Abstract
Background
Oral mechanistic target of rapamycin (mTOR) inhibitors have been shown to reduce visceral tumor volume in tuberous sclerosis complex (TSC) patients.
Objective
To evaluate the cutaneous response to oral sirolimus in TSC patients with an indication for systemic treatment, including long-term effects.
Methods
A retrospective analysis of fourteen adult TSC patients prescribed sirolimus to treat lymphangioleiomyomatosis (LAM) was performed. Serial photographs of angiofibromas, shagreen patches and ungual fibromas taken before, during and after the treatment period were blinded, then assessed using the Physician’s Global Assessment of Clinical Condition (PGA). Microscopic and molecular studies were performed on skin tumors harvested before and during treatment.
Results
Sirolimus significantly improved angiofibromas (median treatment duration: 12 months; median PGA: 4.5 [range 1.5–5]; Wilcoxon signed-rank test, p= 0.018) and shagreen patches (10; 4.5 [3.5–5]; p=0.039), whereas ungual fibromas improved in some patients (6.5; 4.66 [2.75–5]; p=0.109). Clinical, immunohistochemical or molecular evidence of resistance was not observed (range 5 to 64 months of treatment).
Limitations
This was a retrospective analysis limited to adult women with LAM.
Conclusion
Oral sirolimus is an effective long-term therapy for TSC skin tumors, particularly angiofibromas, in patients for whom systemic treatment is indicated.
Introduction
Tuberous sclerosis complex (TSC) is an autosomal dominant neurocutaneous syndrome that leads to benign tumor formation in the brain, kidneys, lungs (i.e. lymphangioleiomyomatosis) and skin. It is caused by mutations in the TSC1 or TSC2 tumor suppressor genes, resulting in hyperactivation of the mechanistic target of rapamycin (mTOR) signaling pathway and subsequent cell cycle dysregulation.
Oral mTOR inhibitors, such as sirolimus (rapamycin) and everolimus, have been shown to reduce neurological, lymphatic, pulmonary and renal disease in TSC patients. 1–12 However, attention has previously focused on internal disease and effects after 6 to 12 months of treatment.
Angiofibromas, shagreen patches and ungual fibromas occur frequently in adult TSC patients13 and can be painful, disfiguring, emotionally distressful, or prone to bleeding. We sought to evaluate objectively the initial and long-term response of skin hamartomas to oral sirolimus, document the mucocutaneous side effects of treatment, and evaluate for resistance to ongoing treatment.
Methods
Patients
Twenty-six women with TSC and lymphangioleiomyomatosis (LAM), a TSC-associated lung disease whose clinical manifestations occur almost exclusively in women, were enrolled at the National Institutes of Health Clinical Center in Bethesda, Maryland. Fourteen patients were prescribed oral sirolimus to treat LAM. Sirolimus was started at 2 mg per day, and then titrated to achieve serum levels between 5–15 ng/ml in accordance to the MILES trial.3 The remaining twelve patients were not treated. Informed consent was obtained according to protocols 00-H-0051, 95-H-0186 and/or 82-H-0032, which were approved by the National Heart, Lung, and Blood Institute Institutional Review Board.
Clinical response of skin lesions
A retrospective analysis of medical records, including dermatology consultation records and skin photography, was performed for each patient. Baseline presence of angiofibromas, shagreen patches or ungual fibromas was documented. Incidence of mucocutaneous or systemic adverse events was also documented. Serial images taken before, during and after the treatment period were scored by two blinded board-certified dermatologists (E.W.C., T.N.D) using the Physician’s Global Assessment of Clinical Condition (PGA).14, 15 According to this seven-point scale, unchanged lesions receive a score of 5. Improvement greater than or equal to 25%, but less than 50% is 4, ≥50% to <75% is 3, ≥75% to <90% is 2, ≥90% to <100% is 1, and 100% is 0. Worsening by greater than 25% is scored as 6.
Blind scoring was achieved by using a database of unlabeled skin photographs compiled by a third party without linkage to patient, treatment status, or date taken. One pair of photographs was created for each patient for right-sided facial angiofibromas, left-sided facial angiofibromas, individual shagreen patches and closely spaced ungual fibromas. For treated patients, the pair consisted of one pre-treatment photograph and one treatment photograph in random order. For non-treated patients (angiofibromas only, due to insufficient sample size for shagreen patches and ungual fibromas), the pair consisted of two photographs taken 1–3 years apart, also in random order. Other analyses compared the first treatment photograph and second treatment photograph, or one treatment photograph and one photograph after treatment cessation (for angiofibromas and shagreen patches only) also arranged in random order. For each pair of photographs, the reviewer was instructed to choose the photograph showing the most severely affected skin lesions and to treat this photograph as a “baseline”. Then, the second photograph was scored with respect to any change from the “baseline” photograph. If the reviewer appreciated a difference of less than 25% between the photos, a score of “5” was assigned. In instances where the more recent photograph was chosen as the most severe photograph by the reviewer, the third party would assign a score of “6” for the pair to denote disease progression. Scores from each pair of right and left-sided angiofibromas, individual shagreen patches and ungual fibromas from each reviewer were averaged to create an overall PGA for each type of skin lesion.
Statistical Analysis
The Wilcoxon signed-rank test was performed to evaluate clinical change in each lesion type before and during treatment, or between first and second treatment visit. The Mann-Whitney U test was performed to detect any difference between skin tumors from treated and untreated patients. The Mann-Whitney U test was also performed to evaluate the difference between treated lesions and lesions after treatment cessation.
Immunohistochemistry
Tissue sections from skin tumors obtained before drug initiation and during sirolimus treatment were incubated with anti-phospho-S6 ribosomal protein (Ser-235/236) or rabbit IgG (Santa Cruz Biotechnology), and stained with ABC-alkaline phosphatase (Vectastain) by methods described in our previous work.16
Western Blot Analysis
Fibroblasts grown from normal skin and skin tumors obtained during the treatment period were plated on 100-mm dishes and were incubated in Dulbecco’s modified Eagle’s medium with or without 10% fetal bovine serum, with or without 2 nM sirolimus for 24 hours. Cells were lysed with protein extraction buffer, separated on 10% Mini-PROTEAN® precast polyacrylamide gels, transferred to polyvinylidene fluoride membranes and immunoblotted with β-actin (Sigma-Aldrich), anti-TSC2 (D93F12), anti-phospho-S6 ribosomal protein (Ser-235/236) and anti-S6 ribosomal protein antibodies (Cell Signaling Technology) as described in previous methods. 17
Results
Patient Characteristics
All twenty-six women met updated diagnostic criteria for TSC.18, 19 Fourteen patients were prescribed oral sirolimus at a median dose of 2 mg daily to treat LAM. Pre-treatment (baseline) photographs were available for eleven patients. Pertinent patient characteristics are summarized in Table I.
Table I.
Patient baseline characteristics of 14 TSC/LAM patients prescribed oral sirolimus.
| Mean age (range) | 37 years (25–52) |
|---|---|
| Sirolimus median daily dose (range) | 2 mg (1–7) |
| Angiofibromas | 9/11* |
| Prior ablative therapy | 4/9 |
| Shagreen patch | 7/11* |
| Ungual fibroma | 5/11* |
Eleven patients had pre-treatment (baseline) skin photographs available for analysis of cutaneous responses to oral sirolimus. Of the three patients without baseline photographs, 3/3 had angiofibromas (1 with prior ablative therapy), 1 had a shagreen patch and 1 had ungual fibromas.
Cutaneous responses to sirolimus therapy
Angiofibromas improved with treatment (Wilcoxon signed-rank test, p= 0.018) (Table II). The clinical difference in angiofibromas between nine treated patients and twelve untreated TSC/LAM patients (mean age=39; median PGA 4.88 [range 4.5–6]) was also significant (Mann-Whitney U test, p=0.015). Shagreen patches improved with treatment (Wilcoxon signed-rank test, p=0.039). Ungual fibromas improved in some patients (4.66 [2.75–5]), although sample size was small (Wilcoxon signed-rank test, p=0.109). There were insufficient numbers of shagreen patches and ungual fibromas in the untreated group for comparison.
Table II.
Change in tuberous sclerosis complex skin tumors during oral sirolimus treatment in 11 TSC/LAM patients.
| Patients with baseline photos (n=11) | Median Treatment Duration | Improvement (%) | Interquartile PGA | Median PGA (range) | Wilcoxon Signed Rank Test |
|---|---|---|---|---|---|
| Angiofibromas (n=9) | 12 months | 7/9 (78) | 3.5–4.75 | 4.5 (1.5–5) | p=.018 |
| Shagreen patch (n=7) | 10 months | 6/7 (71) | 4.25–5 | 4.5 (3.5–5) | p=.039 |
| Ungual fibroma (n=5) | 6.5 months | 3/5 (60) | 4.5–5 | 4.66 (2.75–5) | p=.109 |
PGA; Physician’s Global Assessment of Clinical Condition
When individual patient responses were evaluated for those with a PGA score < 5, angiofibromas improved in 7/9 (78%) patients, shagreen patches improved in 5/7 (71%) patients and ungual fibromas improved in 3/5 (60%) patients. Representative improvement in each type of lesion before and during treatment is displayed in Figure 1.
Figure 1.
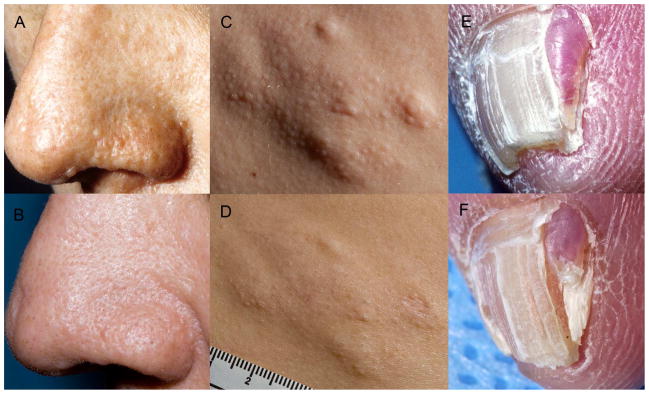
Tuberous Sclerosis Complex. Clinical improvement of angiofibromas, shagreen patch and ungual fibroma in patients treated with oral sirolimus. (A) Multiple skin-colored to pink papules on the nasal ala and alar crease on baseline assessment. (B) After 1 month of oral sirolimus, papules are substantially diminished. (C) Nodular plaque with follicular papules on baseline assessment. (D) Flattening of plaque is noted after 10 months of oral sirolimus. (E) Red, exophytic papule with hyperkeratotic tip on baseline examination. (F) Reduction in size and erythema after 6 months of oral sirolimus.
Change in lesions after treatment cessation
Three patients with angiofibromas and shagreen patches were evaluated at a median of 14 months (range 6–48) after treatment cessation. Angiofibromas did not significantly worsen after cessation of treatment (median PGA 4.75 [range 4.5–4.75]; Mann-Whitney U test, p=0.633); in contrast, shagreen patches worsened after treatment cessation (5.25[5–5.75]; p=0.020).
Evaluation of treatment resistance
Clinical assessment
Six patients were evaluated two or more times during the treatment period (median treatment duration 26 months [range 18–64]). All patients remained stable or continued to improve between first and second treatment visits in angiofibromas [n=6; median PGA 4.75 (range 2–5); Wilcoxon signed-rank test, p=0.461], shagreen patches (4; 4.5 (2.5–4.5); p=0.102] and ungual fibromas [2; 4.5 (4–5)]. Fourteen patients were evaluated 5 to 64 months after sirolimus initiation; during this range of treatment, there was no evidence of disease progression in any patient. Of these, eight patients were taking sirolimus for more than 12 months.
Immunohistochemistry
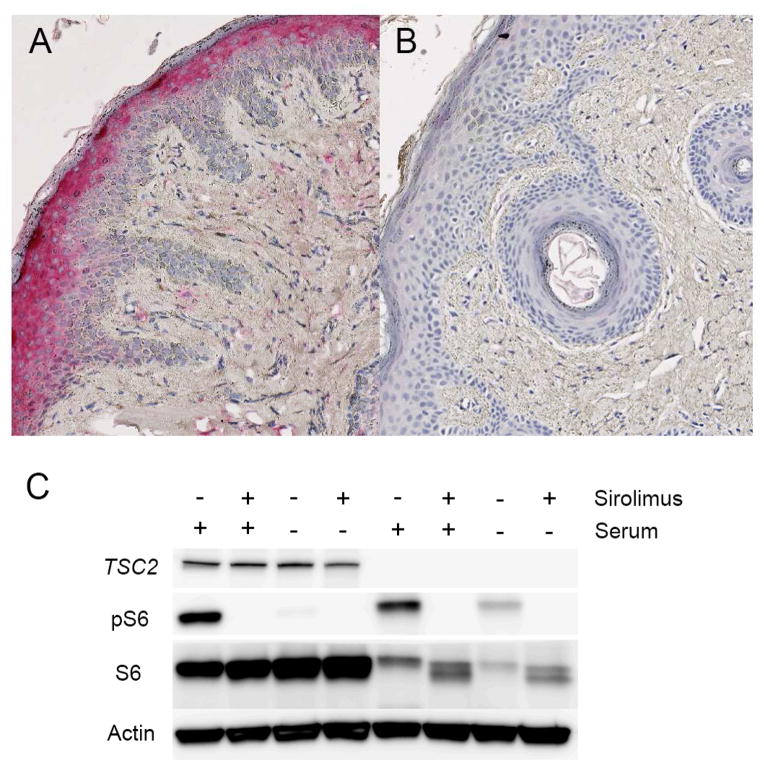
Staining for phosphorylated ribosomal protein S6 (pS6), a marker of mTOR hyperactivation, was prominent in the tumor fibroblasts, epidermis, and adnexal structures in tissue sections from four skin tumors obtained from two patients prior to treatment initiation (Figure 2A), confirming previously published results.16 Following 6 or 10 months of oral sirolimus treatment, an additional four skin tumors were harvested from the same two patients. Tissue sections from these tumors revealed markedly reduced staining (Figure 2B).
Figure 2.
Tuberous Sclerosis Complex. Evaluation for resistance to oral sirolimus in skin tumors. (A) Tissue section from treatment-naïve angiofibroma demonstrates increased staining for phosphorylated ribosomal protein S6 (pS6) in stromal fibroblast-like cells. (B) pS6 staining is decreased in an angiofibroma harvested after 10 months of treatment. (C) Persistent pS6 expression in TSC2-null, serum-starved fibroblasts derived from an angiofibroma harvested after 5 months of treatment. Phosphorylation is blocked when cells are incubated in vitro with sirolimus.
Western Blot Analysis
Similar to treatment naïve samples from our previous work20, TSC2-null skin tumor fibroblasts grown from two angiofibromas and one ungual obtained during in vivo sirolimus treatment (5, 10 or 15 months after treatment initiation) demonstrated constituent expression of pS6 that was undetectable after treatment with sirolimus in vitro (Figure 2C). Prior genetic analysis demonstrated biallelic TSC2 mutations in two of these skin tumors (refer to samples P3T1 and P22T)17.
Adverse Events
Seven of fourteen treated patients (50%) experienced mucocutaneous adverse events. Acne-like lesions affected 6 patients (43%). These were diffusely distributed on the face and scalp of four patients, while the scalp was primarily involved in two patients. Oral ulcers developed in three patients (21%), causing one patient (7%) to temporarily discontinue treatment. Nail fragility affected one patient. Systemic adverse events affected three patients. Pedal edema developed in one patient, elevated blood pressure and headache developed in one patient and unspecified bowel discomfort developed in one patient, leading her to temporarily discontinue treatment.
Discussion
Our blind assessment revealed that TSC skin tumors not only improve following treatment with systemic sirolimus, but also maintain improvement during at least 64 months of treatment. All but one treated patient (91%) demonstrated improvement in at least one skin lesion type, and no patients worsened during treatment. There was no evidence that resistance to sirolimus developed in skin tumors during treatment. Limitations of this study include small sample size, enrichment for adult women with LAM, retrospective assessment of images and between-subjects design for clinical comparison to non-treated angiofibromas.
In our cohort, angiofibromas appeared to respond more favorably to treatment than shagreen patches or ungual fibromas. Patients with angiofibromas demonstrated the greatest interquartile improvement of all three lesions types, with roughly 25–50% betterment. Moreover, angiofibromas improved in more than three-quarters of patients despite a history of prior ablative therapy in nearly one-half. Similarly, in a previous open-label trial of sirolimus for renal tumors that secondarily assessed skin lesions as “improved” or “unchanged”, angiofibromas were found to improve in a greater percentage of patients than shagreen patches or ungual fibromas. 6 It is possible that the anti-angiogenic capacity of sirolimus21 is responsible for the apparent superior benefit of therapy on highly vascular TSC skin tumors.
Unexpectedly, angiofibromas did not seem to worsen after treatment cessation. This is in contrast to worsening of shagreen patches following treatment cessation in our cohort and progression of TSC-associated internal tumors in previous studies.2, 3, 8 It has been observed that angiofibromas worsen during puberty22, likely due to increasing hormonal stimulation; thus, it is possible that decreased erythema or flattening of angiofibromas following treatment was maintained due to stable hormone levels in this post-pubertal cohort.
In follow-up of patients up to 64 months after initiation of oral sirolimus, we did not observe any clinical evidence of treatment resistance. These findings were corroborated on the microscopic and molecular level. Immunohistochemical staining revealed that pS6 immunoreactivity in tumor fibroblast-like cells, the tumor-initiating cells16, 20, is substantially decreased for at least 10 months after treatment initiation. The absence of pS6 positive stromal cells in the treated tumor is not due to elimination of tumor cells, as cells with biallelic mutations in TSC2 could be grown from treated lesions. Instead, tumor cells persisting in the tumor continue to show inhibition of mTOR with sirolimus both in vivo and in vitro. These results are consistent with the known cytostatic rather than cytotoxic effects of sirolimus and are in concordance with the persistence of tumor cells observed during sirolimus treatment in our xenograft model.20 While our results support the hypothesis that TSC-related cutaneous hamartomas remain susceptible to sirolimus years after initiation, development of resistance during prolonged mTOR inhibition has been reported in renal and thyroid cancer. 23, 24
Similar to previous clinical trials of oral mTOR inhibitors3, 6, 8, 9, mucocutaneous adverse events of treatment were common, but generally tolerable. One-half of treated patients experienced at least one mucocutaneous adverse event, most frequently acne-like lesions and oral ulcers. In general, these were only treated if concerning to the patient, as the severity of the adverse events usually decreased with continued treatment. As systemic side effects developed in three patients, the authors do not recommend oral treatment for patients without concomitant internal disease requiring oral therapy. For patients with troublesome skin tumors only, topical sirolimus and more recently, topical everolimus, may be effective but are still under investigation.25, 26
Capsule Summary.
Oral sirolimus reduces internal tumors and lymphangioleiomyomatosis (LAM) in patients with tuberous sclerosis complex (TSC).
During oral sirolimus treatment for LAM, skin tumors improved in nearly all patients; there was no evidence for development of resistance during long-term therapy.
Oral sirolimus instituted to treat internal disease improves TSC skin tumors.
Acknowledgments
We thank Dr. Cara H. Olsen for statistical consultation. J.M. was supported by the Intramural Research Program, National Institutes of Health, National Heart, Lung, and Blood Institute, Bethesda, MD. T.N.D. was supported by NIH R01 AR062080. This research was made possible through the National Institutes of Health (NIH) Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc., The Doris Duke Charitable Foundation, The Alexandria Real Estate Equities, Inc. and Mr. and Mrs. Joel S. Marcus, and the Howard Hughes Medical Institute, as well as other private donors. This research was also made possible through a Doris Duke Charitable Foundation Clinical Research Mentorship grant.
Footnotes
Conflicts of interest:
The authors have no conflicts of interest to declare.
Statement on prior presentation:
This work was presented at the 2015 Society for Investigative Dermatology Annual Meeting, May 6–9, 2015.
IRB status:
Informed consent was obtained according to protocols 00-H-0051, 95-H-0186 and/or 82-H-0032, which were approved by the National Heart, Lung, and Blood Institute Institutional Review Board.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–51. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801–11. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- 3.McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364:1595–606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies DM, de Vries PJ, Johnson SR, McCartney DL, Cox JA, Serra AL, et al. Sirolimus therapy for angiomyolipoma in tuberous sclerosis and sporadic lymphangioleiomyomatosis: a phase 2 trial. Clin Cancer Res. 2011;17:4071–81. doi: 10.1158/1078-0432.CCR-11-0445. [DOI] [PubMed] [Google Scholar]
- 5.Taveira-DaSilva AM, Hathaway O, Stylianou M, Moss J. Changes in lung function and chylous effusions in patients with lymphangioleiomyomatosis treated with sirolimus. Ann Intern Med. 2011;154:797–805. W-292–3. doi: 10.1059/0003-4819-154-12-201106210-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dabora SL, Franz DN, Ashwal S, Sagalowsky A, DiMario FJ, Jr, Miles D, et al. Multicenter phase 2 trial of sirolimus for tuberous sclerosis: kidney angiomyolipomas and other tumors regress and VEGF- D levels decrease. PLoS One. 2011;6:e23379. doi: 10.1371/journal.pone.0023379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krueger DA, Care MM, Agricola K, Tudor C, Mays M, Franz DN. Everolimus long-term safety and efficacy in subependymal giant cell astrocytoma. Neurology. 2013;80:574–80. doi: 10.1212/WNL.0b013e3182815428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franz DN, Belousova E, Sparagana S, Bebin EM, Frost M, Kuperman R, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013;381:125–32. doi: 10.1016/S0140-6736(12)61134-9. [DOI] [PubMed] [Google Scholar]
- 9.Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, Belousova E, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2013;381:817–24. doi: 10.1016/S0140-6736(12)61767-X. [DOI] [PubMed] [Google Scholar]
- 10.Krueger DA, Wilfong AA, Holland-Bouley K, Anderson AE, Agricola K, Tudor C, et al. Everolimus treatment of refractory epilepsy in tuberous sclerosis complex. Ann Neurol. 2013;74:679–87. doi: 10.1002/ana.23960. [DOI] [PubMed] [Google Scholar]
- 11.Kingswood JC, Jozwiak S, Belousova ED, Frost MD, Kuperman RA, Bebin EM, et al. The effect of everolimus on renal angiomyolipoma in patients with tuberous sclerosis complex being treated for subependymal giant cell astrocytoma: subgroup results from the randomized, placebo-controlled, Phase 3 trial EXIST-1. Nephrol Dial Transplant. 2014;29:1203–10. doi: 10.1093/ndt/gfu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franz DN, Belousova E, Sparagana S, Bebin EM, Frost M, Kuperman R, et al. Everolimus for subependymal giant cell astrocytoma in patients with tuberous sclerosis complex: 2-year open-label extension of the randomised EXIST-1 study. Lancet Oncol. 2014;15:1513–20. doi: 10.1016/S1470-2045(14)70489-9. [DOI] [PubMed] [Google Scholar]
- 13.Darling TN, Moss J, Mausner M. Tuberous Sclerosis Complex. Wiley-VCH Verlag GmbH & Co. KGaA; 2010. Dermatologic Manifestations of Tuberous Sclerosis Complex (TSC) pp. 285–309. [Google Scholar]
- 14.Prince HM, McCormack C, Ryan G, Baker C, Rotstein H, Davison J, et al. Bexarotene capsules and gel for previously treated patients with cutaneous T-cell lymphoma: results of the Australian patients treated on phase II trials. Australas J Dermatol. 2001;42:91–7. doi: 10.1046/j.1440-0960.2001.00488.x. [DOI] [PubMed] [Google Scholar]
- 15.Duvic M, Martin AG, Kim Y, Olsen E, Wood GS, Crowley CA, et al. Phase 2 and 3 clinical trial of oral bexarotene (Targretin capsules) for the treatment of refractory or persistent early-stage cutaneous T-cell lymphoma. Arch Dermatol. 2001;137:581–93. [PubMed] [Google Scholar]
- 16.Li S, Takeuchi F, Wang JA, Fan Q, Komurasaki T, Billings EM, et al. Mesenchymal-epithelial interactions involving epiregulin in tuberous sclerosis complex hamartomas. Proc Natl Acad Sci U S A. 2008;105:3539–44. doi: 10.1073/pnas.0712397105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tyburczy ME, Wang JA, Li S, Thangapazham R, Chekaluk Y, Moss J, et al. Sun exposure causes somatic second-hit mutations and angiofibroma development in tuberous sclerosis complex. Hum Mol Genet. 2014;23:2023–9. doi: 10.1093/hmg/ddt597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Northrup H, Krueger DA International Tuberous Sclerosis Complex Consensus G. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 Iinternational Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243–54. doi: 10.1016/j.pediatrneurol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teng JM, Cowen EW, Wataya-Kaneda M, Gosnell ES, Witman PM, Hebert AA, et al. Dermatologic and Dental Aspects of the 2012 International Tuberous Sclerosis Complex Consensus Statements. JAMA Dermatol. 2014 doi: 10.1001/jamadermatol.2014.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S, Thangapazham RL, Wang JA, Rajesh S, Kao TC, Sperling L, et al. Human TSC2-null fibroblast-like cells induce hair follicle neogenesis and hamartoma morphogenesis. Nat Commun. 2011;2:235. doi: 10.1038/ncomms1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frost P, Berlanger E, Mysore V, Hoang B, Shi Y, Gera J, et al. Mammalian target of rapamycin inhibitors induce tumor cell apoptosis in vivo primarily by inhibiting VEGF expression and angiogenesis. J Oncol. 2013;2013:897025. doi: 10.1155/2013/897025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jozwiak S, Schwartz RA, Janniger CK, Michalowicz R, Chmielik J. Skin lesions in children with tuberous sclerosis complex: their prevalence, natural course, and diagnostic significance. Int J Dermatol. 1998;37:911–7. doi: 10.1046/j.1365-4362.1998.00495.x. [DOI] [PubMed] [Google Scholar]
- 23.Carew JS, Kelly KR, Nawrocki ST. Mechanisms of mTOR inhibitor resistance in cancer therapy. Target Oncol. 2011;6:17–27. doi: 10.1007/s11523-011-0167-8. [DOI] [PubMed] [Google Scholar]
- 24.Wagle N, Grabiner BC, Van Allen EM, Amin-Mansour A, Taylor-Weiner A, Rosenberg M, et al. Response and acquired resistance to everolimus in anaplastic thyroid cancer. N Engl J Med. 2014;371:1426–33. doi: 10.1056/NEJMoa1403352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tu J, Foster RS, Bint LJ, Halbert AR. Topical rapamycin for angiofibromas in paediatric patients with tuberous sclerosis: follow up of a pilot study and promising future directions. Australas J Dermatol. 2014;55:63–9. doi: 10.1111/ajd.12125. [DOI] [PubMed] [Google Scholar]
- 26.Dill PE, De Bernardis G, Weber P, Losch U. Topical everolimus for facial angiofibromas in the tuberous sclerosis complex. A first case report. Pediatr Neurol. 2014;51:109–13. doi: 10.1016/j.pediatrneurol.2014.02.016. [DOI] [PubMed] [Google Scholar]