Abstract
Objectives
Within a New York City (NYC) birth cohort, we assessed the associations between polycyclic aromatic hydrocarbon (PAH) and other aromatic DNA adducts and brain derived neurotrophic factor (BDNF) concentrations in umbilical cord blood, and neurodevelopment at age 2 years and whether BDNF is a mediator of the associations between PAH/aromatic-DNA adducts and neurodevelopment.
Methods
PAH/aromatic-DNA adduct concentrations in cord blood were measured in 505 children born to nonsmoking African-American and Dominican women residing in NYC, and a subset was assessed for neurodevelopment at 2 years using the Bayley Scales of Infant Development Mental Development Index (MDI). A spectrum of PAH/aromatic-DNA adducts was measured using the 32P-postlabeling assay; DNA adducts formed by benzo[a]pyrene (B[a]P), a representative PAH, were measured by High Performance Liquid Chromatography (HPLC)/fluorescence. BDNF mature protein in cord blood plasma was quantified by an ELISA. Multivariate regression analysis, adjusting for potential confounders, was conducted.
Results
PAH/aromatic-DNA adduct concentration measured by postlabeling was inversely associated with BDNF concentration (p=0.02) and with MDI scores at 2 years (p=0.04). BDNF level was positively associated with MDI scores (p=0.003). Restricting to subjects having all three measures (PAH/aromatic-DNA adducts by postlabeling, MDI, and BDNF), results were similar but attenuated (p=0.13, p=0.05, p=0.01, respectively). Associations between B[a]P-DNA adducts and BDNF and B[a]P-DNA adducts and MDI at age 2 years were not significant. At age 3 years, the positive association of BDNF with MDI was not observed.
Conclusions
The results at age 2 suggest that prenatal exposure to a spectrum of PAH/aromatic pollutants may adversely affect early neurodevelopment, in part by reducing BDNF levels during the fetal period. However, the same relationship was not seen at age 3.
Keywords: cord blood, DNA adducts, BDNF, neurodevelopment, PAH, 32P-postlabeling
Introduction
Polycyclic aromatic hydrocarbons (PAH) are toxic pollutants released during incomplete combustion of organic material including fossil fuel, tobacco, and certain foods (Bostrom et al., 2002). The prenatal period is known to be a window of susceptibility to many neurotoxicants (Grandjean and Landrigan, 2006; Perera et al., 2004). Laboratory studies exposing experimental animals to PAH during the prenatal and neonatal periods have reported neurodevelopmental and behavioral effects, including impairment of memory and ability to learn (Brown et al., 2007; Wormley et al., 2004b). Our previous studies in the Columbia Center for Children’s Environmental Health (CCCEH) cohort in New York City (NYC) have found associations between various measures of prenatal PAH exposure and adverse cognitive and behavioral outcomes in the children, including delayed development (Perera et al., 2006), reduced IQ (Perera et al., 2009), and attention problems (Perera et al., 2014; Perera et al., 2012). PAH-DNA adducts are widely used indicators of exposure and biologically effective dose of the toxicants (Perera et al., 2004; Phillips and Arlt, 2007; Phillips and Venitt, 2012; Tang et al., 2001).
Brain Derived Neurotrophic Factor (BDNF) plays a major role in early brain development. It is the most widely distributed neurotrophin (Lu et al., 2008) and is critical for the neurological survival and cognitive development of the central nervous system (Cohen-Cory et al., 2010; Numakawa et al., 2010). BDNF is first released as the proBDNF precursor before being cleaved into mature BDNF (mBDNF) (Numakawa et al., 2010). Regulation of BDNF depends on its site of release (Balkowiec and Katz, 2002; Cunha et al., 2010). Following release, BDNF binds to two different transmembrane proteins: tropomyosin- related TrkB receptor and neurotrophin receptor p75, with higher affinity for the TrkB receptor (Cunha et al., 2010). The binding of TrkB receptor triggers three signaling cascades that ultimately phosphorylate and activate the cAMP responsive element binding protein (CREB) transcription factor to encourage gene transcription essential for neuronal development and synaptic plasticity (Cunha et al., 2010; Minichiello, 2009).
Because BDNF and TrkB are expressed in the hippocampus to reinforce and stabilize synaptic connections, BDNF has been widely recognized as a key regulator in long-term potentiation (LTP), one of several phenomena underlying synaptic plasticity, learning, and memory (Kandel, 2004; Lu et al., 2008). BDNF is an active mediator of neuronal processes in the developing and mature brain, promoting differentiation, growth, and survival of neurons during development (Huang and Reichardt, 2003). Studies have shown that higher endogenous levels of BDNF are required to activate multiple signaling cascades that may act concertedly to regulate downstream cellular effects for memory formation and maintenance (Haapasalo et al., 2002).
In a Chinese cohort, we have previously reported that concentrations of cord benzo[a]pyrene (B[a]P)-DNA adducts were inversely associated with both BDNF and with development scores on the Gesell Test at age 2, and BDNF was a positive predictor of development scores in the same cohort(Tang et al., 2014). The findings suggested that BDNF might serve as a potential risk marker in assessing the neurodevelopment effects of fetal exposure to PAH. In an attempt to confirm these findings, we have assessed the relationships between two measures of cord PAH-DNA adducts (the broad spectrum of PAH/aromatic adducts measured by 32P-postlabeling analysis and the specific B[a]P-DNA adduct measured by High Performance Liquid Chromatography [HPLC]/fluorescence), cord BDNF, and cognitive development at age 2 years in our NYC cohort.
Methods
Sample selection
A complete description of the CCCEH NYC cohort and study design appears elsewhere (Perera et al., 2003; Perera et al., 2006). Briefly, 727 African-American and Dominican women residing in Washington Heights, Harlem, or the South Bronx in New York City, USA, were recruited between 1998 and 2006 into a prospective cohort study. To reduce the potential for confounding, we limited enrollment to women who were aged 18–35 years; non–cigarette smokers; nonusers of other tobacco products or illicit drugs; free of diabetes, hypertension, or known HIV; and who initiated prenatal care by the 20th week of pregnancy. The Institutional Review Board of the New York Presbyterian Medical Center approved the study. The present analysis involved the 505 children who had data on at least one measure of cord PAH-DNA adducts and cord BDNF.
Personal interview and HOME inventory
A trained bilingual interviewer administered a 45-minute questionnaire during the last trimester of pregnancy to obtain demographic information, residential history, and health and environmental data such as active and passive smoking. Environmental tobacco smoke (ETS) exposure, self-reported as having at least one smoker in the home, was dichotomized as a yes/no variable (Perera et al., 2003). The questionnaire also elicited information on dietary PAH (consumption of broiled, fried, grilled, or smoked meat), and socioeconomic information related to income and education. Postnatal maternal interviews were re-administered when the child was 6 months old and annually thereafter to determine any changes in residence, exposure to ETS, and other health or environmental conditions. The Home Observation for Measurement of the Environment (HOME) Inventory (Bradley, 1994; Caldwell and Bradley, 1979), administered between the ages of 1 and 4 years, was used to assess the quality of the child home caretaking environment. Self-reported maternal demoralization during pregnancy was measured by the Psychiatric Epidemiology Research Instrument Demoralization Scale (Dohrenwend et al., 1980). We administered the Test of Nonverbal Intelligence-Second Edition (TONI-2), a language-free measure of intelligence (Brown et al., 1990), to the mothers at child age 3 years.
Biomarkers
Umbilical cord blood (30–60 mL) was collected at delivery (Perera et al., 2004). Assays were performed on all samples that were of adequate quantity and quality for analysis, and all samples were run blinded.
The nuclease P1 digestion enhancement procedure of the 32P-postlabeling assay was used to analyze a spectrum of PAH/aromatic DNA adducts in umbilical cord blood samples having a sufficient yield of DNA (≥ 12 μg) (Phillips and Arlt, 2007). The nuclease P1 digestion enrichment procedure favors PAH adducts over aromatic amine adducts. The method can detect adducts in the range of one per 108–109 nucleotides using a 4-μg DNA sample. An aliquot (4 μg) of each DNA sample was analyzed on three separate occasions in batches of between 20 and 28 samples. For each assay, a positive control consisting of DNA modified with B[a]P diol-epoxide was also analyzed.
In contrast to the broad measure of PAH/aromatic-DNA adducts by postlabeling, B[a]P–DNA adducts were analyzed in extracted white blood cell DNA using a HPLC/fluorescence method that detects B[a]P tetraols (Alexandrov et al., 2002; Rojas et al., 2004); the method has been modified as described previously (Tang et al., 2006). 100 μg of DNA were used for each analysis. Total tetraol concentrations were calculated by comparing the samples analyzed with an external calibration curve generated from the fluorescence peak of a known amount of authentic benzo[a]pyrene diol-epoxide (BPDE) tetraol standard each time a set of samples was analyzed. The correlation coefficient was 0.98 and the mean coefficient of variation for analyses repeated on different days was 12%. The detection threshold of BPDE tetraols [r-7,c-10,t-8,t-9-tetrahydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene (B[a]P tetraol I-1) and r-7,t-9,t-10,t-8-tetrahydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene (B[a]P tetraol I-2)] was 0.25 adducts per 108 nucleotides (signal-to-noise ratio > 3) so that, in the present study, with 100 μg DNA, this assay could detect 0.25 adducts per 108 nucleotides. As in prior studies, non-detectable samples were assigned a value of 0.25/2 = 0.125 per 108 nucleotides. The number of samples analyzed for adducts by HPLC was considerably smaller than for adducts by postlabeling because of the greater requirement for DNA in the HPLC method (100μg vs.4μg).
Analysis of plasma levels of mBDNF from umbilical cord blood was performed using the BDNF Emax ImmunoAssay System (Promega) according to the manufacturer’s instruction. The assay specifically detects BDNF, typically with less than 3% cross-reactivity with other related neurotrophic factors (NGF, NT-3 and NT-4) at 100ng/ml, and detects a minimum of 15.6pg/ml of BDNF (Promega # TB257). The coefficient of variation was reported to be 2.2 to 8.8 pg/ml depending on the level of BDNF—high, medium, low, respectively (Promega Corporation, 2009), -
Neurodevelopmental outcomes
Research workers trained in neurodevelopmental testing administered the Bayley Scales of Infant Development- Revised (BSID-II) (Bayley, 1993) to assess cognitive and psychomotor development at 2 and 3 years of age. The BSID-II is a widely used norm referenced developmental test for young children, can be used to diagnose developmental delay, and is known to be sensitive to the developmental effects of toxic exposures such as low level intrauterine lead. Each test yields a developmental quotient (raw score/chronologic age) which generates a mental development index (MDI) score. In addition to this continuous measure, children are classified as normal (> 85), moderately delayed (> 70 and <85), or severely delayed (≤ 70) based on standardized cut-points. Each child was tested under controlled conditions at the CCCEH by a bilingual research assistant who was trained and checked for reliability. In the present study, the inter-rater reliability for the 24-month MDI was r = 0.92, based on double scoring of a random 5% of the sample (Rauh et al., 2004).
Statistical methods
We imputed missing values for covariates, except for adduct values and BDNF concentration, using a multiple imputation method with five imputed datasets, as described (Rubin, 1987). Covariates were retained in the models as potential confounders if they exhibited an association (p ≤ 0.1) with mental development. Covariates in all models included maternal self-report of ETS exposure during pregnancy (yes/no), mother’s intelligence, mother’s completed years of education before birth of the child, maternal prenatal demoralization, child’s sex and ethnicity, and gestational age. Additionally, in analysis of the association between adducts or BDNF and MDI, the quality of the postnatal caretaking environment (HOME), child’s age at administration of the HOME inventory, and the child’s exact age at MDI assessment were included. Dietary PAH was not a predictor of outcomes at p ≤ 0.1 and was not included in the models.
Concentrations of PAH/aromatic adducts by 32P-postlabeling (0–19 adducts per 108 nucleotides), B[a]P-DNA adducts (0.125- 0.72 adducts per 108 nucleotides) and BDNF were analyzed as continuous variables (both measures of adducts were ln-transformed to achieve a mean function that is linear in the transformed scale). MDI scores were analyzed as continuous or categorical (≥ 85 vs. <85) measures, with those children with scores ≥ 85 considered to be normal and those children with scores < 85 considered to be moderately to severely delayed as described in the Bayley Scales of Infant Development (Bayley, 1993)–Revised (BSID-II) manual. Multiple linear regression and logistic regression were used, as appropriate, to test the relationships among cord DNA adduct measures, BDNF, and Bayley MDI scores. When MDI was treated as a continuous outcome using linear regression, the beta estimates referred to the increase in MDI associated with a one log unit increase in adduct or BDNF concentration. When MDI was treated as a dichotomized outcome, beta estimates referred to the odds ratio (OR) of MDI ≥ 85 vs. MDI < 85 associated with a one log unit increase in adduct or BDNF concentration. The MDI score >85 was coded as 1 and <85 as 0 such that an OR <1 implies a lower odds of being categorized as normal.
Children included and those not included due to missing data were compared using two-sample t-test or Chi-square test (Fisher’s exact test for sparse data), as appropriate. Analyses were run first including all subjects having any two measures (either adducts measure, MDI, and/or BDNF) (“unrestricted analysis”) and then including only those subjects having all three measures (either adduct measure, MDI, and BDNF) (“restricted analysis”). The former approach included a larger number of subjects and therefore had more power; the latter approach was more rigorous since all comparisons were made in the same subsample.
To explore whether the effect of adducts on MDI might be mediated, in part, by reduction in the levels of the neurotrophin BDNF and whether the need for a formal mediation analysis was indicated, we compared the results for adducts as a predictor of MDI with results of a model including both adducts and BDNF as independent variables. The value of the mediated effect of adducts on MDI can be initially estimated as the difference in the independent variable coefficients in the two regression models. We then applied a formal test of mediation (Sobel, 1982).
We also conducted an analysis of the smaller subset with data on adducts, BDNF and MDI at age 3 years.
All effect estimates, 95% confidence intervals (CIs), and p-values (α=0.05) were generated using SAS (version 9.1.0.3; SAS Institute Inc., Cary, NC, USA).
Results
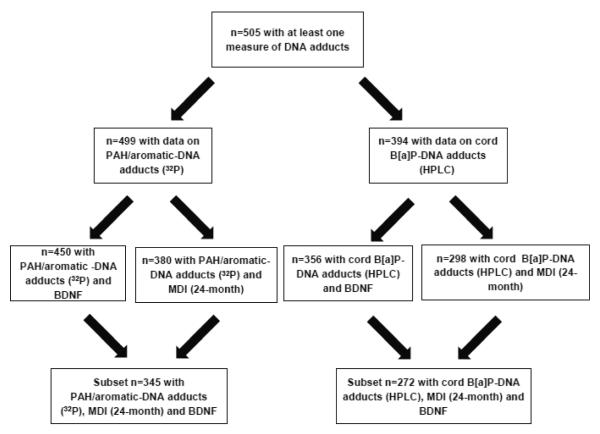
As shown in Figure 1, 505 children had data on at least one measure of cord PAH/aromatic-DNA adducts. Table 1 shows the characteristics of the 505 participants. There were no significant differences in these characteristics between cohort children included in the analyses and the 207 children not included due to missing data, except for gestational age. Children included in the present analysis had a slightly greater gestational age than those not included (p<0.01) (Supplemental Table S1).The mean levels of biomarkers were 2.41 (SD=2.43); 0.23, (SD=0.13), and 1.02 (SD=0.68), for adducts by 32P-postlabeling, adducts by HPLC, and BDNF, respectively. There were 386 children with data on MDI and BDNF, and 345 children had data on DNA adducts by 32P-postlabeling, BDNF and MDI at 2 years. There was a modest correlation between the two measures of adducts (e.g., among the 267 children with 2 yr. MDI, Pearson’s correlation coefficient was 0.19, p=0.0023). There were no significant differences by gender in levels of adducts by 32P-postlabelling or HPLC or levels of BDNF. As expected the MDI scores were significantly higher among the girls (e.g., among the children with 2-year MDI , p<0.0001-0.006).
Figure 1.
Sample Size Flow Chart
Table 1.
Characteristics of children who are included in the present study (with at least one measure of DNA adducts, N=505)
| Variable | Mean ± SD or % |
|---|---|
| Percent with prenatal ETS exposure | 33.5 |
| Maternal TONI score | 20.3 ± 7.6 |
| Percent >= high school education | 62.6 |
| Maternal demoralization score | 1.2 ± 0.6 |
| Percent female | 51.7 |
| Percent African American | 35.1 |
| Gestational age at birth (weeks) | 39.4 ± 1.3 |
| HOME inventory | 39.6 ± 5.5 |
| Age at MDI assessment (months) | 24.1 ± 2.3 |
| Age at HOME assessment (months) | 41.5 ± 9.1 |
The results of multiple regression analysis of the associations between each pair of variables (32P-DNA adducts: MDI, 32P-DNA adducts: BDNF, BDNF: MDI), are shown in Table 2. Adducts and BDNF were treated as continuous variables and MDI as either continuous or dichotomous. Both the unrestricted and restricted analyses are presented. In unrestricted analyses, 32P-DNA adducts were significantly and inversely associated with both continuous MDI score (β=−2.07, p=0.04) and BDNF (β=−0.11, p=0.02) and were significantly associated with the outcome of borderline or clinical delay (OR=0.65, p=0.02). BDNF was significantly associated with MDI both when analyzed as a continuous score (β=2.69, p=0.003) and at the borderline or clinical delay cutoff (OR=1.58, p=0.01). In restricted analyses the results were similar but the associations were attenuated (Table 2). When PAH-DNA adducts and BDNF were both included as independent variables in the regression model, the association between PAH-DNA adducts and MDI was attenuated, suggesting some mediation. A formal test of mediation was not undertaken.
Table 2.
Associations between PAH/Aromatic (32P)-DNA Adducts, BDNF, and MDI at 24 months1
| Adducts and MDI | Adducts and BDNF5 | BDNF and MDI | Adducts on MDI (BDNF in model) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAH-DNA (32P) adducts | N | β adduct | P-value | N | β adduct | P-value | N | β BDNF | P-value | N | β adduct | P-value | |
| MDI continuous |
Unrestricted analysis3 |
380 | −2.07 | 0.04 * | 450 | −0.11 | 0.02 | 386 | 2.69 | 0.003 * | 345 | −1.92 | 0.07 |
| Restricted analysis4 |
345 | −2.10 | 0.05 * | 345 | −0.09 | 0.13 | 345 | 2.54 | 0.01 * | ||||
| N | OR | P-value | N | OR | P-Value | N | OR | P-value | |||||
| MDI dichotomous2 (Odds Ratio, > 85 vs. <85) |
Unrestricted analysis3 |
380 | 0.65 | 0.02 * | 386 | 1.58 | 0.01 * | 345 | 0.72 | 0.10 | |||
| Restricted analysis4 |
345 | 0.71 | 0.08 | 345 | 1.53 | 0.02 * | |||||||
Adjusting for prenatal ETS, child sex, maternal education, child ethnicity, gestational age, quality of the postnatal caretaking environment (HOME), maternal intelligence, child age at assessment, and maternal demoralization. In analysis of the association between adducts and BDNF (both measured in cord blood), age at MDI assessment and HOME were not included.
MDI scores were dichotomized at 85 (< 85 vs. > 85), the cutpoint for moderate developmental delay. MDI score >85 was coded as 1 and <85 as 0 such that an OR <1 implies a lower odds of being categorized as normal.
Unrestricted analysis: including all subjects with any two measures (adducts, MDI, or BDNF)
Restricted analysis: including subjects with all three measures (adducts, MDI, or BDNF)
The results for the unrestricted and restricted analyses only appear once as the MDI score is not included in this analysis.
p-value <0.05
In the corresponding analyses with B[a]P-DNA adducts, none of the associations were significant (data not shown).
When both adducts by 32P-postlabeling and BDNF were included in the same model, there was only a minor change in the two coefficients compared to the separate models. Further, in a formal test of mediation, we did not observe significant mediation.
We followed up with an analysis of the same associations in the smaller subset with MDI data at age 3 years. In the restricted analysis, n=334 children had 32P-DNA adduct, BDNF and MDI data and n=265 B[a]P-DNA adduct, BDNF and MDI data. As in the 2 year dataset, 32P-postlabeling adducts were inversely and significantly associated with BDNF in the unrestricted analysis (p=0.02). The associations between PAH-DNA adducts by 32P-postlabeling and MDI at age 3 years were inverse, albeit not significant (see Supplemental Table 2). Contrary to our hypothesis, the associations between BDNF and 3 year MDI score (continuous measure) were inverse, albeit not with statistical significance. Models restricting to those subjects who had MDI data available at both 2 and 3 years yielded similar results to those described above (data not shown).
Discussion
We have previously reported that PAH (B[a]P)-DNA adducts were inversely associated with BDNF and with the average developmental quotient on the Gesell Developmental Schedules administered at 2 years in a Chinese cohort (Tang et al., 2014). In the present analysis, we sought to determine if similar associations would be observed at age 2 years in a cohort of African-American and Dominican children in NYC exposed to lower levels of air pollution than the Chinese cohort (Perera et al., 2005). Here, we estimated the molecular dose of PAH in two ways: the specific B[a]P-DNA adducts measured by HPLC/fluorescence and the broader spectrum of PAH/aromatic DNA adducts detected by 32P-postlabeling analysis. The nuclease digestion method of adduct enhancement (32P-postlabeling) detects a range of adducts formed by genotoxic PAH, for example benz[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, BaP, indeno [1,2,3-cd]pyrene, benz[a,h]anthracene, and benzo[ghi]perylene; other aromatic and/or hydrophobic adducts may also be included in the quantitation (Phillips and Arlt, 2007). Contrary to our hypothesis, we did not observe the same associations as in the Chinese study between the specific B[a]P-DNA adducts and either BDNF or MDI scores. However, the broader spectrum of PAH/aromatic-DNA adducts by 32P-postlabeling was a significant predictor of lower BDNF concentrations as well as lower MDI scores and a positive predictor of the outcome of borderline or clinical delay at age 2 (Table 2). The lack of consistency with our prior study of B[a]P-DNA adducts may be explained in part by the significantly lower concentrations of cord B[a]P-DNA adducts in the NYC compared to the Chinese cohort (p <0.001) (Perera et al., 2005). The mean level in the NYC cohort was 0.23 per 108 nucleotides, whereas it is 0.31 per 108 nucleotides in the Chinese cohort. In addition, the mix of PAH in air pollution and the proportion represented by B[a]P may differ between the two settings. Unfortunately, we did not have 32P-postlabelling-DNA adduct measurements available in the Chinese cohort.
When we assessed the same associations between PAH/aromatic-DNA adducts by 32P-postlabeling among the subset of children who were evaluated for MDI at age 3, the associations between adducts and MDI and adducts and BDNF were generally similar to the results at age 2 years, although less significant; however, BDNF was negatively associated with MDI score and positively associated with the outcome of borderline or clinical delay. We speculate that factors in the postnatal environment, including exposure to other environmental pollutants, diet, physical activity, and parental stress play an increasing role in the development of the child as he/she grows older and these factors may dilute or override the impact of prenatal BDNF and also affect the concurrent levels of BDNF in the child (Ding et al., 2011; Onishchenko et al., 2008; Smith et al., 1995; Spulber et al., 2010; Stansfield et al., 2012). There may also be additional changes in the child’s environment between these two age timepoints that may contribute to the observed results. Therefore, the benefits of high prenatal BDNF on child development may no longer be apparent at this older age. Interestingly, we found that a number of children’s age-corrected scores increased substantially on the MDI between ages 2 and 3 years and that scores on the HOME Inventory measure were positively correlated with the change in MDI score (p=0.07).
As to the mechanisms involved, a number of PAH and other chemicals detected by the postlabeling assay are not only carcinogenic but neurotoxic. They are capable of exerting their toxicity through a number of genomic and non-genomic mechanisms. PAH not only directly damage DNA via adduct formation, but also disrupt the endocrine system, alter DNA methylation and gene expression, and generate reactive oxygen species (Herbstman et al., 2012; Pereira et al., 2015). Prenatal exposures are of particular concern as PAH readily cross the placenta and damage the fetal brain (Brown et al., 2007; Hood et al., 2000) likely by inducing inflammation, oxidative stress (Saunders et al., 2006), vascular injury (Block and Calderon-Garciduenas, 2009) and, as suggested here, by affecting BDNF levels. Animal models have shown that prenatal PAH exposure impairs subsequent development of behavior, learning, and memory, in part by disrupting glutamate signaling (Brown et al., 2007; Saunders et al., 2006; Saunders et al., 2002; Wormley et al., 2004a), activating glial cells that then become neurotoxic (Dutta et al., 2010) and by reducing neural plasticity (Brown et al., 2007). The prenatal period is highly sensitive to neurotoxic effects of environmental contaminants (Nijland et al., 2008; Rodier, 2004). Because BDNF is an active mediator of neuronal processes in the developing brain, promoting differentiation, growth, and survival of neurons during development (Huang and Reichardt, 2003), it is a potentially important target for PAH. This was suggested by our previous study in a Chinese population (Tang et al., 2014). We observed a weakened association between PAH-DNA adducts and MDI when BDNF was included in the regression model, suggesting that the PAH effect observed at age 2 years may be due, in part, to depression of BDNF levels.
Our results suggest an adverse effect of adducts formed by PAH from traffic emissions and other combustion sources on child development at age 2 years. By design, we eliminated another major source of these contaminants, active smoking during pregnancy, by enrolling only nonsmokers, and we adjusted for ETS exposure in the analysis. We also found that dietary PAH, another major source of PAH, was not a predictor of outcomes at p ≤ 0.1.
The strengths of the analysis include our ability to account for a number of factors other than PAH/aromatic pollutant exposure that are known to affect child neurodevelopment. We were able to draw upon individual prenatal exposure data from biomarkers, medical records and questionnaires. Limitations of this research include the relatively small sample size in the analysis and the lack of adequate data on lead levels to control for this known neurotoxic exposure. In addition, this study did not have the power to assess possible interactions with the genetic variant of BDNF (Val66met) that have been found to affect the gene’s functioning (Egan et al., 2003).
Conclusion
In conclusion, as hypothesized, the data suggest that prenatal exposure to a spectrum of combustion-related pollutants measured as PAH/aromatic DNA adducts by 32P-postlabeling analysis may adversely affect MDI outcomes at ages 2 and 3 years as well as levels of BDNF in the fetus. Contrary to our hypothesis, a more specific measure (B[a]P-DNA adducts) was not a significant predictor of BDNF or MDI at ages 2 or 3 years. Although this study consistently showed a strong positive association between levels of BDNF in cord blood and MDI at 2 years, the same relationship was not seen at age 3 years, possibly due to factors in the postnatal environment that play an increasing role and may dilute or override the impact of prenatal BDNF and also affect the concurrent levels of BDNF in the child.
PAH pollutants are widespread in urban environments in the United States and worldwide, in large part as a result of fossil fuel combustion for energy production, heating, and transportation. Fortunately, it is possible to reduce air pollution concentrations through currently available pollution controls, energy efficiency, alternative energy sources (Wong et al., 2004), and regulatory intervention to remove or control polluting sources (Millman et al., 2008).
Supplementary Material
Highlights.
Cord blood Polycyclic Aromatic Hydrocarbon (PAH)/aromatic-DNA adducts were assayed.
Brain Derived Neurotrophic Factor (BDNF) concentration was measured concurrently.
Associations between biomarkers and neurodevelopment at age 2 years were assessed.
Adduct level was inversely associated with BDNF concentration and neurodevelopment.
BDNF level was positively associated with neurodevelopment scores at age 2 years.
Acknowledgments
Funding Sources:
Funding was provided by the National Institute for Environmental Health Sciences (NIEHS) and the U.S. Environmental Protection Agency (US EPA): NIEHS/EPA P01ES09600/R82702701, NIEHS/EPA P01ES09600/RD832141, NIEHS/EPA P01ES09600/RD834509, NIEHS R01ES08977, the New York Community Trust, Trustees of the Blanchette Hooker Rockefeller Fund, Cancer Research UK, and the John and Wendy Neu Foundation.
Funding from all the institutions listed was used towards the design and conduct of the study; collection, management, analysis, and interpretation of the data; and the preparation, review, and approval of the manuscript. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the US EPA. Further, the US EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no actual or potential competing financial interests.
Human subjects research: All activities were approved by the Institutional Review Boards at the Columbia University Medical Center under human subjects protocol number AAAA-6110, and by the Centers for Disease Control and Prevention.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- Alexandrov K, et al. CYP1A1 and GSTM1 genotypes affect benzo[a]pyrene DNA adducts in smokers’ lung: comparison with aromatic/hydrophobic adduct formation. Carcinogenesis. 2002;23:1969–77. doi: 10.1093/carcin/23.12.1969. [DOI] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J Neurosci. 2002;22:10399–407. doi: 10.1523/JNEUROSCI.22-23-10399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley N. Bayley scales of infant development. 2nd Edition The Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- Block ML, Calderon-Garciduenas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32:506–16. doi: 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom CE, et al. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect. 2002;110:451–88. doi: 10.1289/ehp.110-1241197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RH. The Home Inventory: review and reflections. Adv Child Dev Behav. 1994;25:241–88. doi: 10.1016/s0065-2407(08)60054-3. [DOI] [PubMed] [Google Scholar]
- Brown L, et al. Test of non-verbal intelligence: a language-free measure of cognitive ability. 2nd edition PRO-ED, Inc.; Austin, Tx: 1990. [Google Scholar]
- Brown LA, et al. Down-regulation of early ionotrophic glutamate receptor subunit developmental expression as a mechanism for observed plasticity deficits following gestational exposure to benzo(a)pyrene. Neurotoxicology. 2007;28:965–78. doi: 10.1016/j.neuro.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell BM, Bradley RH. Home observation for measurement of the environment. University of Arkansas Press; Little Rock, AK: 1979. [Google Scholar]
- Cohen-Cory S, et al. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev Neurobiol. 2010;70:271–88. doi: 10.1002/dneu.20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha C, et al. A simple role for BDNF in learning and memory? Front Mol Neurosci. 2010;3:1. doi: 10.3389/neuro.02.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, et al. Exercise influences hippocampal plasticity by modulating brain-derived neurotrophic factor processing. Neuroscience. 2011;192:773–80. doi: 10.1016/j.neuroscience.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrenwend B, et al. Nonspecific psychological distress and other dimensions of psychopathology. Measures for use in the general population. Arch Gen Psychiatr. 1980;37:1229. doi: 10.1001/archpsyc.1980.01780240027003. [DOI] [PubMed] [Google Scholar]
- Dutta K, et al. A common carcinogen benzo[a]pyrene causes neuronal death in mouse via microglial activation. PLoS One. 2010;5:e9984. doi: 10.1371/journal.pone.0009984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–78. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Haapasalo A, et al. Regulation of TRKB surface expression by brain-derived neurotrophic factor and truncated TRKB isoforms. J Biol Chem. 2002;277:43160–7. doi: 10.1074/jbc.M205202200. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, et al. Prenatal exposure to polycyclic aromatic hydrocarbons, benzo[a]pyrene-DNA adducts, and genomic DNA methylation in cord blood. Environmental Health Perspectives. 2012;120:733–8. doi: 10.1289/ehp.1104056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood DB, et al. Modulation in the developmental expression profile of Sp1 subsequent to transplacental exposure of fetal rats to desorbed benzo[a]pyrene following maternal inhalation. Inhal Toxicol. 2000;12:511–35. doi: 10.1080/089583700402897. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–42. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialog between genes and synapses. Biosci Rep. 2004;24:475–522. doi: 10.1007/s10540-005-2742-7. [DOI] [PubMed] [Google Scholar]
- Lu Y, et al. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2008;89:312–23. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman A, et al. Air pollution threatens the health of children in China. Pediatrics. 2008;122:620–8. doi: 10.1542/peds.2007-3143. [DOI] [PubMed] [Google Scholar]
- Minichiello L. TrkB signalling pathways in LTP and learning. Nat Rev Neurosci. 2009;10:850–60. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- Nijland MJ, et al. Prenatal origins of adult disease. Curr Opin Obstet Gynecol. 2008;20:132–8. doi: 10.1097/GCO.0b013e3282f76753. [DOI] [PubMed] [Google Scholar]
- Numakawa T, et al. BDNF function and intracellular signaling in neurons. Histol Histopathol. 2010;25:237–58. doi: 10.14670/HH-25.237. [DOI] [PubMed] [Google Scholar]
- Onishchenko N, et al. Long-lasting depression-like behavior and epigenetic changes of BDNF gene expression induced by perinatal exposure to methylmercury. J Neurochem. 2008;106:1378–87. doi: 10.1111/j.1471-4159.2008.05484.x. [DOI] [PubMed] [Google Scholar]
- Pereira RD, et al. Angiogenesis in the Placenta: The Role of Reactive Oxygen Species Signaling. Biomed Res Int. 2015;2015:814543. doi: 10.1155/2015/814543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, et al. Early-life exposure to polycyclic aromatic hydrocarbons and ADHD behavior problems. Plos One. 2014;9:e111670. doi: 10.1371/journal.pone.0111670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, et al. Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years. Pediatrics. 2009;124:e195–202. doi: 10.1542/peds.2008-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect. 2003;111:201–5. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, et al. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect. 2006;114:1287–92. doi: 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, et al. Biomarkers in maternal and newborn blood indicate heightened fetal susceptibility to procarcinogenic DNA damage. Environ Health Perspect. 2004;112:1133–6. doi: 10.1289/ehp.6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, et al. Prenatal polycyclic aromatic hydrocarbon (PAH) exposure and child behavior at age 6-7 years. Environ Health Perspect. 2012;120:921–6. doi: 10.1289/ehp.1104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, et al. DNA damage from polycyclic aromatic hydrocarbons measured by benzo[a]pyrene-DNA adducts in mothers and newborns from Northern Manhattan, the World Trade Center Area, Poland, and China. Cancer Epidemiol Biomarkers Prev. 2005;14:709–14. doi: 10.1158/1055-9965.EPI-04-0457. [DOI] [PubMed] [Google Scholar]
- Phillips DH, Arlt VM. The 32P-postlabeling assay for DNA adducts. Nat Protoc. 2007;2:2772–81. doi: 10.1038/nprot.2007.394. [DOI] [PubMed] [Google Scholar]
- Phillips DH, Venitt S. DNA and protein adducts in human tissues resulting from exposure to tobacco smoke. Int J Cancer. 2012;131:2733–53. doi: 10.1002/ijc.27827. [DOI] [PubMed] [Google Scholar]
- Promega Corporation . Technical Bulletin. BDNF Emax. ® ImmunoAssay System; USA: 2009. [Google Scholar]
- Rauh VA, et al. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicol Teratol. 2004;26:373–85. doi: 10.1016/j.ntt.2004.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier PM. Environmental causes of central nervous system maldevelopment. Pediatrics. 2004;113:1076–83. [PubMed] [Google Scholar]
- Rojas M, et al. High DNA damage by benzo[a]pyrene 7,8-diol-9,10-epoxide in bronchial epithelial cells from patients with lung cancer: comparison with lung parenchyma. Cancer Lett. 2004;207:157–63. doi: 10.1016/j.canlet.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Rubin DB. Multiple Imputation for Nonresponse in Surveys J. Wiley & Sons; New York: 1987. [Google Scholar]
- Saunders CR, et al. Benzo(a)pyrene-induced acute neurotoxicity in the F-344 rat: role of oxidative stress. J Appl Toxicol. 2006;26:427–38. doi: 10.1002/jat.1157. [DOI] [PubMed] [Google Scholar]
- Saunders CR, et al. Modulation of neurotoxic behavior in F-344 rats by temporal disposition of benzo(a)pyrene. Toxicol Lett. 2002;129:33–45. doi: 10.1016/s0378-4274(01)00467-2. [DOI] [PubMed] [Google Scholar]
- Smith MA, et al. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–77. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel ME. Asymptotic Confidence Intervals for Indirect Effects in Structural Equation Models. Sociological Methodology. 1982;13:290–312. [Google Scholar]
- Spulber S, et al. Effects of maternal smoking and exposure to methylmercury on brain-derived neurotrophic factor concentrations in umbilical cord serum. Toxicol Sci. 2010;117:263–9. doi: 10.1093/toxsci/kfq216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfield KH, et al. Dysregulation of BDNF-TrkB signaling in developing hippocampal neurons by Pb(2+): implications for an environmental basis of neurodevelopmental disorders. Toxicol Sci. 2012;127:277–95. doi: 10.1093/toxsci/kfs090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, et al. Molecular and neurodevelopmental benefits to children of closure of a coal burning power plant in China. Plos One. 2014;9:e91966. doi: 10.1371/journal.pone.0091966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, et al. PAH-DNA Adducts in cord blood and fetal and child development in a Chinese cohort. Environ Health Perspect. 2006;114:1297–1300. doi: 10.1289/ehp.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, et al. Association between carcinogen-DNA adducts in white blood cells and lung cancer risk in the physicians health study. Can Res. 2001;61:6708–12. [PubMed] [Google Scholar]
- Wong EY, et al. Assessing the health benefits of air pollution reduction for children. Environ Health Perspect. 2004;112:226–32. doi: 10.1289/ehp.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormley DD, et al. Inhaled benzo(a)pyrene impairs long-term potentiation in the F1 generation rat dentate gyrus. Cell Mol Biol (Noisy-le-grand) 2004a;50:715–21. [PubMed] [Google Scholar]
- Wormley DD, et al. Environmental contaminant-mixture effects on CNS development, plasticity, and behavior. Toxicol Appl Pharmacol. 2004b;197:49–65. doi: 10.1016/j.taap.2004.01.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.