INTRODUCTION
Psychotic symptoms, hallucinations and delusions, are frequent in patients with Late Onset Alzheimer’s disease (LOAD). Multiple studies suggest that the frequency of psychosis in patients with Late Onset Alzheimer’s Disease (LOAD+P) is close to 50%. With estimates of greater than 13 million Americans affected by LOAD by 2050 (Thies, et al., 2013), LOAD+P would be among the most prevalent psychotic disorders in the United States.
Compared to LOAD patients without psychosis (LOAD-P), patients with LOAD+P demonstrate more severe cognitive and functional deficits (Scarmeas, et al., 2005), more rapid cognitive decline (Sweet, et al., 2012) and are more prone aggressive behaviors (Sweet, et al., 2000). Patients with LOAD+P progress to more advanced stages of disease with worse general health (Bassiony, et al., 2000), greater rates of institutionalization (Lopez, et al., 1999) and increased mortality (Wilson, et al., 2006).
There is strong evidence for familial aggregation of psychosis in patients with LOAD. In a previous study of familial aggregation of LOAD+P in a cohort of patients and their siblings with AD (Sweet, et al., 2002) we showed that the occurrence of psychotic symptoms in siblings with LOAD was three times that of the siblings of patients without psychosis. Additional studies have supported the familial aggregation of LOAD+P (Hollingworth, et al., 2007). The estimated heritability of psychosis in LOAD is 61% when psychosis is defined by the presence of multiple or recurrent psychotic symptoms and is 30% for any single occurrence of a symptom (Bacanu, et al., 2005). Subsequent linkage and association studies to identify genetic variants associated with the combined LOAD+P phenotype have detected linkage to chromosomes 2p, 6q, 7qand 15q (Avramopoulos, et al., 2005, Bacanu, et al., 2002, Go, et al., 2005, Hollingworth, et al., 2007).
Initial efforts to identify genetic variants contributing to LOAD+P used a candidate gene approach, focused on the APOE locus and on genes implicated in alterations in the serotonergic, dopaminergic, and catecholaminergic neurotransmission systems (DeMichele-Sweet and Sweet, 2010). Results for the APOE locus have been inconsistent, but predominantly negative (DeMichele-Sweet and Sweet, 2010). More recently, the first genome wide association study of LOAD+P was reported (Hollingworth, et al., 2012), a meta-analysis of three LOAD GWAS datasets comprising 1299 cases with LOAD+P, 735 with LOAD-P and 5659 unaffected controls. This analysis identified several loci with strong evidence of association with LOAD+P, although no single locus reached the threshold for genome wide significance.
We hypothesized that a distinct genetic mechanism raises risk for LOAD with an increased likelihood of comorbid psychosis. To test this hypothesis, we divided a large set of LOAD families into two groups based on the presence/absence of psychosis during the course of LOAD in at least one individual. Then, we carried out linkage analysis separately within each set of families.
MATERIALS AND METHODS
Study participants
National Institute of Aging Late Onset Alzheimer’s Disease cohort (NIA-LOAD)
Standard Protocol Approvals, Registrations and Patient Consents
Informed consent for the study was obtained for all participants. Recruitment for the NIA-LOAD Study was approved by the relevant institutional review boards of the participating centers. The study was conducted according to the principles expressed in the Declaration of Helsinki.
Description of the recruitment, diagnostic procedures, and the approach to characterization of psychotic symptoms for the NIA-LOAD cohort has been described previously (Sweet, et al., 2010). In brief, qualifying families required a proband and a full sibling, each with a diagnosis of definite or probable Alzheimer disease (McKhann, et al., 1984) with onset after 60 years of age, and at least one additional biologically related family member who was 60 years or older if unaffected or 50 years or older if diagnosed as having AD or mild cognitive impairment (Petersen, et al., 1999). Psychosis was assessed by interview of a knowledgeable informant and rated on the Consortium to Establish a Registry for Alzheimer Disease Behavioral Rating Scale (CBRS) 1996 version (Mack, et al., 1999), the Neuropsychiatric Inventory (Cummings, et al., 1994), and the Neuropsychiatric Inventory Questionnaire (Kaufer, et al., 2000), with slight modification as described previously (Sweet, et al., 2010). We have previously established excellent inter-rater reliability of psychosis using this approach to assessment in this cohort (Sweet, et al., 2010).
Delusions were defined as persistent false beliefs based on incorrect inference about external reality, resistant to persuasion or contrary evidence, and not attributable to social or cultural mores. Hallucinations were defined as sensory perceptions for which there was no basis in reality. Discrete hypnagogic and hypnopompic hallucinations, as well as symptoms occurring only during an episode of delirium, were not rated. To avoid phenocopies due to momentary confusion or misinterpretation by the observer, a delusion or hallucination were defined by its persistence over time. Thus, a given CBRS item is considered evidence of a delusion or hallucination when it was rated as rated as occurring (at least) 3 times in the past month at any visit. Because the frequency of psychosis in LOAD increases in individuals with a Clinical Dementia Rating Scale (CDR) score > 1 (Sweet, et al., 2010), classification as not psychotic required individuals to be free of delusions and hallucinations throughout their illness and to have a CDR > 1. Using this definition, we found that LOAD+P is a highly heritable trait (h2=0.61, SE=0.31) in the NIA-LOAD cohort (see Supplemental methods for a description of heritability analysis), supporting its use for the gene-mapping analyses we conducted.
For the present analysis, we used a sample of 607 families from the NIA-LOAD cohort that was previously used in a genome-wide analysis of familial LOAD (Wijsman, et al., 2011). We furthermore restricted the analysis to families where members had psychosis assessments and also available genome-wide data, leading to a final sample of 1,279 subjects from 263 families. The families were divided into two subgroups based on the presence or absence of psychosis symptoms in LOAD family members. Families with at least one LOAD family member with psychosis were classified as LOAD+P families (n=215 families). Families without any LOAD members with psychosis were classified as LOAD-P families (n=48 families).
Statistical Analysis
Genome-wide genotyping of NIA-LOAD cohort
Genotyping of the study participants was performed using Illumina Human610Quadv1_B BeadChips (Illumina, San Diego, CA, USA) (Wijsman, et al., 2011). Genome-wide linkage analysis was restricted to SNP markers with minor allele frequencies of 5% or higher.
Linkage analysis
To evaluate the evidence for linkage in the LOAD+P and LOAD-P families, we performed non-parametric two-point genome-wide linkage analysis using the Kong and Cox linear model implemented in MERLIN (Abecasis, et al., 2002). Linkage analyses performed in both LOAD+P and LOAD-P families were carried out using sex, age, education and CDR as covariates. We have included CDR score because functional severity of dementia has been associated with the presence of psychosis in patients with LOAD (24),. Evidence for linkage was considered statistically significant in the two-point analysis if the two-point LOD score was ≥ 3.6 (Lander and Kruglyak, 1995). We performed multipoint linkage analysis in regions with significant linkage evidence in the two-point analysis. Because the presence of linkage disequilibrium among SNPs violates an underlying assumption of multipoint linkage analysis software, SNPs were pruned on the basis of a correlation coefficient, r2, of 0.2 using PLINK software (http://pngu.mgh.harvard.edu/~purcell/plink/index.shtml).
Test of linkage heterogeneity
To test for linkage heterogeneity between the LOAD+P and LOAD-P families, we used an approach based on Morton’s heterogeneity test (Morton, 1956). First, we performed linkage analysis in all LOAD+P and LOAD-P families combined, to obtain the maximum LOD score under the null hypothesis of homogeneity (Zmax, combined). Second, we performed linkage analysis within each family subgroup separately, obtained the maximum LOD score for each SNP, Zmax, LOAD+P and Zmax, LOAD-P, and then summed these two maximum values. The chi-square statistic for the test for heterogeneity was computed as: χ2(1)= 4.6 × [(Zmax, LOAD+P + Zmax, LOAD-P) − Zmax, combined].
Linkage power calculation
To estimate the power of our LOAD-P families in detecting linkage, we performed simulations of genotypes for 100 replicates using SLINK (Ott, 1989). We assumed an autosomal dominant inheritance model, recombination fraction ranging 0.0–0.45 and minor allele frequencies ranging from 10% to 40%.
Joint linkage and association analysis
For chromosomal regions that yielded significant two-point LOD scores and multipoint LOD scores ≥ 2.5, we carried out joint linkage and association analysis in a 50kb-region encompassing the linkage peaks using PSEUDOMARKER software (Gertz, et al., 2014). Adjustment for multiple testing based on the 157 SNPs tested on 19q13.12. was carried out using Bonferroni correction (significance threshold P≤ 3.7×10−4).
Generalization Cohort
Because the frequencies of genetic variants can differ substantially between different ethnic groups, it is important to assess whether results obtained in populations of European ancestry can be extended to populations of different ancestry. Hence we assessed whether our results can be generalized using a Caribbean Hispanic (CH) sample, Estudio Familiar de Influencia Genetica en Alzheimer (EFIGA) and Washington Heights Aging Project (WHICAP). Unrelated Caribbean Hispanic subjects were selected from the Washington Heights–Inwood Columbia Aging Project (WHICAP) study and the Estudio Familiar de Influencia Genetica de Alzheimer (EFIGA). WHICAP is a population-based study of elderly individuals residing in New York and EFIGA study recruited Caribbean Hispanic families with multiple members affected with Late Onset Alzheimer’s Disease (LOAD). Both studies followed the same clinical diagnostic methods and both cohorts have been described elsewhere (Mayeux, et al., 2001, Vardarajan, et al., 2014). For analysis purposes, we identified unrelated individuals as psychotic based on the presence of one or more of the following rated as present on the medical history form: i) psychosis identified as the first symptom of dementia, ii) hallucinations (visual, auditory, olfactory or tactile), iii) delusions and iv) receipt of anti-psychotic medication. In this analysis we have included 4,917 study’s participants from three different and independent recruitment waves (CH1, CH2 and CH3).
Genome-wide genotyping
Genome-wide genotyping was done using two high-throughput SNP genotyping platforms: Illumina HumanHap 650Y platform and Omni Express.
Test of SNP association in the CH sample
For generalization purposes, the 19q13.12 region was defined by a total of 246 SNPs that were within the region of interest and genotyped in the three CH datasets. To account for the effects of population substructure, we performed a principal components analysis using EIGENSTRAT software (http://genepath.med.harvard.edu/~reich/EIGENSTRAT.htm). Logistic regression models were conducted using PLINK software (http://pngu.mgh.harvard.edu/~purcell/plink/index.shtml). All analyses were adjusted for sex, age, and the first ten principal components derived from the EIGENSTRAT analysis.
Meta-analysis of the CH datasets
The three independent datasets were combined into a meta-analysis taking into account study specific sample size and direction of the association effect using METAL software (http://www.sph.umich.edu/csg/abecasis/metal/). Adjustment for multiple testing was carried out using Bonferroni correction.
Replication Cohort: University of Pittsburgh Alzheimer Disease Research Center (ADRC)
To further replicate associations in an independent dataset, we used a Non-Hispanic Caucasian (NHC) cohort recruited from the University of Pittsburgh AD Research Center. Characterization of the psychotic symptoms as well as quality control of the ADRC genomic data has been described in detail elsewhere (Hollingworth, et al., 2012). For analysis purposes, a total of 496 LOAD+P and 156 LOAD-P subjects were included.
Brain expression eQTL analyses
To further determine whether associated SNPs affect gene expression levels, we used the publically available dataset from the UK Brain Expression Consortium (UKBEC) which is based on tissue from 12 brain regions from 134 individuals free of neurodegenerative disorders analyzed using the Affymetrix Exon 1.0 ST Array (http://www.braineac.org/).
RESULTS
Characteristics of the different study cohorts (NIA-LOAD, CH1, CH2, CH3 and NHC) are provided in Supplementary Table 3.
The LOAD+P families used for the linkage analysis consisted of 981 individuals from 215 families, including 264 LOAD patients with psychotic symptoms and 279 without psychosis. The average age at evaluation and standard deviation of the LOAD+P family members was 76±7 and 55% were women. The LOAD-P families consisted of 298 individuals from 48 families, including 139 LOAD patients without psychotic symptoms. The age at evaluation of the LOAD-P family members was 76±8 and 53% were females.
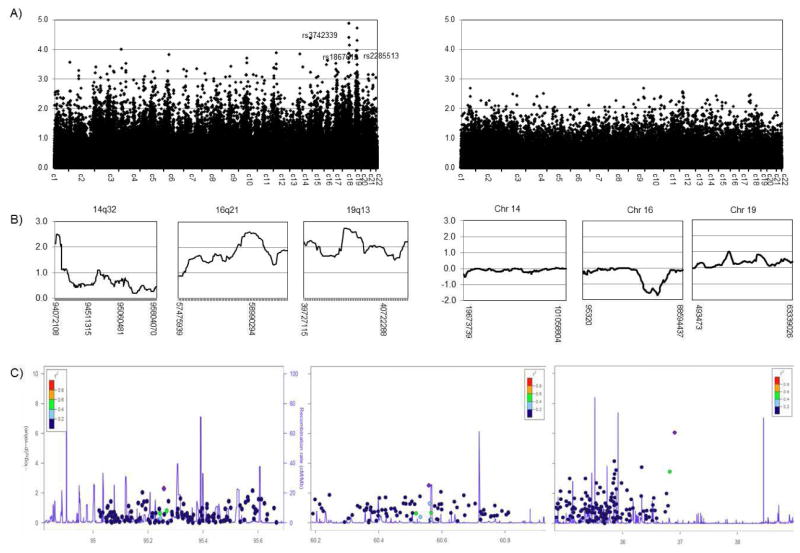
Genome-wide linkage analysis of the LOAD+P families identified several regions with two-point LOD scores ≥ 3.6. The maximum two-point LOD score was observed on chromosome 18q21.32, with LOD=4.9 at rs2332026 (90.18 cM), however multi-point linkage analysis of the region did not yield significant evidence of linkage (maxLOD=2.0 at rs7069001). There were three different chromosomal regions, 14q32, 16q21 and 19q13.12 that yielded 2pt-LOD scores ≥ 3.6 and mpt-LOD scores ≥2.5 which were prioritized for additional analysis (Figure 1A and 1B, Table 1). Joint linkage and association analysis of these three candidate regions (Figure 1C, Table 1) identified SNP marker rs2945988 at 19q13.12 as strongly associated with psychosis (P= 8.7 × 10−7) even after Bonferroni adjustment. Although additional SNPs showed also nominal association with psychosis within the two other regions: rs10139111 at 14q32 (P=0.005) and rs9927943 at 16q21 (P=0.003), they did not survive correction for multiple testing.
Figure 1.
Results of the genome-wide linkage and association analyses. A) Genome-wide non-parametric two-point linkage analysis in LOAD+P (right panel) and LOAD-P families (left panel); X-axis represent each of the analyzed chromosomes and Y–axis correspond to the individual SNP’s LOD score B) Multi-point linkage analysis in chromosomal regions with genome-wide significant (two-point LOD scores evidence of linkage ≥ 3.6) in the LOAD+P families (right panel) and multi-point linkage analysis of the same chromosomes in the LOAD-P families. X-axis represent physical location in bp and Y-axis represent the individual SNP’s LOD score; C) Joint linkage and association analysis in 50kb region encompassing the linkage peak for chromosomal regions multi-point LOD scores ≥ 2.5 in the LOAD+P families. The X-axis represent represents base-pair position (Mb) along the chromosome, the left Y-axis correspond to the recombination rate (cM/Mb) and the right Y-axis correspond to the logarithm of the p-value.
Table 1.
Candidate regions from genome-wide linkage and association analyses of the LOAD+P families.
| Band | SNP | 2pt-LOD | SNP | mpt-LOD | SNP | Pjoint |
|---|---|---|---|---|---|---|
| 14q32 | rs3742339 | 4.4 | rs5508 | 2.5 | rs10139111 | 0.005 |
| 16q21 | rs1867612 | 3.6 | rs9302724 | 2.6 | rs9927943 | 0.003 |
| 19q13.12 | rs2285513 | 3.8 | rs541169 | 2.7 | rs2945988 | 8.7 × 10−7 |
We focused on 19q13.12 signal due to its strong evidence of linkage and association, we and tested linkage heterogeneity. We found statistical evidence for the existence of heterogeneity between LOAD+P and LOAD-P families (P=0.011), further supporting the need for stratifying the family cohort based on the presence or absence of psychotic symptoms among the LOAD patients.
Our simulation results shown that when the linked allele frequency is 37%, the frequency of the minor allele of SNP rs2945988 in the LOAD-P cohort of families, we will have 93% power to detect LOD scores exceeding the Lander and Kruglyak threshold for significant linkage (LOD≥ 3.6).
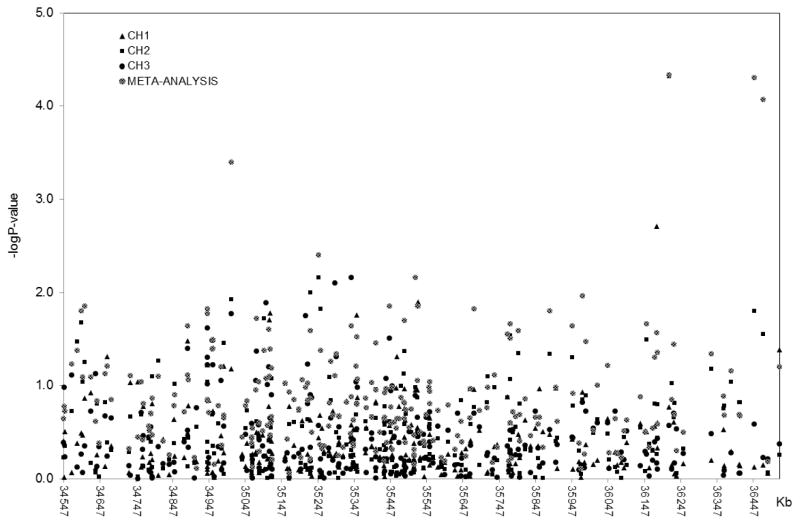
To test whether our findings can be generalizable to another ethnic group, we used a sample of unrelated subjects with Caribbean Hispanic ancestry (CH1, CH2 and CH3) that included 231 LOAD patients with psychotic symptoms and 1,943 without psychosis. Logistic regression analysis (age, sex, and population stratification adjusted) identified several SNPs as strongly associated with the LOAD+P phenotype after adjustment for multiple testing with Bonferroni’s correction (Suppl. Table 1). The strongest signal with the same effect direction in all three CH datasets was found for SNP rs10410711 (Pmeta=5 × 10−5), an intronic variant in ZNF566 gene. Additional variants in this gene also survived multiple testing corrections and achieved significant LOAD+P association: rs10421862, located 24Kb downstream (Pmeta=5 × 10−5) showed same direction of the association in the three CH cohorts and rs10419962 (Pmeta=4.7 × 10−5), 234Kb upstream, showed same direction of the association in CH1 and CH2.
We interrogated 19q13.12 region, defined by a total of 246 SNPs, in an independent Non-Hispanic Caucasian (NHC) dataset. First, we analyzed the results from CH and NHC datasets via trans-ethnic meta-analysis using MANTRA software (Morris, 2011). Results from MANTRA revealed little evidence of heterogeneity in allelic effects of the SNPs (posterior probability of heterogeneity (PPH) < 50%) between the different populations, i.e., the most heterogeneous SNP in terms of allelic effect, rs7249613, has a PPH of 34%. As SNPs in CH and NCH cohorts share a common effect size, we meta-analyzed the results with a fixed-effect model approach using METAL software (http://www.sph.umich.edu/csg/abecasis/metal/). The strongest association with LOAD+P phenotype corresponds to rs10421862 (Pmeta= 1.0 × 10−5), with the same effect direction in CH and NHC datasets.
SNP rs2945988 identified in the NIA-LOAD cohort was not statistically significant in the CH meta-analysis (P=0.063). However, SNPrs2945988 in NIA-LOAD and its closest variant in CH, rs10421862, located 45Kb apart, are in low linkage disequilibrium (r2=0.10), which would be expected based on the different linkage disequilibrium patterns within this chromosomal region between European and Hispanics ancestry populations (Suppl. Figure 2). Nevertheless, the location would suggest that a gene or set of genes in this region are likely to underlie psychosis in patients with LOAD.
Results from eQTL analysis using BRAINEAC showed that 73 genes within the 19q13.12 region had also altered brain expression due to these two 19q13.12 variants, rs2945988 and rs10421862 (Supplementary Table 2). After Bonferroni multiple testing adjustment (P ≤ 6.8×10−4), rs2945988 is a significant gene-level cis-eQTL for ZNF461 (P=2.2 × 10−5) and ZFP82 (P=8.0 × 10−5). Both genes are expressed in brain and codify Krüppel-associated box-containing (KRAB) zinc-finger proteins, the largest family of transcriptional regulators in the human genome (Urrutia, 2003).
DISCUSSION
To identify loci potentially containing genetic variants associated with higher risk of developing psychosis in subjects affected by LOAD, we performed a genome-wide linkage analysis in a sample of NIA-LOAD families in which some members with LOAD also exhibited psychosis symptoms. We observed significant evidence for two-point and multipoint linkage on chromosome 19q13.12 (2ptLOD=3.8 and mpt-LOD=2.7). Further investigation of the linkage signal using joint linkage and association analyses identified SNP rs2945988 as strongly associated with psychosis (Pjoint=8.7 × 10−7). The 19q13.12 association with psychosis was generalized to a sample of unrelated Alzheimer’s patients and controls of Caribbean Hispanic ancestry rs10410711 (Pmeta=5 × 10−5). Further evidence was achieved through the meta-analysis of the Caribbean Hispanics and an independent Non-Hispanic Caucasian cohorts, where another 19q13.12 variant located 24Kb upstream rs10410711, rs10421862, appeared strongly associated with LOAD+P (Pmeta=1.0 × 10−5) with the same effect direction in all datasets.
In the most recent genome-wide linkage analysis of LOAD+P up to date, Hollingworth et al reported chromosomewide and genomewide significant linkage peaks on chromosomes 7 and 15(Hollingworth, et al., 2007). Despite the differences in their study design (linkage analysis was restricted to affected relative pairs) and clinical assessment of psychosis (limited data were available covering the presence, type and severity of psychotic symptoms), they reported suggestive linkage (LOD=1.86) to chromosome 19 at 50cM, being the nearest marker D19S433 (30,417,027–30,417,232), ~5Mb upstream 19q13.12 region.
Recent studies evaluating the genetic contributions to LOAD+P have consisted of GWAS approaches. Hollingworth et al (Hollingworth, et al., 2012) undertook a meta-analysis of three genome-wide association studies (GWASs) to identify loci LOAD+P loci. The possible association between chromosome 19 and LOAD+P is limited to APOE locus (located more than 10Mb apart from the identified 19q13.12 region). However, as previously reported (Demichele-Sweet, et al., 2011), no evidence of association was observed at the APOE locus when analyzing LOAD+P versus LOAD-P. They also identified genetic variants/genes revealed overlap with other psychiatric disorders with psychotic features.
In the first genome-wide study of copy number variation (CNV) to date, Zheng and colleagues (Zheng, et al., 2015) identified a significant duplication in the APC2 gene on 19p13, which was protective against developing LOAD+P. Their results also suggested that the same genetic variants (SNPs, CNVs), may be implicated in different psychiatric disorders (schizophrenia, autism and LOAD+P).
To that end, the identified 19q13.12 variants in this study, rs2945988 and rs10421862 are located within zinc-finger binding proteins, ZNF260 and ZNF566 respectively, with a plausible role as regulators of gene expression. Of interest, several recent genetic association studies have reported that variants in a zinc-finger protein gene, ZNF804A, strongly influence susceptibility to psychosis, schizophrenia and bipolar disorder (Steinberg, et al., 2011).
Additional studies have implicated genomic variation in 19q13.12 as risk factor for neuropsychiatric disorders. Xu and colleges reported a de novo deletion on chromosome 19q13.12 with relatively high penetrance that contributes to the genetic component of schizophrenia (Xu, et al., 2008). A multistage schizophrenia genome-wide association study (Consortium, 2014) identified 128 independent associations spanning 108 conservatively defined loci. Among the significant loci was a SNP on chromosome 19q13.12, mapping 13Mb upstream of our identified region.
Brain eQTL analyses revealed that SNP rs2945988 signifincalty affects gene expression of two KRAB zinc-finger genes (ZNF461 and ZFP82), suggesting transcriptional regulation as possible functional mechanism. Although they represent plausible a priori functional candidates for LOAD+P, there is no guarantee that they are the causal genes, thus it is likely that other loci within this region contribute to psychosis risk in LOAD (see Supplementary Figure 1 for genes in the 19q13.12 linkage region).
Among other potential candidate genes within this region are WDR62 and SNX26, both expressed in brain and highly conserved. Assessment of brain expression patterns also revealed that rs2945988 and rs10421862 nominally affect the expression levels of WDR62 (P=0.012) and SNX26 (P=0.006) genes, respectively.
Mutations in the WD repeat-containing protein 62 gene WDR62 has been reported as the cause of a wide spectrum of severe cerebral cortical malformations. Experiments in human and mouse embryonic brain found that its expression was restricted to neural precursors undergoing mitosis, suggesting that WDR62 is a key protein in neural precursor generation, a process that is uniquely vital to human cerebral cortex growth (Nicholas, et al., 2010).
The sorting nexin 26 gene (SNX26), a brain-enriched Rho GTPase activating protein, is involved in the regulation of dendritic branching and neuronal complexity in the developing brain and also affects synaptic plasticity in mature neurons. Dendritic spines changes are closely associated with various neurological diseases (Kim, et al., 2013).
Our results suggest that the locus at 19q13.12 may influence the risk of developing a form of LOAD associated with psychosis. Our generalization sample consisted of a population-based cohort of Caribbean Hispanic subjects. Because of differences in genetic ancestry between European and Hispanic populations, we would not necessarily expect the same SNPs to be associated with LOAD+P in both populations, although different SNPs in the same region might show association. Our observation of association of LOAD+P with several SNPs in the same region in Caribbean Hispanics and Non-Hispanic Caucasian, despite the differences between European and Hispanic samples in environmental and genetic factors, strengthens the evidence for 19q13.12 as susceptibility locus for psychosis in LOAD patients. Similar generalization approaches have been previously reported in the literature. Graff and colleagues (Graff, et al., 2013) used Hispanic population to investigate common adiposity-related genetic loci previously reported in European descent populations, and provided evidence for the generalization of several BMI and central adiposity loci in Hispanic women.
Although a potential limitation of our study was the lack of detailed assessments of the psychosis phenotype in the Caribbean Hispanic cohort, the evidence for generalization of the linkage signal indicates that the findings were robust and not related to the method of identification of the phenotype. Some of the subjects from the NIA-LOAD family cohort were included in the GWAS meta-analysis conducted by Hollingworth and colleges (Hollingworth, et al., 2012).
The fact that the identified LOAD+P variants affect the brain expression levels of the several genes in 19q13.12 region, suggest that their expression might be transcriptionally regulated. Whether these variants are associated with the development of psychosis in LOAD specifically, or are associated also with psychosis in the absence of LOAD is an interesting question that remains to be investigated. Sequencing of the 19q13.12 region using the most informative families may help to identify variants contributing to susceptibility to LOAD and psychosis.
Supplementary Material
Supplemental. Figure 1. Genes in the 19q13.12 region
Supplemental. Figure 2. Pattern of linkage disequilibrium (LD) in 19q13.12 region. A) The region represents SNPs within 500kbp upstream SNP rs2945988, the strongest SNP identified in the NIA-LOAD cohort joint linkage and association analysis (red rectangle). B) Generalization results in the Caribbean Hispanic (CH) cohorts identified rs10419962 (blue rectangle), located 307Kb upstream of rs2945988, as the strongest LOAD+P associated variant. Results from the meta-analysis of CH and an independent Non-Hispanic Caucasian (NHC) cohorts identified rs10421862 (green rectangle), 45kb upstream of rs2945988, strongly associated with LOAD+P (Pmeta=1.0 × 10−5). As stated in the manuscript, the figure shows the close proximity of the variants though they are not in strong LD.
Suppl. Table 1. Meta-analysis of the 246 SNPs in 19q13.12 region using the Caribbean Hispanics (CH1, CH2 and CH3) and Non-Hispanic Caucasian (NHC) cohorts.
Supplemental Table 2. BRAINEAC Cis-eQTL results (P≤ 0.05) for SNPs rs10421862 and rs2945988 in 19q13.12 region.
Supplemental Table 3- Characteristics of the study cohorts.
Figure 2. Generalization of 19q13.12 region in Caribbean Hispanics (CH) cohorts.
Multivariate regression results from three independent Caribbean Hispanics replication cohorts and their meta-analysis. X-axis represents physical location of the SNP markers in kilobases; Y-axis represents logarithm of the P-value obtained in the analyses.
Psychotic symptoms are frequent in late-onset Alzheimer’s disease (LOAD) patients
Families were classified into Psychotic (LOAD+P) and Non-psychotic families (LOAD-P)
Genome-wide linkage analysis yielded strong evidence of linkage on 19q13.12 in LOAD+P families
Results were generalized to population of different ancestry (Caribbean Hispanics) and replicated in an independent Non-Hispanic Caucasian dataset
Genetic variants in genes on 19q13.12 may influence the susceptibility to psychosis in LOAD patients.
Acknowledgments
Study participants were enrolled under federal grants R01AG041797, U24AG026395, R37AG015473, AG030653, AG041718, AG005133 and U24AG21886 from the NIA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Department of Veterans Affairs, or the United States Government. We would like to acknowledge Dr. Andrew P. Morris for his useful contribution, assisting with the analysis and interpretation of the MANTRA results.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin--rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet. 2002;30(1):97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- Avramopoulos D, Fallin MD, Bassett SS. Linkage to chromosome 14q in Alzheimer’s disease (AD) patients without psychotic symptoms. Am J Med Genet B Neuropsychiatr Genet. 2005;132B(1):9–13. doi: 10.1002/ajmg.b.30074. [DOI] [PubMed] [Google Scholar]
- Bacanu SA, Devlin B, Chowdari KV, DeKosky ST, Nimgaonkar VL, Sweet RA. Linkage analysis of Alzheimer disease with psychosis. Neurology. 2002;59(1):118–20. doi: 10.1212/wnl.59.1.118. [DOI] [PubMed] [Google Scholar]
- Bacanu SA, Devlin B, Chowdari KV, DeKosky ST, Nimgaonkar VL, Sweet RA. Heritability of psychosis in Alzheimer disease. Am J Geriatr Psychiatry. 2005;13(7):624–7. doi: 10.1176/appi.ajgp.13.7.624. [DOI] [PubMed] [Google Scholar]
- Bassiony MM, Steinberg MS, Warren A, Rosenblatt A, Baker AS, Lyketsos CG. Delusions and hallucinations in Alzheimer’s disease: prevalence and clinical correlates. Int J Geriatr Psychiatry. 2000;15(2):99–107. doi: 10.1002/(sici)1099-1166(200002)15:2<99::aid-gps82>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- S.W.G.o.t.P.G Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–7. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–14. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Demichele-Sweet MA, Lopez OL, Sweet RA. Psychosis in Alzheimer’s disease in the national Alzheimer’s disease coordinating center uniform data set: clinical correlates and association with apolipoprotein e. Int J Alzheimers Dis. 2011;2011:926597. doi: 10.4061/2011/926597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMichele-Sweet MA, Sweet RA. Genetics of psychosis in Alzheimer’s disease: a review. J Alzheimers Dis. 2010;19(3):761–80. doi: 10.3233/JAD-2010-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertz EM, Hiekkalinna T, Digabel SL, Audet C, Terwilliger JD, Schaffer AA. PSEUDOMARKER 2.0: efficient computation of likelihoods using NOMAD. BMC Bioinformatics. 2014;15:47. doi: 10.1186/1471-2105-15-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go RC, Perry RT, Wiener H, Bassett SS, Blacker D, Devlin B, Sweet RA. Neuregulin-1 polymorphism in late onset Alzheimer’s disease families with psychoses. Am J Med Genet B Neuropsychiatr Genet. 2005;139B(1):28–32. doi: 10.1002/ajmg.b.30219. [DOI] [PubMed] [Google Scholar]
- Graff M, Fernandez-Rhodes L, Liu S, Carlson C, Wassertheil-Smoller S, Neuhouser M, Reiner A, Kooperberg C, Rampersaud E, Manson JE, Kuller LH, Howard BV, Ochs-Balcom HM, Johnson KC, Vitolins MZ, Sucheston L, Monda K, North KE. Generalization of adiposity genetic loci to US Hispanic women. Nutr Diabetes. 2013;3:e85. doi: 10.1038/nutd.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth P, Hamshere ML, Holmans PA, O’Donovan MC, Sims R, Powell J, Lovestone S, Myers A, DeVrieze FW, Hardy J, Goate A, Owen M, Williams J. Increased familial risk and genomewide significant linkage for Alzheimer’s disease with psychosis. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(7):841–8. doi: 10.1002/ajmg.b.30515. [DOI] [PubMed] [Google Scholar]
- Hollingworth P, Sweet R, Sims R, Harold D, Russo G, Abraham R, Stretton A, Jones N, Gerrish A, Chapman J, Ivanov D, Moskvina V, Lovestone S, Priotsi P, Lupton M, Brayne C, Gill M, Lawlor B, Lynch A, Craig D, McGuinness B, Johnston J, Holmes C, Livingston G, Bass NJ, Gurling H, McQuillin A, Holmans P, Jones L, Devlin B, Klei L, Barmada MM, Demirci FY, DeKosky ST, Lopez OL, Passmore P, Owen MJ, O’Donovan MC, Mayeux R, Kamboh MI, Williams J. Genome-wide association study of Alzheimer’s disease with psychotic symptoms. Mol Psychiatry. 2012;17(12):1316–27. doi: 10.1038/mp.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, Lopez OL, DeKosky ST. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233–9. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- Kim Y, Ha CM, Chang S. SNX26, a GTPase-activating protein for Cdc42, interacts with PSD-95 protein and is involved in activity-dependent dendritic spine formation in mature neurons. J Biol Chem. 2013;288(41):29453–66. doi: 10.1074/jbc.M113.468801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander E, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet. 1995;11(3):241–7. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Wisniewski SR, Becker JT, Boller F, DeKosky ST. Psychiatric medication and abnormal behavior as predictors of progression in probable Alzheimer disease. Arch Neurol. 1999;56(10):1266–72. doi: 10.1001/archneur.56.10.1266. [DOI] [PubMed] [Google Scholar]
- Mack JL, Patterson MB, Tariot PN. Behavior Rating Scale for Dementia: development of test scales and presentation of data for 555 individuals with Alzheimer’s disease. J Geriatr Psychiatry Neurol. 1999;12(4):211–23. doi: 10.1177/089198879901200408. [DOI] [PubMed] [Google Scholar]
- Mayeux R, Small SA, Tang M, Tycko B, Stern Y. Memory performance in healthy elderly without Alzheimer’s disease: effects of time and apolipoprotein-E. Neurobiol Aging. 2001;22(4):683–9. doi: 10.1016/s0197-4580(01)00223-8. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Morris AP. Transethnic meta-analysis of genomewide association studies. Genet Epidemiol. 2011;35(8):809–22. doi: 10.1002/gepi.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton NE. The detection and estimation of linkage between the genes for elliptocytosis and the Rh blood type. Am J Hum Genet. 1956;8(2):80–96. [PMC free article] [PubMed] [Google Scholar]
- Nicholas AK, Khurshid M, Desir J, Carvalho OP, Cox JJ, Thornton G, Kausar R, Ansar M, Ahmad W, Verloes A, Passemard S, Misson JP, Lindsay S, Gergely F, Dobyns WB, Roberts E, Abramowicz M, Woods CG. WDR62 is associated with the spindle pole and is mutated in human microcephaly. Nat Genet. 2010;42(11):1010–4. doi: 10.1038/ng.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott J. Computer-simulation methods in human linkage analysis. Proc Natl Acad Sci U S A. 1989;86(11):4175–8. doi: 10.1073/pnas.86.11.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Brandt J, Albert M, Hadjigeorgiou G, Papadimitriou A, Dubois B, Sarazin M, Devanand D, Honig L, Marder K, Bell K, Wegesin D, Blacker D, Stern Y. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62(10):1601–8. doi: 10.1001/archneur.62.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg S, Mors O, Borglum AD, Gustafsson O, Werge T, Mortensen PB, Andreassen OA, Sigurdsson E, Thorgeirsson TE, Bottcher Y, Olason P, Ophoff RA, Cichon S, Gudjonsdottir IH, Pietilainen OP, Nyegaard M, Tuulio-Henriksson A, Ingason A, Hansen T, Athanasiu L, Suvisaari J, Lonnqvist J, Paunio T, Hartmann A, Jurgens G, Nordentoft M, Hougaard D, Norgaard-Pedersen B, Breuer R, Moller HJ, Giegling I, Glenthoj B, Rasmussen HB, Mattheisen M, Bitter I, Rethelyi JM, Sigmundsson T, Fossdal R, Thorsteinsdottir U, Ruggeri M, Tosato S, Strengman E, Kiemeney LA, Melle I, Djurovic S, Abramova L, Kaleda V, Walshe M, Bramon E, Vassos E, Li T, Fraser G, Walker N, Toulopoulou T, Yoon J, Freimer NB, Cantor RM, Murray R, Kong A, Golimbet V, Jonsson EG, Terenius L, Agartz I, Petursson H, Nothen MM, Rietschel M, Peltonen L, Rujescu D, Collier DA, Stefansson H, St Clair D, Stefansson K. Expanding the range of ZNF804A variants conferring risk of psychosis. Mol Psychiatry. 2011;16(1):59–66. doi: 10.1038/mp.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet RA, Bennett DA, Graff-Radford NR, Mayeux R. Assessment and familial aggregation of psychosis in Alzheimer’s disease from the National Institute on Aging Late Onset Alzheimer’s Disease Family Study. Brain. 2010;133(Pt 4):1155–62. doi: 10.1093/brain/awq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet RA, Hamilton RL, Lopez OL, Klunk WE, Wisniewski SR, Kaufer DI, Healy MT, DeKosky ST. Psychotic symptoms in Alzheimer’s disease are not associated with more severe neuropathologic features. Int Psychogeriatr. 2000;12(4):547–58. doi: 10.1017/s1041610200006657. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Panchalingam K, Pettegrew JW, McClure RJ, Hamilton RL, Lopez OL, Kaufer DI, DeKosky ST, Klunk WE. Psychosis in Alzheimer disease: postmortem magnetic resonance spectroscopy evidence of excess neuronal and membrane phospholipid pathology. Neurobiol Aging. 2002;23(4):547–53. doi: 10.1016/s0197-4580(02)00009-x. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Seltman H, Emanuel JE, Lopez OL, Becker JT, Bis JC, Weamer EA, DeMichele-Sweet MA, Kuller LH. Effect of Alzheimer’s disease risk genes on trajectories of cognitive function in the Cardiovascular Health Study. Am J Psychiatry. 2012;169(9):954–62. doi: 10.1176/appi.ajp.2012.11121815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies W, Bleiler L As Association. 2013 Alzheimer’s disease facts and figures. Alzheimers Dement. 2013;9:208–45. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Urrutia R. KRAB-containing zinc-finger repressor proteins. Genome Biol. 2003;4(10):231. doi: 10.1186/gb-2003-4-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardarajan BN, Faber KM, Bird TD, Bennett DA, Rosenberg R, Boeve BF, Graff-Radford NR, Goate AM, Farlow M, Sweet RA, Lantigua R, Medrano MZ, Ottman R, Schaid DJ, Foroud TM, Mayeux R, Group NLNFS. Age-specific incidence rates for dementia and Alzheimer disease in NIA-LOAD/NCRAD and EFIGA families: National Institute on Aging Genetics Initiative for Late-Onset Alzheimer Disease/National Cell Repository for Alzheimer Disease (NIA-LOAD/NCRAD) and Estudio Familiar de Influencia Genetica en Alzheimer (EFIGA) JAMA Neurol. 2014;71(3):315–23. doi: 10.1001/jamaneurol.2013.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijsman EM, Pankratz ND, Choi Y, Rothstein JH, Faber KM, Cheng R, Lee JH, Bird TD, Bennett DA, Diaz-Arrastia R, Goate AM, Farlow M, Ghetti B, Sweet RA, Foroud TM, Mayeux R. Genome-wide association of familial late-onset Alzheimer’s disease replicates BIN1 and CLU and nominates CUGBP2 in interaction with APOE. PLoS Genet. 2011;7(2):e1001308. doi: 10.1371/journal.pgen.1001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Tang Y, Aggarwal NT, Gilley DW, McCann JJ, Bienias JL, Evans DA. Hallucinations, cognitive decline, and death in Alzheimer’s disease. Neuroepidemiology. 2006;26(2):68–75. doi: 10.1159/000090251. [DOI] [PubMed] [Google Scholar]
- Xu B, Roos JL, Levy S, van Rensburg EJ, Gogos JA, Karayiorgou M. Strong association of de novo copy number mutations with sporadic schizophrenia. Nat Genet. 2008;40(7):880–5. doi: 10.1038/ng.162. [DOI] [PubMed] [Google Scholar]
- Zheng X, Demirci FY, Barmada MM, Richardson GA, Lopez OL, Sweet RA, Kamboh MI, Feingold E. Genome-wide copy-number variation study of psychosis in Alzheimer’s disease. Transl Psychiatry. 2015;5:e574. doi: 10.1038/tp.2015.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental. Figure 1. Genes in the 19q13.12 region
Supplemental. Figure 2. Pattern of linkage disequilibrium (LD) in 19q13.12 region. A) The region represents SNPs within 500kbp upstream SNP rs2945988, the strongest SNP identified in the NIA-LOAD cohort joint linkage and association analysis (red rectangle). B) Generalization results in the Caribbean Hispanic (CH) cohorts identified rs10419962 (blue rectangle), located 307Kb upstream of rs2945988, as the strongest LOAD+P associated variant. Results from the meta-analysis of CH and an independent Non-Hispanic Caucasian (NHC) cohorts identified rs10421862 (green rectangle), 45kb upstream of rs2945988, strongly associated with LOAD+P (Pmeta=1.0 × 10−5). As stated in the manuscript, the figure shows the close proximity of the variants though they are not in strong LD.
Suppl. Table 1. Meta-analysis of the 246 SNPs in 19q13.12 region using the Caribbean Hispanics (CH1, CH2 and CH3) and Non-Hispanic Caucasian (NHC) cohorts.
Supplemental Table 2. BRAINEAC Cis-eQTL results (P≤ 0.05) for SNPs rs10421862 and rs2945988 in 19q13.12 region.
Supplemental Table 3- Characteristics of the study cohorts.