Abstract
The observation of increased hyperphosphorylated tau levels correlating with microglial activation in opiate abusers has been interpreted as predisposition to accelerated Alzheimer disease (AD)-related changes. The present study focused on evaluating additional neurodegeneration-related proteins, including α-synuclein and TDP-43, and p62-positive deposits. We performed a systematic mapping of protein deposits in the brains of 27 individuals with documented heroin addiction (age: 19-40) and compared with 11 controls (age: 15-40). We confirm previous findings that heroin addiction associates with tau hyperphosphorylation in predilection brain areas for aging and AD. Furthermore, we show that this occurs also in areas implicated in the molecular disturbances and in vivo neuronal networks related to heroin abuse. There was, however, no presence of Amyloid-beta deposits. We extend previous findings by showing the lack of TDP-43 or α-synuclein pathology and emphasize the independent effect of the duration of drug use on the appearance of age-related p62-positive neuritic profiles. These observations provide unique insights about neuropathological alterations in the brains of young heroin addicts and have implications about brain aging and the influences of environmental and toxic factors.
Keywords: aging, α-synuclein, heroin, neuropathology, opiate, tau, TDP-43, p62
1. Introduction
Heroin is a commonly abused opiate drug with significant medical and societal consequences. Heroin users show deficits in visual and working memory, processing speed and executive function, as well as disturbances of mood, reward and motor function (Gruber, et al., 2007). In addition to heroin’s known effects on molecular and cellular processing, neuropathological studies report cerebral edema induced by hypoxic-ischemic injury, ischemic neuronal damage and neuronal loss, alterations in gray and white matter morphometry, spongiform leukoencephalopathy, furthermore, deposition of amyloid-beta (Aβ) and hyperphosphorylated tau (Anthony, et al., 2010; Buttner, et al., 2000; Cadet, et al., 2014; Ramage, et al., 2005). Further neuropathological alterations have been related to HIV (human immunodeficiency virus) infection (Ramage, et al., 2005). The observation that increased hyperphosphorylated tau levels correlate with microglial activation in a cohort of HIV negative individuals has been interpreted as reflective of a predisposition to accelerated Alzheimer-changes (Anthony, et al., 2010; Ramage, et al., 2005).
Alzheimer disease (AD) is a frequent neurodegenerative disease (NDD) characterized by the deposition of extracellular plaques composed of Aβ and intracellular neurofibrillary degeneration composed of altered (i.e. hyperphosphorylated) tau protein (Duyckaerts, et al., 2009). Appearance of these pathological alterations follows a hierarchical anatomical pathway in the brain (Braak and Braak, 1991; Thal, et al., 2002). It had until recently been widely accepted that the entorhinal/transentorhinal cortex is the earliest anatomical region to become affected by neurofibrillary degeneration (Braak and Braak, 1991). However, studies in young individuals suggest that select brainstem nuclei such as the noradrenergic locus coeruleus may be affected even earlier in the AD-pathological process (Braak and Del Tredici, 2011; Grinberg, et al., 2009). Further proteins, like α-synuclein or TDP-43, are deposited in various NDDs, like Parkinson disease or subsets of patients with frontotemporal lobar degeneration (FTLD), respectively (Kovacs, et al., 2010). These proteins appear also as concomitant proteinopathies in AD brains (Kovacs, et al., 2010; Kovacs, et al., 2013).
The concept of proteinopathies emphasizes the importance of protein processing systems in NDDs. These systems comprise two major elimination pathways that control the quality of cellular components and maintain cell homeostasis: the ubiquitin-proteasome-system that degrades short-lived proteins in the cytoplasm and nucleus, and the autophagy-lysosome pathway, which digests long-lived proteins and abnormal organelles in the cytoplasm (Nijholt, et al., 2011). Accordingly, the majority of protein deposits associated with NDDs can be detected immunohistochemically using antibodies against ubiquitin and the ubiquitin binding protein p62 (Kuusisto, et al., 2008). Importantly, anti-ubiquitin and anti-p62 labels mature fibrillized protein aggregates, but not early pre-aggregates (Kuusisto, et al., 2003). On the other hand they immunolabel structures in the aging human brain, which are not detectable with antibodies against NDD-associated proteins, including ubiquitin-positive neuritic profiles (Braak, et al., 2013; Dickson, et al., 1992; Dickson, et al., 1990). In addition to anatomical areas highly implicated in AD, there are other regions such as the amygdala, fusiform gyrus, nucleus accumbens and ventral tegmental area (VTA), which are also affected partly in diverse NDDs, that have functional relevance to drug addiction, impulsivity or emotional regulation (Horvath, et al., 2007; Xie, et al., 2011). Indeed, we have also previously shown microscopically detectable loss of dopamine transporter expression in the nucleus accumbens associated with impairment of the VTA in heroin addicts (Horvath, et al., 2007).
Based on the above lines of evidence and various related literature, we hypothesized that heroin addiction may lead to accelerated deposition of NDD-related proteins in different anatomical regions affected in NDDs or related to drug abuse. Therefore, we performed a systematic mapping of pathological alterations and protein deposits in the brains of HIV-negative individuals with documented heroin addiction and compared with age-matched controls.
2. Material and Methods
2.1. Selection of cases
Brain specimens from Caucasian subjects were obtained at autopsy from the Department of Forensic and Insurance Medicine of Semmelweis University (Budapest, Hungary) under approved local ethical guidelines. All cases had a post mortem interval of <24 h. We evaluated levels of common drugs of abuse and their metabolites (including cannabis, cocaine, amphetamine), alcohol, and therapeutic drugs (including benzodiazepines). Subjects were classified into two main case groups according to their cause of death: control and heroin overdose. The heroin group represented a unique drug abuse population with predominant heroin use and with no history of methadone or buprenorphine clinical treatment; information was obtained from family and/or available medical documentation. They were also negative for HIV infection. The control group (sudden death without detectable pathological alterations or with acute myocardial infarction, acute bronchitis, pulmonary embolism, cardiomyopathy, or myocardial fibrosis) had no history of abuse to drugs or of a neuropsychiatric disorder and there were no needle tracks detected in autopsy. Furthermore, the control individuals had negative toxicology for opiates or other drugs of abuse, except alcohol in a single case in which the ethanol concentration was similar to the limited alcohol-positive subjects identified in the heroin group.
2.2 Neuropathology and immunohistochemistry
Formalin fixed, paraffin-embedded tissue blocks (2.5 × 2.0 cm) were evaluated. In addition to Hematoxylin and Eosin, Luxol-Cresyl violet, and Bielschowsky silver staining, the following monoclonal antibodies were used for immunohistochemistry: anti-tau AT8 (pS202/pT205, 1:200, Pierce Biotechnology, Rockford, IL, USA; indicated as pTau), anti-phospho-TDP-43 (pS409/410, 1:2,000, Cosmo Bio, Tokyo, Japan), anti-α-synuclein (1:2,000, clone 5G4 detecting only disease-associated alpha-synuclein (Kovacs, et al., 2014), Roboscreen, Leipzig, Germany), anti-Aβ (1:50, clone 6F/3D, Dako, Glostrup, Denmark), and anti-p62 (1:1,000, BD Transduction, Lexington KY, USA). The DAKO EnVision© detection kit, peroxidase/DAB, rabbit/mouse (Dako, Glostrup, Denmark) was used for visualization of antibody reactions. Examined anatomical regions are summarized in Table 1.
Table 1.
Summary of the examined anatomical regions in the study groups. + indicates that it was examined and − indicates that it was not.
| Nr. of cases |
Antibody |
||||||
|---|---|---|---|---|---|---|---|
| Examined block | Subregions | Her | Co | Tau | pTDP-43 | α-Syn | Aβ |
| Frontal | Cortex | 18 | 10 | + | + | + | + |
| White matter | 18 | 10 | + | + | + | + | |
| Temporal | Cortex | 26 | 9 | + | + | + | + |
| White matter | 26 | 9 | + | + | + | + | |
| Occipital | Cortex | 17 | 6 | + | − | − | + |
| White matter | 17 | 6 | + | − | − | + | |
| Hippocampus + | CA4 | 26 | 11 | + | + | + | + |
| CA2/3 | 26 | 11 | + | + | + | + | |
| CA1 | 26 | 11 | + | + | + | + | |
| Dentate gyrus | 26 | 11 | + | + | + | + | |
| Subiculum | 26 | 11 | + | + | + | + | |
| Entorhinal cortex | 26 | 11 | + | + | + | + | |
| Fusiform Gyrus | 26 | 11 | + | + | + | + | |
| White matter | 26 | 11 | + | + | + | + | |
| Basal ganglia | Caudate nucleus | 24 | 10 | + | + | + | + |
| Putamen | 24 | 10 | + | + | + | + | |
| Accumbens nucleus | 24 | 10 | + | + | + | + | |
| Globus pallidus | 24 | 10 | + | + | + | + | |
| Thalamus | Dorsomedial nucleus | 20 | 11 | + | + | + | + |
| Amygdala | Amygdala | 17 | 7 | + | + | + | + |
| Basal nucleus | Basal nucleus | 17 | 7 | + | + | + | + |
| Cerebellum | Purkinje cells | 2 | 2 | + | − | − | + |
| Granular layer | 2 | 2 | + | − | − | + | |
| Molecular layer | 2 | 2 | + | − | − | + | |
| White matter | 2 | 2 | + | − | − | + | |
| Dentate nucleus | 2 | 2 | + | − | − | + | |
| Mesencephalon | Substantia nigra | 17 | 9 | + | + | + | + |
| Ventral tegmental area | 17 | 9 | + | + | + | + | |
| Dorsal raphe nucleus | 17 | 9 | + | + | + | + | |
| Pons | Locus coeruleus | 4 | 2 | + | + | + | + |
| Pontine base nuclei | 4 | 2 | + | + | + | + | |
| Medulla oblongata | Inferior olives | 3 | 2 | + | + | + | + |
| Dorsal vagus nucleus | 3 | 2 | + | + | + | + | |
| Medullary raphe nuclei | 3 | 2 | + | + | + | + | |
We evaluated (blinded to the case grouping) extracellular and cellular immunoreactivites for Aβ, neuronal or glial cytoplasmic or nuclear inclusions and neurites for pTDP-43, tau, p62, and α-synuclein. We semiquantitatively evaluated p62 immunoreactive grains and neurites in the neuropil (none, few, moderate, many) and we counted the number of p62 immunoreactive nuclear Marinesco bodies in the substantia nigra (total number / 20 consecutively counted neuron with neuromelanin pigment in the cytoplasm and visible nucleus). In addition, we counted the number of tau immunoreactive neuritic profiles and neurons with cytoplasmic immunoreactivity in a 0.3 mm2 area using a counting grid and x40 objective (one large square covers 250 μm × 250 μm; 5 non-overlapping squares were evaluated).
2.3 Statistical analysis
The following variables were entered into the statistical evaluation in addition to the sex of the individuals: age at death, years of heroin use, percent of brain regions with p62 neuropil grains, semiquantitative scores of p62 grains in different anatomical regions, percent of anatomical regions where tau immunoreactive neurites were detected, number of tau-immunoreactive neurites and neuronal immunoreactivities in different anatomical regions. Chi-square, Fisher exact test, and Spearman correlation tests were used to evaluate significant association of variables with heroin addiction. Oneway ANOVA test was used to evaluate differences of the number of tau neurites and semiquantitative scores (p62 immunoreactivity) in different anatomical regions between study groups. SPSS Statistics Version 20 was used for the analysis. P value below 0.05 was considered as significant. Univariate linear model was used to evaluate the interaction between correlations.
3. Results
3.1. Description of study groups
27 heroin addicts (26 men; mean age at death: 26.15 ± 0.98, range 19-40 years) and 11 controls (8 men; 26.45 ± 2.83, range 15-40 years) were examined. Duration of addiction (range 1-13 years) was available for 22 individuals in the heroin study group. Routine neuropathological examination excluded ischemic/hypoxic encephalopathy, inflammatory changes, CNS tumor, and vascular pathology.
3.2. Immunostaining for Aβ, α-synuclein, pTDP-43, and p62
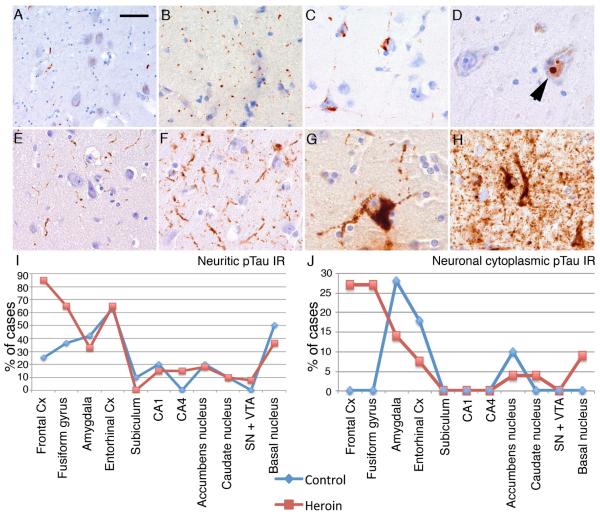
We did not observe any Aβ immunoreactive deposits in the study groups examined. Furthermore, there was a lack of α-synuclein or pTDP-43 immunopositive neurites or cytoplasmic inclusions. Immunostaining for p62 revealed thin neurites in the locus coeruleus, substantia nigra and VTA (Figure 1A) and grain-like structures (Figure 1B), in the basal ganglia, frontal, temporal, and entorhinal cortices and amygdala. These were negative for α-synuclein, pTDP-43 and pTau immunostaining. Cytoplasmic p62 immunoreactivity of single neurons was noted in the basal ganglia. In a single heroin addict case, neurofibrillary tangles in the entorhinal cortex (Figure 1C) were also immunolabeled by anti-p62. In addition, several intranuclear inclusions (Figure 1D), visible also in Hematoxylin and eosin staining, representing Marinesco bodies, were seen in the substantia nigra in both groups.
Figure 1.
Immunostaining for p62 reveals thin neurites and dots in the substantia nigra (A), grain-like structures (B), and neurofibrillary tangles in the entorhinal cortex (C), and intranuclear inclusions (arrowhead) representing Marinesco bodies in the substantia nigra (D). Immunostaining for tau revealed variable amounts of neuritic profiles (E, F) and neuronal cytoplasmic labeling (G) or both (H). Graphical presentation of the percent of cases showing neuritic (I) and neuronal cytoplasmic (J) Tau immunoreactivity in the heroin addict and control group. Bar in “A” represents 100 μm for A, B, E, and F, and 30 μm for C, D, G and H.
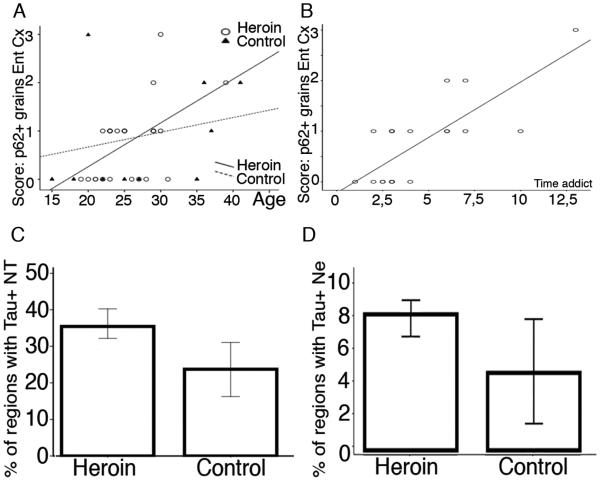
Distribution of p62 immunopositive structures was similar in controls and heroin subjects. The mean percent (± standard error) of anatomical regions (Table 1) showing p62 positive grains was 23.07 ± 3.08 in heroin addicts and was 24.64 ± 6.11 in controls. Semiquantitative scores of p62 neurites/grains in the locus coeuruleus, substantia nigra/VTA and grains in the neuropil in the frontal and entorhinal cortex, amygdala and basal ganglia did not differ significantly between heroin addicts and controls. Instead there was a positive correlation between p62 scores with the age of the individuals in the frontal (controls: R= 0.76, p=0.044; heroin addicts: R= 0.38, p=0.064) and entorhinal cortices (controls: R= 0.42, p=0.2; heroin addicts: R= 0.48, p=0.013) (Figure 2C). Intriguingly, a strong correlation was noted with the duration of heroin use and the semiquantitative score of p62 immunopositive grains in the entorhinal cortex (R=0.68, p=0.001) (Figure 2D), independently from the age of the individual (univariate model, p= 0.54). The proportion of anatomical regions showing p62 immunoreactive structures did not correlate with age either in controls or heroin subjects. Interestingly, p62 immunoreactive Marinesco bodies were less evident in the heroin group (mean ± standard error for heroin addicts: 0.72 ± 0.21; for controls: 2.57 ± 1.06; p=0.018). Their presence correlated with age only in the control subjects (R=0.872, p=0.011).
Figure 2.
The score of p62 positive granular deposits correlate with age in both heroin addicts and controls (A), and further with the duration of heroin addiction (B). Graphic representation of the percent of regions with Tau positive neurites (NT) (C) and neurons (NE) (D) in heroin addicts and controls.
3.3. Immunostaining for pTau
Immunostaining for pTau revealed variable amounts of neuritic profiles (Figure 1E, F), neuronal cytoplasmic labeling (Figure 1G) or both (Figure 1H) in controls and heroin addicts. Fine granular immunostaining of occasional astrocytes were seen in the frontal cortex, basal nucleus of Meynert and fusiform gyrus only in three heroin addicts. Several anatomical differences were evident when evaluating pTau immunostaining; both neuritic and cytoplasmic pTau immunoreactivity involved more areas in heroin addicts. Four controls (out of 11) and 22 heroin addicts (out of 27) showed pTau immunoreactivity in at least the entorhinal cortex or more than one examined anatomical regions (Table 2). Demographic data of these cases did not differ significantly from the subjects (7 controls: 6 men, age range 15-40 years; 5 heroin addicts: all men, age range: 21-40; duration of addiction: 2.5-5 years) which did not show pTau immunoreactivity in the entorhinal cortex or exhibited tau pathology only in one other anatomical area.
Table 2.
Overview of those cases (CO: controls; HER: heroin addicts) which showed pTau (AT8) in at least the entorhinal cortex or more than one examined anatomical regions. White box indicates none; grey coloured box indicates single neuronal cytoplasmic (N) or 1-10/0.3 mm2 fine neuritic (Nt); black box indicates 3 or more neuronal cytoplasmic or >10/0.3 mm2 fine neuritic immunoreactivity.
| Case | Age | Sex | Duration of addiction | Frontal | Temporal | Entorhinal | Fusiform Gyrus | Subiculum | CA1 | CA4 | Caudate nucleus | Accumbens | Basal nucleus | Amygdala | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Nt | N | Nt | N | Nt | N | Nt | N | Nt | N | Nt | N | Nt | N | Nt | N | Nt | N | Nt | N | Nt | ||||
| CO3 | 18 | F | - | na | na | ||||||||||||||||||||
| CO6 | 25 | M | - | na | na | ||||||||||||||||||||
| CO9 | 36 | F | - | na | |||||||||||||||||||||
| CO10 | 37 | M | - | ||||||||||||||||||||||
| HER1 | 19 | M | na | na | |||||||||||||||||||||
| HER2 | 20 | M | 3 | na | |||||||||||||||||||||
| HER4 | 21 | M | 2 | na | |||||||||||||||||||||
| HER6 | 22 | M | 6 | ||||||||||||||||||||||
| HER7 | 23 | M | 2 | na | |||||||||||||||||||||
| HER8 | 23 | M | 3 | na | na | ||||||||||||||||||||
| HER10 | 23 | M | 3 | na | na | ||||||||||||||||||||
| HER11 | 23 | M | 3 | na | na | na | |||||||||||||||||||
| HER13 | 25 | M | 7 | ||||||||||||||||||||||
| HER14 | 25 | M | 3 | ||||||||||||||||||||||
| HER15* | 25 | M | na | ||||||||||||||||||||||
| HER16 | 26 | M | 1 | na | |||||||||||||||||||||
| HER17 | 27 | M | 3 | ||||||||||||||||||||||
| HER18 | 28 | M | 3 | na | na | ||||||||||||||||||||
| HER19 | 29 | F | 10 | na | |||||||||||||||||||||
| HER20 | 29 | M | 7 | na | |||||||||||||||||||||
| HER21 | 29 | M | na | na | na | na | |||||||||||||||||||
| HER22 | 29 | M | na | ||||||||||||||||||||||
| HER23 | 30 | M | 13 | na | na | na | |||||||||||||||||||
| HER24 | 30 | M | na | na | na | na | |||||||||||||||||||
| HER25 | 31 | M | 2,5 | na | na | ||||||||||||||||||||
| HER26** | 39 | M | 6 | ||||||||||||||||||||||
indicates that in that case the locus ceruleus was also available and showed fine neuritic tau immunoreactivity;
indicates that ubiquitinated (p62+ve) neurofibrillary tangles were in the entorhinal cortex; na indicates not available.
In cases HER11, 21, 26 fine granular immunostaining of occasional astrocytes were seen in the frontal cortex, basal nucleus of Meynert and fusiform gyrus, respectively. Age at death and duration of addiction is provided in years.
M: male, F: female. NA: not available.
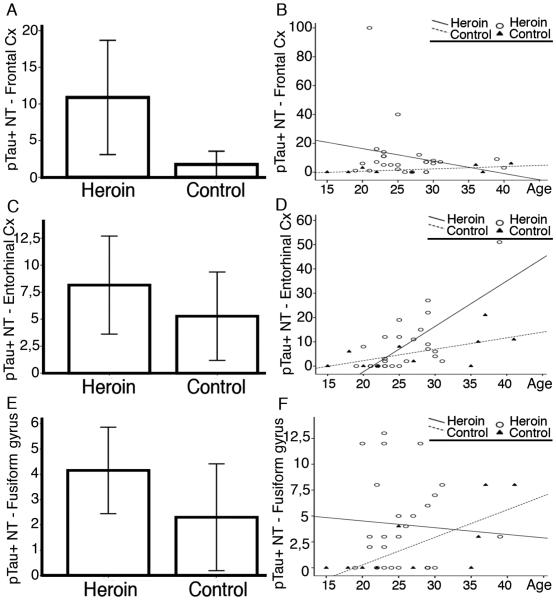
Tau immunoreactive neurites were seen in several brain regions including the locus coeruleus, amygdala, forebrain basal nuclei of Meynert, frontal cortex, entorhinal cortex, CA1 subregions and subiculum, fusiform gyrus, caudate and nucleus accumbens in control individuals; and in addition to these, in the substantia nigra, VTA, and hippocampus CA4 subregion in heroin addicts (Table 2 and Figure 1I). There was a tendency (p=0.084) for more widespread pTau pathology in heroin addicts as indicated by an increased percent of affected anatomical regions (total number 11) with detectable neuritic pTau immunoreactivity (Figure 1I and 2A), independent from the age. The presence of neuritic pTau immunoreactivity was more frequent in the frontal cortex and fusiform gyrus in heroin addicts compared to controls (Fisher exact test p=0.04 and p=0.13, respectively). The locus coeruleus was examined in two controls and four heroin addict cases; fine neuritic tau immunoreactivity was present in all. There was a tendency for the number of neurites to be higher in heroin addicts than controls in the frontal and entorhinal cortex and fusiform gyrus (Figure 3A, C, E), however, this did not reach significance (p= 0.19, 0.44, 0.23, respectively). Excluding two heroin addict individuals, who were outliers with quite abundant neurites in the frontal cortex, showed that the group comparison was significant (p=0.021). The load of neurites correlated well with the age of the individual in the control group (R= 0.65, p=0.078 in the frontal cortex; R= 0.72, p=0.011 in the entorhinal cortex; R= 0.74, p=0.013 in the fusiform gyrus). However, this was strikingly different in heroin addicts, where the neuritic count only in the entorhinal cortex correlated with age (R=0.59, p=0.001). In contrast, the involvement of the frontal cortex [(R=-0.1, p=0.50, even when excluding the two cases with abundant neurites: p=0.83), and fusiform gyrus (R=-0.01, p=0.93)] was independent of age (Figure 3B, D, F). Neurite counts in the fusiform gyrus showed significant inverse correlation with the history of heroin addiction (R= − 0.52, p=0.01).
Figure 3.
Graphic representation of the tau immunoreactive neurites (NT) in heroin addicts and controls (A,C,E) and their correlation with age of the individual (B, D, F) in the frontal (A,B), entorhinal (C, D) and fusiform gyrus (E, F).
Diffuse neuronal cytoplasmic pTau immunolabeling was seen only in the amygdala, entorhinal cortex and nucleus acccumbens in controls, whereas in heroin addicts, in addition to these regions, it was detected in the fusiform gyrus, frontal cortex, forebrain basal nucleus, and caudate nucleus (Table 2 and Figure 1J). Remarkably, evidence of neuronal cytoplasmic pTau immunoreactivity noted in any region (pooled data) was significantly associated with heroin addiction (Fisher exact test, p= 0.036). Neuronal cytoplasmic tau immunoreactivity was more frequently seen in the frontal cortex and fusiform gyrus in heroin addicts (Fischer exact test; p= 0.056). In heroin addicts, neuronal pTau immunoreactivity in the fusiform gyrus and frontal cortex did not associate with its concomitant presence in the entorhinal cortex or hippocampus. Notably, abundant neuronal tau pathology (> 20 immunoreactive neurons/evaluated area) was seen in the amygdala and frontal cortex, less in the fusiform gyrus, but lacking in the entorhinal cortex and hippocampus in a 21-year-old individual (Table 2; HER4). The mean percent of the anatomical regions where neuronal cytoplasmic pTau immunoreactivity was seen per case was nearly double in heroin addicts as in controls, however, this was not significant (p=0.31) (Figure 1J and 2B).
4. Discussion
In the present study we demonstrated differences in the distribution of tau pathology in the brains of HIV-negative heroin addicts as compared to age-matched controls. The major finding of our study is that phosphorylated tau pathology involves more anatomical regions of the brain and shows a higher pathological load in heroin addicts. We extend earlier findings (Anthony, et al., 2010; Ramage, et al., 2005) by showing that there is a lack of α-synuclein and TDP-43 immunoreactivities in heroin users. Furthermore, our study reveals an important contribution of the duration of heroin use on the appearance of age-related p62-positive neuritic profiles.
The neuropathology of heroin abuse is characterized by alterations related to acute and chronic effects. These include cerebrovascular lesions either due to episodes of hypoxia, hypotension, or to cardio-embolic events, but rare pathologies like granulomatous or inflammatory vessel alterations are also reported (Buttner, et al., 2000; Cadet, et al., 2014). In addition, the white matter may also be affected in the form of vascular or spongiform leukoencephalopathy (Buttner, et al., 2000; Cadet, et al., 2014). We detected only mild to moderate degrees of hypoxic/ischemic damage, and therefore, we focused on lesions implicated in NDDs or brain aging. Indeed, accelerated AD-related changes correlating with microglial activation have been reported (Anthony, et al., 2010; Ramage, et al., 2005). We confirmed those findings by demonstrating more widespread deposition of hyperphosphorylated tau in heroin addicts, but we did not detect Aβ deposition (Anthony, et al., 2010). The lack of Aβ pathology may be due to the young age of the subjects currently studied since Aβ deposition has previously been reported to be absent in young individuals (Braak and Del Tredici, 2011). Tau positivity was present mostly as neuropil threads or thin neurites, which has been recognized as an early and characteristic pathological hallmark of AD (Braak, et al., 2006). Importantly, tau pathology correlated with age in the entorhinal cortex, a predilection site in AD both in controls and heroin abusers. This supports the hypothesis that heroin use exaggerates age-related deficits in recall and working memory beyond such impairments normally ascribed to frequently co-occuring HIV-associated neurocognitive disorders (Ersche, et al., 2006; Hauser, et al., 2012). Our study also confirmed the observations of Braak and colleagues that phospho-tau deposits can be detected in young individuals in the locus coeruleus (Braak and Del Tredici, 2011). In contrast, the noted tau hyperphosphorylation pathology in the frontal cortex and fusiform gyrus, which was irrespective of age suggest sensitivity of these cortical regions to the effects of heroin abuse. It must be noted that those cases with around a 3-year history of heroin abuse showed the highest values of tau-immunoreactive neurites. Although acute hypoxia can also promote the phosphorylation of tau (Fang, et al., 2010), we could not find any link to ischemic/hypoxic damage in the fusiform gyrus and frontal cortex.
As reviewed recently (see Cadet, et al., 2014) heroin addiction is associated with profound alterations in brain structure and composition. This includes volume loss or decreased gray matter density including the areas we also examined (i.e. frontal cortex, fusiform area, amygdala). Importantly, in addition to the established role of the frontal cortex in decision-making and other cognitive processes, the fusiform gyrus, which also exhibited neuronal tau pathology, is a component of the brain connectivity network related to impulsivity observed in heroin addicts (Xie, et al., 2011). Accordingly, it can be theorized that anatomical areas, which directly regulate cognitive function may be more prone to tau phosphorylation. The brain reward system, including the nucleus accumbens is the major target of the mesolimbic dopaminergic system, which shows molecular disturbances in heroin addicts (Albertson, et al., 2006; Sillivan, et al., 2013). Apart from the cortical pathologies, tau positive neurites appeared more frequently in the mesencephalon, including the VTA, which has been also implicated in the pathogenesis of heroin addiction (Horvath, et al., 2007). Based on these observation we speculate that tau phosphorylation might be accelerated, even transiently, by heroin abuse in areas implicated in the molecular disturbances and in vivo neuronal networks related to heroin addiction. We theorize that this is multifactorial; as a limitation of our study, we cannot exclude the possibility that the amount of heroin intake has an influence as well. Accordingly, the finding of an inverse correlation between the history of addiction and neuritic counts in the fusiform gyrus merits further studies, including in vivo imaging.
Recently a new term “primary age-related tauopathy” (PART) has been described to designate a pathology that is commonly observed in the brains of aged individuals (Crary, et al., 2014). In PART, neurofibrillary tangles (NFTs), indistinguishable from those in AD with an absence of Aβ plaques, are mostly restricted to structures in the medial temporal lobe, basal forebrain, brainstem, and olfactory areas (bulb and cortex). Remarkably, in our study, as well as two previous investigations focusing on neuronal and neuritic tau pathology in heroin abuse (Anthony, et al., 2010; Ramage, et al., 2005), diffuse neuronal cytoplasmic tau (AT8) immunoreactivity and NFTs were found in several brain regions of heroin addicts without the presence of Aβ plaques. There was a deviation from the PART profile particularly when evaluating neuritic (thread-like) tau pathology (see above); indeed the basal ganglia, thalamus, substantia nigra and VTA, frontal and occipital cortices also showed these immunoreactivities (Anthony, et al., 2010). It is possible that some of the changes observed in heroin abusers are potentially reversible over time (Anthony, et al., 2010), however, it seems that the abused drug as an environmetal toxic substance contributes to the involvement of additional anatomical regions as seen in PART or AD. Our findings in non-heroin addict controls support the notion that tau phosphorylation does occur in young individuals as well. Importantly, we did not observe astroglial tau pathology as seen in primary tauopathies (Kovacs, 2015). There was a lack of thorny astrocytes in the subpial and subependymal regions or clusters of thorny astrocytes in the white or grey matter, which supports the observations that these are associated mainly with the ageing of the brain (Kovacs, 2015).
Our investigation expands the spectrum of proteinopathies examined previously in relation to heroin addition. We were unable to detect any pathologies of phosphorylated TDP-43, a protein associated with sporadic and genetic FTLD, and there was a lack of α-synuclein deposition. We focused on α-synuclein due to our previous observations of elevated VTA α-synuclein mRNA levels and cell loss in the substantia nigra/VTA of heroin addicts (Horvath, et al., 2007) but also wanted to expand our analysis of other brain areas which are functionally important in heroin addiction or related emotional disturbances (i.e. amygdala). However, in contrast to the hyperphosphorylation of tau, our study could not confirm that heroin addiction induces α-synucleinopathy in the examined age-range. Although α-synuclein is highly implicated in Parkinson’s disease, it must be noted that parkinsonian syndromes with encephalopathy after heroin abuse are rare (Deik, et al., 2012).
In addition to neurofibrillary degeneration or amyloid plaque formation, the aging brain shows further alterations such as ubiquitin immunoreactive neuritic profiles in the gray matter (Dickson, et al., 1992; Dickson, et al., 1990). In our study we used anti-p62 (sequestome) immunostaining, a sensitive method to detect these neuritic alterations together with a wide variety of neurodegenerative disease-related protein deposits (Kuusisto, et al., 2008). Recently, Braak et al. reported inclusions resembling Marinesco bodies within cell nuclei of catecholaminergic neurons (Braak, et al., 2013) as well as the dot-like structures previously described by Dickson in specific neuropil areas in humans (Dickson, et al., 1992). Their prevalence significantly increased with advancing age, and they were interpreted as unrelated to proteinaceous aggregate-formation of NDDs. We confirmed these findings and furthermore were able to show that the duration of addiction had an age-independent effect on the presence of p62 positive granules in the entorhinal cortex. In addition to the findings on tau, this provides further evidence that heroin addiction accelerates brain aging. Interestingly, we detected less neurons with p62 positive intranuclear bodies resembling Marinesco-bodies in the mesencephalic neuromelanin-containing neurons in heroin addicts. Accumulation of Marinesco bodies is thought to be an age-related phenomenon and accumulate mostly in vulnerable neurons (Kanaan, et al., 2007). Since we detected extracellular neuromelanin pigment and neuronal loss in these nuclei (Horvath, et al., 2007), the relative lack of Marinesco body containing cells might be due to their early loss.
5. Conclusions
We confirm previous findings that heroin addiction associates with tau hyperphosphorylation, but not with TDP-43 or α-synuclein pathology, in predilection brain areas for aging and AD. Furthermore we demonstrate that this occurs also in areas highly implicated with molecular disturbances and in vivo connectivity networks in heroin abuse. We expand earlier findings on heroin abuse by showing an independent effect of the duration of addiction on the appearance of age-related p62-positive neuritic profiles. These observations expand our knowledge on neuropathological alterations in the brains of heroin addicts and have implications for understanding of how brain aging may be influenced by environmental and toxic factors. Our study also highlights the importance of neuropathological examinations in young individuals, with or without brains insults, that can help provide more insight about early stages in the development of neurodegenerative conditions.
We evaluate neurodegeneration-related proteins in the brains of heroin addicts
We confirm that heroin addiction associates with tau hyperphosphorylation
Tau hyperphosphorylation occurs in areas with heroin-related molecular disturbances
There is a lack of TDP-43 or α-synuclein pathology in the brains of heroin addicts
The duration of addiction has an effect on the appearance of p62-positive profiles
Acknowledgements
Supported by Hungarian Scientific Health Council (ETT-098/2009), National Institutes of Health (DA15446; YLH and EK) and Hungarian Scientific Research Fund (OTKA), and the European Commission's 7th Framework Programme under GA No 278486, "DEVELAGE" for GGK. We are grateful to Mrs. Marianna Lenkeiné for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors report no conflict of interest.
References
- Albertson DN, Schmidt CJ, Kapatos G, Bannon MJ. Distinctive profiles of gene expression in the human nucleus accumbens associated with cocaine and heroin abuse. Neuropsychopharmacology. 2006;31:2304–2312. doi: 10.1038/sj.npp.1301089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony IC, Norrby KE, Dingwall T, Carnie FW, Millar T, Arango JC, Robertson R, Bell JE. Predisposition to accelerated Alzheimer-related changes in the brains of human immunodeficiency virus negative opiate abusers. Brain. 2010;133:3685–3698. doi: 10.1093/brain/awq263. [DOI] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. The pathological process underlying Alzheimer's disease in individuals under thirty. Acta Neuropathol. 2011;121:171–181. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- Braak H, Thal DR, Matschke J, Ghebremedhin E, Del Tredici K. Age-related appearance of dendritic inclusions in catecholaminergic brainstem neurons. Neurobiol. Aging. 2013;34:286–297. doi: 10.1016/j.neurobiolaging.2012.02.031. [DOI] [PubMed] [Google Scholar]
- Buttner A, Mall G, Penning R, Weis S. The neuropathology of heroin abuse. Forensic Sci. Int. 2000;113:435–442. doi: 10.1016/s0379-0738(00)00204-8. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Bisagno V, Milroy CM. Neuropathology of substance use disorders. Acta Neuropathol. 2014;127:91–107. doi: 10.1007/s00401-013-1221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, Arnold SE, Attems J, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Gearing M, Grinberg LT, Hof PR, Hyman BT, Jellinger K, Jicha GA, Kovacs GG, Knopman DS, Kofler J, Kukull WA, Mackenzie IR, Masliah E, McKee A, Montine TJ, Murray ME, Neltner JH, Santa-Maria I, Seeley WW, Serrano-Pozo A, Shelanski ML, Stein T, Takao M, Thal DR, Toledo JB, Troncoso JC, Vonsattel JP, White CL, 3rd, Wisniewski T, Woltjer RL, Yamada M, Nelson PT. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128:755–766. doi: 10.1007/s00401-014-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deik A, Saunders-Pullman R, Luciano MS. Substance abuse and movement disorders: complex interactions and comorbidities. Curr. Drug Abuse Rev. 2012;5:243–253. doi: 10.2174/1874473711205030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Crystal HA, Mattiace LA, Masur DM, Blau AD, Davies P, Yen SH, Aronson MK. Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol. Aging. 1992;13:179–189. doi: 10.1016/0197-4580(92)90027-u. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Wertkin A, Kress Y, Ksiezak-Reding H, Yen SH. Ubiquitin immunoreactive structures in normal human brains. Distribution and developmental aspects. Lab. Invest. 1990;63:87–99. [PubMed] [Google Scholar]
- Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118:5–36. doi: 10.1007/s00401-009-0532-1. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology. 2006;31:1036–1047. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H, Zhang LF, Meng FT, Du X, Zhou JN. Acute hypoxia promote the phosphorylation of tau via ERK pathway. Neurosci Lett. 2010;474:173–177. doi: 10.1016/j.neulet.2010.03.037. [DOI] [PubMed] [Google Scholar]
- Grinberg LT, Rub U, Ferretti RE, Nitrini R, Farfel JM, Polichiso L, Gierga K, Jacob-Filho W, Heinsen H, Brazilian Brain Bank Study, G. The dorsal raphe nucleus shows phospho-tau neurofibrillary changes before the transentorhinal region in Alzheimer's disease. A precocious onset? Neuropathol. Appl. Neurobiol. 2009;35:406–416. doi: 10.1111/j.1365-2990.2009.00997.x. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Silveri MM, Yurgelun-Todd DA. Neuropsychological consequences of opiate use. Neuropsychol. Rev. 2007;17:299–315. doi: 10.1007/s11065-007-9041-y. [DOI] [PubMed] [Google Scholar]
- Hauser KF, Fitting S, Dever SM, Podhaizer EM, Knapp PE. Opiate drug use and the pathophysiology of neuroAIDS. Curr. HIV Res. 2012;10:435–452. doi: 10.2174/157016212802138779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath MC, Kovacs GG, Kovari V, Majtenyi K, Hurd YL, Keller E. Heroin abuse is characterized by discrete mesolimbic dopamine and opioid abnormalities and exaggerated nuclear receptor-related 1 transcriptional decline with age. J. Neurosci. 2007;27:13371–13375. doi: 10.1523/JNEUROSCI.2398-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan NM, Kordower JH, Collier TJ. Age-related accumulation of Marinesco bodies and lipofuscin in rhesus monkey midbrain dopamine neurons: relevance to selective neuronal vulnerability. J. Comp. Neurol. 2007;502:683–700. doi: 10.1002/cne.21333. [DOI] [PubMed] [Google Scholar]
- Kovacs GG. Invited review: Neuropathology of tauopathies: principles and practice. Neuropathol. Appl. Neurobiol. 2015;41:3–23. doi: 10.1111/nan.12208. [DOI] [PubMed] [Google Scholar]
- Kovacs GG, Botond G, Budka H. Protein coding of neurodegenerative dementias: the neuropathological basis of biomarker diagnostics. Acta Neuropathol. 2010;119:389–408. doi: 10.1007/s00401-010-0658-1. [DOI] [PubMed] [Google Scholar]
- Kovacs GG, Breydo L, Green R, Kis V, Puska G, Lorincz P, Perju-Dumbrava L, Giera R, Pirker W, Lutz M, Lachmann I, Budka H, Uversky VN, Molnar K, Laszlo L. Intracellular processing of disease-associated alpha-synuclein in the human brain suggests prion-like cell-to-cell spread. Neurobiol. Dis. 2014;69:76–92. doi: 10.1016/j.nbd.2014.05.020. [DOI] [PubMed] [Google Scholar]
- Kovacs GG, Milenkovic I, Wohrer A, Hoftberger R, Gelpi E, Haberler C, Honigschnabl S, Reiner-Concin A, Heinzl H, Jungwirth S, Krampla W, Fischer P, Budka H. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta Neuropathol. 2013;126:365–384. doi: 10.1007/s00401-013-1157-y. [DOI] [PubMed] [Google Scholar]
- Kuusisto E, Kauppinen T, Alafuzoff I. Use of p62/SQSTM1 antibodies for neuropathological diagnosis. Neuropathol. Appl. Neurobiol. 2008;34:169–180. doi: 10.1111/j.1365-2990.2007.00884.x. [DOI] [PubMed] [Google Scholar]
- Kuusisto E, Parkkinen L, Alafuzoff I. Morphogenesis of Lewy bodies: dissimilar incorporation of alpha-synuclein, ubiquitin, and p62. J. Neuropathol. Exp. Neurol. 2003;62:1241–1253. doi: 10.1093/jnen/62.12.1241. [DOI] [PubMed] [Google Scholar]
- Nijholt DA, De Kimpe L, Elfrink HL, Hoozemans JJ, Scheper W. Removing protein aggregates: the role of proteolysis in neurodegeneration. Curr. Med. Chem. 2011;18:2459–2476. doi: 10.2174/092986711795843236. [DOI] [PubMed] [Google Scholar]
- Ramage SN, Anthony IC, Carnie FW, Busuttil A, Robertson R, Bell JE. Hyperphosphorylated tau and amyloid precursor protein deposition is increased in the brains of young drug abusers. Neuropathol. Appl. Neurobiol. 2005;31:439–448. doi: 10.1111/j.1365-2990.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- Sillivan SE, Whittard JD, Jacobs MM, Ren Y, Mazloom AR, Caputi FF, Horvath M, Keller E, Ma'ayan A, Pan YX, Chiang LW, Hurd YL. ELK1 transcription factor linked to dysregulated striatal mu opioid receptor signaling network and OPRM1 polymorphism in human heroin abusers. Biol. Psych. 2013;74:511–519. doi: 10.1016/j.biopsych.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- Xie C, Li SJ, Shao Y, Fu L, Goveas J, Ye E, Li W, Cohen AD, Chen G, Zhang Z, Yang Z. Identification of hyperactive intrinsic amygdala network connectivity associated with impulsivity in abstinent heroin addicts. Behav. Brain Res. 2011;216:639–646. doi: 10.1016/j.bbr.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]