Abstract
Hypertension is the major risk factor for the development of stroke, coronary artery disease, heart failure and renal disease. The underlying cellular and molecular mechanisms of hypertension are complex and remain largely elusive. MicroRNAs (miRNAs) are short, noncoding RNA fragments of 22–26 nucleotides and regulate protein expression post-transcriptionally by targeting the 3′-untranslated region of mRNA. A growing body of recent research indicates that miRNAs are important in the pathogenesis of arterial hypertension. Herein, we summarize the current knowledge regarding the mechanisms of miRNAs in cardiovascular remodeling, focusing specifically on hypertension. We also review recent progress of the miRNA-based therapeutics including pharmacological and nonpharmacological therapies (such as exercise training) and their potential applications in the management of hypertension.
Keywords: MicroRNA, hypertension, vasculature remodeling, exercise
Introduction
Hypertension, characterized by persistent elevation of systemic blood pressure, is a major risk factor for the development of stroke, coronary artery disease, heart failure and chronic renal failure (http://www.who.int/whr/2002/). According to the most recent health statistics from the World Health Organization (WHO), hypertension is a major public health problem affecting ~40% of adults aged 25 years and over with high levels of associated morbidity and mortality. Hypertension can be classified as either primary (essential) hypertension (90–95%) with no obvious underlying medical cause [1] or secondary hypertension (5–10%) caused by various identifiable medical conditions affecting the kidney, heart or endocrine system. Despite extensive and continuous efforts to understand the pathogenesis of essential hypertension, the underlying cellular and molecular mechanisms remain largely elusive.
MicroRNAs (miRNAs) are short noncoding RNA molecules comprising 22–26 nucleotides that negatively regulate gene expression of their targets at the post-transcriptional levels [2,3]. Since their discovery in 1993, miRNAs have been found to be involved in almost all biologic processes, including cellular proliferation, differentiation, senescence, apoptosis, tumorigenesis and stress response [4]. The recent accumulation of research results indicates that miRNAs are important in the pathogenesis of arterial hypertension. Understanding the processes regulated by miRNAs and identifying the novel miRNA targets in the pathogenesis of hypertension could eventually lead to the development of new treatment approaches for hypertension.
Herein, we summarize the current knowledge about the mechanisms of miRNAs in hypertension, focusing specifically on the role of miRNAs in heart and vasculature remodeling, as well as the molecular mechanisms underlying the pathogenesis of hypertension. We also review recent progress of the miRNA-based therapeutics, including pharmacological and nonpharmacological therapies and their potential applications in the management of hypertension.
Biogenesis and function of miRNAs
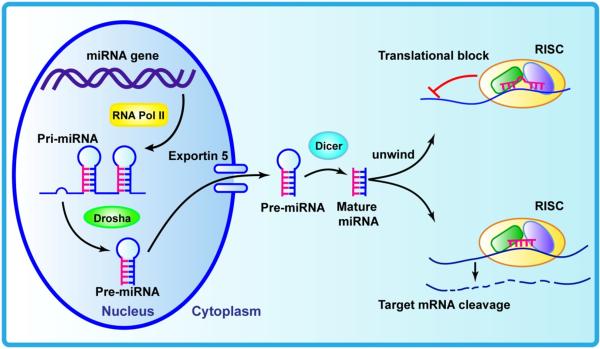
The biogenesis of miRNAs has been extensively reviewed [5-8]. miRNA genes reside in regions of the genome as distinct transcriptional units, as well as in clusters of polycistronic units, carrying the information of several miRNAs [9,10]. Studies suggest that approximately half of known miRNAs reside in non-protein-coding RNAs (introns and exons) or within the intron of protein-coding genes [11]. RNA polymerase II transcribes miRNA genes, generating long primary transcripts (pri-miRNAs). Subsequently, the process to yield mature miRNAs involves two steps. The first step is the generation of precursors (pre-miRNA), which contain a characteristic stem loop structure by Drosha, a member of the RNase III family [12]. This pre-miRNA consists of approximately 70 nucleotides and is produced in the nucleus (Figure 1). The pre-miRNA hairpins are then exported out of the nucleus and into the cytoplasm by the protein exportin 5, and further processed into unstable, ~22-nucleotide miRNA duplex structures (miRNA–miRNA* duplex) by the RNase III protein Dicer [13]. The mature miRNA, which is a single strand of this duplex, is preferentially incorporated into a multiple-protein nuclease complex, the mRNA-induced silencing complex (RISC), which regulates protein expression. The non-RISC-incorporated strand (the complementary strand miRNA*) is subsequently degraded. The mature miRNA guides the RISC complex to its targets. Once a mRNA is targeted by a miRNA its gene expression is downregulated either by mRNA degradation or translational repression, or both [14,15]. In mammals, mature miRNAs bind to the 3′ untranslated region (3′-UTR) of target genes by partial complementarity. The interaction of the 5′ end of miRNAs (a ‘seed region’) with the target mRNA results in the termination of translation of the target genes [16,17]. The miRNA–mRNA interaction is imperfect in nature, which means that one miRNA can target multiple mRNAs, and one mRNA can be targeted by multiple, distinct miRNAs; therefore, miRNAs can significantly alter gene expression regulatory networks [12]. It has been predicted that miRNAs can manage the regulation of at least 60% of protein-coding genes in humans [18]. Growing evidence shows that the epigenetic modification of the genome and post-transcriptional regulation of gene expression by miRNAs are powerful regulatory mechanisms that could significantly influence health and disease.
Figure 1.
Schematic diagram of biogenesis of miRNA. Primary miRNA (pri-miRNA) is transcribed by RNA polymerase II. Drosha cleaves pri-miRNA into pre-miRNA, which is exported to the cytoplasm by exportin 5, and further processed into mature miRNA duplex structures by Dicer. Then one of the single strands (mature miRNA) is incorporated into mRNA-induced silencing complex (RISC) and binds to the 3′-untranslated region (3′-UTR) of target mRNA, following translational repression or mRNA degradation.
Mechanisms of miRNAs in the regulation of normal cardiovascular function
Cardiogenesis
To assess the essential role of miRNAs in cardiogenesis in the mouse heart, a floxed Dicer allele was deleted by using Cre recombinase under the control of the endogenous Nkx2.5 regulatory region, which directs expression in cardiac progenitors at embryonic day 8.5 (E8.5) [s1][19]. This intervention results in developmental anomalies in the mouse heart, including a poorly developed ventricular myocardium and pericardial edema, which ultimately lead to cardiac failure and embryonic lethality by E12.5. Because Dicer is essential for processing pre-miRNAs into the mature form, the early lethality in the Dicer mutant reveals an essential requirement for miRNA function in the developing heart. Another mechanism is the targeted deletion of Dicer using a Nkx2.5-Cre allele (3′-UTR-IRES-Cre), which differs from that previously employed by Zhao et al. [19]. In this approach, the inactivated targets present with subtly different spatiotemporal kinetics, which in turn leads to the disruption in the development of a four-chambered heart and abnormal outflow-tract patterning in mice, ultimately resulting in embryonic lethality by E13.75 [20]. Recently, Peng [s2]et al. demonstrated that specifically inactivating Dicer in the myocardium by crossing cTnt-Cre mice with DicerloxP mice efficiently inactivated target genes in cardiomyocytes at mid-gestation, thus leading to severe myocardial wall defects, including reduced cell proliferation, increased cell death and spongy myocardial wall, and ultimately resulting in the death of all mutants between E14.5 and E16.5 [21]. Mutant hearts showed an upregulation of a dozen contractile proteins such as actinin, cTnC, MHC[s3], myosin light chain [s4](MLC)1V and MLC2V, as well as an increase in transforming growth factor β receptor (TGFBR)1 activity. By contrast, blocking TGFBR1 activity partially rescues the heart from defects caused by the inactivation of Dicer in vivo [21]. Specifically, myocardial inactivation of Dicer under the control of a promoter for the cardiomyocyte structural protein alpha myosin heavy chain (αMHC) in mice resulted in dilated cardiomyopathy, heart failure and postnatal lethality, which was consistent with the disruption in expression of cardiac contractile proteins, as well as changes in sarcomeric structure [22]. Taken together, these studies indicate that Dicer-controlled miRNA maturation has complex roles at different cardiogenesis stages.
Not only is Dicer crucial to cardiogenesis during different gestation, its activity is also important for maintaining normal postnatal cardiac function and structural integrity. Cardiomyocyte-specific Dicer deficiency using an αMHC-Cre line did not result in embryonic lethality. However, it could lead to a dramatic decrease in the expression of mature miRNAs in neonatal hearts that were associated with the phenotypes of dilative cardiomyopathy and heart failure. This results in neonatal death by postnatal day 4 [22]. Conditioning Dicer loss in the older murine myocardium under the control of a tamoxifen-inducible αMHC promoter, Cre-mediated excision of Dicer in 3-week-old mice leads to premature death within 1 week, accompanied by mild ventricular remodeling, dramatic atrial enlargement and mild inflammation. By contrast, in the adult myocardium, loss of Dicer induces a rapid and dramatic biventricular enlargement, accompanied by more-severe histopathological changes, including myocyte hypertrophy, myofiber disarray and ventricular fibrosis [23]. Furthermore, cardiomyocyte-specific genetic knockdown of DGCR8[s5] using MCK[s6]-Cre line expressed under the control of the myosin creatine kinase [24] promoter results in a marked reduction in the thickness of the ventricular myocardium, as well as a disruption of the cardiac conduction system, ultimately leading to dilated cardiomyopathy and premature lethality at two months of age [25]. These results indicate that selected DGCR8-dependent miRNAs have a crucial role in the maintenance of cardiac function. In conclusion, there is growing evidence for the regulatory role of the miRNA biogenesis pathways in the maintenance of normal cardiac function and integrity during embryonic development and adulthood.
In addition to Dicer that has a key role in the regulation of cardiogenesis, other specific miRNAs including miR-1 and miR-133 have been identified as having a crucial role in this process. Zhao and colleagues have demonstrated that the overexpression of miR-1 in mouse embryonic cardiomyocytes results in a decrease in the proliferation of cardiomyocytes, thus leading to the embryonic death at E10.5 [26]. This result was attributed to a decrease in the expression of Hand2, a transcription factor that promotes cardiomyocyte proliferation. By contrast, genetic ablation of miR-1-2 in pure Sv129 background mice exhibited ventricular-septal defects (VSD) in some mutant embryos, which in turn caused an incompletely penetrant embryonic lethality from E15.5 to birth [19]. Interestingly, however, most of the surviving adult miR-1-2 mutants showed normal heart function; heart hyperplasia with a 20% increase in the number of cardiomyocytes and cardiomyocytes undergoing nuclear division was not observed in age-matched wild-type animals [19]. A recent study showed that the phenotype of miR-1-1 knockout (KO) Sv129 mice was similar to that of miR-1-2 KO Sv129 mice by the same targeting strategy [27]. However, a more recent study involving miR-1 double KO mice demonstrated that targeted deletion of miR-1-1 or miR-1-2 caused neither embryonic lethality nor septal defects in Sv129 mice, which was in contrast to the findings of the two former studies of separate miR-1-1 KO or miR-1-2 KO mice [28]. Similarly, mice lacking either miR-133a-1 or miR-133a-2 presented normal cardiac physiology, whereas deletion of both miRNAs resulted in approximately half of the double-mutant embryos or neonates with severe VSDs, resulting in death between P0 and P1 [29]. Only 25% of miR-133a double KO mice that survived to adulthood had developed dilated cardiomyopathy and exhibited an increase in cardiomyocyte proliferation, whereas the specific overexpression of miR-133a in cardiomyocytes led to a decrease in ventricular cardiomyocyte proliferation, and ultimately death at E15.5 secondary to cardiac failure [29]. A more recent genetic knockdown of a single miR-1/133 cluster in mice also did not show any significant cardiac defects; however, only the miR-1/133 double KO mice exhibited severe heart malformations during embryonic development, which resulted in embryonic lethality at day 10.5 [30].
Vascular smooth muscle cell proliferation, differentiation and vascular tone
miRNAs have been clearly demonstrated to have an important role in controlling vascular smooth muscle cell (VSMC) development and function, including proliferation, differentiation and phenotypic switching. Here, we will review some of the most important discoveries regarding miRNAs in the regulation of normal functions of VSMC, including VSMC proliferation, differentiation and vascular tone.
To demonstrate the global role of miRNAs in VSMC development and function, Albinsson et al. first demonstrated that SMC-targeting deletion of exons 1 and 2 of the Dicer gene decreased VSMC proliferation and differentiation, which in turn resulted in thinner vessel walls, impaired contractility and hemorrhage, and ultimately caused embryonic lethality at E16 to E17 [31]. Similarly, disruption of exon 21 of the Dicer gene in mouse VSMCs also exhibited a phenotype of dilated blood vessels and hemorrhage, reduced VSMC proliferation, downregulated expression of VSMC marker genes and a disarray of vascular architecture, with later embryonic death between E14.5 and E15.5 [32]. This minor discrepancy could be attributed to the expression of a truncated Dicer protein following the deletion of exon 21 of the Dicer gene in VSMCs, thus rescuing and mitigating the abnormal phenotype [32]. Moreover, tamoxifen-inducible and SMC-specific deletion of Dicer in postnatal mice exhibited a dramatic reduction in blood pressure as a result of a significant dysfunction in vascular contractility and VSMC differentiation, as well as vascular remodeling [33]. This study supports the idea that miRNA synthesis and turnover have a key role in the regulation of the terminally differentiated state of VSMCs. In addition, conditional deletion of DGCR8 in VSMCs led to blood vessel dilation, with a phenotype of reduced VSMC marker gene expression, decreased cell proliferation and promoted apoptosis, thus resulting in embryonic death between E12.5 and E13.5 [34]. Overall, these studies indicate that DGCR8- [s7]and Dicer-dependent miRNAs are crucial for the maintenance of normal VSMC development, differentiation and contractile functioning during embryonic and adult stages.
In addition, a spectrum of individual miRNAs has been identified to have a key role in regulating the normal function of VSMCs, including VSMC proliferation, differentiation and vascular tone. It has been demonstrated that certain miRNAs, including miR-143, miR-145, miR-328, miR-26a, miR-1, miR-133, miR-132, miR-124, miR-195, miR-424/322, miR-365 and miR-663 alter VSMC phenotype by inhibiting VSMC proliferation and promoting differentiation, whereas miR-21, miR-221, miR-222 and miR-146a promote VSMC proliferation and dedifferentiation. Among these miRNAs, miR-143 and miR-145 are enriched in the vasculature and have a pivotal role in modulating VSMC phenotype. In the setting of in vitro studies, overexpression of miR-143/145 increased the expression of contractile genes and inhibited proliferation of cultured VSMCs [35,36]. In in vivo studies, several miR-143/145 KO mouse models revealed that the loss of miR-143 and miR-145 significantly compromised VSMC contractile protein expression, vascular contractility and blood pressure regulation [35-39]. In a recent study, miR-328 was found to inhibit α1C subunit expression of L-type calcium channels (CaL) and attenuate the pulmonary arterial response to KCl [40]. Furthermore, miR-328 suppressed the insulin growth factor 1 receptor, ultimately leading to apoptosis of pulmonary VSMCs [40].
In addition, several other miRNAs are also shown to be important in suppressing VSMC proliferation. miR-1 has a functional role in promoting SMC differentiation from embryonic stem cells (ESCs), and the induction of miR-1 by myocardin in SMCs inhibits cell proliferation [41,42]. Through loss- and gain-of-function experiments, miR-133 has been shown to be a potent inhibitor of the VSMC phenotypic switch in vitro and in vivo [43]. Similarly, miR-132 and miR-195 can also block VSMC proliferation [44,45]. miR-424/322 overexpression in vitro can inhibit proliferation and migration, as well as promote VSMC differentiation, but without any effect on apoptosis [46]. Exogenous miR-365 overexpression reduces VSMC proliferation and proliferating cell nuclear antigen (PCNA) expression, whereas it enhances the proliferation of VSMCs by various stimuli factors, including angiotensin II, platelet-derived growth factor (PDGF)-BB and serum, leading to the downregulation of miR-365 expression levels, which induces an increase in VSMC proliferation [47]. Overexpression of miR-124 not only inhibits the proliferation of human pulmonary artery smooth muscle cells (PASMCs) but also maintains its differentiated phenotype [48]. Similarly, overexpression of miR-663 increases the expression of human aortic VSMC differentiation marker genes and potently inhibits PDGF-induced VSMC proliferation and migration [49].
miR-21 is the first miRNA recognized as a regulator of the proliferative and contractile phenotype by using different mechanisms. Transforming growth factor (TGF)-β and bone morphogenetic proteins (BMPs) increase miR-21 expression, which in turn promotes VSMC differentiation [50,51]. By contrast, in vitro overexpression of miR-21 increases proliferation and reduces apoptosis in cultured human aortic SMCs [52]. miR-26a also promotes human aortic SMC proliferation, whereas it inhibits cellular differentiation and apoptosis [53]. Similarly, the upregulation of miR-221/222 leads to increased proliferation and migration of VSMCs and reduced expression of SMC contractile marker proteins [54]. In addition, downregulation of miR-221/222 results in a decrease in VSMC proliferation in vitro [54]. miR-146a has also been shown to promote VSMC proliferation in cultured rat VSMCs [55]. In vitro, synthetic miR-24 overexpression imparted detrimental effects on SMC functional capacity, thus inducing apoptosis, migration defects, enhanced autophagy and loss of contractile marker genes [56].
Endothelial cells
Vascular endothelial cells (ECs) are a monolayer of epithelial cells that line the intimal surface of vascular structures and play a key part in the maintenance of normal vascular homeostasis, including vascular development, regulation of vascular tone, VSMC phenotypic switch, vascular barrier, coagulation and fibrinolysis, and leukocyte trafficking [57]. In recent years, by using loss- and gain-of-function in vitro or in vivo methods, several studies have demonstrated that various miRNAs that predominate in ECs play a key part in regulating normal EC functions, including proliferation, apoptosis and migration, which are crucial for the control of normal vascular processes.
Silencing of Dicer in ECs leads to a reduction in the formation of capillary sprouting, migration and proliferation [58,59]. Similarly, deleted Dicer in human microvascular ECs impairs cell migration and tube formation. Downregulation of Dicer in cultured human umbilical vein ECs (HUVECs) by serum withdrawal results in endothelial apoptosis, whereas overexpression of Dicer in HUVECs markedly decreases apoptosis upon serum withdrawal [60]. Postnatal conditional inactivation of Dicer in ECs reduces angiogenic responses to a variety of proangiogenic factor stimuli, including exogenous vascular endothelial growth factor (VEGF), limb ischemia, wound healing and tumors [61]. Taken together, these studies indicate that Dicer-dependent miRNAs have a crucial role in the maintenance of the normal function of ECs, including proliferation, apoptosis, migration and angiogenesis.
miR-126 is an EC-specific proangiogenic miRNA that is essential for the maintenance of vascular integrity and promotion of vessel growth. Targeted deletion of miR-126 in mouse ECs causes a reduction in EC growth, sprouting and adhesion, which in turn results in vascular abnormalities, including vascular leakage, hemorrhaging and embryonic lethality in a subset of mutant mice [62-64]. In vitro, downregulation of miR-126 in ECs promotes the expression of tumor necrosis factor (TNF)α, which stimulates vascular cell adhesion molecule (VCAM)-1 expression, thus enhancing leukocyte adherence to ECs that ultimately leads to vessel inflammation [65]. Overexpression of miR-210 that promotes the formation of capillary-like structures as well as VEGF stimulates migration of normoxic ECs; by contrast, the inactivation of miR-210 inhibits tube formation and migration [66]. miR-130a antagonizes the inhibitory effects of growth arrest homeobox (GAX) and HoxA5 on EC migration, proliferation and tube formation in vitro [67].
miR-221/222 was highly expressed in HUVECs. Overexpression of miR-221/222 in HUVECs inhibits endothelial tube formation, migration and attenuates cell survival [68]. Another study on Dicer deficiency involving the endothelial layer also revealed the antiangiogenic role of miR-221/222 in ECs [58]. These two studies demonstrated that miR-221/222 exerts antiangiogenic effects by using the same mechanism.
miR-92a is highly expressed in rat aortic ECs (RAO-ECs) and HUVECs, and inhibition of miR-92a expression enhances EC proliferation, migration and endothelial sprouting in vitro [69]. Transfection of pre-miR-92a in cultured human coronary ECs inhibited EC migration and proliferation. By contrast, pre-miR-92a imparted no effect on EC apoptosis. Overexpression of pre-miR-92a in ECs resulted in the downregulation of eNOS mRNA expression levels [70].
miR-24 is enriched in ECs [71] and activated by hypoxic conditions via hypoxia-inducible factor (HIF)-1 [72]. Overexpression of miR-24 in cultured HUVECs promotes apoptosis, blocks endothelial capillary network formation on Matrigel® and inhibits endothelial spheroid formation, as well as impairing EC migration and proliferation; whereas miR-24 antagonism had the opposite effect [73].
Overexpression of miR-19a inhibits the proliferation of HUVECs, which controls cell cycle progression, thus resulting in a higher proportion of cells in the G0/G1 phase and a lower proportion of cells in the S phase compared with control cells [74]. Similarly, miR-16 or miR-424 overexpression decreased HUVEC proliferation without significant effects on apoptosis, impaired cord formation under basal conditions and, following stimulation with VEGF or basic fibroblast growth factor (bFGF), resulted in a decrease in bovine aortic EC basal migration and VEGF- or bFGF-induced migration. By contrast, downregulation of these miRNAs showed opposite effects on migration and cord formation [75].
miR-100 is an antiangiogenic miRNA that is highly expressed in ECs. Overexpression of miR-100 in HUVECs by transfecting specific miR-100 precursors resulted in the inhibition of EC network formation on Matrigel® and EC sprouting, as well as a decrease in EC proliferation. Silenced miR-100 by anti-miRs exhibited the opposite phenotype; however, the migratory ability of ECs was not affected by manipulating the expression level of miR-100 [76]. miR-214 overexpression resulted in the inhibition of HUVEC migration, sprout formation and tubule formation on Matrigel® in vitro, whereas anti-miR-214 induced the opposite effects. Interestingly, EC proliferation and viability were not affected by modulating miR-214 levels [77].
Downregulation of miR-132 expression in cultured HUVECs promotes proliferation, viability and migration, but decreases apoptosis. Upregulation of miR-132 increases cell migration and viability, proliferation, and induces apoptosis in HUVECs. miR-132 also promotes TNFα-induced proinflammatory processes of ECs [77].
miRNA targets identified in maintaining cardiovascular function
Cardiomyocytes
In the past decades, a dozen miRNA targets have been identified in cardiomyocytes. These miRNA targets have a crucial role in the regulation of normal cardiac function such as proliferation, apoptosis, contractility, electric conduction and energy metabolism (Table 1). To date, for example, several validated targets of miR-1 have been identified, which include Hand2 [26], Notch ligand Delta [78], Irx5 [19], KCNJ2 [79], connexin 43 [79, 80], monocyte enhancer factor [s8](MEF)2A and calmodulin [81], cyclin-dependent kinase (CDK)9 [82], telokin [27], myocardin [28] and estrogen-related receptor (Err)β [28]. These targets are very important in the regulation of cardiomyocyte proliferation, cardiac cell fate, cardiac conduction, cardiac hypertrophy, myocardial differentiation, smooth muscle gene expression and metabolic control. A recent study showed that KCNJ2 is also a target of miR-26 in the heart [83]. miR-133 also has several validated targets, including RhoA and Cdc42, Nelf-A/WHSC2 [84], SRF [s9]and cyclin D2 [29], and Krüpple-like factor (KLF)15 [85], which take major roles in the regulation of cardiac hypertrophy, expression of SMC genes, cardiomyocyte proliferation and metabolic control. The primary targets of miR-208 are Thrap1, myostatin, Sox6, Purβ and MED13, which are required for the regulation of cardiomyocyte-specifc gene expression, cardiomyocyte hypertrophy, thyroid hormone sensitivity and systemic metabolic control [86-89]. miR-17-92 mainly targets Isl1 and Tbx1 which promote myogenic differentiation in the secondary heart field [90,91]. miR-15 targets are Arl2, phosphoinositide-dependent kinase (PDK)4 and serum- and glucocorticoid-regulated kinase (SGK)1, which regulate the cardiomyocyte mitotic arrest and response to hypoxia [92-94].
Table 1. Role of miRNA in regulating normal function of cardiovascular system.
| Cell type | miRNA | Validate target | Function | Refs |
|---|---|---|---|---|
| Cardiomyocyte | miR-1 | Hand2 | Regulation of cardiomyocyte proliferation | [26] |
| Notch ligand Delta | Regulation of cardiac cell fate | [78] | ||
| MEF2a, Calmodulin |
Negative regulation of cardiac hypertrophy | [81] | ||
| Cdk9 | Repression of myocardial differentiation | [82] | ||
| Irx5, KCNJ2, Connexin 43 |
Regulation of cardiac conduction | [19,79,80] | ||
| Telokin, Myocardin |
Regulation of cardiac contractility | [27] | ||
| Errβ | Metabolic control | [28] | ||
|
| ||||
| miRNA-26 | KCNJ2 | Regulation of cardiac conduction | [83] | |
|
| ||||
| miRNA-133 | RhoA, Cdc42, Nelf-A/WHSC2 |
Regulation of cardiac hypertrophy | [84] | |
| SRF, cyclinD2, | Repression of SMC genes, negative regulation cardiomyocyte | [29] | ||
| KLF15 | proliferation, metabolic control | [29,85] | ||
|
| ||||
| miRNA-208 | Thrap1, myostatin, Sox6, Purβ, MED13 |
Regulation of cardiomyocyte-specifc gene expression, cardiomyocyte hypertrophy, thyroid hormone sensitivity, systemic metabolic control |
[86-89] | |
|
| ||||
| miRNA-17~92 | Isl1, Tbx1 | Promote myogenic differentiation in the secondary heart field | [90,91] | |
|
| ||||
| miRNA-15 | Arl2, PDK4, SGK1 | Regulation of the cardiomyocyte mitotic arrest, response to hypoxia |
[92-94] | |
|
| ||||
| VSMC | miRNA-221/222 | p27kip1, p57kip2, cKit |
Increase proliferation | [54] |
|
| ||||
| miRNA-146a | KLF4 | Increase proliferation | [55] | |
|
| ||||
| miRNA-21 | PTEN, PDCD4 | Increase proliferation, apoptosis | [50,51] | |
|
| ||||
| miRNA-24 | Tribbles-like protein 3, OH-1 |
Inhibiting BMP promote VSMC differentiation, induces VSMC apoptosis, migration |
[56,95] | |
|
| ||||
| miRNA-143/145 | Elk-1, CamKIIδ, KLF4/5, ACE, MRTF-B, Tpm4, Add3, Srgap-1/2, Ssh |
Decrease proliferation, promote differentiation, maintenance of vascular contractility and cytoskeletal dynamics |
[35-37,39,97] | |
|
| ||||
| miRNA-26a | Smad1, Smad4 | Decrease proliferation, promote differentiation | [53] | |
|
| ||||
| miRNA-1 | pim-1 | Decrease proliferation | [42] | |
|
| ||||
| miRNA-663 | JunB/Myl9 | Promoting differentiation, inhibiting proliferation and migration |
[49] | |
|
| ||||
| miRNA-124 | NFATc1, CAMTA1, PTBP1 |
Inhibits the proliferation of HPASMCs | [48] | |
|
| ||||
| miRNA-10a | HADC4 | Promoting retinoic-acid-induced VSMC differentiation | [98] | |
|
| ||||
| miRNA-132 | Lrrfipl | Decrease proliferation | [44] | |
|
| ||||
| miR-322 | Cyclin D1, Calumenin |
Promoting differentiation, decrease proliferation and migration | [46] | |
|
| ||||
| miRNA-195 | Cdc42, CCND1, FGF1 |
Decrease proliferation and migration | [45] | |
|
| ||||
| miRNA-328 | Insulin growth factor 1 receptor, L-type calcium channel-α1C |
Increases PASMCs apoptosis, decrease contraction | [40] | |
|
| ||||
| EC | miR-92a | Itga5, Sirt1, Klf2/4 | Regulation of EC proliferation, migration, sprouting, vessel patterning, neovascularization after ischemia |
[99-101] |
|
| ||||
| miR-132 | Sirt1 | Promotes lipid metabolism-dependent proinflammatory processes |
[77] | |
|
| ||||
| miRNA-100 | mTOR | Decrease proliferation, tube formation and sprouting activity | [76] | |
|
| ||||
| miR-221/222 | cKit | Decreases cell survival, migration, endothelial tube formation | [68] | |
|
| ||||
| miR-410 | VEGF-A | Inhibits oxygen-induced retinal neovascularization | [102] | |
|
| ||||
| miRNA-16, miRNA-424 |
VEGFR2, FGFR1 | Regulation of cell-intrinsic angiogenic activity of EC | [75] | |
|
| ||||
| miR-19a | Cyclin D1 | Decreased EC proliferation | [74] | |
|
| ||||
| miR-126 | PIK3R2, SPRED1 | Regulation of VEGF-dependent EC function | [64,66] | |
|
| ||||
| miR-210 | Ephrin-A3 | Promote ECs survival, migration, tube formation in response to hypoxia |
[66] | |
|
| ||||
| miR-382 | PTEN | Regulation of hypoxia-induced bovine aortic ECs proliferation, migration and tube formation |
[103] | |
Abbreviations:
VSMCs
Several validated miRNA targets have been determined to have a crucial role in the miRNA-based regulation of VSMC proliferation, migration, growth and apoptosis. For example, the negative regulators of cell cycle progression, p27kip1 and p57kip2 [s10]are the targets of miR-221 and miR-222, respectively. cKit is another miR-221 target, which is capable of decreasing SMC differentiation [54]. KLF4 is the target of miR-146a and it promotes VSMC proliferation in culture [55]. Phosphatase and tensin homolog (PTEN) is one of the miR-21 targets that regulate VSMC growth and survival [50]. Programmed cell death (PDCD)4 is a tumor suppressor protein and is another miR-21 target, and miR-21 silencing PDCD4-mediated BMP4 upregulates smooth-muscle-specific contractile proteins.
miR-24 silences Tribbles-like protein 3 expression and increases the Smad ubiquitin ligase Smurf1 expression, resulting in a decrease in Smad1 expression and the inhibition of BMP and promotion of VSMC differentiation [95,96]. A recent study revealed that heme oxygenase 1 (OH-1) was another target of miR-24. The overexpression of miR-24 induces VSMC apoptosis, migration defects and loss of contractile marker genes, and is mediated in part by silencing OH-1 expression [56].
In addition, several miRNAs that silence their targets can inhibit VSMC proliferation, migration, growth and apoptosis, thus maintaining VSMCs in a specific differentiation phenotype of quiescence. For example, KLF4 and KLF5, Elk-1, CamKIIδ, and angiotensin-converting enzyme, myocardin-related transcription factor (MRTF)-B, Tpm4, Add3, Srgap-1/2 and Ssh are miR-143/145 targets [35-37,39,97]. These miR-143/145 targets play a key part in regulating the VSMC phenotypic switch and maintenance of vascular contractility and cytoskeletal dynamics.
Smad1 and Smad4 are two targets of miR-26a in human aortic VSMCs. Repression of these two targets by miR-26a leads to a decrease in proliferation and an increase in differentiation [53]. Similarly, miR-1 mediates the overexpression of myocardin-induced inhibition of human aortic smooth muscle cell proliferation by silencing Pim-1 which is a serine/threonine kinase and promotes VSMC proliferation [42]. miR-663 also plays a crucial part in promoting human VSMC differentiation and in inhibiting proliferation and migration by silencing JunB/Myl9 expression [49]. Moreover, miR-124 inhibits the proliferation of pulmonary arterial hypertension smooth muscle cells (PAHSMCs) by targeting multiple genes, including nuclear factor of activated T cells (NFAT)c1, calmodulin-binding transcription activator (CAMTA)1 and polypyrimidine tract-binding protein (PTBP)1 [48].
The targets of miR-10a include histone deacetylase (HDAC)4 which promotes retinoic-acid-induced VSMC differentiation [98]. miR-132 targets leucine–rich repeat (in Flightless 1) interacting protein (Lrrfip)1, which blocks VSMC proliferation [44]. Cyclin D1 and Ca2+-regulating protein calumenin are direct targets of miR-322, and are negative regulators of VSMC differentiation, proliferation and migration [46]. miR-195 reduces VSMC proliferation and migration by repressing the expression of its target genes, Cdc42, CCND1 and FGF1 [45]. Insulin growth factor 1 receptor and CaL-α1C are two targets of miR-328 that suppress the insulin growth factor 1 receptor, promote apoptosis of pulmonary arterial SMCs and attenuate the KCl-induced PA contraction response by inhibiting CaL-α1C expression [40].
Endothelial cells
miRNA targets also have a crucial role in modulating the normal function of ECs, including proliferation, apoptosis, migration, tube formation and sprouting activity. These functions are essential for governing vascular integrity and angiogenesis. Multiple targets of miR-92a, including integrin-a5 (Itga5), Sirt1, KLF2 and KLF4, are important in the regulation of EC proliferation, migration and sprouting, as well as vessel patterning and neovascularization after ischemia [99-101]. Sirt1 is also a direct target of miR-132 in HUVECs, which results in a decrease in the expression of Sirt1 by miR-132, thus promoting lipid-metabolism-dependent proinflammatory processes in ECs [77].
cKit was identified as the direct target gene of miR-221 and miR-222. miR-221/222 decrease cell survival, migration and endothelial tube formation by repressing the expression levels of cKit [68]. Mammalian target of rapamycin (mTOR) is a direct target of miR-100; silencing mTOR expression by miR-100 blocks proliferation, tube formation and sprouting activity of ECs [76]. In human retinal vascular ECs (HRCECs) and HUVECs, miR-410 targets VEGF-A and inhibits its expression, thus inhibiting oxygen-induced retinal neov[s11]ascularization [102]. miR-16 and miR-424 have important roles in regulating cell-intrinsic angiogenic activity of ECs by targeting VEGF, VEGF receptor (VEGFR)2 and fibroblast growth factor receptor (FGFR)1 [75]. miR-19a, by downregulating its target cyclin D1, arrests the EC cycle at the G1/S transition, thus resulting in a decrease in EC proliferation [74]. miR-126 can negatively target PIK3R2 and SPRED1[s12], which in turn modulates VEGF-dependent EC function [62,64]. Ephrin-A3 is now identified as an important target of miR-210. Inhibition of ephrin-A3 expression by miR-210 promotes EC survival, migration and tube formation in response to hypoxia [66]. In addition, PTEN has been identified as a target gene of miR-382; it represses PTEN expression, thus mediating hypoxia-induced bovine aortic EC proliferation, migration and tube formation [103]. Table 1 summarizes the role of miRNAs in regulating the normal function of the cardiovascular system.
Signature miRNA expression profile of hypertension
The initial stages and progression of hypertension involve miRNAs, and changes in their expression profiles could be used for the diagnosis, prognosis and treatment of a diverse spectrum of hypertension cases. miRNA profiling has shown that these small molecules are dysregulated in hypertension, although the exact involvement and mechanisms behind this observation are currently unclear. Insofar as sustained cell inflammation could induce DNA damage [104,105], researchers have reported that the activation of poly (AKP-ribose) polymerase-1 (PARP-1), a crucial enzyme for DNA repair and cellular stress sensor, resulted from DNA damage during pulmonary arterial hypertension (PAH) and could thus modulate the expression of miR-204 [106]. The complexity of hypertension is manifested in the dysfunction of multiple organs, as well as in its association with other diseases. These relationships thus indicate that conducting miRNA research could be challenging. We have reviewed recent reports on the miRNA mechanisms in different types of hypertension and have summarized the hypertension models or study subjects (the origin of tissue or cells) employed (Table 2).
Table 2. Summary of microRNAs implicated in hypertension.
| microRNA | Hypertension model Study source |
Property | Activity | Targets | Refs |
|---|---|---|---|---|---|
| miR-145 | Hypoxic mice Idiopathic/heritable patient PASMC |
Trigger PAH | Up | KLF4 | [105] |
|
| |||||
| miR-204 | Human nonfamilial PAH lung PASMC EC |
Antiproliferative and promote apoptotic of SMC |
Down | SHP2 | [106,108] |
|
| |||||
| miR-130/301 | Lung from 8 PAH model PASMC EC |
Promote hypertensive phenotype | Up | PPARγ | [107] |
|
| |||||
| miR-21 | Lungs and arteries of hypoxic mice PASMC |
Enhance SMC proliferation and associated proteins, cell migration |
Up | BMPR2, WWP1, YOD1, PDCD4, SPRY2, PPARα |
[109-111] |
| SMC EC of heritable PAH patients | Antiproliferation | Down | BMPR2 | [112] | |
| PH patient lung species Hypoxic mice Hypoxic EC |
Decrease angiogenesis and vasodilatation |
Up | BMPR2, Rho | [112,119] | |
| Hypoxia plus SU5416 | Promote EC apoptosis | Up | PDCD4 | [120] | |
|
| |||||
| miR-206 | Hypoxic rats PASMC |
Enhance SMC proliferation, dedifferentiation |
Down | HIF-1α, Notch-3 | [113,114] |
|
| |||||
| miR-17 | Hypoxic mice Monocrotaline induced rats PASMC |
Increase SMC proliferation | Up | p21 | [109] |
|
| |||||
| miR-193 | Lung tissue and serum from idiopathic PAH patients Monocrotaline rat Hypoxia mouse PASMC |
Inhibit SMC proliferation | Down | Apolipoprotein A-I mimetic peptide |
[116] |
|
| |||||
| miR-328 | Hypoxic mice PASMC |
Suppress SMC apoptosis | Down | L-type calcium channel-α1C, IGF-1R |
[40] |
|
| |||||
| miR-9 | Hypoxic PASMC | Induce SMC phenotype switch | Up | HIF-1α | [117] |
|
| |||||
| miR-424/503 | PAH patient lung Monocrotaline and hypoxic/SU5416 rats |
Inhibit EC proliferation and media to SMC |
Down | FGF2, FGFR1 | [121] |
| EC | Promote cellular quiescence | ||||
|
| |||||
| miR-17/92 | EC | Loss of BMPR2 | Up | BMPR2 | [122] |
|
| |||||
| miR-124 | Human/calves with PAH or IH adventitial fibroblasts |
Attenuate fibroblast proliferation and migration |
Down | PTBP1 MCP-1 |
[24] |
|
| |||||
| miR-126 | PAH patient/monocrotaline PAH rats Skeletal muscle CD31 cells |
Protect skeletal muscle microcirculation and promote angiogenesis |
Down | SPRED-1 | [126] |
| HSC | Reduce intrahepatic vascular resistance and improve microcirculation |
Down | VEGF | [136] | |
|
| |||||
| miR-22 | SHR | Initiate events through Chga to increase BP in brainstem |
Up | Chga | [129] |
|
| |||||
| miR-666 miR-708 |
Cirrhosis mice Hyperosmolality EC |
Mediate osmolar changes | Down | AQP1 | [137] |
|
| |||||
| miR-487 | Ang II H rats Rat/human arterial adventitial fibroblast |
Damage adventitial and medial integrity |
Up | IRS1 | [141] |
|
| |||||
| miR-608 | Human cortices C57B1/6J mice HEK-293T cells |
Modulate parasympathetic and anxiety controlling genes |
- | AchE, CDC42, IL-6 | [145] |
|
| |||||
| miR-210 | Placenta, plasma, JAR cells | Reduce trophoblast invasion | Up | HOXA9, EFNA3 | [159-161] |
|
| |||||
| miR-155 | Blood of hypertension subject | Interplay with AT1R | Down | AT1R | [142] |
| Umbilical vein endothelial cells | Reduce angiotensin-II-induced extracellular signal regulated kinase 1/2 activation |
Down | AT1R | [164] | |
| Placenta | Cause insufficient expression of VEGF in preeclampsia placenta |
Up | CYR61 | [163] | |
|
| |||||
| miR-376c | Placenta, plasma trophoblast |
Increase trophoblast proliferation and invasion |
Down | ALK5, ALK7 | [165] |
Abbreviations:
Pulmonary hypertension
The development of PAH is characterized by the adaptation and remodeling of pulmonary arteries caused by an imbalance in proliferation and apoptosis of the vascular wall. Pulmonary artery ECs, SMCs and fibroblasts are disrupted during pulmonary artery remodeling as induced by various physiological changes, pathological events, as well as environmental stimuli including hypoxia. As an important epigenetic mechanism, miRNAs provide a promising target in the regulation of these molecular processes.
SMCs
Among the three layers of the pulmonary arterial wall, the SMCs in the media represent major pathological changes during PAH progression [107]. SMCs can experience proliferation, apoptosis-resistance and phenotypic switching from contractile to synthetic, during which miRNAs serve as novel modulators in the progression of pulmonary hypertension.
miR-143 and miR-145 are abundantly expressed in the vessel wall and are particularly localized in SMCs [36]. This miRNA family has been widely studied and is recognized for its predominant function of directing SMC differentiation, targeting a network of transcription factors such as KLF4, myocardin and Elk1 [35]. In cultured human primary pulmonary SMCs, miR-143 and miR-145 are necessary for the downregulation of KLF4 transcripts and protein expression by TGF-β and BMP4, which in turn promote the expression of SMC contractile genes [97]. By contrast, an upregulation of miR-145 in lung tissues of mice and patients with idiopathic and heritable PAH was observed upon exposure to hypoxia. Compared with miR-143, miR-145 knockouts and inhibitors induced a significant protection from PAH, thus suggesting a crucial role for miR-145 in the development of PAH [108]. It is in line with the upregulation of miR-145 in SMCs from the pulmonary artery of PAH patients [109].
Network-based bioinformatics prediction programs have identified the miR-130/301 family as the main miRNA that controls PAH pathogenesis. The miR-130/301 family is highly regulated in PAH animal models and human PAH subjects; induction of miR-130/301 promotes the development of PAH, whereas inhibition of miR-130/301 shows the opposite effect [110]. It has been suggested that there might be an integrative function for miR-130/301, with signal transducer and activator of transcription (STAT)3/miR-204 signaling to modulate SMC phenotype switching, whereas in PAH ECs a different network could also be involved [110].
miR-204 is especially confined to SMCs in PAH compared to ECs, bronchi and veins of the lungs. The severity of PAH [s13]correlates with the downregulation of miR-204 that, in turn, by regulating SHP2 expression, activates Src kinase and NFAT, sustains SMC proliferation and suppresses apoptosis in the pulmonary artery [109]. These results were obtained from three in vivo PAH models (nonfamilial PAH patients, hypoxia-induced PAH mice and monocrotaline-induced PAH rats), as well as in cultured human pulmonary artery SMCs. In addition, studies involving PAH patients and monocrotaline-induced PAH rats showed that miR-204 imparted a protective effect from dehydroepiandrosterone (DHEA) by inhibiting the activation of Src/STAT3 in pulmonary artery SMCs [111]. These findings thus suggest that miR-204 acts as hub for PAH artery remodeling.
The upregulation of miR-21 in the lungs of hypoxia-induced PAH mice has also been recently described [112,113]; this stimulatory effect has been predominantly observed in the SMCs of pulmonary arteries. It has been found that blocking miR-21 in the lungs of hypoxia-exposed mice leads to a reduction in the severity of right ventricle hypertrophy and PAH [113]. Similarly, muscularization of the pulmonary arteries was attenuated by this sequestration because of the upregulation of the smooth muscle α-actin, a phenotypic marker for contractile SMCs. In the same study, transfection of miR-21 accelerated cultured human pulmonary SMC proliferation and enhanced the expression of proteins that regulate cell cycle, cell proliferation and apoptosis in SMCs [113]. The role of miR-21 in hypoxia-induced pulmonary artery SMC proliferation was also confirmed by Sarkar et al. [114] in hypoxia and normoxia environments. However, a study of the role of miR-21 in noncanonical BMP pathways involving Smad-8 during PAH development showed that miR-21, along with miR-27a, suppressed SMC and EC proliferation after BMP treatment [115].
Another potential regulator of pulmonary artery SMCs is miR-206 which has been correlated to the increase in right ventricular systolic pressure in hypertensive mice. In in vitro studies, the reduction of miR-206 was responsible for an increase in the proliferation and a reduction in apoptosis in human pulmonary SMCs. By contrast, overexpression of miR-206 resulted in the maintenance of the contractile phenotype of SMCs [116]. The inhibition of Notch-3 by miR-206 provides an explanation for the antihypertension effect. Similarly, in another study that focused on the role of the HIF-1α/Fhl-1 pathway during hypoxia-induced PAH, dysregulation of miR-206 functioned as an early triggering factor for PAH in vivo and in vitro [117].
In hypoxia- and monocrotaline-induced PAH, miR-17 inhibition is effective in reducing right ventricular systolic pressure and total pulmonary vascular resistance index and in improving pulmonary artery acceleration time, compared with miR-21 and miR-92a [112]. In addition, the same study showed that the overexpression of miR-17 resulted in an increase in the proliferation of human pulmonary arterial SMCs. The involvement of the cell cycle regulator p21 by miR-17 might contribute to SMC modulation because p21 is known to suppress the proliferation of SMCs [118].
Transfection of miR-193 in human pulmonary artery SMCs resulted in the inhibition of cell proliferation, whereas knockdown of miR-193 in SMCs of control subjects stimulated proliferation [119]. miR-193 was significantly reduced in the lung tissue and serum from humans and animals with PAH. In hypoxia and primary PAH rat models, miR-9 also promoted SMC proliferation in PAH by interacting with HIF-1α [120].
Downregulation of miR-328 in hypoxia-induced PAH has been reported in a hypoxia-induced PAH animal model, whereas the overexpression of miR-328 could greatly relieve the symptoms, which might be associated with the inhibition of L-type calcium channel-α1C and the suppression of insulin growth factor 1 receptor [40].
ECs
PAH usually occurs with EC damage, accompanied by an increase in the release of growth factors and the activation of signaling pathways that are controlled by miRNAs, ultimately leading to proliferation and migration of SMCs. The dysfunction of miRNAs in the lung tissue during hypoxia-induced PAH and monocrotaline confirmed the results of cultured pulmonary artery ECs that were stimulated by hypoxia and growth factors. miR-22, miR-30 and let-7f were downregulated, whereas miR-322 and miR-451 were upregulated during the development of PAH in both models; miR-21 and let-7a were significantly expressed only in monocrotaline-treated rats [121].
miR-21 and miR-27a imparted antiproliferative effects on pulmonary artery ECs as previously mentioned; the growth rate of ECs after BMP stimulation correlated well with the changes in miR-21 levels [115]. The central role of miR-21 in regulating PAH angiogenesis and vasodilation is also in line with the study that demonstrated its upregulation in PAH animal models and patients and its involvement with BMP and Rho/Rho-kinase signaling in cultured pulmonary artery ECs [122]. A study concerning EC apoptosis contributing to PAH found that the miR-21-mediated PDCD4/caspase-3 pathway promoted endothelial apoptosis in vitro and PAH in vivo [123,124].
PAH rats induced by monocrotaline and hypoxia showed a decreased level of miR-424 in lung tissue and isolated ECs. It has been reported that miR-424 and miR-503 promoted cellular quiescence in ECs and inhibited capacity to induce SMC proliferation in PAH, thus exerting its effects of maintaining vascular homeostasis by mediating the APLN[s14] and FGF2 pathways [124]. Unlike the regulatory effect of miR-130/301 on miR-204, as previously mentioned for SMC, miR-130/301 targeted apelin–miR-424/503–FGF2 signaling in PAH ECs, which was indicative of the importance of miR-130/301 and the diverse miRNA network across disparate cell types in the pathogenesis of PAH [110].
Brock et al. explored the dysregulation of bone morphogenetic protein receptor (BMPR)2 in PAH and its linkage to the loss of BMP3R2 and STAT3 by demonstrating the modulating function of miR-17/92. miR-17-5p and miR-20a directly target BMPR2 and thus transfer the activation of interleukin (IL)-6 signaling induced by STAT3 [125].
Adventitia: fibroblasts
In the adventitia of PAH, fibroproliferative changes and extensive deposition of extracellular matrix proteins have also implicated the regulation of miRNAs as revealed by several studies. During the pulmonary artery remodeling of PAH, adventitial fibroblasts undergo phenotypic switching, including excessive proliferation, migration and fibrotic and inflammatory activation [126,127]. miR-124 shows different expression levels in adventitial fibroblasts from calves and humans with PAH [24], yet showing species-specific discrepancies in PAH rodent models. miR-124 regulates cell proliferation and migration in fibroblasts and the loss of miR-124 might contribute to PAH fibrosis, which could be modulated by histone deacetylation. miR-21 also plays an important part in fibrotic lung disease, and administration of miR-21 antisense probes diminishes the severity of lung fibrosis in mice [128], thus providing a possible target for treating PAH-induced lung fibrosis.
A recent study reported that miRNA might be involved in exercise intolerance induced by PAH because exercise capacity was correlated with muscle capillary density[s15], which was regulated by miR-126 [129]. The effect of miR-126 on improving muscle angiogenesis has been confirmed by in vivo and in vitro experiments. The endothelial miR-126 is also reduced by high glucose concentration, which provides an explanation for impaired angiogenic signaling in patients with diabetes [130]. This role of miR-126 in diabetes is then confirmed by the study that identified the deregulation of miR-126 in the endothelial progenitor cells (EPCs) from patients through Ras/extracellular signal-related kinase (ERK)/VEGF and phosphoinositide 3 kinase (PI3K)/Akt/endothelial nitric oxide synthase (eNOS) pathways [131]. miR-126 is also associated with the dysfunction of EPCs in patients with chronic heart failure [132] and could be used as a biomarker for heart failure [133]. The animal models used for studying human PAH actually differ in terms of molecular mechanisms and therefore cannot present a similar etiology for a different PAH, much less the diverse tissues such as whole lungs, dissected pulmonary arteries or isolated cell lines.
Genetic hypertension
Genetic hypertension is a complex syndrome determined by genetic and environmental factors. Li et al. identified specific miRNA profiling differences in plasma between normal and genetic hypertension patients by using miRNA array analysis, and hcmv-miR-UL112, let-7e and miR-296-5p were validated independently among the 27 differentially expressed miRNAs [134]. Another research group assessed circulating miR-9 and miR-126 by real-time PCR in genetic hypertension patients and found that miR-9 positively correlated with left ventricular mass index and that miR-9 and miR-126 levels increased with blood pressure [135].
We have discussed genetic PAH, but limited research has focused on miRNA mechanisms in genetic hypertension. Friese et al. demonstrated that spontaneous hypertension rats (SHR) receiving miR-22 antagomir showed a reduction in blood pressure [136]; the mechanism behind this antihypertension effect was to reduce the binding of miR-22 to the Chga-3′-UTR, which increased the abundance of Chga protein in the brainstem, thus leading to a decrease in blood pressure.
Other types of hypertension
In this part, we will discuss the miRNAs that are potentially related to other types of hypertension including portal hypertension, salt-sensitive hypertension, angiotensin-II-induced hypertension, anxiety-induced hypertension and gestation-induced hypertension. Even though there is limited evidence involving miRNAs available for a clear etiologic mechanism, more research can result in significant improvement in markers of prediction and diagnosis, prevention or therapies of this disorder.
Portal hypertension
As a major complication of several liver diseases, portal hypertension requires further mechanistic studies involving miRNAs because several key miRNAs have been identified, thus highlighting the need to improve clinical therapies and reduce mortality. The biological behavior of hepatic stellate cells (HSCs) in liver fibrosis might be the major player of angiogenesis; it is closely related to intrahepatic portal hypertension [137,138]. For example, studies showed that miR-21, miR-16, miR-27 and miR-146a had major roles in regulating HSC differentiation, proliferation and apoptosis [139-142]. In addition, Guo et al. confirmed that miR-126 might be used in reducing intrahepatic vascular resistance and in improving hepatic microcirculation [143]. miR-666 and miR-708 influenced the post-transcriptional of aquaporin-1 and subsequently mediated osmolar changes to reverse angiogenesis, fibrosis and portal hypertension [144]. Nevertheless, the research and application of miRNAs directly on relieving portal hypertension have been rarely reported.
Salt-sensitive hypertension
Blood pressure often responds to a high-salt diet and this salt sensitivity varies among individuals, thus indicating genetic differences [145]; miRNA could possibly provide a gene environment explanation for these interindividual differences. Naraba and Iwai established a miRNA library of the kidney of salt-sensitive hypertension rats and found that miRNAs might contribute a limited function to salt-sensitive hypertension, because no statistical difference was observed between Dahl salt-sensitive and Lewis rats subjected to normal or high-salt diets [146]. By contrast, there was a higher level of expression of miR-21 in the kidneys of Dahl rats receiving excessive salt compared with other groups. However, these findings have to be confirmed in other hypertensive Dahl rats.
Angiotensin-II-induced hypertension
Rennin-angiotensin-aldosterone system (RAAS) activity plays a central part in blood pressure regulation. A study involving single nucleotide polymorphisms (SNPs) located in miRNA-binding sites in genes of RAAS emphasized the role of miRNAs in modulating blood pressure, which identified four SNPs that were associated with changing blood pressure in three different genes of RAAS [147]. The gene polymorphisms of RAAS disrupted the binding affinity of miRNA to mRNA and then influenced the expression of these genes.
As the biologically active component of the rennin-angiotensin system, angiotensin II regulates blood pressure and influences cellular functions within the cardiovascular system. Studies on the early development of vascular pathogenesis used angiotensin II to induce chronic hypertension and demonstrated the most robust expression of miR-487b in rat aorta by performing miRNA profiling [148]. By targeting insulin receptor substrate 1, miR-487b has been identified to be responsible for cell death and loss of vascular integrity in medial and adventitial cells[s16].
Angiotensin II type 1 receptor (AT1R) defines the biological function of angiotensin II, which is thought to play a central part in the etiology of hypertension. The interaction between AT1R and miRNA could be responsible for hypertension and cardiovascular complications because the silent polymorphism (+1166A/C SNP) of AT1R was recognized by miR-155. Ceolotto et al. found that AT1R negatively correlated with miR-155 expression and positively with blood pressure, suggesting a potential role of miR-155 in regulating blood pressure [149]. The post-transcriptional regulation on AT1R might be critically associated with miRNA. miR-155 can recognize the 3′-UTR of the human AT1R, and these were co-expressed in CHO cells because transfection with miR-155 inhibitor resulted in an upregulation of AT1R and ERK1/2 activation [150,151].
Anxiety-induced hypertension
Brain function involves specific miRNAs that could affect the maintenance of blood pressure. Hanin et al. has shown that the balance of miR-608 interacting with acetylcholinesterase and other targets, including CDC42 and IL-6, affects stress-stimulated hypertension [152]. Few other studies focused on this issue.
Gestation-induced hypertension
Hypertension disorder is one of the common medical complications during pregnancy [153], and studies concerning the relationship with miRNAs focus on preeclampsia among the categories of gestation-induced hypertension. Even though the causes of preeclampsia are not clear yet, it has been found that removing the placenta could rapidly remit hypertensive symptoms suggesting a key role of the placenta [154]. Results from studies demonstrate that miRNA is deregulated in the human placentas of preeclamptic pregnancies showing the possible involvement in the pathogenesis of preeclampsia [155-158].
In recent years, several studies report that there is a noticeable high expression of miR-210 in placental tissue and maternal plasma with preeclampsia [159,160]. In vitro models of hypoxia could also induce the increase of miR-210 in trophoblast cells [159] and overexpression of miR-210 reduced trophoblast invasion [160]. As studies suggest, the high level of miR-210 during preeclampsia might be responsible for mitochondria dysfunction in the placenta [161]. The essential function of miR-210 in preeclampsia is also validated to target a predominantly expressed steroidogenetic enzyme, along with miR-518c [s17][162]. Another miRNA that has been studied in the development of preeclampsia is miR-155, which is overexpressed in preeclamptic placenta and targets cysteine-rich protein 61 (CYR61), an important early angiogenic factor during pregnancy [163]. By contrast, in the umbilical vein endothelial cells isolated from severe preeclampsia patients mature miR-155 was downregulated and could act as an important contributor to preeclampsia through angiotensin II type 1 receptor [164]. In addition, miR-376c was downregulated in the placenta and plasma of preeclampsia patients and reported to be able to induce trophoblast cell proliferation, migration and invasion [165]. Table 2 summarizes microRNAs implicated in hypertension.
Potential role of miRNAs in therapy and prognostic for hypertension
Pharmacology and nonpharmacology
Antihypertension drugs targeting miRNAs
The application of anti-miRNA, miRNA mimics and related molecules appears to be attractive in treating hypertension. Despite previous therapeutic options that were limited to symptomatic relief, the manipulation of individual miRNAs appears to influence PAH. Several miRNAs have been tested in clinical trials in different studies; the identification of miRNAs as promising therapeutic targets could offer new approaches for targeting of the disease origins and avoiding potentially serious adverse consequences.
Reestablishing the miR-204 level might represent a novel therapeutic approach for PAH. Nebulization of synthetic miR-204 decreased the severity of monocrotaline-induced PAH rats with limited adverse effects as observed; treatment efficacy indicators, including pulmonary arterial thickness and pressure, right ventricle wall thickness and related signaling molecules, were measured invasively [109]. Researchers also focused on the mechanism involving miR-204 and used DHEA to reverse vascular remodeling in the same PAH model [111]. This treatment significantly decreased pulmonary artery pressure and right ventricular hypertrophy; general cardiac function and wall remodeling improved, as confirmed by suppression of proliferation and apoptosis resistance in isolated SMCs.
Gain in miR-193 could rescue PAH in monocrotaline and hypoxia models as observed after intratracheal administration of the mimic delivered to the lung; overexpression of miR-193 mainly localized to pulmonary arteries reduced right ventricular systolic pressure and hypertrophy significantly and also prevented arterial muscularization and remodeling [119].
By targeting the miRNA function in PAH ECs, Kim et al. found that rats subjected to monocrotaline[s18] and hypoxia to induce PAH demonstrated a marked reduction of right ventricular systolic pressures and muscularized microvessels after receiving an intranasal delivery of lentiviral miR-424 and miR-503, suggesting another possible strategy for ameliorating experimental PAH in rodents [124]. By contrast, the therapeutic potentials of antagomirs against specific miRNAs have suggested the need to address the treatment of PAH. Researchers have shown that the sequestration of miR-21 in mice before and after hypoxia exposure diminished pulmonary pressure, vascular remodeling and cardiac hypertrophy [113]. Antagomirs of miR-17, miR-21 and miR-92a were injected into hypoxia-exposed mice; miR-17 and miR-21 inhibition showed lower impacts on right ventricular systolic pressure without increasing system blood pressure. Among these, the application of miR-17 in interfering with pulmonary vessel muscularization and right ventricular remodeling was outstanding. In hypoxia- and monocrotaline-induced PAH models, the miR-17 inhibitor successfully improved hemodynamic parameters and normalized cardiovascular morphology [112]; this therapeutic efficacy might be in part due to the BMPR2 signaling pathway.
The successful use of miR-22 inhibitor in decreasing blood pressure for SHR rats has constituted a viable therapeutic target by modulating the sympathetic activity without any adverse effects on blood plasma [136]. Central sympathetic deactivation is considered as an important strategy for arresting hypertension [166]. Fan et al. used artificial miRNA targeting angiotensin II type 1 receptor in the paraventricular nucleus of SHR successfully to decrease hypertension symptoms and basal sympathetic activity [167].
By contrast, the implementation of miRNAs as antihypertension drugs in patients should be taken with great caution because all the research is limited to animal experiments at the present stage. Injection and intratracheal nebulization are the most common ways for animal intervention; however, the specific function of miRNA could vary between different organs and tissues. The challenges also show up in the complicated network of miRNAs and their target mRNAs involved in hypertension. After all, the clinical development of miRNA as a new therapy for PAH should be imminent.
Exercise training as a nonpharmacological therapy for hypertension
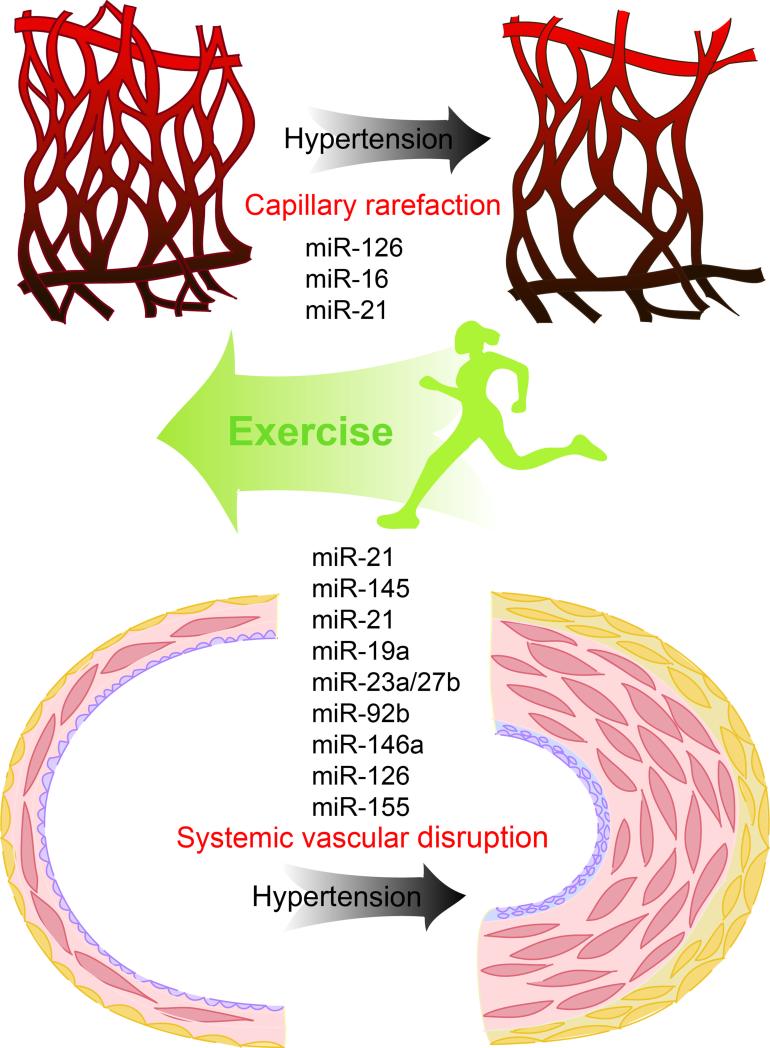
Exercise programs are shown to be effective in preventing and treating hypertension; the lack of physical activity is highly related to cardiovascular morbidity. As a major nonpharmacological approach or to assist pharmacologic therapy, physical exercise has been employed to modulate cardiovascular function and revert vascular remodeling as induced by hypertension through increased mechanical load and metabolic stress. The antihypertensive effects of exercise include the improvement of exercise tolerance, peripheral perfusion, arterial stiffness, autonomic function, endothelial repair, among others [168-172]. Indeed, exercise can produce clinical benefits in resistant hypertension [173] and, even in an old age group, exercise training could be an important nonpharmacological strategy [174]. Among the disparate aspects of mechanisms through which blood pressure control is improved by exercise, endothelial function, SMC phenotype switching and peripheral capillary rarefaction will be discussed in terms of their potential relationship with miRNAs (Figure 2).
Figure 2.
Potential miRNAs involved in hypertension regulated by exercise training. Exercise training could have effects on the vascular miRs and thus modulate systemic vascular remodeling and peripheral capillary rarefaction induced by hypertension.
Evidence indicates that hemodynamic signals produced by physical activity modulate a particular EC phenotype and exert beneficial effects on the cardiovascular system [175,176]. Weber et al. have demonstrated that unidirectional shear stress regulates the expression of miRNAs in ECs and that the elevated level of miR-21 influences endothelial apoptosis through eNOS phosphorylation and nitric oxide production [177], and oscillatory shear stress also induces the expression of miR-21 in vascular ECs [178]. Other endothelial miRNAs such as miR-19a, miR23a/27b and miR92b also respond to mechanical stress [74,100,179].
The sensitivity of miR-21 to mechanical forces was also observed in cultured human aortic SMCs; elevated mechanical stretch upregulated the expression of miR-21, inducing SMC proliferation and apoptosis [52]. Exercise training could guide the phenotype of SMCs as being the main target cells during altered hemodynamics of the vessel wall, and miRNAs are involved in the cellular response to diverse mechanical or biochemical cues. A study has revealed that miR-26a regulates human airway SMC hypertrophy via its target gene GSK-3β as being a mechanosensitive gene under the stimulation of stretch [180]. However, most of the results concerning the mechanical stress on cell functions were from in vitro studies and could hardly mimic the exact influences of pathological stimuli and physical activity on vessels. The unpublished data from our research group have shown that aerobic exercise might produce an opposite shift in SMCs under the pathological conditions such as hypertension. No study has elucidated the possible relationship between miRNA mechanisms and exercise-induced SMC phenotype changing. However, Hu et al. have identified miRNA profiles in an altered SMC phenotype by mechanical stretching using microarray and have identified the central role of miR-145 in this process [181].
Even though delineating the miRNA mechanisms through which hypertensive vascular function or remodeling benefit from exercise is still in the early stages, other vascular pathological alterations have been reported to be mitigated through miRNA, demonstrating the protective effects of exercise. Increased miR-146a and miR-126 and reduced miR-155 were induced by 12-week aerobic exercise on the aorta of mice with atherosclerosis [182]. Healthy rats subjected to 10-week swimming training showed increased cardiac capillary fiber ratio along with elevation of miR-126 expression, and this promoting angiogenesis effect might be regulated by MAPK and PI3K/Akt/eNOS signaling through targeting Spred-1 and PI3KR2 by miR-126 [183], which has been reported as an endothelial-specific modulator on vascular integrity, angiogenesis and repair [s19][62,64]. Hypertension could typically affect the capillary bed of skeletal muscle, thus the rarefaction of arterioles and capillaries reduces the transcapillary fluid exchange and prevents overperfusion of the terminal vascular bed, which contributes to total peripheral resistance in hypertension [184,185]. Exercise training could prevent microvascular rarefaction induced by hypertension; indeed, exercised and nonexercised muscle respond to physical training by promoting arteriole and venule growth and proliferation [185]. Fernandes et al. have reported that the balance between angiogenic and apoptotic factors might be associated with the elevation of muscle miR-16, miR-21 and miR-126 by 10 weeks of swimming [186].
Role of circulating miRNAs in prognostics for hypertension
Although substantial progress has been made in recent years for cardiovascular diseases (CVDs) such as hypertension in diagnosis, treatment and prognosis, new diagnostic and therapeutic strategies are very much needed in clinical practice. Accumulating evidence suggests that miRNAs are promising novel biomarkers for the early detection of CVDs. In 2008, miRNAs were first discovered to circulate in the bloodstream. So far, they have been known to present in the circulation in all blood components, including plasma, platelets, red blood cells and nucleated blood cells [67,187]. Because they have many important features, circulating miRNAs now have been considered as important blood-based biomarkers for CVDs. These distinct features indicate: (i) they are stable in the circulation; (ii) their sequences are evolutionarily conserved; (iii) their expression is often tissue- or pathology-specific; and (iv) their detection is based on sequence-specific amplification [188]. These attractive qualities suggest that circulating miRNAs might not only be novel biomarkers for early diagnosis but could also be new drug targets for CVDs [2,3].
Recently, numerous studies have shown that plasma levels of many miRNAs are significantly changed in CVDs, including essential hypertension [189]. A recent study compared the miRNA expression levels in plasma samples from 13 hypertensive patients and five healthy control subjects by using microarray-based miRNA expression profiling. The results showed that 27 miRNAs were differentially expressed. The expressions of selected miRNAs (miR-296-5p, let-7e and hcmv-miR-UL112) were validated independently in plasma samples from hypertensive patients and control subjects. Among these miRNAs, hcmv-miR-UL112, a human cytomegalovirus (HCMV)-encoded miRNA, is highly expressed in patients with hypertension [134]. The results showed a correlation between the HCMV titers and the levels of hcmv-miR-UL112 in plasma. Recently, in three independent cohorts, the plasma level of hsa-miR-505, a previously reported tumor suppressive miRNA, was found significantly elevated in hypertensive patients. Hsa-miR-505 is a novel circulating signature of hypertension, which could have a role in angiogenesis. The results indicate that hsa-miR-505 could also be a potential target for intervention of hypertension [190]. In another prospective longitudinal cohort study of pregnant women who are at first trimester (10–13 weeks), relative quantification of placental-specific C19MC miRNAs (miR-516-5p, miR-517*, miR-518b, miR-520a*, miR-520h, miR-525 and miR-526a) was determined in 28 normal pregnancies and 18 pregnancies that developed gestational hypertension [191]. The result showed that the upregulation of miR-516-5p, miR-517*, miR-520h and miR-518b was associated with a risk of later development of gestational hypertension. First trimester screening of extracellular miR-520h alone or in combination with miR-518b identified a significant proportion of women with subsequent gestational hypertension. Chronic thromboembolic pulmonary hypertension (CTEPH) is a progressive disease characterized by misguided thrombolysis and remodeling of pulmonary arteries. A recent study showed that CTEPH patients had an aberrant miRNA signature, in which let-7b, one of the downregulated antioncogenic miRNAs in the signature, was validated to decrease to about 0.25-fold in CTEPH patients [192]. The results suggested that reduced let-7b might be involved in the pathogenesis of CTEPH by affecting ET-1 expression and the function of pulmonary arterial ECs and VSMCs.
Methods of detection circulating miRNAs
As promising candidates for hypertensive biomarkers, detecting circulating miRNAs with potential high specificity and sensitivity is easily implemented and cost-efficient, opening a new page for diagnosis and therapy associated with hypertension. However, to ensure the accuracy, further identification should be designed to combine several miRNAs [188] and tested by reliable methods because of the possible interaction from unrelated organs and their limited content in the blood.
Among the difficulties in determining circulating miRNAs, to isolate sufficient and qualified miRNAs from serum or plasma requires the following steps. There are available kits developed specifically for isolating miRNAs, and researchers have suggested slight protocol modifications to resolve low quantity of miRNAs from samples such as blood. It suggests that repeating the phenol–chloroform extraction might generate more miRNAs [193].
Several methodologies, including microarray, qPCR, northern blotting and in situ hybridization [25] are used to detect circulating miRNAs. By applying microarray, the global circulating miRNAs profiling hypertension could be obtained based on the sequence complementarity. Real time qPCR is the most commonly performed method and is suitable for relatively high-throughput detection. Data normalization in circulating miRNAs targeting hypertension emerges as another special challenge. Reference genes such as U6, U87 and cel-miR-39 are often used as the internal control, but none has been generally validated and some have been queried about stability in serum of patients with urological malignancies [189,194]. The research team of Sayed et al. offered the usage of miR-156 in normalizing the qPCR data to achieve reproducible results [189]. Other researchers preferred not to use an endogenous control under the identical conditions within the experiment [117,195]. An obvious advantage of northern blotting over the other methods is its ability to probe pre-miRNA that might be related to hypertension [195] and using locked nucleic-acid-modified oligonucleotide probes might be more sensitive for investigating circulating miRNAs with limited abundance [196]. In summary, the multiplexed detection of circulating miRNAs should be combined to compensate the traits according to the study goal and specific miRNA features.
Concluding remarks
miRNAs, short noncoding RNAs, act to fine-tune various biological processes to maintain homeostasis; they also play a key part in the pathogenesis of arterial hypertension. Through the degradation and translational inhibition of their target transcripts, miRNAs influence various aspects of cell physiology by negatively regulating gene expression. Although progress in the understanding of the role of miRNAs in hypertension has been advanced in recent years, much remains to be explored. With further characterization, elucidating the function and detecting the methods of miRNAs as well as the feasibility of miRNA delivery in humans could provide us with new diagnostic, prognostic and therapeutic targets for the treatment of arterial high blood pressure. Undoubtedly, there will be a rapid growth in understanding the molecular processes regulated by miRNAs in the coming years and, consequently, the potential to harness miRNA biology for the improvement of human health, prevention and cure of human diseases, which could represent the next frontier in medicine.
Highlights.
Hypertension is a major risk factor for cardiovascular and renal disease
MicroRNAs are important in the pathogenesis of hypertension
MicroRNAs provide sensitive biomarkers and novel therapeutic targets of hypertension
Acknowledgments
This work was supported in part by National Natural Science Foundation of China 31371201 (L.S.), Beijing Natural Science Foundation 5132017 (L.S.), Chinese Universities Scientific Fund 2015 (L.S.), ‘12th Five-Year Plan’ (2012BAK21B03) (L.S.) and US National Institutes of Health Grants HL110125 (L.Z.), HD031226 (L.Z.), HL118861 (L.Z.). We apologize to all authors whose work could not be cited because of space limitations.
Biographies

Lubo Zhang
Dr Zhang is Professor of Pharmacology and Physiology and Director of the Center for Perinatal Biology at Loma Linda University School of Medicine. He was the President of the Western Pharmacology Society in 2008. He has been a member, in various study sections of grant review, of the US National Institutes of Health and American Heart Association for more than 15 years. Dr Zhang is the author or coauthor of over 500 scientific articles, book chapters and abstracts. His research interests focus on the molecular and epigenetic mechanisms in the regulation of uteroplacental circulation and developmental programming of health and disease.

Lijun Shi
Dr Shi is Professor of Exercise Physiology at Beijing Sport University in China. She has worked in the Center for Perinatal Biology in Loma Linda University and Harbor-UCLA Medical Center as a visiting scientist during 2007–2009 and 2003–2004. Her research interests focus on the molecular mechanisms in the regulation of cardiocerebrovascular functions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Carretero OA, Oparil S. Essential hypertension. Part I: definition and etiology. Circulation. 2000;101:329–335. doi: 10.1161/01.cir.101.3.329. [DOI] [PubMed] [Google Scholar]
- 2.Kosaka N, et al. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010;101:2087–2092. doi: 10.1111/j.1349-7006.2010.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu DC, et al. Circulating microRNAs: potential biomarkers for cancer. Int. J. Mol. Sci. 2011;12:2055–2063. doi: 10.3390/ijms12032055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee RC, et al. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 5.Eulalio A, et al. Getting to the root of miRNA-mediated gene silencing. Cell. 2008;132:9–14. doi: 10.1016/j.cell.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 6.Fazi F, Nervi C. MicroRNA: basic mechanisms and transcriptional regulatory networks for cell fate determination. Cardiovasc. Res. 2008;79:553–561. doi: 10.1093/cvr/cvn151. [DOI] [PubMed] [Google Scholar]
- 7.Makeyev EV, Maniatis T. Multilevel regulation of gene expression by microRNAs. Science. 2008;319:1789–1790. doi: 10.1126/science.1152326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song MA, et al. Differential expression of microRNAs in ischemic heart disease. Drug Discov. Today. 2015;20:223–235[s20]. doi: 10.1016/j.drudis.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagos-Quintana M, et al. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 10.Lee Y, et al. MicroRNA maturation: stepwise processing and subcellular localization. EMBO J. 2002;21:4663–4670. doi: 10.1093/emboj/cdf476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez A, et al. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14:1902–1910. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung HJ, Suh Y. MicroRNA in aging: from discovery to biology. Curr. Genomics. 2012;13:548–557. doi: 10.2174/138920212803251436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sontheimer EJ. Assembly and function of RNA silencing complexes. Nat. Rev. Mol. Cell Biol. 2005;6:127–138. doi: 10.1038/nrm1568. [DOI] [PubMed] [Google Scholar]
- 14.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 16.Yamakuchi M. MicroRNAs in vascular biology. Int. J. Vasc. Med. 2012;2012:794898. doi: 10.1155/2012/794898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filipowicz W, et al. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat. Rev. Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 18.Friedman RC, et al. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y, et al. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell. 2007;129:303–317. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 20.Saxena A, Tabin CJ. miRNA-processing enzyme Dicer is necessary for cardiac outflow tract alignment and chamber septation. Proc. Natl. Acad. Sci. U. S. A. 2010;107:87–91. doi: 10.1073/pnas.0912870107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng Y, et al. Critical roles of miRNA-mediated regulation of TGFbeta signalling during mouse cardiogenesis. Cardiovasc. Res. 2014;103:258–267. doi: 10.1093/cvr/cvu126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen JF, et al. Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2111–2116. doi: 10.1073/pnas.0710228105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.da Costa Martins PA, et al. Conditional dicer gene deletion in the postnatal myocardium provokes spontaneous cardiac remodeling. Circulation. 2008;118:1567–1576. doi: 10.1161/CIRCULATIONAHA.108.769984. [DOI] [PubMed] [Google Scholar]
- 24.Wang D, et al. MicroRNA-124 controls the proliferative, migratory, and inflammatory phenotype of pulmonary vascular fibroblasts. Circ. Res. 2014;114:67–78. doi: 10.1161/CIRCRESAHA.114.301633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao PK, et al. Loss of cardiac microRNA-mediated regulation leads to dilated cardiomyopathy and heart failure. Circ. Res. 2009;105:585–594. doi: 10.1161/CIRCRESAHA.109.200451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y, et al. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436:214–220. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 27.Heidersbach A, et al. microRNA-1 regulates sarcomere formation and suppresses smooth muscle gene expression in the mammalian heart. Elife. 2013;2:e01323. doi: 10.7554/eLife.01323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei Y, et al. Multifaceted roles of miR-1s in repressing the fetal gene program in the heart. Cell Res. 2014;24:278–292. doi: 10.1038/cr.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu N, et al. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev. 2008;22:3242–3254. doi: 10.1101/gad.1738708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wystub K, et al. miR-1/133a clusters cooperatively specify the cardiomyogenic lineage by adjustment of myocardin levels during embryonic heart development. PLoS Genet. 2013;9:e1003793. doi: 10.1371/journal.pgen.1003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albinsson S, et al. MicroRNAs are necessary for vascular smooth muscle growth, differentiation, and function. Arterioscler. Thromb. Vasc. Biol. 2010;30:1118–1126. doi: 10.1161/ATVBAHA.109.200873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan Y, et al. Conditional deletion of Dicer in vascular smooth muscle cells leads to the developmental delay and embryonic mortality. Biochem. Biophys. Res. Commun. 2011;408:369–374. doi: 10.1016/j.bbrc.2011.02.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albinsson S, et al. Smooth muscle miRNAs are critical for post-natal regulation of blood pressure and vascular function. PLoS One. 2011;6:e18869. doi: 10.1371/journal.pone.0018869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Z, et al. DiGeorge syndrome critical region 8 (DGCR8) protein-mediated microRNA biogenesis is essential for vascular smooth muscle cell development in mice. J. Biol. Chem. 2012;287:19018–19028. doi: 10.1074/jbc.M112.351791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cordes KR, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng Y, et al. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ. Res. 2009;105:158–166. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boettger T, et al. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J. Clin. Invest. 2009;119:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elia L, et al. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ. 2009;16:1590–1598. doi: 10.1038/cdd.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xin M, et al. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009;23:2166–2178. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo L, et al. The microRNA-328 regulates hypoxic pulmonary hypertension by targeting at insulin growth factor 1 receptor and L-type calcium channel-alpha1C. Hypertension. 2012;59:1006–1013. doi: 10.1161/HYPERTENSIONAHA.111.185413. [DOI] [PubMed] [Google Scholar]
- 41.Xie C, et al. MicroRNA-1 regulates smooth muscle cell differentiation by repressing Kruppel-like factor 4. Stem Cells Dev. 2011;20:205–210. doi: 10.1089/scd.2010.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J, et al. Induction of microRNA-1 by myocardin in smooth muscle cells inhibits cell proliferation. Arterioscler. Thromb. Vasc. Biol. 2011;31:368–375. doi: 10.1161/ATVBAHA.110.218149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torella D, et al. MicroRNA-133 controls vascular smooth muscle cell phenotypic switch in vitro and vascular remodeling in vivo. Circ. Res. 2011;109:880–893. doi: 10.1161/CIRCRESAHA.111.240150. [DOI] [PubMed] [Google Scholar]
- 44.Choe N, et al. The microRNA miR-132 targets Lrrfip1 to block vascular smooth muscle cell proliferation and neointimal hyperplasia. Atherosclerosis. 2013;229:348–355. doi: 10.1016/j.atherosclerosis.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Wang YS, et al. MicroRNA-195 regulates vascular smooth muscle cell phenotype and prevents neointimal formation. Cardiovasc. Res. 2012;95:517–526. doi: 10.1093/cvr/cvs223. [DOI] [PubMed] [Google Scholar]
- 46.Merlet E, et al. miR-424/322 regulates vascular smooth muscle cell phenotype and neointimal formation in the rat. Cardiovasc. Res. 2013;98:458–468. doi: 10.1093/cvr/cvt045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim MH, et al. MicroRNA-365 inhibits the proliferation of vascular smooth muscle cells by targeting cyclin D1. J. Cell. Biochem. 2014;115:1752–1761. doi: 10.1002/jcb.24841. [DOI] [PubMed] [Google Scholar]
- 48.Kang K, et al. MicroRNA-124 suppresses the transactivation of nuclear factor of activated T cells by targeting multiple genes and inhibits the proliferation of pulmonary artery smooth muscle cells. J. Biol. Chem. 2013;288:25414–25427. doi: 10.1074/jbc.M113.460287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li P, et al. MicroRNA-663 regulates human vascular smooth muscle cell phenotypic switch and vascular neointimal formation. Circ. Res. 2013;113:1117–1127. doi: 10.1161/CIRCRESAHA.113.301306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ji R, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ. Res. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 51.Davis BN, et al. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song J, et al. Mechanical stretch modulates microRNA 21 expression, participating in proliferation and apoptosis in cultured human aortic smooth muscle cells. PLoS One. 2012;7:e47657. doi: 10.1371/journal.pone.0047657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leeper NJ, et al. MicroRNA-26a is a novel regulator of vascular smooth muscle cell function. J. Cell. Physiol. 2011;226:1035–1043. doi: 10.1002/jcp.22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu X, et al. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ. Res. 2009;104:476–487. doi: 10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun SG, et al. miR-146a and Kruppel-like factor 4 form a feedback loop to participate in vascular smooth muscle cell proliferation. EMBO Rep. 2011;12:56–62. doi: 10.1038/embor.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fiedler J, et al. Functional microRNA library screening identifies the hypoxamir miR-24 as a potent regulator of smooth muscle cell proliferation and vascularization. Antioxid. Redox. Signal. 2014;21:1167–1176. doi: 10.1089/ars.2013.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol. Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 58.Suarez Y, et al. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ. Res. 2007;100:1164–1173. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- 59.Kuehbacher A, et al. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ. Res. 2007;101:59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- 60.Asada S, et al. Downregulation of Dicer expression by serum withdrawal sensitizes human endothelial cells to apoptosis. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H2512–2521. doi: 10.1152/ajpheart.00233.2008. [DOI] [PubMed] [Google Scholar]
- 61.Suarez Y, et al. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 2008;105:14082–14087. doi: 10.1073/pnas.0804597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fish JE, et al. miR-126 regulates angiogenic signaling and vascular integrity. Dev. Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuhnert F, et al. Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development. 2008;135:3989–3993. doi: 10.1242/dev.029736. [DOI] [PubMed] [Google Scholar]
- 64.Wang S, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harris TA, et al. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc. Natl. Acad. Sci. U. S. A. 2008;105:1516–1521. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fasanaro P, et al. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J. Biol. Chem. 2008;283:15878–15883. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Y, Gorski DH. Regulation of angiogenesis through a microRNA (miR-130a) that down-regulates antiangiogenic homeobox genes GAX and HOXA5. Blood. 2008;111:1217–1226. doi: 10.1182/blood-2007-07-104133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poliseno L, et al. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108:3068–3071. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- 69.Iaconetti C, et al. Inhibition of miR-92a increases endothelial proliferation and migration in vitro as well as reduces neointimal proliferation in vivo after vascular injury. Basic Res. Cardiol. 2012;107:296. doi: 10.1007/s00395-012-0296-y. [DOI] [PubMed] [Google Scholar]
- 70.Daniel JM, et al. Inhibition of miR-92a improves re-endothelialization and prevents neointima formation following vascular injury. Cardiovasc. Res. 2014;103:564–572. doi: 10.1093/cvr/cvu162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou Q, et al. Regulation of angiogenesis and choroidal neovascularization by members of microRNA-23~27~24 clusters. Proc. Natl. Acad. Sci. U. S. A. 2011;108:8287–8292. doi: 10.1073/pnas.1105254108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kulshreshtha R, et al. A microRNA signature of hypoxia. Mol. Cell Biol. 2007;27:1859–1867. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fiedler J, et al. MicroRNA-24 regulates vascularity after myocardial infarction. Circulation. 2011;124:720–730. doi: 10.1161/CIRCULATIONAHA.111.039008. [DOI] [PubMed] [Google Scholar]
- 74.Qin X, et al. MicroRNA-19a mediates the suppressive effect of laminar flow on cyclin D1 expression in human umbilical vein endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 2010;107:3240–3244. doi: 10.1073/pnas.0914882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chamorro-Jorganes A, et al. MicroRNA-16 and microRNA-424 regulate cell-autonomous angiogenic functions in endothelial cells via targeting vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1. Arterioscler. Thromb. Vasc. Biol. 2011;31:2595–2606. doi: 10.1161/ATVBAHA.111.236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grundmann S, et al. MicroRNA-100 regulates neovascularization by suppression of mammalian target of rapamycin in endothelial and vascular smooth muscle cells. Circulation. 2011;123:999–1009. doi: 10.1161/CIRCULATIONAHA.110.000323. [DOI] [PubMed] [Google Scholar]
- 77.Zhang L, et al. MiR-132 inhibits expression of SIRT1 and induces proinflammatory processes of vascular endothelial inflammation through blockade of the SREBP-1c metabolic pathway. Cardiovasc. Drugs Ther. 2014;28:303–311. doi: 10.1007/s10557-014-6533-x. [DOI] [PubMed] [Google Scholar]
- 78.Kwon C, et al. MicroRNA1 influences cardiac differentiation in Drosophila and regulates Notch signaling. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18986–18991. doi: 10.1073/pnas.0509535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang B, et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat. Med. 2007;13:486–491. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 80.Curcio A, et al. MicroRNA-1 downregulation increases connexin 43 displacement and induces ventricular tachyarrhythmias in rodent hypertrophic hearts. PLoS One. 2013;8:e70158. doi: 10.1371/journal.pone.0070158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ikeda S, et al. MicroRNA-1 negatively regulates expression of the hypertrophy-associated calmodulin and Mef2a genes. Mol. Cell Biol. 2009;29:2193–2204. doi: 10.1128/MCB.01222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takaya T, et al. MicroRNA-1 and MicroRNA-133 in spontaneous myocardial differentiation of mouse embryonic stem cells. Circ. J. 2009;73:1492–1497. doi: 10.1253/circj.cj-08-1032. [DOI] [PubMed] [Google Scholar]
- 83.Luo X, et al. MicroRNA-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation. J. Clin. Invest. 2013;123:1939–1951. doi: 10.1172/JCI62185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Care A, et al. MicroRNA-133 controls cardiac hypertrophy. Nat. Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 85.Horie T, et al. MicroRNA-133 regulates the expression of GLUT4 by targeting KLF15 and is involved in metabolic control in cardiac myocytes. Biochem. Biophys. Res. Commun. 2009;389:315–320. doi: 10.1016/j.bbrc.2009.08.136. [DOI] [PubMed] [Google Scholar]
- 86.Callis TE, et al. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J. Clin. Invest. 2009;119:2772–2786. doi: 10.1172/JCI36154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Rooij E, et al. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 88.van Rooij E, et al. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev. Cell. 2009;17:662–673. doi: 10.1016/j.devcel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grueter CE, et al. A cardiac microRNA governs systemic energy homeostasis by regulation of MED13. Cell. 2012;149:671–683. doi: 10.1016/j.cell.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ventura A, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132:875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang J, et al. Bmp signaling regulates myocardial differentiation from cardiac progenitors through a MicroRNA-mediated mechanism. Dev. Cell. 2010;19:903–912. doi: 10.1016/j.devcel.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Porrello ER, et al. MiR-15 family regulates postnatal mitotic arrest of cardiomyocytes. Circ. Res. 2011;109:670–679. doi: 10.1161/CIRCRESAHA.111.248880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nishi H, et al. MicroRNA-15b modulates cellular ATP levels and degenerates mitochondria via Arl2 in neonatal rat cardiac myocytes. J. Biol. Chem. 2010;285:4920–4930. doi: 10.1074/jbc.M109.082610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hullinger TG, et al. Inhibition of miR-15 protects against cardiac ischemic injury. Circ. Res. 2012;110:71–81. doi: 10.1161/CIRCRESAHA.111.244442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chan MC, et al. Molecular basis for antagonism between PDGF and the TGFbeta family of signalling pathways by control of miR-24 expression. EMBO J. 2010;29:559–573. doi: 10.1038/emboj.2009.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chan MC, et al. A novel regulatory mechanism of the bone morphogenetic protein (BMP) signaling pathway involving the carboxyl-terminal tail domain of BMP type II receptor. Mol. Cell Biol. 2007;27:5776–5789. doi: 10.1128/MCB.00218-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Davis-Dusenbery BN, et al. down-regulation of Kruppel-like factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-beta and bone morphogenetic protein 4. J. Biol. Chem. 2011;286:28097–28110. doi: 10.1074/jbc.M111.236950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang H, et al. miR-10a contributes to retinoid acid-induced smooth muscle cell differentiation. J. Biol. Chem. 2010;285:9383–9389. doi: 10.1074/jbc.M109.095612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Doebele C, et al. Members of the microRNA-17-92 cluster exhibit a cell-intrinsic antiangiogenic function in endothelial cells. Blood. 2010;115:4944–4950. doi: 10.1182/blood-2010-01-264812. [DOI] [PubMed] [Google Scholar]
- 100.Wu W, et al. Flow-dependent regulation of Kruppel-like factor 2 is mediated by microRNA-92a. Circulation. 2011;124:633–641. doi: 10.1161/CIRCULATIONAHA.110.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fang Y, Davies PF. Site-specific microRNA-92a regulation of Kruppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler. Thromb. Vasc. Biol. 2012;32:979–987. doi: 10.1161/ATVBAHA.111.244053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen N, et al. MicroRNA-410 reduces the expression of vascular endothelial growth factor and inhibits oxygen-induced retinal neovascularization. PLoS One. 2014;9:e95665. doi: 10.1371/journal.pone.0095665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Seok JK, et al. MicroRNA-382 induced by HIF-1alpha is an angiogenic miR targeting the tumor suppressor phosphatase and tensin homolog. Nucleic Acids Res. 2014;42:8062–8072. doi: 10.1093/nar/gku515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gorenne I, et al. Vascular smooth muscle cell sirtuin 1 protects against DNA damage and inhibits atherosclerosis. Circulation. 2013;127:386–396. doi: 10.1161/CIRCULATIONAHA.112.124404. [DOI] [PubMed] [Google Scholar]
- 105.Fehsel K, et al. Analysis of TNF alpha-induced DNA strand breaks at the single cell level. Am. J. Pathol. 1991;139:251–254. [PMC free article] [PubMed] [Google Scholar]
- 106.Meloche J, et al. Role for DNA damage signaling in pulmonary arterial hypertension. Circulation. 2014;129:786–797. doi: 10.1161/CIRCULATIONAHA.113.006167. [DOI] [PubMed] [Google Scholar]
- 107.Meyrick B, et al. Development of Crotalaria pulmonary hypertension: hemodynamic and structural study. Am. J. Physiol. 1980;239:H692–702. doi: 10.1152/ajpheart.1980.239.5.H692. [DOI] [PubMed] [Google Scholar]
- 108.Caruso P, et al. A role for miR-145 in pulmonary arterial hypertension: evidence from mouse models and patient samples. Circ. Res. 2012;111:290–300. doi: 10.1161/CIRCRESAHA.112.267591. [DOI] [PubMed] [Google Scholar]
- 109.Courboulin A, et al. Role for miR-204 in human pulmonary arterial hypertension. J. Exp. Med. 2011;208:535–548. doi: 10.1084/jem.20101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bertero T, et al. Systems-level regulation of microRNA networks by miR-130/301 promotes pulmonary hypertension. J. Clin. Invest. 2014;124:3514–3528. doi: 10.1172/JCI74773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Paulin R, et al. Dehydroepiandrosterone inhibits the Src/STAT3 constitutive activation in pulmonary arterial hypertension. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H1798–1809. doi: 10.1152/ajpheart.00654.2011. [DOI] [PubMed] [Google Scholar]
- 112.Pullamsetti SS, et al. Inhibition of microRNA-17 improves lung and heart function in experimental pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2012;185:409–419. doi: 10.1164/rccm.201106-1093OC. [DOI] [PubMed] [Google Scholar]
- 113.Yang S, et al. miR-21 regulates chronic hypoxia-induced pulmonary vascular remodeling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012;302:L521–529. doi: 10.1152/ajplung.00316.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sarkar J, et al. MicroRNA-21 plays a role in hypoxia-mediated pulmonary artery smooth muscle cell proliferation and migration. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;299:L861–871. doi: 10.1152/ajplung.00201.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Drake KM, et al. Altered MicroRNA processing in heritable pulmonary arterial hypertension: an important role for Smad-8. Am. J. Respir. Crit. Care Med. 2011;184:1400–1408. doi: 10.1164/rccm.201106-1130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jalali S, et al. Mir-206 regulates pulmonary artery smooth muscle cell proliferation and differentiation. PLoS One. 2012;7:e46808. doi: 10.1371/journal.pone.0046808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yue J, et al. MicroRNA-206 is involved in hypoxia-induced pulmonary hypertension through targeting of the HIF-1alpha/Fhl-1 pathway. Lab. Invest. 2013;93:748–759. doi: 10.1038/labinvest.2013.63. [DOI] [PubMed] [Google Scholar]
- 118.Chang MW, et al. Adenovirus-mediated over-expression of the cyclin/cyclin-dependent kinase inhibitor, p21 inhibits vascular smooth muscle cell proliferation and neointima formation in the rat carotid artery model of balloon angioplasty. J. Clin. Invest. 1995;96:2260–2268. doi: 10.1172/JCI118281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sharma S, et al. Apolipoprotein A-I mimetic peptide 4F rescues pulmonary hypertension by inducing microRNA-193-3p. Circulation. 2014;130:776–785. doi: 10.1161/CIRCULATIONAHA.114.007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shan F, et al. HIF-1 alpha-induced up-regulation of miR-9 contributes to phenotypic modulation in pulmonary artery smooth muscle cells during hypoxia. J. Cell. Physiol. 2014;229:1511–1520. doi: 10.1002/jcp.24593. [DOI] [PubMed] [Google Scholar]
- 121.Caruso P, et al. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler. Thromb. Vasc. Biol. 2010;30:716–723. doi: 10.1161/ATVBAHA.109.202028. [DOI] [PubMed] [Google Scholar]
- 122.Parikh VN, et al. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation. 2012;125:1520–1532. doi: 10.1161/CIRCULATIONAHA.111.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.White K, et al. Endothelial apoptosis in pulmonary hypertension is controlled by a microRNA/programmed cell death 4/caspase-3 axis. Hypertension. 2014;64:185–194. doi: 10.1161/HYPERTENSIONAHA.113.03037. [DOI] [PubMed] [Google Scholar]
- 124.Kim J, et al. An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nat. Med. 2013;19:74–82. doi: 10.1038/nm.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brock M, et al. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ. Res. 2009;104:1184–1191. doi: 10.1161/CIRCRESAHA.109.197491. [DOI] [PubMed] [Google Scholar]
- 126.Coen M, et al. Myofibroblast-mediated adventitial remodeling: an underestimated player in arterial pathology. Arterioscler. Thromb. Vasc. Biol. 2011;31:2391–2396. doi: 10.1161/ATVBAHA.111.231548. [DOI] [PubMed] [Google Scholar]
- 127.Stenmark KR, et al. The adventitia: essential regulator of vascular wall structure and function. Annu. Rev. Physiol. 2013;75:23–47. doi: 10.1146/annurev-physiol-030212-183802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu G, et al. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J. Exp. Med. 2010;207:1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Potus F, et al. Impaired angiogenesis and peripheral muscle microcirculation loss contribute to exercise intolerance in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2014;190:318–328. doi: 10.1164/rccm.201402-0383OC. [DOI] [PubMed] [Google Scholar]
- 130.Zampetaki A, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ. Res. 2010;107:810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- 131.Meng S, et al. Downregulation of microRNA-126 in endothelial progenitor cells from diabetes patients, impairs their functional properties, via target gene Spred-1. J. Mol. Cell. Cardiol. 2012;53:64–72. doi: 10.1016/j.yjmcc.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 132.Qiang L, et al. Expression of miR-126 and miR-508-5p in endothelial progenitor cells is associated with the prognosis of chronic heart failure patients. Int. J. Cardiol. 2013;168:2082–2088. doi: 10.1016/j.ijcard.2013.01.160. [DOI] [PubMed] [Google Scholar]
- 133.Fukushima Y, et al. Assessment of plasma miRNAs in congestive heart failure. Circ. J. 2011;75:336–340. doi: 10.1253/circj.cj-10-0457. [DOI] [PubMed] [Google Scholar]
- 134.Li S, et al. Signature microRNA expression profile of essential hypertension and its novel link to human cytomegalovirus infection. Circulation. 2011;124:175–184. doi: 10.1161/CIRCULATIONAHA.110.012237. [DOI] [PubMed] [Google Scholar]
- 135.Kontaraki JE, et al. MicroRNA-9 and microRNA-126 expression levels in patients with essential hypertension: potential markers of target-organ damage. J. Am. Soc. Hypertens. 2014;8:368–375. doi: 10.1016/j.jash.2014.03.324. [DOI] [PubMed] [Google Scholar]
- 136.Friese RS, et al. MicroRNA-22 and promoter motif polymorphisms at the Chga locus in genetic hypertension: functional and therapeutic implications for gene expression and the pathogenesis of hypertension. Hum. Mol. Genet. 2013;22:3624–3640. doi: 10.1093/hmg/ddt213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Thabut D, Shah V. Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension? J. Hepatol. 2010;53:976–980. doi: 10.1016/j.jhep.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 138.Zhao Y, et al. Hepatic stellate cells produce vascular endothelial growth factor via phospho-p44/42 mitogen-activated protein kinase/cyclooxygenase-2 pathway. Mol. Cell. Biochem. 2012;359:217–223. doi: 10.1007/s11010-011-1016-x. [DOI] [PubMed] [Google Scholar]
- 139.Guo CJ, et al. Effects of upregulated expression of microRNA-16 on biological properties of culture-activated hepatic stellate cells. Apoptosis. 2009;14:1331–1340. doi: 10.1007/s10495-009-0401-3. [DOI] [PubMed] [Google Scholar]
- 140.He Y, et al. MicroRNA-146a modulates TGF-beta1-induced hepatic stellate cell proliferation by targeting SMAD4. Cell Signal. 2012;24:1923–1930. doi: 10.1016/j.cellsig.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 141.Ji J, et al. Over-expressed microRNA-27a and 27b influence fat accumulation and cell proliferation during rat hepatic stellate cell activation. FEBS Lett. 2009;583:759–766. doi: 10.1016/j.febslet.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 142.Wei J, et al. MicroRNA-21 activates hepatic stellate cells via PTEN/Akt signaling. Biomed. Pharmacother. 2013;67:387–392. doi: 10.1016/j.biopha.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 143.Guo CJ, et al. Dynamic expression of miR-126* and its effects on proliferation and contraction of hepatic stellate cells. FEBS Lett. 2013;587:3792–3801. doi: 10.1016/j.febslet.2013.09.047. [DOI] [PubMed] [Google Scholar]
- 144.Huebert RC, et al. Aquaporin-1 promotes angiogenesis, fibrosis, and portal hypertension through mechanisms dependent on osmotically sensitive microRNAs. Am. J. Pathol. 2011;179:1851–1860. doi: 10.1016/j.ajpath.2011.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Luft FC, et al. Genetic influences on the response to dietary salt reduction, acute salt loading, or salt depletion in humans. J. Cardiovasc. Pharmacol. 1988;12(Suppl. 3):S49–55. [PubMed] [Google Scholar]
- 146.Naraba H, Iwai N. Assessment of the microRNA system in salt-sensitive hypertension. Hypertens. Res. 2005;28:819–826. doi: 10.1291/hypres.28.819. [DOI] [PubMed] [Google Scholar]
- 147.Nossent AY, et al. SNPs in microRNA binding sites in 3′-UTRs of RAAS genes influence arterial blood pressure and risk of myocardial infarction. Am. J. Hypertens. 2011;24:999–1006. doi: 10.1038/ajh.2011.92. [DOI] [PubMed] [Google Scholar]
- 148.Nossent AY, et al. The 14q32 microRNA-487b targets the antiapoptotic insulin receptor substrate 1 in hypertension-induced remodeling of the aorta. Ann. Surg. 2013;258:743–751. doi: 10.1097/SLA.0b013e3182a6aac0. discussion 752-743. [DOI] [PubMed] [Google Scholar]
- 149.Ceolotto G, et al. Interplay between miR-155, AT1R A1166C polymorphism, and AT1R expression in young untreated hypertensives. Am. J. Hypertens. 2011;24:241–246. doi: 10.1038/ajh.2010.211. [DOI] [PubMed] [Google Scholar]
- 150.Martin MM, et al. The human angiotensin II type 1 receptor +1166 A/C polymorphism attenuates microRNA-155 binding. J. Biol. Chem. 2007;282:24262–24269. doi: 10.1074/jbc.M701050200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 151.Martin MM, et al. MicroRNA-155 regulates human angiotensin II type 1 receptor expression in fibroblasts. J. Biol. Chem. 2006;281:18277–18284. doi: 10.1074/jbc.M601496200. [DOI] [PubMed] [Google Scholar]
- 152.Hanin G, et al. Competing targets of microRNA-608 affect anxiety and hypertension. Hum. Mol. Genet. 2014;23:4569–4580. doi: 10.1093/hmg/ddu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am. J. Obstet. Gynecol. 2000;183:S1–22. [No authors listed] [PubMed] [Google Scholar]
- 154.Redman CW. Current topic: pre-eclampsia and the placenta. Placenta. 1991;12:301–308. doi: 10.1016/0143-4004(91)90339-h. [DOI] [PubMed] [Google Scholar]
- 155.Pineles BL, et al. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am. J. Obstet. Gynecol. 2007;196:e261–266. doi: 10.1016/j.ajog.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 156.Zhu XM, et al. Differential expression profile of microRNAs in human placentas from preeclamptic pregnancies vs normal pregnancies. Am. J. Obstet. Gynecol. 2009;200:e661–667. doi: 10.1016/j.ajog.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 157.Enquobahrie DA, et al. Placental microRNA expression in pregnancies complicated by preeclampsia. Am. J. Obstet. Gynecol. 2011;204:e112–121. doi: 10.1016/j.ajog.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mayor-Lynn K, et al. Expression profile of microRNAs and mRNAs in human placentas from pregnancies complicated by preeclampsia and preterm labor. Reprod. Sci. 2011;18:46–56. doi: 10.1177/1933719110374115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee DC, et al. miR-210 targets iron-sulfur cluster scaffold homologue in human trophoblast cell lines: siderosis of interstitial trophoblasts as a novel pathology of preterm preeclampsia and small-for-gestational-age pregnancies. Am. J. Pathol. 2011;179:590–602. doi: 10.1016/j.ajpath.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Anton L, et al. miR-210 inhibits trophoblast invasion and is a serum biomarker for preeclampsia. Am. J. Pathol. 2013;183:1437–1445. doi: 10.1016/j.ajpath.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Muralimanoharan S, et al. MIR-210 modulates mitochondrial respiration in placenta with preeclampsia. Placenta. 2012;33:816–823. doi: 10.1016/j.placenta.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ishibashi O, et al. Hydroxysteroid (17-beta) dehydrogenase 1 is dysregulated by miR-210 and miR-518c that are aberrantly expressed in preeclamptic placentas: a novel marker for predicting preeclampsia. Hypertension. 2012;59:265–273. doi: 10.1161/HYPERTENSIONAHA.111.180232. [DOI] [PubMed] [Google Scholar]
- 163.Zhang Y, et al. MicroRNA-155 contributes to preeclampsia by down-regulating CYR61. Am. J. Obstet. Gynecol. 2010;202:e461–467. doi: 10.1016/j.ajog.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 164.Cheng W, et al. microRNA-155 regulates angiotensin II type 1 receptor expression in umbilical vein endothelial cells from severely pre-eclamptic pregnant women. Int. J. Mol. Med. 2011;27:393–399. doi: 10.3892/ijmm.2011.598. [DOI] [PubMed] [Google Scholar]
- 165.Fu G, et al. MicroRNA-376c impairs transforming growth factor-beta and nodal signaling to promote trophoblast cell proliferation and invasion. Hypertension. 2013;61:864–872. doi: 10.1161/HYPERTENSIONAHA.111.203489. [DOI] [PubMed] [Google Scholar]
- 166.Fisher JP, Fadel PJ. Therapeutic strategies for targeting excessive central sympathetic activation in human hypertension. Exp. Physiol. 2010;95:572–580. doi: 10.1113/expphysiol.2009.047332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fan ZD, et al. Artificial microRNA interference targeting AT(1a) receptors in paraventricular nucleus attenuates hypertension in rats. Gene Ther. 2012;19:810–817. doi: 10.1038/gt.2011.145. [DOI] [PubMed] [Google Scholar]
- 168.Oliveira NL, et al. The effects of exercise training on arterial stiffness in coronary artery disease patients: a state-of-the-art review. Clin. Physiol. Funct. Imaging. 2014;34:254–262. doi: 10.1111/cpf.12093. [DOI] [PubMed] [Google Scholar]
- 169.Ribeiro F, et al. Is exercise training an effective therapy targeting endothelial dysfunction and vascular wall inflammation? Int. J. Cardiol. 2010;141:214–221. doi: 10.1016/j.ijcard.2009.09.548. [DOI] [PubMed] [Google Scholar]
- 170.Gielen S, et al. Exercise training in coronary artery disease and coronary vasomotion. Circulation. 2001;103:E1–6. doi: 10.1161/01.cir.103.1.e1. [DOI] [PubMed] [Google Scholar]
- 171.Linke A, et al. Exercise and the coronary circulation-alterations and adaptations in coronary artery disease. Prog. Cardiovasc. Dis. 2006;48:270–284. doi: 10.1016/j.pcad.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 172.Ribeiro F, et al. Effects of exercise training on endothelial progenitor cells in cardiovascular disease: a systematic review. Am. J. Phys. Med. Rehabil. 2013;92:1020–1030. doi: 10.1097/PHM.0b013e31829b4c4f. [DOI] [PubMed] [Google Scholar]
- 173.Ribeiro F, et al. Exercise training in the management of patients with resistant hypertension. World J. Cardiol. 2015;7:47–51. doi: 10.4330/wjc.v7.i2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Oliveira J, et al. Postaerobic exercise blood pressure reduction in very old persons with hypertension. J. Geriatr. Phys. Ther. 2015 doi: 10.1519/JPT.0000000000000049. PMID: 25760278. [DOI] [PubMed] [Google Scholar]
- 175.Hambrecht R, et al. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107:3152–3158. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- 176.Laughlin MH, et al. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J. Appl. Physiol. (1985) 2008;104:588–600. doi: 10.1152/japplphysiol.01096.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Weber M, et al. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochem. Biophys. Res. Commun. 2010;393:643–648. doi: 10.1016/j.bbrc.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhou J, et al. MicroRNA-21 targets peroxisome proliferators-activated receptor-alpha in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc. Natl. Acad. Sci. U. S. A. 2011;108:10355–10360. doi: 10.1073/pnas.1107052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.He J, et al. The feedback regulation of PI3K-miR-19a, and MAPK-miR-23b/27b in endothelial cells under shear stress. Molecules. 2012;18:1–13. doi: 10.3390/molecules18010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mohamed JS, et al. Mechanical stretch up-regulates microRNA-26a and induces human airway smooth muscle hypertrophy by suppressing glycogen synthase kinase-3beta. J. Biol. Chem. 2010;285:29336–29347. doi: 10.1074/jbc.M110.101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hu B, et al. Mechanical stretch suppresses microRNA-145 expression by activating extracellular signal-regulated kinase 1/2 and upregulating angiotensin-converting enzyme to alter vascular smooth muscle cell phenotype. PLoS One. 2014;9:e96338. doi: 10.1371/journal.pone.0096338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wu XD, et al. Effect of aerobic exercise on miRNA-TLR4 signaling in atherosclerosis. Int. J. Sports. Med. 2014;35:344–350. doi: 10.1055/s-0033-1349075. [DOI] [PubMed] [Google Scholar]
- 183.Da Silva ND, Jr, et al. Swimming training in rats increases cardiac microRNA-126 expression and angiogenesis. Med. Sci. Sports Exerc. 2012;44:1453–1462. doi: 10.1249/MSS.0b013e31824e8a36. [DOI] [PubMed] [Google Scholar]
- 184.Greene AS, et al. Microvascular rarefaction and tissue vascular resistance in hypertension. Am. J. Physiol. 1989;256:H126–131. doi: 10.1152/ajpheart.1989.256.1.H126. [DOI] [PubMed] [Google Scholar]
- 185.Melo RM, et al. Training-induced, pressure-lowering effect in SHR: wide effects on circulatory profile of exercised and nonexercised muscles. Hypertension. 2003;42:851–857. doi: 10.1161/01.HYP.0000086201.27420.33. [DOI] [PubMed] [Google Scholar]
- 186.Fernandes T, et al. Exercise training prevents the microvascular rarefaction in hypertension balancing angiogenic and apoptotic factors: role of microRNAs-16, -21, and -126. Hypertension. 2012;59:513–520. doi: 10.1161/HYPERTENSIONAHA.111.185801. [DOI] [PubMed] [Google Scholar]
- 187.Mitchell PS, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. U. S. A. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tijsen AJ, et al. Circulating microRNAs as diagnostic biomarkers for cardiovascular diseases. Am. J. Physiol. Heart Circ. Physiol. 2012;303:H1085–1095. doi: 10.1152/ajpheart.00191.2012. [DOI] [PubMed] [Google Scholar]
- 189.Sayed AS, et al. Diagnosis, prognosis and therapeutic role of circulating miRNAs in cardiovascular diseases. Heart Lung Circ. 2014;23:503–510. doi: 10.1016/j.hlc.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 190.Yang Q, et al. MicroRNA-505 identified from patients with essential hypertension impairs endothelial cell migration and tube formation. Int. J. Cardiol. 2014;177:925–934. doi: 10.1016/j.ijcard.2014.09.204. [DOI] [PubMed] [Google Scholar]
- 191.Hromadnikova I, et al. First trimester screening of circulating C19MC microRNAs can predict subsequent onset of gestational hypertension. PLoS One. 2014;9:e113735. doi: 10.1371/journal.pone.0113735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Guo L, et al. Differentially expressed plasma microRNAs and the potential regulatory function of Let-7b in chronic thromboembolic pulmonary hypertension. PLoS One. 2014;9:e101055. doi: 10.1371/journal.pone.0101055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Burgos KL, et al. Identification of extracellular miRNA in human cerebrospinal fluid by next-generation sequencing. RNA. 2013;19:712–722. doi: 10.1261/rna.036863.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sanders I, et al. Evaluation of reference genes for the analysis of serum miRNA in patients with prostate cancer, bladder cancer and renal cell carcinoma. Int. J. Urol. 2012;19:1017–1025. doi: 10.1111/j.1442-2042.2012.03082.x. [DOI] [PubMed] [Google Scholar]
- 195.van Rooij E. The art of microRNA research. Circ. Res. 2011;108:219–234. doi: 10.1161/CIRCRESAHA.110.227496. [DOI] [PubMed] [Google Scholar]
- 196.Valoczi A, et al. Sensitive and specific detection of microRNAs by northern blot analysis using LNA-modified oligonucleotide probes. Nucleic Acids Res. 2004;32:e175. doi: 10.1093/nar/gnh171. [DOI] [PMC free article] [PubMed] [Google Scholar]