO1 activity in serum and culture supernatants
Analysis of Trp and Kyn in the serum samples and cell supernatants was done as reported in details elsewhere (35, 39). IDO1 activity is expressed as the ratio Kyn/Trp × 1,000.
Statistics
Statistical analysis was performed by using the parametric T-Test and nonparametric Mann-Whitney-U-Test. Correlations were calculated with the Pearson test. A p-value <0.05 was considered as statistically significant. Data were calculated with SPSS software (SPSS 20.0 and 22.0) for windows; SPSS, Inc, Chicago, IL, USA). Results are shown as means ± SD or boxplots with medians, 25th and 75th quartiles, minimum and maximum of distribution. Asterisks and circles mark outliers.
Results
Increased tryptophan degradation activity in serum of AD patients presenting with acute EH
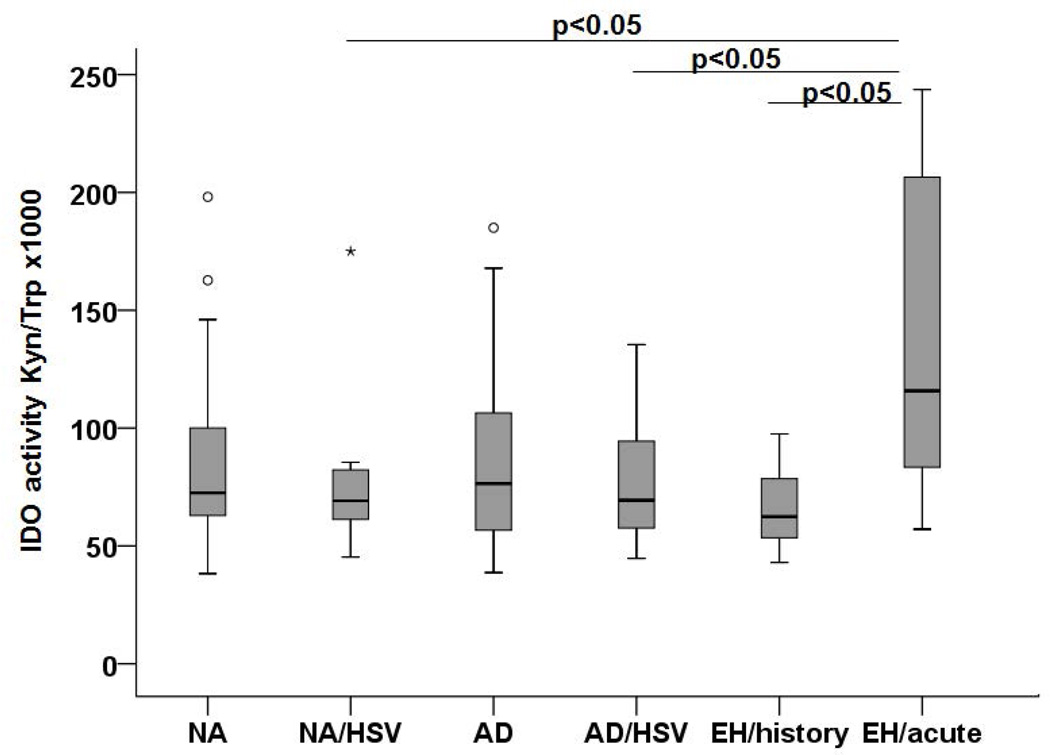
In a first approach, we explored the levels of Trp degradation in the serum of our patients and control individuals. When comparing NA and AD (excluding all individuals with HSV-1 infections and/or EH), there was no significant difference suggesting that AD per se is not characterized by an altered Trp degradation (data not shown). Similarly, there was no difference between individuals suffering from recurrent HSV-1 infections and those without, within the group of control or AD patients. In contrast, a significantly higher level of Trp degradation was found in the serum of AD patients with acute EH when compared to all other groups with HSV infections (Figure 1). From these experiments, we concluded that there is evidence for an involvement of IDO/TDO expressing cells during acute episodes of EH in AD patients, raising the question of the source of IDO1 expression and activity in this condition.
Figure 1. IDO activity in serum of patients and controls.
IDO activity expressed as ratio of Kyn and Trp levels × 1,000 was detected by HPLC in serum of NA controls (n=26), NA/HSV (n=12), AD (n=24), AD/HSV (n=17); AD/HSV/EH history (n=12) and AD/HSV/EH acute patients (n=11). A p-value < 0.05 was considered statistically significant and is indicated accordingly.
IDO1 expression and activity in freshly isolated blood plasmacytoid cells and monocytes
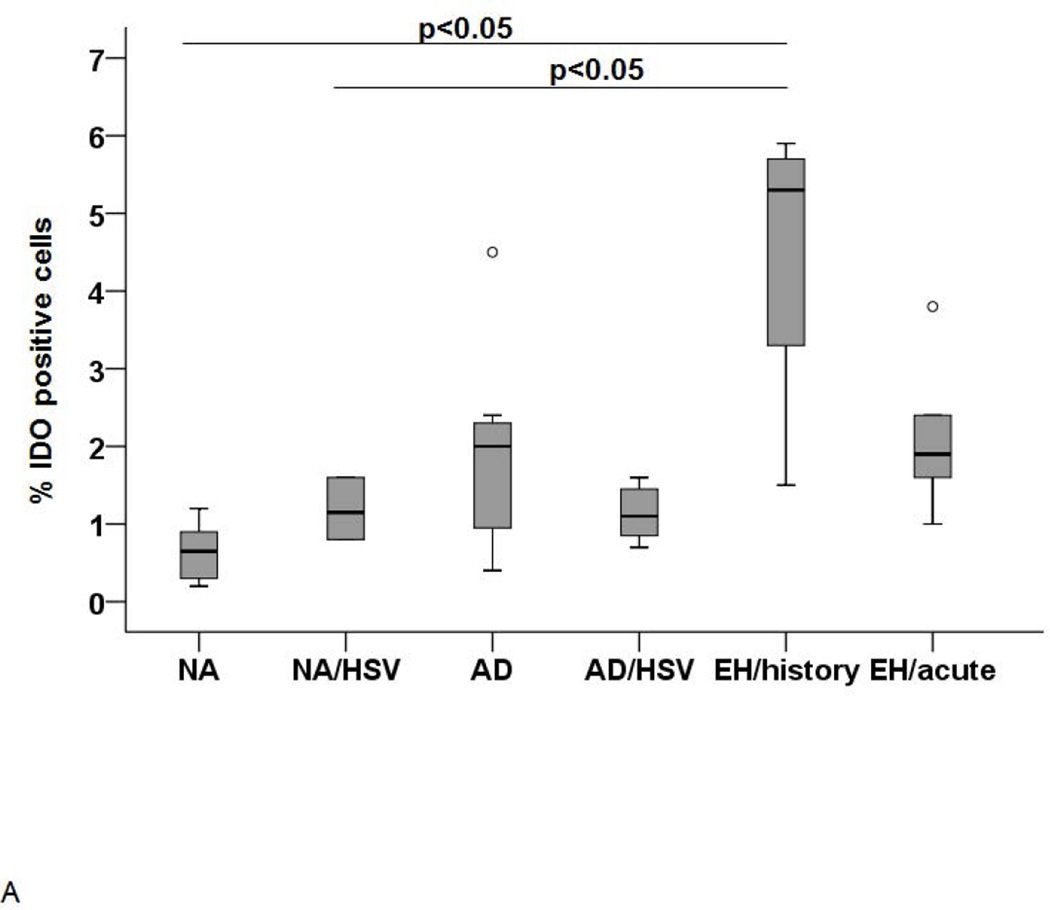
The next set of experiments aimed to identify whether circulating plasmacytoid and myeloid cells may represent a source of the Trp degradation activity described above. Therefore, pDC were analyzed by flow cytometry after intracellular staining. Thereby, the overall IDO1 expression in pDC was low (≤ 2% positive cells) and a significantly (p<0.05) higher expression was observed in AD patients with history of EH (Figure 2A). Hence, although this finding confirms that pDC are a source of IDO1, its relevance for the increased Trp degradation activity seen in the serum in the acute EH episode remains unlikely.
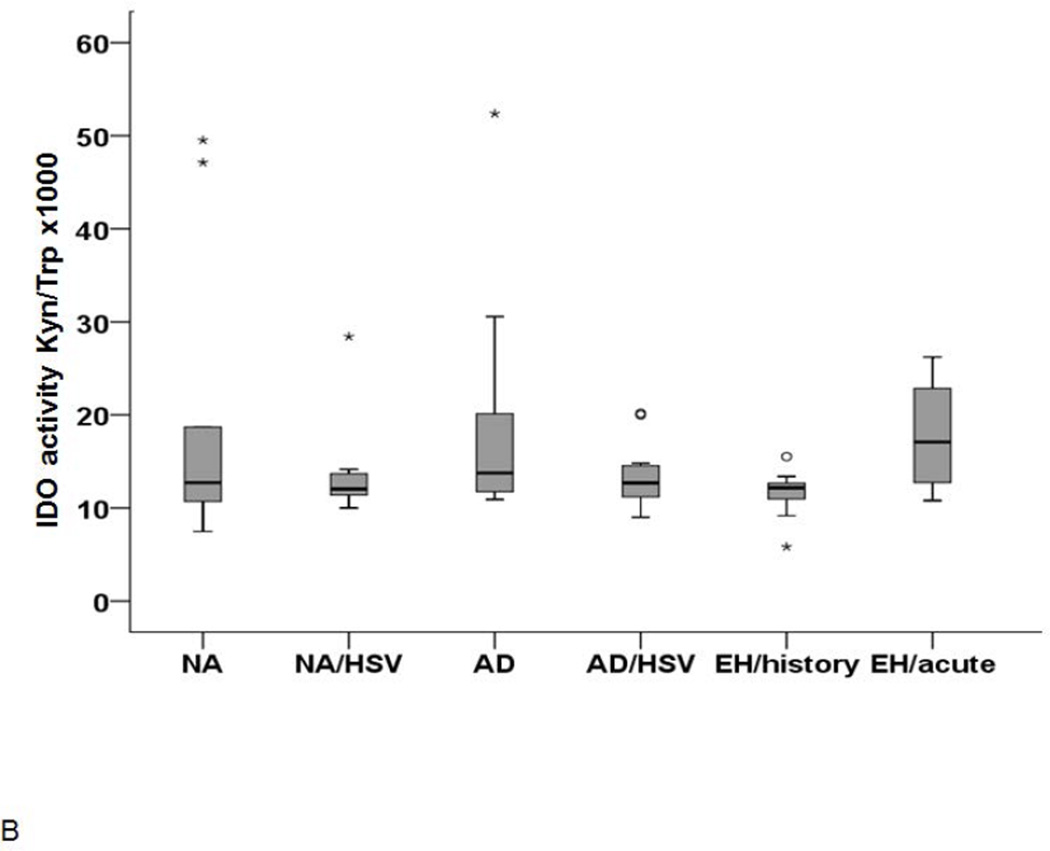
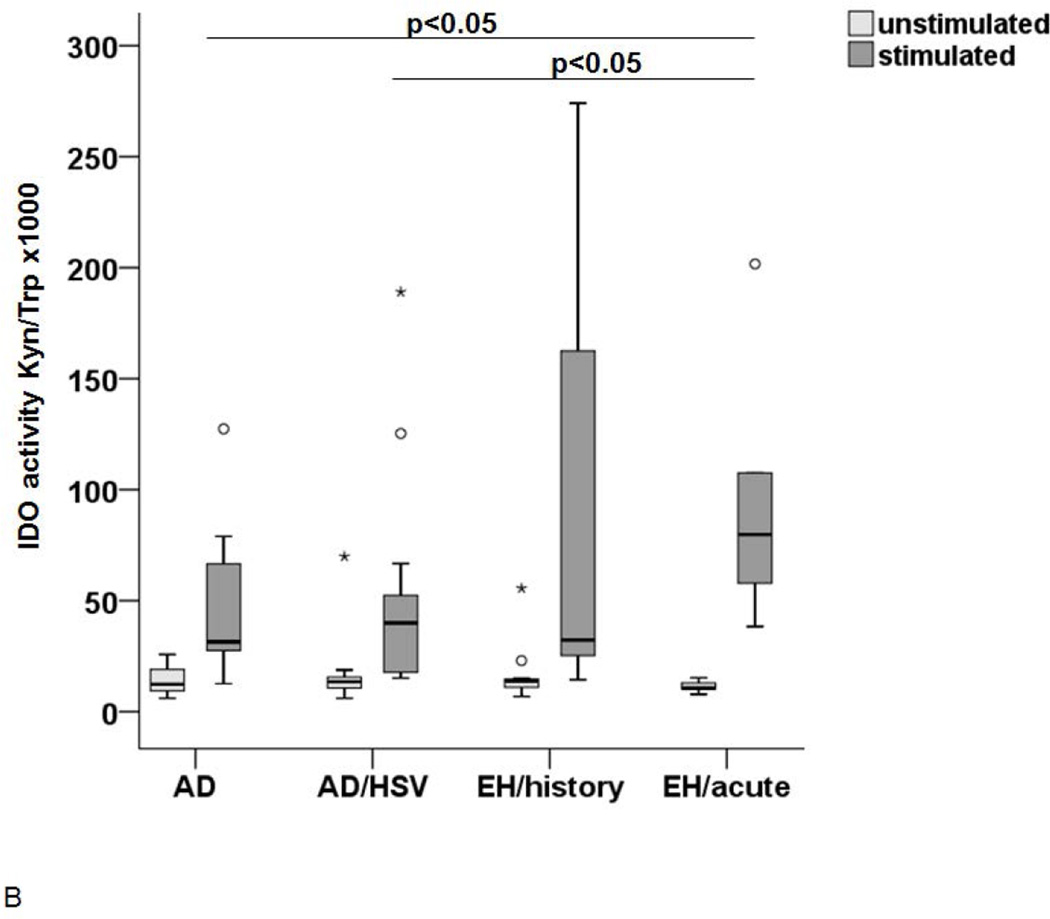
Figure 2. IDO expression in pDC (A) and IDO activity in monocytes (B).
(A) IDO expression in pDC as determined by FACS analysis. Significantly higher IDO expression was found in AD/HSV/EH history (n=4) but not AD/HSV/EH acute patients (n=5) p-value <0.05. (B) IDO activity in the supernatants of monocytes from AD patients with acute EH analyzed by HPLC. IDO activity is expressed as ratio of Kyn and Trp levels × 1,000.
Monocytes are considered as the circulating precursor cells for tissue myeloid dendritic cells and important players in the defence against HSV infection. Therefore, we explored the IDO1 expression and function in freshly isolated monocytes. As for pDC, the overall expression was low (range from 2.5 to 5.6 %). Although there was a trend for a higher IDO1 expression in monocytes from AD patients with recurrent HSV infections and patients with acute EH, the difference was not statistically significant (data not shown). However, the supernatants of monocytes from AD patients with acute EH had higher IDO1 activity compared to the other groups (Figure 2B).
IDO1 expression and activity upon maturation of Langerhans cells
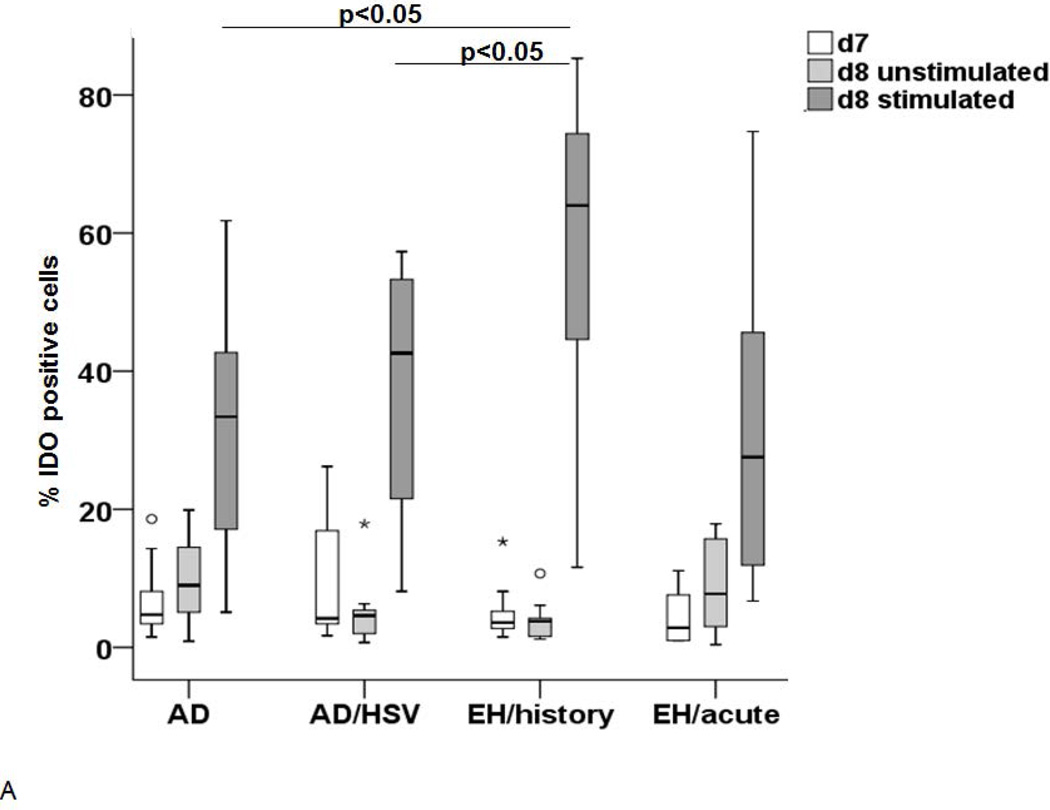
The above reported experiments suggest that, despite the low IDO1 expression, monocytes have been either primed in vivo or alternatively have an intrinsic capacity to produce IDO1 when differentiated into DC. Therefore, the IDO1 expression and function were investigated in LC-like DC generated from circulating monocytes of these patients and controls as a surrogate for their tissue LC. After in vitro generation, the cells were investigated either as immature LC-like DC (at day 7) or after further 24–48h culture in the presence or absence of a defined cytokine cocktail known to induce their maturation (39, 42). As shown in Figure 3A, the IDO1 expression was low in all conditions at day 7, but was significantly increased in mature LC-like DC at day 8. This increase in expression was particularly pronounced in cells from patients with history of EH. As expected, the IDO1 activity was also increased in mature LC-like DC but the highest activity was measured in the supernatants from cells obtained from patients with acute EH (Figure 3B).
Figure 3. IDO expression (A) and activity (B) in LC-like DC after maturation.
(A) Immature LC-like DC were stimulated with 10ng/ml IL-1β, 5ng/ml IL-6 and 10ng/ml TNF-α for 24h and analyzed by flow cytometry.
IDO expression is shown in immature LC on day 7 (white), unstimulated cells on day 8 (light grey) and stimulated LC on day 8 (dark grey). The increase was most pronounced in AD/HSV/EH history patients (n=9), IDO expression was significantly higher in this group than in the other groups, p-value <0.05. (B) IDO activity in unstimulated control LC (light grey) and LC after incubation in 100µM Trp medium with the above mentioned cytokines for 48h (dark grey).
Viral signal induces an exaggerated IDO1 expression and activity in Langerhans cells from AD patients with EH
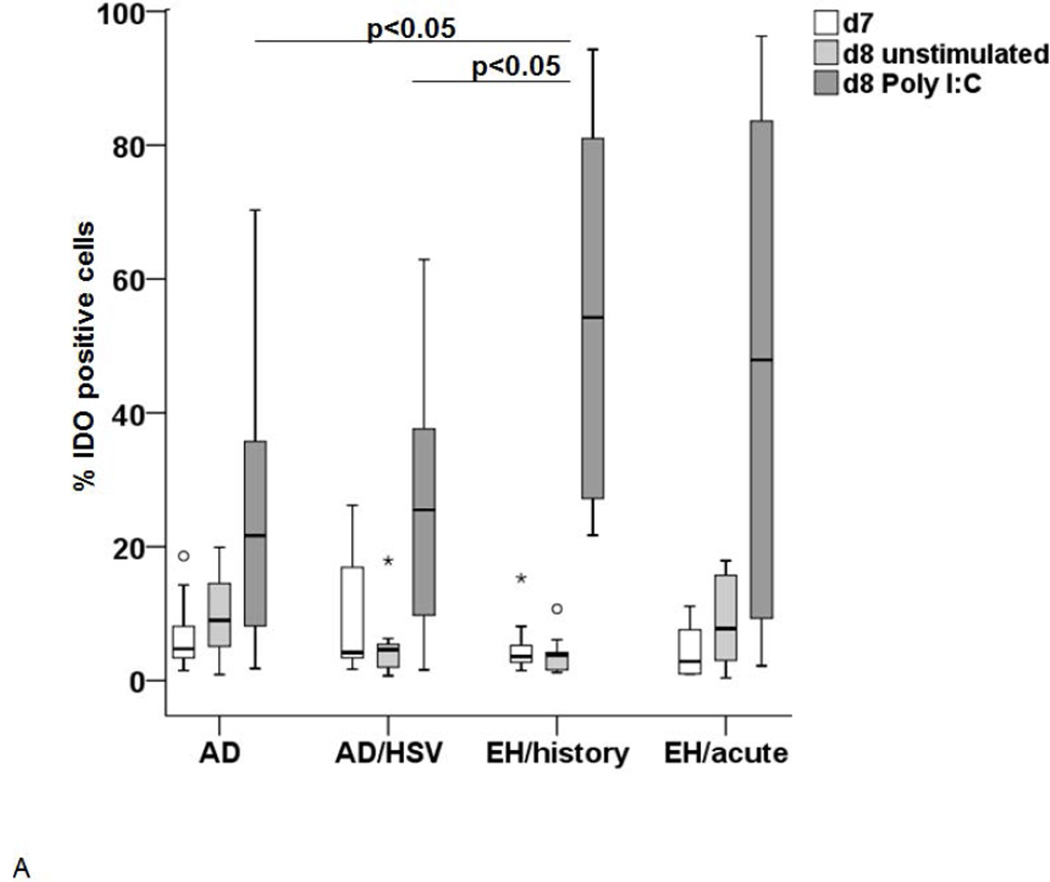
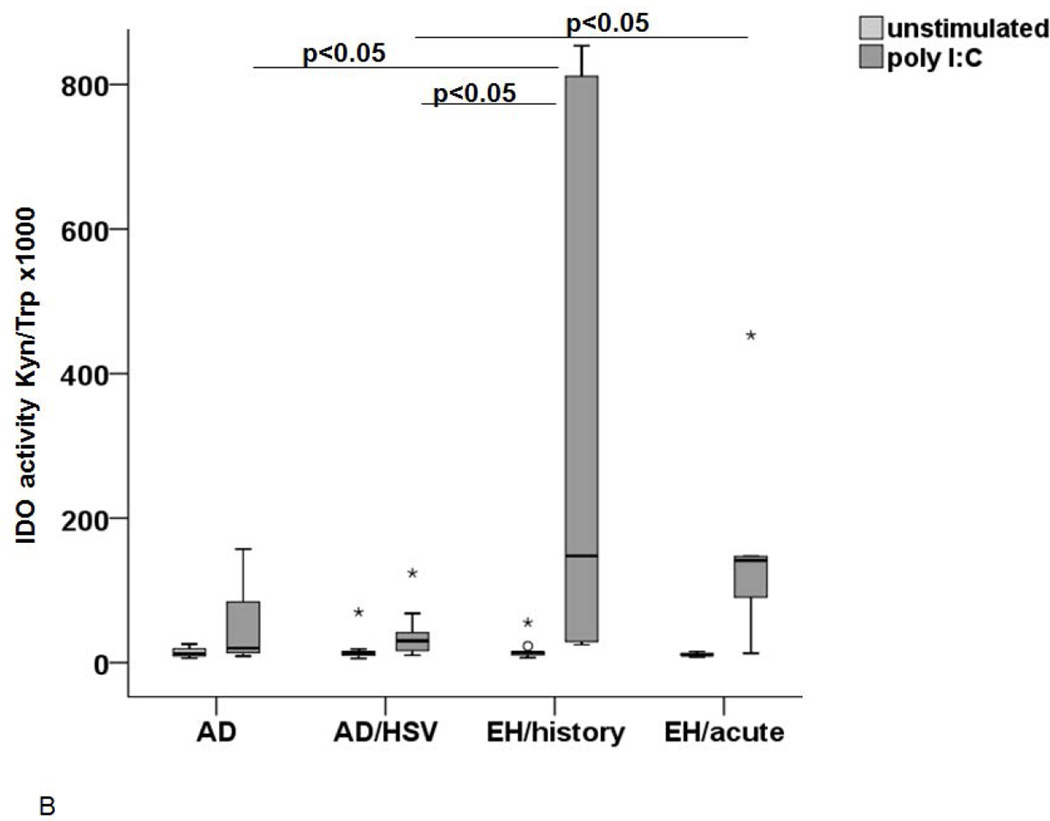
In a next set of experiments, we subjected immature LC-like DC to stimulation with Poly I:C, mimicking a viral infection. As shown on Figure 4A, this stimulation lead to an increase in IDO1 expression in these cells from all groups, but significantly more pronounced in AD patients with history of EH. IDO1 activity in the supernatants was significantly higher in patients with acute EH and history of EH (Figure 4B), but unexpectedly also in non-atopic patients with recurrent HSV infections (data not shown).
Figure 4. IDO expression and activity after stimulation with microbial stimuli.
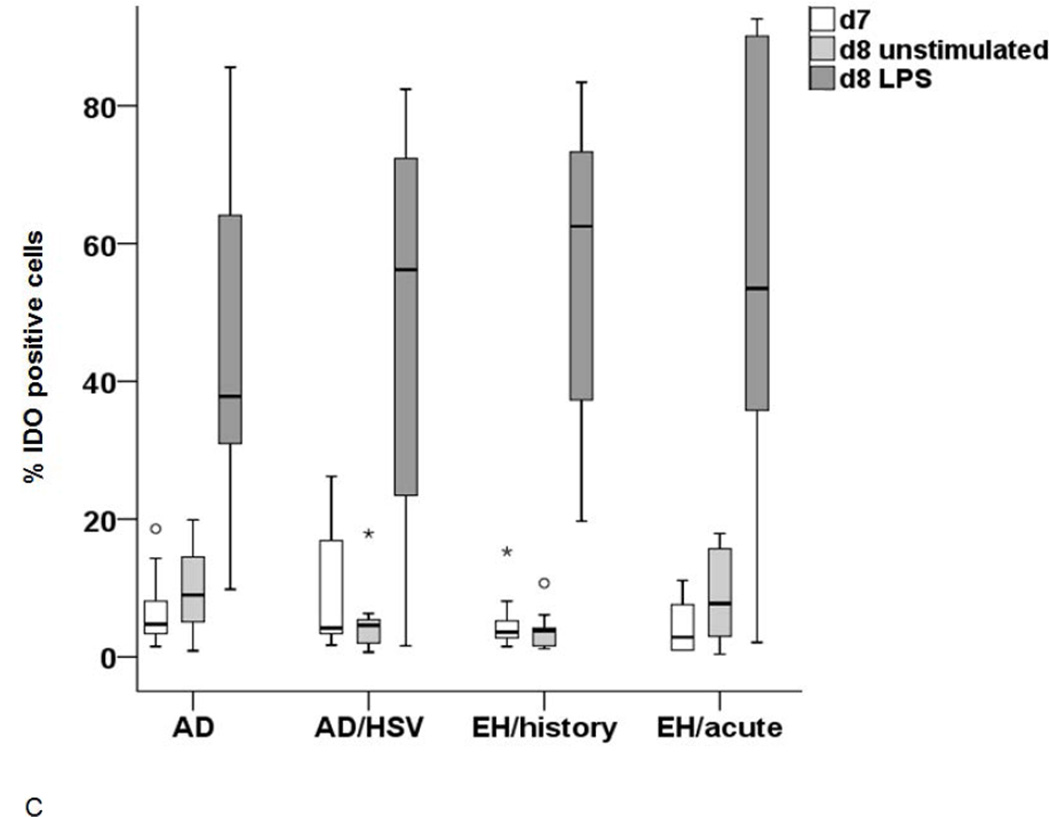
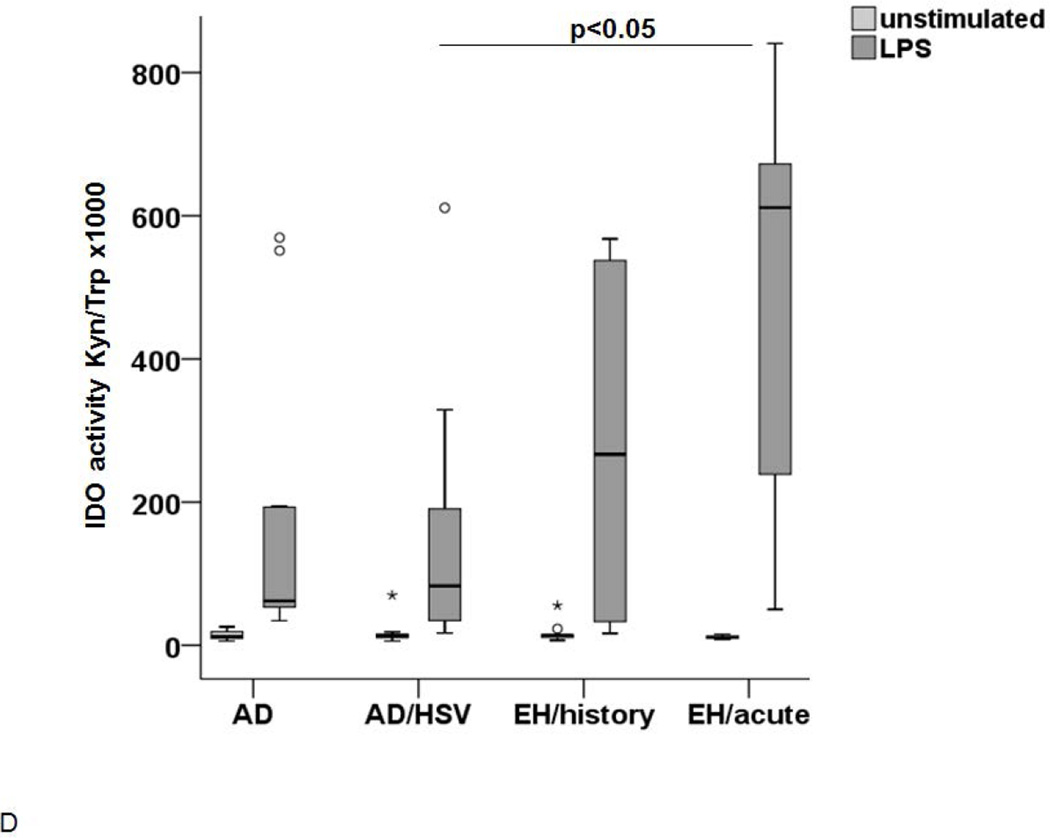
The IDO expression (A and C) and activity (B and D) was measured in LC upon stimulation with either Poly I:C (A and B) or LPS (C and D). (A) IDO expression in immature d7 cells (white), unstimulated cells on d8 (light grey) and after stimulation with Poly I:C 8 (dark grey). Significant differences between the stimulated cells (p<0.05) are marked appropriately. (B) IDO activity in supernatants of control LC (light grey) and LC stimulated with Poly I:C (dark grey) incubated in 100µM Trp medium. A p-value <0.05 is noted accordingly as statistically significant. (C) IDO expression in immature LC-like DC (d7 white) unstimulated d8 cells (light grey) and cells stimulated with LPS (dark grey) as analyzed by flow cytometry. (D) IDO activity (Kyn/Trp ×1000) in supernatants of LC after stimulation with LPS (light grey) in comparison to unstimulated controls (white).
In order to explore whether the phenomenon observed is specific to stimulation mimicking viral infection, we cultured the immature cells for 24h to 48h with LPS and measured the IDO1 expression and activity. As observed with Poly I:C, LC-like DC expressed an increased level of IDO1 in all groups. However, the increase tended to be higher in AD patients with HSV infections (Figure 4C). Most importantly, the IDO1 activity in the supernatants was not significantly increased in AD patients with EH history but only in acute EH (Figure 4D). The IDO1 activity after Poly I:C or LPS stimulation correlated to the expression of the enzyme for all groups except for the non-atopic controls (see supplementary material).
Discussion
The Kyn pathway has been suspected to be operative in a number of distinct diseases including allergic conditions and AD where a decreased IDO1 activity has been reported (43). In the present study, we could not find any significant differences comparing the Trp and Kyn levels of AD patients with those of healthy non-atopic controls. This might be due to the fact that we have carefully phenotyped our patients and focused on a particular group of patients with AD as a representative of atopic disorders. Alternatively, the cause might be attributable to the limited number of selected patients examined herein and the fact that AD is to be considered as a heterogeneous disease with a complex genetic and immunologic background.
EH is a severe viral complication which seems to affect only a subgroup of AD patients. In this report we have confirmed that pDC are a potential source of IDO1 activity but they do not seem to represent the major origin of the observed increased Trp degradation in our patients with acute episode of EH. This may also be the case in lesional skin of AD where pDC have been shown to be absent (14). Thus, either hepatic TDO and/or some peripheral sources of IDO1 or IDO2 activity could explain the phenomenon observed in AD patients with acute EH. With this regard, we have shown herein that monocytes and myeloid DC such as LC may represent a significant source of IDO1 activity during EH episodes. However, it remains unclear whether the observed increased IDO1 activity is the cause or the result of the HSV spreading on the skin. If it is the result, it may represent an attempt to control exaggerated HSV infection and/ or the damage done to the tissue by immune cells during the course of this infection. On the other hand, exaggerated IDO1 may also contribute to the severe inflammation typically seen in these patients since this enzyme participates in a delicate balance between immunostimulatory and immunosuppressive signals mediated by DC. Since microbial agents need up to 40-fold higher Trp concentrations than T cells, an exaggerated IDO1 activity may in a first step block the pathogens and in a second step during ongoing activation block also T- cell proliferation and IFN production. This has been postulated previously (30) and represents a deleterious mechanism for efficient fighting against HSV. Therefore, the overactivation we observed in our LC may correspond to a similar phenomenon described in pDC and other cells which may locally or systemically contribute to inhibit antiviral T–cell response (44, 45) in chronic viral infections and might already be initiated during the acute episodes of EH.
The most unexpected finding is the exaggerated IDO1 profile in LC-like DC from AD patients with history of EH when the cells are exposed to viral stimulation while after exposure to bacterial signals only AD patients with acute EH show a significant increase of IDO1 activity. This strongly argues for a specific predisposition in DC from these individuals when challenged by viral signals. Several mutually non-excluding explanations can be considered. Firstly, this predisposition is genetically determined and would explain the increased vulnerability of a subgroup of AD patients to HSV. Indeed, a number of single nucleotide polymorphisms in the gene encoding IDO1 have been described in relationship with infectious conditions (46, 47). Secondly, the overactivation of LC-like DC in these patients could be the result of an in vivo priming of the monocytes for an increased sensitivity to microbial signals. However, based on the fact that most of the patients with history of EH had their episodes from several months up to several years before the time point we collected the blood samples, this would not be compatible with the limited half-life time of circulating monocytes which does not exceed a few days. Thirdly, another explanation may be related to a kind of “memory” of previous episodes preparing the DC for subsequent EH episodes. This “memory” could be explained by an epigenetic regulation of the IDO1 gene in myeloid lineage as it has been described in Rheumatoid arthritis (48) and would potentially offer the opportunity to act therapeutically using agents able to modulate the methylation status of the responsible genes.
Whatever the reasons for the observed exaggerated IDO1 activity of LC in AD patients with EH may be, this feature seems specific for this subgroup of AD patients. Hence it may represent an interesting biomarker for the identification of patients at risk to develop severe viral complications such as EH but possibly also eczema vaccinatum, a life-threatening condition occurring in AD patients in the context of small pox vaccination.
In conclusion, we have shown for the first time that AD patients with EH are characterized by an exaggerated IDO1 activity in myeloid DC secondary to viral signals. This phenomenon may represent a possible explanation for the lack of efficient anti-viral defense seen in these patients and could potentially be considered as an interesting predictive biomarker for patients at risk to develop severe viral complications.
Supplementary Material
Acknowledgements
We thank Dr. K. Iwamoto, Dr. M. Brenk, Dr. M. Reckmeyer, H. Wilms and K. Brendes (all Department of Dermatology and Allergy, University of Bonn) for excellent assistance.
This work was supported in part by the NIH/NIAID contract N01 AI40029 and by the Christine-Kühne Center of Allergy Research and Education (CK-CARE)
Abbrevations
- AD
atopic dermatitis
- APC
allophycocyanin
- DC
dendritic cells
- EH
Eczema herpeticum
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HSV
herpes simplex virus
- hTGF-β1
human transforming growth factor beta1
- IDO
Indoleamine 2,3-dioxygenase
- IL
Interleukin
- Kyn
Kynurenine
- LC
Langerhans cells
- LPS
lipopolysaccharide
- mAb
monoclonal antibody
- pDC
plasmacytoid dendritic cells
- PE
Phycoerythrin
- Poly I:C
polyriboinosinic-polyribocytidilic acid
- TNF-alpha
tumor-necrosis factor-alpha
- Trp
Tryptophan
References
- 1.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358(14):1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 2.Eyerich K, Novak N. Immunology of atopic eczema: overcoming the Th1/Th2 paradigm. Allergy. 2013;68:974–982. doi: 10.1111/all.12184. [DOI] [PubMed] [Google Scholar]
- 3.Howell MD, Parker ML, Mustelin T, Ranade K. Past, present, and future for biologic intervention in atopic dermatitis. Allergy. 2015 doi: 10.1111/all.12632. [DOI] [PubMed] [Google Scholar]
- 4.Bieber T, Straeter B. Off-label prescriptions for atopic dermatitis in Europe. Allergy. 2015;70:6–11. doi: 10.1111/all.12498. [DOI] [PubMed] [Google Scholar]
- 5.Simon D, Bieber T. Systemic therapy for atopic dermatitis. Allergy. 2014;69:46–55. doi: 10.1111/all.12339. [DOI] [PubMed] [Google Scholar]
- 6.Hinz T, Zaccaro D, Byron M, Brendes K, Krieg T, Novak N, et al. Atopic dermo-respiratory syndrome is a correlate of eczema herpeticum. Allergy. 2011;66:925–933. doi: 10.1111/j.1398-9995.2010.02538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wollenberg A. Eczema herpeticum. Chem Immunol Allergy. 2012;96:89–95. doi: 10.1159/000331892. [DOI] [PubMed] [Google Scholar]
- 8.Wollenberg A, Zoch C, Wetzel S, Plewig G, Przybilla B. Predisposing factors and clinical features of eczema herpeticum: a retrospective analysis of 100 cases. J Am Acad Dermatol. 2003;49:198–205. doi: 10.1067/s0190-9622(03)00896-x. [DOI] [PubMed] [Google Scholar]
- 9.Peng WM, Jenneck C, Bussmann C, Bogdanow M, Hart J, Leung DY, et al. Risk factors of atopic dermatitis patients for eczema herpeticum. J Invest Dermatol. 2007;127:1261–1263. doi: 10.1038/sj.jid.5700657. [DOI] [PubMed] [Google Scholar]
- 10.Gao PS, Rafaels NM, Hand T, Murray T, Boguniewicz M, Hata T, et al. Filaggrin mutations that confer risk of atopic dermatitis confer greater risk for eczema herpeticum. J Allergy Clin Immunol. 2009;124:507–513. doi: 10.1016/j.jaci.2009.07.034. 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung DY, Gao PS, Grigoryev DN, Rafaels NM, Streib JE, Howell MD, et al. Human atopic dermatitis complicated by eczema herpeticum is associated with abnormalities in IFN-gamma response. J Allergy Clin Immunol. 2011;127:965–973. doi: 10.1016/j.jaci.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barchet W, Cella M, Colonna M. Plasmacytoid dendritic cells--virus experts of innate immunity. Semin Immunol. 2005;17:253–261. doi: 10.1016/j.smim.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 14.Wollenberg A, Wagner M, Gunther S, Towarowski A, Tuma E, Moderer M, et al. Plasmacytoid dendritic cells: a new cutaneous dendritic cell subset with distinct role in inflammatory skin diseases. J Invest Dermatol. 2002;119:1096–1102. doi: 10.1046/j.1523-1747.2002.19515.x. [DOI] [PubMed] [Google Scholar]
- 15.Huch JH, Cunningham AL, Arvin AM, Nasr N, Santegoets SJ, Slobedman E, et al. Impact of varicella-zoster virus on dendritic cell subsets in human skin during natural infection. J Virol. 2010;84:4060–4072. doi: 10.1128/JVI.01450-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunningham AL, Abendroth A, Jones C, Nasr N, Turville S. Viruses and Langerhans cells. Immunol Cell Biol. 2010;88:416–423. doi: 10.1038/icb.2010.42. [DOI] [PubMed] [Google Scholar]
- 17.Bosnjak L, Jones CA, Abendroth A, Cunningham AL. Dendritic cell biology in herpesvirus infections. Viral Immunol. 2005;18:419–433. doi: 10.1089/vim.2005.18.419. [DOI] [PubMed] [Google Scholar]
- 18.Ball HJ, Jusof FF, Bakmiwewa SM, Hunt NH, Yuasa HJ. Tryptophan-catabolizing enzymes - party of three. Front Immunol. 2014;5:485. doi: 10.3389/fimmu.2014.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137–143. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fallarino F, Grohmann U, Puccetti P. Indoleamine 2,3-dioxygenase: from catalyst to signaling function. Eur J Immunol. 2012;42:1932–1937. doi: 10.1002/eji.201242572. [DOI] [PubMed] [Google Scholar]
- 21.Harden JL, Egilmez NK. Indoleamine 2,3-dioxygenase and dendritic cell tolerogenicity. Immunol Invest. 2012;41:738–764. doi: 10.3109/08820139.2012.676122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boasso A, Herbeuval JP, Hardy AW, Anderson SA, Dolan MJ, Fuchs D, et al. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood. 2007;109:3351–3359. doi: 10.1182/blood-2006-07-034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Bubnoff D, Bausinger H, Matz H, Koch S, Hacker G, Takikawa O, et al. Human epidermal langerhans cells express the immunoregulatory enzyme indoleamine 2,3-dioxygenase. J Invest Dermatol. 2004;123:298–304. doi: 10.1111/j.0022-202X.2004.23217.x. [DOI] [PubMed] [Google Scholar]
- 24.Fallarino F, Puccetti P. Toll-like receptor 9-mediated induction of the immunosuppressive pathway of tryptophan catabolism. Eur J Immunol. 2006;36:8–11. doi: 10.1002/eji.200535667. [DOI] [PubMed] [Google Scholar]
- 25.MacKenzie CR, Heseler K, Muller A, Daubener W. Role of indoleamine 2,3-dioxygenase in antimicrobial defence and immuno-regulation: tryptophan depletion versus production of toxic kynurenines. Curr Drug Metab. 2007;8:237–244. doi: 10.2174/138920007780362518. [DOI] [PubMed] [Google Scholar]
- 26.Meisel R, Brockers S, Heseler K, Degistirici O, Bulle H, Woite C, et al. Human but not murine multipotent mesenchymal stromal cells exhibit broad-spectrum antimicrobial effector function mediated by indoleamine 2,3-dioxygenase. Leukemia. 2011;25:648–654. doi: 10.1038/leu.2010.310. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt SK, Ebel S, Keil E, Woite C, Ernst JF, Benzin AE, et al. Regulation of IDO activity by oxygen supply: inhibitory effects on antimicrobial and immunoregulatory functions. PLoS One. 2013;8:e63301. doi: 10.1371/journal.pone.0063301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nino-Castro A, Abdullah Z, Popov A, Thabet Y, Beyer M, Knolle P, et al. The IDO1-induced kynurenines play a major role in the antimicrobial effect of human myeloid cells against Listeria monocytogenes. Innate Immun. 2014;20:401–411. doi: 10.1177/1753425913496442. [DOI] [PubMed] [Google Scholar]
- 29.Narui K, Noguchi N, Saito A, Kakimi K, Motomura N, Kubo K, et al. Anti-infectious activity of tryptophan metabolites in the L-tryptophan-L-kynurenine pathway. Biol Pharm Bull. 2009;32:41–44. doi: 10.1248/bpb.32.41. [DOI] [PubMed] [Google Scholar]
- 30.Muller A, Heseler K, Schmidt SK, Spekker K, Mackenzie CR, Daubener W. The missing link between indoleamine 2,3-dioxygenase mediated antibacterial and immunoregulatory effects. J Cell Mol Med. 2009;13:1125–1135. doi: 10.1111/j.1582-4934.2008.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt SV, Schultze JL. New Insights into IDO Biology in Bacterial and Viral Infections. Front Immunol. 2014;5:384. doi: 10.3389/fimmu.2014.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams O, Besken K, Oberdorfer C, MacKenzie CR, Russing D, Daubener W. Inhibition of human herpes simplex virus type 2 by interferon gamma and tumor necrosis factor alpha is mediated by indoleamine 2,3-dioxygenase. Microbes Infect. 2004;6:806–812. doi: 10.1016/j.micinf.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Liu WL, Lin YH, Xiao H, Xing S, Chen H, Chi PD, et al. Epstein-Barr virus infection induces indoleamine 2,3-dioxygenase expression in human monocyte-derived macrophages through p38/mitogen-activated protein kinase and NF-kappaB pathways: impairment in T cell functions. J Virol. 2014;88:6660–6671. doi: 10.1128/JVI.03678-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meisel R, Heseler K, Nau J, Schmidt SK, Leineweber M, Pudelko S, et al. Cytomegalovirus infection impairs immunosuppressive and antimicrobial effector functions of human multipotent mesenchymal stromal cells. Mediators Inflamm. 2014:898630. doi: 10.1155/2014/898630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Bubnoff D, Fimmers R, Bogdanow M, Matz H, Koch S, Bieber T. Asymptomatic atopy is associated with increased indoleamine 2,3-dioxygenase activity and interleukin-10 production during seasonal allergen exposure. Clin Exp Allergy. 2004;34:1056–1063. doi: 10.1111/j.1365-2222.2004.01984.x. [DOI] [PubMed] [Google Scholar]
- 36.Maneechotesuwan K, Ekjiratrakul W, Kasetsinsombat K, Wongkajornsilp A, Barnes PJ. Statins enhance the anti-inflammatory effects of inhaled corticosteroids in asthmatic patients through increased induction of indoleamine 2, 3-dioxygenase. J Allergy Clin Immunol. 2010;126:754–762. doi: 10.1016/j.jaci.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Kawasaki H, Chang HW, Tseng HC, Hsu SC, Yang SJ, Hung CH, et al. A tryptophan metabolite, kynurenine, promotes mast cell activation through aryl hydrocarbon receptor. Allergy. 2014;69:445–452. doi: 10.1111/all.12346. [DOI] [PubMed] [Google Scholar]
- 38.Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12:870–878. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 39.Von Bubnoff D, Scheler M, Wilms H, Fimmers R, Bieber T. Identification of IDO-positive and IDO-negative human dendritic cells after activation by various proinflammatory stimuli. J Immunol. 2011;186:6701–6709. doi: 10.4049/jimmunol.1003151. [DOI] [PubMed] [Google Scholar]
- 40.Novak N, Bieber T, Katoh N. Engagement of Fc epsilon RI on human monocytes induces the production of IL-10 and prevents their differentiation in dendritic cells. J Immunol. 2001;167:797–804. doi: 10.4049/jimmunol.167.2.797. [DOI] [PubMed] [Google Scholar]
- 41.Geissmann F, Prost C, Monnet JP, Dy M, Brousse N, Hermine O. Transforming growth factor beta1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J Exp Med. 1998;187:961–966. doi: 10.1084/jem.187.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jonuleit H, Kuhn U, Muller G, Steinbrink K, Paragnik L, Schmitt E, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 43.Raitala A, Karjalainen J, Oja SS, Kosunen TU, Hurme M. Indoleamine 2,3-dioxygenase (IDO) activity is lower in atopic than in non-atopic individuals and is enhanced by environmental factors protecting from atopy. Mol Immunol. 2006;43:1054–1056. doi: 10.1016/j.molimm.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 44.Larrea E, Riezu-Boj JI, Gil-Guerrero L, Casares N, Aldabe R, Sarobe P, et al. Upregulation of indoleamine 2,3-dioxygenase in hepatitis C virus infection. J Virol. 2007;81:3662–3666. doi: 10.1128/JVI.02248-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boasso A, Royle CM, Doumazos S, Aquino VN, Biasin M, Piacentini L, et al. Overactivation of plasmacytoid dendritic cells inhibits antiviral T-cell responses: a model for HIV immunopathogenesis. Blood. 2011;118:5152–5162. doi: 10.1182/blood-2011-03-344218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coutinho LG, Christen S, Bellac CL, Fontes F, de Souza F, Grandgirard D, et al. The kynurenine pathway is involved in bacterial meningitis. J Neuroinflammation. 2014;11:169. doi: 10.1186/s12974-014-0169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Souza FR, Fontes FL, da Silva TA, Coutinho LG, Leib SL, Agnez-Lima LF. Association of kynurenine aminotransferase II gene C401T polymorphism with immune response in patients with meningitis. BMC Med Genet. 2011;12:51. doi: 10.1186/1471-2350-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xue ZT, Sjogren HO, Salford LG, Widegren B. An epigenetic mechanism for high, synergistic expression of indoleamine 2,3-dioxygenase 1 (IDO1) by combined treatment with zebularine and IFN-gamma: potential therapeutic use in autoimmune diseases. Mol Immunol. 2012;51:101–111. doi: 10.1016/j.molimm.2012.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.