Abstract
Background
Mid-frontal and mid-lateral (F3/F4 and F7/F8) EEG asymmetry has been associated with motivation and affect. We examined alpha EEG asymmetry in depressed and healthy participants before and after Behavioral Activation treatment for depression; examined the association between alpha EEG asymmetry and motivational systems and affect; and evaluated the utility of alpha EEG asymmetry in predicting remission.
Methods
Depressed (n = 37) and healthy participants (n = 35) were assessed before and after treatment using a clinical interview, a task to measure baseline EEG, and questionnaires of behavioral activation and inhibition, avoidance, and affect.
Results
Alpha EEG asymmetry was significantly higher in depressed than healthy participants at pre-treatment, positively correlated with negative affect and behavioral inhibition, and inversely correlated with lower behavioral activation sensitivity.
Conclusions
Heightened alpha EEG asymmetry in depressed participants was significantly associated with increased behavioral inhibition and negative emotion and was independent of clinical remission.
Keywords: alpha EEG asymmetry, Behavioral Activation treatment, major depression, approach-related motivation, withdrawal-related motivation, avoidance
Introduction
Available evidence from studies on biologic mechanisms suggests that identifying neurophysiologic markers of major depression may advance efforts to identify underlying factors that characterize depression (Allen, Iacono, Depue, & Arbisi, 1993; Sutton & Davidson, 1997). Much of the focus has been on the role of the frontal cortex, which has been linked with impairment of information processing and with potential markers of treatment response (Cook et al., 2013). Specifically, research has focused on resting electroencepholagraphic activity in the alpha band (8–12Hz), which reflects a reliable index of relaxed wakefulness in the brain when an individual has their eyes closed. Alpha activity may be suppressed or ‘desynchronized’ when individuals open their eyes, engage in mental activity, become alert, or conversely, when drowsy (Pizzagalli, 2007). Alpha activity in healthy samples has been associated with emotional experience (Allen, Urry, Hitt, & Coan, 2004; Maxwell & Davidson, 2007; Tenke & Kayser, 2005) and internalized attention during the practice of meditation (Aftanas & Golocheikine, 2001). Moreover, greater alpha activity has been inversely associated with cognitive function and attention (Dujardin et al., 1993; Gevins & Smith, 2000; Ray & Cole, 1985; Rugg & Dickens, 1982).
Increased alpha activity has been conceptualized a psychophysiological trait-like characteristic distinguishable in current and remitted depressed participants from healthy participants (Brenner et al., 1986; Pollock & Schneider, 1989). Notably, frontal alpha EEG asymmetry, defined as the difference in alpha activity over right vs. left hemisphere of the brain, has been observed such that higher scores indicate greater relative left activation (i.e., increased right alpha activity). More specifically, data has shown that current and remitted depression is associated with increased left (versus right) alpha activity, which corresponds to decreased left (versus right) hemispheric activation, though this pattern has not always been reported (Allen et al., 1993; Debener et al., 2000; Gotlib, Ranganath, & Rosenfeld, 1998; Henriques & Davidson, 1991; Mathersul, Williams, Hopkinson, & Kemp, 2008; Rosenfeld, Baehr, Baehr, Gotlib, & Ranganath, 1996; Stewart, Bismark, Towers, Coan, & Allen, 2010; Thibodeau, Jorgensen, & Kim, 2006). The magnitude of average effects associated with studies of depressed and healthy participants on resting frontal alpha EEG asymmetry indicates mean weighted effect sizes by Cz reference scheme of κ = 16, and mean weighted effects of r = .29 (95% CI = .24, .34). Also, mean weighted effects linked with the mid-frontal (F3/4) site were bigger than the lateral frontal (F7/8; Thibodeau et al., 2006). However, several studies have not been able to differentiate depressed from healthy participants in alpha EEG asymmetry (Davidson, Pizzagalli, Nitschke, & Putnam, 2002; Reid, Duke, & Allen, 1998).
Such inconsistent results may be explained by different measures of depression, smaller patient samples with heterogeneous symptoms, and varied recruitment strategies. Specifically, inadvertent enrolling of bipolar versus unipolar patients may have altered results (Lieber & Prichep, 1988) in which each diagnosis may have a unique neurophysiologic signature that is not necessarily observed in milder depression or sufficiently measured using a cursory assessment of depression symptoms (e.g., self-reports of depression). Also, consistent with the idea that alpha asymmetry is a reliable and stable psychophysiological marker of depression versus a state-specific factor (Coan & Allen, 2003; Sutton & Davidson, 1997), Allen and colleagues’ careful research (Allen et al., 2004) found high internal consistency coefficients for alpha asymmetry at frontal regions (F34, F78, and FTC12) across reference schemes ranging from .86 to .89, with a median of .89 at baseline, and from .61 to .92, with a median of .86 across five assessment sessions. Further, Tomarken, Davidson, Wheeler, and Kinney (1992) evaluated the psychometric properties of frontal EEG asymmetry in 85 college students twice (separated by three weeks), and observed that EEG asymmetry assessed at mid-frontal and anterior temporal regions, under both Cz-referenced and computer averaged ears referenced data, showed high internal consistency (Cronbach’s alphas ranging from .81 to .92) with acceptable test-retest stability, showing intraclass correlations ranging from .53 to .72 (Tomarken, Davidson, Wheeler, & Kinney, 1992). Finally, in support of the contention that frontal EEG asymmetry represents a stable neurophysiological marker, data has shown that frontal EEG asymmetry has 60% stable trait variance (Hagemann, Naumann, Thayer, & Bartussek, 2002), potentially serving as a vulnerability to first onset of depression (Nusslock et al., 2011) and predictive of reoccurrence (Allen, McKnight, Moreno, Demaree, & Delgado, 2009).
Alpha activity has been associated with approach- and withdrawal-related motivation measured by the Behavioral Activation System and Behavioral Inhibition System Scales (Gray, 1970). Specifically, less relative left frontal alpha activity (greater left activation) has been associated with heightened behavioral activation sensitivity, or an increased motivation to approach when goal-directed action is indicated (Coan & Allen, 2003; Sutton & Davidson, 1997). Also, stimuli intended to elicit approach-oriented responses, including reward cues and anger-evoking stimuli, have been associated with lower relative left frontal alpha activity (Coan & Allen, 2004; Harmon-Jones & Allen, 1998). These data support an association between behavioral activation sensitivity in the context of both pleasant and unpleasant (i.e., including anger-inducing) stimuli (Harmon-Jones & Allen, 1998). The link between alpha activity and BAS scores may also be explained by decreased right frontal activation, as opposed to increased left frontal activation, still indicating decreased relative left alpha activity (Coan & Allen, 2003). Conversely, less alpha activity over the right prefrontal regions (greater relative right activation) has been associated with measures of behavioral inhibition (Buss, Davidson, Kalin, & Goldsmith, 2004; Shackman, McMenamin, Maxwell, Greischar, & Davidson, 2009). The consistency and strength of association between EEG asymmetry and behavioral activation and inhibition sensitivity suggests that they strongly relate to an individual’s tendency to approach or avoid. Minimal research, however, has linked frontal EEG asymmetry to avoidance behavior in depressed individuals before and after a treatment designed to modify behavioral avoidance.
Finally, as frontal EEG asymmetry has been linked with affect, data have indicated an association between greater relative left prefrontal activation (i.e., less alpha activity) with positive affect measured by the Positive and Negative Affect Schedule (PANAS; Tomarken, Davidson, Wheeler, & Doss, 1992). Conversely, greater relative right prefrontal activation (i.e., more alpha activity) has been associated with negative personality factors (Sutton & Davidson, 1997) and more intense reactions to negative films (Tomarken, Davidson, & Henriques, 1990) in addition to trait anxiety and negative emotions (Coan & Allen, 2003). This differential association between frontal EEG asymmetry and positive and negative affect may indicate a biological substrate of affect (Sutton & Davidson, 1997).
Alpha activity over the occipital sites has predicted treatment response (Bruder et al., 2008). Also, there has been support for alpha power as a state-independent neurophysiologic characteristic in treatment-seeking samples early in treatment. Higher alpha-theta ratios derived from frontal QEEG assessments at pre-treatment and Week 1 of escitalopram treatment have predicted 8-week outcomes (Leuchter et al., 2009a), as well as differential response to antidepressants (Leuchter et al., 2009b). They have also predicted shorter time to remission during treatment with significantly more depressed patients attaining sustained remission (Cook et al., 2013). Additionally, significantly greater alpha amplitude in eyes closed versus eyes open conditions has predicted response to antidepressants (Tenke et al., 2011). However, frontal EEG asymmetry has been observed to change in response to interventions, such as Cognitive Behavioral Treatment (CBT) for Post-Traumatic Stress Disorder (PTSD; Rabe, Zoellner, Beauducel, Maercker, & Karl, 2008), which found a greater reduction in relative right anterior activation in the participants receiving CBT when compared to a Wait-list condition. Significant findings revealed a shift in hemispheric asymmetry, mainly due to an effect of a decreased alpha activity in the right [t(16) = 2.33, p = .03] but not the left anterior region [t(16) = 0.69, p = .50]. Conversely, participants in the Wait-list condition exhibited no shift in hemispheric activity over time. However, with an intervention using mindfulness meditation with suicidal adults, Barnhofer and colleagues (2007) showed significant pre to post-decreases in F4/F3 asymmetry scores in the treatment as usual group, whereas no significant pre to post changes were evident in the Mindfulness Behavioral Cognitive Treatment (MBCT) group (Barnhofer et al., 2007). In summary, there are inconsistent findings regarding the stability and independence of cortical activity with and without interventions for depression and no study of frontal EEG asymmetry before and after treatment for depression.
Behavioral Activation treatment (BA) is an evidence-based treatment for depression (Dimidjian, Barrera, Martell, Munoz, & Lewinsohn, 2011; Martell, Dimidjian, & Herman-Dunn, 2010) and aims to alter inhibitory and exploratory behavior and modify cognitive activity (Jacobson et al., 1996). BA for major depression proposes that symptom reduction occurs via monitoring daily activities to identify patterns of low reward and mood, assessing and assigning tasks that generate pleasure and competence, understanding and reducing unproductive avoidance, and improving skill deficits to promote action towards treatment goals (Cuijpers, van Straten, & Warmerdam, 2007; Daughters et al., 2008; Dimidjian et al., 2006; Jacobson et al., 1996). There has been minimal research to clarify the extent to which BA may influence frontal EEG asymmetry in depressed patients, and the extent to which frontal EEG asymmetry underlies the changes in behavioral inhibition and activation, cognitive and behavioral avoidance, and positive and negative affect that occur during BA.
This study aimed to test the extent to which depressed, relative to healthy participants, show greater alpha EEG asymmetry and whether there are unique associations between frontal alpha EEG asymmetry, motivation, and affect among depressed versus healthy participants. This study also aimed to test whether frontal alpha EEG asymmetry would not change with BA treatment in depressed participants in an attempt to clarify conflicting results from prior findings, but that pre-treatment frontal alpha EEG asymmetry, controlling for motivation and affect, would predict post-treatment depression severity. We hypothesized:
Depressed relative to healthy participants will exhibit significantly greater frontal alpha EEG asymmetry scores at pre-treatment.
Frontal alpha EEG asymmetry will not be correlated with depressive severity, but will be correlated with Behavioral Inhibition Sensitivity, negative affect, and self-reported avoidance in depressed participants at pre-treatment.
Given the research on the stability of frontal alpha EEG asymmetry, symptomatic and remitted depressed patients will differ from healthy (never-disordered) participants at post-treatment.
Based on findings from aforementioned treatment studies on predictive utility of frontal alpha EEG asymmetry, pre-treatment frontal alpha EEG asymmetry will predict remission status at post-treatment in depressed participants.
Methods and Materials
Participants
Participants were 72 males and females (aged 18–65, M = 35.65y, SD = 13.05y), including 37 participants diagnosed with current major depressive disorder per the Structured Clinical Interview for the DSM-IV Axis I Disorders, (SCID; First, Spitzer, Gibbon, & Williams, 2002) and a score ≥ 24 on the Inventory of Depressive Symptomatology-Clinician-Rated, (IDS-C; Rush et al., 2003; Rush et al., 1986). Thirty-five healthy participants were enrolled with no lifetime history or current presentation of psychiatric symptoms per the SCID and a score ≤ 11 on the IDS-C. To enroll, all participants had to be medically healthy, unmedicated with no recent medication wash out, and between ages 18 and 65 years. Depressed participants were excluded if they had current diagnoses of bipolar I or II, psychosis, obsessive-compulsive disorder, substance abuse/dependence, borderline, schizoid, schizotypal, or antisocial personality disorders. There was available EEG data at pre-treatment for 35 depressed and 37 healthy participants. At post-treatment, EEG data was available for 29 depressed and 26 healthy participants.
Procedure
Participants were initially screened via phone to ensure eligibility, and then invited to the laboratory for two occasions, separated by one week. In the first session, participants provided informed consent, passed a toxicology urine screen, and completed the clinical interview and self-report questionnaires. On the day of the psychophysiological assessment (second session), participants were asked to come with their hair washed and free of styling products. They were also asked to refrain from smoking or drinking caffeine within two hours of testing. Upon arrival, participants were asked to sign a consent form and take a urine toxicology screen, and were then seated in a comfortable chair in a light and sound attenuated room. Participants completed a second toxicology urine screen and the EEG assessment, in which sensors were applied, high quality data was assured, and 8 1-minute periods of resting EEG data were collected, half with of eyes open (O) and half with eyes-closed (C) (order counterbalanced that coordinated with event sequence in raw EEG). For the EEG assessment, participants were seated in a sound-attenuated room in a recliner. Compensation was offered upon completion. Thereafter, depressed participants were scheduled for their first of 16 treatment sessions to start the following week, and healthy participants were evaluated prospectively for 16 weeks. Compensation and debriefing was offered upon study completion.
Measures
Trained clinical psychology masters and doctoral students conducted psychiatric evaluations. All evaluators were supervised to prevent rater drift.
Structured Clinical Interview for the DSM-IV Axis I Disorders, Outpatient Version (SCID-I, First et al., 2002)
The SCID is a semi-structured interview that collects demographic information (age, years of education, marital and employment status), as well as clinical data (severity of depression, lifetime and current DSM-IV Axis I diagnoses). The SCID has adequate inter-rater reliability with kappa values for modules reported to be between .7 and 1 (First, Spitzer, Gibbon, & Williams, 1995). Our evaluators underwent a training program with SCID training tapes (Spitzer, Williams, Gibbon, & First, 1989), formal training, observing and demonstrating SCID competency, and then co-rating and reviewing SCID interviews. Our reliability checks of five separate tapes yielded κ≥0.8 for the Mood and Anxiety modules.
Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II; First, Gibbon, & Spitzer, 1997)
The SCID-II is a 47-item self-report measure in which participants give ‘yes’ or ‘no’ responses to items probing for symptoms of Axis II disorders. All ‘yes’ responses are further probed by clinical interview to exclude those meeting criteria for Antisocial, Borderline, Schizotypal, or Schizoid Personality Disorder.
Longitudinal Follow-up Evaluation (LIFE; Keller et al., 1987)
The LIFE is a semi-structured retrospective interview focused on psychiatric status (e.g., onset, offset, duration of symptoms). The LIFE captures weekly status of DSM-IV depressive symptoms, asking the rater to assign a Psychiatric Status Rating (PSR) for each week. Data is recorded using the PSRs to reflect minimal symptoms (PSRs 1–2) to moderate to severe symptoms (PSRs 3–6).
Inventory of Depressive Symptomatology – Clinician-Rated (IDS-C; Rush, Carmody, & Reimitz, 2000; Rush et al., 1986)
IDS-C is a validated 30-item clinician-rated measure that assesses DSM-IV symptom domains for MDD, including mood, cognitive, psychomotor, and vegetative, to diagnose a Major Depressive Episode and commonly associated symptoms (e.g., anxiety, somatization). Inter-rater reliability estimates from this study’s sample yielded a Krippendorff’s alpha ratio of .87 for IDS-C total scores. Cronbach alpha values for our sample were .62 for the depressed group and .56 for the healthy group.
Inventory of Depressive Symptomatology – Self-Rated (IDS-SR; J. A. Rush et al., 1986
The IDS-SR is a 30-item patient self-rated measure that corresponds to the IDS-C, used for the purpose of comparing subjective and clinician-rated depression severity. Convergent validity with the IDS-C in our sample was strong, with correlations of .96 at pre-treatment and .91 at post-treatment. Cronbach alpha estimates for our sample were .73 for the depressed group and .59 for the healthy group.
Behavioral Inhibition System/Behavioral Activation System (BIS/BAS scales; Carver & White, 1994)
The BIS/BAS is a 20-item self-report form. Seven items measure the Behavioral Inhibition System (i.e., aversive motivation) and 13 items measure the Behavioral Activation System (i.e., appetitive motivation). Responses are recorded using a four-point Likert scale ranging from strong agreement (1 = very true for me) to strong disagreement (4 = very false for me). Behavioral Inhibition is measured using a single subscale relevant to punishment sensitivity. The BAS scale contains three subscales: (1) Drive, (2) Reward Responsiveness, and (3) Fun Seeking, representing behavioral, affective, and combined behavioral-affective motivational responding to reward. Cronbach alpha estimates in our sample were equivalent for healthy and depressed participants on BIS (.78 and .77, respectively), BAS Reward (.85 and .61, respectively), BAS Drive (.83 and .75, respectively), and BAS Fun Seeking (.75 and .53).
Positive Affect and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988)
The PANAS is a 20-item self-report measure, divided into two ten-item subscales and measures activation of positive and negative affect. The PANAS consists of a list of 20 emotion states. The rater is asked to indicate the extent to which the rater has experienced this emotion in the preceding seven days, using a five-item Likert scale ranging from ‘Very slightly or Not at all’ to ‘Extremely’. The subscales of the PANAS have been shown to be reliable: Cronbach alphas were .89 for positive affect and .85 for negative affect in a normative sample (Crawford & Henry, 2004). In our sample, Cronbach alpha estimates were .86 for positive affect in the depressed group and .87 in the healthy group. For negative affect, internal consistency estimates were .85 in the depressed group and .92 in the healthy group.
Cognitive Behavioral Avoidance Scale (CBAS; Ottenbreit & Dobson, 2004)
The Cognitive Behavioral Avoidance Scale is a 31 item self-report measure that assesses four reliable factors reflecting combinations of cognitive/behavioral and social/nonsocial dimensions of avoidance. Participants are asked to identify the extent to which they use strategies to respond to situations and problems, using a rating system (1 = not at all true to 5 = extremely true). The cognitive social subscale items represent avoidance or passivity of addressing relationship problems, waiting for social tension to change, and failing to address tension in friendships. The behavioral social dimension refers to social withdrawal during social activities, avoiding opportunities to meet with the opposite sex, and not responding to invitations to be social. The CBAS has been shown to be a valid and reliable measure of avoidance in an undergraduate student population (Ottenbreit & Dobson, 2004). Cronbach alphas were generally equivalent for the depressed and healthy participants on the behavioral social avoidance subscale (.81 and .51, respectively), on the behavioral nonsocial subscale (.74 and .67, respectively), on the cognitive social avoidance subscale (.78 and .79), and on the cognitive nonsocial avoidance subscale (.89 and .84, respectively).
EEG Recording, Reduction, and Analyses
EEG was recorded for 8 minutes while participants were awake and relaxed (Mindware Technologies, Gahanna, OH). Using a stretch-lycra cap with Ag/AgCl electrodes, continuous EEG was collected over 20 scalp sites using the International 10–20 system (AF3/AF4, F3/F4, F7/F8, FC1/FC2, FC5/FC6, C3/C4, T7/T8, P3/P4, P7/P8, O1/O2, referenced to M1 with M2 as an active channel and the area between Fz and Fpz as the participant ground; bandpass 0.01–100 Hz with 60 Hz notch; impedances < 5kΩ with homologs within ± 1kΩ; digitization rate 500 Hz). Offline, a digital average mastoid reference, (M1+M2)/2, was performed.
Following data recording, a technologist who was uninvolved in data collection and blind to participant identity visually inspected the EEG data for eye movements and muscle artifacts, and data with artifacts were rejected from all data channels. Then, we applied a principal-component-based spatial filter that was designed to remove ocular artifact components while sparing frontal EEG components (Ford, Sands & Lew, 2004; as implemented in EMSE 5.5.1, 2013). Power spectra were derived by means of a fast Fourier transform with a Hamming window (50% overlap) for each two second epoch within each baseline type (eyes open, eyes closed) for each channel for each participant, thus EEG finding are specific to both open and closed eyes. Thus, each one minute baseline type contained 59 overlapping two-second epochs. The median power spectrum, which is robust with respect to outliers, was obtained by rank ordering the 59 Fourier transformed magnitudes at each 0.5 Hz frequency bin and each channel and then selecting the median (middle) value. This processing was conducted using a 50% trimmed mean in EMSE version 5.5.1 (Source Signal Imaging, Inc., La Mesa, CA). Half Hertz frequency bins in the 8–13 Hz range were averaged to produce band average median alpha power.
Frontal EEG asymmetry scores were calculated over the midfrontal sites, subtracting the natural log of the alpha power of the electrode in the left hemisphere (F3 or F7) from that of the right frontal electrode (F4 or F8). A higher score thus reflected greater relative left versus right frontal activation (e.g., relatively higher right alpha activity; Coan & Allen, 2004; Lindsley & Wicke, 1974). However, we were interested in asymmetry scores in general, and therefore, the absolute value of this difference score was taken. Using the natural log transformation is commonly used in EEG asymmetry research as EEG power appears to be positively skewed (Tomarken, Davidson, Wheeler, & Doss, 1992). The choice of sites was based on previous research (i.e., F3/F4; Shackman et al., 2009). Mean Cronbach’s alpha internal consistency estimates for resting alpha asymmetry as a function of length of recording (eight 1 minute recordings) were 0.96 and 0.95 for F4 and F3 at pre- and post-treatment in the depressed group and 0.95 and 0.94 in the healthy sample.
Treatment
Weekly sessions for 16 consecutive weeks and a BA patient workbook (Addis & Martell, 2004) were offered. Clinicians were offered two clinician handbooks (Martell, Addis, & Jacobson, 2001; Martell et al., 2010), training, supervision, and integrity reviews by off-site experts. Patients learned the BA model of depression, monitored daily activities, assessed pleasure and competence, assigned or scheduled activities that promoted mastery or pleasure, rehearsal of scheduled activities, clarified patterns of avoidance, identified goals, reduced rumination, and modified skill deficits. Clinicians included postdoctoral fellows in clinical psychology (n = 2) and licensed clinical psychologists (n = 2) trained with one pilot case and then were assessed for competency thereafter. Study clinicians underwent competency reviews by off-site BA experts, Drs. Christopher Martell and Ruth Herman-Dunn. These experts reviewed 10% of audiotaped treatment sessions and issued independent ratings using the Behavioral Activation Treatment Scale (BATS; Jacobson et al., 1996), a 16-item measure designed to assess core competencies of BA (Dimidjian et al., 2006; Jacobson et al., 1996), using ratings assigned on a scale of 1 to 6 (1 = Poor, 6 = Excellent). Clinicians achieved competency ratings over the minimum requirement of 60 (M = 68.26, SD = 5.21, Range = 63–75). Finally, clinicians exhibited strong adherence to BA, per assessments conducted by trained evaluators (KHF, DH) using the BA items from the Collaborative Study Psychotherapy Rating Scale (CSPRS; Hollon et al., 1998). The adherence measure outlined 28 items, rated on a 0–7 scale. After establishing inter-rater reliability (.86), adherence scores on 20% of the completer sample were generated (M = 4.96, SD =.37).
Statistical Analyses
Analyses of demographic and clinical characteristics at pre-treatment were conducted using one sample t-tests or Analysis of Variance (ANOVA) for continuous variables and Chi-square tests of independence for categorical variables. In the presence of small or empty cells in the tests of categorical variables, the Chi-square test was replaced by Fisher’s exact test. Analyses included partial and full completers using the definitions from prior BA trials (Dimidjian et al., 2006; Jacobson et al., 1996). Partial completers (attended ≥ 5 – 11 sessions, n = 4) and completers (attended ≥ 12 sessions, n = 32) completed an average of 13.43 (SD = 1.68), or 84%, of the 16 available sessions. Analyses differentiating change in BA treatment defined remission as IDS-C ≤ 11 at post-treatment and at PSR of 1 or 2 at post-treatment (Frank et al., 1991).
A one way ANOVA to examine differences in alpha EEG asymmetry between the depressed and healthy groups at pretreatment was conducted. Analyses were two-tailed at the .05 level of significance. Two separate repeated measures ANOVAs to test group effects (depressed versus healthy) over time (pre- and post-treatment status) focused on F4-F3 alpha asymmetry and F8-F7 alpha asymmetry. This was conducted to retain the intent to treat sample. Pearson correlations were conducted to test the total alpha power at each site (F3, F4, F7 and F8) at pre- and at post-treatment with the scores on the BIS, BAS subscales, CBAS, and PANAS. All correlations were computed within diagnostic groups (depressed and healthy). Finally, logistic regressions were computed to evaluate the predictive use of pre-treatment F4-F3 and F8-F7 alpha asymmetry scores to identify participants who remit after a course of BA treatment. Alpha asymmetry scores were entered in separate logistic regressions as predictor variables for the categorical dependent variable of remission measured at post-treatment.
Results
Demographic and Clinical Characteristics
Demographics are presented in Table 1. Of the depressed participants (Mage = 36, SDage = 12, Rangeage = 22–62), 70% were female (n = 26) and 41% had completed college (n = 15). Over half of the participants were Caucasian (n = 22; 59.5%), a third were African American (n = 11; 29.7%) and the rest were Hispanic (n = 4; 10.8%).
Table 1.
Demographic and Clinical Characteristics
| MDD (n=37) | HV (n=35) | Relevant Statistic | p value | |||
|---|---|---|---|---|---|---|
| n | (%) | (%) | ||||
| Sex | ||||||
| Male | 11 | (29.7) | 16 | (45.7) | 1.96 | .16 |
| Female | 26 | (70.3) | 19 | (54.3) | ||
| Race | ||||||
| Caucasian | 22 | (59.5) | 19 | (54.3) | ||
| Black | 11 | (29.7) | 11 | (31.4) | 5.97 | .11 |
| Hispanic | 4 | (10.8) | 1 | (2.9) | ||
| Other | 0 | (0.0) | 4 | (11.4) | ||
| Education | ||||||
| Under 7 years of school | 0 | (0.0) | 1 | (2.9) | ||
| Partial high school | 1 | (2.7) | 0 | (0.0) | ||
| High school graduate | 2 | (5.4) | 1 | (2.9) | 5.22 | .39 |
| Partial college | 14 | (37.8) | 14 | (40.0) | ||
| College graduate | 15 | (40.5) | 18 | (51.4) | ||
| Graduate training | 5 | (13.5) | 1 | (2.9) | ||
| Marital Status | ||||||
| Never married | 27 | (73.0) | 24 | (68.6) | ||
| Married | 2 | (5.4) | 6 | (17.1) | ||
| Separated | 1 | (2.7) | 0 | (0.0) | 3.52 | .47 |
| Divorced | 6 | (16.2) | 4 | (11.4) | ||
| Common Law | 1 | (2.7) | 1 | (2.9) | ||
| Employment | ||||||
| Unemployed | 14 | (37.8) | 6 | (17.1) | ||
| Employed | 18 | (48.6) | 19 | (54.3) | 7.27 | .06 |
| Full-time student | 5 | (13.5) | 6 | (17.1) | ||
| Retired | 0 | (0.0) | 4 | (11.4) | ||
|
| ||||||
| M | (SD) | M | (SD) | |||
|
| ||||||
| Age | ||||||
| Years | 36.2 | (12.4) | 35.1 | (13.7) | .13 | .72 |
Chi-square analyses revealed no group differences on demographic characteristics between healthy and depressed groups (χgender = 1.9, ns; χethnicity = 5.9, ns; χemployment= 7.2, ns; χmarital status= 3.5, ns). Group differences were found on severity of depression at pre-treatment, FIDS-R (1, 71) = 366.1, p < 0.01; MHealthy = 3.1 (SE = 0.5, 95% CI: 2.1, 4.1), MDepressed = 33.2 (SE = 1.4, 95% CI: 30.2, 36.2), and at post-treatment, FIDS-C (1, 71) = 529.7, p < 0.01, MHealthy = 2.2 (SE = 0.4, 95% CI: 1.3, 3.1); MDepressed = 33.7 (SE = 1.2, 95% CI: 31.1, 36.1). The average age of onset of depression was 30.73 years (SD = 23.3) and 67% reported recurrent depression. Slightly less than half of participants (n = 15, 40.5%) had received treatment prior to study enrollment. Within this sample, 8 participants received consultation for treatment or described participating in a brief period of treatment (21.6%), 3 participants received continuous treatment for six months or several brief periods (8.1%), and 4 participants received continuous treatment lasting one year or more or numerous brief periods (10.8%). All participants were unmedicated at the start of treatment.
The mean and variance of scores on self-reported scales in healthy and depressed participants are in Table 2. Depressed participants showed significantly less behavioral activation sensitivity, more behavioral inhibition sensitivity, greater negative affect, decreased positive affect.
Table 2.
Clinical Measures at Pre- and Post-Treatment
| Measure | MDD (n = 37)a | HV (n = 35)b | F(df) | p value | ||
|---|---|---|---|---|---|---|
| M | SD | M | SD | |||
| IDS-C | ||||||
| Pre-treatment | 33.73 | 7.69 | 2.26 | 2.58 | F(1, 71) = 529.78 | < .001 |
| Post-treatment | 12.81 | 8.60 | 2.24 | 2.77 | F(1, 63) = 44.83 | < .001 |
| IDS-SR | ||||||
| Pre-treatment | 33.19 | 8.82 | 3.17 | 2.97 | F(1, 71) = 366.12 | < .001 |
| Post-treatment | 15.26 | 10.69 | 3.03 | 4.64 | F(1, 63) = 35.98 | < .001 |
| BIS | ||||||
| Pre-treatment | 20.97 | 3.57 | 15.8 | 3.55 | F(1, 71) = 38.00 | < .001 |
| Post-treatment | 20.06 | 4.33 | 15.55 | 3.76 | F(1, 63) = 19.96 | < .001 |
| BAS Reward | ||||||
| Pre-treatment | 14.81 | 3.23 | 17.54 | 1.74 | F(1, 71) = 19.65 | < .001 |
| Post-treatment | 15.97 | 3.77 | 17.45 | 1.94 | F(1, 63) = 4.00 | .05 |
| BAS Drive | ||||||
| Pre-treatment | 8.92 | 2.94 | 11.26 | 2.33 | F(1, 71) = 13.89 | < .001 |
| Post-treatment | 9.68 | 2.94 | 12.15 | 2.40 | F(1, 63) = 13.69 | < .001 |
| BAS Fun | ||||||
| Pre-treatment | 9.89 | 2.32 | 12.00 | 1.91 | F(1, 71) = 17.62 | < .001 |
| Post-treatment | 11.32 | 2.80 | 12.33 | 2.34 | F(1, 63) = 2.47 | .12 |
| PANAS-PA | ||||||
| Pre-treatment | 18.24 | 6.12 | 38.74 | 5.80 | F(1, 71) = 212.36 | <.001 |
| Post-treatment | 31.42 | 8.68 | 38.55 | 7.33 | F(1, 63) = 12.65 | <.001 |
| PANAS-NA | ||||||
| Pre-treatment | 27.57 | 8.33 | 12.06 | 3.86 | F(1, 71) = 100.73 | < .001 |
| Post-treatment | 21.32 | 7.40 | 13.24 | 4.96 | F(1, 63) = 26.62 | < .001 |
| CBAS-Cog-S | ||||||
| Pre-treatment | 15.92 | 5.60 | 9.63 | 3.53 | F(1, 71) = 32.04 | < .001 |
| Post-treatment | 12.81 | 4.51 | 9.70 | 3.29 | F(1, 63) = 10.00 | < .01 |
| CBAS Cog-NS | ||||||
| Pre-treatment | 22.89 | 9.19 | 14.20 | 5.71 | F(1, 71) = 22.94 | < .001 |
| Post-treatment | 20.00 | 8.33 | 14.09 | 5.60 | F(1, 63) = 11.22 | < .001 |
| CBAS Beh-S | ||||||
| Pre-treatment | 19.41 | 6.87 | 11.49 | 2.96 | F(1, 71) = 39.57 | < .001 |
| Post-treatment | 16.16 | 6.70 | 10.85 | 3.02 | F(1, 63) = 17.07 | < .001 |
| CBAS-Beh-NS | ||||||
| Pre-treatment | 14.16 | 4.72 | 8.43 | 2.74 | F(1, 71) = 39.14 | < .001 |
| Post-treatment | 11.87 | 4.46 | 8.15 | 2.21 | F(1, 63) = 18.24 | < .001 |
Note: IDS-C = Inventory of Depressive Symptomatology – Clinician; IDS – SR = Inventory of Depressive Symptomatology – Self-Report; BIS = Behavioral Inhibition System, BAS Reward = Behavioral Activation System Reward Subscale, BAS Drive = Behavioral Activation System Drive Subscale, BAS Fun = Behavioral Activation System Fun Seeking Subscale, PANAS-PA = Positive Affect Negative Affect Scale Positive Affect Subscale, PANAS-NA = Positive Affect Negative Affect Scale Negative Affect Subscale. CBAS-Cog-S = Cognitive Behavioral Avoidance Scale Cognitive Social Subscale, CBAS-Cog-NS = Cognitive Behavioral Avoidance Scale Cognitive
Nonsocial Subscale, CBAS-Beh-S = Cognitive Behavioral Avoidance Scale Behavioral Social Subscale, CBAS-Beh-NS = Cognitive Behavioral Avoidance Scale Behavioral Nonsocial Subscale.
Post-treatment n = 31,
Post-treatment n = 33
Frontal Alpha EEG Asymmetry Differences
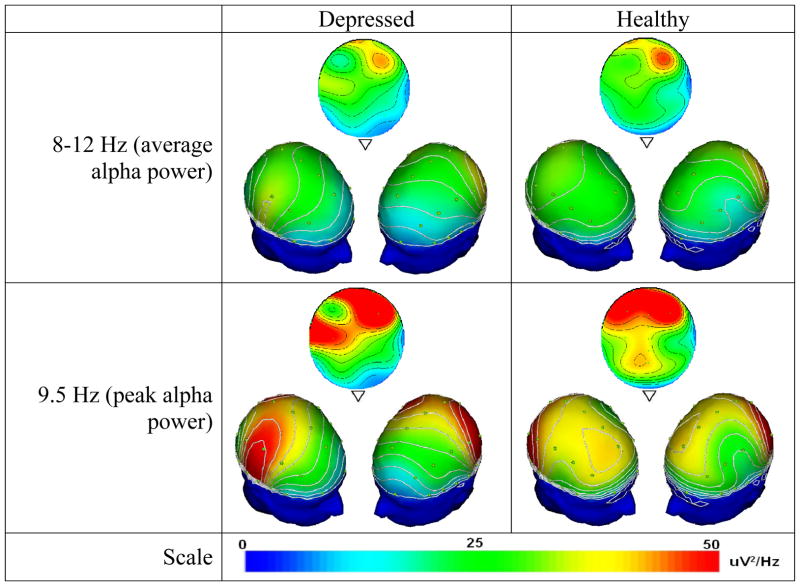
Table 3 outlines means and standard deviations of alpha power at F3, F4, F7, F8, and alpha asymmetry in depressed and healthy groups. A one way ANOVA to examine differences in alpha EEG asymmetry by two groups (depressed v. healthy) at pretreatment indicated that depressed participants showed a significantly higher F4-F3 alpha asymmetry (M = 0.43, SE = 0.12, 95% CI: 0.18, 0.67) than the healthy participants (M = 0.16, SE = 0.05, 95% CI: 0.18, 0.67). No differences were found for F8-F7 alpha asymmetry and for absolute alpha power at each site at pre-treatment. Figure 1 shows that depressed participants have a more diffuse pattern of alpha activation from the anterior to posterior part of the brain unlike healthy participants who retain focused frontal right alpha activity. The contrast between groups appears most strongly at the 9.5 Hz peak, although it remains evident via the contour lines after averaging across 8–12 Hz.
Table 3.
EEG Estimates for Sites and Asymmetry Scores
| EEG measure | MDD (n = 37)a | HV (n = 35)b | F(df) | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | SE | 95% CI | M | SD | SE | 95% CI | |||
| F3 | ||||||||||
| Pre-treatment | −0.26 | 0.86 | 0.14 | −0.54, 0.03 | −0.37 | 0.91 | 0.15 | −0.69, −0.06 | F(1, 71) = 0.31 | .58 |
| Post-treatment | −0.02 | 1.38 | 0.26 | −0.54, 0.51 | −0.34 | 0.89 | 0.16 | −0.68, 0.00 | F(1, 57) = 1.09 | .30 |
| F4 | ||||||||||
| Pre-treatment | −0.36 | 0.98 | 0.16 | −0.69, −0.04 | −0.37 | 0.89 | 0.15 | −0.67, −0.06 | F(1, 71) = 0. | .98 |
| Post-treatment | 0.01 | 1.42 | 0.26 | −0.53, 0.55 | 0.31 | 0.80 | 0.15 | −0.62, 0.01 | F(1, 57) = 1.16 | .29 |
| F7 | ||||||||||
| Pre-treatment | −0.39 | 1.08 | 0.18 | −0.75, −0.03 | −0.38 | 0.87 | 0.15 | −0.68, −0.08 | F(1, 71) = 0.00 | .95 |
| Post-treatment | 0.06 | 1.07 | 0.20 | −0.35, 0.47 | −0.26 | 0.73 | 0.14 | −0.53, 0.02 | F(1, 57) = 1.68 | 0.20 |
| F8 | ||||||||||
| Pre-treatment | −0.26 | 0.78 | 0.13 | −0.52, −0.00 | −0.35 | 0.83 | 0.14 | −0.63, −0.06 | F(1, 71) = 0.19 | .66 |
| Post-treatment | 0.09 | 1.28 | 0.24 | −0.40, 0.58 | −0.27 | 0.74 | 0.14 | −0.55, 0.01 | F(1, 57) = 1.78 | .19 |
| F4 – F3c | ||||||||||
| Pre-treatment | 0.43 | 0.73 | 0.12 | 0.18, 0.67 | 0.16 | 0.27 | 0.05 | 0.06, 0.25 | F(1, 71) = 4.26 | .04* |
| Post-treatment | 0.22 | 0.19 | 0.03 | 0.15, 0.29 | 0.13 | 0.10 | 0.02 | 0.09, 0.18 | F(1, 54) = 4.41 | .04* |
| F8 – F7c | ||||||||||
| Pre-treatment | 0.33 | 0.59 | 0.10 | 0.14, 0.53 | 0.17 | 0.18 | 0.03 | 0.11, 0.24 | F(1, 71) = 2.41 | .13 |
| Post-treatment | 0.39 | 0.41 | 0.08 | 0.23, 0.55 | 0.24 | 0.18 | 0.03 | 0.17, 0.31 | F(1, 56) = 3.22 | .08 |
Note:
p < .05
Post-treatment for F3/F4 n = 29, for F7/F8 n = 28
Post-treatment for F3/F4 n = 26, for F7/F8 n = 29
Absolute values
Figure 1.
Topographical distributions of 8–12 Hz band average alpha power (top) and 9.5 Hz peak alpha power (bottom) for depressed (left) and healthy (right) participants, showing a top view stretched across all recording sites, along with two 3D-rotated views to display frontal laterality. Depressed participants assessed at pretreatment show a more diffuse anterior-to-posterior pattern of alpha activation (note contour lines) unlike healthy participants who retain focused right frontal activity. Both groups have a peak frequency at 9.5 Hz, at which the noted contrast is particularly evident. Contour lines are spaced at intervals of 5 μV2/Hz.
Two separate repeated measures analysis of variance (ANOVA) to test group effects (depressed versus healthy) over time (pre- and post-treatment status) focused on F4-F3 alpha asymmetry and F8-F7 alpha asymmetry. Results indicated that there was no significant interaction effect between group and time in F4-F3 alpha asymmetry F(1,53) = 0.88, p = 1.44, η2 = 0.01). Similarly, there was no main group effect, F(1,53) = 3.64, p = 0.62, η2 = 0.06), and no main effect of time F(1,53) = 2.23, p = 0.14, η2 = 0.04). Results for the F8-F7 alpha asymmetry showed no significant interaction effect between group and time F(1,53) = 1.35, p = 0.24, η2 = 0.02), no main effect for group F (1,53) = 3.80, p = 0.56), but there was a significant main effect for time, F(1, 53) = 6.67, p = .013, η2 = 0.11). Given that 67% of the depressed sample reported recurrent depression, we conducted GLM Repeated Measures with episode (single, recurrent) as between-subjects factor and F4-F3 alpha asymmetry as within-subjects factor. Results indicated that there was no significant interaction effect between group and time in F4-F3 alpha asymmetry F(1,25) = 1.26, p = 0.27, η2 = 0.04). Similarly, there was no main group effect, F(1,25) = 0.49, p = 0.48, η2 = 0.02), and no main effect of time F(1,25) = 2.82, p = 0.10, η2 = 0.10).
Additionally, correlation between F4-F3 alpha asymmetry at pre and post-treatment was not significant in the depressed subgroup with recurrent depression (r = 0.07, p = 0.71) and also statistically not significant in the single depression subgroup (r = 0.14, p = 0.37).
Correlational Analyses Between EEG Asymmetry, Depression, and Behavioral Measures
The healthy group exhibited no significant correlations between F4-F3 and F8-F7 alpha asymmetry indices and depression severity and behavioral measures at pre- and at post-treatment. Table 4 shows bivariate correlations between frontal EEG activity and measures of depression, avoidance, and approach at pre- and end-treatment in the depressed sample. The depressed group exhibited significant positive correlations between F4-F3 alpha EEG asymmetry and BIS, r (37) = 0.41, p = 0.05 and negative affect, r (37) = 0.39, p = 0.05, as well a significant inverse correlation between F4-F3 alpha EEG asymmetry and BAS Fun Seeking, r (37) = −0.41, p = 0.05 at pre-treatment. No significant correlations were observed between F4-F3 alpha asymmetry, depressive severity, and behavioral measures at post-treatment. In the depressed group, no significant correlations were observed between F8-F7 alpha asymmetry and behavioral measures at pre-treatment. However, significant positive correlations were observed between F8-F7 alpha asymmetry and BIS r (31) = 0.44, p = 0.05 and CBAS behavioral nonsocial avoidance subscale, r (31) = 0.44, p = 0.05 at post-treatment. No significant correlation was observed with depressive severity.
Table 4.
Bivariate Correlations Between Frontal Activity, Depression, Approach, and Avoidance in the Depressed Group
| Pre F4-F3 | Post F4-F3 | Pre IDS-SR | Post IDS-SR | Pre BIS | Post BIS | Pre BAS-Reward | Post BAS-Reward | Pre BAS-Drive | Post BAS-Drive | Pre BAS-Fun | Post BAS-Fun | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | .07 | .25 | −.12 | .41* | .34 | .22 | .16 | .19 | .06 | −.41* | −.19 |
| p | .72 | .14 | .54 | .01 | .06 | .18 | .38 | .25 | .74 | .01 | .31 | |
| 2 | .06 | − .20 | −.02 | −.02 | −.00 | .11 | .05 | −.04 | .07 | −.03 | ||
| p | .75 | .29 | .93 | .94 | .99 | .58 | .79 | .85 | .71 | .89 | ||
| 3 | .28 | .14 | .00 | −.01 | .11 | .04 | −.10 | −.36* | −.04 | |||
| p | .10 | .38 | .99 | .95 | .55 | .81 | .56 | .02 | .84 | |||
| 4 | −.08 | .14 | −.21 | −.18 | −.16 | .05 | −.33 | .14 | ||||
| p | .65 | .45 | .23 | .31 | .37 | .77 | .06 | .44 | ||||
| 5 | .44** | .54** | .05 | .20 | −.23 | .13 | −.12 | |||||
| p | .01 | .00 | .80 | .21 | .19 | .41 | .51 | |||||
| 6 | .29 | .10 | −.11 | −.22 | −.08 | .11 | ||||||
| p | .10 | .56 | .54 | .21 | .64 | .55 | ||||||
| 7 | .16 | .57** | −.13 | .51** | −.06 | |||||||
| p | .36 | .00 | .45 | .00 | .72 | |||||||
| 8 | .44** | .55** | .14 | .68** | ||||||||
| p | .01 | .00 | .43 | .00 | ||||||||
| 9 | .37* | .42** | .15 | |||||||||
| p | .03 | .01 | .40 | |||||||||
| 10 | .05 | .47** | ||||||||||
| p | .78 | .01 | ||||||||||
| 11 | .12 | |||||||||||
| p | .51 |
Note: Pre-tx= Pre-treatment; End-tx= End-Treatment; IDS-C = Inventory of Depressive Symptomatology – Clinician; IDS – SR = Inventory of Depressive Symptomatology –Self-Report; BIS = Behavioral Inhibition System, BAS Reward = Behavioral Activation System Reward Subscale, BAS Drive = Behavioral Activation System Drive Subscale, BAS Fun = Behavioral Activation System Fun Seeking Subscale, PANAS-PA = Positive Affect Negative Affect Scale Positive Affect Subscale, PANAS-NA = Positive Affect Negative Affect Scale Negative Affect Subscale. CBAS-Cog-S = Cognitive Behavioral Avoidance Scale Cognitive Social Subscale, CBAS-Cog-NS = Cognitive Behavioral Avoidance Scale Cognitive Nonsocial Subscale, CBAS-Beh-S = Cognitive Behavioral Avoidance Scale Behavioral Social Subscale, CBAS-Beh-NS = Cognitive Behavioral Avoidance Scale Behavioral Nonsocial Subscale.
p < .05,
p < .001
Utility of Alpha Asymmetry in Predicting BA Remission
The predictive use of pre-treatment alpha asymmetry for determining remission status at post-treatment was ascertained using logistic regression analyses. Results from the two logistic regression analyses using F4-F3 and F8-F7 alpha asymmetry did not predict group status (remitter, non-remitter, healthy). Multiple regression analyses indicated pre-treatment F4-F3 alpha asymmetry predicted negative affect at post-treatment, R = 0.43, Rsquare = 0.18, Radjusted = .16, SE = 6.75, F(1, 28) = 6.61, p = 0.01. We further examined this finding by using pre-treatment negative affect and F4-F3 alpha asymmetry as predictors of negative affect at end of treatment to test whether negative affect was stable over time in the depressed group and pre-treatment F4-F3 asymmetry remained a significant predictor. In this model, we obtained R = 0 .43, Rsquare = 0.18, Radjusted = 0.13, SE = 6.90 (F(1, 28) = 3.23, p = 0.05) with pre-treatment frontal alpha asymmetry as a significant predictor (β = 0.44, p = 0.02, 95% CI: 0.81, 9.29) whereas pre-treatment negative affect was not significant (β = −0.04, p = 0.80, 95% CI: −0.37, 0.29).
Discussion
The current study was designed to investigate (a) differences in frontal alpha EEG asymmetry in depressed participants relative to healthy controls, (b) the effect of BA treatment on frontal alpha EEG asymmetry after treatment, (c) the strength and direction of associations between frontal alpha EEG asymmetry and motivational systems, behavioral avoidance, and affective disposition, and (d) the predictive utility of alpha EEG asymmetry in determining remission status after 16 weeks of BA treatment. Results showed that at pre-treatment, depressed, relative to healthy participants, exhibited significantly greater frontal alpha EEG asymmetry. Across treatment, there was an absence of change in frontal alpha EEG asymmetry in healthy and depressed patients, even when patients reported a significant alpha reduction of depressive symptoms at post-treatment. In terms of correlations, among depressed participants at pre-treatment, alpha EEG asymmetry showed a significant positive correlation with behavioral inhibition and negative affect, and a significant inverse correlation with behavioral activation sensitivity (fun-seeking motivation). In comparison, at post-treatment, depressed participants with higher frontal alpha EEG asymmetry showed a positive correlation with behavioral inhibition and behavioral avoidance. No correlation was observed at pre- and at post-treatment between alpha EEG asymmetry and depression severity. Finally, alpha EEG asymmetry status at pre-treatment did not predict treatment response at post-treatment, though it was predictive of negative affect at post-treatment.
The performance of resting frontal alpha EEG asymmetry from this study suggests that this neurophysiologic characteristic may be a stable, trait-like characteristic independent of depression status. Many of the results from this study echo prior research showing that alpha asymmetry distinguishes depressed from healthy individuals, and that this dimension of cortical activity remains steady over time in healthy individuals and among depressed individuals who report symptom reduction. In addition to being a stable trait associated with behavioral avoidance and behavioral inhibition sensitivity in depressed participants, frontal alpha EEG asymmetry was evidently unalterable by behavioral activation treatment strategies (see also Allen et al., 2004), but it does not rule out the possibility that the presence of hemispheric asymmetry in remitters may change with stability of the remission.
The results of this study support the preferential expression of behavioral inhibition sensitivity and avoidance and negative affect with frontal alpha EEG asymmetry, consistent with previous research outlining neuropsychological function of approach- and withdrawal-related behavior in depression (Coan & Allen, 2003; Shackman et al., 2009). The results from this study also offer support to the concept of frontal alpha EEG asymmetry related to inhibition sensitivity (Kemp, Gordon, Rush, & Williams, 2008; Shankman, Klein, Tenke, & Bruder, 2007). Furthermore, frontal alpha EEG asymmetry and behavioral inhibition sensitivity were tightly linked before and after treatment.
In the current BA model, a functional analyses is often used to ascertain the context and consequence of depression symptoms (Ferster, 1973), as well as how to modify avoidance and context to generate treatment response. Reversing behavioral inhibition, as well as promoting behavioral activation systems, could potentially represent BA-specific mechanisms. Also, according to the cognitive model (Beck, 2008), behavioral strategies are designed to modify dysfunctional attitudes, and when cognitions are modified early in treatment, there is subsequent clinical improvement (DeRubeis et al., 1990; Kwon & Oei, 2003). BA treatment does not focus on tasks requiring patients to directly modify attentional performance, potentially helping patients to practice and subsequently increase alpha power, but rather patients are asked to focus on behavioral modification of avoidance, which may not sufficiently modify frontal alpha EEG asymmetry. The use of BA, which aims to alter behavioral and cognitive avoidance, and does not specifically target cognitive activity and motivational substrates of inhibition and activation sensitivity, therefore may have limited the predictive utility of alpha EEG asymmetry in BA.
Clinically, the findings of this study indicate that depressed individuals are likely to show frontal asymmetry, which increases the tendency for the individual to report higher negative affect and behavioral inhibition sensitivity. Moreover, the lack of change in cortical activity does not correspond to the individual’s response to treatment. If BA were viewed as an opportunity to modify cortical activity, the individual’s pre-treatment status would not necessarily limit the potential for achieving recovery, offering clinical information to patients who potentially may wish to know if pre-treatment alpha EEG asymmetry influences the propensity to remit with BA. Despite an interest in improving early prediction of response to BA, very few studies have yet examined the extent to which change in specific patient-related characteristics, separately and in combination, serve as putative mediators of treatment response, and none, thus far, have examined alpha EEG asymmetry as a predictor of outcome. Understanding how frontal alpha EEG asymmetry predicts negative affect at post-treatment may advance our knowledge of potential predictors of relapse among those fortunate enough to experience remission.
In terms of limitations of the study, inferences cannot be made about which hemisphere was influenced (i.e., reduced left or increased right) due to the use of asymmetry scores in addition to type of measurement (i.e., absolute value). Furthermore, frontal alpha EEG asymmetry was measured while the participant was resting, and it may differ during a cognitive task (Coan, Allen, & McKnight, 2006). Specifically, alpha EEG synchronization during mental relaxation may not offer a sufficient test of capability as might be observed during task performance (Coan et al., 2006) given that decreased alpha power during cognitive tasks represents the proportion of cortical neural activity involved in the task (Dujardin et al., 1993; Gevins & Smith, 2000; Gevins et al., 1998). Also, because the study did not include a group of untreated depressed participants, we cannot determine if untreated depressed participants over time would exhibit similar features with the alpha asymmetry as observed in the remitted and depressed subgroups. Also, the anterior asymmetries in the healthy participants may be generated by the reduction of alpha at the left central/temporal sites, aligning with an asymmetric mu rhythm in sensorimotor regions (e.g., contraction of right-handed muscles) (Pineda, 2005), Mu rhythm has frequencies in the 8–13Hz band, which have been associated with greater left than right frontal activity and increased self-reported approach affect (Harmon-Jones, 2006). Because we did not use a formal measure of handedness, rather we asked the participants to confirm that they were dominant right-handed, it is possible that mu rhythms explain the alpha asymmetry difference. Also, though all participants did not use a substance (e.g., alcohol, nicotine, or caffeine) within three hours of the tasks, there is the potential that some of these individuals were smokers, which can influence EEG asymmetry and mood in depression (Knott et al., 2012). Finally, though frontal alpha EEG asymmetry represents a neurophysiologic indicator of cognitive activity, there is no accompanying measure to assess cognitive activity. The use of neural-event related potentials (ERP) to valenced stimuli may offer new data to clarify the neural substrates of altered activity in depressed participants relative to healthy controls. Strengths of this study include the reliance of treatment-seeking depressed patients who were unmedicated at the time of enrollment, and we ensured that depressed participants endorsed moderate severity of illness.
In conclusion, the data from this study has shown that depressed relative to healthy participants show a greater frontal alpha EEG asymmetry before they enrolled into BA treatment, demonstrating a more diffuse pattern of activation from the anterior to posterior part of the brain unlike healthy participants who retain focused frontal right activity. Also, higher frontal alpha EEG asymmetry does not appear to underlie clinical severity before and after treatment, rather higher behavioral inhibition and negative affect along with lower behavioral activation sensitivity (fun-seeking motivation) at pre-treatment. The specific functions of EEG asymmetry where we observed diffuse pattern of activation remains to be determined, though continued study of the frontal alpha EEG asymmetry index may advance efforts to identify neurophysiologic markers of major depression.
Highlights.
We test for alpha EEG asymmetry differences in depressed and healthy adults, as well as for predictive utility of alpha EEG asymmetry of treatment response to Behavioral Activation for depression.
Alpha EEG asymmetry was significantly higher in depressed than healthy participants at pre-treatment.
Alpha EEG asymmetry was positively correlated with negative affect and behavioral inhibition, and inversely correlated with lower behavioral activation sensitivity.
Heightened alpha EEG asymmetry in depressed participants may reflect a stable neurophysiologic characteristic independent of depressive severity.
Footnotes
Financial Disclosures: The preparation for this manuscript was supported by Grant R21 MH082133-01A1 (Gollan: PI) from the National Institute of Mental Health. The funding source did not have any involvement in the design, data collection, analysis, or interpretation of this study. Drs. Denada Hoxha, Jon Sutton, Jordana Segal-Goldstein, Patrick Nowlin served as research clinicians. Sarah Getch and Kallio Hunnicutt-Ferguson served as coordinators. Bjorn Hanson, Angel Buchanan, Shandra Brown, Justin Birnholz, Noah Yulish, Michal Rischall, and Lindsey Sankin conducted phone screens and clinical interviews. Bjorn Hanson and Justin Birnholz conducted adherence ratings. Rebecca Shor and Sara Polis conducted physiological assessments. Ruth Herman Dunn and Christopher Martell provided off-site BA competency reviews. Demetrios Voreades, Applications Engineer from Source Signal Imaging, Inc. generated the topographies using the EMSE 5.5.1 program.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis ME, Martell C. Overcoming depression one step at a time: The new behavioral activation approach to getting your life back. New York, NY: New Harbinger Press; 2004. [Google Scholar]
- Aftanas LI, Golocheikine SA. Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: high-resolution EEG investigation of meditation. Neuroscience Letters. 2001;310(1):57–60. doi: 10.1016/S0304-3940(01)02094-8. [DOI] [PubMed] [Google Scholar]
- Allen JJ, Iacono WG, Depue RA, Arbisi P. Regional electroencephalographic asymmetries in bipolar seasonal affective disorder before and after exposure to bright light. Biological Psychiatry. 1993;33(8–9):642–646. doi: 10.1016/0006-3223(93)90104-L. [DOI] [PubMed] [Google Scholar]
- Allen JJ, McKnight KM, Moreno FA, Demaree HA, Delgado PL. Alteration of frontal EEG asymmetry during tryptophan depletion predicts future depression. Journal of Affective Disorders. 2009;115(1–2):189–195. doi: 10.1016/j.jad.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JJ, Urry HL, Hitt SK, Coan JA. The stability of resting frontal electroencephalographic asymmetry in depression. Psychophysiology. 2004;41(2):269–280. doi: 10.1111/j.1469-8986.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- Barnhofer T, Duggan D, Crane C, Hepburn S, Fennell MJ, Williams JM. Effects of meditation on frontal alpha-asymmetry in previously suicidal individuals. Neuroreport. 2007;18(7):709–712. doi: 10.1097/WNR.0b013e3280d943cd. [DOI] [PubMed] [Google Scholar]
- Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. American Journal of Psychiatry. 2008;165(8):969–977. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- Brenner RP, Ulrich RF, Spiker DG, Sclabassi RJ, Reynolds CF, 3rd, Marin RS, Boller F. Computerized EEG spectral analysis in elderly normal, demented and depressed subjects. Electroencephalography and Clinical Neurophysiology. 1986;64(6):483–492. doi: 10.1016/0013-4694(86)90184-7. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Sedoruk JP, Stewart JW, McGrath PJ, Quitkin FM, Tenke CE. Electroencephalographic alpha measures predict therapeutic response to a selective serotonin reuptake inhibitor antidepressant: pre- and post-treatment findings. Biological Psychiatry. 2008;63(12):1171–1177. doi: 10.1016/j.biopsych.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA, Davidson RJ, Kalin NH, Goldsmith HH. Context-specific freezing and associated physiological reactivity as a dysregulated fear response. Developmental Psychology. 2004;40(4):583–594. doi: 10.1037/0012-1649.40.4.583. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology. 1994;67(2):319–333. doi: 10.1037/0022-3514.67.2.319. [DOI] [Google Scholar]
- Coan JA, Allen JJ. Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology. 2003;40(1):106–114. doi: 10.1111/1469-8986.00011. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJ. Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology. 2004;67(1–2):7–49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJ, McKnight PE. A capability model of individual differences in frontal EEG asymmetry. Biological Psychology. 2006;72(2):198–207. doi: 10.1016/j.biopsycho.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook IA, Hunter AM, Gilmer WS, Iosifescu DV, Zisook S, Burgoyne KS, Leuchter AF. Quantitative electroencephalogram biomarkers for predicting likelihood and speed of achieving sustained remission in major depression: a report from the biomarkers for rapid identification of treatment effectiveness in major depression (BRITE-MD) trial. Journal of Clinical Psychiatry. 2013;74(1):51–56. doi: 10.4088/JCP.10m06813. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Henry JD. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. British Journal of Clinical Psychology. 2004;43(Pt 3):245–265. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, van Straten A, Warmerdam L. Behavioral activation treatments of depression: a meta-analysis. Clinical Psychology Review. 2007;27(3):318–326. doi: 10.1016/j.cpr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Daughters SB, Braun AR, Sargeant MN, Reynolds EK, Hopko DR, Blanco C, Lejuez CW. Effectiveness of a brief behavioral treatment for inner-city illicit drug users with elevated depressive symptoms: the life enhancement treatment for substance use (LETS Act!) Journal of Clinical Psychiatry. 2008;69(1):122–129. doi: 10.4088/JCP.v69n0116. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Parsing affective space: Perspectives from neuropsychology and psychophysiology. Neuropsychology. 1993;7(4):464–475. doi: 10.1037/0894-4105.7.4.464. [DOI] [Google Scholar]
- Davidson RJ. What does the prefrontal cortex “do” in affect: perspectives on frontal EEG asymmetry research. Biological Psychology. 2004;67(1–2):219–233. doi: 10.1016/j.biopsycho.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annual Review of Psychology. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Debener S, Beauducel A, Nessler D, Brocke B, Heilemann H, Kayser J. Is resting anterior EEG alpha asymmetry a trait marker for depression? Findings for healthy adults and clinically depressed patients. Neuropsychobiology. 2000;41(1):31–37. doi: 10.1159/000026630. [DOI] [PubMed] [Google Scholar]
- DeRubeis RJ, Evans MD, Hollon SD, Garvey MJ, Grove WM, Tuason VB. How does cognitive therapy work? Cognitive change and symptom change in cognitive therapy and pharmacotherapy for depression. Journal of Consulting and Clinical Psychology. 1990;58(6):862–869. doi: 10.1037/0022-006X.58.6.862. [DOI] [PubMed] [Google Scholar]
- Dimidjian S, Barrera M, Jr, Martell C, Munoz RF, Lewinsohn PM. The origins and current status of behavioral activation treatments for depression. Annual Review of Clinical Psychology. 2011;7:1–38. doi: 10.1146/annurev-clinpsy-032210-104535. [DOI] [PubMed] [Google Scholar]
- Dimidjian S, Hollon SD, Dobson KS, Schmaling KB, Kohlenberg RJ, Addis ME, Jacobson NS. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. Journal of Consulting and Clinical Psychology. 2006;74(4):658–670. doi: 10.1037/0022-006X.74.4.658. [DOI] [PubMed] [Google Scholar]
- Dujardin K, Derambure P, Defebvre L, Bourriez JL, Jacquesson JM, Guieu JD. Evaluation of event-related desynchronization (ERD) during a recognition task: effect of attention. Electroencephalography and Clinical Neurophysiology. 1993;86(5):353–356. doi: 10.1016/0013-4694(93)90049-2. [DOI] [PubMed] [Google Scholar]
- EMSE 5.5.1 [computer software and User Manual] Source Signal Imaging, Inc; La Mesa, CA USA: 2013. [Google Scholar]
- Ferster CB. A functional anlysis of depression. American Psychologist. 1973;28(10):857–870. doi: 10.1037/h0035605. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL. Structured clinical interview for DSM-IV Axis II personality disorders. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for the DSM-IV Axis I Disorders (SCID-I/P, version 2) New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P W/PSYCHOTIC SCREEN) New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 2002. [Google Scholar]
- Ford MR, Sands S, Lew HL. Overview of artifact reduction and removal in evoked potential and event-related potential recordings. Physical Medicine and Rehabilitation Clinics of North America. 2004;15(1):1–17. doi: 10.1016/s1047-9651(03)00125-6. [DOI] [PubMed] [Google Scholar]
- Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Weissman MM. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Archive of General Psychiatry. 1991;48(9):851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME. Neurophysiological measures of working memory and individual differences in cognitive ability and cognitive style. Cerebral Cortex. 2000;10(9):829–839. doi: 10.1093/cercor/10.9.829. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, Leong H, McEvoy L, Whitfield S, Du R, Rush G. Monitoring working memory load during computer-based tasks with EEG pattern recognition methods. Human Factors. 1998;40(1):79–91. doi: 10.1518/001872098779480578. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Ranganath C, Rosenfeld JP. Frontal EEG alpha asymmetry, depression, and cognitive functioning. Cognition and Emotion. 1998;12:449–478. doi: 10.1080/026999398379673. [DOI] [Google Scholar]
- Gray JA. The psychophysiological basis of introversion-extraversion. Behaviour Research and Therapy. 1970;8(3):249–266. doi: 10.1016/0005-7967(70)90069-0. [DOI] [PubMed] [Google Scholar]
- Hagemann D, Naumann E, Thayer JF, Bartussek D. Does resting electroencephalograph asymmetry reflect a trait? an application of latent state-trait theory. Journal of Personality and Social Psychology. 2002;82(4):619–641. doi: 10.1037/0022-3514.82.4.619. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E. Unilateral right-hand contractions cause contralateral alpha power suppression and approach motivational affective experience. Psychophysiology. 2006;43:695–603. doi: 10.1111/j.1469-8986.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJ. Anger and frontal brain activity: EEG asymmetry consistent with approach motivation despite negative affective valence. Journal of Personality and Social Psychology. 1998;74(5):1310–1316. doi: 10.1037/0022-3514.74.5.1310. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. Journal of Abnormal Psychology. 1991;100(4):535–545. doi: 10.1037/0021-843X.100.4.535. [DOI] [PubMed] [Google Scholar]
- Hollon SD, Evans MD, Auerbach A, DeRubeis RJ, Elkin I, Piasecki J. Development of a system for rating therapies for depression: Differentiating cognitive therapy, interpersonal therapy, and clinical management pharmacotherapy. University of Minnesota; Twin Cities Campus: 1998. [Google Scholar]
- Jacobson NS, Dobson KS, Truax PA, Addis ME, Koerner K, Gollan JK, Prince SE. A component analysis of cognitive-behavioral treatment for depression. Journal of Consulting and Clinical Psychology. 1996;64(2):295–304. doi: 10.1037/0022-006X.64.2.295. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, Andreasen NC. The Longitudinal Interval Follow-up Evaluation. A comprehensive method for assessing outcome in prospective longitudinal studies. Archives of General Psychiatry. 1987;44(6):540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Gordon E, Rush AJ, Williams LM. Improving the prediction of treatment response in depression: integration of clinical, cognitive, psychophysiological, neuroimaging, and genetic measures. CNS Spectrums. 2008;13(12):1066–1086. doi: 10.1017/s1092852900017120. quiz 1087-1068. [DOI] [PubMed] [Google Scholar]
- Knott V, Thompson A, Shah D, Ilivitsky V. Neural expression of nicotine’s antidepressant properties during tryptophan depletion: An EEG study in healthy volunteers at risk for depression. Biological Psychology. 2012;91(2):190–200. doi: 10.1016/j.biopsycho.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Kwon SM, Oei TP. Cognitive change processes in a group cognitive behavior therapy of depression. Journal of Behavior Therapy and Experimental Psychiatry. 2003;34(1):73–85. doi: 10.1016/S0005-7916(03)00021-1. [DOI] [PubMed] [Google Scholar]
- Leuchter AF, Cook IA, Gilmer WS, Marangell LB, Burgoyne KS, Howland RH, Greenwald S. Effectiveness of a quantitative electroencephalographic biomarker for predicting differential response or remission with escitalopram and bupropion in major depressive disorder. Psychiatry Research. 2009a;169(2):132–138. doi: 10.1016/j.psychres.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Leuchter AF, Cook IA, Marangell LB, Gilmer WS, Burgoyne KS, Howland RH, Greenwald S. Comparative effectiveness of biomarkers and clinical indicators for predicting outcomes of SSRI treatment in Major Depressive Disorder: results of the BRITE-MD study. Psychiatry Research. 2009b;169(2):124–131. doi: 10.1016/j.psychres.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Lieber AL, Prichep LS. Diagnosis and subtyping of depressive disorders by quantitative electroencephalography: I. Discriminant analysis of selected variables in untreated depressives. Hillside Journal of Clinical Psychiatry. 1988;10(1):71–83. [PubMed] [Google Scholar]
- Lindsley DB, Wicke JD. The electroencephalogram: Autonomous electrical activity in man and animals. Bioelectric Recording Techniques. 1974;1(part B):3–83. [Google Scholar]
- Martell C, Addis ME, Jacobson NS. Depression in context: Strategies for guided action. New York, NY: W. W. Norton & Co; 2001. [Google Scholar]
- Martell C, Dimidjian S, Herman-Dunn R. Behavioral Activation for depression: A clinician’s guide. New York, NY: The Guilford Press; 2010. [Google Scholar]
- Mathersul D, Williams LM, Hopkinson PJ, Kemp AH. Investigating models of affect: relationships among EEG alpha asymmetry, depression, and anxiety. Emotion. 2008;8(4):560–572. doi: 10.1037/a0012811. [DOI] [PubMed] [Google Scholar]
- Maxwell JS, Davidson RJ. Emotion as motion: asymmetries in approach and avoidant actions. Psychological Science. 2007;18(12):1113–1119. doi: 10.1111/j.1467-9280.2007.02033.x. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Shackman AJ, Harmon-Jones E, Alloy LB, Coan JA, Abramson LY. Cognitive vulnerability and frontal brain asymmetry: common predictors of first prospective depressive episode. Jouranl of Abnormal Psychology. 2011;120(2):497–503. doi: 10.1037/a0022940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenbreit ND, Dobson KS. Avoidance and depression: the construction of the cognitive-behavioral avoidance scale. Behaviour Research and Therapy. 2004;42(3):293–313. doi: 10.1016/S0005-7967(03)00140-2. [DOI] [PubMed] [Google Scholar]
- Pineda JA. The functional significance of mu rhythms: translating “seeing” and “hearing” into “doing”. Brain Research Reviews. 2005;50(1):57–68. doi: 10.1016/j.brainresrev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D. Electroencephalography and high-density electrophysiological source localization. In: Caccioppo JT, Tassinary LG, Berntson G, editors. Handbook of psychophysiology. 3. Cambridge, UK: Cambridge University Press; 2007. pp. 56–84. [Google Scholar]
- Pollock VE, Schneider LS. Topographic electroencephalographic alpha in recovered depressed elderly. Journal of Abnormal Psychology. 1989;98(3):268–273. doi: 10.1037/0021-843X.98.3.268. [DOI] [PubMed] [Google Scholar]
- Rabe S, Zoellner T, Beauducel A, Maercker A, Karl A. Changes in brain electrical activity after cognitive behavioral therapy for posttraumatic stress disorder in patients injured in motor vehicle accidents. Psychosomatic Medicine. 2008;70(1):13–19. doi: 10.1097/PSY.0b013e31815aa325. [DOI] [PubMed] [Google Scholar]
- Ray WJ, Cole HW. EEG alpha activity reflects attentional demands, and beta activity reflects emotional and cognitive processes. Science. 1985;228(4700):750–752. doi: 10.1126/science.3992243. [DOI] [PubMed] [Google Scholar]
- Reid SA, Duke LM, Allen JJ. Resting frontal electroencephalographic asymmetry in depression: inconsistencies suggest the need to identify mediating factors. Psychophysiology. 1998;35(4):389–404. doi: 10.1017/S0048577298970986. [DOI] [PubMed] [Google Scholar]
- Rosenfeld JP, Baehr E, Baehr R, Gotlib IH, Ranganath C. Preliminary evidence that daily changes in frontal alpha asymmetry correlate with changes in affect in therapy sessions. International Journal of Psychophysiology. 1996;23(1–2):137–141. doi: 10.1016/0167-8760(96)00037-2. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Dickens AM. Dissociation of alpha and theta activity as a function of verbal and visuospatial tasks. Electroencephalography and Clinical Neurophysiology. 1982;53(2):201–207. doi: 10.1016/0013-4694(82)90024-4. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Carmody TJ, Reimitz PE. The Inventory of Depressive Symptomatology (IDS): Clinician (IDS-C) and Self-Report (IDS-SR) ratings of depressive symptoms. International Journal of Methods in Psychiatric Research. 2000;9(2):45–59. doi: 10.1002/mpr.79. [DOI] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Keller MB. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological Psychiatry. 2003;54(5):573–583. doi: 10.1016/S0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Rush JA, Giles DE, Schlesser MA, Fulton CL, Weissenburger J, Burns C. The inventory for depressive symptomatology (IDS): Preliminary findings. Psychiatry Research. 1986;18:65–87. doi: 10.1016/0165-1781(86)90060-0. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23(6):695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, McMenamin BW, Maxwell JS, Greischar LL, Davidson RJ. Right dorsolateral prefrontal cortical activity and behavioral inhibition. Psychological Science. 2009;20(12):1500–1506. doi: 10.1111/j.1467-9280.2009.02476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Klein DN, Tenke CE, Bruder GE. Reward sensitivity in depression: a biobehavioral study. Journal of Abnormal Psychology. 2007;116(1):95–104. doi: 10.1037/0021-843X.116.1.95. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. DSM-III Case Book. Washington, DC: American Psychiatric Press; 1989. [Google Scholar]
- Stewart JL, Bismark AW, Towers DN, Coan JA, Allen JJ. Resting frontal EEG asymmetry as an endophenotype for depression risk: sex-specific patterns of frontal brain asymmetry. Journal of Abnormal Psychology. 2010;119(3):502–512. doi: 10.1037/a0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychological Science. 1997;8(3):204–210. doi: 10.1111/j.1467-9280.1997.tb00413.x. [DOI] [Google Scholar]
- Tenke CE, Kayser J. Reference-free quantification of EEG spectra: combining current source density (CSD) and frequency principal components analysis (fPCA) Clinical Neurophysiology. 2005;116(12):2826–2846. doi: 10.1016/j.clinph.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J, Manna CG, Fekri S, Kroppmann CJ, Schaller JD, Bruder GE. Current source density measures of electroencephalographic alpha predict antidepressant treatment response. Biological Psychiatry. 2011;70(4):388–394. doi: 10.1016/j.biopsych.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: a meta-analytic review. Journal of Abnormal Psychology. 2006;115(4):715–729. doi: 10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Henriques JB. Resting frontal brain asymmetry predicts affective responses to films. Journal of Personality and Social Psychology. 1990;59(4):791–801. doi: 10.1037/0022-3514.59.4.791. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, Doss RC. Individual differences in anterior brain asymmetry and fundamental dimensions of emotion. Journal of Personality and Social Psychology. 1992;62(4):676–687. doi: 10.1037/0022-3514.62.4.676. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, Kinney L. Psychometric properties of resting anterior EEG asymmetry: temporal stability and internal consistency. Psychophysiology. 1992;29(5):576–592. doi: 10.1111/j.1469-8986.1992.tb02034.x. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]