Abstract
Human papillomaviruses (HPVs) are small double-stranded DNA viruses that pose significant public health concerns as the causative agent of approximately 5% of worldwide cancers. The HPV oncogenes E6 and E7 play key roles in carcinogenesis. In the last 15 years there has been a significant increase in the incidence of HPV-related head and neck cancers arising primarily in the oropharynx. Patients with HPV-positive head and neck cancers (HNCs) have a significantly improved prognosis compared to those with HPV-negative disease. In this review we will discuss data suggesting how HPV oncogenes modulate both the intrinsic radiation sensitivity of HNCs and also have important effects upon the tumor microenvironment. Together, these findings contribute to the improved outcomes seen in patients with HPV-positive HNC.
Introduction
Early viral discovery efforts by Shope and Hurst described an agent that could be transmitted from one animal to another, was of a defined size that enabled it to be filtered, caused the growth of benign papillomas, and ultimately resulted in the identification of the papillomavirus family [1]. This discovery of human papillomavirus (HPV) in 1956, led to the finding that this pathogen causes unrestrained epithelial proliferations including papillomas, warts, condylomas, and carcinomas [2]. During the second half of the 20th century, HPV has subsequently been shown to be the cause of squamous cell carcinomas (SCCs) arising in multiple anatomic locations including the squamous epithelium of the uterine cervix, vulva, vagina, penis, anal canal, and head and neck (in particular the oropharynx). Radiation plays a key role in the curative treatment of each of these cancers and growing evidence suggests that the function of the papillomavirus proteins may play an important role in the relative increased sensitivity of these cancers to radiation, via the modulation of DNA damage response and alteration of the tumor microenvironment. Herein, we briefly review the role of HPV in cancer development and describe work by a number of groups to elucidate mechanisms underlying the significantly improved outcomes seen in patients with HPV-positive tumors.
Human papillomavirus and carcinogenesis
The HPV genome encodes approximately 8,000 base pairs of double-stranded DNA. The virus is non-enveloped and different viral subtypes are classified on the basis of their L1 capsid protein into nearly 200 unique subtypes (see http://pave.niaid.nih.gov/ for the most current listing). HPVs can be sub-classified into cutaneous or mucosal subtypes based on their specific tissue tropism [3] or can be separated into low-risk and high-risk types based on their ability to cause malignant transformation and induce cancer. The high risk subtypes (Table 1) can cause cancers of the uterine cervix, anus, vagina, vulva, penis, and head and neck [4].
Table 1.
Association of HPV subtypes with mucosal or skin carcinomas.
| Category | HPV types | |
|---|---|---|
| Mucosal | Group 1: Carcinogenic to humans | 16, 18, 31, 33, 45, 51, 52 |
|
| ||
| Group 2A: Probably carcinogenic to humans | 68 | |
|
| ||
| Group 2B: Possiblity carcinogenic to humans | 26, 53, 64, 65, 66, 67, 69, 70, 73, 82 | |
|
| ||
| Carcinomas of Skin | 5, 8; less commonly 14, 17, 20, 47 | |
|
|
||
The viral proteins encoded by the HPV genome (Fig. 1) regulate the viral life cycle [5]. The viral capsid proteins L1 and L2 play no known role in carcinogenesis but are the immunologic targets of current HPV vaccines such as Gardasil or Cervarix; in the tumor cells of many HPV-associated cancers expression of these proteins is lost, thus limiting the value of these vaccines for cancer treatment [6]. Viral genome replication is controlled by the early genes E1 and E2 which also regulate the transcription of other viral genes [5]. A splice variant of the E1^E4 mRNA transcript encodes the E4 protein which facilitates viral particle release and may play a role in G2 arrest [7]. Most important to the clinical oncologist, three HPV oncogenes, E5, E6, and E7 drive unrestrained cellular proliferation to as a required feature of viral amplification but also play key roles in carcinogenesis by promoting proliferation and inducing genomic instability [8–10].
Figure 1. HPV-16.
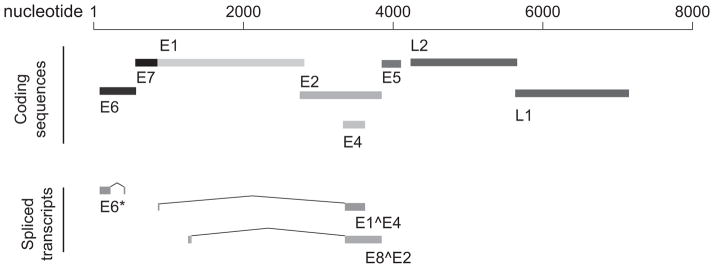
HPV-16 is a 7905 bp genome that encodes eight proteins and at least 11 mRNA transcripts. E6 and E7 are the primary HPV oncogenes and play critical roles in oncogenesis via their interactions with the cellular proteins p53 and RB, among others.
The E7 protein enhances proliferation of HPV-infected cells by targeting members of the pocket protein family for degradation, most notably retinoblastoma 1 (Rb) [9, 11]. Rb plays an important role in preventing excessive cell growth by inhibiting cell cycle progression [12]. A higher avidity interaction between high-risk E7 and Rb appears to promote Rb’s degradation in the more oncogenic HPV subtypes [9]. Acting alone, E7-driven proliferation can result in a p53-dependent anti-proliferative response. This function is countered by HPV E6 mediated degradation of p53 via activation of the ubiquitin ligase E6AP [8, 13]. E6 and E7 act in concert to inhibit apoptosis, promote unrestrained cell proliferation, and play key roles in the promotion of genomic instability [2, 8, 14, 15], all critical factors in HPV-mediated carcinogenesis. E6 and E7 cooperate to promote chromosomal segregation errors and aneuploidy [15] while E7 induces centrosome synthesis via CDK2 activity [16]. Beyond these historically well-described functions, it is now clear that both E6 and E7 interact with a multitude of additional cellular proteins that may play additional roles in carcinogenesis [17, 18]. Finally, while much less is known about its function, E5 can cooperate with E6 and E7 and plays a minor role in transformation [10].
HPV AS AN INDICATOR OF TREATMENT RESPONSE
As cervical cancer is overwhelmingly associated with HPV positivity, it is difficult to assess the role of the viral oncoproteins in contrast to non-viral associated malignancies in that setting. The mixed etiology of head and neck cancer (HNC), however, provides an opportunity to study the influence of HPV on clinical outcomes. HPV-status is now a well-accepted prognostic biomarker in HNC and appears to have similar value in anal cancer [19–22]. Two recent meta-analyses by O’Rorke and Petrelli both confirm a remarkable survival advantage for patients with HPV-positive HNC as compared to those with HPV-negative disease (HR 0.46; 95% CI, 0.37–0.57 and HR = 0.33; 95% CI, 0.27–0.40, respectively) [23, 24]. Tobacco abuse appears to be a modifying factor as patients with HPV-positive HNC and significant tobacco abuse histories have outcomes intermediate to those in HPV-positive non-smokers or traditional HPV-negative (i.e. tobacco and/or alcohol associated) HNCs [25–28]. Due to these differences, clinical trials in HNC currently stratify patients on the basis of HPV status (including tobacco use) or are designed specifically for HPV-positive or HPV-negative patients. Ongoing efforts in HNC are focused on decreasing the intensity of therapy while maintaining excellent cure rates (see [29]) and may provide important insights that can be later applied to other HPV-associated malignancies.
HPV and RADIATION SENSITIVITY
Since being first postulated [30], evidence has grown that enhanced sensitivity to radiation in HPV-positive HNC is an important contributor to the improved prognosis of these patients [31]. A number of both epidemiological and mechanistic hypotheses have been proposed to explain the improved outcomes consistently seen in HPV-positive HNC patients. One of the simplest is that patients with HPV-positive HNC are typically younger and healthier than those with HPV-negative disease [25, 32, 33]. This makes them better able to tolerate therapy and less likely to die from comorbid illnesses. While this may be true, even within well-matched cohorts of patients (i.e. similar age, performance status, and disease stage), those with HPV-positive cancers have significantly improved outcomes compared to those with HPV-negative disease.
Over the last few years, we and others have demonstrated that both HPV-16 positive HNC cell lines [34–37] and oral epithelial cells engineered to express the HPV-16 E6 oncoprotein [34, 38], have greater intrinsic sensitivity to radiation (i.e. lower survival fractions over a range of radiation doses) than HPV-negative cells. While to date only a limited number of trials have reported outcomes comparing HPV+ and HPV− patients treated with radiation monotherapy, in these reports, tumor HPV status was highly prognostic for improved local control and overall survival [39, 40]. This result is consistent with increased intrinsic sensitivity to radiation demonstrated in the lab in cell line and mouse studies. Several complementary mechanisms appear to be at work to explain these profound differences. Following radiation, residual wild-type p53 not yet degraded by HPV-E6 activates a canonical p53 transcriptional program resulting in cell cycle arrest and apoptosis [34, 36]. HPV-positive tumors also exhibit impaired double-strand break repair capacity that may influence radiosensitivity [35, 41] that may be related to p16-mediated impairment of homologous recombination-mediated DNA repair [37]. A finding common to several of these studies is a profound and sustained G2 arrest induced by radiation in HPV positive tumors [34–37].
A number of other factors also likely play a role in the improved outcomes seen in patients with HPV-positive HNC. For example, in the DAHANCA 5 trial, HPV-negative tumors demonstrated a larger benefit to hypoxic modification than HPV-positive tumors, a finding that correlated with an increase in hypoxia markers in HPV-negative tumors [42, 43]. This data led to the hypothesis that increased hypoxia in HPV-negative tumors may be partially responsible for the differential in response to radiation compared to less hypoxic HPV-positive tumors. The hypoxia/HPV link remains unsettled, however, as contrasting data from several other groups has failed to demonstrate any correlation between imaging markers of hypoxia and HPV status [44, 45]. It may be that the increased radiation sensitivity seen in HPV-positive HNCs compensates for the presence of hypoxia in these tumors at current radiation doses, but that as radiation doses are decreased the impact of hypoxia will again become evident. In fact, work by Sorensen et al has demonstrated that HPV-positive cells demonstrate a similar oxygen enhancement ratio as HPV-negative cells [46].
An increasing body of evidence suggests that HPV-positive tumors may provide a more immunologically rich environment that may also affect tumor control. Tumor infiltrating T cells are increased in HPV-positive tumors [47–50], and are shifted from naïve to memory or effector T cells with greater frequency compared to patients with HPV-negative tumors [51]. Similarly, programmed death-1 (PD-1) positive tumor infiltrating lymphocytes are more common in HPV-positive tumors [52]. Several groups have also reported the detection of circulating anti-HPV16 antibodies and circulating and tumor-infiltrating HPV-specific T cells in patients with HPV-positive HNC, a finding that is correlated with improved clinical outcome [53–55]. In fact, Ward and colleagues found that patients with HPV-positive tumors with high levels of tumor infiltrating lymphocytes have increased survival compared to patients with HPV-positive tumors with low levels of tumor infiltrating lymphocytes (3-yr DSS 96% vs. 59%), a group which had a similar prognosis as patients with HPV-negative tumors [56].
Potentially necessary for the development of cancer in this more robust immune environment, HPV-positive tumors have also been reported to employ several immune evasion strategies. HPV-positive cancers exhibit impaired immune effector recognition and block immune-mediated cell death via reduction of HLA and FasL expression, promote an immune suppressive cytokine milieu, and recruit immunosuppressive regulatory T cells, myeloid-derived suppressor cells, and tumor-associated macrophages [49, 53, 57–59].
It has been hypothesized that radiation disrupts this tolerogenic phenotype, essentially “reawakening” the immune response against HPV-positive tumors. Radiation increases the percentage of activated circulating CD8+ and CD4+ lymphocytes, a finding that correlates with improved survival [60, 61]. Further data supporting an important role for the immune system in HPV-positive cancers comes from in vivo studies by Spanos and colleagues who demonstrated that control of HPV-positive mouse tumors with radiation was significantly better than that of HPV-negative tumors, but only in the presence of an intact immune system in the animals [62]. In addition, low-dose radiation therapy has been found to greatly enhance the antitumor response to DNA vaccination in E7-expressing tumor-bearing mice, with increased frequency of peripheral and infiltrating E7-specific CD8+ T cells, enhanced tumor cell susceptibility to CTL-mediated lysis, and improved survival of mice treated with vaccination and radiation therapy compared to either therapy alone [63]. Several clinical studies are poised to provide much needed evidence regarding the role of the immune response in HPV-positive cancers and how radiation may promote immune-mediated tumor clearance.
CONCLUSIONS
HPV has been a growing cause of HNC. While current treatment for patients with HPV-associated disease does not differ from those with HPV-negative cancers, it is likely that ongoing clinical trials will soon provide data to guide the personalization of treatment based on HPV-status of their cancers. A growing body of basic and translational studies describe a mechanistic basis for the improved outcomes seen in HPV-positive patients and point to key roles for microenvironmental differences in therapeutic response. Additional details that emerge over the coming years will further define the molecular underpinnings of HPV-mediated altered radiation sensitivity and how HPV effects on the tumor microenvironment can be used to personalize therapy for these patients. Better understanding of these mechanisms will ultimately enhance the efficacy of radiation in the treatment of these cancers.
Acknowledgments
This work was supported in part by NIH/NCI CA160639 (RJK), a Jimmy V Foundation for Cancer Research Scholar Award (RJK) and a PhRMA Foundation Translational Medicine and Therapeutics Postdoctoral Fellowship (AS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shope RE, Hurst EW. Infectious Papillomatosis of Rabbits : With a Note on the Histopathology. J Exp Med. 1933;58:607–24. doi: 10.1084/jem.58.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore PS, Chang Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat Rev Cancer. 2010;10:878–89. doi: 10.1038/nrc2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzich JA, Ghim SJ, Palmer-Hill FJ, et al. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci U S A. 1995;92:11553–7. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 1995;64:1–378. [PMC free article] [PubMed] [Google Scholar]
- 5.Hebner CM, Laimins LA. Human papillomaviruses: basic mechanisms of pathogenesis and oncogenicity. Rev Med Virol. 2006;16:83–97. doi: 10.1002/rmv.488. [DOI] [PubMed] [Google Scholar]
- 6.Horvath CA, Boulet GA, Renoux VM, Delvenne PO, Bogers JP. Mechanisms of cell entry by human papillomaviruses: an overview. Virol J. 2010;7:11. doi: 10.1186/1743-422X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knight GL, Pugh AG, Yates E, et al. A cyclin-binding motif in human papillomavirus type 18 (HPV18) E1^E4 is necessary for association with CDK-cyclin complexes and G2/M cell cycle arrest of keratinocytes, but is not required for differentiation-dependent viral genome amplification or L1 capsid protein expression. Virology. 2011;412:196–210. doi: 10.1016/j.virol.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–36. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 9.Munger K, Werness BA, Dyson N, Phelps WC, Harlow E, Howley PM. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989;8:4099–105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiMaio D, Mattoon D. Mechanisms of cell transformation by papillomavirus E5 proteins. Oncogene. 2001;20:7866–73. doi: 10.1038/sj.onc.1204915. [DOI] [PubMed] [Google Scholar]
- 11.Song S, Liem A, Miller JA, Lambert PF. Human papillomavirus types 16 E6 and E7 contribute differently to carcinogenesis. Virology. 2000;267:141–50. doi: 10.1006/viro.1999.0106. [DOI] [PubMed] [Google Scholar]
- 12.Indovina P, Marcelli E, Casini N, Rizzo V, Giordano A. Emerging roles of RB family: new defense mechanisms against tumor progression. J Cell Physiol. 2013;228:525–35. doi: 10.1002/jcp.24170. [DOI] [PubMed] [Google Scholar]
- 13.Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–9. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 14.Fan X, Liu Y, Heilman SA, Chen JJ. Human papillomavirus E7 induces rereplication in response to DNA damage. J Virol. 2013;87:1200–10. doi: 10.1128/JVI.02038-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duensing S, Lee LY, Duensing A, et al. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci U S A. 2000;97:10002–7. doi: 10.1073/pnas.170093297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duensing A, Liu Y, Tseng M, Malumbres M, Barbacid M, Duensing S. Cyclin-dependent kinase 2 is dispensable for normal centrosome duplication but required for oncogene-induced centrosome overduplication. Oncogene. 2006;25:2943–9. doi: 10.1038/sj.onc.1209310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White EA, Kramer RE, Tan MJ, Hayes SD, Harper JW, Howley PM. Comprehensive analysis of host cellular interactions with human papillomavirus E6 proteins identifies new E6 binding partners and reflects viral diversity. J Virol. 2012;86:13174–86. doi: 10.1128/JVI.02172-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huh KW, DeMasi J, Ogawa H, Nakatani Y, Howley PM, Munger K. Association of the human papillomavirus type 16 E7 oncoprotein with the 600-kDa retinoblastoma protein-associated factor, p600. Proc Natl Acad Sci U S A. 2005;102:11492–7. doi: 10.1073/pnas.0505337102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodel F, Wieland U, Fraunholz I, et al. Human papillomavirus DNA load and p16INK4a expression predict for local control in patients with anal squamous cell carcinoma treated with chemoradiotherapy. Int J Cancer. 2015;136:278–88. doi: 10.1002/ijc.28979. [DOI] [PubMed] [Google Scholar]
- 20.Ravenda PS, Magni E, Botteri E, et al. Prognostic value of human papillomavirus in anal squamous cell carcinoma. Cancer Chemother Pharmacol. 2014;74:1033–8. doi: 10.1007/s00280-014-2582-x. [DOI] [PubMed] [Google Scholar]
- 21.Serup-Hansen E, Linnemann D, Skovrider-Ruminski W, Hogdall E, Geertsen PF, Havsteen H. Human papillomavirus genotyping and p16 expression as prognostic factors for patients with American Joint Committee on Cancer stages I to III carcinoma of the anal canal. J Clin Oncol. 2014;32:1812–7. doi: 10.1200/JCO.2013.52.3464. [DOI] [PubMed] [Google Scholar]
- 22.Roldan Urgoiti GB, Gustafson K, Klimowicz AC, Petrillo SK, Magliocco AM, Doll CM. The prognostic value of HPV status and p16 expression in patients with carcinoma of the anal canal. PLoS One. 2014;9:e108790. doi: 10.1371/journal.pone.0108790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Rorke MA, Ellison MV, Murray LJ, Moran M, James J, Anderson LA. Human papillomavirus related head and neck cancer survival: a systematic review and meta-analysis. Oral Oncol. 2012;48:1191–201. doi: 10.1016/j.oraloncology.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Petrelli F, Sarti E, Barni S. Predictive value of human papillomavirus in oropharyngeal carcinoma treated with radiotherapy: An updated systematic review and meta-analysis of 30 trials. Head Neck. 2014;36:750–9. doi: 10.1002/hed.23351. [DOI] [PubMed] [Google Scholar]
- 25.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith EM, Rubenstein LM, Haugen TH, Pawlita M, Turek LP. Complex etiology underlies risk and survival in head and neck cancer human papillomavirus, tobacco, and alcohol: a case for multifactor disease. J Oncol. 2012;2012:571862. doi: 10.1155/2012/571862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang SH, Xu W, Waldron J, et al. Refining American Joint Committee on Cancer/Union for International Cancer Control TNM stage and prognostic groups for human papillomavirus-related oropharyngeal carcinomas. J Clin Oncol. 2015;33:836–45. doi: 10.1200/JCO.2014.58.6412. [DOI] [PubMed] [Google Scholar]
- 28.O’Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31:543–50. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 29.Kimple RJ, Harari PM. Is radiation dose reduction the right answer for HPV-positive head and neck cancer? Oral Oncol. 2013;50:560–4. doi: 10.1016/j.oraloncology.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–20. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 31.Lassen P. The role of Human papillomavirus in head and neck cancer and the impact on radiotherapy outcome. Radiother Oncol. 2010;95:371–80. doi: 10.1016/j.radonc.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 32.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–9. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 33.Deboni AL, Giordani AJ, Lopes NN, et al. Long-term oral effects in patients treated with radiochemotherapy for head and neck cancer. Support Care Cancer. 2012;20:2903–11. doi: 10.1007/s00520-012-1418-7. [DOI] [PubMed] [Google Scholar]
- 34.Kimple RJ, Smith MA, Blitzer GC, et al. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res. 2013;73:4791–800. doi: 10.1158/0008-5472.CAN-13-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rieckmann T, Tribius S, Grob TJ, et al. HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother Oncol. 2013;107:242–6. doi: 10.1016/j.radonc.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Arenz A, Ziemann F, Mayer C, et al. Increased radiosensitivity of HPV-positive head and neck cancer cell lines due to cell cycle dysregulation and induction of apoptosis. Strahlenther Onkol. 2014;190:839–46. doi: 10.1007/s00066-014-0605-5. [DOI] [PubMed] [Google Scholar]
- 37.Dok R, Kalev P, Van Limbergen EJ, et al. p16INK4a impairs homologous recombination-mediated DNA repair in human papillomavirus-positive head and neck tumors. Cancer Res. 2014;74:1739–51. doi: 10.1158/0008-5472.CAN-13-2479. [DOI] [PubMed] [Google Scholar]
- 38.Pang E, Delic NC, Hong A, Zhang M, Rose BR, Lyons JG. Radiosensitization of oropharyngeal squamous cell carcinoma cells by human papillomavirus 16 oncoprotein E6 *I. Int J Radiat Oncol Biol Phys. 2011;79:860–5. doi: 10.1016/j.ijrobp.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 39.Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:1992–8. doi: 10.1200/JCO.2008.20.2853. [DOI] [PubMed] [Google Scholar]
- 40.O’Sullivan B, Huang SH, Perez-Ordonez B, et al. Outcomes of HPV-related oropharyngeal cancer patients treated by radiotherapy alone using altered fractionation. Radiother Oncol. 2012;103:49–56. doi: 10.1016/j.radonc.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 41.Park JW, Nickel KP, Torres AD, Lee D, Lambert PF, Kimple RJ. Human papillomavirus type 16 E7 oncoprotein causes a delay in repair of DNA damage. Radiother Oncol. 2014;113:337–44. doi: 10.1016/j.radonc.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Overgaard J, Eriksen JG, Nordsmark M, et al. Plasma osteopontin, hypoxia, and response to the hypoxia sensitiser nimorazole in radiotherapy of head and neck cancer: results from the DAHANCA 5 randomised double-blind placebo-controlled trial. Lancet Oncol. 2005;6:757–64. doi: 10.1016/S1470-2045(05)70292-8. [DOI] [PubMed] [Google Scholar]
- 43.Toustrup K, Sorensen BS, Lassen P, et al. Gene expression classifier predicts for hypoxic modification of radiotherapy with nimorazole in squamous cell carcinomas of the head and neck. Radiother Oncol. 2012;102:122–9. doi: 10.1016/j.radonc.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 44.Mortensen LS, Johansen J, Kallehauge J, et al. FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: results from the DAHANCA 24 trial. Radiother Oncol. 2012;105:14–20. doi: 10.1016/j.radonc.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 45.Jansen JF, Carlson DL, Lu Y, et al. Correlation of a priori DCE-MRI and (1)H-MRS data with molecular markers in neck nodal metastases: Initial analysis. Oral Oncol. 2012;48:717–22. doi: 10.1016/j.oraloncology.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorensen BS, Busk M, Olthof N, et al. Radiosensitivity and effect of hypoxia in HPV positive head and neck cancer cells. Radiother Oncol. 2013;108:500–5. doi: 10.1016/j.radonc.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 47.Kong CS, Narasimhan B, Cao H, et al. The relationship between human papillomavirus status and other molecular prognostic markers in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2009;74:553–61. doi: 10.1016/j.ijrobp.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jung AC, Guihard S, Krugell S, et al. CD8-alpha T-cell infiltration in human papillomavirus-related oropharyngeal carcinoma correlates with improved patient prognosis. Int J Cancer. 2013;132:E26–36. doi: 10.1002/ijc.27776. [DOI] [PubMed] [Google Scholar]
- 49.Nasman A, Romanitan M, Nordfors C, et al. Tumor infiltrating CD8+ and Foxp3+ lymphocytes correlate to clinical outcome and human papillomavirus (HPV) status in tonsillar cancer. PloS one. 2012;7:e38711. doi: 10.1371/journal.pone.0038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nordfors C, Grun N, Tertipis N, et al. CD8+ and CD4+ tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. Eur J Cancer. 2013;49:2522–30. doi: 10.1016/j.ejca.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 51.Turksma AW, Bontkes HJ, van den Heuvel H, et al. Effector memory T-cell frequencies in relation to tumour stage, location and HPV status in HNSCC patients. Oral Dis. 2013;19:577–84. doi: 10.1111/odi.12037. [DOI] [PubMed] [Google Scholar]
- 52.Badoual C, Hans S, Merillon N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128–38. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 53.Heusinkveld M, Goedemans R, Briet RJ, et al. Systemic and local human papillomavirus 16-specific T-cell immunity in patients with head and neck cancer. Int J Cancer. 2012;131:E74–85. doi: 10.1002/ijc.26497. [DOI] [PubMed] [Google Scholar]
- 54.Hoffmann TK, Arsov C, Schirlau K, et al. T cells specific for HPV16 E7 epitopes in patients with squamous cell carcinoma of the oropharynx. Int J Cancer. 2006;118:1984–91. doi: 10.1002/ijc.21565. [DOI] [PubMed] [Google Scholar]
- 55.Albers A, Abe K, Hunt J, et al. Antitumor activity of human papillomavirus type 16 E7-specific T cells against virally infected squamous cell carcinoma of the head and neck. Cancer Res. 2005;65:11146–55. doi: 10.1158/0008-5472.CAN-05-0772. [DOI] [PubMed] [Google Scholar]
- 56.Ward MJ, Thirdborough SM, Mellows T, et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br J Cancer. 2014;110:489–500. doi: 10.1038/bjc.2013.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watanabe Y, Katou F, Ohtani H, Nakayama T, Yoshie O, Hashimoto K. Tumor-infiltrating lymphocytes, particularly the balance between CD8(+) T cells and CCR4(+) regulatory T cells, affect the survival of patients with oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:744–52. doi: 10.1016/j.tripleo.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 58.Strauss L, Bergmann C, Gooding W, Johnson JT, Whiteside TL. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:6301–11. doi: 10.1158/1078-0432.CCR-07-1403. [DOI] [PubMed] [Google Scholar]
- 59.Grabowska AK, Riemer AB. The invisible enemy - how human papillomaviruses avoid recognition and clearance by the host immune system. Open Virol J. 2012;6:249–56. doi: 10.2174/1874357901206010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miyazaki K, Mizutani H, Katabuchi H, Fukuma K, Fujisaki S, Okamura H. Activated (HLA− DR+) T-lymphocyte subsets in cervical carcinoma and effects of radiotherapy and immunotherapy with sizofiran on cell-mediated immunity and survival. Gynecol Oncol. 1995;56:412–20. doi: 10.1006/gyno.1995.1073. [DOI] [PubMed] [Google Scholar]
- 61.Eric A, Juranic Z, Tisma N, et al. Radiotherapy-induced changes of peripheral blood lymphocyte subpopulations in cervical cancer patients: relationship to clinical response. J BUON. 2009;14:79–83. [PubMed] [Google Scholar]
- 62.Spanos WC, Nowicki P, Lee DW, et al. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135:1137–46. doi: 10.1001/archoto.2009.159. [DOI] [PubMed] [Google Scholar]
- 63.Tseng CW, Trimble C, Zeng Q, et al. Low-dose radiation enhances therapeutic HPV DNA vaccination in tumor-bearing hosts. Cancer Immunol Immunother. 2009;58:737–48. doi: 10.1007/s00262-008-0596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


