Abstract
Epicardial adipose tissue (EAT) has been recognized as a sensitive marker of cardiometabolic risk. Recent evidence suggests efficacy of long-term statin therapy in reducing EAT in patients with coronary artery disease. Whether short-term statin therapy is associated with changes in the volume of EAT is currently unknown. A cohort of atrial fibrillation (AF) patients undergoing pulmonary vein isolation was randomized to receive either 80 mg/day of atorvastatin (n=38, 32 males, age 56 ± 11 years) or placebo (n=41, 33 males, age 56 ± 10 years) for a 3-month period. EAT volume was assessed by cardiac computed tomography at baseline and at follow-up. Patients randomized to statin treatment exhibited a modest but significant decrease in median EAT volume (baseline vs follow-up: 92.3(62.0–133.3) vs 86.9(64.1–124.8) cm3, p < 0.05) while median EAT remained unchanged in the placebo group (81.9(55.5–110.9) vs 81.3(57.1–110.5) cm3, p = NS). Changes in median systemic inflammatory markers and lipid profile were also seen with statin treatment: C-reactive protein (2.4(0.7–3.7) vs 1.1(0.5–2.7) mg/L, p < 0.05), total cholesterol (186(162.5–201) vs 123(99–162.5) mg/dL, p < 0.001), and low density lipoprotein-cholesterol (116(96.5–132.5) vs 56(40.5–81) mg/dL, p < 0.001) diminished, while median body mass index did not change (27.8(25–30) vs 27.6(25.7–30.5) kg/m2, p = NS). No variations occurred in the placebo group. In conclusion, short-term intensive statin therapy significantly reduced the volume of EAT in AF patients.
Keywords: Atrial fibrillation, Cardiometabolic risk, Epicardial adipose tissue, Statin
Epicardial adipose tissue (EAT) is a locally active endocrine organ covering 80% of the heart surface, and is located between the myocardium and visceral pericardium.1 Due to its anatomical proximity and same microcirculation epicardial tissue has been proposed to locally influence adjacent myocardium.2,3 Statins improve cardiometabolic status in individuals with cardiovascular risk factors as well as in patients with overt cardiovascular diseases.4,5 Recent evidence suggests efficacy of long-term statin therapy in reducing EAT in patients with coronary artery disease.6 Whether the volume of EAT changes in atrial fibrillation (AF) patients treated with statins for a short time period is not known. Therefore using a randomized, double-blind, placebo-controlled study design, we examined changes in EAT volume before and after 3 months of statin treatment.
Methods
We reviewed computed tomography (CT) images of patients included in a clinical trial investigating the effects of intensive statin treatment on AF recurrence in patients undergoing pulmonary vein isolation (PVI) (NCT00579098). The original prospective study was conducted at Mayo Clinic between January 2008 and December 2009 and showed no difference between patients treated with statin compared to patients in the placebo arm in terms of AF recurrence after PVI.7 Eligible patients were individuals aged ≥ 18 years with a clinical indication for a left atrial ablation procedure for AF. The definition and classification of AF used in this study were based on published guidelines from the American College of Cardiology–American Heart Association and the European Society of Cardiology.8,9 Patients with known malignancy, inflammatory diseases, surgery, trauma, or myocardial infarction in the previous month, patients with contraindications to statin therapy, elevated liver enzymes >2-fold than the upper limit of normal, patients using statin, niacin, or fibrates at the time of randomization, and patients with an indication for statin therapy per published guidelines were excluded.10 One-hundred and twenty five patients (98 males; aged 57 ± 10 years) were enrolled in the study and randomized in a 1:1 ratio to receive either atorvastatin (80 mg/day) or placebo starting on the first postoperative day and continuing for 3 months following the ablation procedure. Randomization was completed using a simple randomization schedule generated by SAS statistical software (SAS institute, Cary, NC). Only the statistician and pharmacist had access to the randomization schedule until the study was completed. Informed written consent was obtained from each patient and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Mayo Clinic Institutional Review Board.
All subjects underwent standard transthoracic echocardiography on the day prior to the ablation procedure. Measurements were performed according to the recommendations of the American Society of Echocardiography.11 Medical history and anthropometric measures were collected prior to intervention. Presence of hypertension, coronary artery disease, diabetes and obstructive sleep apnea was determined by the presence of clinical diagnosis based on medical records. The CHA2DS2-VASc was calculated assigning 2 points to patients ≥75 years old or those with a previous stroke or transient ischemic attack. A single point is assigned for each of female sex, heart failure, hypertension, age 65 to 74, diabetes, and vascular disease (myocardial infarction, aortic plaque, peripheral artery disease).12 Fasting venous blood samples for C-reactive protein (CRP), total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol and triglycerides were taken before initiation of the therapy and at the 3-month follow-up visit.
To identify the pulmonary vein stenosis, all subjects underwent a CT or magnetic resonance imaging (MRI) scan within 30 days before the intervention and 3 months after the procedure. For the purpose of the current analysis, we reviewed all CT scans of adequate quality to measure EAT. Patients who underwent MRI (n =13) and patients with incomplete CT data were excluded (n =33). Cardiac CT angiography was acquired using a 64-slice CT (Sensation, Siemens Medical Solutions, Erlangen, Germany). ECG-referenced scans were obtained after intravenous administration of contrast material (Omnipaque 350). Acquisitions were conducted on the entire heart area in the head-to-feet direction. Scan parameters were as follows: collimation 64×0.625 mm, gantry rotation time of 0.5 s, tube voltage of 120 kV, and tube current of 850 mA. Because pulmonary vein assessment was the primary clinical goal, 1.5-mm-thick images were reconstructed.
Epicardial fat quantification was conducted by a dedicated offline workstation (Aquarius 3D Workstation, TeraRecon Inc, San Mateo, CA). Image reconstructions were performed at 75% of the R-R interval. The superior heart limit slice was selected at the split of pulmonary artery. The inferior heart boundary was set as the most inferior slice of the myocardium. Epicardial fat was defined as the entire adipose tissue outside of the myocardium enclosed by the visceral pericardium. All epicardial fat measurements were performed by an experienced reader blinded to the randomization status. Pericardial contours were generated by spine interpolation through several controls which were placed manually. When necessary, the reader made manual adjustments through the scan volumes to account for interpolating errors. Fat voxels were identified using threshold attenuation values of −250 to −30 HU.13 The intraclass correlation coefficient (ICC) for intra-rater reliability was 0.99 (95% CI, 0.99–1.0), while ICC for inter-rater reliability was 0.98 (95% CI, 0.98–0.99), thus indicating excellent reproducibility of EAT measurements. Consistent with the protocol followed for assessing EAT on the entire cohort, readers were blinded to the study participant trial arm and to the results of the previous measures.
Normally distributed data are expressed as mean (SD) while median and inter-quartile ranges (IQR) are provided for skewed variables. Categorical variables are presented as percentages. Wilcoxon rank sums tests and Fisher exact tests were used to compare the baseline characteristics of continuous and categorical measures between the study arms (atorvastatin vs placebo), respectively. Changes from pre- to post-treatment in EAT, lipid profile, CRP and body mass index (BMI) within each treatment group were tested by means of Wilcoxon signed rank tests. Between-group comparisons on delta values were performed with Wilcoxon rank sums tests. To examine the relationship between baseline EAT and clinical and laboratory parameters, univariate and multivariate regression analyses were run. Log-transformation was applied to improve normality when needed and the variance inflation factor was calculated to detect multicollinearity. The significance level was set at <0.05 for all tests. The JMP 9.0.3 statistical package was used for data management and analysis.
Results
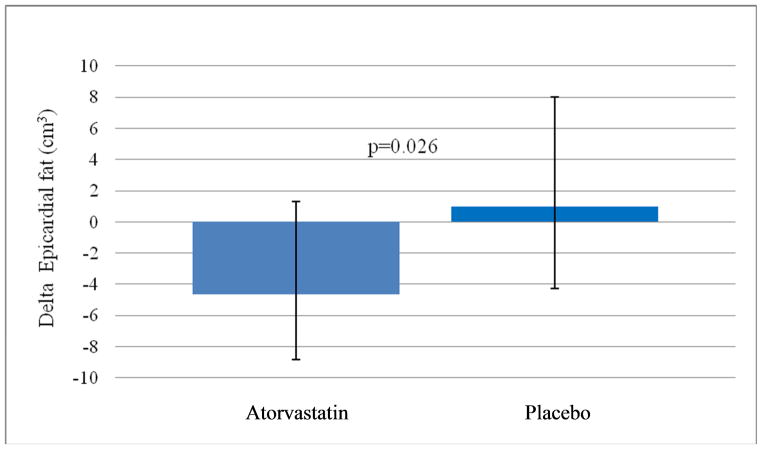
Of the 79 patients included in this study, 38 received atorvastatin and 41 placebo. As summarized in Table 1 and Table 2, there were no significant differences in baseline characteristics between the study groups. As for the entire study cohort,7 atorvastatin treatment did not reduce 3-month AF recurrences in the population subset included in this study (log-rank test for group difference in the survival curves, p = 0.677). Patients randomized to atorvastatin exhibited a significant reduction in median EAT volume after 3 months of therapy [delta −4.6 (−8.9 to 1.3) cm3, p < 0.05] (absolute values are provided in Table 2). Expected decreases in median CRP [delta −0.4 (−1.8 to 0.2) mg/L, p < 0.05], total cholesterol [delta −61 (−77 to −32) mg/dL, p < 0.001], and LDL-cholesterol [delta −55 (−63 to −37) mg/dL, p < 0.001] levels were also observed, while BMI, HDL-cholesterol and triglycerides did not change appreciably. In the placebo group total cholesterol [delta 14 (−5 to 29) mg/dL, p < 0.01] and triglycerides [delta 24 (−13 to 50) mg/dL, p < 0.05] increased significantly, while EAT and other measures remained unchanged. The between group comparison of EAT volume changes is shown in Figure 1. In comparison to patients with paroxysmal AF, patients with persistent AF had significantly higher baseline median EAT volume (100.4(75.1–131.3) vs 81.8(57–108.2) cm3, p < 0.05).
Table 1.
Baseline characteristics in Atorvastatin and Placebo groups.
| Characteristic | Atorvastatin (n=38) | Placebo (n=41) |
|---|---|---|
| Age (years) | 56 ± 11 | 56 ± 10 |
| Men | 32 (84 %) | 33 (81 %) |
| Hypertension | 12 (32 %) | 8 (20 %) |
| Coronary artery disease | 0 | 0 |
| Diabetes mellitus | 1 (3 %) | 0 |
| Obstructive sleep apnea | 10 (26 %) | 6 (15 %) |
| Current smokers | 4 (11 %) | 2 (5 %) |
| Left ventricle ejection fraction (%) | 0.57 ± 0.11 | 0.60 ± 0.07 |
| History of atrial fibrillation (years) | 5.4 ± 4.9 | 4.8 ± 4.2 |
| Left atrium volume index (cc/m2) | 39 (29–43) | 36 (30–41) |
| Paroxysmal atrial fibrillation | 25 (66 %) | 35 (85 %) |
| CHA2DS2-VASc | 1 (0–1.25) | 0 (0–1) |
Table 2.
Body mass index, epicardial adipose tissue and laboratory parameters at baseline and at follow-up.
| Atorvastatin (n=38) | Placebo (n=41) | |||
|---|---|---|---|---|
|
| ||||
| Characteristic | Baseline | Follow-up | Baseline | Follow-up |
| Body mass index (kg/m2) | 28 (26–32) | 27 (27–32) | 28 (25–30) | 28 (26–31) |
| C-reactive protein (mg/L) | 2.4 (0.7–3.7) | 1.1 (0.5–2.7)* | 1.3 (0.7–2.1) | 1.3 (0.5–2.6) |
| Total Cholesterol (mg/dL) | 186 (163–201) | 123 (99–163)‡ | 190 (173–210) | 204 (183–223)† |
| Low-density lipoprotein Cholesterol (mg/dL) | 116 (97–133) | 56 (41–81)‡ | 122 (107–133) | 123 (110–146) |
| High-density lipoprotein Cholesterol (mg/dL) | 45 (37–54) | 48 (37–59) | 47 (38–56) | 46 (39–60) |
| Triglycerides (mg/dL) | 101 (74–150) | 83 (68–122) | 116 (74–144) | 118 (99–175)* |
| Epicardial adipose tissue (cm3) | 92 (62–133) | 87 (64–125)* | 82 (56–111) | 81 (57–111) |
There were no significant differences in baseline characteristics between the two study groups. Within-group comparisons:
p < 0.05;
p < 0.01;
p < 0.001.
Figure 1. Between-group changes in epicardial adipose tissue volume after treatment.
Data are presented as median (IQR). P-value is calculated by Wilcoxon rank sum test.
Correlation analysis showed that baseline EAT volume was associated with BMI, left atrium volume index, HDL-cholesterol, CRP, and triglycerides (Table 3). When a multivariate regression was modeled including statistically significant univariate predictors, only BMI (standardized beta coefficient = 0.302, p < 0.01) and HDL-cholesterol (beta= −0.297, p < 0.01) remained significant and independent determinants of EAT volume (adjusted R2 = 0.39, F = 10.68, p < 0.0001).
Table 3.
Univariate correlation coefficients between baseline epicardial adipose tissue and patient characteristics.
| Variable | Pearson’s r | P-value |
|---|---|---|
| Age | 0.12 | 0.29 |
| History of atrial fibrillation | 0.14 | 0.24 |
| Body mass index | 0.54 | <0.0001 |
| Left atrium volume index | 0.26 | 0.02 |
| C-reactive protein | 0.29 | 0.008 |
| Total Cholesterol | −0.001 | 0.99 |
| Low-density lipoprotein Cholesterol | −0.01 | 0.95 |
| High-density lipoprotein Cholesterol | −0.46 | <0.0001 |
| Triglycerides | 0.33 | 0.003 |
Discussion
Our data show that intensive atorvastatin therapy in AF patients who underwent PVI is associated with significant decreases in EAT in 3 months. Although statins are most well-known for their lipid-lowering effect and site of maximum action in liver, emerging evidence suggestive of potential anti-inflammatory and antioxidant effects indicates that non-liver tissues may be targeted to exert their beneficial metabolic effects.14
The effects of statins on adipose tissue have been addressed in several in-vitro studies.15,16 Khan et al.17 showed a decrease of adipose tissue in statin-treated mice compared to a vehicle-treated group; histologically, a reduction of adipocytes was evident. Krysiak et al.18 documented an association between attenuated adipokine release in human visceral adipose tissue and statin therapy. With regard to clinical studies, Alexopoulos et al.6 compared the effect of low-dose versus high-dose statin therapy on EAT volume in hyperlipidemic post-menopausal women and observed that high-dose atorvastatin therapy for a year significantly decreased EAT volume. Our findings on AF patients are in agreement with these results. In addition, it is noteworthy that we observed a substantial reduction in EAT volume even after a shorter period (3 months) of statin therapy.
Importantly, we demonstrate for the first time, that these effects are present in men as well, and are not specific to post-menopausal women. Furthermore, we can reasonably exclude that the changes in EAT were secondary to variations in weight as we did not see any significant change in BMI in either group over the observed time period. However, we also noted that baseline EAT volume correlated with BMI and HDL-cholesterol levels. This is consistent with prior research demonstrating that EAT thickness is associated with clinical characteristics of the metabolic syndrome, including waist circumference, high blood pressure, and LDL- cholesterol levels, and increases linearly with the increase in the number of metabolic syndrome components.19
The present work has several strengths. First, we selected a relatively healthy cohort of AF patients without any previous indication for statin therapy. Second, unlike other studies on this topic which are mainly cross-sectional, the longitudinal nature of our study enabled us to assess changes in epicardial fat in AF patients following PVI. Nonetheless, some limitations need to be acknowledged. In particular, our study was limited by the relatively small sample consisting of only patients undergoing PVI for AF. Moreover, the modest follow-up period limits generalizability of our observations. Whether our findings can be extended to AF patients without indications for PVI as well as to the general population has yet to be determined. A large, prospective cohort study would allow an investigation of the effects of prolonged statin therapy on EAT and its long-term clinical implications. Also, the low number of female patients in our study cohort precluded us from evaluating gender differences in EAT changes and other variables of interest. In mitigation this allowed us to demonstrate that statin-associated reductions in EAT occur in men and are not limited to women.
Acknowledgments
Dr Soucek and Dr Ruzek were supported by the European Regional Development Fund, Project FNUSA-ICRC [CZ.1.05/1.1.00/02.0123], the European Social Fund, and the State Budget of the Czech Republic. This work was further supported by the National Institutes of Health [Grant R01HL114024].
Footnotes
Disclosures
Dr. Somers has served as a consultant for Resmed, Pricewaterhouse Coopers, Sorin Inc., GlaxoSmithKline, Philips, Rhonda Gray, U-Health and Respicardia. All of the other authors have no potential conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–543. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 2.Sacks HS, Fain JN, Holman B, Chary A, Parks F, Karas J, Optican R, Bahouth SW, Garrett E, Wolf RY, Carter RA, Robbins T, Wolford D, Samaha J. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab. 2009;94:3611–3615. doi: 10.1210/jc.2009-0571. [DOI] [PubMed] [Google Scholar]
- 3.Iacobellis G, Malavazos AE, Corsi MM. Epicardial fat: from the biomolecular aspects to the clinical practice. Int J Biochem Cell Biol. 2011;43:1651–1654. doi: 10.1016/j.biocel.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Brugts JJ, Yetgin T, Hoeks SE, Gotto AM, Shepherd J, Westendorp RG, de Craen AJ, Knopp RH, Nakamura H, Ridker P, van Domburg R, Deckers JW. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ. 2009;338:b2376. doi: 10.1136/bmj.b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 6.Alexopoulos N, Melek BH, Arepalli CD, Hartlage GR, Chen Z, Kim S, Stillman AE, Raggi P. Effect of intensive versus moderate lipid-lowering therapy on epicardial adipose tissue in hyperlipidemic post-menopausal women: a substudy of the BELLES Trial (Beyond Endorsed Lipid Lowering with EBT Scanning) J Am Coll Cardiol. 2013;61:1956–1961. doi: 10.1016/j.jacc.2012.12.051. [DOI] [PubMed] [Google Scholar]
- 7.Suleiman M, Koestler C, Lerman A, Lopez-Jimenez F, Herges R, Hodge D, Bradley D, Cha YM, Brady PA, Munger TM, Asirvatham SJ, Packer DL, Friedman PA. Atorvastatin for prevention of atrial fibrillation recurrence following pulmonary vein isolation: a double-blind, placebo-controlled, randomized trial. Heart Rhythm. 2012;9:172–178. doi: 10.1016/j.hrthm.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL American College of Cardiology/American Heart Association Task Force; European Society of Cardiology Committee for Practice Guidelines; European Heart Rhythm Association; Heart Rhythm Society. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 guidelines for the management of patients with atrial fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;5:114, e257–e354. [Google Scholar]
- 9.European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 10.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 11.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W American Society of Echocardiography’s Nomenclature and Standards Committee; Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography, European Society of Cardiology. . Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 13.Alexopoulos N, McLean DS, Janik M, Arepalli CD, Stillman AE, Raggi P. Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis. 2010;210:150–154. doi: 10.1016/j.atherosclerosis.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 14.Mihos CG, Pineda AM, Santana O. Cardiovascular effects of statins, beyond lipid-lowering properties. Pharmacol Res. 2014;88:12–19. doi: 10.1016/j.phrs.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Nishio E, Tomiyama K, Nakata H, Watanabe Y. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitor impairs cell differentiation in cultured adipogenic cells (3T3-L1) Eur J Pharmacol. 1996;301:203–206. doi: 10.1016/0014-2999(96)00063-5. [DOI] [PubMed] [Google Scholar]
- 16.Tomiyama K, Nishio E, Watanabe Y. Both wortmannin and simvastatin inhibit the adipogenesis in 3T3-L1 cells during the late phase of differentiation. Jpn J Pharmacol. 1999;80:375–378. doi: 10.1254/jjp.80.375. [DOI] [PubMed] [Google Scholar]
- 17.Khan T, Hamilton MP, Mundy DI, Chua SC, Scherer PE. Impact of simvastatin on adipose tissue: pleiotropic effects in vivo. Endocrinology. 2009;150:5262–5272. doi: 10.1210/en.2009-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krysiak R, Labuzek K, Okopien B. Effect of atorvastatin and fenofibric acid on adipokine release from visceral and subcutaneous adipose tissue of patients with mixed dyslipidemia and normolipidemic subjects. Pharmacol Rep. 2009;61:1134–1145. doi: 10.1016/s1734-1140(09)70176-8. [DOI] [PubMed] [Google Scholar]
- 19.Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, Di Mario U, Leonetti F. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88:5163–5168. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]