Abstract
In this study, we isolated an environmental clone of Ochrobactrum intermedium, strain 2745-2, from the formation water of Changqing oilfield in Shanxi, China, which can degrade crude oil. Strain 2745-2 is aerobic and rod-shaped with optimum growth at 42 °C and pH 5.5. We sequenced the genome and found a single chromosome of 4 800 175 bp, with a G+C content of 57.63%. Sixty RNAs and 4737 protein-coding genes were identified: many of the genes are responsible for the degradation, emulsification, and metabolizing of crude oil. A comparative genomic analysis with related clinical strains (M86, 229E, and LMG3301T) showed that genes involved in virulence, disease, defense, phages, prophages, transposable elements, plasmids, and antibiotic resistance are also present in strain 2745-2.
Keywords: Comparative genome, Ochrobactrum intermedium, Oil degradation, Pathogen
1. Introduction
Oil contamination is a worldwide problem, which is growing more serious with economic development; its effects are long lasting and remediation is difficult. Several methods of oil degradation have been developed, the method with the longest history being land farming, which is “low-tech” but time consuming (Genouw et al., 1994). Physical methods such as surface heating are more efficient but energy consuming (Edelstein et al., 1994). Microbial oil degradation shows promise of being sustainable and environmentally friendly, and the screening of potential oil degrading microorganisms is becoming increasingly important. Bacteria from different habitats, such as soil (Jesubunmi, 2014; Kumar et al., 2014; Pham et al., 2014) and the ocean (Hazen et al., 2010; Hassanshahian et al., 2014), are screened for their oil degrading properties. These bacteria are then used individually or in a mixture (Creencia et al., 2014; Silva et al., 2015).
In our previous studies, several strains of bacteria, which have the ability to degrade crude oil, were isolated from the formation water of Chinese oilfields (She et al., 2011; 2014; Zhang et al., 2012; 2014; Zheng et al., 2014). In this study, we isolated from the Changqing oilfield a strain which has rarely been isolated from an oilfield, Ochrobactrum intermedium strain 2745-2. O. intermedium was first described in 1998 with five strains formerly known as members of Ochrobactrum anthropi (Holmes et al., 1988; Velasco et al., 1998). The name, O. intermedium, indicates an intermediate position between O. anthropi and Brucella spp. (Velasco et al., 1998). O. anthropi is an emerging opportunistic pathogen in immunocompromised patients (Mudshingkar et al., 2013) and members of Brucella are pathogens causing brucellosis which is a common zoonotic infection globally (Dean et al., 2012). Strains of O. intermedium are associated with both human beings and the environment. Some strains are pathogens which cause infection (Möller et al., 1999; Apisarnthanarak et al., 2005); some live in environments polluted by chromium (Kavita and Keharia, 2012), lead (Waranusantigul et al., 2011), and tobacco waste (Yuan et al., 2007), etc.
As a human pathogen and environmental bacterium, O. intermedium attracts a lot of interest. From a database survey, we found three draft genome sequences within O. intermedium, two of which (strains M86 and 229E) have been published (Kulkarni et al., 2013; 2014). Strains M86, 229E, and LMG3301T were isolated from a stomach biopsy and blood taken from a non-ulcer dyspeptic individual from India. Thus, all of these three strains are associated with humans; no environmental strain had been sequenced before our study. Comparative genomic analysis is needed between human and environmental isolates of O. intermedium to give us a better understanding of the mechanisms by which it adapts to its environment.
Here, we describe the classification and features of O. intermedium strain 2745-2, together with its genome sequence and the comparative genomic study we conducted with its clinical relatives, strains LMG3301T, 229E, and M86. The aims of this work are to investigate the oil-degrading genes of strain 2745-2 and to find the distinction and similarities among the genomes and genes that reflect adaptation to specific environments.
2. Materials and methods
2.1. Sampling and isolation of oil degrading bacteria
A water sample was collected from an oil producing well in Changqing oilfield, Shanxi Province, China, in 2012. The sample was stored immediately at 4 °C. Oil degrading bacteria were isolated using sterile crude oil as the medium. After incubation, the culture was spread on LB agar plates containing 5.0 g/L yeast extract (Difco, USA), 10.0 g/L NaCl, 10.0 g/L tryptone, and 20.0 g/L agar (Difco, USA) to select the single clones. One strain (2745-2) was further characterized. It was cultured in LB medium and genomic DNA was extracted using QIAamp DNA Mini Kit (Qiagen, Germany) following the manufacturer’s instructions. 16S ribosomal RNA (rRNA) was amplified by polymerase chain reaction (PCR) using the primers as follows: 27F (5'-AGA GTT TGA TCC TGG CTC AG-3') and 1492R (5'-GGT TAC CTT GTT ACG ACT T-3').
2.2. Phylogenetic tree construction
16S rRNA nucleotide sequence analysis was conducted using the BLASTN program against the national center for biotechnology information (NCBI)-nucleotide collection (nr/nt) database. Sequences were aligned by the CLUSTALW (Larkin et al., 2007). A Neighbor-Joining phylogenetic tree based on the Tamura-Nei model was constructed using MEGA6 software (Tamura et al., 2013).
2.3. Characterization of strain 2745-2
Cell morphology of strain 2745-2 was examined using a scanning electron micrograph (Quanta 200, FEI Co., USA). The temperature range, pH range, and NaCl range for growth were determined using methods described before (Cheng et al., 2015). Gram-reaction was carried out according to Bergey’s manual (Holt et al., 1994). Tests for H2S production and indole production were conducted using the method described by Mata et al. (2002). Hydrolase of starch, gelatin, and casein were tested. Single carbon source utilization tests were performed using D-glucose, maltose, lactose, D-galactose, rhamnose, raffinose, sorbitol, glycerol, cellobiose, sucrose, tetradecane, and hexadecane. Resistance to ampicillin, erythromycin, tetracycline, kanamycin, and gentamicin were tested.
2.4. Whole genome sequencing
Strain 2745-2 was cultivated aerobically in LB medium, pH 5.5 at 42 °C overnight. Genomic DNA was extracted using the method described by Marmur and Doty (1962). The resulting genomic DNA was then measured using gel electrophoresis 0.7% (7 g/L) agarose with λ-Hind III digest DNA as the marker (TaKaRa, Dalian, China). The concentration of the genomic DNA was measured by NanoDrop™ 1000 spectrophotometer (Thermo Fisher Scientific Inc., USA). Genomic DNA sequencing was performed using Illumina HiSeq2000 with Solexa paired-end sequencing strategy. One DNA library (500 bp insert size with Illumina adapter at both ends) was generated and detected by Agilent DNA analyzer 2100 (Agilent Technologies, USA).
2.5. Sequence assembly and annotation
Clean reads were assembled into scaffolds using the Velvet version 1.2.07 (Zerbino and Birney, 2008). We then used PAGIT flow (Swain et al., 2012) to prolong the initial contigs and correct sequencing errors.
The transfer RNAs (tRNAs) and rRNAs were identified using tRNAscan-SE (Lowe and Eddy, 1997), RNAmmer (Lagesen et al., 2007), and Rfam database (Griffiths-Jones et al., 2003; Burge et al., 2012). The genome annotation was predicted using the RAST server online (Aziz et al., 2008). Predicted genes were blast against the Clusters of Orthologous Groups (COGs) database (Tatusov et al., 2000; 2001). We applied the PHAST program to predict the prophages and putative phage-like elements in the genome (Zhou et al., 2011).
2.6. Comparative genomic analysis
The genome sequences of O. intermedium M86, 229E, and LMG3301T were downloaded from the NCBI database under the accession numbers AOGE00000000.1, ASXJ00000000.1, and ACQA 00000000.1, respectively (Kulkarni et al., 2013; 2014). All these genomes were annotated by the RAST on-line server, which was also used for subsystem annotations (Aziz et al., 2008). Contigs were re-ordered using the Mauve program (Darling et al., 2010). Blasts of the three genomes together with strain 2745-2 were performed using the BLAST+program (Camacho et al., 2009). The BLAST Ring Image Generator (BRIG) was used for genome alignment visualization (Alikhan et al., 2011).
2.7. Nucleotide sequence accession number
The genome sequence of O. intermedium 2745-2 has been deposited in GenBank with the accession number JFHY00000000.1.
3. Results
3.1. Phylogenetic analysis and characterization of strain 2745-2
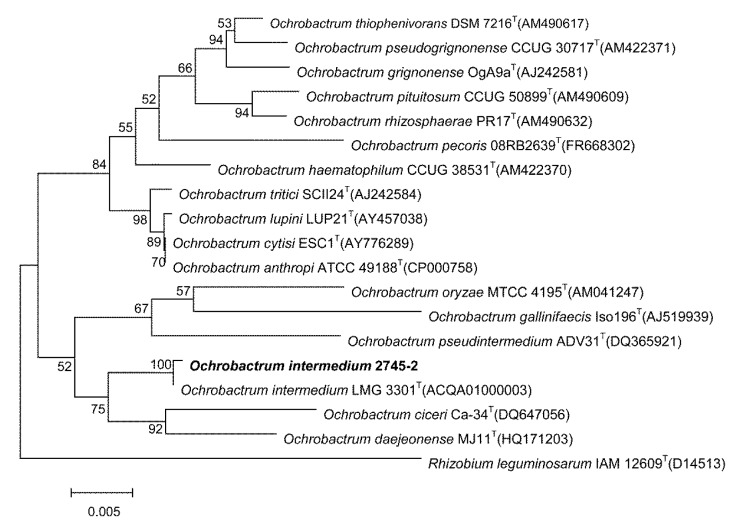
Neighbor-Joining phylogenetic analysis indicated the taxonomic status of 2745-2, which is clearly classified into the same branch as O. intermedium LMG3301T. Rhizobium leguminosarum IAM 12609T was used as an out group (Fig. 1).
Fig. 1.
Phylogenetic tree presenting the position of Ochrobactrum intermedium strain 2745-2
GenBank accession numbers for 16S rRNA genes of the strains used in this phylogenetic tree are shown following the organism names. The bootstrap values on the branching nodes were calculated on 1000 replications. Rhizobium leguminosarum IAM 12609T was used as an out group. The scale bar indicated 0.005 substitutions per nucleotide position
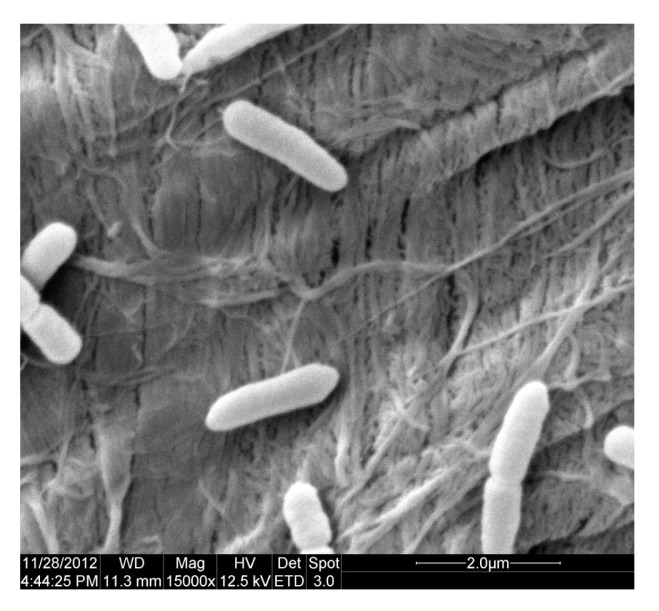
Strain 2745-2 was capable of growing at 15–45 °C and pH 5.5–9.0 with optimum conditions being 42 °C and pH 5.5. Cells are straight rods, 0.6–0.9 μm in diameter and 1.7–5.3 μm long (Fig. 2). Colonies grown at 42 °C on LB agar plate are gray, circular, and convex. H2S and indole are produced. Gelatin and casein are hydrolyzed, but not starch. Lactose, rhamnose, tetradecane, and hexadecane are used as the carbon source, while D-glucose, maltose, D-galactose, raffinose, sorbitol, glycerol, cellobiose, and sucrose are not used. An antimicrobial susceptibility test showed that strain 2745-2 is resistant to ampicillin, erythromycin, tetracycline, kanamycin, and gentamicin.
Fig. 2.
Scanning electron micrograph of cells of Ochrobactrum intermedium strain 2745-2
Cells were grown at 42 °C in LB broth for 16 h (about early stationary phase)
3.2. Genome features
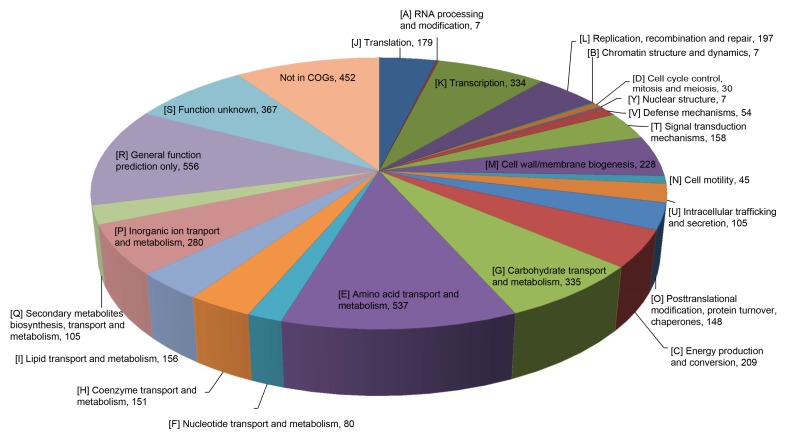
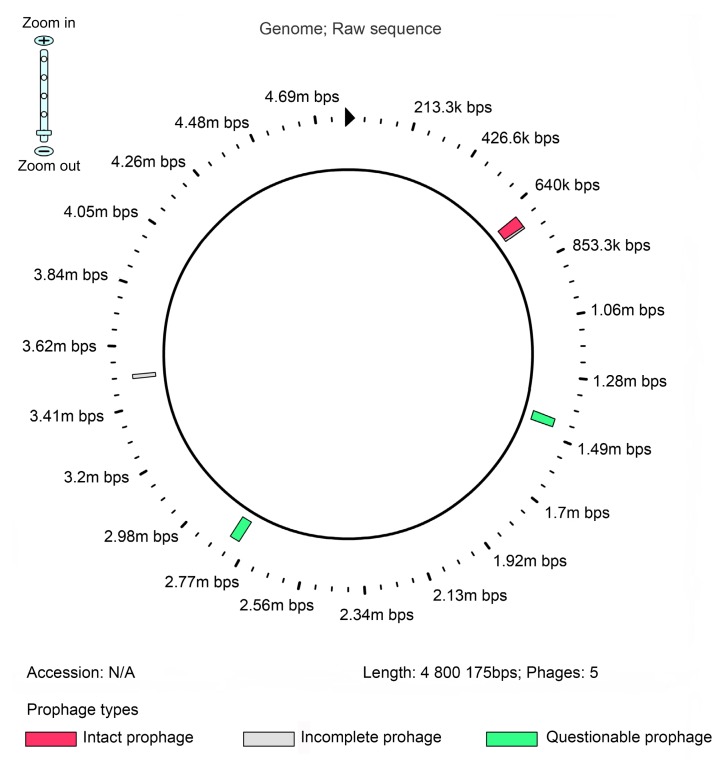
The draft genome size of O. intermedium 2745-2 was 4 800 175 bp with a G+C content of 57.62%. The draft genome contains 4737 coding sequences (CDSs) and 60 RNAs including two complete rRNA operons. Detailed information on the genome is summarized in Table 1. A total of 4285 genes were categorized into COGs functional groups (Fig. 3). Five prophage regions have been identified (Fig. 4), including one intact, two incomplete, and two questionable regions (Table 2).
Table 1.
Detailed information of the draft genome sequence of Ochrobactrum intermedium strain 2745-2
| Size (bp) | G+C content (bp) | Coding region (bp) | Gene number |
|||
| Total | RNA | Protein-coding | COGs | |||
| 4 800 175 | 2 766 132 | 4 145 190 | 4797 | 60 | 4737 | 4285 |
Fig. 3.
Distribution of the genes associated with COG functional categories in Ochrobactrum intermedium strain 2745-2
Fig. 4.
Genomic view of prophage regions identified in the genome of strain 2745-2
Table 2.
Summary of prophage regions in strain 2745-2
| Region | Region length (kb) | Completeness | CDS | Specific keyword |
| 1 | 51.0 | Intact | 47 | Terminase, portal, plate, tail |
| 2 | 10.3 | Incomplete | 16 | Tail |
| 3 | 35.3 | Questionable | 32 | Integrase, terminase, portal, head, capsid |
| 4 | 40.1 | Questionable | 54 | Terminase, portal, head, capsid, tail |
| 5 | 15.5 | Incomplete | 20 | Terminase, capsid, head, tail |
3.3. Comparative genomic analysis
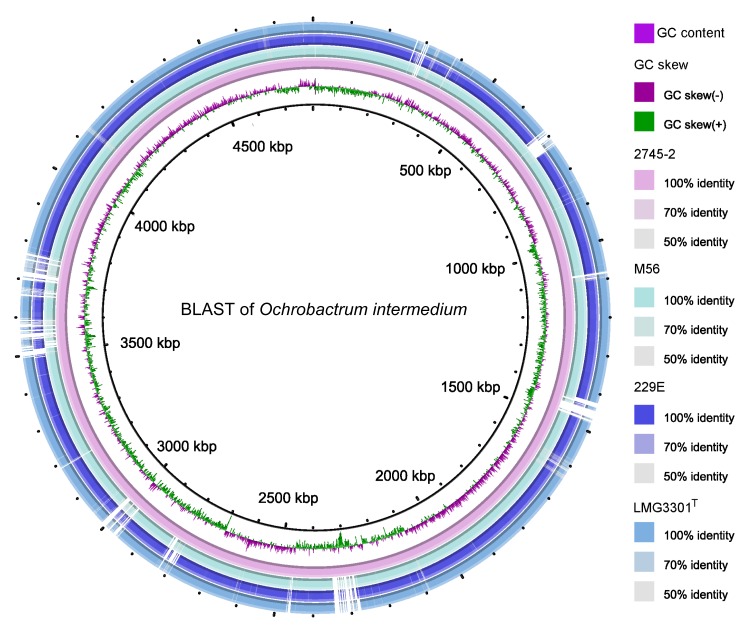
Comparative genomic analyses of O. intermedium M86, 229E, LMG3301T, and 2745-2 were conducted. The isolation source and genomic statistics are shown in Table 3. Comparisons of subsystem features between the four genomes revealed some distinctions between 2745-2 and the other three strains (Table 4). The numbers of genes involved in virulence, disease, and defense were significantly lower in 2745-2 than in the other strains. Genes involved in secondary metabolism were higher in 2745-2. This difference in gene numbers may be due to the different habitat of 2745-2. BLAST of nucleotide sequence between 2745-2 and the other three strains was performed and the identities were visualized (Fig. 5).
Table 3.
Comparison between the genome statistics of four strains of Ochrobactrum intermedium
| Strain | Isolation source | Accession No. | Size (Mb) | GC (%) | Number |
|||
| Contig | Subsystem | CDS | RNA | |||||
| 2745-2 | Formation water | JFHY00000000.1 | 4.80 | 57.6 | 95 | 440 | 4737 | 60 |
| M86 | Gastric biopsy | AOGE00000000.1 | 5.19 | 57.9 | 148 | 480 | 5473 | 66 |
| 229E | Stomach biopsy | ASXJ00000000.1 | 4.81 | 57.9 | 378 | 468 | 5610 | 58 |
| LMG3301T | Blood | ACQA00000000.1 | 4.73 | 57.7 | 4 | 474 | 4723 | 70 |
Table 4.
Comparisons between subsystem features of four strains of Ochrobactrum intermedium
| Subsystem feature | Number of genes |
|||
| 2745-2 | M86 | 229E | LMG3301T | |
| Cofactors, vitamins, prosthetic groups, pigments | 224 | 276 | 305 | 275 |
| Cell wall and capsule | 111 | 107 | 134 | 111 |
| Virulence, disease, and defense | 77 | 102 | 112 | 94 |
| Potassium metabolism | 15 | 18 | 24 | 17 |
| Photosynthesis | 0 | 0 | 0 | 0 |
| Miscellaneous | 55 | 63 | 76 | 63 |
| Phages, prophages, transposable elements, plasmids | 19 | 38 | 0 | 15 |
| Membrane transport | 180 | 254 | 281 | 226 |
| Iron acquisition and metabolism | 41 | 55 | 80 | 56 |
| RNA metabolism | 127 | 145 | 189 | 157 |
| Nucleosides and nucleotides | 100 | 109 | 121 | 110 |
| Protein metabolism | 207 | 273 | 305 | 265 |
| Cell division and cell cycle | 16 | 30 | 30 | 28 |
| Motility and chemotaxis | 77 | 84 | 78 | 88 |
| Regulation and cell signaling | 70 | 82 | 84 | 75 |
| Secondary metabolism | 9 | 4 | 4 | 4 |
| DNA metabolism | 98 | 123 | 124 | 107 |
| Fatty acids, lipids, and isoprenoids | 113 | 153 | 169 | 153 |
| Nitrogen metabolism | 35 | 38 | 51 | 38 |
| Dormancy and sporulation | 2 | 2 | 3 | 1 |
| Respiration | 126 | 134 | 157 | 134 |
| Stress response | 119 | 131 | 146 | 128 |
| Metabolism of aromatic compounds | 30 | 32 | 42 | 32 |
| Amino acids and derivatives | 409 | 522 | 618 | 503 |
| Sulfur metabolism | 11 | 54 | 65 | 54 |
| Phosphorus metabolism | 43 | 53 | 57 | 49 |
| Carbohydrates | 440 | 496 | 580 | 484 |
Fig. 5.
BLAST visualization of Ochrobactrum intermedium genomes
The rings illustrate a shared identity with the four strains
4. Discussion
4.1. Crude oil degradation related genes
Crude oil is a mixture of hydrocarbons of various molecular weights. Many bacteria in nature have been found to be capable of degrading crude oil (Dawar and Aggarwal, 2015; Lincoln et al., 2015), using it as their sole carbon source. Strain 2745-2 is one of them. Experiments showed that this strain can use tetradecane and hexadecane, the main compounds in crude oil, as its carbon source. Tetradecane and hexadecane are alkanes which can be oxidized by alkane hydroxylases, such as AlkB and P450. Two alkB genes and one P450 gene were found in the genome of 2745-2. alkB encodes a protein named alkane 1-monooxygenase, which is the key enzyme in the degradation of alkanes. Genes encoding for 2-polyprenylphenol hydroxylase and alkaline phosphatase, which are responsible for degrading aminobenzoate, were found in the genome. Genes of 2-haloalkanoic acid dehalogenase and alcohol dehydrogenase for chloroalkane and chloroalkene degrading exist in the genome. Furthermore, 2745-2 also contains all genes involved in the assembly of flagella which allows the bacterium to move to the oil-water interface the degradation process. Many other oil-degrading bacteria also have suits of flagella assembly-related genes and it is believed that these genes can also benefit emulsification of the hydrocarbon in crude oil (Das et al., 2015). Strain 2745-2 also contains genes encoding for phosphomannomutase, acyl transferase, glycosyl transferase, rhamnosyltransferase, glucose-1-phosphate thymidylyltransferase, dTDP-glucose 4,6-dehydratase, dTDP-4-dehydrorhamnose 3,5-epimerase, dTDP-4-dehydrorhamnose reductase, and N-acyl-L-homoserine lactone synthase. These enzymes are involved in the synthesis of rhamnolipids, a class of glycolipid, which work as bacterial surfactants by reducing the surface tension, critical micelle concentration, and interfacial tension, and increasing the emulsification and solubility of hydrocarbons in mixtures such as crude oil (Das et al., 2015). All these genes reflect the ability of strain 2745-2 to degrade crude oil.
4.2. Pathogen potential of strain 2745-2
Previous studies on the genome of two strains, O. intermedium M86 and 229E, identified many gene clusters related to virulence (Kulkarni et al., 2013; 2014). In our study, the genomes of three strains of O. intermedium, which are available in the public database (M86, 229E, and LMG3301T), were compared with the genome of strain 2745-2. All the strains have an average genomic size of 4.8 Mb except M86 (Table 3). 2745-2 is an environmental strain and the other three are clinical. There are fewer genes involved in virulence, disease, and defense in strain 2745-2 compared with the others (Table 4). However, 2745-2 contains several genes that are related to phages, prophages, transposable elements, and plasmids. Furthermore, we identified five prophage regions in 2745-2. One region is an intact phage with a length of 51 kb, which encodes for phage-like and hypothetical proteins (Fig. 4). It is believed that the phage-like sequences improve the cell adhesion and the ability to acquire antibiotic resistance, properties that can enable bacteria to survive in new environments and become pathogens (Casjens, 2003; Zhou et al., 2011). The clinical isolates of O. intermedium, which are related to pathogens such as O. anthropi and Brucella spp., display a high level of resistance to forms of β-lactam antibiotics (Teyssier et al., 2005) and are considered to be pathogens. Although 2745-2 was isolated from a non-clinical environment, it was found to be resistant to ampicillin and the genomic annotation results showed the presence of several β-lactamase genes that provide resistance to β-lactam antibiotics. With all these properties, strain 2745-2 may have the potential to be a pathogen.
5. Conclusions
As the first environmentally-derived strain of O. intermedium whose genome has been sequenced, strain 2745-2 is giving us a new perspective on its adaption to the environment. Genes involved in crude oil degradation are annotated in its genome, reflecting its ability to degrade crude oil. Further comparative genomic studies between 2745-2 and strains isolated from clinical samples will give us a better understanding of the adaption and evolution of environmental bacteria into host pathogens.
Footnotes
Project supported by the National High-Tech R & D Program (863) of China (No. 2013AA064402), the National Natural Science Foundation of China (Nos. 81301461 and 51474034), the Zhejiang Provincial Natural Science Foundation of China (No. LQ13H190002), and the Scientific Research Foundation of Zhejiang Provincial Health Bureau (No. 2012KYB083), China
Compliance with ethics guidelines: Lu-jun CHAI, Xia-wei JIANG, Fan ZHANG, Bei-wen ZHENG, Fu-chang SHU, Zheng-liang WANG, Qing-feng CUI, Han-ping DONG, Zhong-zhi ZHANG, Du-jie HOU, and Yue-hui SHE declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Alikhan NF, Petty NK, Zakour NLB, et al. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics. 2011;12(1):402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apisarnthanarak A, Kiratisin P, Mundy LM. Evaluation of Ochrobactrum intermedium bacteremia in a patient with bladder cancer. Diagn Micr Infec Dis. 2005;53(2):153–155. doi: 10.1016/j.diagmicrobio.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Aziz RK, Bartels D, Best AA, et al. The RAST Server: rapid annotations using subsystems technology. BMC Genomics. 2008;9(1):75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burge SW, Daub J, Eberhardt R, et al. Rfam 11.0: 10 years of RNA families. Nucleic Acids Res. 2012;41(D1):D226–D232. doi: 10.1093/nar/gks1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camacho C, Coulouris G, Avagyan V, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10(1):421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casjens S. Prophages and bacterial genomics: what have we learned so far. Mol Microbio. 2003;49(2):277–300. doi: 10.1046/j.1365-2958.2003.03580.x. [DOI] [PubMed] [Google Scholar]
- 7.Cheng H, Zhang S, Huo YY, et al. Gilvimarinus polysaccharolyticus sp. nov., an agar-digesting bacterium isolated from seaweed, and emended description of the genus Gilvimarinus . Int J Syst Evol Microbiol. 2015;65(Pt 2):562–569. doi: 10.1099/ijs.0.065078-0. [DOI] [PubMed] [Google Scholar]
- 8.Creencia AR, Mendoza BC, Migo VP, et al. Degradation of residual jatropha oil by a promising lipase-producing bacterial consortium. Philipp J Sci. 2014;143(1):73–78. [Google Scholar]
- 9.Darling AE, Mau B, Perna NT. ProgressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE. 2010;5(6):e11147. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das D, Baruah R, Roy AS, et al. Complete genome sequence analysis of Pseudomonas aeruginosa N002 reveals its genetic adaptation for crude oil degradation. Genomics. 2015;105(3):182–190. doi: 10.1016/j.ygeno.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Dawar C, Aggarwal RK. Draft genome sequence of hydrocarbon-degrading Pseudomonas putida strain KG-4, isolated from soil samples collected from Krishna Godavari Basin in India. Genome Announc. 2015;3(3):e00590–e00615. doi: 10.1128/genomeA.00590-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dean AS, Crump L, Greter H, et al. Global burden of human brucellosis: a systematic review of disease frequency. PLoS Negl Trop Dis. 2012;6(10):e1865. doi: 10.1371/journal.pntd.0001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edelstein W, Iben I, Mueller O, et al. Radiofrequency ground heating for soil remediation: science and engineering. Environ Prog. 1994;13(4):247–252. doi: 10.1002/ep.670130413. [DOI] [Google Scholar]
- 14.Genouw G, de Naeyer F, van Meenen P, et al. Degradation of oil sludge by landfarming–a case-study at the Ghent harbour. Biodegradation. 1994;5(1):37–46. doi: 10.1007/BF00695212. [DOI] [Google Scholar]
- 15.Griffiths-Jones S, Bateman A, Marshall M, et al. Rfam: an RNA family database. Nucleic Acids Res. 2003;31(1):439–441. doi: 10.1093/nar/gkg006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassanshahian M, Zeynalipour MS, Musa FH. Isolation and characterization of crude oil degrading bacteria from the Persian Gulf (Khorramshahr provenance) Mar Pollut Bull. 2014;82(1-2):39–44. doi: 10.1016/j.marpolbul.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 17.Hazen TC, Dubinsky EA, DeSantis TZ, et al. Deep-sea oil plume enriches indigenous oil-degrading bacteria. Science. 2010;330(6001):204–208. doi: 10.1126/science.1195979. [DOI] [PubMed] [Google Scholar]
- 18.Holmes B, Popoff M, Kiredjian M, et al. Ochrobactrum anthropi gen. nov., sp. nov. from human clinical specimens and previously known as group Vd. Int J Syst Bacteriol. 1988;38(4):406–416. doi: 10.1099/0020771338-4-406. [DOI] [Google Scholar]
- 19.Holt JG, Krieg NR, Sneath PH, et al. Bergey’s Manual of Determinative Bacteriology. 9th Edition. Baltimore: Williams and Wilkins; 1994. [Google Scholar]
- 20.Jesubunmi CO. Isolation of oil-degrading microorganisms in spent engine oil-contaminated soil. J Biol Agric Healthcare. 2014;4(25):191–195. [Google Scholar]
- 21.Kavita B, Keharia H. Reduction of hexavalent chromium by Ochrobactrum intermedium BCR400 isolated from a chromium-contaminated soil. 3 Biotech. 2012;2(1):79–87. doi: 10.1007/s13205-011-0038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulkarni G, Dhotre D, Dharne M, et al. Draft genome of Ochrobactrum intermedium strain M86 isolated from non-ulcer dyspeptic individual from India. Gut Pathog. 2013;5:7. doi: 10.1186/1757-4749-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulkarni G, Shetty S, Dharne M, et al. Genome sequencing analysis reveals virulence-related gene content of Ochrobactrum intermedium strain 229E, a urease-positive strain isolated from the human gastric niche. FEMS Microbiol Lett. 2014;359(1):12–15. doi: 10.1111/1574-6968.12549. [DOI] [PubMed] [Google Scholar]
- 24.Kumar V, Singh S, Manhas A, et al. Bioremediation of petroleum hydrocarbon by using Pseudomonas species isolated from petroleum contaminated soil. Analysis. 2014;30(4):1771–1776. doi: 10.13005/ojc/300436. [DOI] [Google Scholar]
- 25.Lagesen K, Hallin P, Rødland EA, et al. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007;35(9):3100–3108. doi: 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larkin MA, Blackshields G, Brown N, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 27.Lincoln SA, Hamilton TL, Juárez AGV, et al. Draft genome sequence of the piezotolerant and crude oil-degrading bacterium Rhodococcus qingshengii strain TUHH-12. Genome Announc. 2015;3(2):e00268–e00315. doi: 10.1128/genomeA.00268-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25(5):955–964. doi: 10.1093/nar/25.5.0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marmur J, Doty P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962;5(1):109–118. doi: 10.1016/S0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- 30.Mata JA, Martínez-Cánovas J, Quesada E, et al. A detailed phenotypic characterisation of the type strains of Halomonas species. Syst Appl Microbiol. 2002;25(3):360–375. doi: 10.1078/0723-2020-00122. [DOI] [PubMed] [Google Scholar]
- 31.Möller LV, Arends JP, Harmsen HJ, et al. Ochrobactrum intermedium infection after liver transplantation. J Clin Microbiol. 1999;37(1):241–244. doi: 10.1128/jcm.37.1.241-244.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mudshingkar S, Choure A, Palewar M, et al. Ochrobactrum anthropi: an unusual pathogen: are we missing them. Indian J Med. Microbiol. 2013;31(3):306–308. doi: 10.4103/0255-0857.115664. [DOI] [PubMed] [Google Scholar]
- 33.Pham VH, Kim J, Jeong SW. Enhanced isolation and culture of highly efficient psychrophilic oil degrading bacteria from oil-contaminated soils in South Korea. J Environ Biol. 2014;35(6):1145–1149. [PubMed] [Google Scholar]
- 34.She YH, Zhang F, Xia JJ, et al. Investigation of biosurfactant-producing indigenous microorganisms that enhance residue oil recovery in an oil reservoir after polymer flooding. Appl Biochem Biotech. 2011;163(2):223–234. doi: 10.1007/s12010-010-9032-y. [DOI] [PubMed] [Google Scholar]
- 35.She YH, Wu WQ, Hang CC, et al. Genome sequence of Brevibacillus agri strain 5-2, isolated from the formation water of petroleum reservoir. Mar Genomics. 2014;18:123–125. doi: 10.1016/j.margen.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Silva DSP, de Lima Cavalcanti D, de Melo EJV, et al. Bio-removal of diesel oil through a microbial consortium isolated from a polluted environment. Int Biodeter Biodegr. 2015;97:85–89. doi: 10.1016/j.ibiod.2014.09.021. [DOI] [Google Scholar]
- 37.Swain MT, Tsai IJ, Assefa SA, et al. A post-assembly genome-improvement toolkit (PAGIT) to obtain annotated genomes from contigs. Nat Protoc. 2012;7(7):1260–1284. doi: 10.1038/nprot.2012.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamura K, Stecher G, Peterson D, et al. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tatusov RL, Galperin MY, Natale DA, et al. The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000;28(1):33–36. doi: 10.1093/nar/28.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tatusov RL, Natale DA, Garkavtsev IV, et al. The COG database: new developments in phylogenetic classification of proteins from complete genomes. Nucleic Acids Res. 2001;29(1):22–28. doi: 10.1093/nar/29.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teyssier C, Marchandin H, Jean-Pierre H, et al. Molecular and phenotypic features for identification of the opportunistic pathogens Ochrobactrum spp. J Med Microbiol. 2005;54(10):945–953. doi: 10.1099/jmm.0.46116-0. [DOI] [PubMed] [Google Scholar]
- 42.Velasco J, Romero C, López-Goñi I, et al. Evaluation of the relatedness of Brucella spp. and Ochrobactrum anthropi and description of Ochrobactrum intermedium sp. nov., a new species with a closer relationship to Brucella spp. Int J Syst Bacteriol. 1998;48(3):759–768. doi: 10.1099/00207713-48-3-759. [DOI] [PubMed] [Google Scholar]
- 43.Waranusantigul P, Lee H, Kruatrachue M, et al. Isolation and characterization of lead-tolerant Ochrobactrum intermedium and its role in enhancing lead accumulation by Eucalyptus camaldulensis . Chemosphere. 2011;85(4):584–590. doi: 10.1016/j.chemosphere.2011.06.086. [DOI] [PubMed] [Google Scholar]
- 44.Yuan Y, Lu Z, Huang L, et al. Biodegradation of nicotine from tobacco waste extract by Ochrobactrum intermedium DN2. J Ind Microbiol Biotechnol. 2007;34(8):567–570. doi: 10.1007/s10295-007-0212-x. [DOI] [PubMed] [Google Scholar]
- 45.Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18(5):821–829. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang F, She Y, Chai L, et al. Microbial diversity in long-term water-flooded oil reservoirs with different in situ temperatures in China. Sci Rep. 2012;2:760. doi: 10.1038/srep00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang F, Jiang X, Chai L, et al. Permanent draft genome sequence of Bacillus flexus strain T6186-2, a multidrug-resistant bacterium isolated from a deep subsurface oil reservoir. Mar Genomics. 2014;18:135–137. doi: 10.1016/j.margen.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 48.Zheng B, Zhang F, Chai L, et al. Permanent draft genome sequence of Geobacillus thermocatenulatus strain GS-1. Mar Genomics. 2014;18:129–131. doi: 10.1016/j.margen.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Zhou Y, Liang Y, Lynch KH, et al. PHAST: a fast phage search tool. Nucleic Acids Res. 2011;39(Suppl. 2):W347–W352. doi: 10.1093/nar/gkr485. [DOI] [PMC free article] [PubMed] [Google Scholar]