Abstract
Objective
To obtain normative data for the normal laboratory beagle cornea using high-resolution in vivo confocal microscopy (IVCM).
Animals studied
Sixteen eyes of 8 healthy young female intact beagles.
Procedures
The central cornea was imaged using IVCM. Mixed effects linear regression was used for statistical analysis.
Results
IVCM allowed detailed visualization and quantification of epithelial cells (superficial epithelial cell diameter: 43.25 ± 6.64 μm, basal cell diameter: 4.43 ± 0.67 μm), and nerve fibers (subepithelial nerve fiber diameter: 2.38 ± 0.69 μm, anterior stromal nerve fiber diameter: 16.93 ± 4.55 μm). Keratocyte density (anterior stroma 993.38 ± 134.24 cells/mm2, posterior stroma 789.38 ± 87.13 cells/mm2) and endothelial cell density (2815.18 ± 212.59 cells/mm2) were also measured.
Conclusion
High-resolution IVCM provides detailed non-invasive evaluation of the cornea in the normal laboratory beagle.
Keywords: Cornea, endothelial cell density, confocal microscopy, corneal nerves, canine, laboratory beagle
INTRODUCTION
Anterior segment imaging is a rapidly developing field in ophthalmology. In vivo confocal microscopy (IVCM) is a noninvasive, real-time diagnostic modality, which provides high-resolution imaging of all corneal layers by using an optical imaging system where both the illumination (condenser) and observation (objective) systems are focused on a single focal point.(1) IVCM has been utilized for assessing normal morphologic features of the cornea in several species(2-5) and has led to significant enhancement of our understanding of the living healthy and diseased cornea. For example, IVCM has been proven to be an efficient tool to confirm the clinical diagnosis of fungal keratitis in humans, alpacas and horses and in corneal neuropathies associated with conditions such as dry eye and diabetes.(1, 6-10) IVCM allows for assessment of structures that would otherwise require histological examination and allows the examiner to evaluate and focus on cells in different depths of the cornea.(4, 11) For example, IVCM is currently the only diagnostic modality commercially available to evaluate corneal nerves in vivo. A limited number of IVCM studies of the canine anterior segment have been published.(4, 12, 13) However, none of these studies have focused on the laboratory beagle despite its wide use in the development of ophthalmic drugs and devices. Thus the purpose of this study was to report normative IVCM data for the laboratory beagle.
METHODS
All aspects of the study were approved by the Institutional Animal Care and Use Committees of the University of California-Davis and were performed according to the Association for Research in Vision and Ophthalmology resolution on the use of animals in research. Prior to study entry, all dogs underwent a complete physical examination and only systemically healthy dogs were included in the study. All dogs also received a detailed ophthalmic examination, including digital slit lamp biomicroscopy, indirect ophthalmoscopy, tear film break up time (TFBUT)(14), Schirmer tear test 1 (STT-1)(15), intraocular pressure (IOP)(16) and corneal sensitivity measurement using Cochet-Bonnet aesthesiometry(17-19). In addition, all dogs had their corneas stained with fluorescein and Rose Bengal and only dogs assessed as having normal eyes were used in the study. All baseline exams were done an hour or longer prior to imaging. None of the dogs had received topical eye medications prior to entry into the study. All IVCM measurements were obtained by the same person (DEC), highly skilled in the technique, to minimize variability.
Eight healthy female laboratory beagles (16 eyes) with a mean ± SD age of 0.52 ± 0.12 years and body weight of 6.83 ± 1.40 kg were included in the study. The dogs received dexmedetomidine (2.5-5.0 μg/kg) intramuscularly (IM) or intravenously (IV) as needed for sedation prior to imaging. Animals were placed in sternal recumbency for all imaging, and balanced salt solution (BSS; Akorn Inc., Buffalo Grove, IL, USA) solution was applied to the eyes as needed to prevent corneal desiccation. One drop of proparacaine 0.5% was instilled in both eyes prior to imaging for topical anesthesia. A Barraquer eyelid speculum was used to keep the eyes open on the sedated dogs as needed. Then, IVCM (ConfoScan 4; Nidek Technologies, Gamagori, Japan) with a 40x/0.75 objective lens was used to image the central cornea of each eye. An eye gel of 0.3% hypromellose/carbomer 980 (GenTeal® gel; Novartis Ophthalmics, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA) was placed on the tip of the objective lens as an optical coupling medium and the lens was manually advanced until the gel contacted the central surface of the cornea. Automatic, full scans were performed without autoalignment and 350 images/scan were collected for each eye. Three repeated measurements, located in the central cornea, were obtained for each structure. Superficial and basal epithelial and endothelial cells were identified by their previously described characteristic appearance in the dog.(4, 12, 13) Stromal images adjacent to epithelium and endothelium were utilized to identify keratocytes in the anterior and posterior stroma, respectively. Manual counts and cell area measurements were performed using the ConfoScan 4 NAVIS imaging software (Rev. 1.2, version 1.2.0, June 28th, 2006). Three images per location were analyzed, and these results were averaged. For anterior and posterior keratocyte cell density measurement the region of interest (ROI) was kept at 0.05mm2 for each image and for the endothelial cell density counts the ROI was kept at 0.02 mm2. For all cell counts, the cells touching the borderlines were counted only along the upper and right border. Those cells touching the left and lower border were omitted from analysis.
Statistics
Mixed effects linear regression was used to evaluate the main effects of eye (nested within individual), corneal location (nested within eye), replicates (nested within corneal location), and method of measurement, as well as their pairwise statistical interactions, on the outcome variables. Analyses were performed using Stata/IC 12.1 (StataCorp LP, College Station, TX, USA). All measurements were expressed as mean ± standard deviation. For all analyses, values of P ≤ 0.05 were considered significant.
RESULTS
All dogs examined were normal on physical and ophthalmic examination, including an appropriate TFBUT (16.1 ± 4.82 s), STT-1 (21.3 ± 1.98 mm/min), IOP (18.3 ± 2.96 mmHg), and central corneal sensitivity as measured by Cochet-Bonnet aesthesiometry (2.05 ± 0.622 g/mm2); all corneas were fluorescein and Rose Bengal stain negative.
High quality, high-resolution in vivo images of the canine cornea were obtained. Specifically, corneal superficial and basal epithelium (Figure 1 and 2) and stroma including nerves, keratocytes, and pre-Descemet's fibers (Figures 3-5), as well as endothelium (Figure 6) were successfully imaged and evaluated using IVCM.
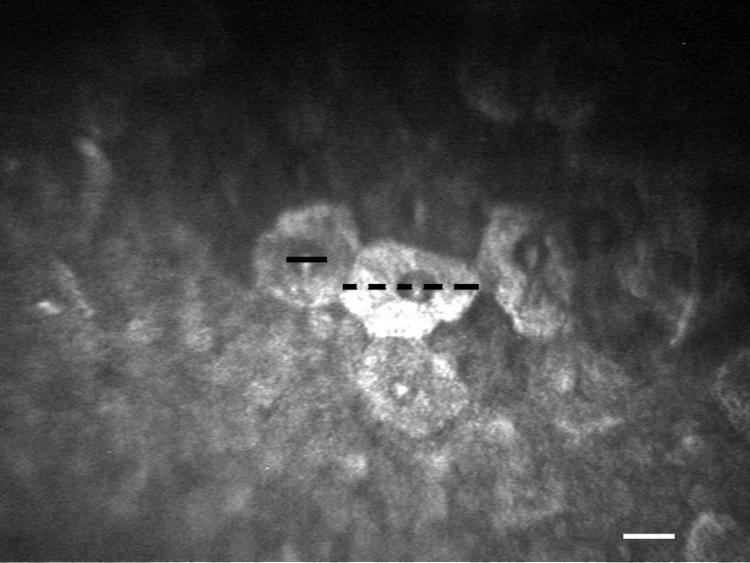
Figure 1.
IVCM of the cornea in a young healthy intact female beagle. Superficial epithelial cell diameter (black dotted line) and their nuclei diameter (black line) were measured. White scale bar = 20 μm.
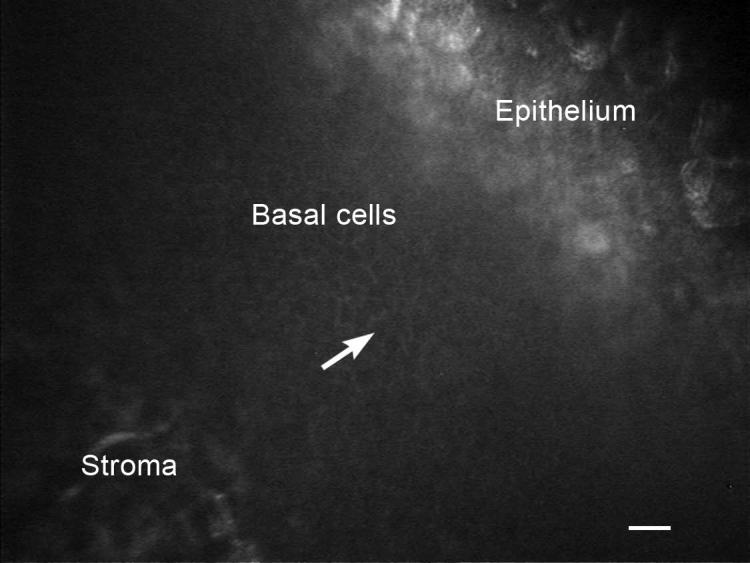
Figure 2.
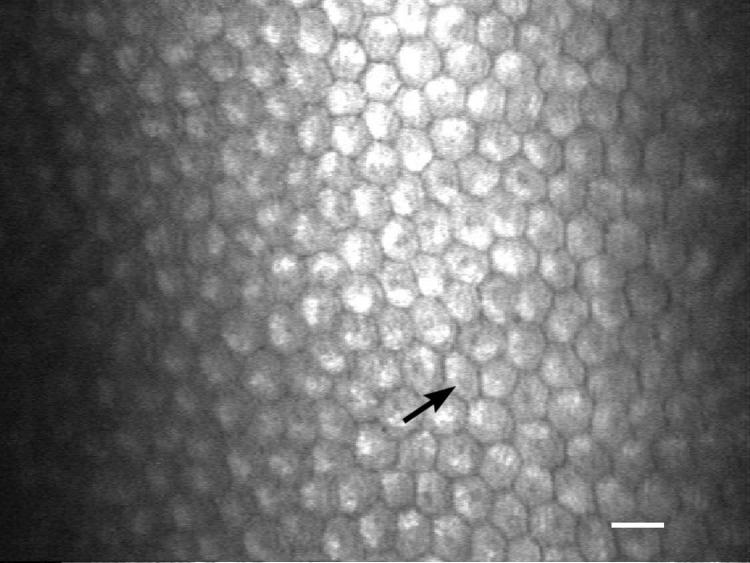
IVCM of the cornea in a young healthy intact female beagle. The white arrow is pointing to a corneal basal cell. White scale bar = 20 μm.
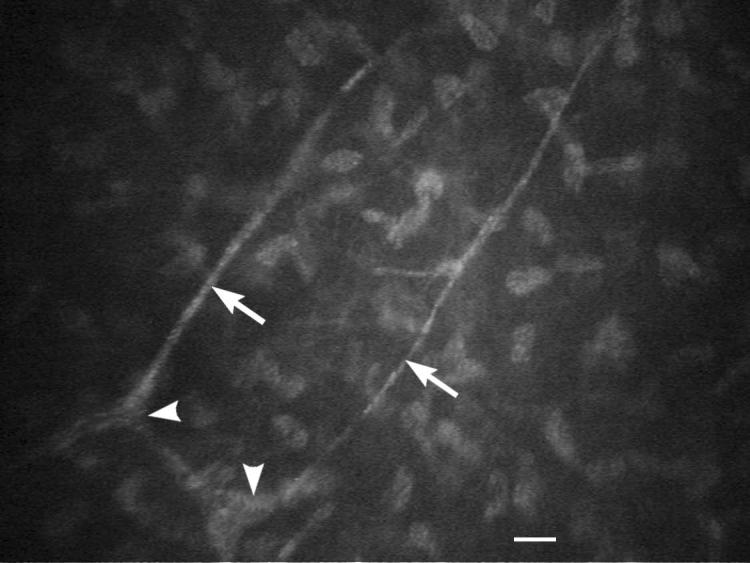
Figure 3.
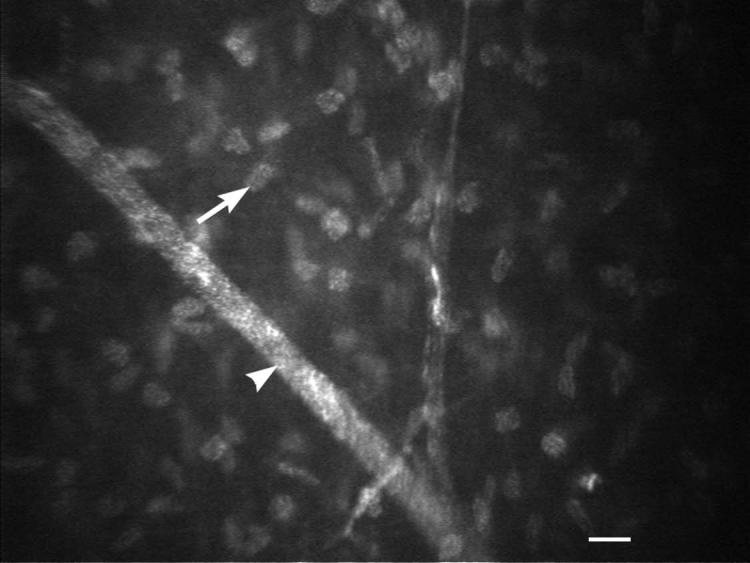
IVCM of the subepithelial nerve plexus in young healthy intact female beagle (white arrowheads). The nerve fiber thickness of the subepithelial nerve plexus (white arrows) was measured. White scale bar = 20 μm.
Figure 5.
IVCM of the posterior corneal stroma and pre-Descemet's fibers in a young intact female beagle. Density of keratocytes, keratocyte nucleus (white arrow) diameter, and pre-Descemet's fibers (white arrowhead) were measured. White scale bar = 20 μm.
Figure 6.
IVCM of the corneal endothelium young healthy intact female beagle. Note the relatively uniform size (black arrow) and density between the endothelial cells. Endothelial cell density and diameter was measured. White scale bar = 20 μm.
There were no significant differences between replicates for all measurements obtained. Unless otherwise mentioned in the following, there were no significant differences between eyes for the measurements obtained (P > 0.05).
As measured by IVCM, superficial and basal epithelial cells possessed the largest and smallest cross sectional diameters (Table 1). Corneal nerve fiber bundles within the subepithelial plexus were much thinner in diameter in comparison to those posteriorly in the anterior stroma, at 2.38 ± 0.69 μm and 16.93 ± 4.55 μm, respectively. Keratocyte cell density was significantly greater (P < 0.001) while keratocyte nuclei were significantly smaller (P < 0.001) in the anterior versus posterior stroma (Table 1). Pre-Descemet's stromal fibers were 2.14 ± 0.50 μm thick. Endothelial cells had the greatest density of all corneal cell types measured with a size intermediate between superficial and basal epithelial cells (Table 1). There was a significant difference (P = 0.007) between eyes in terms of anterior corneal stromal cell density at 1032.68 ± 163.43 cells/mm2 and 954.09 ± 83.04 cells/mm2 for the right and left eye, respectively. In addition the subepithelial nerve plexus thickness was significantly different (P = 0.002) between eyes at 2.16 ± 0.58 µm for the right eye and 2.60 ± 0.73 μm for the left eye.
Table 1.
IVCM of the central cornea of healthy female laboratory beagles (Mean ± SD)
| Cell Density (cells/mm2) | Cell Diameter (μm) | Nuclei Diameter (μm) | Thickness (μm) | |
|---|---|---|---|---|
| Superficial epithelium | n/a | 43.25 ± 6.64* (OD: 42.43 ± 5.61; OS: 44.07 ± 7.57) | 14.19 ± 2.55* (OD: 14.14 ± 3.02; OS: 14.23 ± 2.03) | n/a |
| Basal epithelium | n/a | 4.43 ± 0.67* (OD: 4.31 ± 0.56; OS: 4.55 ± 0.76) | n/a | n/a |
| Anterior stromal keratocytes | 993.38 ± 134.24* (OD:1032.68 ± 163.43; OS: 954.09 ± 83.04)‡ | n/a | 18.80 ± 3.11* (OD: 18.25 ± 3.16; OS: 19.35 ± 3.03) | n/a |
| Posterior stromal keratocytes | 789.38 ± 87.13* (OD: 775.76 ± 82.61; OS: 803.00 ± 91.12) | n/a | 27.21 ± 2.89†* (OD: 27.15 ± 2.82; OS: 27.26 ± 3.02) | n/a |
| Endothelium | 2815.18 ± 212.59 (OD: 2780.58 ± 256.89; OS: 2849.78 ± 154.48) | 20.53 ± 2.06*(OD: 20.94 ± 1.87; OS: 20.13 ± 2.19) | n/a | n/a |
| Subepithelial nerve plexus fibers | n/a | n/a | n/a | 2.38 ± 0.69§,‡ (OD: 2.16 ± 0.58; OS: 2.60 ± 0.73) |
| Anterior stromal nerve fibers | n/a | n/a | n/a | 16.93 ± 4.55§ (OD: 17.71 ± 4.58; OS: 16.16 ± 4.47) |
| Predescemet's fibers | n/a | n/a | n/a | 2.14 ± 0.50 (OD: 2.24 ± 0.51; OS: 2.05 ± 0.49) |
IVCM = In Vivo Confocal Microscopy
Statistically significant difference (p ≤ 0.05)
Statistically significant difference (p ≤ 0.05)
Statistically significant difference (p ≤ 0.05)
Statistically significant difference between eyes (p ≤ 0.05)
n/a = Not available (not measured)
OD = Oculus Dexter
OS = Oculus Sinister
DISCUSSION
This study demonstrated that high-quality IVCM images could be obtained for analysis from sedated laboratory beagles. We demonstrated that keratocyte density was significantly greater and keratocyte nuclei size was significantly smaller in the anterior stroma in comparison to the posterior stroma. This is consistent with previous reports in humans, rabbits, and dogs.(4, 5, 20) Endothelial cell density was similar (2815.18 ± 212.59 cells/mm2) to previously reported values in young dogs measured by specular microscopy (approximately 2500 and 2816 ± 187 SD cells/mm2, respectively).(21, 22) However, the endothelial cell densities reported in the present study were less than those reported in a study of dogs less than 1 year of age using a Heidelberg Retina Tomograph II unit with a Rostock Cornea Module (3641 ± 752 cells/mm2).(4) This difference is likely due to difference in age and breeds (different breeds were included in the previously published study) though it can not be ruled out that differences in IVCM units used between studies contributed to differences in the data reported.(4)
In vivo confocal microscopy is the only commercially available diagnostic modality capable of evaluating corneal nerves in vivo and that can be used to evaluate nerves in neuropathies affecting the cornea.(8, 12, 13, 23) We were able to obtain high quality images of corneal nerves. The values and morphology were comparable to previous studies assessing canine corneal innervation,(4, 12, 24) as well as to normal humans.(25-28) We expect this imaging technique will be useful in evaluating canine models for corneal neuropathies associated with conditions such as KCS and diabetes mellitus.
In the present study, there was a significant difference between eyes in two measurements, including anterior stromal keratocyte density and subepithelial plexus thickness. These numerical differences were small and are unlikely to be clinically important. The pre-Descemet's stromal fibers found in this study were similar to those found in young dogs and cats in a previous study.(4) The authors of that study suggested that these fibers represent pre-elastic (i.e., oxytalan) fibers similar to those found in corneas of juvenile humans and kittens.(29, 30) The clinical significance of these distinct fibers is currently unknown. A limitation of this study is a small sample size using only young female subjects. Further normative studies of different dog populations are warranted.
In conclusion, we have generated normative data of the central cornea of the young, healthy, female laboratory beagle using IVCM. High-resolution IVCM enabled detailed noninvasive evaluation of in vivo canine cornea. This study provides normative values with which to evaluate data obtained from dogs with spontaneous corneal diseases, induced models of anterior segment disease and in evaluation of ophthalmic therapeutics and devices.
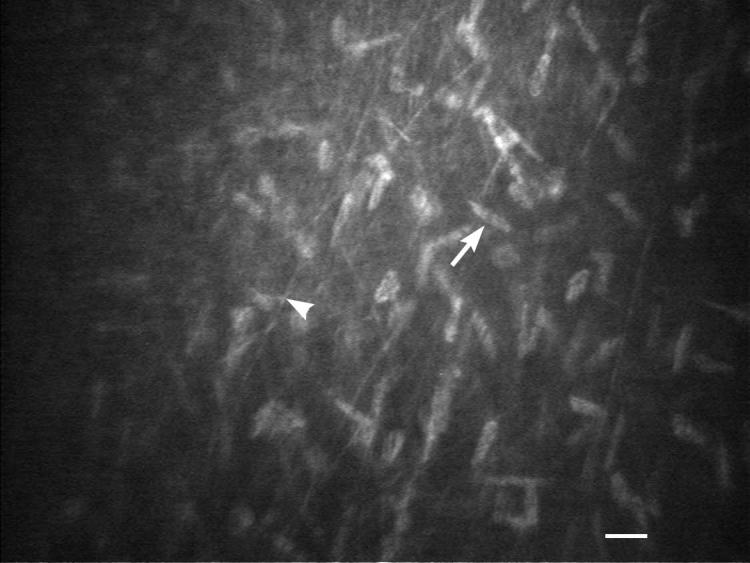
Figure 4.
IVCM of the anterior corneal stroma and nerve in a young intact female beagle. Density of keratocytes, keratocyte nucleus (white arrow) diameter and anterior stromal nerve (white arrowhead) thickness were measured. White scale bar = 20 μm.
Acknowledgments
Supported by grants from the American College of Veterinary Ophthalmologists (ACVO) Vision For Animals Foundation, UC Davis Center for Companion Animal Health, the National Institute of Health, K08EY021142 (SMT), R01EY019970 (CJM), R01EY016134 (CJM) and P30EY12576, and an unrestricted gift from Research to Prevent Blindness.
REFERENCES
- 1.Jalbert I, Stapleton F, Papas E, Sweeney DF, Coroneo M. In vivo confocal microscopy of the human cornea. The British journal of ophthalmology. 2003;87(2):225–36. doi: 10.1136/bjo.87.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seyed-Razavi Y, Chinnery HR, McMenamin PG. A novel association between resident tissue macrophages and nerves in the peripheral stroma of the murine cornea. Investigative ophthalmology & visual science. 2014;55(3):1313–20. doi: 10.1167/iovs.13-12995. [DOI] [PubMed] [Google Scholar]
- 3.Ledbetter EC, Scarlett JM. In vivo confocal microscopy of the normal equine cornea and limbus. Veterinary ophthalmology. 2009;12(Suppl 1):57–64. doi: 10.1111/j.1463-5224.2009.00730.x. [DOI] [PubMed] [Google Scholar]
- 4.Kafarnik C, Fritsche J, Reese S. In vivo confocal microscopy in the normal corneas of cats, dogs and birds. Veterinary ophthalmology. 2007;10(4):222–30. doi: 10.1111/j.1463-5224.2007.00543.x. [DOI] [PubMed] [Google Scholar]
- 5.Reichard M, Hovakimyan M, Wree A, Meyer-Lindenberg A, Nolte I, Junghans C, et al. Comparative in vivo confocal microscopical study of the cornea anatomy of different laboratory animals. Current eye research. 2010;35(12):1072–80. doi: 10.3109/02713683.2010.513796. [DOI] [PubMed] [Google Scholar]
- 6.Ledbetter EC, Irby NL, Kim SG. In vivo confocal microscopy of equine fungal keratitis. Veterinary ophthalmology. 2011;14(1):1–9. doi: 10.1111/j.1463-5224.2010.00822.x. [DOI] [PubMed] [Google Scholar]
- 7.Patel DV, McGhee CN. Contemporary in vivo confocal microscopy of the living human cornea using white light and laser scanning techniques: a major review. Clinical & experimental ophthalmology. 2007;35(1):71–88. doi: 10.1111/j.1442-9071.2007.01423.x. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed A, Bril V, Orszag A, Paulson J, Yeung E, Ngo M, et al. Detection of diabetic sensorimotor polyneuropathy by corneal confocal microscopy in type 1 diabetes: a concurrent validity study. Diabetes care. 2012;35(4):821–8. doi: 10.2337/dc11-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benitez-Del-Castillo JM, Acosta MC, Wassfi MA, Diaz-Valle D, Gegundez JA, Fernandez C, et al. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Investigative ophthalmology & visual science. 2007;48(1):173–81. doi: 10.1167/iovs.06-0127. [DOI] [PubMed] [Google Scholar]
- 10.Ledbetter EC, Montgomery KW, Landry MP, Kice NC. Characterization of fungal keratitis in alpacas: 11 cases (2003-2012). Journal of the American Veterinary Medical Association. 2013;243(11):1616–22. doi: 10.2460/javma.243.11.1616. [DOI] [PubMed] [Google Scholar]
- 11.Bohnke M, Masters BR. Confocal microscopy of the cornea. Progress in retinal and eye research. 1999;18(5):553–628. doi: 10.1016/s1350-9462(98)00028-7. [DOI] [PubMed] [Google Scholar]
- 12.Kafarnik C, Fritsche J, Reese S. Corneal innervation in mesocephalic and brachycephalic dogs and cats: assessment using in vivo confocal microscopy. Veterinary ophthalmology. 2008;11(6):363–7. doi: 10.1111/j.1463-5224.2008.00659.x. [DOI] [PubMed] [Google Scholar]
- 13.Ledbetter EC, Marfurt CF, Dubielzig RR. Metaherpetic corneal disease in a dog associated with partial limbal stem cell deficiency and neurotrophic keratitis. Veterinary ophthalmology. 2013;16(4):282–8. doi: 10.1111/j.1463-5224.2012.01064.x. [DOI] [PubMed] [Google Scholar]
- 14.Moore CP. Qualitative tear film disease. The Veterinary clinics of North America Small animal practice. 1990;20(3):565–81. doi: 10.1016/s0195-5616(90)50071-6. [DOI] [PubMed] [Google Scholar]
- 15.Margadant DL, Kirkby K, Andrew SE, Gelatt KN. Effect of topical tropicamide on tear production as measured by Schirmer's tear test in normal dogs and cats. Veterinary ophthalmology. 2003;6(4):315–20. doi: 10.1111/j.1463-5224.2003.00313.x. [DOI] [PubMed] [Google Scholar]
- 16.Park YW, Jeong MB, Kim TH, Ahn JS, Ahn JT, Park SA, et al. Effect of central corneal thickness on intraocular pressure with the rebound tonometer and the applanation tonometer in normal dogs. Veterinary ophthalmology. 2011;14(3):169–73. doi: 10.1111/j.1463-5224.2010.00859.x. [DOI] [PubMed] [Google Scholar]
- 17.Good KL, Maggs DJ, Hollingsworth SR, Scagliotti RH, Nelson RW. Corneal sensitivity in dogs with diabetes mellitus. American journal of veterinary research. 2003;64(1):7–11. doi: 10.2460/ajvr.2003.64.7. [DOI] [PubMed] [Google Scholar]
- 18.Wieser B, Tichy A, Nell B. Correlation between corneal sensitivity and quantity of reflex tearing in cows, horses, goats, sheep, dogs, cats, rabbits, and guinea pigs. Veterinary ophthalmology. 2013;16(4):251–62. doi: 10.1111/j.1463-5224.2012.01069.x. [DOI] [PubMed] [Google Scholar]
- 19.Murphy CJ, Marfurt CF, McDermott A, Bentley E, Abrams GA, Reid TW, et al. Spontaneous chronic corneal epithelial defects (SCCED) in dogs: clinical features, innervation, and effect of topical SP, with or without IGF-1. Investigative ophthalmology & visual science. 2001;42(10):2252–61. [PubMed] [Google Scholar]
- 20.Weed KH, MacEwen CJ, Cox A, McGhee CN. Quantitative analysis of corneal microstructure in keratoconus utilising in vivo confocal microscopy. Eye (Lond) 2007;21(5):614–23. doi: 10.1038/sj.eye.6702286. [DOI] [PubMed] [Google Scholar]
- 21.Stapleton S, Peiffer RL., Jr Specular microscopic observations of the clinically normal canine corneal endothelium. American journal of veterinary research. 1979;40(12):1803–4. [PubMed] [Google Scholar]
- 22.Gwin RM, Lerner I, Warren JK, Gum G. Decrease in canine corneal endothelial cell density and increase in corneal thickness as functions of age. Investigative ophthalmology & visual science. 1982;22(2):267–71. [PubMed] [Google Scholar]
- 23.Cruzat A, Pavan-Langston D, Hamrah P. In vivo confocal microscopy of corneal nerves: analysis and clinical correlation. Seminars in ophthalmology. 2010;25(5-6):171–7. doi: 10.3109/08820538.2010.518133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marfurt CF, Murphy CJ, Florczak JL. Morphology and neurochemistry of canine corneal innervation. Investigative ophthalmology & visual science. 2001;42(10):2242–51. [PubMed] [Google Scholar]
- 25.Mocan MC, Durukan I, Irkec M, Orhan M. Morphologic alterations of both the stromal and subbasal nerves in the corneas of patients with diabetes. Cornea. 2006;25(7):769–73. doi: 10.1097/01.ico.0000224640.58848.54. [DOI] [PubMed] [Google Scholar]
- 26.Marfurt CF, Cox J, Deek S, Dvorscak L. Anatomy of the human corneal innervation. Experimental eye research. 2010;90(4):478–92. doi: 10.1016/j.exer.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira-Soto L, Efron N. Morphology of corneal nerves using confocal microscopy. Cornea. 2001;20(4):374–84. doi: 10.1097/00003226-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Chiou AG, Beuerman RW, Kaufman SC, Kaufman HE. Confocal microscopy in lattice corneal dystrophy. Graefe's archive for clinical and experimental ophthalmology. 1999;237(8):697–701. doi: 10.1007/s004170050299. [DOI] [PubMed] [Google Scholar]
- 29.Alexander RA, Garner A. Oxytalan fibre formation in the cornea: a light and electron microscopical study. Histopathology. 1977;1(3):189–99. doi: 10.1111/j.1365-2559.1977.tb01658.x. [DOI] [PubMed] [Google Scholar]
- 30.Carrington SD, Alexander RA, Grierson I. Elastic and related fibres in the normal cornea and limbus of the domestic cat. Journal of anatomy. 1984;139(Pt 2):319–32. [PMC free article] [PubMed] [Google Scholar]