Abstract
The phenomenon of so called renal “ischemic preconditioning”, whereby an initial ischemic insult induces resistance against subsequent kidney damage, has been well established in the experimental literature. However, a clinically applicable way to safely recapitulate this state has not been defined. We hypothesized that a unique combination of agents (nitrited myoglobin + Sn protoporphyrin) can achieve these ends by safely, and synergistically, increasing cytoprotective proteins (e.g., HO-1, IL-10, haptoglobin) in kidney cells. To test this hypothesis, CD-1 mice received 1 mg N-Mgb and 1 µmole SnPP, either alone or in combination. Renal cortical HO-1, haptoglobin, and IL-10 gene expression (mRNA, protein levels) were determined 4 and 18 hrs later. Cytoresistance to three forms of AKI were assessed (glycerol- induced rhabdomyolysis; maleate nephrotoxicity; post-ischemic AKI progression to CKD). To ascertain whether cytoresistance might emerge in extra-renal organs, hepatic HO-1, IL-10, and haptoglobin levels were also measured, and resistance to 25 min of hepatic ischemia-reperfusion injury and hepatotoxicity (intraperitoneal glycerol injection) was sought. N-Mgb + SnPP induced additive or synergistic increases in renal HO-1, haptoglobin, and IL-10 mRNA/protein levels (up to 20 fold) without inducing any apparent renal or extra-renal damage. By 18 hrs post treatment, marked, or complete, protection against glycerol-induced AKI, maleate- induced AKI, and post ischemic AKI progression to CKD had emerged. Combined N-Mgb+SnPP was more protective than was either agent alone (assessed in glycerol model). N-Mgb+SnPP also up-regulated cytoprotective pathways in liver, and induced marked protection against both hepatic ischemia-reperfusion and toxic liver damage. In sum, we posit that “preconditioning” with combined N-Mgb+SnPP administration represents a promising approach for protecting against diverse forms of renal and non renal (hepatic) forms of tissue damage.
Keywords: acute kidney injury, ischemia, nephrotoxicity, preconditioning, heme oxygenase 1, haptoglobin, IL-10
INTRODUCTION
Acute kidney injury (AKI) is a well recognized risk factor for morbidity, mortality, and the initiation of chronic kidney disease (1–5). However, no proven ways to prevent AKI currently exist. It is well established that following an initial bout of ischemic or toxic injury, the kidney develops marked resistance to subsequent damage (e.g. ref. 6–10). This phenomenon, which is mediated, in part, by an up-regulation of cytoprotective and anti-inflammatory ‘stress’ proteins (eg., heme oxygenase 1, ferritin, haptoglobin, hemopexin, alpha 1 antitrypsin, IL-10) (11–23), has been referred to as “ischemic preconditioning” or the “acquired cytoresistance” state.
Given the profound protective nature of acquired cytoresistance, investigators have sought ways to safely recapitulate it in humans. Notable in this regard is so called “remote preconditioning”, whereby recurrent bouts of upper and/or lower limb ischemia are induced by recurrently inflating and deflating blood pressure cuffs (24–26). The goal to release unknown tissue ‘conditioning factors’ into the systemic circulation that will trigger protective tissue responses (e.g., in brain, heart, liver, and kidney). Despite its appeal, this approach has had only questionable success (24–26), likely due to the following: 1) the ‘factors’ released from post ischemic limbs that might induce ‘preconditioning’, and how much ‘factor’ release is required to induce this state, are unknown; 2) the cellular pathways by which such ‘factors’ impact distant organs to induce cytoresistance have not been defined; and 3) it is impossible to judge whether the desired ‘pre-conditioning’ actually develops in any given individual.
We have sought an alternative approach for inducing acquired cytoresistance. To this end, we have developed a pharmacologic regimen, consisting of low dose nitrited-myoglobin (N-Mgb), + Sn protoporphyrin (SnPP), which markedly, and synergistically up-regulate a host of renal tubular cell cytoprotectants (e.g., heme oxygenase 1, haptoglobin, IL-10) in the absence of obvious renal or extra-renal toxicities. Within 18 hrs of agent administration, striking resistance to nephrotoxic AKI, ATP depletion-induced AKI, and post- AKI progression to CKD result. In addition, hepatic protection against post-ischemic and toxic injury are expressed. Lastly, we observed that the induction of this cytoresistant state can be gauged non-invasively by using plasma heme oxygenase 1 (HO-1) and haptoglobin levels as ‘biomarkers’ of its induction. The experiments and results that have led to these conclusions form the basis of this report.
METHODS
General approach
Animals
All experiments were conducted using male CD-1 mice (35–45 grams; Charles River Laboratories, Wilmington, MA). They were housed under standard vivarium conditions with free food and water access throughout. The employed protocols were approved by the Institution’s IACUC according to NIH guidelines.
Cytoresistance inducing reagents
Horse skeletal muscle (Sigma; #Mb0360), was used as the primary cytoresistance inducing agent. However, because Mgb has an inherent nephrotoxic potential (particularly under conditions of volume depletion and aciduria), two approaches were utilized to mitigate Mgb’s potential adverse effects:
First, the Mgb was converted into a nitrited form by the addition of equimolar Na nitrite. In this regard, nitrite is an ambidendrate molecule that directly binds 1:1 to myoglobin Fe either via its oxygen or nitrogen component (27,28). Noteworthy is that Fe is the dominant mediator of Mgb’s cytotoxic effect (29,30), and this toxicity is substantially reduced by prior nitrite binding (31–35). In addition to its ability to decrease Fe mediated toxicity, nitrite has been implicated as a mediator of ‘remote preconditioning’, possibly through nitric oxide (NO) generation (31). In this regard, heme proteins directly reduce nitrite, with resultant NO. production (37–39). Thus, by binding nitrite directly to Mgb, Mgb injection with subsequent proximal tubule endocytic uptake, results in direct proximal tubule nitrite / NO targeting; and
Second, in order for heme Fe to induce most of its cytotoxicity, it must first be released from its sequestration site within the porphyrin ring. This Fe release is mediated via porphyrin ring cleavage via heme oxygenase 1 (HO-1). Hence, to attenuate potential Mgb cytotoxicity, we administered it along with a transient HO-1 inhibitor, tin protoporphyrin (SnPP). In this regard, we have previously reported that SnPP addition to Mgb- exposed cultured proximal tubule (HK-2) cells, or to in vivo heme loaded mouse proximal tubule segments, reduces Mgb toxicity by as much as 85% (30). This cytoprotective action is further indicated by observations that SnPP may mitigate post ischemic ARF (36,37).
Impact of Mgb, SnPP, and Mgb+SnPP on renal cortical heme signaling
We postulated the following: 1) that N-Mgb would be a potent signaling molecule, leading to the up-regulation of heme responsive element (HRE) / redox sensitive genes which evoke cytoprotective activities (e.g. HO-1, haptoglobin, hemopexin, IL-10); 2) SnPP can independently up-regulate such genes; and 3) when administered together, N-Mgb+SnPP co-administration can lead to additive or synergistic HRE signaling. The following experiment tested these hypotheses.
Thirty two mice were divided into 4 equal groups: 1) control mice; 2) 1 mg N-Mgb injection (as a bolus through the tail vein); 3) 1 µmole SnPP (via tail vein); and 4) combined N-Mgb+SnPP. After either 4 hrs (n,16) or 18 hrs (n,16), the mice were deeply anesthetized with pentobarbital (50 mg/Kg), the abdomen was opened through a midline abdominal incision, and the kidneys were removed. To determine potential effects on extra-renal organs, a liver lobe as well as the heart were also removed. The tissues were iced and then extracted for protein and RNA (RNeasy Plus Mini; Qiagen, Valencia, CA) and subjected to ELISA and RT-PCR for HO-1, haptoglobin, and IL-10 protein and mRNAs (11,20). As an assessment of renal function, control mice and the 18 hr post N-Mgb+SnPP treated mice had BUN and plasma creatinine levels measured. Additinally, tranverse 10% formalin fixed kidney sections (3µM) were cut and stained with hematoxylin and eosin and PAS to further assess potential injury.
Assessments of cytoresistance
Glycerol model of rhabdomyolysis- induced AKI
Twenty mice were divided into 4 equal groups (controls, N-Mgb, SnPP, or N-Mgb + SnPP tail vein injections), as noted above. Approximately 18 hrs later, the mice were briefly anesthetized with isoflurane and immediately injected with 9 mL/Kg of 50% glycerol, administered in equally divided doses into each hind limb. At 18 hrs post glycerol injection, the mice were anesthetized with pentobarbital, the abdomen was opened, a blood sample for BUN/creatinine assessments was obtained from the vena cava, and the kidneys were resected. The control post glycerol group and the N-Mgb+SnPP pre-treated glycerol group had kidney sections stained with with H and E, as noted above.
Maleate model of AKI
When injected into rodents, maleate undergoes selective proximal tubule uptake via organic anion transporters and induces profound, proximal tubule-specific, ATP depletion (38,39). This culminates in severe AKI. The following experiment assessed whether N-Mgb+SnPP pre-treatment can protect against this form of renal damage. Twelve mice were divided into two equal groups, which received either N-Mgb-SnPP or vehicle injection. Eighteen hrs later, they all received an IP injection of Na maleate (600 mg/Kg) (39). Approximately 18 hrs later, the mice were anesthetized, and terminal blood samples were obtained from the inferior vena cava for BUN and creatinine measurements.
Post- ischemic AKI progression to chronic kidney disease (CKD)
Following 30 min of unilateral renal ischemia, the damaged kidney undergoes a transition to CKD, manifested by progressive tubular dropout, interstitial inflammation, and fibrosis, culminating in an approximate 40% loss of renal mass (kidney weight) (40,41). To ascertain whether N-Mgb-SnPP treatment could mitigate post- ischemic disease progression, 6 mice were pre-treated with these agents and 18 hrs later they were anesthetized with pentobarbital and subjected to 30 min of left renal pedicle occlusion performed through a midline abdominal incision at a body temperature of 37°C. The right kidney was left untouched. Following the period of renal ischemia, the vascular clamp was removed and complete reperfusion was confirmed by loss of renal cyanosis. The mice were then sutured and allowed to recover from anesthesia. Six mice, subjected to the same surgical protocol, but without N-Mgb/SnPP pre-treatment, served as controls. Two weeks later, the abdominal incision was re-opened and the kidneys were resected. Relative degrees of renal injury between the two groups was assessed by loss of left renal mass (as determined by renal wet weight) vs. weight of left kidneys from six normal mice. Renal cortical NGAL mRNA and protein levels were also assessed as additional markers of renal damage.
Dose–response relationships following intraperitoneal N-Mgb-SnPP injections
To assess whether a slower delivery rate of N-Mgb-SnPP (vs. via IV bolus injection) also induces an up-regulation of cytoprotective proteins and renal cytoresistance, mice were injected with either 0, 1, 2.5, or 5 mg of N-Mgb + the standard SnPP dose (1 umole) (3 mice each; 1 mL saline vehicle). Eighteen hrs later, the mice were anesthetized with pentobarbital, a blood sample was obtained, and the kidneys were resected to determine HO-1 and haptoglobin mRNA and protein levels. To test whether plasma HO-1/ haptoglobin levels rose and reflected degrees of renal HO-1 and haptoglobin up-regulation, plasma HO-1 and haptoglobin levels were also determined.
To assess whether the above determined plasma and kidney HO-1 and haptoglobin levels correlated with degrees of cytoresistance, additional mice were injected with either 0, 2.5, or 5 mg of N-Mgb + I umole SnPP (n, 3 mice per group). Eighteen hrs later, all mice were subjected to the glycerol AKI model, as noted above. Severities of AKI were determined 18 hrs post glycerol injection by BUN / plasma creatinine analyses.
Hepatic ischemia experiments
Fourteen mice were subjected to our previously published partial hepatic ischemia model which is conducted by occluding blood flow (at the portal triad) to three of 5 hepatic lobes x 25 min (42). Half of the mice were pre-treated 18 hrs earlier with 1 mg N-Mgb + 1 umole SnPP, as noted above. Reperfusion following the ischemic period was assessed by restoration of normal hepatic color in the three involved liver lobes. Eighteen hrs later, the mice were re-anesthetizedl, the abdominal cavity was re-opened, and a terminal blood sample was obtained for plasma ALT and LDH levels as markers of post-ischemic liver damage.
Hepatotoxic injury
To assess whether N-Mgb-SnPP can protect against a toxic form of liver injury, 12 anesthetized mice received injections of 50% glycerol. The glycerol (8 mg/Kg) was given via an IP injection to favor hepatocellular uptake via the portal circulation. Half of the mice had been pre-treated 18 hrs earlier with N-Mgb-SnPP (1 mg Mgb/1umole SnPP), as noted above. Four hrs post IP glycerol injection, while the mice were still anesthetized, they were sacrificed via transection of the abdominal vena cava. The extent of acute hepatic injury was gauged by plasma ALT concentrations.
Calculations and Statistics
All values are given as means ± 1 SEM. Statistical comparisons were made by unpaired Student’s t test. If multiple comparisons were made, the Bonferroni correction was applied. The severity of renal histologic injury in the glycerol AKI model was graded on a 1+ to 4+ scale (least to most severe tubular necrosis and cast formation observed). The histologic results were compared by Wilcoxon Rank Sum test. Statistical significance was taken as a p value of <0.05.
RESULTS
Renal function and histology following IV N-Mgb + SnPP injection
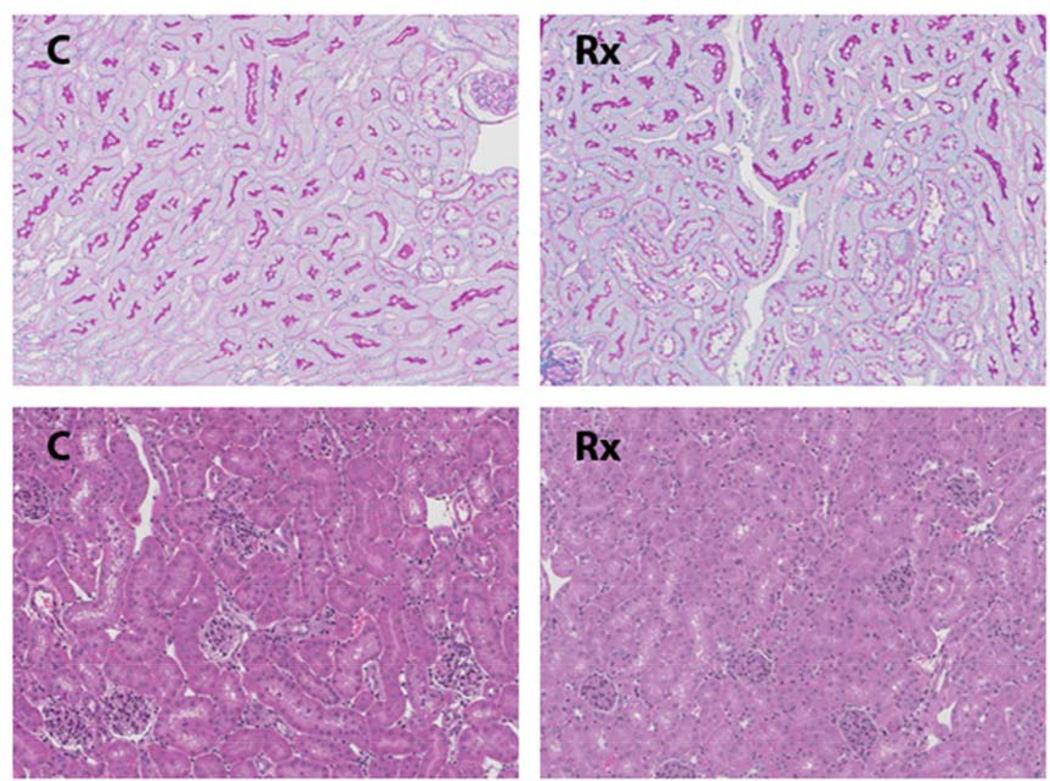
Neither BUN nor plasma creatinine elevations were observed at 18 hrs post IV injection of 1 mg N-Mgb + SnPP (BUNs 22±3 vs. 25±3 mg/dl; creatinines, 0.32±0.03 vs. 0.30±0.04 mg dl; controls vs. Mgb/SnPP treatment respectively). Furthermore, there was no evidence of renal morphologic injury, as evidenced by either PAS or H&E staining. In particular, no evidence of tubular necrosis or heme cast formation was apparent in the treatment group (see Fig.1) The proximal tubular brush border, as depicted by PAS staining, remained entirely intact (upper two panels). In this regard, brush border blebbing/sluffing into proximal tubule lumina are judged to be highly sensitive light microscopic markers of tubular damage (43,44) Thus, the above data indicated that the IV N-Mgb-SnPP treatment was well tolerated by the kidney.
Figure 1. Renal histology 18 hrs following N-Mgb-SnPP administration.
The top two panels are PAS stained kidney sections from a control mouse ( C ) and a mouse 18 hr post N-Mgb-SnPP treatment (Rx). The tubular epithelium from treated mice maintains a normal histologic appearance with a completely intact brush border (dark staining of luminal membrane). The bottom two panels depict H and E stained sections. No histologic injury is apparent with N-Mgb-SnPP pre-treatment.
Impact of IV N-Mgb and SnPP, alone and in combination, on HO-1, IL-10, and haptoglobin expression
Renal HO:1 assessments
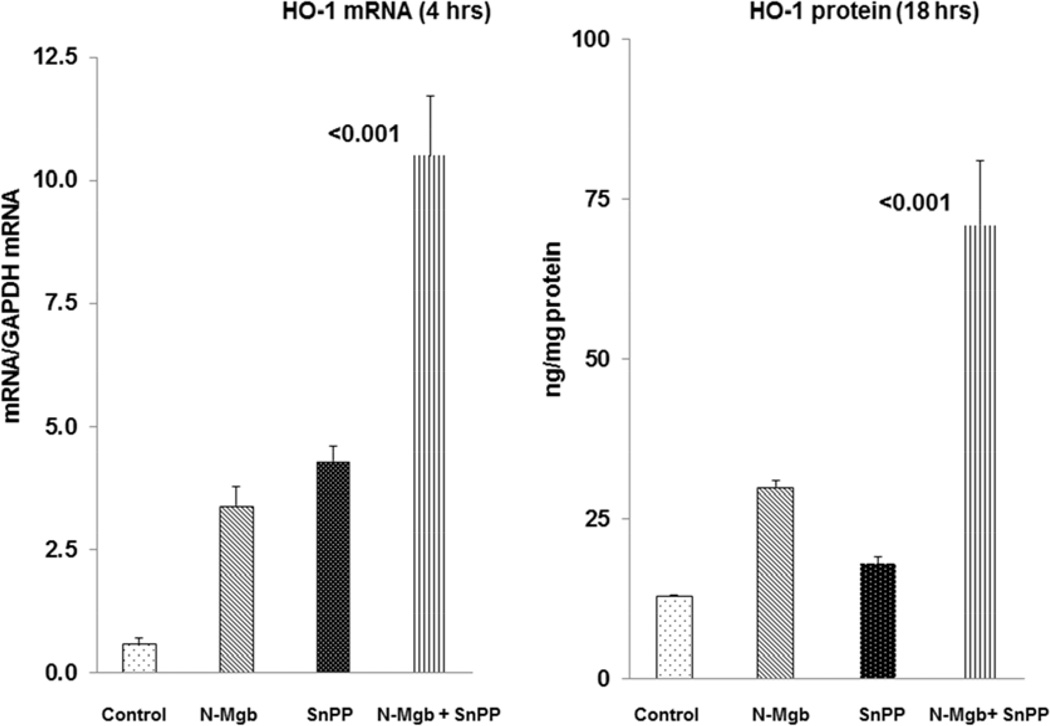
As shown in Fig. 2, left panel, N-Mgb alone and SnPP alone caused only modest increases in 4 hr HO-1 mRNA levels. In contrast, a 20 fold increase in HO-1 mRNA was observed at 4 hrs post combined N-Mgb+SnPP injection. By 18 hrs post treatment, these mRNA increases translated into marked HO-1 protein elevations, being ∼7 fold higher than control values. In contrast, only relatively small HO-1 protein increases were observed with N-Mgb alone or SnPP alone at the 18 hr time point. In sum, these 4 hr mRNA and 18 hr HO-1 protein increases indicate a synergistic effect of N-Mgb+SnPP on renal cortical HO-1 gene expression. [Additional HO-1 mRNA (18 hrs) and protein data (4 hrs) are presented in Table 1].
Figure 2. Synergistic induction of HO-1 with N-Mgb-SnPP treatment of normal mice.
The left hand panel depicts HO-1 mRNA levels 4 hrs post treatment. Whereas N-Mgb alone and SnPP alone induced modest mRNA increases, a 20 fold HO-1 mRNA increase is seen with combined agent administration. At 18 hrs post administration, a synergistic HO-1 protein increase was observed. (p values vs. controls).
Table 1.
Indvidual renal cortical mRNA and protein levels induced by the test agents administered alone or in combination at 4 hrs (Table 1a) and 18 hrs (Table 1b) post injection.
| a. Kidney mRNA and protein values 4 hrs post Rx | ||||
|---|---|---|---|---|
| Kidney mRNA | Control | N-Mgb | SnPP | SnPP+N-Mgb |
| HO-1 | 0.61±0 1 | 3.38±0.38 | 4.38±0.28 | 10.5±1.23 <0.0001 |
| Haptoglobin | 0.20±0.06 | 1.94±0.31 | 0.19±0.01 | 5.45±1.16 <0.005 |
| IL-10 | 0.56±0.20 | 1.45±0.6 | 0.39±0.15 | 5.18±1.23 <0.005 |
| Kidney Protein | Control | N-Mgb | SnPP | SnPP+N-Mgb |
| HO-1 protein | 13.3.±1.3 | 36.1±2.9 | 22.9±2.6 | 55.5±3.7 <0.001 |
| Haptoglobin | 7.9±1.9 | 17.6±1 | 13.1±1.3 | 18.9±2.7 <0.001 |
| IL-10 | 304±29 | 265±54 | 550±86 | 838±63 <0.001 |
| b. Kidney mRNA and protein values 18 hrs post Rx | ||||
|---|---|---|---|---|
| Kidney mRNA cortex |
Control | N-Mgb | SnPP | SnPP+N-Mgb |
| HO-1 | 0.61±0 1 | 0.58±0.18 | 0.63±0.06 | 1.45±0.61 (NS) |
| Haptoglobin | 0.20±0.06 | 0.24± 0.04 | 0.23±0.08 | 0.4±0.19 (NS) |
| IL-10 | 0.56±0.20 | 0.43±0.10 | 0.18±0.09 | 0.57±0.13 (NS) |
| Kidney Protein | Control | N-Mgb | SnPP | SnPP+N-Mgb |
| HO-1 | 13.3±1.3 | 30±6 | 18±2 | 71±10 <0.001 |
| Haptoglobin | 10.3±2.7 | 183.8±20.4 | 53.5±17.2 | 203±8 <0.0001 |
| IL-10 | 304±29 | 292±54 | 454±51 | 840±60 <0.001 |
Renal IL-10 assessments
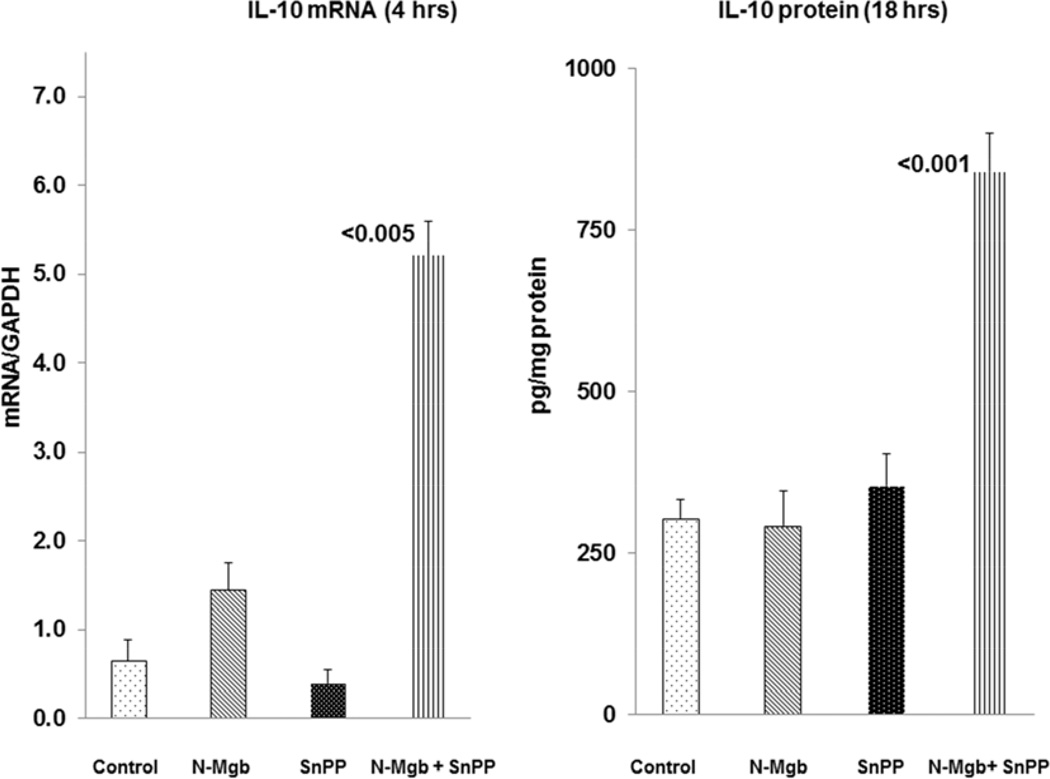
As shown in Fig 3, left, N-Mgb alone and SnPP alone had either minimal or no impact on IL-10 mRNA levels at the 4 hr time point. In contrast, combined N-Mgb+SnPP caused a 10 fold IL-10 mRNA increase at 4 hrs post injection. Corresponding with these results was the absence of IL-10 protein increases at 18 hrs post N-Mgb alone or SnPP injection alone. Conversely, a >2 fold increase in IL-10 protein was seen at 18 hrs post combined N-Mgb-SnPP injection. [Additional IL-10 mRNA (18 hrs) and protein data (4 hrs) are presented in Table 1].
Figure 3. Synergistic induction of IL-10 with N-Mgb-SnPP treatment of normal mice.
The left hand panel depicts IL-10 mRNA levels at 4 hrs post treatment. N-Mgb alone and SnPP alone evoked either minimal or no IL-10 mRNA increases. However, with combined administration, an approximate 10 fold IL-10 mRNA increase was observed. As shown in the right hand panel, only the combined treatment induced IL-10 protein increases, as assessed at the 18 hr time point. (p values vs. controls).
Renal cortical haptoglobin assessments
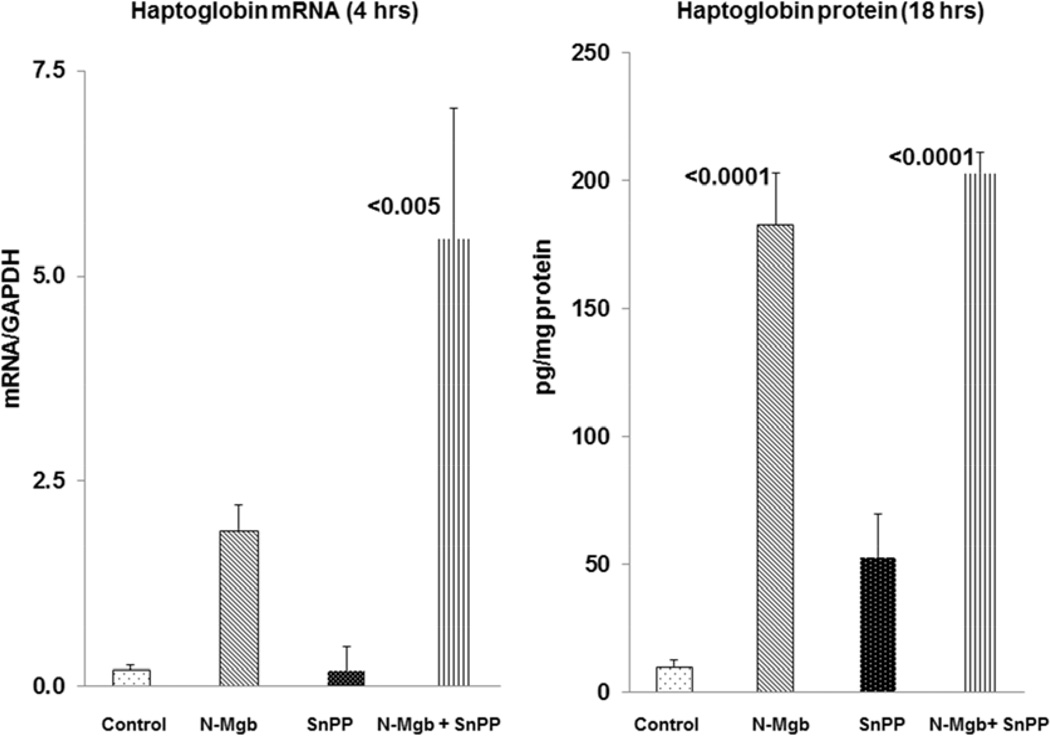
As with HO-1 and IL-10, the combination of N-Mgb+SnPP induced the greatest haptoglobin mRNA increases at 4 hrs post agent injection (Fig. 4). At 18 hrs post injections, a massive (∼20 fold) increase in renal cortical haptoglobin protein levels was observed in response to N-Mgb-SnPP injection. However, the haptoglobin protein levels were comparably increased with N-Mgb alone at the 18hr time point.. This implies that it was the N-Mgb component of the combined therapy that drove the 18 hr haptoglobin protein elevations. [Given this massive increase, it is conceivable that no added increase could be induced by the combination therapy]. Additional haptoglobin mRNA (18 hrs) and protein data (4 hrs) are presented in Table 1.
Figure 4. Haptoglobin mRNA and protein expression following N-Mgb-SnPP administration to normal mice.
Combined N-Mgb + SnPP evoked far greater haptoglobin mRNA increases at 4 hrs post injection than did either agent alone. However, by 18 hrs post agent administration, N-Mgb alone and N-Mgb+SnPP induced comparable haptoglobin protein increases. This suggests that N-Mgb accounted for the haptoglobin increases that were produced by combined N-Mgb + SnPP administration. (p values vs. controls).
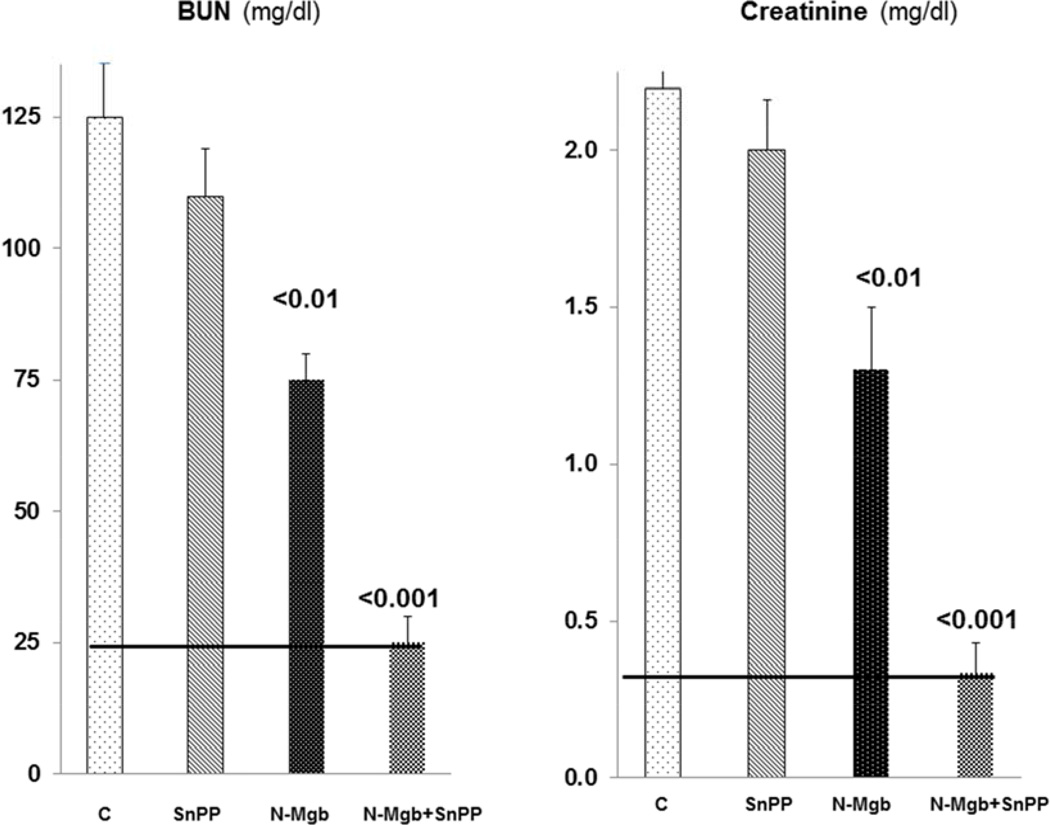
Impact of IV N-Mgb alone, SnPP alone, and N-Mgb+SnPP on the severity of the glycerol AKI model
As shown in Fig. 5, pre-treatment with SnPP alone had virtually no effect on the severity of glycerol- induced AKI. N-Mgb alone induced modest protection, as judged by BUN/creatinine levels. However, when N-Mgb+SnPP were used together, complete functional protection was observed (BUN/creatinine levels remained at normal values). Coinciding histologic protection was also observed (3.5±0.25 vs. 1.25±0.25 histologic scores for the glycerol vs. the N-Mgb+SnPP treated group; p<0.05). In this regard, the untreated glycerol group manifested widespread tubular necrosis and cast formation, as previously depicted (42). These changes were virtually absent in the N-Mgb+SnPP pre-treatment group.
Figure 5. Degrees of protection induced by test agents in the glycerol model of rhabdomyolysis- induced AKI.
Control mice ( C ) developed marked AKI, as denoted by BUN and creatinine concentrations. SnPP conferred no significant protection, whereas N-Mgb induced a modest protective effect. Conversely, combined N-Mgb-SnPP administration conferred complete functional protection, as gauged by normal BUN and creatinine levels at 18 hrs post glycerol administration (normal levels are depicted by the solid horizontal line).
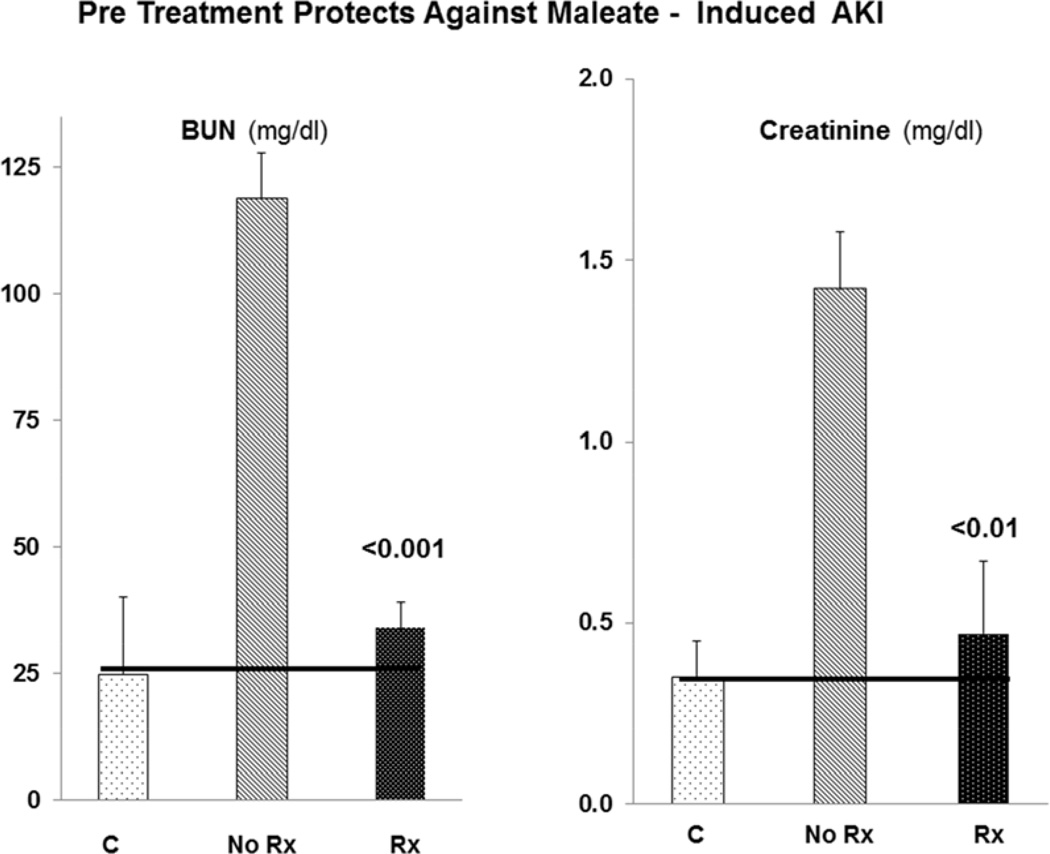
Maleate AKI model
As depicted in Fig. 6, maleate injection caused severe renal injury, as denoted by marked BUN / creatinine increases. Pre-treatment with N-Mgb + SnPP conferred almost complete protection against this injury, as denoted by near normal BUN/creatinine levels.
Figure 6. Combined N-Mgb + SnPP (“Rx”) confers marked protection against the maleate model of AKI.
Maleate injection caused marked BUN and creatinine increases. Combined N-Mgb + SnPP conferred a near complete protective effect (horizontal lines represent normal BUN and creatinine concentrations). P value vs. no treatment (no Rx).
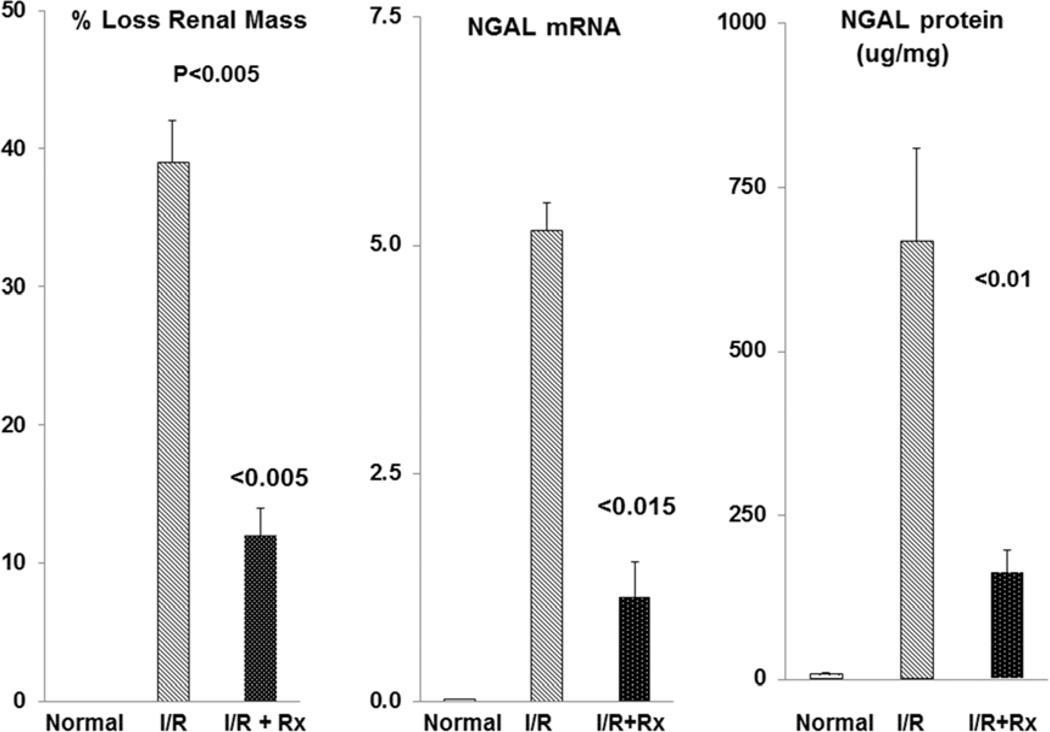
Post-ischemic AKI model
By 2 weeks post ischemia, a 38% reduction in post-ischemic left renal mass was observed in the control unilateral ischemia mice (Fig. 7). In contrast, only a 12% reduction was seen in the mice which had received prophylactic N-Mgb+SnPP treatment (p<0.005). This reduction in renal injury was also denoted by marked reductions in NGAL mRNA and protein levels in the N-Mgb-SnPP pre-treatment group ( Fig. 7).
Figure 7. N-Mgb+SnPP (Rx) treatment confers protection against unilateral ischemia-reperfusion (I/R)- induced progressive kidney disease.
Mice were subjected to 30 min of left renal ischemia with or without N-Mgb-SnPP pre-treatment 18 hrs earlier. At 2 weeks post ischemia, I/R led to a 38% reduction in renal mass (gauged by kidney weight). Pre-treatment (Rx) conferred significant protection, as gauged by only a 12% reduction in renal mass. Protection was also implied by marked reductions in NGAL mRNA and protein levels in the 2 week post ischemic left kidneys.
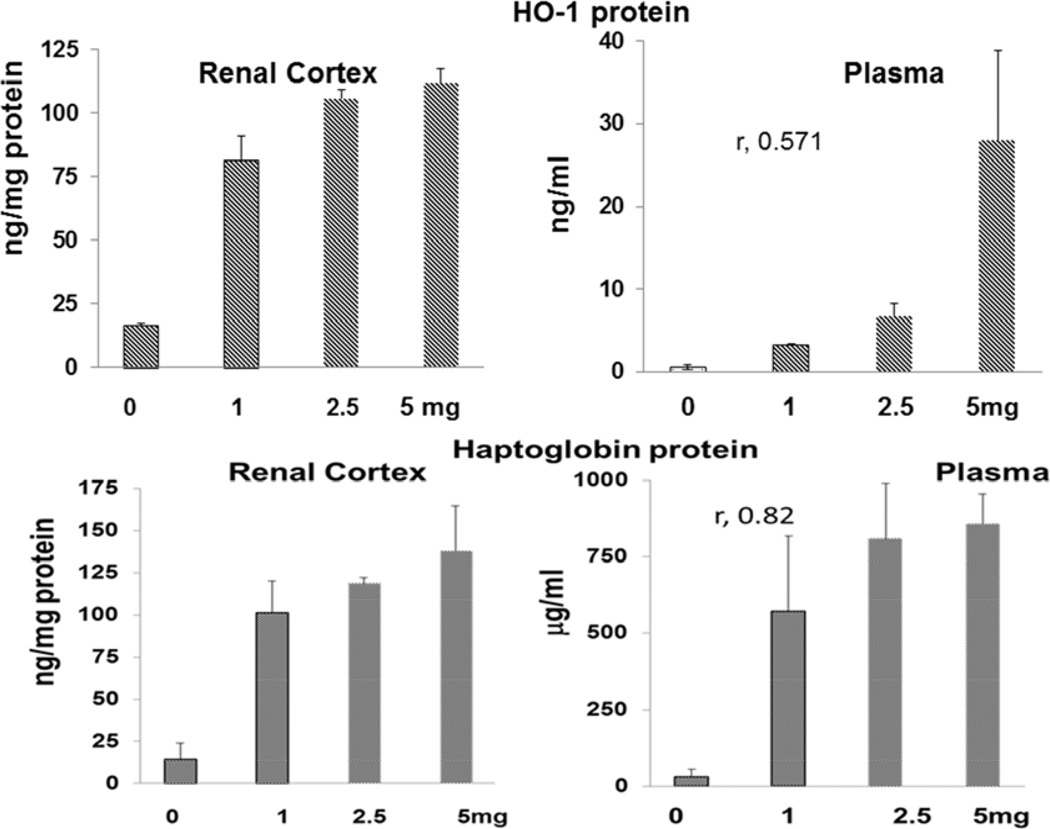
Dose - response relationships between renal cortical and plasma HO-1 / haptoglobin levels and degrees of protection against glycerol-induced AKI
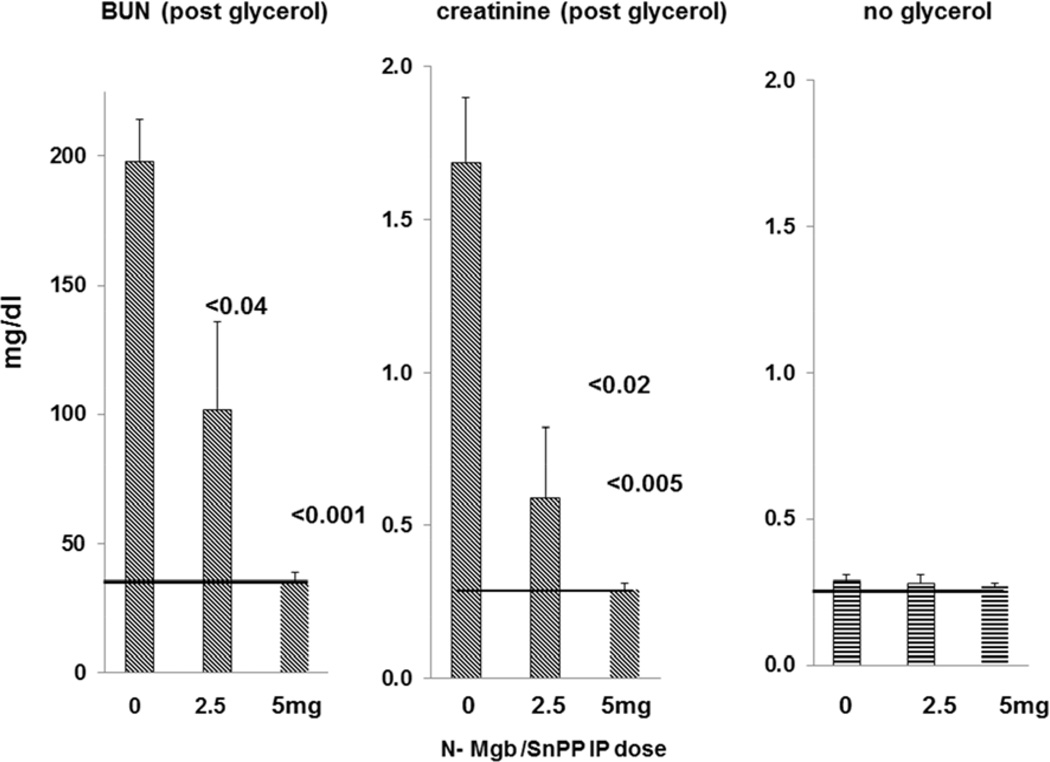
As shown in Fig. 8, progressive increases in IP N-Mgb dosages into normal mice produced progressive increases in renal cortical and plasma HO-1 and haptoglobin levels. Significant correlations between plasma and renal cortical HO-1 and haptoglobin levels were observed (e.g. r, 0.82 between renal cortical and plasma haptoglobin levels). Furthermore, these increasing N-Mgb doses were associated with progressive (50%; 100%) protection against the glycerol AKI model (Fig. 9). Thus, these data imply that the degree of plasma HO-1 or haptoglobin increases can serve as biomarkers of N-Mgb-SnPP- induced renal gene induction and the degrees of resistance to subsequent ARF. Despite administering 5 mg of N-Mgb (+ standard SnPP dose), no evidence renal injury (normal BUNs, creatinines; see figure 9, right) was observed.
Figure 8. Dose - response relationship between increasing doses of N-Mgb (with a fixed dose of SnPP, 1 umole) and HO-1 / haptoglobin protein levels in renal cortex and plasma.
Increasing doses of N-Mgb, administered IP, led to increasing levels of each protein in plasma and renal cortex. The correlation coefficients for plasma vs. renal concentrations were 0.57 and 0.82 for HO-1 and haptoglobin, respectively. The correlations between the dose administered and plasma protein levels were r, 0.85 and r, 0.75 for HO-1 and haptoglobin, respectively.
Figure 9. Dose – response relationship between administered IP dose of N-Mgb vs. BUN / creatinine concentrations at 18 hrs post glycerol administration.
Increasing doses of N-Mgb (with a fixed dose of SnPP; 1 µmole) led to increasing degrees of protection against glycerol- induced AKI. When analyzed with the results given in Figure 8, strong direct relationships between plasma and renal cortical HO-1 / haptoglobin concentrations and. degrees of protection against glycerol- induced AKI are apparent. The horizontal lines indicate normal BUN and creatinine concentrations. As shown at the right, the administered doses were well tolerated by the kidney, as evidenced by maintenance of normal 18 hr plasma creatinine concentrations across the tested dosage range.
Liver assessments
Hepatic HO-1, IL-10, and haptoglobin expression in liver in response to N-Mgb/SnPP injections
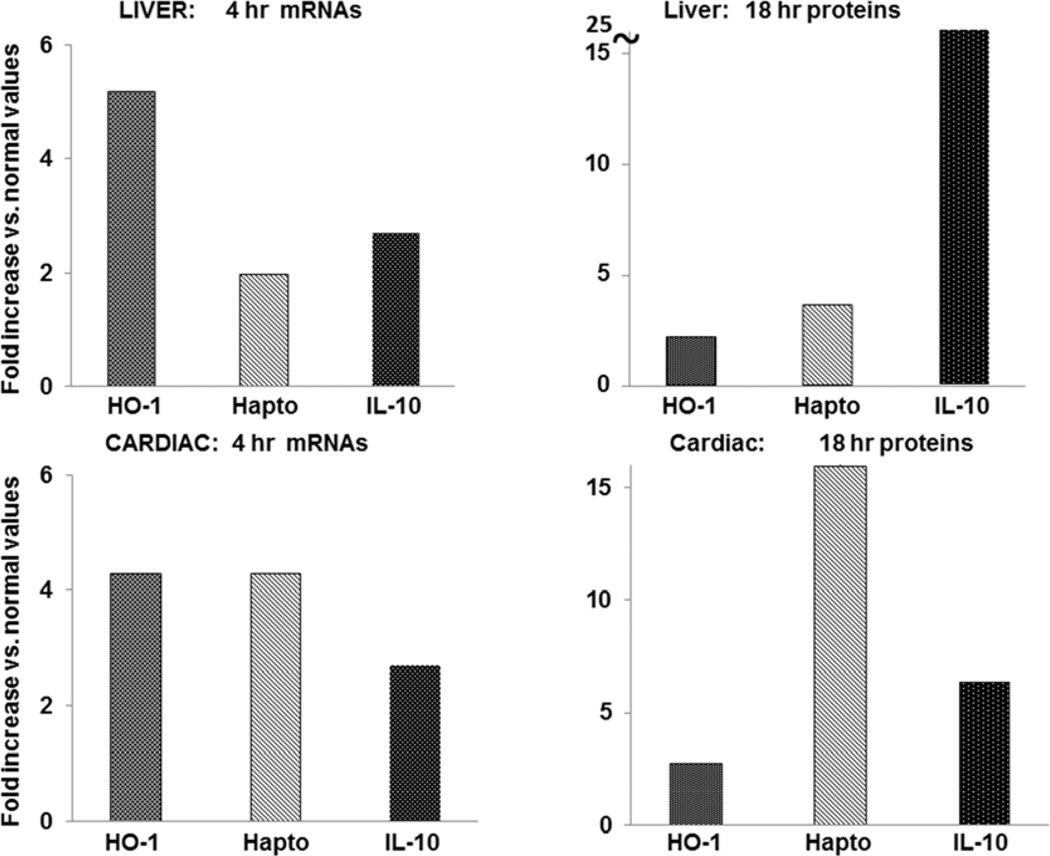
The fold increases over control values for HO-1, IL-10 and haptoglobin mRNAs (4 hrs) and for protein levels (18 hrs) are depicted in Fig. 10, top. Marked increases in each were observed. Individual values for each agent alone or in combination are given in Table 2. At 4 hrs post injection, hepatic HO-1, IL-10, and haptoglobin mRNA levels were significantly higher with combined N-Mgb+SnPP injection vs. either agent alone (Table 2). This translated into greater hepatic HO-1, IL-10 and haptoglobin protein increases, as assessed at the 18 hr time point (p<0.001 for each protein, vs control tissues).
Figure 10. Up-regulation of HO-1, haptoglobin (hapto), and IL-10 gene expression in liver and heart with combined N-Mgb/SnPP treatment.
The degrees of elevation with treatment, expressed as a fold increase over control values are presented. Each was increased in both liver (top two panels) and heart (bottom two panels). Individual values are given in Tables 2 and 3.
Table 2.
Indvidual liver mRNA and protein levels induced by the test agents administered alone or in combination at 4 hrs (Table 1a) and 18 hrs (Table 1b) post injection.
| a: Liver mRNA and protein levels 4 hrs post Rx | ||||
|---|---|---|---|---|
| Liver mRNA 4 hrs post Rx |
control | N-Mgb | SnPP | SnPP+N-Mgb |
| HO-1 | 1.1±0.12 | 1.83±0.33 | 3.15±.25 | 5.66±0.29 <0.001 |
| Haptoglobin | 0.5±0.1 | 0.98±0.1 | 0.32±0.02 | 1.01±0.12 <0.01 |
| IL-10 | 0.06±0.0.02 | 0.67±0.46 | 0.14±0.1 | 1.12±0.64 <0.001 |
|
Liver protein 4 hrs post Rx |
control | N-Mgb | SnPP | SnPP+N-Mgb |
| HO-1 protein | 10.2±1.2 | 17±2 | 7.4±0.7 | 15.1±1.4 <0.025 |
| Haptoglobin | 115.3±9.6 | 359.5±39.6 | 130.0±8.9 | 351.0±40.7 <0.001 |
| IL-10 | 314±36 | 286±32 | 485±47 | 412±34 =0.1 |
| b: Liver mRNA and protein levels 18 hrs post Rx | ||||
|---|---|---|---|---|
|
Liver mRNA 18 hrs post Rx |
control | N-Mgb | SnPP | SnPP+N-Mgb |
| HO-1 | 1.1 ±0.12 | 0.82±0.07 | 2.56±0.21 | 2.86±0.4 <0.001 |
| Haptoglobin | 0.5±0.1 | 0.91±0.07 | 0.65±0.17 | 1.19±0.4 <0.05 |
| IL-10 | 0.06±0.02 | 0.06±0.02 | 0.05±0.01 | 0.06±0.02 (NS) |
|
Liver protein 18 hrs post Rx |
control | N-Mgb | SnPP | SnPP+N-Mgb |
| HO-1 protein | 10.2±1.2 | 14.9±0.3 | 18±2.5 | 22.9±1.2 <0.0001 |
| Haptoglobin | 115.3±9.6 | 358.8±32.8 | 212.0±63.6 | 410±64.3 <0.001 |
| IL-10 | 314±36 | 5988±549 | 8198±725 | 8015±225 <0.001 |
Hepatic ischemic model
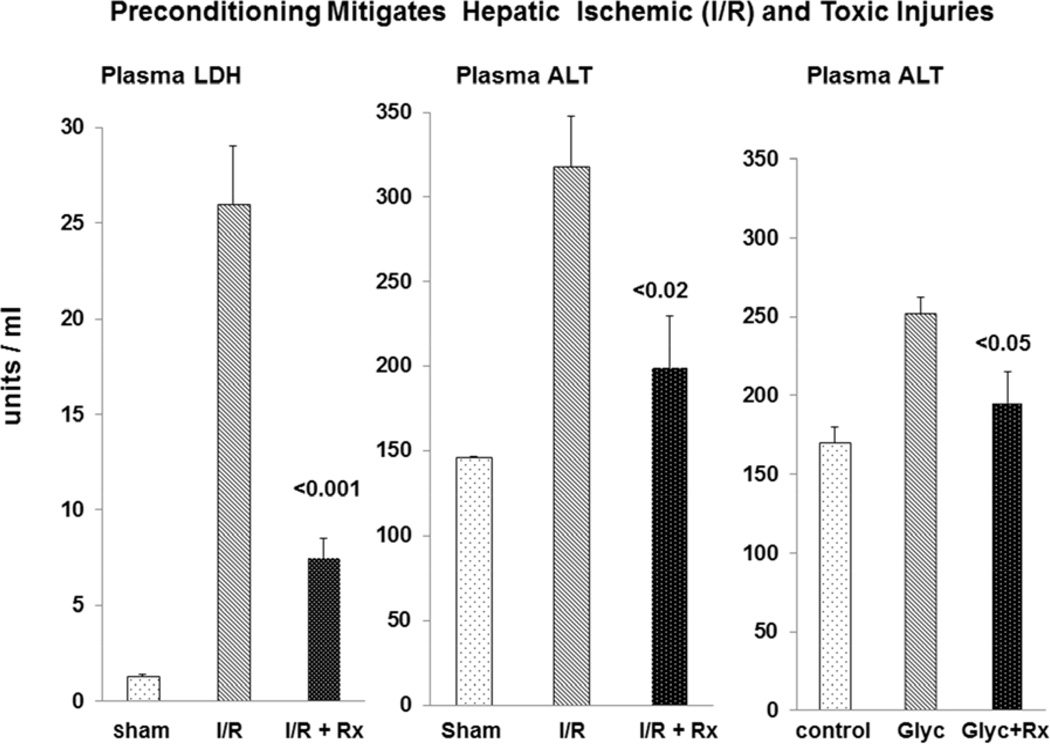
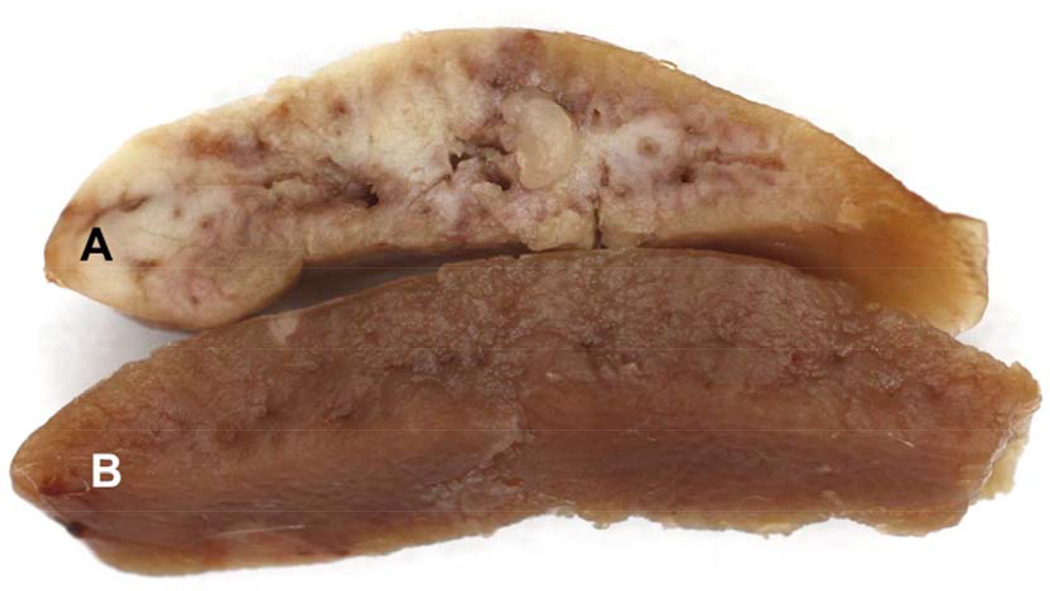
Hepatic ischemia induced marked elevations in plasma ALT and LDH concentrations (see Fig. 11). The LDH and ALT increases were reduced by ∼75% and 50% respectively with N-Mgb+SnPP pre-treatment (corresponding with the hepatic HO-1, haptoglobin, and IL-10 protein increases; Table 2). As shown in Fig. 12, widespread necrosis was observed in gross sections of post ischemic liver (A). Pre-treatment with N-Mgb+SnPP led to a much more normal gross hepatic appearance (B).
Figure 11. Preconditioning with N-Mgb-SnPP mitigates post- ischemic liver injury and hepatotoxic injury.
Degrees of hepatic ischemic-reperfusion injury were judged by plasma lactate dehydrogenase (LDH) and alanine aminotransferase (ALT) levels. Pre-treatment with N-Mgb-SnPP significantly decreased both LDH and ALT concentrations (left and middle panels). It also decreased the extent of hepatotoxic injury induced by intraperitoneal glycerol injection (right hand panel).
Figure 12. Gross appearance of liver sections obtained at 18 hr post ischemia without (A) and with (B) N-Mgb-SnPP pre-treatment.
Liver ischemia evoked extensive gross necrosis, as evidenced by whitish-grey liver appearance (A). However, with N-Mgb-SnPP pre-treatment, the liver retained a near normal appearance (B).
Hepatotoxic injury
As shown at the right hand panel of Fig 11, N-Mgb+SnPP treatment also reduced the extent of IP glycerol- induced liver injury, as assessed by plasma ALT levels.
Cardiac HO-1, IL-10, and haptoglobin mRNA and protein levels
As shown at the bottom of Fig. 10, combined N-Mgb + SnPP induced 3–4 fold increases in HO-1, haptoglobin, and IL-10 mRNAs at 4 hrs, and up to 3–15 fold increases in their protein levels at the 18 hr time point. Individual values are given in Table 3. In general, far greater mRNA and protein increases were observed with combined agent administration vs. either agent alone.
Table 3.
Indvidual cardiac mRNA and protein levels induced by the test agents administered alone or in combination at 4 hrs (Table 1a) and 18 hrs (Table 1b) post injection.
| a: Cardiac mRNA and protein levels 4 hrs post Rx | ||||
|---|---|---|---|---|
| Cardiac mRNA 4 hrs post Rx |
Control | N-Mgb | SnPP | SnPP+N-Mgb |
| HO-1 | 0.07±0.01 | 0.09±0.01 | 0.14±0.01 | 0.3±0.01 <0.001 |
| Haptoglobin | 0.31±.0.07 | 0.75±0.10 | 0.52±0.21 | 1.32±0.22 <0.002 |
| IL-10 | 0.36±0.10 | 0.86±0.11 | 0.58±0.16 | 0.96±0.22 <0.04 |
|
Cardiac protein 4 hrs post Rx |
control | N-Mgb | SnPP | SnPP+N-Mgb |
| HO-1 | 1.50±08 | 1.64±0.19 | 1.310.13 | 1.54±0.09 (NS) |
| Haptoglobin | 15.6±5.0 | 40.4±8.6 | 19.1±2.1 | 29.4±4.1 <0.1 |
| IL-10 | 8.1 ±5 | 21±12 | 15.8±10 | 41±14 <0.035 |
| b: Cardiac mRNA and protein levels 18 hrs post Rx | ||||
|---|---|---|---|---|
| Cardiac mRNA 18 hrs post Rx |
control | N-Mgb | SnPP | SnPP+N-Mgb |
| HO-1 | 0.07±0.01 | 0.08±0.01 | 0.09±0.03 | 0.24±0.07 <0.005 |
| Haptoglobin | 0.31±.0.07 | 0.53±0.21 | 0.59±0.17 | 1.28±0.2 <0.002 |
| IL-10 | 0.36±0.10 | 0.67±0.10 | 0.53±0.10 | 0.64±0.14 =0.1 |
|
Cardiac protein 18 hrs post Rx |
control | N-Mgb | SnPP | SnPP+N-Mgb |
| HO-1 | 1.5±0.08 | 1.9.7±0.37 | 2.67±0.4 | 4.2±.98 <0.01 |
| Haptoglobin | 15.6±5.0 | 249.6±15.0 | 96.1±36.2 | 250.7±37.8 <0.001 |
| IL-10 | 8±5 | 14.5±10 | 23.4±14 | 51.4±19 <0.035 |
DISCUSSION
In 1992, Nath et al (14) demonstrated that hemoglobin administration in the rat can induce marked protection against subsequent (24 hrs later) glycerol-mediated rhabdomyolysis-induced ARF. This protective response was ascribed to heme- mediated HO-1 up-regulation, based on two pivotal observations: 1) heme pre-treatment markedly increased renal HO-1 mRNA, protein, and enzyme activity; and 2) the glycerol model was markedly worsened by administering the potent HO-1 inhibitor, SnPP, at the time of (and after) glycerol injection. Since these seminal observations, the role of HO-1 as a potent anti-oxidant / anti-inflammatory molecule has been well established in multiple models of AKI (e.g. cisplatin, renal ischemia, endotoxemia; reviewed in ref. 45). Furthermore, its protective effects have been extensively described in diverse forms of extra-renal tissue damage (e.g., brain, liver, heart, organ transplantation) (46–52). However, less certain than the existence of HO’s protective actions is the exact mechanism by which that protection is effected. Because HO-1 cleavage of the prophyrin ring releases highly toxic catalytic Fe (which exerts direct adverse effects) (30), it is now believed that secondary consequences of increased HO-1 activity are involved. These include the generation of the anti-oxidants biliverdin and bilirubin, cytoprotective carbon monoxide production, and increased tissue (H) ferritin levels, with its great capacity for catalytic Fe binding (13,45). Additional complexities in interpreting HO-1 involvement in cytoprotection stem from the fact that HO-1 inducers (e.g. heme), also up-regulate a number of other cytoprotective pathways, e.g., haptoglobin (20), hemopexin (15), alpha 1 antitrypsin (21), and IL-10 (as shown in the current report). This complicates interpretation of HO-1 effects on tissue injury given the presence of multiple up-regulated tissue protective proteins.
The interplay of SnPP and HO-1 is also complex. First, as a competitive inhibitor of HO-1, SnPP administration can secondarily increase HO-1 mRNA and protein levels either by enzyme ‘feedback inhibition’ or by the induction of a mild pro-oxidant state with counterbalancing HO-1 production (e.g. ref. 36). Second, the Sn moiety of SnPP may independently up- regulate HO-1 via direct pro-oxidant effects (53). Third, whenever considering the effects of SnPP, it is important to recognize that secondary HO-1 induction could potentially be offset by SNPP- induced HO-1 inhibition. However, it is noteworthy that SnPP has a relatively short half-life (∼2–4 hrs; ref. 54). Thus, delayed HO-1 increases (e.g. 18 hrs post glycerol administration or N-Mgb-SnPP treatment) should be free to exert its biologic effects due to prior SnPP elimination. This concept is supported by observations that at 24 hrs post SnPP administration, up-regulated HO-1 was able to exert a cytoprotective effect (e.g., against ischemic ARF; ref. 36, 37).
In light of the above considerations, we hypothesized that a combination of N-Mgb + SnPP might induce either additive, or synergistic, increases in HO-1, as well as in other redox sensitive cytoprotective proteins (e.g., haptoglobin, IL-10). We tested this hypothesis by measuring HO-1, haptoglobin, and IL-10 mRNA and protein levels at 4 and 18 hrs post N-Mgb, SnPP, or N-Mgb+SnPP injection. As shown in figures 2–4, combined therapy generally induced synergistic or additive responses. For example, at 4 hrs post injection, a 20 fold increase in HO-1 mRNA was observed, more than doubling the increases seen with either N-Mgb or SnPP alone. At 18 hrs, this early mRNA increase translated into ∼7 fold HO-1 protein increases. Qualitatively similar results were observed with IL-10. Particularly noteworthy was a massive (20 fold) increase in haptoglobin protein at 18 hrs post N-Mgb-SnPP administration. However, in this case, it appeared that it was N-Mgb, rather than an N-Mgb-SnPP interaction, was largely responsible, given that N-Mgb alone vs. N-Mgb+SNPP induced comparable renal haptoglobin increases. Clearly, additional cytoprotective redox sensitive proteins, other than HO-1, IL-10 and haptoglobin, may also have been induced by our N-Mgb-SnPP protocol (e.g. α1 antitrypsin, hemopexin, hepcidin) (11–23). Thus, it seems logical that multiple cytoprotective proteins could act in concert to mitigate cell injury responses.
Having observed dramatic renal cortical increases in cytoprotective proteins following N-Mgb-SnPP administration, we tested the latter’s effectiveness in protecting against three forms of AKI. Figure 5 depicts the results in the glycerol model. As shown, SnPP administration alone induced no significant reductions in BUN or creatinine levels. When N-Mgb alone was administered, a modest protective effect was observed. However, when administered together, N-Mgb + SnPP pre-treatment evoked essentially complete functoinal protection, as indicated by normal BUN and plasma creatinine concentrations at 18 hrs post glycerol injection. Furthermore, near normal renal histology was observed. Thus, these findings underscore the principle that synergistic increases in cytoprotective proteins can translate into synergistic protection against ARF.
To further explore the scope of N-Mgb-SnPP mediated protection, we utilized the maleate model of proximal tubular ATP depletion- mediated ARF. Again, dramatic / near complete protection was observed (Fig. 6). Because it is now well recognized that AKI can initiate the onset of progressive chronic renal disease, we assessed whether N-Mgb-SnPP pre-treatment could abrogate this process in our previously published unilateral post ischemic renal injury model (41,42) in which an approximate 40% loss of renal mass normally results in 2 weeks. As shown in Fig. 7, post ischemic injury was markedly attenuated with N-Mgb-SnPP pre-treatment, as evidenced by a reduction in renal mass loss from 38% to 12%, and marked reductions in NGAL mRNA and protein levels. Thus, in each of three heterogeneous AKI models, dramatic protection was observed.
Although the kidney has the largest exposure to the two test agents (e.g. via rapid filtration, Mgb endocytosis), virtually all cells are transiently exposed to them following their IV injection. Furthermore, protoporphyrins can bind to and be taken up by a variety of cells (55). Thus, we questioned whether our N-Mgb-SnPP regimen might also up regulate protective responses in extra-renal organs. Indeed, this was the case, given that both hepatic and cardiac tissues manifested HO-1, IL-10 and haptoglobin mRNA/protein increases at both 4 and 18 hrs post N-Mgb-SnPP injection (as presented in Tables 2 and 3). That N-Mgb alone could induce a response in extra-renal tissues was surprising, given that megalin-cubulin mediated endocytosis is thought to be a renal specific pathway. This either suggests potential extra-renal uptake, possibly via scavenger receptors (56), or that while present in the microcirculation, N-Mgb and SnPP are able to activate intracellular cytoprotective genes. To test whether extra-renal protection might, we assessed the impact of N-Mgb +SnPP pre-treatment on the extent of post-ischemic hepatic injury. As presented in Fig. 10, marked reductions in both LDH and ALT plasma concentrations were observed. Furthermore, obvious protection was indicated by the gross appearance of post ischemic hepatic tissues (Fig. 11). To further test for hepatic resistance to injury, mice were subjected to an IP injection of glycerol, which upon reaching the liver through the portal circulation, induces modest hepatic damage. Within 4 hrs of IP glycerol injection, increases in plasma ALT resulted, which were largely abrogated by prior N-Mgb-SnPP injection. We have not yet accessed whether N-Mgb-SnPP treatment also induces protection against myocardial ischemia due to the complexity of mouse myocardial ischemia models (57). Thus, the full extent of N-Mgb-SnPP’s protective actions remain subjects for future investigation.
We have previously demonstrated that plasma levels of either haptoglobin or HO-1 can serve as biomarkers of renal cortical haptoglobin and HO-1 increments in the setting of ARF (20,58). Thus, we questioned whether plasma haptoglobin and HO-1 might also serve as biomarkers for induction of these proteins in kidney following N-Mgb-SnPP administration and for the emergence of the cytoresistant state. This indeed was the case. As shown in Figs. 8, increasing doses of N-Mgb-SnPP induced dose dependent increases in plasma haptoglobin and HO-1 levels, and these increases directly correlated with renal cortical haptoglobin and HO-1 content. Furthermore, striking inverse relationships between N-Mgb-SnPP-induced plasma HO-1 / plasma haptoglobin increases, and post glycerol BUN concentrations were observed (r, −0.79 / r, −0.71 for BUN vs. plasma HO-1 / haptoglobin levels, respectively). Thus, plasma HO-1 / haptoglobin increments would likely confirm biologic activity of N-Mgb-SnPP in clinical trials, and the degrees of HO-1 / haptoglobin plasma elevation might also be predictve of degrees of resistance to subsequent AKI.
A obvious concern with applying this prophylactic strategy in patients is potential renal and/or extra-renal toxicities. However, in this regard, it is noteworthy that SnPP has already been shown to be well tolerated in humans (e.g., ref 54,59,60). Furthermore, we have previously documented in a cell culture system that nitrition markedly reduces myoglobin’s cytotoxic effects by approximately 75% (unpublished data). To assess the potential in vivo margin of safety for N-Mgb + SnPP, in data not shown, we tested the maximal amount of N-Mgb that could be given to mice before nephrotoxicity was observed. Up to 25 times the employed N-Mgb dose (with a constant SnPP dose) could be administered (over 2 hrs) without induction of nephrotoxicity (normal BUNs and creatinines, 18 hrs later). Finally, neither our standard N-Mgb-SnPP dosage (1 mg N-Mgb / 1 umole SnPP), nor 5 mg IP N-Mgb (Fig. 9), induced overt renal injury (18 hr BUN/creatinines, or histology), nor did it raise hepatic ALT or cardiac troponin levels. In concert, these data suggest the potential for clinical application, assuming a similar safety profile exists in normal human subjects and in patients with pre-existent renal disease.
Despite the extensive studies reported above, important questions remain to be addressed. A partial list of questions includes the following: First, can N-Mgb-SnPP be used as a therapeutic, and not just a prophylactic agent, such that its administration after the induction of AKI can mitigate disease progression or enhance renal recovery rates? Second, given that N-Mgb-SnPP up-regulates a number of cytoprotective proteins, which one(s) are dominant in mediating the above described protective effects? Third, what is the full scope of renal and extra-renal tissue injuries that can be prevented? For example, might N-Mgb-SnPP mitigate radiocontrast toxicity as well as myocardial ischemia-reperfusion injury during balloon angioplasty or cardiopulmonary bypass surgery? Fourth, would there be potential applications for tissue protection in the setting of renal or extra-renal transplantation? These are just some of the basic and translational issues that remain to be defined.
In conclusion, administration of nitrited myoglobin, along with an inhibitor of its degradation (SnPP), leads to dramatic increases in a number of cytoprotective proteins in kidney. The potency of this response is indicated by observations that the documented renal HO-1 protein increases were ∼15x greater than that which has been achieved with bardoxolone methyl, a well recognized Nrf-2 mediated HO-1 inducer (61). Within 18 hrs of its administration, N-Mgb+SnPP evoked dramatic protection against three diverse models of AKI: glycerol-induced rhabdomyolysis, maleate- induced proximal tubule ATP depletion; and post-ischemic AKI progression to CKD. Surprisingly, N-Mgb+SnPP administration also induced synergistic increases in cytoprotective proteins in liver, leading to dramatic protection against hepatic ischemic-reperfusion injury and hepatotoxicity. Finally, N-Mgb + SnPP can up-regulate cytoprotective proteins in heart, suggesting the potential for cardiac protection, and thus, broad ranging cytoprotective effects. Of note, each of these responses was induced in the absence of discernable renal, hepatic, or cardiac toxicity. Thus, these data suggest that N-Mgb+SnPP co-administration could potentially develop into a clinical prophylactic strategy for protecting against both renal, as well as extra-renal, injuries, such as may result during cardiopulmonary bypass, aortic aneurysm repair, or other complex, high risk surgeries. Thus, this strategy could potentially meet a number of significant unmet clinical needs.
ACKNOWLEDGMENTS
This work was supported by a grant (DK38432) from the National Institutes of Health.
The author thanks Ms. Ali CM Johnson and Ms. Kirsten B Frostad for their superb technical support during work on this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- The author has no financial or personal relationship with organizations that could potentially be perceived as influencing the described research.
- The author has read the journal's policy on disclosure of potential conflicts of interest.
REFERENCES
- 1.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17:1135–1142. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 3.Liangos O, Wald R, O'Bell JW, Price L, Pereira BJ, Jaber BL. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1:43–51. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 4.Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, Ray JG. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302:1179–1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg A, Kogan E, Hammerman H, Markiewicz W, Aronson D. The impact of transient and persistent acute kidney injury on long-term outcomes after acute myocardial infarction. Kidney Int. 2009;76:900–906. doi: 10.1038/ki.2009.295. [DOI] [PubMed] [Google Scholar]
- 6.Honda IK, Hishida A, Ikuma K, Yonemura K. Acquired resistance to acute renal failure: Editorial Review. Kidney Int. 1987;31:1233–1238. doi: 10.1038/ki.1987.136. [DOI] [PubMed] [Google Scholar]
- 7.Zager RA, Baltes LA, Sharma HM, Jurkowitz MS. Responses of the ischemic acute renal failure kidney to additional ischemic events. Kidney Int. 1984;26:689–700. doi: 10.1038/ki.1984.204. [DOI] [PubMed] [Google Scholar]
- 8.Zager RA, Burkhart KM, Gmur DJ. Postischemic proximal tubular resistance to oxidant stress and Ca2+ ionophore-induced attack. Implications for reperfusion injury. Lab Invest. 1995;72:592–600. [PubMed] [Google Scholar]
- 9.Zager RA. Heme protein-induced tubular cytoresistance: expression at the plasma membrane level. Kidney Int. 1995;47:1336–1345. doi: 10.1038/ki.1995.189. [DOI] [PubMed] [Google Scholar]
- 10.Zager RA. Obstruction of proximal tubules initiates cytoresistance against hypoxic damage. Kidney Int. 1995;47:628–637. doi: 10.1038/ki.1995.80. [DOI] [PubMed] [Google Scholar]
- 11.Zager RA, Johnson AC, Becker K. Renal cortical pyruvate depletion during AKI. J Am Soc Nephrol. 2014;25:998–1012. doi: 10.1681/ASN.2013070791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt SM, Miyaji T, McLeroy P, Nibhanupudy B, Li S, Star RA. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int. 2001;60:2118–2128. doi: 10.1046/j.1523-1755.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 13.Zarjou A, Bolisetty S, Joseph R, Traylor A, Apostolov EO, Arosio P, Balla J, Verlander J, Darshan D, Kuhn LC, Agarwal A. Proximal tubule H-ferritin mediates iron trafficking in acute kidney injury. J Clin Invest. 2013;123:4423–4434. doi: 10.1172/JCI67867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, Rosenberg ME. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992;90:267–270. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zager RA, Johnson AC, Becker K. Renal cortical hemopexin accumulation in response to acute kidney injury. Am J Physiol 2012. 2012;303:F1460–F1472. doi: 10.1152/ajprenal.00426.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fink MP. Hemopexin: newest member of the anti-inflammatory mediator club. J Leukoc Biol. 2009;86:203–204. doi: 10.1189/jlb.0309137. [DOI] [PubMed] [Google Scholar]
- 17.Arredouani MS, Kasran A, Vanoirbeek JA, Berger FG, Baumann H, Ceuppens JL. Haptoglobin dampens endotoxin-induced inflammatory effects both in vitro and in vivo. Immunology. 2005;114:263–271. doi: 10.1111/j.1365-2567.2004.02071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blum S, Asaf R, Guetta J, Miller-Lotan R, Asleh R, Kremer R, Levy NS, Berger FG, Aronson D, Fu X, Zhang R, Hazen SL, Levy AP. Haptoglobin genotype determines myocardial infarct size in diabetic mice. J Am Coll Cardiol. 2007;49:82–87. doi: 10.1016/j.jacc.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 19.Galicia G, Maes W, Verbinnen B, Kasran A, Bullens D, Arredouani M, Ceuppens JL. Haptoglobin deficiency facilitates the development of autoimmune inflammation. Eur J Immunol. 2009;39:3404–3412. doi: 10.1002/eji.200939291. [DOI] [PubMed] [Google Scholar]
- 20.Zager RA, Vijayan A, Johnson AC. Proximal tubule haptoglobin gene activation is an integral component of the acute kidney injury "stress response". Am J Physiol. 2012;303:F139–F148. doi: 10.1152/ajprenal.00168.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zager RA, Johnson AC, Frostad KB. Rapid renal alpha-1 antitrypsin gene induction in experimental and clinical acute kidney injury. PLoS One. 2014 May 21;9(5):e9838. doi: 10.1371/journal.pone.0098380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunt JM, Tuder R. Alpha 1 anti-trypsin: one protein, many functions. Curr Mol Med. 2012;12:827–835. doi: 10.2174/156652412801318755. [DOI] [PubMed] [Google Scholar]
- 23.Janciauskiene SM, Nita IM, Stevens T. Alpha1-antitrypsin exerts in vitro anti-inflammatory activity in human monocytes by elevating cAMP. J Biol Chem. 2007;282:8573–8582. doi: 10.1074/jbc.M607976200. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y, Lang XB, Zhang P, Lv R, Wang YF, Chen JH. Remote ischemic preconditioning for prevention of acute kidney injury: a meta-analysis of randomized controlled trials. Am J Kidney Dis. 2014;64:574–583. doi: 10.1053/j.ajkd.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 25.Mohd H, Yasin NA, Herbison P, Saxena P, Praporski S, Konstantinov IE. The role of remote ischemic preconditioning in organ protection after cardiac surgery: a meta-analysis. J Surg Res. 2014;186:207–216. doi: 10.1016/j.jss.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Li G, Yu C, Li Y. The role of remote ischemic preconditioning on postoperative kidney injury in patients undergoing cardiac and vascular interventions. J Cardiothorac Surg. doi: 10.1186/1749-8090-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cotton FA, Wilkinson G, Murillo CA. Advanced Inorganic Chemistry. Wiley-Interscience; 1999. p. 1355. [Google Scholar]
- 28.Silaghi-Dumitrescu R, Svistunenko DA, Cioloboc D, Bischin C, Scurtu F, Cooper CE. Nitrite binding to globins: linkage isomerism, EPR silence and reductive chemistry. Nitric Oxide. 2014;42C:32–39. doi: 10.1016/j.niox.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zager RA, Foerder CA. Effects of inorganic iron and myoglobin on in vitro proximal tubular lipid peroxidation and cytotoxicity. J Clin Invest. 1992;89:989–995. doi: 10.1172/JCI115682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zager RA, Burkhart K. Myoglobin toxicity in proximal human kidney cells: roles of Fe, Ca2+, H2O2, and terminal mitochondrial electron transport. Kidney Int. 1997;51:728–738. doi: 10.1038/ki.1997.104. [DOI] [PubMed] [Google Scholar]
- 31.Rassaf T, Totzeck M, Hendgen-Cotta UB, Shiva S, Heusch G. Kelm: Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res. 2014;114:1601–1610. doi: 10.1161/CIRCRESAHA.114.303822. [DOI] [PubMed] [Google Scholar]
- 32.Totzeck M, Hendgen-Cotta UB, Luedike P. Nitrite regulates hypoxic vasodilation via myoglobin-dependent nitric oxide generation. Circulation. 2012;126:325–334. doi: 10.1161/CIRCULATIONAHA.111.087155. [DOI] [PMC free article] [PubMed] [Google Scholar]; J Clin Invest. 1992 Mar;89(3):989–995. doi: 10.1172/JCI115682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Totzeck M, Hendgen-Cotta UB, Kelm M, Rassaf T. Crosstalk between nitrite, myoglobin and reactive oxygen species to regulate vasodilation under hypoxia. PLoS One. 2014;22(9):e105951. doi: 10.1371/journal.pone.0105951. [DOI] [PMC free article] [PubMed] [Google Scholar]; 37 Rassaf T, Totzeck M, Hendgen-Cotta UB, Shiva S, Heusch G. Kelm: Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res. 2014;114:1601–610. doi: 10.1161/CIRCRESAHA.114.303822. [DOI] [PubMed] [Google Scholar]
- 34.Totzeck M, Hendgen-Cotta UB, Luedike P. Nitrite regulates hypoxic vasodilation via myoglobin-dependent nitric oxide generation. Circulation. 2012;126:325–334. doi: 10.1161/CIRCULATIONAHA.111.087155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hendgen-Cotta UB, Merx MW, Shiva S, et al. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2008;105:10256–10261. doi: 10.1073/pnas.0801336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaizu T, Tamaki T, Tanaka M, Uchida Y, Tsuchihashi S, Kawamura A, Kakita A. Preconditioning with tin-protoporphyrin IX attenuates ischemia/reperfusion injury in the rat kidney. Kidney Int. 2003;63:1393–403. doi: 10.1046/j.1523-1755.2003.00882.x. [DOI] [PubMed] [Google Scholar]
- 37.Juncos JP, Grande JP, Murali N, Croatt AJ, Juncos LA, Hebbel RP, Katusic ZS, Nath KA. Anomalous renal effects of tin protoporphyrin in a murine model of sickle cell disease. Am J Pathol. 2006;169:21–31. doi: 10.2353/ajpath.2006.051195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kellerman PS. Exogenous adenosine triphosphate (ATP) preserves proximal tubule microfilament structure and function in vivo in a maleic acid model of ATP depletion. J Clin Invest. 1993;92:1940–1949. doi: 10.1172/JCI116787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zager RA, Johnson AC, Naito M, Bomsztyk K. Maleate nephrotoxicity: mechanisms of injury and correlates with ischemic/hypoxic tubular cell death. Am J Physiol. 2008;294:F187–F197. doi: 10.1152/ajprenal.00434.2007. [DOI] [PubMed] [Google Scholar]
- 40.Zager RA, Johnson AC, Becker K. Acute unilateral ischemic renal injury induces progressive renal inflammation, lipid accumulation, histone modification, and "end-stage" kidney disease. Am J Physiol. 2011;301:F1334–F1345. doi: 10.1152/ajprenal.00431.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zager RA, Johnson AC, Andress D, Becker K. Progressive endothelin-1 gene activation initiates chronic/end-stage renal disease following experimental ischemic/reperfusion injury. Kidney Int. 2013;84:703–712. doi: 10.1038/ki.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zager RA, Johnson AC, Frostad KB. Acute hepatic ischemic-reperfusion injury induces a renal cortical "stress response," renal "cytoresistance," and an endotoxin hyperresponsive state. Am J Physiol Renal Physiol. 2014;307:F856–F868. doi: 10.1152/ajprenal.00378.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venkatachalam MA, Bernard DB, Donohoe JF, Levinsky NG. Ischemic damage and repair in the rat proximal tubule: differences among the S1, S2, and S3 segments. Kidney Int. 1978;14:31–49. doi: 10.1038/ki.1978.87. [DOI] [PubMed] [Google Scholar]
- 44.Donohoe JF, Venkatachalam MA, Bernard DB, Levinsky NG. Tubular leakage and obstruction after renal ischemia: structural-functional correlations. Kidney Int. 1978;13:208–222. doi: 10.1038/ki.1978.31. [DOI] [PubMed] [Google Scholar]
- 45.Nath KA. Heme oxygenase and acute kidney injury. Curr Opin Nephrol Hypertension. 2014;23:17–24. doi: 10.1097/01.mnh.0000437613.88158.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kusmic C, Barsanti C, Matteucci M, Vesentini N, Pelosi G, Abraham NG, L'Abbate A. Up-regulation of heme oxygenase-1 after infarct initiation reduces mortality, infarct size and left ventricular remodeling: experimental evidence and proof of concept. J Transl Med. 2014 Apr 5;12:89. doi: 10.1186/1479-5876-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Czibik G, Derumeaux G, Sawaki D, Valen G, Motterlini R. Heme oxygenase-1: an emerging therapeutic target to curb cardiac pathology. Cardiovasc Pathol. 2014;23:231–137. doi: 10.1007/s00395-014-0450-9. [DOI] [PubMed] [Google Scholar]
- 48.Sharp FR, Zhan X, Liu DZ. Heat shock proteins in the brain: role of Hsp70, Hsp 27, and HO-1 (Hsp32) and their therapeutic potential. Transl Stroke Res. 2013;6:685–692. doi: 10.1007/s12975-013-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le LL, Li XY, Meng D, Liang QJ, Wang XH, Li N, Quan J, Xiang M, Jiang M, Sun J, Chen SF. Heme oxygenase-1 mediated memorial and revivable protective effect of ischemic preconditioning on brain injury. CNS Neurosci Ther. 2013;12:963–968. doi: 10.1111/cns.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang HF, Zeng Z, Wang KH, Zhang HY, Wang S, Zhou WX, Wang ZB, Xu WG, Duan J. Heme oxygenase-1 protects rat liver against warm ischemia/reperfusion injury via TLR2/TLR4-triggered signaling pathways. World J Gastroenterol. 2013;21:2937–2948. doi: 10.3748/wjg.v21.i10.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu A, Fang H, Wei W, Dirsch O, Dahmen U. Ischemic preconditioning protects against liver ischemia/reperfusion injury via heme oxygenase-1-mediated autophagy. Crit Care Med. 2014;42:e762–e771. doi: 10.1097/CCM.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 52.Wszola M, Kwiatkowski A, Domagala P, Wirkowska A, Bieniasz M, Diuwe P, Rafał K, Durlik M, Chmura A. Preservation of kidneys by machine perfusion influences gene expression and may limit ischemia/reperfusion injury. Prog Transplant. 2014;1:19–26. doi: 10.7182/pit2014384. [DOI] [PubMed] [Google Scholar]
- 53.Barrera-Oviedo D, Carranza-Pérez MG, Candelario-Mota MT, Mendoza-Patiño N, Maldonado PD, Pedraza-Chaverrí J. Protective effect of SnCl2 on K2Cr2O7-induced toxicity in LLC-PK1 cells. Ren Fail. 2013;35:132–137. doi: 10.3109/0886022X.2012.736071. [DOI] [PubMed] [Google Scholar]
- 54.Berglund L, Angelin B, Blomstrand R, Drummond G, Kappas A. Sn-protoporphyrin lowers serum bilirubin levels, decreases biliary bilirubin output, enhances biliary heme excretion and potently inhibits hepatic heme oxygenase activity in normal human subjects. Hepatology. 1988;8:625–631. doi: 10.1002/hep.1840080331. [DOI] [PubMed] [Google Scholar]
- 55.Anderson KE, Simionatto CS, Drummond GS, Kappas A. Tissue distribution and disposition of tin-protoporphyrin, a potent competitive inhibitor of heme oxygenase. J Pharmacol Exp Ther. 1984;228:327–333. [PubMed] [Google Scholar]
- 56.Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nature Reviews. 2013;13:621–634. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- 57.Xiang SY, Vanhoutte D, Del Re DP, Xiang SY, Vanhoutte D, Del Re DP, Purcell NH, Ling H, Banerjee I, Bossuyt J, Lang RA, Zheng Y, Matkovich SJ, Miyamoto S, Molkentin JD, Dorn GW, Brown JH. RhoA protects the mouse heart against ischemia/reperfusion injury. J Clin Invest. 2011;121:3269–3276. doi: 10.1172/JCI44371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zager RA, Johnson AC, Becker K. Plasma and urinary heme oxygenase-1 in AKI. J Am Soc Nephrol. 2012;23:1048–2057. doi: 10.1681/ASN.2011121147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kappas A, Drummond GS, Manola T, Petmezaki S, Valaes T. Sn-protoporphyrin use in the management of hyperbilirubinemia in term newborns with direct Coombs-positive ABO incompatibility. Pediatrics. 1988;81:485–497. [PubMed] [Google Scholar]
- 60.Reddy P, Najundaswamy S, Mehta R, Petrova A, Hegyi T. Tin-mesoporphyrin in the treatment of severe hyperbilirubinemia in a very-low-birth-weight infant. J Perinatol. 2003;23:507–512. doi: 10.1038/sj.jp.7210943. [DOI] [PubMed] [Google Scholar]
- 61.Wu QQ, Wang Y, Senitko M, Meyer C, Wigley WC, Ferguson DA, Grossman E, Chen J, Zhou XJ, Hartono J, Winterberg P, Chen B, Agarwal A, Lu CY. Bardoxolone methyl (BARD) ameliorates ischemic AKI and increases expression of protective genes Nrf2, PPARγ, and HO-1. Am J Physiol. 2011;300:F1180–F1192. doi: 10.1152/ajprenal.00353.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]