Abstract
Ipilimumab, an antibody that blocks cytotoxic T lymphocyte-associated antigen-4 (CTLA-4; CD152), was approved by the Food and Drug Administration (FDA) in 2011 for the treatment of unresectable stage III or IV malignant melanoma. Although the addition of this particular immunotherapy has broadened treatment options, immune-related adverse events (irAEs) are associated with ipilimumab therapy, including dermatologic effects, colitis and diarrhea, endocrine effects, hepatotoxicity, ocular effects, renal effects, neurologic effects, and others. In this article, a critical evaluation of the underlying mechanisms of irAEs associated with anti-CTLA-4 therapy is presented. Additionally, potentially beneficial effects of combinational therapies to alleviate ipilimumab-induced irAEs in malignant melanoma are discussed. Future research is warranted to elucidate the efficacy of such combination therapies as well as specific biomarkers that would help to predict a clinical response to ipilimumab in patients with malignant melanoma.
Keywords: Ipilimumab, CTLA-4, malignant melanoma, immune-related adverse events
Introduction
Melanoma, an increasingly prevalent cutaneous malignancy, is projected to cause 9,710 deaths in the U.S. in 2014 (1). Whereas early-detected melanoma can generally be cured with wide excision (and possibly a lymph node biopsy), advanced stages of melanoma often require systemic treatment. Hence, localized melanoma has a much more favorable five-year relative survival rate (up to 98%) than regional melanoma (62%) and distant melanoma (16%) based on the stage at diagnosis (1).
There are now several treatments for metastatic melanoma (2). First, there is chemotherapy with dacarbazine or temozolomide. There is also targeted therapy with BRAF (VRAF murine sarcoma viral oncogene homolog B) inhibitors (vemurafenib and dabrafenib), and inhibitors of mitogen-activated protein kinase enzymes MEK1 and MEK2 (trametinib). Lastly, there is immunotherapy with interferon (IFN)α-2b, interleukin-2 (IL-2), and an anti- cytotoxic T-lymphocyte associated protein-4 (CTLA-4; CD152) antibody, ipilimumab. Additional methods of immunotherapy include anti-programmed cell death protein-1 (PD-1; CD279) antibodies, lambrolizumab (3,4), now known as pembrolizumab (MK-3475) (5, 6), nivolumab (7, 8), as well as the anti-PD-1 ligand (PD-L1; CD274) antibodies, BMS936559 (9) and MPDL3280A (10). This review article primarily focuses on immunotherapy, specifically with ipilimumab, and also discusses combinational therapies with several of the agents discussed above.
Inhibition of Checkpoint with Antibodies against CTLA-4, PD-1, and PD-L1
Cellular mechanisms of action of CTLA-4
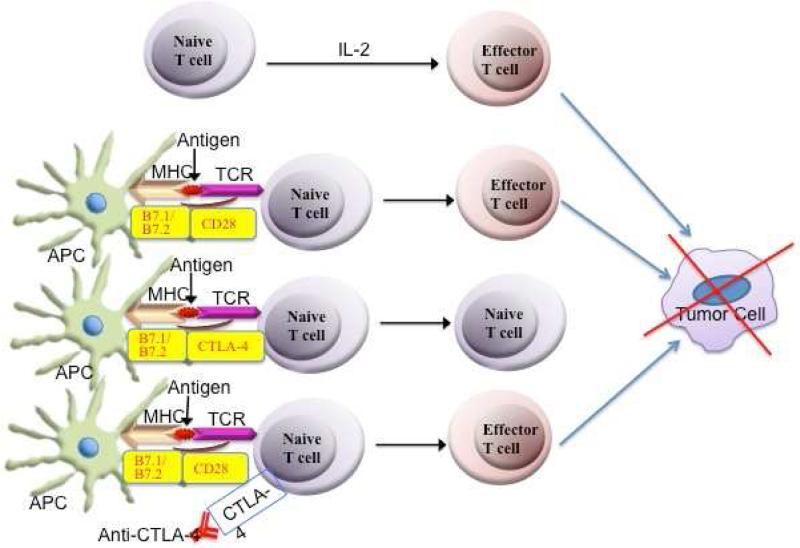
Anti-CTLA-4 antibodies augment tumor-specific cellular immunity by interrupting a negative signaling mechanism that inhibits cytotoxic T cells. In order for a naive T cell to become activated, two receptor-ligand interactions must occur (Figure 1). First, the T cell receptor (TCR) binds to a major histocompatibility complex (MHC) molecule and an antigen on an antigen-presenting cell (APC). Second, there must be a co-stimulatory signal in the form of CD28 on the T cell interacting with B7.1 (CD80) and B7.2 (CD86) on the APC. CTLA-4 serves as a checkpoint in the immune system by binding to B7.1 and B7.2 with greater affinity than CD28 (11). This in turn compromises the co-stimulatory signal that must occur for a naive T cell to become activated, resulting in decreased IL-2 secretion and decreased expression of the IL-2 receptor. Thus, anti-CTLA-4 antibodies act as checkpoint inhibitors and better allow for the patient's own effector T cells to kill melanoma tumor cells.
Figure 1.
Schematic diagram showing the generation of effector T cells and the killing of the tumor cell. Normally, naïve T cell differentiate into effector T cells in response to IL-2. Also, antigen is presented to naïve T cells by antigen presenting cell (APC) via MHCII and T cell receptor and this process is enhanced by the interaction of co-stimulatory molecules whereby CD28 molecule on T cells interacts with B7.1 (CD80) and B7.2 (CD86) on APCs, resulting into the generation of effector T cells to kill tumor cells. However, CTLA-4 binds with B7.1/B7.2 with greater affinity than CD28 and thus inhibits the differentiation of naïve T cells into effector T cells. Blocking CTLA-4 with anti-CTLA-4 antibody will allow CD28 to interact with B7.1/B7.2 to generate effector T cells and promote killing of tumor cells.
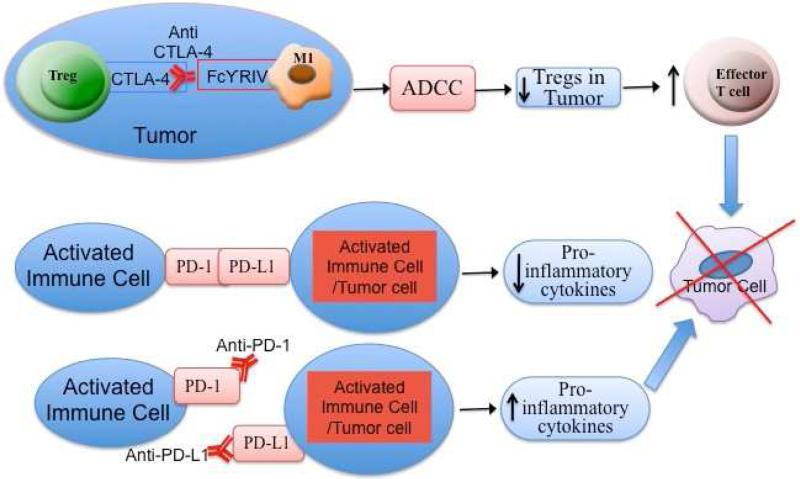
In addition to inhibiting this co-stimulatory signal, CTLA-4 is highly expressed on regulatory T cells (Tregs), which serve to down-regulate cell-mediated immunity. For example, the intratumoral ratio of effector T cells to Tregs with the use of anti-CTLA-4 antibodies has been investigated recently (12). Treatment with anti-CTLA-4 antibodies increases the expression of effector and regulatory T cells in the lymph nodes. However, in melanoma tumor lesions, anti-CTLA-4 antibody treatment depletes Tregs through an FcγR-dependent mechanism, resulting in increased intratumoral Teff/Tregs (Figure 1). There is a selective reduction in Tregs in melanoma tumors for several reasons. First, tumor-induced regulatory T cells expressing CTLA-4 are abundant in the tumor microenvironment. Second, a particular fragment crystallizable receptor (FcR) on macrophages within the tumor, called FcγRIV, is involved in the depletion of these Tregs. Macrophages with FcγRIV interact with anti-CTLA-4 antibodies, which bind to CTLA-4 on Tregs. Macrophages then deplete these Tregs via antibody-dependent cell-mediated cytotoxicity (ADCC) (Figure 2). Therefore, future research is warranted to further evaluate and compare the tumor microenvironment in malignant melanoma patients in order to predict the efficacy of anti-CTLA-4 treatment. Tumors with increased macrophages, or macrophages with increased expression of FcγRIV, could respond better to ipilimumab.
Figure 2.
Effect of anti-CTLA-4 antibody and anti-PD1 and anti-PD-L1 on tumor cells. CTLA-4 is highly expressed on T-regulatory cells (Tregs). Macrophages with FcγRIV interact with anti-CTLA-4 antibodies, which bind to CTLA-4 on Tregs. Macrophages then deplete the Tregs in the tumor via antibody-dependent cell-mediated cytotoxicity (ADCC) to increase the density of effector T cells to kill tumor cells. PD-1 is similar to CTLA-4 in that PD-1 attenuates effector T cell responses. The PD-1 binds to it primary ligand, PD-L1 (also known as B7-H1 or CD274). When PD-1 interacts with its ligands, pro-inflammatory cytokines are diminished, facilitating tumor cells to escape from host immune response. Blocking of either PD-1 by anti-PD-1 antibody or PD-L1 by anti-PD-L1 antibody would enhance pro-inflammatory cytokines to facilitate killing of tumor cells.
Cellular mechanism of action of PD-1, PD-L1, and PD-L2
PD-1 is similar to CTLA-4 in that PD-1 attenuates effector T cell responses. PD-1 finds expression on activated T and B cells, Tregs, and natural killer (NK) cells (13). The primary ligand for PD-1, PD-L1 (also known as B7-H1 or CD274), is found on activated immune cells and tumor cells. A second ligand, PD-L2 (also known as B7-DC or CD273), is found mainly on dendritic cells (antigen-presenting cells) and in a few tumor cell lines. When PD-1 interacts with its ligands, pro-inflammatory cytokines are diminished (Figure 2). Consequently, tumor cells expressing PD-L1 and PD-L2 have the ability to escape from host immune responses (14).
Blockade of the interaction between PD-1 and its ligands has received much attention recently in immunotherapy research. For example, phase I clinical trials involving nivolumab (anti-PD-1, formerly known as BMS936558) (7, 8) and the anti-PD-L1 antibodies, BMS936559 (9) and MPDL3280A (10) have demonstrated longer-lasting beneficial responses in patients with malignant melanoma, renal-cell cancer, and non-small-cell lung cancer. Ongoing phase III trials with nivolumab continue to demonstrate durable objective responses in patients with malignant melanoma (15). Furthermore, a phase I study involving lambrolizumab (anti-PD-1, formerly known as MK-345, now known as pembrolizumab) has shown a high rate of sustained tumor regression in malignant melanoma, with primarily grade 1 or grade 2 toxic effects (3). Pembrolizumab (MK-3475) has now been approved by the FDA for breakthrough therapy for malignant melanoma based on several randomized clinical trials (5, 6). Further clinical trials are necessary to elucidate the safety and efficacy of these antibodies, as well as the possibility of combining them with ipilimumab, which will be further discussed in a subsequent section of this article.
Phase III Clinical Trials with Ipilimumab and Tremelimumab
There are currently two different anti-CTLA-4 antibodies: ipilimumab, an immunoglobulin G1 (IgG1) antibody, and tremelimumab, an IgG2 antibody. Tremelimumab has been effective against melanoma tumor cells in phase I and phase II studies, with the most recent phase II study demonstrating a durable objective response rate of 6.6% in patients receiving tremelimumab (16). However, a phase III study comparing tremelimumab to standard-of-care chemotherapy failed to detect a significant advantage (17). Thus, continued research will help to elucidate its potential role in treating malignant melanoma.
In 2011, the US Food and Drug Administration (FDA) approved ipilimumab for the treatment of malignant melanoma in pre-treated and treatment-naive adult patients due to increased survival in several phase III studies (18, 19). The MDX010-20 study compared malignant melanoma patients in three treatment groups (18). A gp100 vaccine was given in two of these groups because melanoma cells express this particular peptide, inducing an immune response. The dose of ipilimumab was 3 mg per kg of body weight, every 3 weeks for up to 4 treatments. This is the dosage approved by the FDA. Whereas median survival was only 6.4 months in patients given the vaccine alone, it was 10.1 months in the ipilimumab alone group and 10.0 months in the ipilimumab plus vaccine group.
The second phase III study demonstrated 47% survival at 1 year in malignant melanoma patients treated with ipilimumab (10 mg per kilogram) and dacarbazine (850 mg per square meter of body-surface area), versus 36% survival in patients treated with dacarbazine (850 mg per square meter) and a placebo (19). It is important to note that the ipilimumab dose in this second phase III study is significantly higher than the FDA approved dose in the first study, which contributes to the differences in the severity of irAEs discussed in the following section.
Immune-related Adverse Effects in Phase III Clinical Trials
Despite increased overall survival, both phase III studies involving ipilimumab reported grade 3 or grade 4 immune-related adverse effects (irAEs) due to ipilimumab-induced upregulation of the immune system. This scoring system is derived from the National Cancer Institute's Common Terminology Criteria for Adverse Events. Whereas grade 3 indicates severe but not life-threatening events, grade 4 implicates life-threatening consequences.
In the first study, irAEs were evident in 10% to 15% of patients treated with ipilimumab, compared to 3% in the group that only received the gp100 vaccine (18). Of the 14 deaths that were related to the study drugs (2.1%), 7 were associated with irAEs. The skin and gastrointestinal tract were most commonly affected. Diarrhea occurred in 27-31% of patients receiving ipilimumab. Pruritus, rash, or vitiligo occurred in 43.5% of patients.
Outcomes of these particular patients have recently been analyzed by a separate group of authors (20). Of the patients eligible for analysis who received ipilimumab, 20% survived at least 2 years and 16% survived at least 3 years. In these survivors, safety was comparable with the overall study population, and irAEs generally subsided once treatment ended. With the exception of one patient, the irAEs reported in 6 of 78 (8%) patients were grade 1 or 2. Thus, new-onset long-term irAEs of ipilimumab therapy occur infrequently and are not as severe as the short-term side effects.
In the second phase III study, grade 3 or 4 irAEs occurred in 56.3% of patients receiving ipilimumab plus dacarbazine, versus 27.5% of patients receiving dacarbazine plus placebo (19). Of note, this second study reports no drug-related deaths or gastrointestinal perforations in patients who received ipilimumab plus dacarbazine. Furthermore, there was more hepatotoxicity reported in this study (in approximately 20% of patients treated with ipilimumab plus dacarbazine) and less diarrhea (approximately 2%). This may be due to the fact that dacarbazine itself can cause hepatotoxicity, which can be further accentuated by ipilimumab.
IrAEs in Various Organ Systems
Since ipilimumab significantly enhances cytotoxic T cells throughout the body, autoimmune toxicity can arise due to weakening of self-tolerance. As shown in Table 1, irAEs can arise in the skin, gastrointestinal tract, endocrine system (in the form of hypophysitis and thyroiditis), liver, eye, kidney, nervous system, pancreas, and others. Overall, irAEs have been reported in up to 64% of patients receiving ipilimumab (21).
Table 1.
Immune-related adverse effects (irAEs) in various organ systems associated with ipilimumab treatment.
| irAE | Overall Incidence (27) | Incidence of irAEs ≥ grade 3 (27) | Onset | Basic mechanism or associated findings | Treatment in addition to discontinuation of ipilimumab |
|---|---|---|---|---|---|
| Dermatologic effects (primarily involving a maculopapular rash) | 65% | < 3% | 2-3 weeks (28) | • Perivascular lymphocytic infiltrate into the epidermis (24, 25). • Melan-A-specific CD8+ T cells into the dermis (24, 30). • Sarcoidosis-type skin reaction (34-38). • Vitiligo (31, 32). |
Topical corticosteroids with antipruritic agents. Systemic corticosteroids if necessary. |
| Colitis/diarrhea | 33% | 10% | 6-7 weeks (28) | • Altered antibodies to enteric flora, T cell infiltration, and increased calprotectin (47). • Depletion of Tregs (48). • Perforation of the gastrointestinal tract is rare (45, 46). |
Systemic corticosteroids. Infliximab if necessary (48). Ocular evaluation (25). |
| Endocrine effects (hypophysitis, thyroiditis and adrenalitis) | < 5% | < 3% | 6-9 weeks (28) | • Enlarged pituitary causes a mass effect and hormone deficiencies. • Hypophysitis may be due to a type 2 hypersensitivity reaction (52). • Thyroiditis and hypothyroidism (63). • Graves’ disease (hyperthyroidism) (69). • Adrenalitis (65). |
Systemic corticosteroids. Hormone replacement therapy if necessary. |
| Hepatotoxicity | <2% | 1% | 6-7 weeks (28) | Damage to the hepatocytes or to the bile ducts occurs (67). | Systemic corticosteroids. |
| Ocular effects | 1.3% | 0.4% | variable | Graves’ ophthalmopathy (69,70); orbital inflammatory syndrome (71); optic neuropathy (72); and conjunctivitis, scleritis, and uveitis (25). | Corticosteroid eye drops and/or systemic corticosteroids (73). |
| Renal effects | 6 reported cases (77) | NA | variable | • Acute granulomatous tubulointerstitial nephritis (77). • Lupus nephritis (77). |
Systemic corticosteroids. |
| Neurologic effects | 0.1% | None | variable | Guillian-Barré syndrome (80); meningo-radiculo-nevritis (81); cerebral edema (82); aseptic meningitis (26); temporal arteritis (68); posterior reversible encephalopathy syndrome (83); peripheral neuropathy (84); Tolosa-Hunt syndrome (21); enteric neuropathy (85, 86); Multiple Sclerosis exacerbations (87); and chronic inflammatory demyelinating polyneuropathy (CIDP), transverse myelitis (TM), and myasthenia gravis–type syndrome (88). | Systemic corticosteroids. Possibly plasmapheresis, or intravenous immunoglobulin (IVIG). |
Note: Numbers in the parentheses show the reference number for individual studies and/or findings.
Therefore, it is important for clinicians to be aware of these potential complications, and treat irAEs effectively with high-dose systemic corticosteroids and other immunosuppressive agents. Although various review articles have detailed algorithms for clinically managing irAEs (21-25), this review primarily focuses on the mechanisms behind irAEs. Interestingly, the use of corticosteroids during ipilimumab therapy has not been shown to adversely affect treatment outcomes (26). However, future research comparing the anti-tumor response in patients requiring immunosuppressive agents vs. patients not requiring immunosuppressive agents would help elucidate this point and further guide clinical practice because the severity of irAEs may be related to the efficacy of ipilimumab for that individual
Integumentary irAEs
Dermatologic involvement is fairly common with ipilimumab treatment and has been seen in approximately 65% of patients (27). Rash is generally the first irAE to manifest, and it usually appears after the first or second dose of ipilimumab, which correlates to about 2-3 weeks after starting treatment (23, 25, 28). This rash is different from rashes seen with other anticancer agents, including erlotinib, cetuximab, panitumumab, vandetanib, and pertuxumab (24). The rash with ipilimumab is usually maculopapular, erythematous, reticular, and edematous with or without pruritus (21-24).
Pruritus associated with ipilimumab use is the direct result of CTLA-4 inhibition, resulting in activation of the immune system, specifically amplified T cell recognition of self-antigens (29). Histological analyses of the rashes have shown a perivascular lymphocytic and sometimes eosinophilic infiltrate that extends into the epidermis (24, 25). There have also been reports of Melan-A-specific CD8+ T cells infiltrating into the dermis (24, 30). In this case, the rash was more pronounced around nevi, indicating that the inflammatory response was directed against the melanocytes (24, 30).
The proposed mechanism for the appearance of the vitiligo after ipilimumab use is related to activation of melanoma-associated antigen-specific CD4+ T cells and melan-A specific CD8+ T cells as a result of the CTLA-4 blockade (31, 32). These cells target melanocytes, causing vitiligo. On histology, the vitiligo rash shows polymorphonuclear cells and deposition of antibody (33).
A combination of cutaneous and pulmonary sarcoidosis-type reaction after ipilimumab use has been observed (34), which demonstrated a sarcoidal granulomatous dermatitis on biopsy. Ipilimumab-induced sarcoidosis has also been described by other authors (35-38), with skin and pulmonary lesions resolving or improving after discontinuation of ipilimumab. Sarcoidosis is an important adverse effect for clinicians to be aware of because it can mimic tumor progression and distant metastasis (39, 40).
Currently, the mechanism for the sarcoidosis-type skin reaction is not completely understood. However, it is known that sarcoidosis is a disease of Th1 cells, activated macrophages with elevated levels of IL-2, and interferon-γ. Because CTLA-4 normally reduces IL-2 production, the use of ipilimumab can be related to elevated levels of IL-2, providing an explanation for this skin reaction.
Other less common skin reactions include Stevens-Johnson syndrome, prurigo, acneiform rash, lichenoid exanthema, pyoderma gangrenosum-like ulceration, skin toxicity in areas after radiation, photosensitivity reaction, a drug rash with eosinophilia and systemic symptoms (DRESS) (22) and Sweet syndrome (41, 42). A single case of ipilimumab-induced dermatomyositis has also been reported (43).
The rash related to ipilimumab use is usually not severe, with less than 3% of patients experiencing severe reactions (27). Management for mild reactions includes the use of topical corticosteroids such as betamethasone 0.1% cream with antipruritic agents including polidocanol (32). For the more severe skin reactions, systemic corticosteroids such as prednisone (1 - 2 mg/kg) may be necessary (31, 32).
Gastrointestinal irAEs
Especially in the first few months of treatment, colitis and diarrhea are the most common irAEs reported with ipilimumab treatment, as demonstrated by a recent systematic review article (44). In the first phase III clinical trial previously discussed, five of the seven deaths due to irAEs occurred due to colitis/diarrhea (18). Therefore, this can be a very serious irAE, particularly in rare cases in which perforation of the gastrointestinal tract occurs due to excess inflammation of the colonic wall, as described in several cases requiring colectomy due to severe perforation (45, 46).
Ipilimumab-induced colitis/diarrhea involves several underlying mechanisms, which could not be prevented with prophylactic budesonide in a recent study (47). First, ipilimumab treatment gives rise to altered levels of antibodies to enteric flora. Depending on the particular antibody of interest, antibody titers were increased, decreased, or both. Second, biopsies of patients receiving ipilimumab demonstrated increased infiltration of T cells into gastric mucosa. Lastly, there were increased levels of fecal calprotectin, a biomarker of inflammation derived from neutrophils. However, calprotectin levels did not prove to be a reliable predictor of the onset of diarrhea/colitis in patients receiving ipilimumab. These three different patterns of change were found to be distinct from that of inflammatory bowel disease (IBD). Although the pathological mechanism differs, treatment for IBD and ipilimumab-induced colitis is similar, involving corticosteroids and infliximab.
Other authors have suggested that ipilimumab causes colitis via depletion of Foxp3+ Tregs, which diminishes the effectiveness of Tregs in down-regulating the immune response, ultimately favoring gut inflammation (48). In patients receiving ipilimumab, Foxp3+ Tregs accounted for only 0.2% of total CD3+ cells, whereas cytotoxic granzyme B+ T cells accounted for 81%. This alteration in the balance of effector and regulatory T cells favors inflammation, which could help to explain the colitis and diarrhea.
A recent case report discusses the occurrence of ipilimumab-induced colitis in a patient who acquired a diarrheal illness triggered by Salmonella enteridites (49). This case of colitis was severe so ipilimumab had to be discontinued. Due to the temporal relationship between this infection and the onset of colitis, there could be a causal relationship. Furthermore, prophylactic antibiotics could potentially prevent the onset of colitis in patients receiving ipilimumab. Because it is still unclear whether infectious or enteric bacteria play a role in in onset of ipilimumab-induced colitis, more research in this area is needed.
Ipilimumab-induced colitis is treated via drug withdrawal and systemic corticosteroids that must be slowly tapered per patient improvement. Infliximab, a monoclonal antibody against tumor necrosis factor-alpha (TNF-α), can be used if systemic steroids fail to cause improvement. It has been suggested that infliximab helps to reverse ipilimumab-induced colitis by enhancing Foxp3+ regulatory T cells, thereby down-regulating the excess inflammation associated with this particular irAE (48). This is a logical explanation based on the mechanism of action of ipilimumab (Figure 2). Since ipilimumab-induced colitis has been associated with ocular inflammation, it has been suggested that colitis patients should have an eye evaluation (25).
It should also be noted that a rare gastrointestinal complication involves ipilimumab-induced acute pancreatitis (21, 50).
Endocrine irAEs
A rare yet serious irAE associated with ipilimumab use is hypophysitis. It is one of the only irAEs that is potentially irreversible (25, 51). Hypophysitis has been found to occur in less than 5% of patients treated with ipilimumab (52, 53). However, it has been seen in up to 17% of patients treated with escalating doses of ipilimumab (54). It usually develops within 7-12 weeks after starting treatment (28, 32, 52, 55, 56). It presents with headache, visual changes, fatigue, weakness, anorexia, nausea, loss of libido, labile moods, insomnia, temperature intolerance and hyponatremia. These symptoms are the result of the enlarged pituitary gland causing a mass effect and hormonal deficiencies that result from damage to the pituitary gland (32, 52, 57, 58).
The diagnosis of hypophysitis is made from clinical, laboratory and radiologic data. Laboratory tests may show altered levels of adrenocorticotropic hormone (ACTH), cortisol, thyroid stimulating hormone (TSH), free thyroxine (T4), growth hormone (GH), prolactin, insulin-like growth factor-1 (IGF-1), follicle stimulating hormone (FSH), luteinizing hormone (LH) and electrolytes (25, 59). This in turn can lead to secondary adrenal insufficiency, hypothyroidism or hyperthyroidism, and hypogonadism. Gadolinium contrast enhanced magnetic resonance imaging (MRI) will usually show symmetric enlargement of the pituitary gland, thickening of the infundibulum and homogenous enhancement (58, 60, 61). Imaging is important when a patient presents with signs of possible hypophysitis in order to rule out brain metastases or pituitary adenoma, which could present similarly (61). It is also important to distinguish hypophysitis from non-secreting pituitary adenoma as this condition may be treated with surgery while hypophysitis is not (60).
A recent murine study shows significant progress in elucidating the mechanism of action of the damage (52). Because CLTA-4 antigen is expressed in pituitary tissues, these authors have proposed a type 2 hypersensitivity reaction as a cause of damage to the pituitary gland. Upon administration of ipilimumab, immune complexes are formed in the pituitary containing CTLA-4 antigen and CTLA-4 antibody. There is subsequent binding of complement component C1q to the Fc (Fragment, crystallizable) region of the CTLA-4 antibody and activation of the classical complement cascade, leading to the production of C3, C3d, C4d, recruitment of additional inflammatory cells and subsequent tissue damage. Further evidence to support this hypothesis is that patients treated with tremelimumab, of the IgG2 subclass, do not develop hypophysitis as often as those treated with ipilimumab, of the IgG1 subclass. IgG1 is known to activate the classical pathway more potently than IgG2. Therefore, the ability to fix complement plays a significant role in the development of irAEs.
Treatment for hypophysitis may include high dose corticosteroids such as dexamethasone (25), however a recent study reported no improvement with corticosteroids in the clinical outcome (62). However, all patients require corticosteroid replacement but the controversy is whether high-dose steroids are beneficial. Therefore, the impact of corticosteroids on the treatment of ipilimumab-induced hypophysitis deserves further investigation due to this conflicting evidence. Finally, hormone replacement is often necessary and may be required long term as the pituitary damage may be permanent (25).
In addition to hypophysitis, other aspects of the endocrine system, such as the thyroid gland, can be affected. For example, in a comprehensive retrospective review, the overall incidence of hypophysitis after ipilimumab treatment was 8%, and that of hypothyroidism or thyroiditis 6% (63). When ipilimumab was combined with nivolumab (anti-PD-1), the incidence of hypothyroidism or thyroiditis increased to 22%, and that of hypophysitis increased to 9%. Not only has ipilimumab been associated with hypothyroidism, but ipilimumab-induced Graves’ disease (involving hyperthyroidism) has also been described (64). Therefore, thyroid hormone can be increased or decreased with ipilimumab treatment.
Rarely, ipilimumab-induced adrenalitis with primary adrenal insufficiency has been observed as well (65).
Hepatic irAEs
Typically, hepatic irAEs are rare and not life-threatening. Ipilimumab-treated patients with increased transaminase or bilirubin levels generally are asymptomatic and may show normal liver imaging. In the first phase III clinical trial of ipilimumab, only 0.8% of patients experienced elevation of aspartate aminotransferase (AST) and 1.5% of patients experienced elevation of alanine aminotransferase (ALT) (18). The second clinical trial reported elevated transaminase levels in approximately 33% of patients treated with ipilimumab plus dacarbazine, possibly due to the liver toxicity associated with dacarbazine, as previously mentioned (19).
However, in a retrospective review of 11 malignant melanoma patients treated with ipilimumab, 6 patients (approximately 55%) had elevated AST and ALT when treated with the FDA-approved dose alone (66). Although the sample size of 11 is small, there is a significant difference in the hepatotoxicity rate in this study compared to the study by Hodi et al. (18). Therefore, depending on the patient population, the incidence of hepatic irAEs can differ. Further studies should elucidate the variables that can cause such differences, and clinicians should continue to monitor hepatic function during ipilimumab therapy.
Ipilimumab-induced hepatitis may either manifest as an acute hepatitis pattern, involving damage to hepatocytes, or as a biliary pattern, involving damage to bile ducts (67). The acute hepatitis pattern has been described as panlobular, with areas of necrosis as well as perivenular infiltrate with endothelialitis. The biliary pattern primarily consists of bile ductular proliferation of mononuclear cells and mild mixed portal inflammation.
Liver issues caused by ipilimumab are generally corrected easily, but it is important to note that there has been a case of fulminant hepatitis causing mortality (68). Generally, corticosteroids can be used to reverse ipilimumab-induced hepatitis, similar to other irAEs.
Ocular irAEs
Ipilimumab-induced ocular irAEs include Graves’ ophthalmopathy with normal thyroid levels (64, 65), bilateral orbital inflammatory syndrome (71) and bilateral optic neuropathy (72). Conjunctivitis, scleritis, and uveitis have also been described with ipilimumab treatment (25). Ocular corticosteroid drops are usually used for treatment, unless the case is severe enough to require systemic corticosteroids (73).
Renal irAEs
Renal involvement during ipilimumab treatment is rare. The two main types of ipilimumab-induced renal damage are acute kidney injury due mainly to acute granulomatous tubulointerstitial nephritis (21, 74-77) and lupus nephritis (78). Although the mechanism for renal damage is not fully understood, the two proposed mechanisms of damage will be discussed here.
The first mechanism involves renal damage by infiltration of inflammatory cells, involving cell-mediated immunity presenting as interstitial nephritis (77). Several cases have shown granulomatous interstitial nephritis on renal biopsy. Additionally, in this type of reaction, there are often extra-renal manifestations such as rash, fever, lymphadenopathy, and eosinophilia as seen in DRESS syndrome. Upon reporting a case of interstitial nephritis after use of tremelimumab, researchers found it difficult to determine whether inflammatory cells infiltrating the kidney were due to an immune reaction from drug antigens or due to a drug-induced autoimmunity and loss of tolerance to self-antigens (79). Further research in distinguishing the cause of inflammatory cellular infiltrates causing interstitial nephritis is needed.
The second mechanism involves renal damage by an autoimmune reaction, which presents as lupus nephritis (77). Circulating anti-double stranded DNA antibodies and glomerular IgG, C3 and C1q deposit in the kidneys causing damage (77). Renal biopsy in this case shows lupus membranous nephropathy (77, 78).
Treatment for renal irAEs is early administration of corticosteroids (76, 77) and discontinuation of ipilimumab as necessary (76). There is currently no grading system for renal involvement like there is for other irAEs as renal involvement is a rare. However, clinicians should continue to monitor kidney function while patients undergo ipilimumab treatment.
irAEs associated with the nervous system
Ipilimumab-associated irAEs associated with the nervous system are very rare, with an overall incidence of approximately 0.1% (27). Patients typically present with complaints of headache, dizziness, lethargy, weakness, and neuropathy. The majority of these irAEs are grade 1 or 2, and a wide spectrum of neurologic issues has been reported. For example, cases have involved Guillian-Barré syndrome (80), meningo-radiculo-nevritis (81), cerebral edema associated with convulsions/seizures in patients with brain metastasis (82), aseptic meningitis with evidence of lymphocytosis in the cerebrospinal fluid (26), temporal arteritis (68), posterior reversible encephalopathy syndrome (83) and peripheral neuropathy (84). Tolosa-Hunt syndrome, which involves severe headaches, extraocular palsies, and ophthalmoplegia, has been reported after Ipilimumab use (21). Several authors have reported enteric neuropathy due to ipilimumab treatment (85, 86), and it is important to note that occlusive enteric neuropathy may mimic ipilimumab-induced colitis (86).
Ipilimumab should be used with caution in patients with multiple sclerosis, because it can cause clinical relapses in previously stable multiple sclerosis patients (87). Moreover, one group of authors reported ipilimumab-induced chronic inflammatory demyelinating polyneuropathy (CIDP), transverse myelitis (TM), and concurrent myositis and myasthenia gravis–type syndrome in three different patients within 1 to 2 weeks of treatment (88). The patient with TM improved with high-dose intravenous steroids, whereas those with CIPD and myasthenia gravis-type syndrome improved with plasmapheresis. Another treatment option for ipilimumab-induced neurologic irAEs involves intravenous immunoglobulin (IVIG). Two cases of ipilimumab-induced myasthenia gravis have been recently described, related to exuberant T cell activation by ipilimumab (89).
Opportunistic infections as irAEs
The first case of an opportunistic infection with Aspergillus fumigatus pneumonia in a patient receiving ipilimumab has recently been reported (90). These authors also note that other ipilimumab-induced opportunistic infections have occurred at their institution, including Fournier's gangrene and cytomegalovirus viremia.
Hematological irAEs
Hematology-related irAEs include ipilimumab-induced pancytopenia (91), thrombocytopenia (92), red cell aplasia (93), and autoimmune neutropenia and anemia (94, 95). Lastly, Hemophilia A has been acquired two months after the induction of Ipilimumab therapy, as evidenced by the presence of factor VIII inhibition (96).
The correlation between irAEs and tumoral response
Although all of these irAEs can be devastating, the tumor cells in patients with irAEs may have an overall enhanced response to ipilimumab treatment. One study found that the objective response rate in patients receiving ipilimumab and gp100 peptide vaccination was 36% among patients with grade 3 to 4 irAEs, compared to 5% in patients who did not experience serious side effects (97). The specific reason behind this trend deserves further exploration in the future.
Combination therapies
Due to the presence of these irAEs related to monotherapy with ipilimumab, there has been much focus upon utilizing combinations of immunotherapeutic agents in order to enhance different aspects of the immune response against melanoma tumor cells (Figure 2). Employing therapeutic options with complementary roles continues to deserve further attention.
Combining ipilimumab with nivolumab
A recent phase I clinical trial has found that combination therapy with ipilimumab and nivolumab (an anti-PD-1 antibody) has resulted in increased tumor regression with a manageable safety profile (53). This study consisted of two groups. In the concurrent-regimen group, both drugs were received every 3 weeks for 4 doses, followed by nivolumab alone every 3 weeks for 4 doses. Then both drugs were given every 12 weeks for up to 8 doses. In the sequenced-regimen group, nivolumab was given every 2 weeks for up to 48 doses in patients who had previously received ipilimumab. Whereas an objective response occurred in 20% of patients in the sequenced-regimen group, an objective response occurred in 53% of patients in the concurrent-regimen group, and tumors were reduced by at least 80%. This illustrates the fact that non-responders to anti-CTLA4 antibodies can still respond to anti-PD-1 antibodies, but not as effectively as patients receiving combination therapy. It is important to note that grade 3 or 4 adverse events occurred in 53% of patients in the concurrent-regimen group, versus 18% in the sequenced-regimen group. Although these adverse events were generally reversible with treatment, severe irAEs continue to be a concern with this particular combination therapy.
Combining ipilimumab with IL-2
The combination of IL-2 with ipilimumab has been shown to be beneficial in mice with melanoma in a recent study (98). Compared to a placebo or monotherapy, the growth of melanoma tumors was significantly reduced with this combination therapy. The mechanism behind this involves increased tumor-specific CD8+ T cells and decreased regulatory CD4+ T cells in the tumor microenvironment. It has been suggested that Tregs are redirected out of the tumor and into nearby lymph nodes. This correlates with a previously discussed publication (12). More research about the combination of IL-2 and ipilimumab in humans deserves our attention in the future.
Combining ipilimumab with granulocyte-macrophage colony-stimulating factor (GM-CSF)
In a recent phase II clinical trial, combining ipilimumab with sargramostim, GM-CSF, lengthened overall survival and decreased treatment-associated toxicity (99). Whereas 44.9% of patients receiving combination therapy experienced grade 3 to 5 adverse events, 58.3% of patients receiving ipilimumab alone did. Gastrointestinal and pulmonary toxicities were especially diminished with combination therapy. As of December 2012, median overall survival for the combination therapy group was 17.5 months (versus 12.7 months for the ipilimumab-alone group). However, there were no significant differences in progression-free survival (PFS), possibly because treatment-induced inflammation at the tumor site may have been misinterpreted as tumor progression. Although more research is needed regarding the mechanism, possibilities include increased recruitment of dendritic cells and macrophages and enhanced depletion of Tregs in melanoma tumors.
Combining ipilimumab with talimogene laherparepvec (T-VEC)
Recent research has focused upon the combination of ipilimumab with T-VEC, an oncolytic vaccine derived from herpes simplex virus-1 (HSV-1) that is injected directly into melanoma lesions. In a phase III clinical trial involving patients with stage IIIB/C or IV melanoma, biweekly intralesional T-VEC resulted in a 6 month durable response rate of 16.3%, versus 2.1% with the use of subcutaneous GM-CSF (100). Due to these results, a phase 1b/2 study has investigated the utilization of T-VEC as a priming regimen when added to ipilimumab in 18 patients (101). The combination of T-VEC and ipilimumab gives rise to a higher overall response rate (56%) and complete response (33%) than either agent alone. Grade 3 or 4 irAEs occurred in 3 patients. Phase II trials involving this particular combination therapy would be beneficial in the future.
Future directions
Overall, the recent use of ipilimumab is a groundbreaking development and provides clinicians enhanced treatment options. However, the morbidity of irAEs associated with ipilimumab therapy must be balanced against the mortality associated with malignant melanoma. Because irAEs can be so detrimental, it would be helpful to know in advance whether or not patients would respond favorably to ipilimumab before initiating therapy.
One manner in which to predict a beneficial response involves biomarkers. For example, seropositivity for NY-ESO-1, a cancer/testis antigen that finds expression in some patients, has been shown to increase the likelihood of clinical benefit following ipilimumab treatment when compared to seronegative malignant melanoma patients (102). In seropositive patients, clinical benefit to ipilimumab was more likely if patients expressed peripheral CD8+ T-cell responses to NY-ESO-1.
A recent study found that lactate dehydrogenase (LDH) could also potentially be used as a biomarker in order to predict clinical response to ipilimumab in advance (103). In patients who received ipilimumab in the Netherlands and the United Kingdom, baseline serum LDH was found to be the best predictor of overall survival. In patients with baseline LDH levels more than twice the upper limit of normal, a long-term benefit with ipilimumab therapy was less likely to occur.
Malignant melanoma patients with certain genotypes have been found to respond more favorably to ipilimumab than others. After analyzing 6 CTLA-4 single nucleotide polymorphisms (SNPs) (−1661A>G, −1577G>A, −658C>T, −319C>T, +49A>G, and CT60G>A) in malignant melanoma patients, a recent study found that the −1577G/A and CT60G/A genotypes had better overall survival and response to ipilimumab treatment (104). Different SNPs alter CTLA-4 expression, so response to ipilimumab can differ depending on the genotype. Of note, there was no correlation between SNPs and the frequency or severity of ipilimumab-induced irAEs. One significant limitation to this study is related to the small sample size of 14 Italian patients with malignant melanoma and 45 healthy controls. Further research would be helpful in this field
Activating NRAS mutations, which are found in 15-20% of melanomas, have also been studied in 229 patients with melanoma treated with immune therapies (IL-2, ipilimumab, anti-PD-1 therapy, and anti-PD-L1 therapy) (105). Clinical outcomes were superior in the NRAS-mutant cohort than other cohorts, as demonstrated by improved response to first-line immune therapy, response to any line of immune therapy, clinical benefit, and progression-free survival. This clinical benefit was particularly evident with anti-PD-1/PD-L1 therapy.
In a recent study, sequencing of the genomes of 64 patients treated with CTLA-4 blockade (with ipilimumab or tremelimumab) was performed on melanoma tumors and matched blood supplies (106). Whereas 11 of these patients had long-term clinical benefits from therapy, 13 had minimal benefit or no benefit at all. A neoantigen landscape that was specifically represented in tumors responding strongly to CTLA-4 blockade, and these tumors elicited an antitumor response by the immune system that was further augmented by CTLA-4 blockade. These findings were formulated in the discovery set of patients and confirmed in a subsequent validation set of 39 patients.
Although biomarkers need validation in larger clinical studies, this recent focus upon pre-assessment of patients in order to predict response to treatment is a positive development. In the era of personalized medicine, biomarkers or specific patient characteristics should allow more effective assessment of the risks and benefits of ipilimumab treatment.
Key points.
Ipilimumab, approved by the FDA in 2011 for unresectable stage III or IV malignant melanoma, is an antibody against CTLA-4 that has improved overall survival in several phase III clinical trials.
Ipilimumab enhances the ability of immune cells to eradicate melanoma tumor cells by inhibiting the interaction between CTLA-4 and B7.1 and B7.2, thereby enhancing effector T cells.
Ipilimumab also gives rise to decreased Treg expression within melanoma tumors, leading to decreased inhibition of the cellular immune response.
Immune-related adverse events (irAEs) associated with ipilimumab arise in multiple organ systems, with the most common being colitis/diarrhea and skin rashes.
Treatment algorithms for irAEs generally involve discontinuing ipilimumab and administering systemic corticosteroids.
Future research should further investigate the efficacy of combination therapies of ipilimumab with anti-PD-1 antibodies, IL-2, GM-CSF, and/or T-VEC to treat malignant melanoma.
In order to improve patient selection for ipilimumab treatment, future research should focus on biomarkers for predicting a favorable response.
ACKNOWLEDGEMENTS
This work was supported by research grants, R01HL116042, R01HL112597 and R01HL120659 from the Office of the Director, National Institutes of Health (NIH) and the National Heart, Lung and Blood Institute of the NIH, USA to DK Agrawal. All authors have read the journal's policy on conflicts of interest and the authorship agreement and the manuscript has been reviewed and approved by all authors in this article. No editorial support or writing assistance was utilized in the production of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLAIMER
The content of this review is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Karimkhani C, Gonzalez R, Dellavalle RP. A review of novel therapies for melanoma. Am J Clin Dermatol. 2014;15(4):323–337. doi: 10.1007/s40257-014-0083-7. [DOI] [PubMed] [Google Scholar]
- 3.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipson EJ, Sharfman WH, Drake CG, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19(1):462–8. doi: 10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribas A, Hodi FS, Kefford R, et al. Efficacy and safety of the anti-PD-1 monoclonal antibody MK-3475 in 411 patients (pts) with melanoma (MEL). J Clin Oncol (meeting abstracts) 2014;32(18_suppl):LBA9000. [Google Scholar]
- 6.Hamid O, Robert C, Ribas A, et al. Randomized comparison of two doses of the anti-PD-1 monoclonal antibody MK-3475 for ipilimumab-refractory (IPI-R) and ipi-naive (IPI-N) melanoma (MEL). J Clin Oncol (meeting abstracts) 2014;32(15_suppl):3000. [Google Scholar]
- 7.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipson EJ, Drake CG. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res. 2011;17(22):6958–62. doi: 10.1158/1078-0432.CCR-11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simpson TR, Li F, Muntalvo-Ortiz W, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210(9):1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page DB, Postow MA, Callahan MK, Allison JP, Wolchok JD. Immune modulation in cancer with antibodies. Annu Rev Med. 2014;65(1):185–202. doi: 10.1146/annurev-med-092012-112807. [DOI] [PubMed] [Google Scholar]
- 14.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99(19):12293–97. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber JS, D'Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomized, controlled, open-label phase 3 trial. Lancet Oncol. 2015;16(4):375–84. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 16.Kirkwood JM, Lorigan P, Hersey P, et al. Phase II trial of tremelimumab (CP-675,206) in patients with advanced refractory or relapsed melanoma. Clin Cancer Res. 2010;16(3):1042–1048. doi: 10.1158/1078-0432.CCR-09-2033. [DOI] [PubMed] [Google Scholar]
- 17.Ribas A, Kefford R. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. Marshall M et al. J Clin Oncol. 2013;31(5):616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 20.McDermott D, Haanen J, Chen T-T, Lorigan P, O'Day S. Efficacy and safety of ipilimumab in metastatic melanoma patients surviving more than 2 years following treatment in a phase III trial (MDX010-20). Ann Oncol. 2013;24(10):2694–8. doi: 10.1093/annonc/mdt291. [DOI] [PubMed] [Google Scholar]
- 21.Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: An analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8(1):e53745. doi: 10.1371/journal.pone.0053745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Della Vittoria Scarpati G, Fusciello C, Perri F, et al. Ipilimumab in the treatment of metastatic melanoma: management of adverse events. Onco Targets Ther. 2014;7(1):203–9. doi: 10.2147/OTT.S57335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fecher LA, Agarwala SS, Hodi FS, Weber JS. Ipilimumab and its toxicities: A multidisciplinary approach. The Oncologist. 2013;18:733–743. doi: 10.1634/theoncologist.2012-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacouture ME, Wolchok JD, Yosipovitch G, et al. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71(1):161–9. doi: 10.1016/j.jaad.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 25.Tarhini A. Immune-mediated adverse events associated with ipilimumab CTLA-4 blockade therapy: The underlying mechanisms and clinical management. Scientifica. 2013 Apr 17; doi: 10.1155/2013/857519. [E Pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang JC, Hughes M, Kammula U, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J immunother. 2007;30(8):825–30. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ibrahim R, Berman D, de Pril V, et al. Ipilimumab safety profile: Summary of findings from completed trials in advanced melanoma. J Clin Oncol. 2011;29 abstract no. 8583. [Google Scholar]
- 28.Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin. Oncol. 2012;30(21):2691–7. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 29.Fischer A, Rosen AC, Ensslin CJ, Wu S, Lacouture ME. Pruritis to anticancer agents targeting the EGFR, BRAF, and CTLA-4. Dermatol Ther. 2013;26:135–148. doi: 10.1111/dth.12027. [DOI] [PubMed] [Google Scholar]
- 30.Klein O, Ebert LM, Nicholaou T, et al. Melan-A-specific cytotoxic T cells are associated with tumor regression and autoimmunity following treatment with anti-CTLA-4. Clin Cancer Res. 2009;15(7):2507–13. doi: 10.1158/1078-0432.CCR-08-2424. [DOI] [PubMed] [Google Scholar]
- 31.Choi JN. Dermatologic adverse events to chemotherapeutic agents, Part 2: BRAF inhibitors, MEK inhibitors, and ipilimumab. Semin Cutan Med Surg. 2014;33:40–48. doi: 10.12788/j.sder.0061. [DOI] [PubMed] [Google Scholar]
- 32.Kähler KC, Hauschild A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges. 2011;9:277–286. doi: 10.1111/j.1610-0387.2010.07568.x. [DOI] [PubMed] [Google Scholar]
- 33.Tosti G, Cocorocchio E, Pennacchioli E. Anti-cytotoxic T lymphocyte antigen-4 antibodies in melanoma. Clin Cosmet Investig Dermatol. 2013;6:245–256. doi: 10.2147/CCID.S24246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reule RB, North JP. Cutaneous and pulmonary sarcoidosis-like reaction associated with ipilimumab. J Am Acad Dermatol. 2013;69(5):272–3. doi: 10.1016/j.jaad.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 35.Vogel WV, Guislain A, Kvistborg P, et al. Ipilimumab-induced sarcoidosis in a patient with metastatic melanoma undergoing complete remission. J Clin Oncol. 2012;30(2):e7–e10. doi: 10.1200/JCO.2011.37.9693. [DOI] [PubMed] [Google Scholar]
- 36.Eckert A, Schoeffler A, Dalle S, et al. Anti-CTLA4 monoclonal antibody induced sarcoidosis in a metastatic melanoma patient. Dermatology. 2009;218(1):69–70. doi: 10.1159/000161122. [DOI] [PubMed] [Google Scholar]
- 37.Wilgenhof S, Neyns B. Anti-CTLA-4 antibody-induced Guillain-Barre syndrome in a melanoma patient. Ann Oncol. 2011;22(4):991–993. doi: 10.1093/annonc/mdr028. [DOI] [PubMed] [Google Scholar]
- 38.Tissot C, Carsin A, Freymond N, Pacheco Y, Devouassoux G. Sarcoidosis complicating anti-cytotoxic T-lymphocyte-associated antigen-4 monoclonal antibody biotherapy. Eur Respir J. 2013;41(1):246–247. doi: 10.1183/09031936.00107912. [DOI] [PubMed] [Google Scholar]
- 39.Anderson R, Norgaard P, Al-Jailawi MK, Svane IM. Late development of splenic sarcoidosis-like lesions in a patient with metastatic melanoma and long-lasting clinical response to ipilimumab. Oncoimmunology. 2014;3(8):e954506. doi: 10.4161/21624011.2014.954506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy KP, Kennedy MP, Barry JE, O'Regan KN, Power DG. New-onset mediastinal and central nervous system sarcoidosis in a patient with metastatic melanoma undergoing CTLA4 monoclonoal antibody treatment. Oncol Res Treat. 2014;37(6):351–3. doi: 10.1159/000362614. [DOI] [PubMed] [Google Scholar]
- 41.Gormley R, Wanat K, Elenitsas R, et al. Ipilimumab-associated Sweet syndrome in a melanoma patient. J Am Acad Dermatol. 2014;71(5):e211–3. doi: 10.1016/j.jaad.2014.06.042. [DOI] [PubMed] [Google Scholar]
- 42.Kyllo RL, Parker MK, Rosman I, Musiek AC. Ipilimumab-associated Sweet syndrome in a patient with high-risk melanoma. J Am Acad Dermatol. 2014;70:e85–e86. doi: 10.1016/j.jaad.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 43.Sheik AS, Goddard AL, Luke JJ, et al. Drug-associated dermatomyositis following ipilimumab therapy: a novel immune-mediated adverse event associated with cytotoxic T-lymphocyte antigen 4 blockade. JAMA Dermatol. 2015;151(2):195–9. doi: 10.1001/jamadermatol.2014.2233. [DOI] [PubMed] [Google Scholar]
- 44.Ma C, Armstrong AW. Severe adverse events from the treatment of advanced melanoma: a systematic review of severe side effects associated with ipilimumab, vemurafenib, interferon alfa-2b and interleukin-2. J Dermatol treat. 2014;25(5):401–8. doi: 10.3109/09546634.2013.813897. [DOI] [PubMed] [Google Scholar]
- 45.Burdine L, Lai K, Laryea JA. Ipilimumab-induced colonic perforation. J Surg Case Rep. 2014;2014(3):rju010. doi: 10.1093/jscr/rju010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitchell KA, Kluger H, Sznol M, Hartman DJ. Ipilimumab-induced perforating colitis. J Clin Gastroenterol. 2013;47(9):781–5. doi: 10.1097/MCG.0b013e31828f1d51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berman D, Parker SM, Siegel J, et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 2010;10(11):11. [PMC free article] [PubMed] [Google Scholar]
- 48.Nancey S, Boschetti G, Cotte E, et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab is associated with a profound long-lasting depletion of Foxp3+ regulatory T cells: a mechanistic explanation for ipilimumab-induced severe enterocolitis? Inflamm. Bowel Dis. 2012;18(8):E1598–1600. doi: 10.1002/ibd.21927. [DOI] [PubMed] [Google Scholar]
- 49.McCutcheon JL, McClain CM, Puzanov I, Smith TA. Infectious colitis associated with ipilimumab therapy. Gastroenterol Res. 2014;7(1):28–31. doi: 10.14740/gr594e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Di Giacomo AM, Danielli R, Guidoboni M, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol, Immunother. 2009;58(8):1297–1306. doi: 10.1007/s00262-008-0642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blansfield JA, Beck KE, Tran K, et al. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J Immunother. 2005;28(6):593–8. doi: 10.1097/01.cji.0000178913.41256.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iwama S, De Remigis A, Callahan MK, et al. Pituitary expression of CLTA-4 mediates hypophysitis secondary to administration of CLTA-4 blocking antibody. Sci Transl Med. 2014;6(230):1–11. doi: 10.1126/scitranslmed.3008002. [DOI] [PubMed] [Google Scholar]
- 53.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):127–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maker AV, Yang JC, Sherry RM, et al. Intrapatient dose escalation of anti-CTLA-4 antibody in patients with metastatic melanoma. J Immunother. 2006;29(4):455–463. doi: 10.1097/01.cji.0000208259.73167.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Faje AT, Sullivan R, Lawrence D, et al. Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab. 2014;99(11):4078–85. doi: 10.1210/jc.2014-2306. [DOI] [PubMed] [Google Scholar]
- 56.Albarel F, Gaudy C, Castinetti F, et al. Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti-CTLA-4 antibody in melanoma. Eur J Endocrinol. 2015;172(2):195–204. doi: 10.1530/EJE-14-0845. [DOI] [PubMed] [Google Scholar]
- 57.Juszczak A, Gupta A, Karavitaki N, Middleton MR, Grossman AB. Ipilimumab: a novel immunomodulating therapy causing autoimmune hypophysitis: a case report and review. Eur J Endocrinol. 2012;167:1–5. doi: 10.1530/EJE-12-0167. [DOI] [PubMed] [Google Scholar]
- 58.Torino F, Barnabeli A, De Vecchis L, Salvatori R, Corsello SM. Hypophysitis induced by monoclonal antibodies to cytotoxic T lymphocyte antigen 4: Challenges from a new cause of a rare disease. The Oncologist. 2012;17:525–535. doi: 10.1634/theoncologist.2011-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Corsello SM, Barnabei A, Paolo Marchetti, et al. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab. 2013;98(4):1361–1374. doi: 10.1210/jc.2012-4075. [DOI] [PubMed] [Google Scholar]
- 60.Chodakiewitz Y, Brown S, Boxermann JL, Brody JM, Rogg JM. Ipilimumab treatment associated pituitary hypophysitis: Clinical presentation and imaging diagnosis. Clin Neurol Neurosurg. 2014;125:125–130. doi: 10.1016/j.clineuro.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 61.Anderson A, Bhatia V. Ipilimumab immune-related adverse reactions: a case report. S D Med. 2013;66(8):315–7. [PubMed] [Google Scholar]
- 62.Min L, Hodi FS, Giobbie-Hurder, et al. Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: a retrospective cohort study. Clin Cancer Res. 2015;21(4):749–55. doi: 10.1158/1078-0432.CCR-14-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014;21(4):371–381. doi: 10.1530/ERC-13-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borodic G, Hinkle DM. Reply Re: “Drug-induced Graves disease from CTLA-4 receptor suppression.”. Opthal Plast Reconstr Surg. 2013;29(3):241. doi: 10.1097/IOP.0b013e31828957c3. [DOI] [PubMed] [Google Scholar]
- 65.Min L, Ibrahim N. Ipilimumab-induced autoimmune adrenalitis. Lancet Diabetes Endocrinol. 2013;1(3):e15. doi: 10.1016/S2213-8587(13)70031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bernardo SG, Moskalenko M, Pan M, et al. Elevated rates of transaminitis during ipilimumab therapy for metastatic melanoma. Melanoma Res. 2013;23(1):47–54. doi: 10.1097/CMR.0b013e32835c7e68. [DOI] [PubMed] [Google Scholar]
- 67.Kim KW, Ramaiya NH, Krajewski KM, et al. Ipilimumab associated hepatitis: Imaging and clinicopathologic findings. Invest new drugs. 2013;31(4):1071–77. doi: 10.1007/s10637-013-9939-6. [DOI] [PubMed] [Google Scholar]
- 68.O'Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21(8):1712–17. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 69.Borodic G, Hinkle DM, Cia Y. Drug-induced graves disease from CTLA-4 receptor suppression. Ophthal Plast Reconstr Surg. 2011;27(4):e87–e88. doi: 10.1097/IOP.0b013e3181ef72a1. [DOI] [PubMed] [Google Scholar]
- 70.McElnea E, Ni Mhealoid A, Moran S, et al. Thyroid-like ophthalmopathy in a euthyroid patient receiving ipilimumab. Orbit. 2014;22(6):424–7. doi: 10.3109/01676830.2014.949792. [DOI] [PubMed] [Google Scholar]
- 71.Henderson AD, Thomas DA. A case report of orbital inflammatory syndrome secondary to ipilimumab. Ophthalmic Plast Rec. 2014 doi: 10.1097/IOP.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 72.Yeh OL, Francis CE. Ipilimumab-associated bilateral optic neuropathy. J Neuroophthalmol. 2015 Feb 2; doi: 10.1097/WNO.0000000000000217. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 73.Lemech C, Arkenau HT. Novel treatments for metastatic cutaneous melanoma and the management of emergent toxicities. Clinical Medicine Insights, Oncology. 2012;6(1):53–66. doi: 10.4137/CMO.S5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beck KE, Blansfield JA, Tran KQ, et al. Entercolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283–9. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Forde PM, Rock K, Wilson G, O'Bryne KJ. Ipilimumab-induced immune-related renal failure - A case report. Anticancer Res. 2012;32(10):4607–8. [PubMed] [Google Scholar]
- 76.Thajudeen B, Madhrira M, Bracamonte E, Cranmer LD. Ipilimumab granulomatous interstitial nephritis. Am J Ther. 2013 Sep 24; doi: 10.1097/MJT.0b013e3182a32ddc. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 77.Izzedine H, Gueutin V, Gharbi C, et al. Kidney injuries related to ipilimumab. Invest New Drugs. 2014;32(4):769–773. doi: 10.1007/s10637-014-0092-7. [DOI] [PubMed] [Google Scholar]
- 78.Fadel F, Karoui KE, Knebelmann B. Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med. 2009;361(2):211–2. doi: 10.1056/NEJMc0904283. [DOI] [PubMed] [Google Scholar]
- 79.Jolly EC, Clatworthy MR, Lawrence C, Nathan PD, Farrington K. Anti-CTLA-4 (CD 152) monoclonal antibody-induced autoimmune interstitial nephritis. NDT Plus. 2009;2(4):300–2. doi: 10.1093/ndtplus/sfp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wilgenhof S, Morlion V, Seghers AC, et al. Sarcoidosis in a patient with metastatic melanoma sequentially treated with anti-CTLA-4 monoclonal antibody and selective BRAF inhibitor. Anticancer Res. 2012;32(4):1355–59. [PubMed] [Google Scholar]
- 81.Bompaire F, Mateus C, Taillia H, et al. Severe meningo-radiculo-nevritis associated with ipilimumab. Invest New Drugs. 2012;30(6):2407–2410. doi: 10.1007/s10637-011-9787-1. [DOI] [PubMed] [Google Scholar]
- 82.Weber JS, Amin A, Minor D, et al. Safety and clinical activity of ipilimumab in melanoma patients with brain metastases: retrospective analysis of data from a phase 2 trial. Melanoma Res. 2011;21(6):530–534. doi: 10.1097/CMR.0b013e32834d3d88. [DOI] [PubMed] [Google Scholar]
- 83.Maur M, Tomasello C, Frassoldati A, Dieci MV, Barbieri E, Conte P. Posterior reversible encephalopathy syndrome during ipilimumab therapy for malignant melanoma. J Clin Oncol. 2012;30(6):e76–e78. doi: 10.1200/JCO.2011.38.7886. [DOI] [PubMed] [Google Scholar]
- 84.Thaipisuttikul I, Chapman P, Avila EK. Peripheral neuropathy associated with ipilimumab: a report of 2 cases. I Immunother. 2015;38(2):77–9. doi: 10.1097/CJI.0000000000000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bhatia S, Huber BR, Upton MP, Thompson JA. Inflammatory enteric neuropathy with severe constipation after ipilimumab treatment for melanoma: a case report. J Immunother. 2009;32(2):203–205. doi: 10.1097/CJI.0b013e318193a206. [DOI] [PubMed] [Google Scholar]
- 86.Gaudy-Marqueste C, Monestier S, Franques J, Cantais E, Richard M-A, Grob J-J. A severe case of ipilimumab-induced guillain-barré syndrome revealed by an occlusive enteric neuropathy: A differential diagnosis for ipilimumab-induced colitis. J Immunother. 2013;36(1):77–78. doi: 10.1097/CJI.0b013e31827807dd. [DOI] [PubMed] [Google Scholar]
- 87.Gettings EJ, Hackett CT, Scott TF. Severe relapse in a multiple sclerosis patient associated with ipilimumab treatment of melanoma. Multiple Sclerosis. 2014 doi: 10.1177/1352458514549403. [DOI] [PubMed] [Google Scholar]
- 88.Liao B, Shroff S, Kamiya-Matsuoka C, Tummala S. Atypical neurological complications of ipilimumab therapy in patients with metastatic melanoma. Neuro Oncol. 2014;16(4):589–593. doi: 10.1093/neuonc/nou001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Johnson DB, Saranga-Perry V, Lavin PJ, et al. Myasthenia gravis induced by ipilimumab in patients with metastatic melanoma. J Clin Oncol. 2014 Apr 28; doi: 10.1200/JCO.2013.51.1683. [E Pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kyi C, Hellmann MD, Wolchok JD, Chapman PB, Postow MA. Opportunistic infections in patients treated with immunotherapy for cancer. J Immunother Cancer. 2014;2(1):19. doi: 10.1186/2051-1426-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Di Giacomo AM, Danielli R, Calabrò L, et al. Ipilimumab experience in heavily pretreated patients with melanoma in an expanded access program at the University Hospital of Siena (Italy). Cancer Immunol, Immunother. 2011;60(4):467–477. doi: 10.1007/s00262-010-0958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kopecký J, Trojanová P, Kubeček O, Kopecký O. Treatment possibilities of ipilimumab-induced thrombocytopenia--case study and literature review. Jpn J Clin Oncol. 2015;45(1):1–4. doi: 10.1093/jjco/hyu222. [DOI] [PubMed] [Google Scholar]
- 93.Gordon IO, Wade T, Chin K, Dickstein J, Gajewski TF. Immune-mediated red cell aplasia after anti-CTLA-4 immunotherapy for metastatic melanoma. Cancer Immunol, Immunother. 2009;58(8):1351–1353. doi: 10.1007/s00262-008-0627-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Akhtari M, Waller EK, Jaye DL, et al. Neutropenia in a patient treated with ipilimumab (anti-CTLA-4 antibody). J Immunother. 2009;32(3):322–324. doi: 10.1097/CJI.0b013e31819aa40b. [DOI] [PubMed] [Google Scholar]
- 95.Simeone E, Grimaldi AM, Esposito A, et al. Serious haematologial toxicity during and after ipilimumab treatment: a case series. J Med Case Rep. 2014;8:240. doi: 10.1186/1752-1947-8-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Delyon J, Mateus C, Lambert T. Hemophilia A induced by ipilimumab. N Engl J Med. 2011;365(18):1747–48. doi: 10.1056/NEJMc1110923. [DOI] [PubMed] [Google Scholar]
- 97.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23(25):6043–53. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Broucek J, Hughes T, Huelsmann E, et al. Combination immunotherapy with anti-CTLA-4 and interleukin-2 redirects regulatory T cells into tumor-draining lymph nodes and expands anti-tumor CD8+ T cells in the tumor microenvironment. J Immunother Canc. 2014;2(Suppl 3):97. [Google Scholar]
- 99.Hodi FS, Lee S, McDermott DF, et al. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: A randomized clinical trial. JAMA. 2014;312(17):1744–1753. doi: 10.1001/jama.2014.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Andtbacka RHI, Collichio FA, Amatruda T, et al. OPTiM: A randomized phase III trial of talimogene laherparepvec (T-Vec) versus subcutaneous (SC) granulocyte-macrophage colony-stimulating factor (GM-CSF) for the treatment (tx) of unresected stage IIIB/C and IV melanoma. J Clin Oncol. 2013;31(Suppl) abstract LBA9008. [Google Scholar]
- 101.Puzanov I, Milhem MM, Andtbacka RHI, et al. Primary analysis of a phase 1b multicenter trial to evaluate safety and efficacy of talimogene laherparepvec (T-VEC) and ipilimumab (ipi) in previously untreated, unresected stage IIIB-IV melanoma. J Clin Oncol. 2014;32(15)(suppl 9029) [Google Scholar]
- 102.Yuan J, Adamow M, Ginsberg BA, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci USA. 2011;108(40):16723–8. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kelderman S, Heemskerk B, van Tinteren H, et al. Lactate dehydrognease as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol Immun. 2014;63(5):449–458. doi: 10.1007/s00262-014-1528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Queirolo P, Morabito A, Laurent S, et al. Association of CTLA-4 polymorphisms with improved overall survival in melanoma patients treated with CTLA-4 blockade: A pilot study. Cancer Invest. 2013;31(5):336–345. doi: 10.3109/07357907.2013.793699. [DOI] [PubMed] [Google Scholar]
- 105.Johnson DB, Lovly CM, Flavin M, et al. Impact of NRAS Mutations for Patients with Advanced Melanoma Treated with Immune Therapies. Cancer Immunol Res. 2015;3(3):288–95. doi: 10.1158/2326-6066.CIR-14-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Snyder A, Makarov V, Merghoub T. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–99. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]