Abstract
Blood-brain barrier (BBB) is a highly complex physical barrier determining what substances are allowed to enter the brain. Support vector machine (SVM) is a kernel-based machine learning method that is widely used in QSAR study. For a successful SVM model, the kernel parameters for SVM and feature subset selection are the most important factors affecting prediction accuracy. In most studies, they are treated as two independent problems, but it has been proven that they could affect each other. We designed and implemented genetic algorithm (GA) to optimize kernel parameters and feature subset selection for SVM regression and applied it to the BBB penetration prediction. The results show that our GA/SVM model is more accurate than other currently available log BB models. Therefore, to optimize both SVM parameters and feature subset simultaneously with genetic algorithm is a better approach than other methods that treat the two problems separately. Analysis of our log BB model suggests that carboxylic acid group, polar surface area (PSA)/hydrogen-bonding ability, lipophilicity, and molecular charge play important role in BBB penetration. Among those properties relevant to BBB penetration, lipophilicity could enhance the BBB penetration while all the others are negatively correlated with BBB penetration.
1. Introduction
The blood-brain barrier (BBB) plays important roles in separating the central nervous system (CNS) from circulating blood and maintaining brain homeostasis. BBB penetration, which may be desired or not depending on the therapeutic target, is a critical character in chemical toxicological studies and in drug design. Compounds can cross the BBB by passive diffusion or by means of a variety of catalyzed transport systems that can carry compounds into the brain (carrier-mediated transport, receptor-mediated transcytosis) or out of the brain (active efflux). Various parameters are used for predicting BBB penetration such as CNS+/−, logBB, and logPS. CNS+/− is a qualitative property denoting the compound's activity (CNS+) or inactivity (CNS−) against a CNS target with its BBB penetration [1]. The problem with CNS+/− datasets is that CNS activity implies BBB permeation, while CNS inactivity might be due to factors other than nonpermeation, such as the fact that compounds might be rapidly metabolized or effluxed from the brain. Log BB, which is defined as logarithm of Brain/Blood partitioning ratio at steady state [2], is by far the most widely used parameter for BBB penetration. However, this parameter may also result in misleading conclusions because it ignores the main parts of process of permeability [3]. Log PS, which is defined as the logarithm of permeability-surface area product reflecting the rate of brain permeation, is superior to but more difficult to measure compared to logBB [4]. In vivo brain uptake methods may be the most reliable evaluation of BBB penetration. However, the low-throughput, expensive, and labor-intensive characteristics make these methods inapplicable in early drug discovery stages. For these reasons, in vitro and in silico methods have been introduced. As there is no one in vitro model which can mimic all properties of the in vivo BBB, developing more reliable models remains challenging [4]. So far, a great number of in silico BBB models have been developed and thoroughly reviewed [2, 3, 5–9]. Because of the high complex nature of the BBB, most computational models only account for passive diffusion.
Initial studies were focusing on making correlation between BBB permeability of small set of compounds and simple descriptors and then revealed “rules of thumb.” These rough models reflect some important relationships between BBB penetration and properties of compounds but have a problem of oversimplification [10, 11]. As the accumulation of new data, various more sophisticated models were reported to predict BBB permeability. Classification models [12] which were used widely explored for distinguishing between the molecules capable of being across the BBB and those restricted to periphery. These models often developed by using the same dataset of about 1500 drugs compiled by Adenot and Lahana [13], which is the largest single homogeneous up-to-date source of qualitative data published. Some others [12, 14] distinguish molecules based on a certain logBB threshold. However, the main problem is the threshold which is subjectively determined and not unified. Most quantitative models were developed by building QSAR models [10, 15–18]. Since different datasets and validation methods were used, it is difficult to compare the performance of these models [19]. Recently, Carpenter et al. [20] developed a new model predicting the BBB penetration using molecular dynamic simulations and received good results, providing new thread of BBB permeability prediction. Here we focused on logBB models of BBB penetration by passive diffusion.
Various data mining methods have been employed in BBB penetration models, such as multiple linear regression [21, 22], partial least squares (PLS) regression [13, 23], recursive partitioning [23, 24], neural network [25–27], and support vector machine (SVM) [28–30]. SVM, which was originally developed by Vapnik and coworkers [31], has been extensively used and consistently achieves similar or superior performance compared to other machine learning methods [32]. Its main idea is to map data points to a high dimension space with a kernel function, and then these data points can be separated by a hyper plane.
For a successful SVM model, kernel parameters of SVM and feature subset selection are the two most important factors affecting the prediction accuracy. Various strategies have been adopted for the two problems. Grid-based algorithm is one of the most straightforward strategies for parameter optimization, which discretizes the parameters and then systematically searches every grid point to find a best combination of the parameters [33]. However, its use is limited due to the computational complexity and time-consumption. Gradient-based methods [34, 35] are also widely used, which require the kernel function and the scoring function differentiable to assess the performance of the parameters. Evolutionary method [36] has also been used and achieved promising results. As for the feature selection, genetic algorithms- (GA-) based [37–41], F-score based feature recursive elimination [42], and many other methods [43–47] have been employed. Most of these methods focus on feature selection or parameters optimization separately [45]. However, the choice of feature subset influences the appropriate kernel parameters and vice versa [48]. Hence the proper way seems to address the two problems simultaneously. GA [41], immune clonal algorithm (ICA) [49], and Bayesian approach [50] have been recently used for simultaneously feature selection and parameters optimization for SVM on general classification problems. In our study, GA was used to do parameter optimization and feature subset selection simultaneously, and an SVM regression model was developed for the blood-brain barrier penetration prediction.
2. Methods
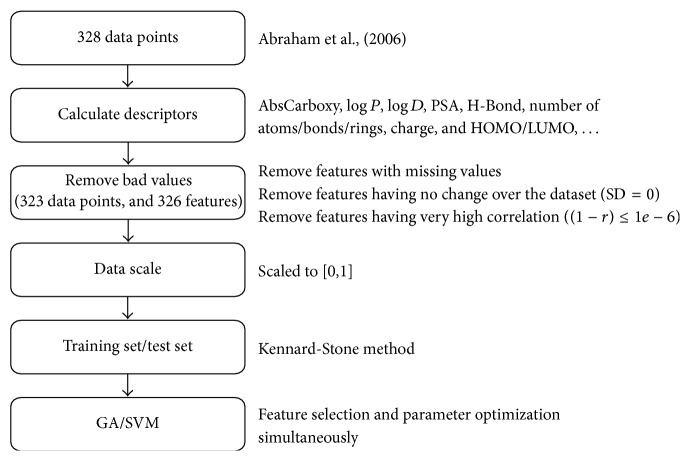
The workflow used in this study for BBB penetration prediction is illustrated in Figure 1.
Figure 1.
Workflow of GA/SVM model for BBB penetration prediction.
2.1. Dataset and Molecular Descriptors
The logBB dataset used in this study was compiled by Abraham et al. [51], which was a combination of both in vivo and in vitro data, including 302 substances (328 data points). Abraham et al. applied linear free energy relationship (LFER) to the dataset and obtained good correlation between logBB values and LFER descriptors plus two indicator variables [51]. CODESSA [52] could not calculate descriptors for the first 5 gases ([Ar], [Kr], [Ne], [Rn], and [Xe]) of the original dataset, and they were excluded from the dataset. The final dataset contained 297 compounds (323 data points). The indicator variables of I v and AbsCarboxy used in Abraham's study [51] were retained in this study. I v was defined as I v = 1 for the in vitro data and I v = 0 for the in vivo data. AbsCarboxy was an indicator for carboxylic acid (AbsCarboxy = 1 for carboxylic acid, otherwise AbsCarboxy = 0).
The initial structures in SMILES format were imported to Marvin [53] and exported in MDL MOL format. AM1 method in AMPAC [54] was used for optimization plus frequencies and thermodynamic properties calculation. The generated output files were used by CODESSA to calculate a large number of constitutional, topological, geometrical, electrostatic, quantum-chemical, and thermodynamic descriptors. Marvin was also used to calculate some physicochemical properties of the compound, including logP, logD, polar surface area (PSA), polarizability, and refractivity. All these descriptors and properties were used as candidate features in later modeling.
Features with missing values or having no change across the data set were removed. If the correlation coefficient of two features is higher than a specified cutoff value (0.999999 used here), then one of them is randomly chosen and removed. The cutoff value used here is very high because very high variable correlation does not mean absence of variable complementarity [55]. A total number of 326 descriptors were left for further analysis. However, many highly correlated features have very similar physicochemical meanings. In our final analysis, similar features were put together by their physicochemical meaning, which we hope could unveil some underlying molecular properties that determine the BBB penetration.
The dataset was then split into training set and test set using the Kennard-Stone method [56], which selects a subset of representative data points uniformly distributed in the sample space [57]. At start, the Kennard-Stone method chooses the data point that is the closest to the center of the dataset measured by Euclidean distance. After that, from all remaining data points, the data point that is the furthest from those already selected is added to the training set. This process continues until the size of the training set reaches specified size. 260 data points were selected as training set and the other 63 were used as test set.
2.2. SVM Regression
Details about SVM regression can be found in literatures [58–60]. As in other multivariate statistical models, the performance of SVM regression depends on the combination of several parameters. In general, C is a regularization parameter that controls the tradeoff between training error and model complexity. If C is too large, the model will have a high penalty for nonseparable points and may store too many support vectors and get overfitting. If it is too small, the model may have underfitting. Parameter ε controls the width of the ε-insensitive zone, used to fit the training data. The value of ε can affect the number of the support vectors used to construct the regression function. The bigger ε is, the fewer support vectors are selected. On the other hand, bigger ε-values result in more flat estimates. Hence, both C and ε-values affect model complexity (but in a different way). The kernel type is another important parameter. In SVM regression, radial basis function (RBF) (1) was the most commonly used kernel function for its better generalization ability, less number of parameters, and less numerical difficulties [33] and was used in this study. Parameter γ in RBF controls the amplitude of the RBF kernel and therefore controls the generalization ability of SVM regression. The LIBSVM package (version 2.81) [61] was used in this study for SVM regression calculation, taking the form
| (1) |
where x i and x j are training vectors (i ≠ j, x i ≠ x j) and γ is kernel parameter.
2.3. Genetic Algorithms
Genetic algorithms (GA) [41] are stochastic optimization and search method that mimics biological evolution as a problem-solving strategy. They are very flexible and attractive for optimization problems.
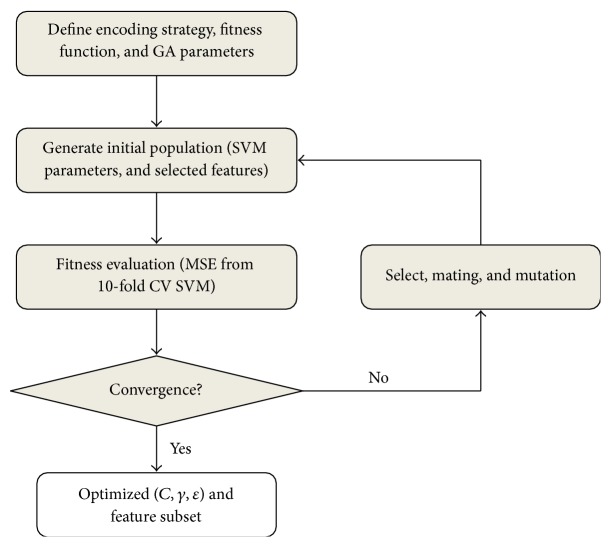
Given a specific problem to solve, the input to the GA is a set of potential solutions to that problem, encoded in some fashion, and a fitness function that allows each candidate to be quantitatively evaluated (Figure 2). Selection, mating, and mutation just mimic the natural process. For each generation, individuals are selected for reproduction according to their fitness values. Favorable individuals have a better chance to be selected for reproduction and the offspring have chance to mutate to keep diversity, while the unfavorable individuals are less likely to survive. After each generation, whether the evolution is converged or the termination criteria are met is checked; if yes, job is done; if not, the evolution goes into next generation. After many generations, good individuals will dominate the population, and we will get solutions that are good enough for our problem.
Figure 2.
Workflow of genetic algorithms.
First, in order to solve a problem with GA, each individual in the population should be represented by a chromosome. In our study, since the parameter optimization and feature subset selection should be addressed simultaneously, the chromosome is a combination of parameter genes and feature gene (Figure 3), where f n is an integer in the range of [1, N] and N is the number of candidate features for model construction. A chromosome represents an individual in genetic algorithms and parameters contained in chromosome could be used for SVM modeling. Left part of the chromosome is the parameter genes, of which C, γ, and ε all are float genes. The feature gene is an array of integers, and each integer represents a feature.
Figure 3.
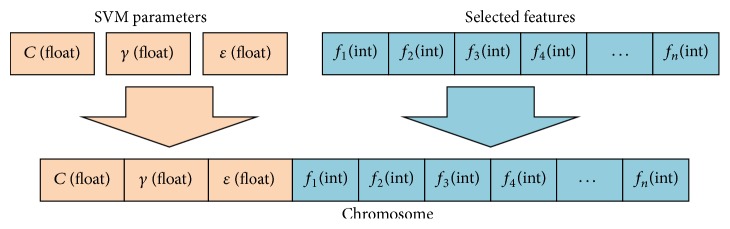
Encoding of the chromosome.
Fitness function can be seen as a ruler, which was used to quantitatively evaluate and compare each candidate. In our study, the mean squared error (MSE) of 10-fold cross validation (CV) for SVM was used as fitness function, and smaller fitness value indicated better individual. Given a training set containing n compounds, (x 1, y 1),…, (x n, y n), x i is descriptor vector of compound i and x i ∈ R n. y i is the logBB value and y i ∈ {−1, +1}. The objective function can be calculated by
| (2) |
α i and α i ∗ are Lagronia factors, K(x i, x) is kernel of radial basis function. The MSE of 10-fold CV for SVM was calculated by
| (3) |
where n is the number of all data points, is the predicted value, and X i is the experiment value.
Tournament selection was used as the selection strategy in GA, which selected the best 1 from 3 randomly chosen candidates. The advantage of tournament selection over roulette wheel selection is that tournament selection does not need to sort the whole population by fitness value.
Since there are different types of genes in a chromosome, different mating strategies were used for different types of genes (Figure 4):
| (4) |
where β uniformly distributed random number on the interval [−0.25,1.25], p n is the value of parent gene, and V new is the value of child gene.
Figure 4.
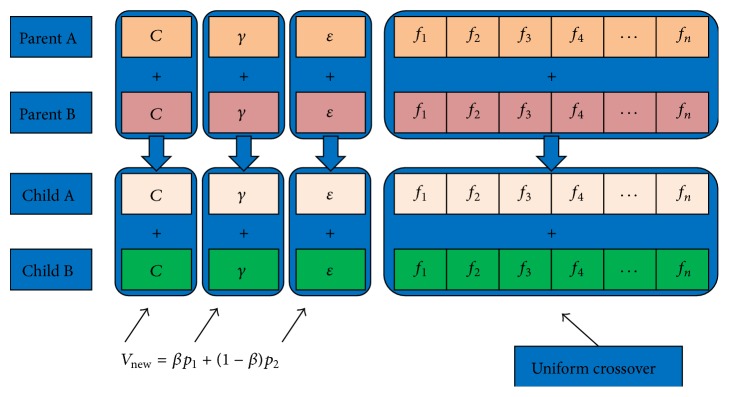
Mating strategy of GA.
For float genes, the new value is a linear combination of the parents (4). For feature gene, uniform crossover is used: each element of the child gene is selected randomly from the corresponding items of parents.
Again, different mutation strategies were used for different types of genes. For float genes, the values were randomly mutated upward or downward. The new value was given by
| (5) |
where β was a random number distributed in [0, 1], V and V new are values before and after mutation, and V min and V max are the minimum and maximum values allowed for a gene.
For feature gene, several points were first randomly chosen for mutation, and then a random number in [1, N] (N is the total number of features) was chosen as new feature while avoiding duplicate features. The GA was terminated when the evolution reached 1000 generations. In our pilot study (data not shown), 1000 generations were enough for the GA to converge. The other parameters for GA were as follows: population size 100, cross rate 0.8, mutation rate 0.1, elite size 2, and number of new individuals in each generation 8.
3. Results and Discussion
3.1. GA/SVM Performance
GA was run with different number of features from 4 to 15. For each number of features, GA was run 50 times, and the best model was chosen for further analysis. From Table 1 and Figure 5, the overall trend of the GA showed the following: (1) the accuracy of the model increased with the number of the features; (2) the accuracy of the model on training set was better than the accuracy on the test set, which was then better than the accuracy of cross validation.
Table 1.
Performance comparison of models with different number of features.
| Number of features | Training (CV = 10) | Prediction/r 2 | Parameters of SVM | ||||
|---|---|---|---|---|---|---|---|
| MSE | r 2 | Test set | Training set | C | γ | ε | |
| 4 | 0.1197 | 0.674 | 0.722 | 0.740 | 38.8833 | 0.6081 | 0.1491 |
| 5 | 0.1042 | 0.715 | 0.770 | 0.805 | 16.3419 | 0.7973 | 0.2743 |
| 6 | 0.0945 | 0.744 | 0.840 | 0.829 | 13.3573 | 0.7158 | 0.1513 |
| 7 | 0.0959 | 0.74 | 0.821 | 0.843 | 34.3067 | 0.5218 | 0.1595 |
| 8 | 0.0883 | 0.761 | 0.834 | 0.883 | 60.9596 | 0.5871 | 0.2357 |
| 9 | 0.0815 | 0.777 | 0.847 | 0.864 | 3.7770 | 0.8764 | 0.1663 |
| 10 | 0.0823 | 0.776 | 0.858 | 0.903 | 15.2236 | 0.6247 | 0.1434 |
| 11 | 0.0714 | 0.804 | 0.861 | 0.891 | 5.6937 | 0.6531 | 0.1573 |
| 12 | 0.0780 | 0.787 | 0.864 | 0.905 | 7.2787 | 0.7428 | 0.1515 |
| 13 | 0.0817 | 0.778 | 0.862 | 0.922 | 4.1957 | 0.7791 | 0.1574 |
| 14 | 0.0812 | 0.778 | 0.882 | 0.917 | 14.8391 | 0.5002 | 0.2054 |
| 15 | 0.0734 | 0.799 | 0.870 | 0.919 | 4.9915 | 0.5231 | 0.1077 |
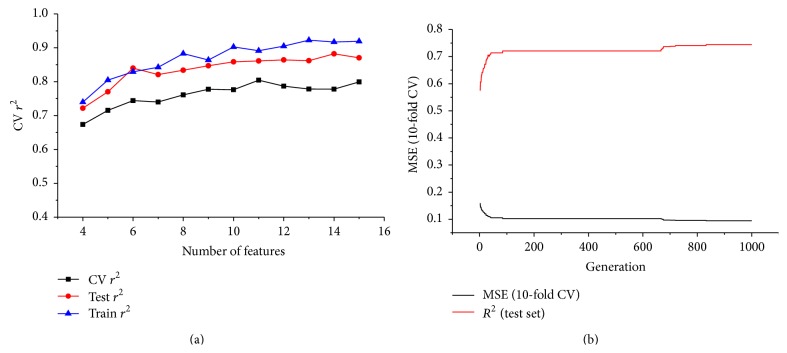
Figure 5.
(a) Performance comparison of models with different number of features. (b) Evolution of the best 6-feature model.
As the feature number increases, the complexity also increases, which will often increase the probability of overfitting. A complex model is also difficult to interpret and apply in practical use, so generally speaking, we need to find a balance between the accuracy and complexity of the model. It is observed that (Table 1, Figure 5(a)) the prediction accuracy (r 2 = 0.744) of cross validation (n = 10) of the 6-feature model was similar to that of the Abraham's model (r 2 = 0.75) which used all 328 data points (Table 2). As the number of features increased from 6 to 15, the prediction accuracy on training set increased from 0.829 to 0.919, while the prediction accuracy on test set only slightly increased from 0.840 to 0.870 accordingly. Take all these into consideration, the 6-feature model (Table 1, Figure 5(a)) was chosen as our final model (Figure 6), of which the prediction accuracy on both test set and training set was similar (r train 2 − r test 2 = 0.11) and high enough (>0.82).
Table 2.
Comparison of most relevant QSAR studies on BBB permeability.
| Descriptors | N train | N test | Methods | r train 2 | Predictive accuracy on test set | Reference |
|---|---|---|---|---|---|---|
| Δlop P, logP,and logP cyc | 20 | — | Linear Regression | 0.69 | — | Young et al. [77] |
| Excess molar refraction, dipolarity/polarisability, H-bond acidity, and basicity Solute McGowan volume |
148 | 30 | LFER | 0.75 | r test 2 = 0.73 | Platts et al. [66] |
| ΔG W° | 55 | — | Linear Regression | 0.82 | — | Lombardo et al. [78] |
| PSA, the octanol/water partition coefficient, and the conformational flexibility | 56 | 7 | MLR | 0.85 | r test 2 = 0.80 | Iyer et al. [79] |
| CODESSA/DRAGON (482) | 200 | 110 | PLS SVM |
0.83 0.97 |
r
test
2 = 0.81 r test 2 = 0.96 |
Golmohammadi et al. [62] |
| Molecular (CODESSA-PRO) descriptors (5) | 113 | 19 | MLR | 0.78 | r test 2 = 0.77 | Katritzky et al. [15] |
| Molecular fragment (ISIDA) descriptors | 112 | 19 | MLR | 0.90 | r test 2 = 0.83 | Katritzky et al. [15] |
| PSA, logP, the number of H-bond acceptors, E-state, and VSA | 144 | 10 | Combinatorial QSAR (KNN SVM) |
0.91 | r test 2 = 0.8 | Zhang et al. [17] |
| Abraham solute descriptors and indicators | 328 | — | LFER | 0.75 | — | Abraham et al. [51] |
| Abraham solute descriptors and indicators | 164 | 164 | LFER | 0.71 | s = 0.25, MAE = 0.20 | Abraham et al. [51] |
| CODESSA/Marvin/indicator (6) | 260 | 63 | GA based SVM | 0.83 | r test 2 = 0.84, RMSE = 0.23 | This research, GA/SVM, final model C = 13.3573, γ = 0.715761, ε = 0.151289 |
| CODESSA/Marvin/indicator (236) | 260 | 63 | GA based SVM | 0.97 | r test 2 = 0.55, RMSE = 0.31 | This research, Grid/SVM C = 8.0, γ = 0.015625, ε = 0.0625 |
| CODESSA/Marvin/indicator (6) | 260 | 63 | GA based SVM | 0.86 | r test 2 = 0.58, RMSE = 0.29 | This research, Grid/SVM C = 8.0, γ = 1.0, ε = 0.125 |
Figure 6.
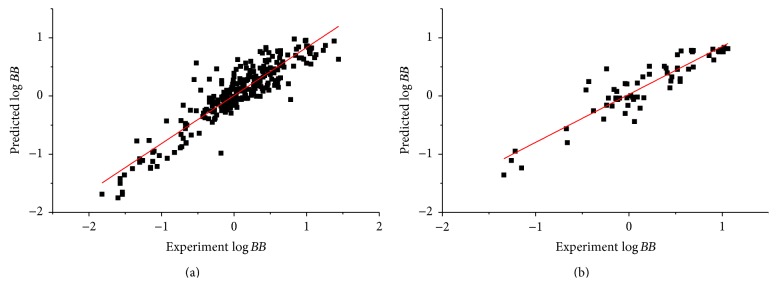
Prediction accuracy of the final model on training set (a) and test set (b).
Figure 5(b) showed the evolution of the prediction performance of the model (MSE and r test 2). In the first 100 generations, the MSE decreased very fast, followed with a platform stage from about 100 to 650 generations. Another decrease occurred at about 650 generations, and then the evolution became stable at about 850 generations. The models did not improve much in the last 150 generations, which may imply a convergence.
A tabular presentation of relevant studies regarding the prediction of the blood-brain distribution is shown in Table 2. These models were constructed by using different statistical learning methods, yielding different prediction capability with R test 2 ranging from 0.5 to over 0.9. Generally, regression by SVM appears to be more robust than traditional linear approaches such as PLS and MLR, with respect to the nonlinear effects induced by multiple potentially cooperative factors governing the BBB permeability. For example, the SVM model by Golmohammadi et al. [62] yielded the highest R test 2 on a test set containing 110 molecules. However, it should be noted that direct comparison with results from previous studies is usually inappropriate because of differences in their datasets. In this study, a combination of both in vivo and in vitro data compiled by Abraham et al. [51] was used for developing BBB prediction model, which is of high data quality and covers large chemical diversity space. In addition to the data source, kernel parameter optimization and feature selection are two crucial factors influencing the prediction accuracy of SVM models. To reduce the computational cost, most of the existing models addressed the feature selection and parameter optimization procedures separately. In this study, we used a GA scheme to perform the kernel parameter optimization and feature selection simultaneously, which is more efficient at searching the optimal feature subset space.
Abraham's model [51] is the best model that is currently publicly available. A comparison of our models with Abraham's models was shown in Table 2. In Table 2, the last 3 rows are models in our study. The same dataset was used in Abraham's research and this study, but the data set was split into different training set and test set (our model: train/test = 260/63; Abraham's model: training/test = 164/164). 7 variables were used in Abraham's model, compared to 6 in our final model. The r 2 values for training set in Abraham's 164/164 model and 328/0 model were 0.71 and 0.75, respectively, compared with 0.83 for our model. It has to be noted that the size of our training set (260) is bigger than Abraham's (164).
Our model was also compared with grid method implemented with Python toolkit (grid.py) shipped with libsvm [61] for parameter optimization. First, since grid method cannot be used to select feature subset, all 326 features were used to construct a BBB prediction model. The prediction accuracy of the training set was very high (r train 2 = 0.97) but that of the test set was disappointing (r test 2 = 0.55). Then we used the same feature set as our final model (6 features). The result was slightly better, but still too bad for test set prediction (r train 2 = 0.86, r test 2 = 0.58).
So compared with the grid-based method, our GA-based method could get better accuracy with fewer features, which suggested that GA could get much better combination of parameters and feature subset. This was also observed in other's study [48].
3.2. Feature Analysis
An examination of the descriptors used in the model could provide an insight into the molecular properties that are most relevant to BBB penetration. Table 3 showed the features used in the final 6-feature model and their meanings. In order to explore the relative importance and the underlying molecular properties of the descriptors, the most frequently used features in all 50 6-feature models were analyzed. Table 4 showed the top 10 most frequently used features. Interestingly, AbsCarboxy, an indicator of the existence of carboxylic acid was the most significant property. Some features with similar meaning were also found to occur in the models, such as PSA related features (M_PSA_7.4, M_PSA_7.0, and M_PSA_6.5), and H-bond related descriptors (number 267, 268, and 138). So we decided to put similar descriptors into the same group (Figure 7) to find the underlying properties affecting BBB penetration.
Table 3.
Features used in the final model.
| Name | Meaning |
|---|---|
| M_logP | logP (Marvin) |
| HA_dependent_HDSA-2_[Zefirov's_PC] | H-bond donor surface area related (CODESSA) |
| M_PSA_7.4 | PSA at pH 7.4 (Marvin) |
| AbsCarboxy | Carboxylic acid indicator (Abraham) |
| HA_dependent_HDCA-2/SQRT(TMSA)_[Zefirov's_PC] | H-bond donor charged area related (CODESSA) |
| Average_Complementary_Information_content_(order_0) | Topology descriptor (CODESSA) |
Table 4.
The most frequently used features for all 6-feature modelsa.
| Number | Feature name | Occurrence (50 models) |
Meaning |
|---|---|---|---|
| 11 | AbsCarboxy | 36 | Indicator for carboxylic acid† |
|
| |||
| 268 | ESP-FHASA_Fractional_HASA_(HASA/TMSA)_Quantum-Chemical_PC | 14 | H-acceptor surface area/total molecular surface area# |
|
| |||
| 101 | Topographic_electronic_index_(all_bonds)_Zefirov's_PC | 12 | Topological electronic index for all bonded pairs of atomsb‡ |
|
| |||
| 8 | M_PSA_7.4 | 11 | PSA at pH 7.4c§ |
|
| |||
| 267 | ESP-HASA_H-acceptors_surface_area_Quantum-Chemical_PC | 10 | H-acceptor surface area# |
|
| |||
| 5 | delta_logD | 9 | logD (pH 6.5) − logD (pH 7.4)d,e∧ |
|
| |||
| 7 | M_PSA_7.0 | 9 | PSA at pH 7.0§ |
|
| |||
| 138 | HA_dependent_HDCA-2_[Zefirov's_PC] | 9 | H-donors charged surface area# |
|
| |||
| 6 | M_PSA_6.5 | 8 | PSA at pH 6.5§ |
|
| |||
| 1 | M_logP | 7 | logP ∧ |
aRows with the same symbol could be categorized into the same group.
bTopological electronic index is a feature to characterize the distribution of molecular charge: T = ∑(i<j) NB(|q i − q j|/r 2 ij), where q i is net charge on ith atom and r ij is the distance between two bonded atoms.
c7.4 is the pH in blood.
d6.5 is the pH in intestine.
e D is the ratio of the sum of the concentrations of all species of a compound in octanol to the sum of the concentrations of all species of the compound in water. For neutral compounds, logD is equal to logP.
Figure 7.
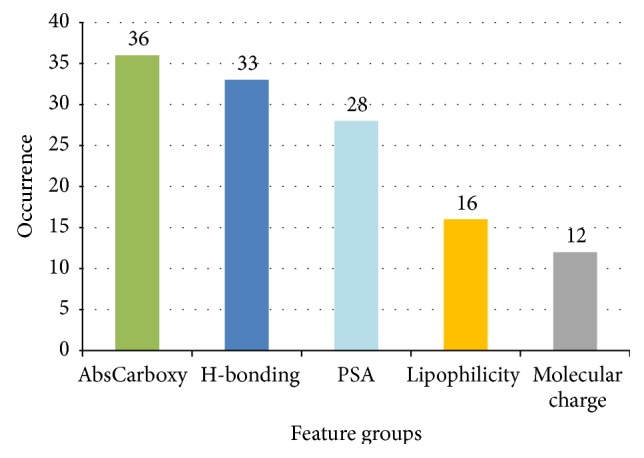
Top features for all 6-feature models (50 in all).
According to the associated molecular properties of the features, those frequently used features are categorized into 5 groups: AbsCarboxy (indicator of carboxylic acid), H-bonding (H-bonding ability, including H-bond donor/acceptor related features), PSA (molecular polar surface area related features), lipophilicity (including M_logP and delta_logD), and molecular charge (including charge and topological electronic index related features).
The following is observed:
-
(1)
Interestingly, AbsCarboxy was also the most significant feature, which occurred 36 times in total 50 models. This may indicate that the carboxylic acid group plays an important role in the BBB penetration, which is consistent with the study of Abraham et al. [51].
-
(2)
H-bonding (H-bond donor/acceptor) related surface area, polar surface area, logP related features, and topological electronic index related features are also significant.
In order to further confirm the previous finding, all top 10 models from features = 4 to 15 were further analyzed, and the result was almost the same (Table 5, Figure 8). The top 10 most frequently used feature sets were very similar. Compared to the 6-feature models, the only difference was that H-bond related feature number 267 is replaced with PSA related feature number 167.
Table 5.
Most frequently used features for all top models (number of features range from 4 to 15)∗.
| Number | Descriptor name | Occurrence (120 models) |
Meaning |
|---|---|---|---|
| 11 | AbsCarboxy | 84 | Indicator for carboxylic acid† |
|
| |||
| 5 | delta_logD | 35 | logD (pH 6.5) − logD (pH 7.4)∧ |
|
| |||
| 7 | M_PSA_7.0 | 32 | PSA at pH 7.0§ |
|
| |||
| 1 | M_logP | 28 | logP ∧ |
|
| |||
| 138 | HA_dependent_HDCA-2_[Zefirov's_PC] | 27 | H-donors charged surface area# |
|
| |||
| 268 | ESP-FHASA_Fractional_HASA_(HASA/TMSA)_Quantum-Chemical_PC | 27 | H-acceptor surface area/total molecular surface area# |
|
| |||
| 6 | M_PSA_6.5 | 25 | PSA at pH 6.5§ |
|
| |||
| 167 | PPSA-1_Partial_positive_surface_area_[Quantum-Chemical_PC] | 25 | Partial positive surface area§ |
|
| |||
| 101 | Topographic_electronic_index_(all_bonds)_Zefirov's_PC | 23 | Topological electronic index for all bonded pairs of atoms‡ |
|
| |||
| 8 | M_PSA_7.4 | 21 | PSA at pH 7.4§ |
∗Rows with the same symbol could be categorized into the same group.
Figure 8.
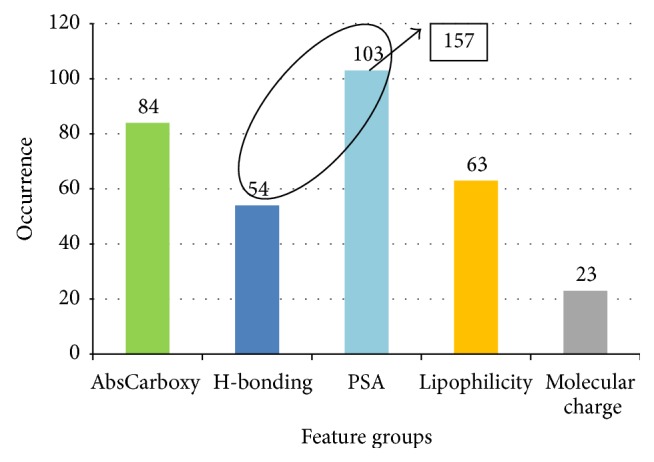
The most frequently used features for all top models.
Again, the descriptors were analyzed by group. The composites of the groups were almost the same. Given that there are 4 descriptors in PSA group, AbsCarboxy was also the most significant property, followed by PSA, lipophilicity, and H-bonding having similar occurences, then followed by molecular charge with relatively low frequencies. The high consistency suggested that these groups of features had signficant impact on the BBB penetration ability.
PSA and H-bonding descriptors are highly relevant properties: PSA is the molecular areas contributed by polar atoms (nitrogen, sulphur, oxygen, and phosphorus), and most of the time these polar atoms can be H-bond acceptor or donor. If PSA and H-bonding were merged into one group (n = 157), they will become the most significant property group of features.
3.3. Properties Relevant to BBB Penetration
3.3.1. Carboxylic Acid Group
It was proposed by Abraham et al. [51] that carboxylic acid group played an important role in BBB penetration. While it was commonly believed that the most important molecular properties related to BBB penetration were H-bonding ability, lipophilicity, and molecular charge [63, 64]. However, our study confirmed Abraham's conclusion and showed that the importance of carboxylic acid group in BBB penetration could be underestimated.
In the models by Abraham et al. [51], the indicator variable of carboxylic acid group has the largest negative coefficient, indicating its importance in BBB penetration, and is consistent with observations in our model that the indicator of carboxylic acid group is the most frequently used descriptor. Zhao et al. [23] tried to classify compounds into BBB positive or BBB negative groups using H-bonding related descriptors, and the indicator of carboxylic acid group was also found to be important in their model. Furthermore, our results are consistent with the fact that basic molecules have a better BBB penetration than the acid molecules [10].
The carboxylic acid group may affect the BBB penetration through molecular charge interactions since in most cases the carboxylic acid group will exist in the ionized form carrying a negative charge. The carboxylic acid group could also affect the BBB penetration by forming H-bond with BBB and hence weaken the BBB penetration abilities of molecules.
Abraham et al. suggested that the presence of carboxylic acid group which acted to hinder BBB penetration was not only simply due to the intrinsic hydrogen bonding and polarity properties of neutral acids [51]. There were some other ways in which the carboxylic acid groups could affect the BBB penetration, such as acidic drugs which could bind to albumin [65], the ionization of the carboxylic acid groups which could increase the excess molar refraction and hydrogen bonding basicity, and finally the carboxylic acid groups which may be removed from brain by some efflux mechanism [66].
3.3.2. Polar Surface Area and H-Bonding Ability
As pointed out in our previous analysis, PSA and H-bonding ability are actually two highly correlated properties. If these two groups are merged, they will be the most significant group of properties, even more significant than the carboxylic acid indicator (Figure 8). Norinder and Haeberlein [6] concluded that hydrogen bonding term is a cornerstone in BBB penetration prediction. In Zhao et al.'s study [23], PSA, AbsCarboxy, number of H-bonding donors, and positively charged form fraction at pH 7.4 were all treated as H-bonding descriptors and were found to be important in the final model.
Furthermore, almost all published models make use of molecular polar and/or H-bonding ability related descriptors, such as PSA [23, 67, 68], high-charged PSA [22], number of hydrogen donors and acceptors [23, 69], and hydrogen bond acidity/basicity [23]. And, in these models, PSA and/or H-bond ability are all negatively correlated with logBB, which is in agreement with Abraham et al.'s study [51] in which the coefficients of the H-bond acidity and H-bond basicity are both negative.
After review of many previous works, Norinder and Haeberlein [6] proposed that if the sum of number of nitrogen and oxygen atoms (N + O) in a molecule was five or less, it had a high chance of entering the brain. As we all know, nitrogen and oxygen atoms have great impact on PSA and H-bonding. Norinder and Haeberlein [6] also concluded that BBB penetration could be increased by lowering the overall hydrogen bonding ability of a compound, such as by encouraging intramolecular hydrogen bonding. After an analysis of the CNS activity of 125 marketed drugs, van de Waterbeemd et al. [70] suggested that the upper limit for PSA in a molecule that is desired to penetrate the brain should be around 90 Å2, while Kelder et al. [71] analyzed the PSA distribution of 776 orally administered CNS drugs that have reached at least phase II studies and suggested that the upper limit should be 60–70 Å2.
Having in mind that molecules mainly cross the BBB by passive diffusion, we think it may be because molecules with strong H-bonding ability have a greater tendency to form H-bonds with the polar environment (the blood), hence weakening their ability to cross the BBB by passive diffusion.
3.3.3. Lipophilicity
Lipophilicity is another property widely recognized as being important in BBB penetration, and most of the current models utilize features related to lipophilicity [22, 64, 67, 72]. Lipophilicity was thought to be positively correlated with logBB; that is, increase the lipophilicity of a molecule will increase the BBB penetration of the molecule. Norinder and Haeberlein [6] also proposed that if logP − (N + O) > 0, then logBB was positive. Van de Waterbeemd et al. [70] suggested that the logD of the molecule should be in [1, 3] for good BBB penetration. These observations are consistent with the fact that the lipid bilayer is lipophilic in nature, and lipophilic molecules could cross the BBB and get into the brain more easily than hydrophobic molecules.
3.4. Molecular Charge
From the viewpoint of computational chemistry, the distribution of molecular charge is a very important property that affects the molecule properties greatly. It is the uncharged form that can pass the BBB by passive diffusion. Fischer et al. [73] have shown that acid molecules with pK a < 4 and basic molecules with pK a > 10 could not cross the BBB by passive diffusion. Under physiological conditions (pH = 7.4), acid molecules with pK a < 4 and basic molecules with pK a > 10 will be ionized completely and carry net charges. As mentioned in our previous analysis, the carboxylic acid group may affect the BBB penetration through molecular charge interactions. Mahar Doan et al. [74] compared physicochemical properties of 93 CNS (n = 48) and non-CNS (n = 45) drugs and showed that 0 of 48 CNS drugs have a negative charge and CNS drugs tend to have less positive charge. These are reasonably consistent with the study of Abraham et al. [51] in which the coefficient of the carboxylic acid indicator is negative.
It has to be noted that, all these molecular properties are not independent, and they are related to each other. For example, the carboxylic acid group is related to both PSA and H-bonding ability, for the O atom in the carboxylic acid group is a polar atom and has a strong ability to form H-bonds; the carboxylic acid group, which carries charge under most conditions, is also related to molecular charge. The PSA/H-bonding ability is also correlated to molecular charge, because in many cases atoms could contribute to PSA or form H-bonds which could probably carry charges. Lipophilicity is also related to molecular charge.
We can get a conclusion that the most important properties for a molecule to penetrate BBB are carboxylic acid group, PSA/H-bonding ability, lipophilicity, and charge. BBB penetration is positively correlated with the lipophilicity and negatively correlated with the other three properties. A comparison of the physicochemical properties of 48 CNS drugs and 45 non-CNS suggested that compared to non-CNS drugs, CNS drugs tend to be more lipophilic and more rigid and have fewer hydrogen-bond donors, fewer charges, and lower PSA (<80 Å2) [74], which is in reasonable consistency with our finding except that the molecular flexibility is not important in our model.
There are some other properties utilized in some existing models, such as molecular weight, molecular shape, and molecular flexibility. It is suggested by van de Waterbeemd et al. [70] that molecular weight should be less than 450 for good BBB penetration, while Hou and Xu [22] suggested that the influence of molecular bulkiness would be obvious when the size of the molecule was larger than a threshold and found that molecular weight made a negative contribution to the BBB penetration when the molecular weight is greater 360. This is not widely observed in other studies. In Zhao et al.'s study [23], molecular weight was found to be not important compared to hydrogen bond properties.
Lobell et al. [68] proposed that spherical shapes have a small advantage compared with rod-like shapes with regard to BBB penetration, they attributed this to the membranes that are largely made from rod-shaped molecules and rod-like shape may become more easily trapped within membrane without exiting into the brain compartment. However, in Rose et al.'s model [75] based on electrotopological state descriptors showed that BBB penetration increased with less sketch branching. Crivori et al. [76] tried to correlate descriptors derived from 3D molecular fields and BBB penetration and concluded that the size and shape descriptors had no marked impact on BBB penetration.
Iyer et al. [67] found that increasing the solute conformational flexibility would increase logBB, while in the study of Mahar Doan et al. [74], CNS drugs tend to be more rigid. However, the roles of molecular weight, molecular shape, and molecular flexibility in BBB penetration seem to be still unclear and not well received. Further studies are still needed.
4. Conclusion
In this study, we have developed a GA/SVM model for the BBB penetration prediction, which utilized GA to do kernel parameters optimization and feature selection simultaneously for SVM regression. The results showed that our method could get better performance than addressing the two problems separately. The same GA/SVM method can be extended to be used on other QSAR modeling applications.
In addition, the most important properties (carboxylic acid group, PSA/H-bond ability, lipophilicity, and molecular charge) governing the BBB penetration were illustrated through analyzing the SVM model. The carboxylic acid group and PSA/H-bond ability have the strongest effect. The existence of carboxylic acid group (AbsCarboxy), PSA/H-bonding and molecular charge is all negatively correlated with BBB penetration ability, while the lipophilicity enhances the BBB penetration ability.
The BBB penetration is a highly complex process and is a result of many cooperative effects. In order to clarify the factors that affect the BBB penetration, further efforts are needed to investigate the mechanistic nature of the BBB, and, as pointed out by Goodwin and Clark [7], the most fundamental need is for more high quality data, both in vivo and in vitro, upon which the next generation of predictive model can be built.
Acknowledgments
The authors gratefully acknowledge financial support from the Hi-Tech Research and Development Program of China (Grant 2014AA01A302 to Mingyue Zheng and Grant 2012AA020308 to Xiaomin Luo), National Natural Science Foundation of China (Grants 21210003 and 81230076 to Hualiang Jiang and Grant 81430084 to Kaixian Chen), and National S&T Major Project (Grant 2012ZX09301-001-002 to Xiaomin Luo).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Daqing Zhang, Jianfeng Xiao, and Nannan Zhou contributed equally to this work.
References
- 1.Lanevskij K., Dapkunas J., Juska L., Japertas P., Didziapetris R. QSAR analysis of blood-brain distribution: the influence of plasma and brain tissue binding. Journal of Pharmaceutical Sciences. 2011;100(6):2147–2160. doi: 10.1002/jps.22442. [DOI] [PubMed] [Google Scholar]
- 2.Lanevskij K., Japertas P., Didziapetris R. Improving the prediction of drug disposition in the brain. Expert Opinion on Drug Metabolism & Toxicology. 2013;9(4):473–486. doi: 10.1517/17425255.2013.754423. [DOI] [PubMed] [Google Scholar]
- 3.Mehdipour A. R., Hamidi M. Brain drug targeting: a computational approach for overcoming blood-brain barrier. Drug Discovery Today. 2009;14(21-22):1030–1036. doi: 10.1016/j.drudis.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Bicker J., Alves G., Fortuna A., Falcão A. Blood-brain barrier models and their relevance for a successful development of CNS drug delivery systems: a review. European Journal of Pharmaceutics and Biopharmaceutics. 2014;87(3):409–432. doi: 10.1016/j.ejpb.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Nagpal K., Singh S. K., Mishra D. N. Drug targeting to brain: a systematic approach to study the factors, parameters and approaches for prediction of permeability of drugs across BBB. Expert Opinion on Drug Delivery. 2013;10(7):927–955. doi: 10.1517/17425247.2013.762354. [DOI] [PubMed] [Google Scholar]
- 6.Norinder U., Haeberlein M. Computational approaches to the prediction of the blood-brain distribution. Advanced Drug Delivery Reviews. 2002;54(3):291–313. doi: 10.1016/S0169-409X(02)00005-4. [DOI] [PubMed] [Google Scholar]
- 7.Goodwin J. T., Clark D. E. In silico predictions of blood-brain barrier penetration: considerations to ‘keep in mind’. Journal of Pharmacology and Experimental Therapeutics. 2005;315(2):477–483. doi: 10.1124/jpet.104.075705. [DOI] [PubMed] [Google Scholar]
- 8.Nicolazzo J. A., Charman S. A., Charman W. N. Methods to assess drug permeability across the blood-brain barrier. Journal of Pharmacy and Pharmacology. 2006;58(3):281–293. doi: 10.1211/jpp.58.3.0001. [DOI] [PubMed] [Google Scholar]
- 9.Di L., Kerns E. H., Carter G. T. Strategies to assess blood-brain barrier penetration. Expert Opinion on Drug Discovery. 2008;3(6):677–687. doi: 10.1517/17460441.3.6.677. [DOI] [PubMed] [Google Scholar]
- 10.Fan Y., Unwalla R., Denny R. A., et al. Insights for predicting blood-brain barrier penetration of CNS targeted molecules using QSPR approaches. Journal of Chemical Information and Modeling. 2010;50(6):1123–1133. doi: 10.1021/ci900384c. [DOI] [PubMed] [Google Scholar]
- 11.Abraham M. H. The factors that influence permeation across the blood-brain barrier. European Journal of Medicinal Chemistry. 2004;39(3):235–240. doi: 10.1016/j.ejmech.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Martins I. F., Teixeira A. L., Pinheiro L., Falcao A. O. A bayesian approach to in silico blood-brain barrier penetration modeling. Journal of Chemical Information and Modeling. 2012;52(6):1686–1697. doi: 10.1021/ci300124c. [DOI] [PubMed] [Google Scholar]
- 13.Adenot M., Lahana R. Blood-brain barrier permeation models: discriminating between potential CNS and non-CNS drugs including P-glycoprotein substrates. Journal of Chemical Information and Computer Sciences. 2004;44(1):239–248. doi: 10.1021/ci034205d. [DOI] [PubMed] [Google Scholar]
- 14.Muehlbacher M., Spitzer G. M., Liedl K. R., Kornhuber J. Qualitative prediction of blood-brain barrier permeability on a large and refined dataset. Journal of Computer-Aided Molecular Design. 2011;25(12):1095–1106. doi: 10.1007/s10822-011-9478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katritzky A. R., Kuanar M., Slavov S., et al. Correlation of blood-brain penetration using structural descriptors. Bioorganic & Medicinal Chemistry. 2006;14(14):4888–4917. doi: 10.1016/j.bmc.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Obrezanova O., Gola J. M. R., Champness E. J., Segall M. D. Automatic QSAR modeling of ADME properties: blood-brain barrier penetration and aqueous solubility. Journal of Computer-Aided Molecular Design. 2008;22(6-7):431–440. doi: 10.1007/s10822-008-9193-8. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L., Zhu H., Oprea T. I., Golbraikh A., Tropsha A. QSAR modeling of the blood–brain barrier permeability for diverse organic compounds. Pharmaceutical Research. 2008;25(8):1902–1914. doi: 10.1007/s11095-008-9609-0. [DOI] [PubMed] [Google Scholar]
- 18.van Damme S., Langenaeker W., Bultinck P. Prediction of blood-brain partitioning: a model based on ab initio calculated quantum chemical descriptors. Journal of Molecular Graphics and Modelling. 2008;26(8):1223–1236. doi: 10.1016/j.jmgm.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Konovalov D. A., Coomans D., Deconinck E., Heyden Y. V. Benchmarking of QSAR models for blood-brain barrier permeation. Journal of Chemical Information and Modeling. 2007;47(4):1648–1656. doi: 10.1021/ci700100f. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter T. S., Kirshner D. A., Lau E. Y., Wong S. E., Nilmeier J. P., Lightstone F. C. A method to predict blood-brain barrier permeability of drug-like compounds using molecular dynamics simulations. Biophysical Journal. 2014;107(3):630–641. doi: 10.1016/j.bpj.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zah J., Terre'Blanche G., Erasmus E., Malan S. F. Physicochemical prediction of a brain-blood distribution profile in polycyclic amines. Bioorganic and Medicinal Chemistry. 2003;11(17):3569–3578. doi: 10.1016/S0968-0896(03)00365-1. [DOI] [PubMed] [Google Scholar]
- 22.Hou T. J., Xu X. J. ADME evaluation in drug discovery. 3. Modeling blood-brain barrier partitioning using simple molecular descriptors. Journal of Chemical Information and Computer Sciences. 2003;43(6):2137–2152. doi: 10.1021/ci034134i. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y. H., Abraham M. H., Ibrahim A., et al. Predicting penetration across the blood-brain barrier from simple descriptors and fragmentation schemes. Journal of Chemical Information and Modeling. 2007;47(1):170–175. doi: 10.1021/ci600312d. [DOI] [PubMed] [Google Scholar]
- 24.Mente S. R., Lombardo F. A recursive-partitioning model for blood-brain barrier permeation. Journal of Computer-Aided Molecular Design. 2005;19(7):465–481. doi: 10.1007/s10822-005-9001-7. [DOI] [PubMed] [Google Scholar]
- 25.Garg P., Verma J. In silico prediction of blood brain barrier permeability: an artificial neural network model. Journal of Chemical Information and Modeling. 2006;46(1):289–297. doi: 10.1021/ci050303i. [DOI] [PubMed] [Google Scholar]
- 26.Guerra A., Páez J. A., Campillo N. E. Artificial neural networks in ADMET modeling: prediction of blood-brain barrier permeation. QSAR & Combinatorial Science. 2008;27(5):586–594. doi: 10.1002/qsar.200710019. [DOI] [Google Scholar]
- 27.Wang Z., Yan A., Yuan Q. Classification of blood-brain barrier permeation by Kohonen's self-organizing neural network (KohNN) and support vector machine (SVM) QSAR & Combinatorial Science. 2009;28(9):989–994. doi: 10.1002/qsar.200960008. [DOI] [Google Scholar]
- 28.Yan A., Liang H., Chong Y., Nie X., Yu C. In-silico prediction of blood-brain barrier permeability. SAR and QSAR in Environmental Research. 2013;24(1):61–74. doi: 10.1080/1062936X.2012.729224. [DOI] [PubMed] [Google Scholar]
- 29.Shen J., Cheng F., Xu Y., Li W., Tang Y. Estimation of ADME properties with substructure pattern recognition. Journal of Chemical Information and Modeling. 2010;50(6):1034–1041. doi: 10.1021/ci100104j. [DOI] [PubMed] [Google Scholar]
- 30.Golmohammadi H., Dashtbozorgi Z., Acree W. E., Jr. Quantitative structure-activity relationship prediction of blood-to-brain partitioning behavior using support vector machine. European Journal of Pharmaceutical Sciences. 2012;47(2):421–429. doi: 10.1016/j.ejps.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 31.Vapnik V. N. The Nature of Statistical Learning Theory. New York, NY, USA: Springer; 1995. [DOI] [Google Scholar]
- 32.Heikamp K., Bajorath J. Support vector machines for drug discovery. Expert Opinion on Drug Discovery. 2014;9(1):93–104. doi: 10.1517/17460441.2014.866943. [DOI] [PubMed] [Google Scholar]
- 33.Hsu C. W., Chang C. C., Lin C. J. A Practical Guide to Support Vector Classication. Taipei, Taiwan: National Taiwan University; 2006. [Google Scholar]
- 34.Chapelle O., Vapnik V., Bousquet O., Mukherjee S. Choosing multiple parameters for support vector machines. Machine Learning. 2002;46(1–3):131–159. doi: 10.1023/a:1012450327387. [DOI] [Google Scholar]
- 35.Chung K.-M., Kao W.-C., Sun C.-L., Wang L.-L., Lin C.-J. Radius margin bounds for support vector machines with the RBF kernel. Neural Computation. 2003;15(11):2643–2681. doi: 10.1162/089976603322385108. [DOI] [PubMed] [Google Scholar]
- 36.Friedrichs F., Igel C. Evolutionary tuning of multiple SVM parameters. Neurocomputing. 2005;64(1–4):107–117. doi: 10.1016/j.neucom.2004.11.022. [DOI] [Google Scholar]
- 37.Yang J. H., Honavar V. Feature subset selection using genetic algorithm. IEEE Intelligent Systems & Their Applications. 1998;13(2):44–48. doi: 10.1109/5254.671091. [DOI] [Google Scholar]
- 38.Raymer M. L., Punch W. F., Goodman E. D., Kuhn L. A., Jain A. K. Dimensionality reduction using genetic algorithms. IEEE Transactions on Evolutionary Computation. 2000;4(2):164–171. doi: 10.1109/4235.850656. [DOI] [Google Scholar]
- 39.Salcedo-Sanz S., Prado-Cumplido M., Perez-Cruz F., Bousono-Calzon C. Artificial Neural Networks—Icann 2002. Vol. 2415. Springer; 2002. Feature selection via genetic optimization; pp. 547–552. [Google Scholar]
- 40.Wang Z. Q., Zhang D. X. Advances in Neural Networks—ISNN 2006. part 1. Vol. 3971. Berlin, Germany: Springer; 2006. Feature selection in text classification via SVM and LSI; pp. 1381–1386. (Lecture Notes in Computer Science). [DOI] [Google Scholar]
- 41.Fernandez M., Caballero J., Fernandez L., Sarai A. Genetic algorithm optimization in drug design QSAR: bayesian-regularized genetic neural networks (BRGNN) and genetic algorithm-optimized support vectors machines (GA-SVM) Molecular Diversity. 2011;15(1):269–289. doi: 10.1007/s11030-010-9234-9. [DOI] [PubMed] [Google Scholar]
- 42.Guyon I., Weston J., Barnhill S., Vapnik V. Gene selection for cancer classification using support vector machines. Machine Learning. 2002;46(1–3):389–422. doi: 10.1023/a:1012487302797. [DOI] [Google Scholar]
- 43.Kohavi R., John G. H. Wrappers for feature subset selection. Artificial Intelligence. 1997;97(1-2):273–324. doi: 10.1016/S0004-3702(97)00043-X. [DOI] [Google Scholar]
- 44.Mao K. Z. Feature subset selection for support vector machines through discriminative function pruning analysis. IEEE Transactions on Systems, Man, and Cybernetics, Part B: Cybernetics. 2004;34(1):60–67. doi: 10.1109/tsmcb.2002.805808. [DOI] [PubMed] [Google Scholar]
- 45.Shen K.-Q., Ong C.-J., Li X.-P., Wilder-Smith E. P. V. Feature selection via sensitivity analysis of SVM probabilistic outputs. Machine Learning. 2008;70(1):1–20. doi: 10.1007/s10994-007-5025-7. [DOI] [Google Scholar]
- 46.Chen Y.-W., Lin C.-J. Feature Extraction. Vol. 207. Berlin, Germany: Springer; 2006. Combining SVMs with various feature selection strategies; pp. 315–324. (Studies in Fuzziness and Soft Computing). [DOI] [Google Scholar]
- 47.Weston J., Mukherjee S., Chapelle O., Pontil M., Vapnik V. Advances in Neural Information Processing Systems 13. MIT Press; 2000. Feature selection for SVMs; pp. 668–674. [Google Scholar]
- 48.Huang C.-L., Wang C.-J. A GA-based feature selection and parameters optimizationfor support vector machines. Expert Systems with Applications. 2006;31(2):231–240. doi: 10.1016/j.eswa.2005.09.024. [DOI] [Google Scholar]
- 49.Zhang X. R., Jiao L. C. Advances in Natural Computation. Vol. 3611. Berlin, Germany: Springer; 2005. Simultaneous feature selection and parameters optimization for SVM by immune clonal algorithm; pp. 905–912. (Lecture Notes in Computer Science). [DOI] [Google Scholar]
- 50.Gold C., Holub A., Sollich P. Bayesian approach to feature selection and parameter tuning for support vector machine classifiers. Neural Networks. 2005;18(5-6):693–701. doi: 10.1016/j.neunet.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 51.Abraham M. H., Ibrahim A., Zhao Y., Agree W. E., Jr. A data base for partition of volatile organic compounds and drugs from blood/plasma/serum to brain, and an LFER analysis of the data. Journal of Pharmaceutical Sciences. 2006;95(10):2091–2100. doi: 10.1002/jps.20595. [DOI] [PubMed] [Google Scholar]
- 52.Katritzky A. R., Lobanov V. S., Karelson M. CODESSA: Reference Manual. University of Florida; 1996. [Google Scholar]
- 53. Marvin 4.0.5, ChemAxon, 2006, http://www.chemaxon.com/
- 54. AMPAC 8, 1992–2004 Semichem, Shawnee, Kan, USA.
- 55.Guyon I., Elisseeff A. An introduction to variable and feature selection. The Journal of Machine Learning Research. 2003;3:1157–1182. [Google Scholar]
- 56.Kennard R. W., Stone L. A. Computer aided design of experiments. Technometrics. 1969;11(1):137–148. doi: 10.1080/00401706.1969.10490666. [DOI] [Google Scholar]
- 57.Daszykowski M., Walczak B., Massart D. L. Representative subset selection. Analytica Chimica Acta. 2002;468(1):91–103. doi: 10.1016/S0003-2670(02)00651-7. [DOI] [Google Scholar]
- 58.Collobert R., Bengio S. SVMTorch: support vector machines for large-scale regression problems. Journal of Machine Learning Research. 2001;1(2):143–160. doi: 10.1162/15324430152733142. [DOI] [Google Scholar]
- 59.Smola A. J., Scholkopf B. A tutorial on support vector regression. Statistics and Computing. 2004;14(3):199–222. doi: 10.1023/b:stco.0000035301.49549.88. [DOI] [Google Scholar]
- 60.Brereton R. G., Lloyd G. R. Support Vector Machines for classification and regression. Analyst. 2010;135(2):230–267. doi: 10.1039/b918972f. [DOI] [PubMed] [Google Scholar]
- 61.Chang C. C., Lin C. J. LIBSVM: a library for support vector machines. 2001.
- 62.Golmohammadi H., Dashtbozorgi Z., Acree W. E., Jr. Quantitative structure–activity relationship prediction of blood-to-brain partitioning behavior using support vector machine. European Journal of Pharmaceutical Sciences. 2012;47(2):421–429. doi: 10.1016/j.ejps.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 63.Clark D. E. In silico prediction of blood-brain barrier permeation. Drug Discovery Today. 2003;8(20):927–933. doi: 10.1016/s1359-6446(03)02827-7. [DOI] [PubMed] [Google Scholar]
- 64.Vilar S., Chakrabarti M., Costanzi S. Prediction of passive blood-brain partitioning: straightforward and effective classification models based on in silico derived physicochemical descriptors. Journal of Molecular Graphics and Modelling. 2010;28(8):899–903. doi: 10.1016/j.jmgm.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herve F., Urien S., Albengres E., Duche J.-C., Tillement J.-P. Drug binding in plasma. A summary of recent trends in the study of drug and hormone binding. Clinical Pharmacokinetics. 1994;26(1):44–58. doi: 10.2165/00003088-199426010-00004. [DOI] [PubMed] [Google Scholar]
- 66.Platts J. A., Abraham M. H., Zhao Y. H., Hersey A., Ijaz L., Butina D. Correlation and prediction of a large blood-brain distribution data set—an LFER study. European Journal of Medicinal Chemistry. 2001;36(9):719–730. doi: 10.1016/s0223-5234(01)01269-7. [DOI] [PubMed] [Google Scholar]
- 67.Iyer M., Mishra R., Han Y., Hopfinger A. J. Predicting blood-brain barrier partitioning of organic molecules using membrane-interaction QSAR analysis. Pharmaceutical Research. 2002;19(11):1611–1621. doi: 10.1023/a:1020792909928. [DOI] [PubMed] [Google Scholar]
- 68.Lobell M., Molnár L., Keserü G. M. Recent advances in the prediction of blood-brain partitioning from molecular structure. Journal of Pharmaceutical Sciences. 2003;92(2):360–370. doi: 10.1002/jps.10282. [DOI] [PubMed] [Google Scholar]
- 69.Kaznessis Y. N., Snow M. E., Blankley C. J. Prediction of blood-brain partitioning using Monte Carlo simulations of molecules in water. Journal of Computer-Aided Molecular Design. 2001;15(8):697–708. doi: 10.1023/a:1012240703377. [DOI] [PubMed] [Google Scholar]
- 70.van de Waterbeemd H., Camenisch G., Folkers G., Chretien J. R., Raevsky O. A. Estimation of blood-brain barrier crossing of drugs using molecular size and shape, and H-bonding descriptors. Journal of Drug Targeting. 1998;6(2):151–165. doi: 10.3109/10611869808997889. [DOI] [PubMed] [Google Scholar]
- 71.Kelder J., Grootenhuis P. D. J., Bayada D. M., Delbressine L. P. C., Ploemen J.-P. Polar molecular surface as a dominating determinant for oral absorption and brain penetration of drugs. Pharmaceutical Research. 1999;16(10):1514–1519. doi: 10.1023/A:1015040217741. [DOI] [PubMed] [Google Scholar]
- 72.Ooms F., Weber P., Carrupt P.-A., Testa B. A simple model to predict blood-brain barrier permeation from 3D molecular fields. Biochimica et Biophysica Acta—Molecular Basis of Disease. 2002;1587(2-3):118–125. doi: 10.1016/s0925-4439(02)00074-1. [DOI] [PubMed] [Google Scholar]
- 73.Fischer H., Gottschlich R., Seelig A. Blood-brain barrier permeation: molecular parameters governing passive diffusion. Journal of Membrane Biology. 1998;165(3):201–211. doi: 10.1007/s002329900434. [DOI] [PubMed] [Google Scholar]
- 74.Mahar Doan K. M., Humphreys J. E., Webster L. O., et al. Passive permeability and P-glycoprotein-mediated efflux differentiate central nervous system (CNS) and non-CNS marketed drugs. Journal of Pharmacology and Experimental Therapeutics. 2002;303(3):1029–1037. doi: 10.1124/jpet.102.039255. [DOI] [PubMed] [Google Scholar]
- 75.Rose K., Hall L. H., Kier L. B. Modeling blood-brain barrier partitioning using the electrotopological state. Journal of Chemical Information and Computer Sciences. 2002;42(3):651–666. doi: 10.1021/ci010127n. [DOI] [PubMed] [Google Scholar]
- 76.Crivori P., Cruciani G., Carrupt P.-A., Testa B. Predicting blood-brain barrier permeation from three-dimensional molecular structure. Journal of Medicinal Chemistry. 2000;43(11):2204–2216. doi: 10.1021/jm990968. [DOI] [PubMed] [Google Scholar]
- 77.Young R. C., Mitchell R. C., Brown T. H., et al. Development of a new physicochemical model for brain penetration and its application to the design of centrally acting H2 receptor histamine antagonists. Journal of Medicinal Chemistry. 1988;31(3):656–671. doi: 10.1021/jm00398a028. [DOI] [PubMed] [Google Scholar]
- 78.Lombardo F., Blake J. F., Curatolo W. J. Computation of brain-blood partitioning of organic solutes via free energy calculations. Journal of Medicinal Chemistry. 1996;39(24):4750–4755. doi: 10.1021/jm960163r. [DOI] [PubMed] [Google Scholar]
- 79.Iyer M., Mishra R., Han Y., Hopfinger A. J. Predicting blood–brain barrier partitioning of organic molecules using membrane–interaction QSAR analysis. Pharmaceutical Research. 2002;19(11):1611–1621. doi: 10.1023/a:1020792909928. [DOI] [PubMed] [Google Scholar]