Abstract
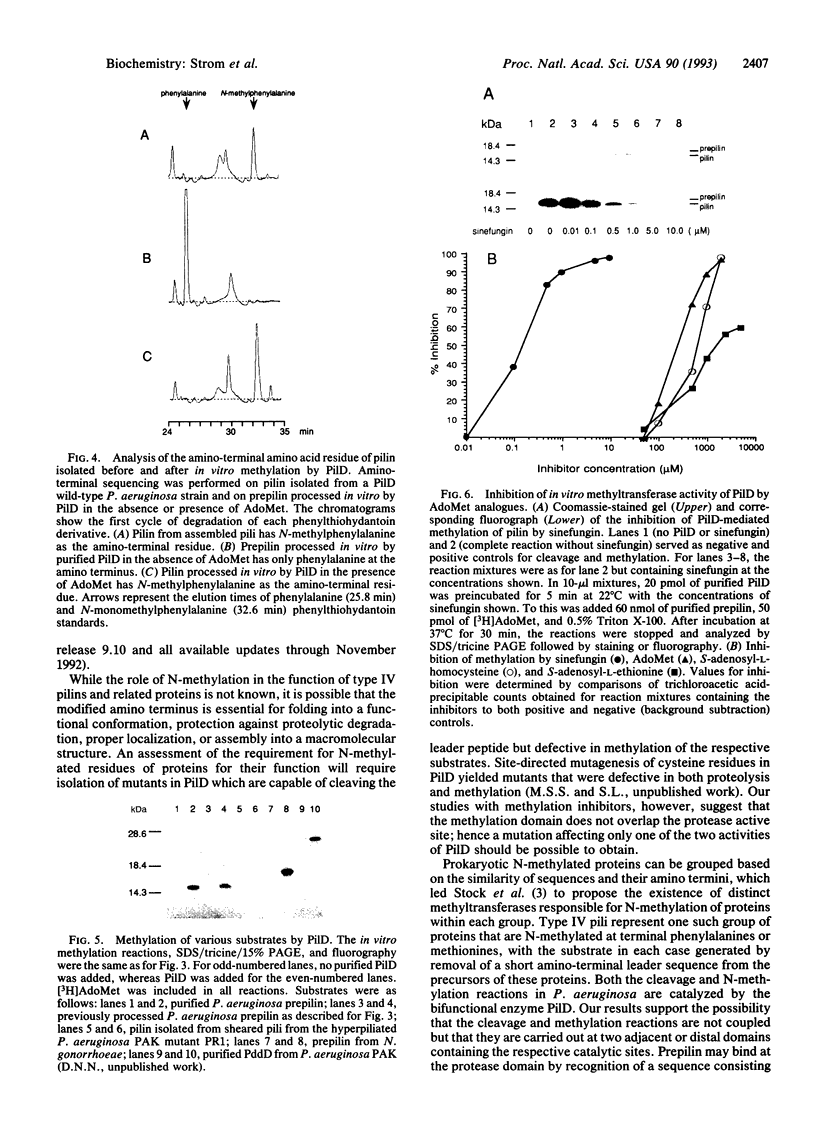
Precursors of the type IV pilins of a number of bacterial pathogens, as well as related proteins involved in extracellular protein export and DNA uptake, are synthesized with short basic leader sequences. Maturation of these proteins involves two consecutive posttranslational modifications. The leader sequence is first proteolytically removed by specialized endopeptidases, of which the prototype is encoded by the pilD gene of Pseudomonas aeruginosa. Subsequently, the amino termini of these proteins are methylated. Here we demonstrate that PilD, in addition to cleaving the amino-terminal leader sequences of prepilin, also catalyzes N-methylation of the amino-terminal phenylalanine of the mature pilin, using S-adenosyl-L-methionine as a methyl donor. Thus, to our knowledge, PilD is the first characterized bacterial N-methyltransferase. Complete inhibition of N-methylation, but not peptide cleavage, by structural analogues of S-adenosyl-L-methionine suggests that PilD is a bifunctional enzyme with proteolytic and methylation activities carried out within two distinct active sites.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano M., Breitling R., Dubnau D. A. Nucleotide sequence and genetic organization of the Bacillus subtilis comG operon. J Bacteriol. 1989 Oct;171(10):5386–5404. doi: 10.1128/jb.171.10.5386-5404.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bally M., Ball G., Badere A., Lazdunski A. Protein secretion in Pseudomonas aeruginosa: the xcpA gene encodes an integral inner membrane protein homologous to Klebsiella pneumoniae secretion function protein PulO. J Bacteriol. 1991 Jan;173(2):479–486. doi: 10.1128/jb.173.2.479-486.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bally M., Filloux A., Akrim M., Ball G., Lazdunski A., Tommassen J. Protein secretion in Pseudomonas aeruginosa: characterization of seven xcp genes and processing of secretory apparatus components by prepilin peptidase. Mol Microbiol. 1992 May;6(9):1121–1131. doi: 10.1111/j.1365-2958.1992.tb01550.x. [DOI] [PubMed] [Google Scholar]
- Brauer D., Wittmann-Liebold B. The primary structure of the initiation factor IF-3 from Escherichia coli. FEBS Lett. 1977 Jul 15;79(2):269–275. doi: 10.1016/0014-5793(77)80801-6. [DOI] [PubMed] [Google Scholar]
- Brosius J., Chen R. The primary structure of protein L16 located at the peptidyltransferase center of Escherichia coli ribosomes. FEBS Lett. 1976 Sep 15;68(1):105–109. doi: 10.1016/0014-5793(76)80415-2. [DOI] [PubMed] [Google Scholar]
- Chen R., Brosius J., Wittmann-Liebold B. Occurrence of methylated amino acids as N-termini of proteins from Escherichia coli ribosomes. J Mol Biol. 1977 Apr;111(2):173–181. doi: 10.1016/s0022-2836(77)80121-6. [DOI] [PubMed] [Google Scholar]
- Clarke S. Protein carboxyl methyltransferases: two distinct classes of enzymes. Annu Rev Biochem. 1985;54:479–506. doi: 10.1146/annurev.bi.54.070185.002403. [DOI] [PubMed] [Google Scholar]
- Dognin M. J., Wittmann-Liebold B. The primary structure of L11, the most heavily methylated protein from Escherichia coli ribosomes. FEBS Lett. 1977 Dec 15;84(2):342–346. doi: 10.1016/0014-5793(77)80721-7. [DOI] [PubMed] [Google Scholar]
- Dow J. M., Daniels M. J., Dums F., Turner P. C., Gough C. Genetic and biochemical analysis of protein export from Xanthomonas campestris. J Cell Sci Suppl. 1989;11:59–72. doi: 10.1242/jcs.1989.supplement_11.5. [DOI] [PubMed] [Google Scholar]
- Dums F., Dow J. M., Daniels M. J. Structural characterization of protein secretion genes of the bacterial phytopathogen Xanthomonas campestris pathovar campestris: relatedness to secretion systems of other gram-negative bacteria. Mol Gen Genet. 1991 Oct;229(3):357–364. doi: 10.1007/BF00267456. [DOI] [PubMed] [Google Scholar]
- He S. Y., Lindeberg M., Chatterjee A. K., Collmer A. Cloned Erwinia chrysanthemi out genes enable Escherichia coli to selectively secrete a diverse family of heterologous proteins to its milieu. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1079–1083. doi: 10.1073/pnas.88.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K., Lory S. Characterization of Pseudomonas aeruginosa mutants with altered piliation. J Bacteriol. 1987 Dec;169(12):5663–5667. doi: 10.1128/jb.169.12.5663-5667.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs C. F., Schoolnik G., Koomey J. M., Hardy J., Rothbard J., Falkow S. Cloning and sequencing of a Moraxella bovis pilin gene. J Bacteriol. 1985 Jul;163(1):132–139. doi: 10.1128/jb.163.1.132-139.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- McKern N. M., O'Donnell I. J., Inglis A. S., Stewart D. J., Clark B. L. Amino acid sequence of pilin from Bacteroides nodosus (strain 198), the causative organism of ovine footrot. FEBS Lett. 1983 Nov 28;164(1):149–153. doi: 10.1016/0014-5793(83)80039-8. [DOI] [PubMed] [Google Scholar]
- Meyer T. F., Billyard E., Haas R., Storzbach S., So M. Pilus genes of Neisseria gonorrheae: chromosomal organization and DNA sequence. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6110–6114. doi: 10.1073/pnas.81.19.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan S., Aghion J., Guillen N., Dubnau D. Molecular cloning and characterization of comC, a late competence gene of Bacillus subtilis. J Bacteriol. 1989 Nov;171(11):6043–6051. doi: 10.1128/jb.171.11.6043-6051.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn D. N., Lory S. Components of the protein-excretion apparatus of Pseudomonas aeruginosa are processed by the type IV prepilin peptidase. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):47–51. doi: 10.1073/pnas.89.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn D. N., Lory S. Product of the Pseudomonas aeruginosa gene pilD is a prepilin leader peptidase. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3281–3285. doi: 10.1073/pnas.88.8.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn D., Bergman S., Lory S. Products of three accessory genes, pilB, pilC, and pilD, are required for biogenesis of Pseudomonas aeruginosa pili. J Bacteriol. 1990 Jun;172(6):2911–2919. doi: 10.1128/jb.172.6.2911-2919.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Dupuy B. An enzyme with type IV prepilin peptidase activity is required to process components of the general extracellular protein secretion pathway of Klebsiella oxytoca. Mol Microbiol. 1992 Mar;6(6):751–760. doi: 10.1111/j.1365-2958.1992.tb01525.x. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Reyss I. Five genes at the 3' end of the Klebsiella pneumoniae pulC operon are required for pullulanase secretion. Mol Microbiol. 1990 Mar;4(3):365–379. doi: 10.1111/j.1365-2958.1990.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Reyss I., Pugsley A. P. Five additional genes in the pulC-O operon of the gram-negative bacterium Klebsiella oxytoca UNF5023 which are required for pullulanase secretion. Mol Gen Genet. 1990 Jul;222(2-3):176–184. doi: 10.1007/BF00633815. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Shaw C. E., Taylor R. K. Vibrio cholerae O395 tcpA pilin gene sequence and comparison of predicted protein structural features to those of type 4 pilins. Infect Immun. 1990 Sep;58(9):3042–3049. doi: 10.1128/iai.58.9.3042-3049.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock A., Clarke S., Clarke C., Stock J. N-terminal methylation of proteins: structure, function and specificity. FEBS Lett. 1987 Aug 10;220(1):8–14. doi: 10.1016/0014-5793(87)80866-9. [DOI] [PubMed] [Google Scholar]
- Stock A. N-methylmethionine at the amino terminus of a protein required for bacterial chemotaxis. Adv Exp Med Biol. 1988;231:387–399. doi: 10.1007/978-1-4684-9042-8_31. [DOI] [PubMed] [Google Scholar]
- Stock A., Schaeffer E., Koshland D. E., Jr, Stock J. A second type of protein methylation reaction in bacterial chemotaxis. J Biol Chem. 1987 Jun 15;262(17):8011–8014. [PubMed] [Google Scholar]
- Stock J. B., Clarke S., Koshland D. E., Jr The protein carboxylmethyltransferase involved in Escherichia coli and Salmonella typhimurium chemotaxis. Methods Enzymol. 1984;106:310–321. doi: 10.1016/0076-6879(84)06031-6. [DOI] [PubMed] [Google Scholar]
- Strom M. S., Lory S. Amino acid substitutions in pilin of Pseudomonas aeruginosa. Effect on leader peptide cleavage, amino-terminal methylation, and pilus assembly. J Biol Chem. 1991 Jan 25;266(3):1656–1664. [PubMed] [Google Scholar]
- Strom M. S., Lory S. Kinetics and sequence specificity of processing of prepilin by PilD, the type IV leader peptidase of Pseudomonas aeruginosa. J Bacteriol. 1992 Nov;174(22):7345–7351. doi: 10.1128/jb.174.22.7345-7351.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom M. S., Nunn D., Lory S. Multiple roles of the pilus biogenesis protein pilD: involvement of pilD in excretion of enzymes from Pseudomonas aeruginosa. J Bacteriol. 1991 Feb;173(3):1175–1180. doi: 10.1128/jb.173.3.1175-1180.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tønjum T., Marrs C. F., Rozsa F., Bøvre K. The type 4 pilin of Moraxella nonliquefaciens exhibits unique similarities with the pilins of Neisseria gonorrhoeae and Dichelobacter (Bacteroides) nodosus. J Gen Microbiol. 1991 Oct;137(10):2483–2490. doi: 10.1099/00221287-137-10-2483. [DOI] [PubMed] [Google Scholar]