Abstract
Recent evidence indicates that thyroid hormones may be closely linked to cognition among adults. We investigated associations between thyroid hormones and longitudinal cognitive change, within and outside of reference ranges, stratifying by sex and race. This longitudinal study used data from the Healthy Aging in Neighborhoods of Diversity Across the Lifespan (HANDLS) study, set in Baltimore City, MD, 2004-2013, on adults aged 30-64y at baseline visit, with a length of follow-up between visits 1 and 2 ranging from <1 year – 8 years; Mean±SD: 4.64±0.93. The final analytic sample sizes ranged from 1,486-1,602 participants with 1.6-1.7 visits/participant (total visits: 2,496-2,757), depending on the cognitive test. Eleven cognitive test scores spanning domains of learning/memory, language/verbal, attention, visuo-spatial/visuo-construction, psychomotor speed, executive function, and mental status were used. Mixed-effects regression models were conducted, interacting time of follow-up with several thyroid exposures. Whites performed better than African-Americans, with only four cognitive test scores of eleven declining significantly over-time. Importantly, above reference range thyroid stimulating hormone (vs. reference range, TSHarr) was linked to faster rates of decline on the Digits Span Backwards test, reflecting working memory (TSHarr×Time γ±SE:-0.14±0.05, P=0.006) and clock-command, a test of visuo-spational/visuo-construction abilities (TSHarr×Time γ±SE:-0.10±0.04, P=0.004). The latter finding was replicated when comparing normal thyroid function to “subclinical hypothyroidism”. Within reference ranges, a higher TSH was related to faster decline on the clock-command test scores in women. In sum, higher baseline TSH was associated with faster cognitive decline over-time among urban US adults, specifically in domains of working memory and visuo-spatial/visuo-construction abilities.
Keywords: Thyroid hormones, cognitive function, longitudinal studies, aging
Introduction
Cognitive impairment, a principal cause for functional disability among the elderly, can lead to dementing illness over time mainly in the form of Alzheimer’s Disease (AD). In fact, the prevalence of AD is expected to rise, reaching 100 million worldwide by 2050, with 1 in 85 persons potentially living with AD.(Alzheimer’s Association, 2009) Thus, it is important to uncover some of the modifiable risk factors that would prevent or delay cognitive impairment, the hallmark of AD and other dementing illnesses.
Among those modifiable factors, hormonal influence on cognition is increasingly gaining interest among researchers in the field. Altered thyroid function is well-known to co-occur with psychological and cognitive changes in adults.(Samuels, 2014) However, it is uncertain which type of disordered function affects cognition, to what extent, among which sub-groups and for which domains of cognition. With advances in the neurosciences, it is now possible to use validated neurocognitive tests reflecting specific cognitive domains and mapped directly to specific brain regions.(Samuels, 2014) Moreover, four categories of thyroid dysfunction are commonly studied in the literature, based on laboratory testing of free thyroxine (fT4), triiodothyronine (T3), and thyroid stimulating hormone (TSH) levels. (Samuels, 2014) Those can be summarized as follow: (A) Overt hypothyroidism: low-serum fT4 coupled with elevated serum TSH; (B) Overt thyrotoxicosis: high-serum fT4 and/or T3 and suppressed TSH level; (C) subclinical hypothyroidism (elevated TSH, normal fT4) and (D) subclinical thyrotoxicosis (suppressed TSH, normal fT4 and T3).(Samuels, 2014) Despite the common use of those groupings for clinical purposes, thyroid function and dysfunction is often thought of as a continuum, thus the importance of examining effects of each of the hormonal factors separately. Some of the domains commonly affected by thyroid dysfunction include memory, executive function and attention/concentration. Many of those cognitive deficits may be completely or partially reversed by administration of levothyroxine (L-T4).(Bono, et al., 2004)
Emerging evidence from animal studies and clinical observations suggests that thyroid hormones are crucial to a well-functioning central nervous system, and that those hormones may play a role for structural and functional development of the brain early on, including brain areas that regulate mood and cognition.(Koromilas, et al., 2010) In fact, hypothyroidism causes a condition termed pseudo-dementia, a progressive non-degenerative cognitive impairment characterized by slower thought processes.(Dugbartey, 1998) Studies also show that thyroid hormones continue to modulate the function of the adult brain, which explains the tight regulation of thyroid hormone transport into the brain, region-specific T4 to T3 conversion as well as T3 receptor levels.(Ceballos, et al., 2009) Epidemiological studies indicated that thyroid dysfunction whether hypo- or hyper-thyroidism (overt or subclinical) increases the risk of cognitive impairment,(Beydoun, et al., 2013,Bono, et al., 2004,Correia, et al., 2009,Miller, et al., 2006,Munte, et al., 2001) although the evidence is still sparse.(Almeida, et al., 2007,Ceresini, et al., 2009,de Jongh, et al., 2011,Formiga, et al., 2014,Joffe, et al., 2013,Kramer, et al., 2009,Parle, et al., 2010,Samuels, et al., 2007,Wijsman, et al., 2013) It is less well-known how thyroid hormone fluctuations within normal ranges can affect cognitive outcomes in the general population, particularly when studies have examined cognitive performance among middle-aged adults.(Beydoun, et al., 2013,Beydoun, et al., 2012,Grigorova and Sherwin, 2012,van Boxtel, et al., 2004)
Limited research has systematically tested the associations between thyroid hormones (both outside and within normal ranges) and cognitive change over-time in a large sample of middle-age adults. Thus, we describe the relationships between variations in thyroid hormones and longitudinal cognitive change in a large socioeconomically diverse bi-racial population of adult men and women. Due to the strong evidence of differential thyroid function by sex as well as by race (Aoki, et al., 2007), we stratified part of the analysis by those two socio-demographic factors.
Materials and Methods
Database
Initiated in 2004, the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study is an ongoing prospective cohort study that used area probability sampling to recruit a socioeconomically diverse and representative sample of African Americans and whites (30-64 years old) residing in Baltimore, Maryland.(Evans, et al., 2010) Written informed consent was obtained from all participants who were provided with a protocol booklet in layman’s terms and a video explaining all study procedures including future re-contacts. Materials’ approval was completed by MedStar Institutional Review Board. The present study used longitudinal HANDLS data from baseline and the first follow-up examination (visit 2 ended in 2013). Time between examination visits 1 (Wave 1) and 2 (Wave 3) ranged from <1y to ~8y, with a mean of 4.64±0.93y.
Study subjects
Initially, 3,720 participants were recruited, of whom 2,630 had baseline complete data on one of the measure of mental status (Mini-Mental State Examination (MMSE)). Of those 2,045 had non-missing dietary data that were used to compute the 2010-Healthy Eating Index (2010-HEI), while 2,077 had complete data on CES-D total score. In addition, thyroid hormone exposures were available for ~2,500 participants of whom 2,296-2,381 were within the reference ranges. Available and reliable cognitive data varied by cognitive test ranging from N=2,088 for California Verbal learning test-free delayed recall (CVLT-DFR) to 2,700 for Clock, Command test at visit 1. At the follow-up visit, those sample sizes were reduced to a range of 1,846 (CVLT-DFR) to 2,139 (Animal Fluency). When combining waves in the final analytic models, samples of participants with complete data on outcomes at either visit, as well as exposures and covariates at baseline were reduced to a range of N=1,486-1,602 with a mean repeat of 1.6-1.7 visits/participant and a total number of visits ranging from 2,496 to 2,757. As is discussed in further details in the “Statistical analysis” section, possible sample selectivity was corrected by using a 2-stage Heckman selection approach.(Heckman, 1979)
Cognitive assessment
Cognitive assessment consisted of 7 tests with 11 test scores covering 7 domains (Mental status, attention, learning/memory, executive function, visuo-spatial/visuo-construction ability, psychomotor speed, language/verbal): the Mini-Mental State Examination (MMSE), the California Verbal Learning Test (CVLT) immediate (List A) and Delayed Free Recall (DFR), Digit Span Forward and Backwards tests (DS-F and DS-B), the Benton Visual Retention Test (BVRT), Animal Fluency test (AF), Brief Test of Attention (BTA), Trails A and B and the Clock Drawing Test (CDT) (See Appendix I for full description of tests and scores). Only individual test scores were used in the analysis rather than cognitive domains. All participants were judged capable of informed consent and were probed for their understanding of the protocol. Although no formal dementia diagnoses were performed, all participants were administered mental status tests, which they completed successfully. In every case, low mental status performance was due to poor literacy skills with no other signs of dementia.
Thyroid hormone assessment
Several assays for thyroid hormone assessment were completed at Quest Diagnostics labs (http://www.questdiagnostics.com/home.html). First, Immunochemiluminometric (ICMA) thyroid stimulating hormone (TSH) assays (TSH-ICMA; Advia-centaur XP, Siemens) were conducted with a 0.01–0.02 mU/L sensitivity.(Ross, 1988) Reference range for TSH among adults aged 20+y is 0.4-4.5 mU/L (http://www.questdiagnostics.com/testcenter/TestDetail.action?ntc=899), with an inter-individual coefficient of variation of 32%. Total thyroxine (tT4) was measured using ICMA (AU 5400, Beckman-Coulter) with a 0.8 μg/dL sensitivity and a reference range of 4.8-10.4 μg/dL. (http://www.questdiagnostics.com/testcenter/TestDetail.action?ntc=17733) Measurements of free thyroxine (fT4) concentration were also conducted using ICMA (Advia-centaur XP, Siemens), non-dialysis, with a sensitivity of 0.1 ng/dL, and a reference range of 0.8-1.8 ng/dL.(http://www.questdiagnostics.com/testcenter/TestDetail.action?ntc=866) Triiodothyronine (T3) percent uptake (T3pu) is used to estimate thyroxin binding globulin (TBG) availability, a protein carrying most of serum T3 and T4. TBG is known to have an inverse relationship with T3pu), with a lower TBG (or higher T3pu) suggestive of possible hyperthyroidism or thyrotoxicosis. T3 (%uptake) was also measured by ICMA (AU 5400, Beckman-Coulter) and had a reference range of 24-37%.(Baskin, et al., 2002) Using fT4 and TSH criteria, thyroid dysfunction status was defined as follows: (A) Overt hypothyroidism: low-serum fT4 coupled with elevated serum TSH; (B) Overt thyrotoxicosis: high-serum fT4 and suppressed TSH level; (C) subclinical hypothyroidism (elevated TSH, normal fT4) and (D) subclinical thyrotoxicosis (suppressed TSH and normal fT4); (E) Other type of dysfunction which were compared to (N) Normal TSH and fT4 levels. The distribution of reference ranges, abnormal values and thyroid dysfunction groups are presented in Table 1.
Table 1.
Selected baseline (Visit 1) study participant characteristics by sex and race/ethnicity for HANDLS participants with complete and reliable baseline MMSE scores (n=2,630) a
| All | Men | Women | Whites | African-Americans | P b | ||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| 45.4±1.7 | 54.5±1.7 | 36.2±1.5 | 63.8±1.5 | ||||
| N=2,630 | N=1,142 | N=1,488 | N=1,118 | N=1,512 | Men vs. women |
Whites vs. African- Americans |
|
| Age at baseline | 47.0±0.3 | 47.2±0.4 | 46.9±0.4 | 46.7±0.4 | 47.2±0.4 | 0.63 | 0.41 |
| (N=2,630) | (N=1,142) | (N=1,487) | (N=1,118) | (N=1,512) | |||
|
| |||||||
| Married, % | 35.0±1.7 | 38.9±2.5 | 31.8±2.2 | 44.9±2.3 | 29.6±2.2 | 0.03 | <0.001 |
| (N=2,447) | (N=1,061) | (N=1,386) | (N=1,018) | (N=1,429) | |||
|
| |||||||
| Education, % | |||||||
| <HS | 4.3±0.6 | 4.5±0.8 | 4.1±0.7 | 5.5±0.9 | 3.7±0.7 | 0.70 | <0.001 |
| HS | 52.8±1.7 | 54.0±2.4 | 51.7±2.3 | 40.2±2.0 | 59.9±2.4 | ||
| >HS | 38.5±1.7 | 36.6±2.4 | 40.1±2.3 | 46.6±2.1 | 33.9±2.3 | ||
| Missing | 4.4±0.8 | 4.9±1.3 | 4.1±1.0 | 7.7±1.0 | 2.6±1.1 | ||
| (N=2,630) | (N=1,142) | (N=1,488) | (N=1,118) | (N=1,512) | |||
|
| |||||||
| Literacy (WRAT score) | 43.2±0.3 | 43.0±0.4 | 43.3±0.3 | 46.7±0.3 | 41.2±0.3 | 0.48 | <0.001 |
| (N=2,616) | (N=1,136) | (N=1,480) | (N=1,114) | (N=1,502) | |||
|
| |||||||
| PIR <125%, % | 19.6±1.0 | 16.4±1.0 | 22.2±1.5 | 12.3±0.8 | 23.7±1.5 | 0.003 | <0.001 |
| (N=2,630) | (N=1,142) | (N=1,488) | (N=1,118) | (N=1,512) | |||
|
| |||||||
| Current smoking status, % | |||||||
| Currently smoking | 43.7±1.7 | 49.7±2.4 | 38.7±2.3 | 36.0±2.0 | 46.3±2.3 | 0.007 | <0.001 |
| Missing | 4.9±1.7 | 3.7±1.0 | 6.0±1.3 | 3.6±0.7 | 5.7±1.2 | ||
| (N=2,630) | (N=1,142) | (N=1,488) | (N=1,118) | (N=1,512) | |||
|
| |||||||
| Current use of illicit drugs, % | |||||||
| Used any type | 61.8±1.6 | 72.3±2.0 | 53.0±2.3 | 55.5±2.1 | 65.3±2.2 | <0.001 | <0.001 |
| Missing | 7.8±0.8 | 6.6±1.0 | 8.7±2.3 | 11.0±1.3 | 6.0±1.0 | ||
| (N=2,630) | (N=1,142) | (N=1,488) | (N=1,117) | (N=1,511) | |||
|
| |||||||
| Body mass index, kg.m−2 | 29.7±0.3 | 28.1±0.3 | 31.1±0.4 | 29.2±0.3 | 30.0±0.4 | <0.001 | 0.10 |
| (N=2,630) | (N=1,142) | (N=1,488) | (N=1,118) | (N=1,512) | |||
|
| |||||||
| HEI-2010 total score | 43.8±0.4 | 43.0±0.5 | 44.4±0.6 | 45.1±0.6 | 43.0±0.5 | 0.07 | 0.008 |
| (N=2,045) | (N=875) | (N=1,170) | (N=865) | (N=1,180) | |||
|
| |||||||
| Depressive symptoms | |||||||
| CES-D score | 10.5±0.3 | 9.6±0.3 | 11.2±0.4 | 9.9±0.4 | 10.7±0.4 | <0.001 | 0.96 |
| CES-D score ≥16 (EDS), % | 22.1±1.5 | 17.8±2.1 | 25.6±2.2 | 23.5±2.1 | 21.5±2.0 | 0.010 | 0.48 |
| (N=2,077) | (N=892) | (N=1,187) | (N=823) | (N=1,255) | |||
|
| |||||||
| Anti-depressant use, % | 12.3±0.1 | 8.1±0.1 | 15.7±0.2 | 18.2±0.2 | 9.1±0.1 | <0.001 | <0.001 |
| (N=2,399) | (N=1,045) | (N=1,354) | (N=1,015) | (N=1,384) | |||
|
| |||||||
| Thyroid hormones, within reference range c | |||||||
| TSH, mU/L | 1.73±0.03 | 1.68±0.04 | 1.78±0.05 | 1.90±0.04 | 1.63±0.05 | 0.12 | <0.001 |
| (N=2,296) | (N=1,018) | (N=1,278) | (N=986) | (N=1,310) | |||
| Free T4, μg/dL | 1.13±0.01 | 1.14±0.01 | 1.12±0.01 | 1.14±0.01 | 1.12±0.01 | 0.038 | 0.07 |
| (N=2,381) | (N=1,030) | (N=1,351) | (N=1,048) | (N=1,333) | |||
| Total T4, ng/dL | 7.53±0.04 | 7.51±0.07 | 7.55±0.05 | 7.57±0.05 | 7.51±0.06 | 0.63 | 0.52 |
| (N=2,232) | (N=963) | (N=1,269) | (N=995) | (N=1,237) | |||
| T3, %uptake | 30.50±0.10 | 30.94±0.16 | 30.13±0.13 | 30.52±0.12 | 30.48±0.15 | <0.001 | 0.85 |
| (N=2,310) | (N=984) | (N=1,326) | (N=1,042) | (N=1,268) | |||
|
| |||||||
|
Thyroid hormones, above or below reference
range |
|||||||
|
| |||||||
| TSH, mU/L | |||||||
| <0.4 | 3.0±0.5 | 2.7±0.7 | 3.3±0.7 | 1.2±0.4 | 4.2±0.8 | 0.013 | <0.001 |
| > 4.5 | 3.4±0.4 | 1.9±0.4 | 4.6±0.7 | 5.9±0.8 | 1.8±0.4 | ||
| (N=2,497) | (N=1,079) | (N=1,418) | (N=1,089) | (N=1,408) | |||
| Free T4, μg/dL | |||||||
| <0.8 | 4.6±0.6 | 5.5±1.2 | 3.9±0.7 | 5.0±0.9 | 4.4±0.9 | 0.12 | 0.78 |
| >1.8 | 0.3±0.1 | 0.0±0.0 | 0.4±0.1 | 0.2±0.1 | 0.3±0.1 | ||
| (N=2,502) | (N=1,082) | (N=1,420) | (N=1,089) | (N=1,413) | |||
| Total T4, ng/dL | |||||||
| <4.8 | 3.3±0.6 | 4.2±1.1 | 2.5±0.7 | 3.7±0.9 | 3.0±0.8 | 0.042 | 0.002 |
| >10.4 | 8.7±1.1 | 6.2±1.2 | 10.8±1.8 | 4.6±0.9 | 11.2±1.7 | ||
| (N=2,504) | (N=1,082) | (N=1,422) | (N=1,089) | (N=1,415) | |||
| T3, %uptake | |||||||
| <24% | 5.6±0.8 | 5.2±1.0 | 6.0±1.3 | 3.3±1.0 | 7.1±1.3 | 0.09 | 0.026 |
| > 37% | 1.7±0.4 | 2.6±0.7 | 0.9±0.3 | 1.7±0.7 | 1.7±0.4 | ||
| (N=2,504) | (N=1,082) | (N=1,422) | (N=1,089) | (N=1,415) | |||
| Thyroid function categories | |||||||
|
| |||||||
| Normal | 89.8±0.9 | 90.7±1.4 | 89.0±1.2 | 89.1±1.2 | 90.2±1.2 | 0.017 | <0.001 |
| Overt hypothyroidism | 0.7±0.2 | 0.5±0.2 | 0.8±0.2 | 1.1±0.3 | 0.5±0.2 | ||
| Overt thyrotoxicosis | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.1±0.1 | ||
| Sub-clinical hypothyroidism | 2.7±0.4 | 1.4±0.3 | 3.8±0.7 | 4.9±0.8 | 1.4±0.4 | ||
| Sub-clinical thyrotoxicosis | 2.8±0.5 | 2.5±0.7 | 3.2±0.7 | 1.1±0.4 | 3.9±0.8 | ||
| Other thyroid dysfunction | 3.9±0.6 | 4.9±1.1 | 3.1±0.6 | 3.9±0.9 | 4.0±0.8 | ||
| (N=2,497) | (N=1,079) | (N=1,418) | (N=1,089) | (N=1,408) | |||
Abbreviation: BVRT=Benton Visual Retention Test; CES-D=Center for Epidemiologic Studies-Depression; CVLT=California Verbal Learning Test; EDS=Elevated Depressive Symptoms; MMSE=Mini-Mental State Examination; PIR=poverty income ratio; T3=triiodothyronine; T4=thyroxine; TSH=Thyroid stimulating hormone; WRAT=Wide Range Achievement Test.
Values are weighted mean±SEM or percent±SEP.
P-value was based on linear regression models when row variable is continuous (svy:reg) and design-based F-test when row variable is categorical (svy:tab).
TSH, free T4 (fT4) and total T4 (tT4) values outside the reference range were excluded in this analysis (See methods section for reference ranges).
Covariates
Many variables were included in the analyses namely age, sex, self-reported race (White vs. African American), marital status, educational attainment (<High School (HS); HS, >HS), poverty income ratio (PIR<125% for “poor”), measured body mass index (BMI, kg/m2), current drug use (opiates, marijuana or cocaine” vs. none), smoking status (“current” vs. “never or former) and the Wide Range Achievement Test letter and word reading (WRAT) subtotal score to measure literacy (See Appendix I) The 20-item Center for Epidemiologic Studies Depression Scale (CES-D) scale was used to assess affective, depressed mood. Baseline CES-D total score was used in our analyses, with CES-D≥16 labeled as “elevated depressive symptoms” (EDS). (See Appendix I) Moreover, overall dietary quality as measured by the Healthy Eating Index (HEI-2010) based on two 24-hr recalls administered in HANDLS baseline visit was also included in the analyses, due to its potentially confounding effect between thyroid hormonal function and cognitive performance and/or decline.(Beydoun, et al., 2014,Fontana, et al., 2006,van de Rest, et al., 2015) The steps for calculating HEI-2010 are provided by the National Cancer Institute’s Applied Research website (http://appliedresearch.cancer.gov/tools/hei/tools.html) as well as the HANDLS website (http://handls.nih.gov/06Coll-dataDoc.htm). Worth of noting that total and component HEI-2010 scores were calculated for each recall day (day 1 and day 2) and then averaged to obtain the mean HEI-2010 total and component scores, thus combining both days. In the present study, only total HEI-2010 score was considered. Use of anti-depressants was included as a covariate in a sensitivity analysis.
Statistical analysis
Stata release 13.0 was used to conduct all analyses. First, using survey commands, we applied MRV exam sampling weights in the descriptive parts of the analysis, to obtain population estimates of means, proportions and regression coefficients, given unequal probability of sampling from the target Baltimore city population. (Lohr, 1999) Means across binary variables were compared using linear regression models that accounted for those sampling weights (svy:reg), whereas design-based F-tests were conducted to test associations between categorical variables using cross-tabulations between those variables while accounting for those same weights (svy:tab).
Second, mixed-effects linear regression models on 11 continuous cognitive test score(s) comparing above and below reference ranges to the reference range of thyroid hormones were conducted (Models 1-4). Interactions by sex or race were not tested, given the expected lower statistical power for those categorical exposures, when compared to continuous exposures.
Third, similar mixed-effects regression on 11 continuous cognitive test score(s) were used to examine associations between the four continuous thyroid hormone exposure variables within reference ranges (also termed Models 1-4) and cognitive performance over-time, controlling for potential confounders. Moderating effects of sex and race were tested by adding interaction terms to the multivariable mixed-effects regression models (3-way interactions Time×exposure×sex or Time×exposure×race) and stratifying by sex or race or both, though separately, when interactions with sex and/or race are deemed significant.
Finally, thyroid dysfunction status was examined in a similar way by comparing the four categories of dysfunction to the normal category defined by fT4 and TSH levels (See Thyroid hormone assessment section). Appendix II describes the mixed-effects regression modeling approach used in detail.
To minimize potential selection bias in mixed-effects regression models (due to the nonrandom selection of participants with complete data from the target study population), a 2-stage Heckman selection model was constructed, by running a probit model to compute an inverse mills ratio at the first stage (derived from the predicted probability of being selected, conditional on the covariates in the probit model, mainly baseline age, sex, race, poverty status and education), as was done in an earlier study. (Heckman, 1979) This inverse mills ratio was then entered as covariate in the mixed-effects regression model at the second stage, as was done in a previous study.(Beydoun, et al., 2013) Due to possible collinearity between the inverse mills ratio and the common covariates entered in both the mixed-effects regression model and the probit model, poverty status was eliminated from the mixed-effects regression in a sensitivity analysis. In a second sensitivity analysis, use of anti-depressants was included as an additional covariate in the mixed-effects regression models.
In all analyses, a type I error of 0.05 was considered for main effects whereas a p<0.10 was deemed significant for interaction terms,(Selvin, 2004), prior to correcting for multiple testing. A familywise Bonferroni procedure was used to correct for multiple testing by accounting only for cognitive tests and assuming that hormonal exposures related to separate substantive hypotheses.(Hochberg, 1987) Therefore, for main effects, p<0.004 (0.05/11) was considered significant. Due to their lower statistical power compared to main effects, interaction terms had their critical p-values reduced to (0.10/11=0.009).
Results
Table 1 displays baseline (visit 1) characteristics among participants with complete and dependable MMSE scores, by sex and race. Compared to men, women had lower income, education and literacy, were less likely to be married, to be current smokers or illicit drug users and had an overall higher BMI and CES-D total score. Both mean fT4 and T3pu within the reference range were lower in women than in men, while both proportions >reference and <reference for TSH were higher in women. Racial differences were also noted for socio-demographic, lifestyle and thyroid hormonal exposures, although no difference by race was observed in terms of CES-D scores or BMI. Specifically, compared to Whites, African-Americans were less likely to be married, had lower income, education and literacy, but were more likely to be currently smoking or using illicit drugs, to have poorer quality diet, and had a lower TSH level within the reference range. African-Americans were also more likely to have sub-optimal TSH values (4.2% in African-Americans vs. 1.2% in Whites), with the reverse being observed for above-reference range values (1.8% in African-Americans vs. 5.9% in Whites). An above-reference range tT4 was more likely in African-Americans (11.2% vs. 4.6%), who were also more likely to have a sub-optimal T3 %uptake (7.1% vs. 3.3%). Thyroid function status varied by sex and race, with a significantly larger proportion of Whites fitting the “subclinical hypothyroidism” compared to African-Americans. In contrast, “subclinical thyrotoxicosis” was more prevalent in African-Americans. Both types of dysfunctions were more prevalent in women than in men.
Table 2 shows that in addition to persistent racial differences in cognitive performance across the two visits with poorer performance found in African-Americans, only 4 of 11 cognitive tests changed between visits, with consistent indication of over-time cognitive decline in 3 of the 4. In particular, verbal and visual memory scores declined in both sexes and racial groups, while MMSE scores reflecting mental status improved over time possibly due to learning, particularly among Whites.
Table 2.
Cognitive performance test scores at visits 1 and 2, by sex and race/ethnicity for HANDLS participants with complete and reliable baseline MMSE scoresa
| All | Men | Women | Whites | African-Americans | |
|---|---|---|---|---|---|
| Mini-Mental State Exam, total score | |||||
| Visit 1 | 27.83±0.07 | 27.71±0.10 | 27.94±0.09 | 28.43±0.07 | 27.50±0.10 c |
| (N=2,630) | (N=1,142) | (N=1,488) | (N=1,118) | (N=1,512) | |
| Visit 2 | 28.04±0.06 | 27.96±0.10 | 28.10±0.08 | 28.65±0.06 | 27.70±0.09c |
| (N=1,934) | (N=775) | (N=1,159) | (N=767) | (N=1,167) | |
|
| |||||
| P (Visit2-Visit1) | 0.028 | 0.08 | 0.19 | 0.022 | 0.12 |
|
| |||||
| California Verbal Learning Test (CVLT), List A | |||||
| Visit 1 | 24.99±0.26 | 23.53±0.39 | 26.26±0.34 b | 26.96±0.36 | 23.93±0.35 c |
| (N=2,172) | (N=939) | (N=1,233) | (N=895) | (N=1,277) | |
| Visit 2 | 20.08±0.26 | 18.73±0.37 | 21.12±0.37 b | 22.55±0.40 | 18.72±0.33 c |
| (N=1,976) | (N=817) | (N=1,159) | (N=781) | (N=1,195) | |
|
| |||||
| P (Visit2-Visit1) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
|
| |||||
| CVLT, free delayed recall | |||||
|
| |||||
| Visit 1 | 7.34±0.12 | 6.83±0.17 | 7.78±0.16 b | 8.36±0.16 | 6.79±0.15 c |
| (N=2,088) | (N=900) | (N=1,188) | (N=863) | (N=1,225) | |
| Visit 2 | 5.82±0.13 | 5.34±0.19 | 6.18±0.17 b | 7.20±0.20 | 5.09±0.15 c |
| (N=1,846) | (N=759) | (N=1,087) | (N=719) | (N=1,127) | |
|
| |||||
| P (Visit2-Visit1) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
|
| |||||
| Benton Visual Retention Test | |||||
|
| |||||
| Visit 1 | 5.66±0.16 | 5.20±0.23 | 6.05±0.23 b | 5.01±0.18 | 6.03±0.23 c |
| (N=2,594) | (N=1,129) | (N=1,465) | (N=1,108) | (N=1,486) | |
| Visit 2 | 7.65±0.18 | 7.20±0.26 | 7.99±0.24 b | 6.24±0.22 | 8.42±0.25 c |
| (N=2,085) | (N=861) | (N=1,224) | (N=816) | (N=1,269) | |
|
| |||||
| P (Visit2-Visit1) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
|
| |||||
| Brief Test of Attention | |||||
|
| |||||
| Visit 1 | 6.72±0.08 | 6.57±0.11 | 6.84±0.12 | 7.47±0.09 | 6.30±0.12 c |
| (N=2,247) | (N=980) | (N=1,267) | (N=930) | (N=1,317) | |
| Visit 2 | 6.64±0.09 | 6.54±0.14 | 6.72±0.12 | 7.21±0.10 | 6.33±0.13 c |
| (N=1,907) | (N=789) | (N=1,118) | (N=772) | (N=1,135) | |
|
| |||||
| P (Visit2-Visit1) | 0.55 | 0.89 | 0.48 | 0.06 | 0.84 |
|
| |||||
| Animal Fluency | |||||
| Visit 1 | 19.19±0.20 | 19.79±0.29 | 18.68±0.27 b | 21.25±0.30 | 18.02±0.25 c |
| (N=2,695) | (N=1,177) | (N=1,518) | (N=1,136) | (N=1,559) | |
| Visit 2 | 19.46±0.24 | 20.06±0.38 | 18.99±0.30 b | 21.66±0.32 | 18.28±0.31 c |
| (N=2,139) | (N=895) | (N=1,244) | (N=838) | (N=1,300) | |
|
| |||||
| P (Visit2-Visit1) | 0.38 | 0.57 | 0.45 | 0.35 | 0.52 |
|
| |||||
| Digits Span, Forward | |||||
| Visit 1 | 7.42±0.07 | 7.48±0.11 | 7.37±0.10 | 8.06±0.10 | 7.06±0.10 c |
| (N=2,579) | (N=1,127) | (N=1,452) | (N=1,092) | (N=1,487) | |
| Visit 2 | 7.50±0.09 | 7.55±0.14 | 7.46±0.12 | 8.24±0.12 | 7.10±0.12 c |
| (N=1,971) | (N=829) | (N=1,142) | (N=760) | (N=1,211) | |
|
| |||||
| P (Visit2-Visit1) | 0.52 | 0.71 | 0.59 | 0.24 | 0.79 |
|
| |||||
| Digits Span, Backward | |||||
| Visit 1 | 5.79±0.07 | 5.78±0.11 | 5.79±0.10 | 6.69±0.10 | 5.26±0.10 c |
| (N=2,561) | (N=1,121) | (N=1,440) | (N=1,091) | (N=1,470) | |
| Visit 2 | 5.78±0.08 | 5.75±0.12 | 5.80±0.10 | 6.70±0.12 | 5.30±0.10 c |
| (N=1,965) | (N=824) | (N=1,141) | (N=755) | (N=1,210) | |
|
| |||||
| P (Visit2-Visit1) | 0.96 | 0.87 | 0.93 | 0.93 | 0.80 |
|
| |||||
| Clock, command | |||||
| Visit 1 | 8.79±0.04 | 8.87±0.06 | 8.73±0.06 | 9.04±0.05 | 8.65±0.06 c |
| (N=2,700) | (N=1,179) | (N=1,521) | (N=1,144) | (N=1,556) | |
| Visit 2 | 8.78±0.05 | 8.82±0.06 | 8.75±0.07 | 9.04±0.05 | 8.64±0.07 c |
| (N=2,104) | (N=873) | (N=1,231) | (N=829) | (N=1,275) | |
|
| |||||
| P (Visit2-Visit1) | 0.87 | 0.61 | 0.80 | 0.97 | 0.90 |
|
| |||||
| Trailmaking test, Part A | |||||
| Visit 1 | 34.86±0.59 | 34.97±0.70 | 34.77±0.91 | 29.58±0.50 | 37.94±0.89 c |
| (N=2,557) | (N=1,096) | (N=1,461) | (N=1,094) | (N=1,463) | |
| Visit 2 | 36.48±1.39 | 37.29±1.64 | 35.88±2.10 | 29.89±0.71 | 40.06±2.11 c |
| (N=1,874) | (N=763) | (N=1,111) | (N=774) | (N=1,100) | |
|
| |||||
| P (Visit2-Visit1) | 0.28 | 0.19 | 0.63 | 0.72 | 0.36 |
|
| |||||
| Trailmaking test, Part B | |||||
| Visit 1 | 138.77±4.57 | 143.11±7.55 | 135.22±5.55 | 92.56±3.80 | 165.71±6.77 c |
| (N=2,556) | (N=1,096) | (N=1,460) | (N=1,094) | (N=1,462) | |
| Visit 2 | 127.87±5.79 | 130.30±8.89 | 126.06±7.64 | 77.22±2.30 | 155.91±8.59 c |
| (N=1,728) | (N=705) | (N=1,023) | (N=724) | (N=1,004) | |
|
| |||||
| P (Visit2-Visit1) | 0.14 | 0.27 | 0.33 | 0.001 | 0.37 |
Most cognitive test scores were in the direction of higher score=better performance, except for BVRT (total errors), and Trailmaking Test both parts (expressed in seconds).
p<0.05 for null hypothesis of no difference in means of cognitive test scores by sex within each visit, Wald test from svy: reg command.
p<0.05 for null hypothesis of no difference in means of cognitive test scores by race within each visit, Wald test from svy: reg command.
Table 3 displays associations between the four thyroid hormone exposures (comparing suboptimal and above reference levels to within reference range) and longitudinal cognitive change in four separate models, based on multiple mixed-effects regression analyses. After correction for multiple testing (Type I error corrected to 0.004 for main effects), sub-optimal tT4 was associated with better performance in AF (Model 3, below reference range vs. reference (BRRVR), γ±SEE: +2.08±0.70, p=0.003) at baseline. When examining cognitive change (Type I error corrected to 0.009), none of the associations survived multiple testing correction. However, when comparing participants above reference ranges to those within (Model 1, ARRVRR×Time), above reference range TSH was linked to faster rates of decline on DS-B, a test of working memory (γ±SEE: −0.14±0.05, P=0.006) and Clock-command, at test of visuo-spatial and visuo-construction abilities (γ±SEE: −0.10±0.04, P=0.004).
Table 3.
Longitudinal cognitive change by thyroid hormonal status: mixed-effects linear regression models
| Intercept | Time | Below reference range vs. reference (BRRVRR) |
(BRRVRR)×Time | Above reference range vs. reference (ARRVRR) |
(ARRVRR)×Time | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| γ ±SEE | P | γ ±SEE | P | γ ±SEE | P | γ±SEE | P | γ±SEE | P | γ±SEE | P | |
| Mini-Mental State Exam, total score | ||||||||||||
| Model 1: TSH(N=1,580; N’=2,592) | 26.8±0.2 | <0.001 | +0.09±0.05 | 0.08 | −0.05±0.25 | 0.83 | −0.01±0.06 | 0.83 | +0.26±0.19 | 0.18 | −0.03±0.05 | 0.62 |
| Model 2: fT4(N=1,583; N’=2,597) | 26.9±0.2 | <0.001 | +0.09±0.05 | 0.10 | +0.05±0.20 | 0.78 | −0.10±0.06 | 0.08 | −1.02±0.64 | 0.11 | −0.14±0.16 | 0.38 |
| Model 3: tT4(N=1,585; N,=2,598) | 26.9±0.2 | <0.001 | +0.09±0.05 | 0.10 | −0.11±0.25 | 0.66 | −0.00±0.07 | 0.97 | −0.22±0.16 | 0.16 | −0.00±0.04 | 0.91 |
| Model 4: T3pu (N=1,585; N’=2,598) | 26.9±0.2 | <0.001 | +0.09±0.05 | 0.10 | −0.30±0.18 | 0.10 | +0.01±0.05 | 0.92 | +0.05±0.31 | 0.87 | −0.07±0.08 | 0.39 |
| California Verbal Learning Test (CVLT), List A | ||||||||||||
| Model 1: TSH(N=1,515; N’=2,376) | 25.8±0.7 | <0.001 | −1.28±0.16 | <0.001 | −1.41±0.92 | 0.13 | +0.26±0.21 | 0.21 | −0.56±0.70 | 0.43 | +0.12±0.17 | 0.47 |
| Model 2: fT4(N=1,518; N’=2,382) | 25.7±0.7 | <0.001 | −1.25±0.16 | <0.001 | −1.22±0.69 | 0.08 | +0.06±0.18 | 0.72 | −2.91±2.35 | 0.22 | −0.71±0.58 | 0.22 |
| Model 3: tT4(N=1,518; N’=2,381) | 25.7±0.7 | <0.001 | −1.25±0.16 | <0.001 | +0.30±0.91 | 0.75 | +0.01±0.22 | 0.97 | +0.10±0.58 | 0.86 | −0.11±0.14 | 0.41 |
| Model 4: T3pu (N=1,518; N’=2,381) | 25.7±0.7 | <0.001 | −1.25±0.16 | <0.001 | −0.71±0.65 | 0.27 | −0.17±0.16 | 0.29 | +0.69±1.19 | 0.56 | +0.06±0.27 | 0.56 |
| CVLT, free delayed recall | ||||||||||||
| Model 1: TSH(N=1,486; n’=2,275) | 7.96±0.33 | <0.001 | −0.40±0.08 | <0.001 | +0.40±0.44 | 0.36 | −0.06±0.11 | 0.60 | +0.11±0.33 | 0.73 | +0.03±0.08 | 0.69 |
| Model 2: fT4(N=1,489; N’=2,281) | 7.96±0.33 | <0.001 | −0.40±0.08 | <0.001 | −0.87±0.32 | 0.007 | +0.12±0.09 | 0.18 | −0.88±1.11 | 0.42 | −0.34±0.29 | 0.25 |
| Model 3: tT4(N=1,489; N’=2,280) | 7.95±0.33 | <0.001 | −0.40±0.08 | <0.001 | −0.27±0.43 | 0.52 | +0.12±0.11 | 0.27 | +0.27±0.28 | 0.34 | −0.12±0.07 | 0.07 |
| Model 4: T3pu (N=1,489; N’=2,280) | 7.96±0.33 | <0.001 | −0.39±0.08 | <0.001 | −0.09±0.31 | 0.55 | −0.09±0.08 | 0.26 | +0.20±0.56 | 0.72 | +0.01±0.13 | 0.90 |
| Benton Visual Retention Test | ||||||||||||
| Model 1: TSH(N=1,594; N’=2,674) | 8.90±0.52 | <0.001 | +0.40±0.13 | <0.001 | −0.73±0.69 | 0.29 | +0.21±0.16 | 0.18 | +0.57±0.53 | 0.28 | −0.14±0.13 | 0.29 |
| Model 2: fT4(N=1,597; N’=2,680) | 8.94±0.51 | <0.001 | +0.38±0.13 | 0.003 | +0.08±0.53 | 0.88 | −0.08±0.14 | 0.57 | +1.08±1.72 | 0.53 | −0.15±0.41 | 0.72 |
| Model 3: tT4(N=1,599; N’=2,682) | 8.93±0.51 | <0.001 | +0.37±0.13 | 0.003 | −0.10±0.66 | 0.89 | −0.08±0.17 | 0.64 | +0.30±0.42 | 0.49 | +0.14±0.10 | 0.18 |
| Model 4: T3pu (N=1,599; N’=2,682) | 8.89±0.52 | <0.001 | +0.37±0.13 | 0.003 | +0.55±0.49 | 0.27 | +0.13±0.12 | 0.30 | +0.59±0.84 | 0.48 | −0.03±0.21 | 0.87 |
| Brief Test of Attention | ||||||||||||
| Model 1: TSH(N=1,546; N’=2,496) | 6.48±0.24 | <0.001 | −0.09±0.06 | 0.12 | −0.07±0.32 | 0.82 | +0.06±0.06 | 0.57 | −0.50±0.24 | 0.034 | +0.06±0.06 | 0.30 |
| Model 2: fT4(N=1,549; N’=2,502) | 6.42±0.24 | <0.001 | −0.08±0.06 | 0.17 | −0.56±0.24 | 0.018 | +0.06±0.07 | 0.35 | +0.01±0.80 | 0.99 | −0.05±0.22 | 0.80 |
| Model 3: tT4(N=1,549; N’=2,501) | 6.43±0.24 | <0.001 | −0.09±0.06 | 0.14 | −0.77±0.31 | 0.013 | +0.19±0.08 | 0.023 | −0.03±0.19 | 0.88 | +0.08±0.05 | 0.09 |
| Model 4: T3pu (N=1,549; N’=2,501) | 6.42±0.24 | <0.001 | −0.08±0.06 | 0.19 | +0.09±0.23 | 0.68 | −0.05±0.06 | 0.38 | −0.14±0.38 | 0.71 | −0.02±0.09 | 0.80 |
| Animal Fluency | ||||||||||||
| Model 1: TSH(N=1,599; N’=2,749) | +17.4±0.6 | <0.001 | −0.08±0.12 | 0.51 | −0.03±0.73 | 0.96 | −0.24±0.04 | 0.10 | +0.74±0.55 | 0.18 | −0.02±0.12 | 0.86 |
| Model 2: fT4(N=1,602; N’=2,755) | +17.4±0.6 | <0.001 | −0.08±0.12 | 0.47 | +0.11±0.56 | 0.85 | −0.19±0.12 | 0.14 | −1.86±1.83 | 0.31 | −0.37±0.38 | 0.33 |
| Model 3: tT4(N=1,604; N’=2,757) | +17.4±0.6 | <0.001 | −0.08±0.12 | 0.48 | +2.08±0.70 | 0.003 | −0.13±0.16 | 0.41 | +0.53±0.44 | 0.23 | −0.05±0.09 | 0.61 |
| Model 4: T3pu (N=1,604; N’=2,757) | +17.5±0.6 | <0.001 | −0.09±0.12 | 0.43 | −0.64±0.51 | 0.21 | +0.09±0.11 | 0.41 | +0.03±0.88 | 0.98 | −0.23±0.18 | 0.22 |
| Digits Span, Forward | ||||||||||||
| Model 1: TSH(N=1,594; N’=2,627) | +6.81±0.22 | <0.001 | +0.02±0.05 | 0.71 | −0.13±0.29 | 0.65 | −0.01±0.06 | 0.91 | +0.08±0.22 | 0.70 | +0.01±0.05 | 0.77 |
| Model 2: fT4(N=1,597; N’=2,632) | +6.81±0.22 | <0.001 | +0.02±0.05 | 0.68 | −0.42±0.22 | 0.06 | +0.01±0.05 | 0.80 | −1.05±0.76 | 0.17 | −0.15±0.19 | 0.41 |
| Model 3: tT4(N=1,598; N’=2,632) | +6.82±0.22 | <0.001 | +0.02±0.05 | 0.71 | −0.01±0.28 | 0.98 | −0.06±0.07 | 0.36 | −0.06±0.18 | 0.75 | +0.01±0.04 | 0.80 |
| Model 4: T3pu (N=1,598; N’=2,632) | +6.85±0.22 | <0.001 | +0.02±0.05 | 0.76 | −0.35±0.21 | 0.09 | +0.05±0.05 | 0.28 | +0.55±0.35 | 0.11 | −0.11±0.08 | 0.13 |
| Digits Span, Backward | ||||||||||||
| Model 1: TSH(N=1,593; N’=2,612) | +1.73±4.59 | 0.71 | +0.97±1.08 | 0.37 | +0.24±0.27 | 0.38 | −0.13±0.06 | 0.038 | −0.07±0.21 | 0.74 | −0.14±0.05 | 0.006 |
| Model 2: fT4(N=1,596; N’=2,617) | +1.87±4.59 | 0.68 | +0.98±1.09 | 0.37 | −0.37±0.21 | 0.07 | +0.05±0.05 | 0.36 | +0.42±0.72 | 0.56 | −0.20±0.19 | 0.28 |
| Model 3: tT4(N=1,597; N’=2,617) | +1.20±4.60 | 0.26 | +1.12±1.09 | 0.31 | −0.25±0.27 | 0..36 | −0.01±0.07 | 0.92 | +0.03±0.17 | 0.85 | −0.08±0.04 | 0.06 |
| Model 4: T3pu (N=1,597; N’=2,617) | +1.37±4.58 | 0.77 | +0.88±1.08 | 0.42 | −0.33±0.19 | 0.09 | +0.01±0.05 | 0.83 | −0.05±0.33 | 0.89 | +0.18±0.08 | 0.018 |
| Clock, command | ||||||||||||
| Model 1: TSH(N=1,597; N’=2,745) | +8.82±0.13 | <0.001 | −0.08±0.04 | 0.031 | +0.22±0.18 | 0.22 | −0.01±0.05 | 0.87 | +0.07±0.13 | 0.61 | −0.10±0.04 | 0.004 |
| Model 2: fT4(N=1,600; N’=2,751) | +8.82±0.13 | <0.001 | −0.09±0.04 | 0.012 | +0.01±0.14 | 0.94 | +0.01±0.04 | 0.72 | +0.39±0.44 | 0.37 | −0.03±0.12 | 0.80 |
| Model 3: tT4(N=1,602; N’=2,753) | +8.81±0.13 | <0.001 | −0.09±0.04 | 0.013 | −0.20±0.17 | 0.23 | +0.12±0.05 | 0.015 | −0.02±0.11 | 0.87 | +0.01±0.03 | 0.85 |
| Model 4: T3pu (N=1,602; N’=2,753) | +8.82±0.12 | <0.001 | −0.09±0.04 | 0.016 | −0.03±0.12 | 0.83 | −0.05±0.04 | 0.20 | −0.24±0.21 | 0.25 | +0.02±0.06 | 0.77 |
| Trailmaking test, Part A | ||||||||||||
| Model 1: TSH(N=1,563; N’=2,639) | +35.1±3.9 | <0.001 | +2.22±1.13 | 0.049 | −6.16±5.03 | 0.22 | +1.26±1.41 | 0.37 | −3.81±3.86 | 0.32 | +0.24±1.13 | 0.84 |
| Model 2: fT4(N=1,566; N’=2,645) | +34.70±3.9 | <0.001 | +2.27±1.12 | 0.044 | −1.71±3.87 | 0.66 | +0.81±1.22 | 0.51 | −8.50±12.3 | 0.49 | +0.09±3.61 | 0.98 |
| Model 3: tT4(N=1,568; N’=2,648) | +35.00±3.86 | <0.001 | +2.24±1.12 | 0.045 | +0.20±4.90 | 0.97 | +2.73±1.53 | 0.07 | −4.45±3.09 | 0.15 | +0.30±0.91 | 0.74 |
| Model 4: T3pu (N=1,568; N’=2,648) | +35.03±3.88 | <0.001 | +2.27±1.13 | 0.044 | −3.11±3.56 | 0.38 | −0.62±1.07 | 0.56 | −4.63±5.92 | 0.43 | +0.05±1.77 | 0.98 |
| Trailmaking test, Part B | ||||||||||||
| Model 1: TSH(N=1,551; N’=2,546) | +208.2±52.7 | <0.001 | +2.82±12.4 | 0.82 | +38.0±20.2 | 0.06 | +0.14±4.03 | 0.97 | −11.1±15.5 | 0.47 | −0.46±3.35 | 0.89 |
| Model 2: fT4(N=1,554; N’=2,551) | +205.2±52.9 | <0.001 | +2.11±12.4 | 0.87 | −13.0±15.5 | 0.40 | +2.53±3.42 | 0.46 | +16.4±49.9 | 0.74 | −2.22±11.49 | 0.85 |
| Model 3: tT4(N=1,556; N’=2,554) | +206.8±52.9 | <0.001 | +3.45±12.4 | 0.78 | −15.7±19.6 | 0.42 | +0.32±4.46 | 0.94 | +8.9±15.5 | 0.47 | +2.59±2.66 | 0.33 |
| Model 4: T3pu (N=1,556; N’=2,554) | +202.2±53.0 | <0.001 | +3.14±12.4 | 0.80 | +9.34±14.4 | 0.52 | −1.83±3.13 | 0.56 | +36.2±24.1 | 0.13 | −3.96±5.50 | 0.47 |
Abbreviation: ARRVRR=Above reference range vs. reference range; BRRVRR=Below reference range vs. reference range; BVRT=Benton Visual Retention Test; CES-D=Center for Epidemiologic Studies-Depression; CVLT=California Verbal Learning Test; HANDLS=Healthy Aging in Neighborhoods of Diversity across the Life Span; MMSE=Mini-Mental State Examination; N=number of participants; N’=number of visits; TSH=Thyroid Stimulating Hormone; T3pu= % uptake of triiodothyronine; fT4= free thyroxine; tT4= total thyroxine; WRAT=Wide Range Achievement Test.
Multiple mixed-effects linear regression models adjusted for baseline age, sex, race/ethnicity, marital status, education, WRAT total score, poverty income ratio, current smoking status, current use of illicit drugs, body mass index, and 2010-HEI.
Most cognitive test scores were in the direction of higher score=better performance, except for BVRT (total errors), and Trailmaking Test both parts (expressed in seconds).
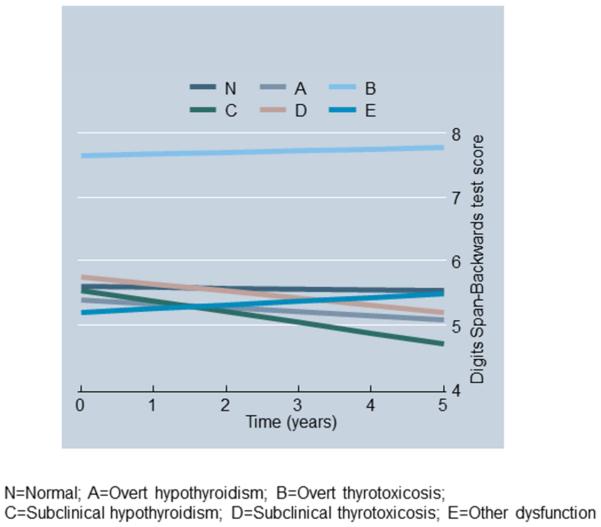
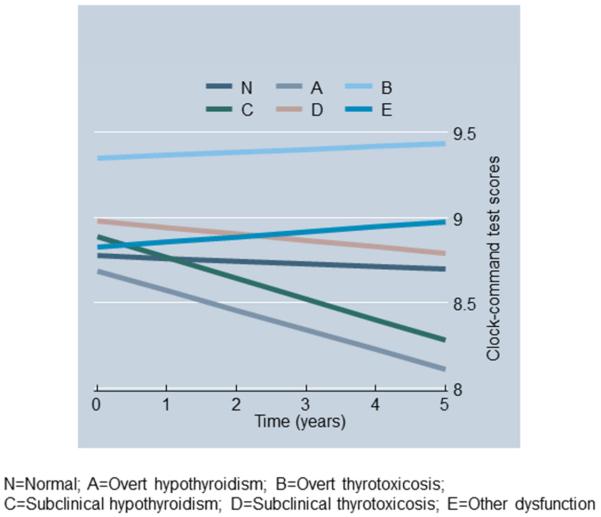
Figures 1A-1B show predictive margins from two mixed-effects regression models whereby the outcomes were DS-B and Clock-command test scores and the key predictor was thyroid function status, controlling for the same covariates as in Table 3. In both models, “sub-clinical hypothyroidism” (category C, see Methods) compared to the “normal” thyroid function category (Thyroid_st_CN) was linked to a faster rate of cognitive decline over-time (P<0.009 for Time×Thyroid_st_CN). In particular, sub-clinical hypothyroidism was associated with 14-15% poorer cognitive performance on DS-B after 5y compared to baseline and ~7% poorer performance on clock-command compared to baseline. The corresponding decline for “normal” thyroid function was <1% in both cases.
Fig 1a.
Predictive margins of Digits Span-Backwards test scores over-time from mixed-effects regression model by thyroid function status
Fig 1b.
Predictive margins of Clock-command test scores over-time from mixed-effects regression model by thyroid function status
Within reference range (Table 4), the higher the TSH level, a faster rate of decline was noted in clock-command scores among women (Model 1: TSH×Time γ±SEE:−0.03±0.01, P=0.008; P=0.009 for 3-way interaction TSH×Time×Male). Although other statistically significant 3-way interactions were detected, none of the stratum-specific effects survived correction for multiple testing. Despite the positive relationship between sub-optimal fT4 and AF test scores at baseline (Table 3) within reference ranges, both higher fT4 and tT4 levels were marginally but positively related to AF at baseline (Models 2-3, THWRR, 0.004<p<0.05). T3pu (Model 4) was not associated with cognitive performance or decline outside or within reference ranges.
Table 4.
Longitudinal cognitive change by thyroid hormonal level within reference range: mixed-effects linear regression models
| Intercept | Time |
Thyroid hormone within
reference range (THWRR) |
(THWRR)×Time | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| γ ±SEE | P | γ ±SEE | P | γ ±SEE | P | γ ±SEE | P | |
| Mini-Mental State Exam, total score | ||||||||
| Model 1: TSH(N=1,452; N’=2,376) | +27.0±0.2 | <0.001 | +0.06±0.06 | 0.35 | −0.10±0.05 | 0.07 | +0.01±0.01 | 0.44 |
| Model 2: fT4(N=1,500; N’=2,465) | +27.5±0.4 | <0.001 | +0.09±0.09 | 0.35 | −0.58±0.27 | 0.029 | −0.00±0.07 | 0.99 |
| Model 3: tT4(N=1,406; N’=2,314) | +27.1±0.3 | <0.001 | −0.02±0.09 | 0.80 | −0.02±0.04 | 0.66 | +0.01±0.01 | 0.13 |
| Model 4: T3pu (N=1,462; N’=2,403) | +27.0±0.5 | <0.001 | +0.13±0.13 | 0.32 | −0.00±0.00 | 0.85 | −0.00±0.00 | 0.63 |
| California Verbal Learning Test (CVLT), List A | ||||||||
| Model 1: TSH(N=1,393; N’=2,183) | +26.8±0.8 | <0.001 | −1.42±0.19 | <0.001 | −0.42±0.19 | 0.029 | +0.03±0.05 | 0.47 |
| Model 2: fT4(N=1,438; N’=2,255) | +24.0±1.3 | <0.001 | −0.98±0.30 | 0.001 | +1.60±0.97 | 0.10 | −0.25±0.22 | 0.26 |
| Model 3: tT4(N=1,397; N’=2,195) | +25.0±1.3 | <0.001 | −1.08±0.29 | <0.001 | +0.14±0.13 | 0.28 | −0.02±0.03 | 0.57 |
| Model 4: T3pu (N=1,397; N’=2,195) | +25.1±1.8 | <0.001 | −1.31±0.41 | 0.002 | +0.02±0.06 | 0.66 | +0.00±0.01 | 0.86 |
| CVLT, free delayed recall | ||||||||
| Model 1: TSH(N=1,364; N’=2,091) | +8.25±0.39 | <0.001 | −0.42±0.09 | <0.001 | −0.13±0.09 | 0.17 | +0.01±0.02 | 0.56 |
| Model 2: fT4(N=1,411; N’=2,158) | +6.85±0.60 | <0.001 | −0.21±0.14 | 0.15 | +1.05±0.46 | 0.022 | −0.18±0.11 | 0.11 |
| Model 3: tT4(N=1,326; N’=2,036) | +7.49±0.59 | <0.001 | −0.30±0.14 | 0.031 | +0.08±0.06 | 0.21 | −0.01±0.01 | 0.50 |
| Model 4: T3pu (N=1,373; N’=2,107) | +7.55±0.85 | <0.001 | −0.46±0.20 | 0.023 | +0.02±0.03 | 0.56 | +0.00±0.01 | 0.75 |
| Benton Visual Retention Test | ||||||||
| Model 1: TSH(N=1,467; N’=2,456) | +8.63±0.61 | <0.001 | +0.41±0.15 | 0.007 | +0.19±0.14 | 0.19 | −0.02±0.03 | 0.51 |
| Model 2: fT4(N=1,515; N’=2,545) | +8.62±0.94 | <0.001 | +0.23±0.23 | 0.30 | +0.31±0.72 | 0.66 | +0.11±0.17 | 0.53 |
| fT4×Time×male: γ ±SEE:−0.93±0.35, p=0.009 | ||||||||
| Men | +5.69±1.49 | <0.001 | +1.00±0.35 | 0.005 | +1.92±1.15 | 0.10 | −0.48±0.27 | 0.07 |
| Women | +9.64±1.25 | <0.001 | −0.23±0.30 | 0.45 | −0.40±0.93 | 0.67 | +0.46±0.22 | 0.039 |
| Model 3: tT4(N=1,422; N’=2,393) | +9.03±0.94 | <0.001 | +0.40±0.22 | 0.07 | −0.05±0.10 | 0.59 | −0.00±0.02 | 0.91 |
| tT4×Time×male: γ ±SEE:−0.09±0.04, p=0.045 | ||||||||
| Men | +6.91±1.37 | <0.001 | +0.80±0.31 | 0.011 | +0.11±0.15 | 0.45 | −0.05±0.03 | 0.13 |
| Women | +10.30±1.27 | <0.001 | +0.00±0.30 | 1.00 | −0.20±0.13 | 0.13 | +0.04±0.03 | 0.16 |
| tT4×Time×AA: γ ±SEE:−0.13±0.05, p=0.004 | ||||||||
| Whites | +9.65±1.29 | <0.001 | −0.48±0.31 | 0.12 | −0.23±0.14 | 0.09 | +0.08±0.03 | 0.010 |
| AA | +8.90±1.32 | <0.001 | +1.24±0.31 | <0.001 | +0.06±0.13 | 0.67 | −0.06±0.03 | 0.06 |
| Model 4: T3pu (N=1,478; N’=2,483) | +8.90±1.31 | <0.001 | +0.67±0.32 | 0.040 | −0.01±0.04 | 0.80 | −0.01±0.01 | 0.35 |
| Brief Test of Attention | ||||||||
| Model 1: TSH(N=1,468; N’=2,376) | +6.22±0.43 | <0.001 | +0.03±0.10 | 0.75 | +0.15±0.33 | 0.65 | −0.09±0.08 | 0.26 |
| Model 2: fT4(N=1,376; N=2,229) | +6.14±0.43 | <0.001 | +0.03±0.10 | 0.74 | +0.05±0.04 | 0.25 | −0.01±0.01 | 0.18 |
| Model 3: tT4(N=1,376; N’=2,229) | +6.14±0.43 | <0.001 | +0.03±0.10 | 0.75 | +0.05±0.04 | 0.25 | −0.01±0.01 | 0.18 |
| Model 4: T3pu (N=1,427; N’=2,308) | +6.27±0.61 | <0.001 | −0.13±0.15 | 0.40 | +0.00±0.02 | 0.89 | −0.00±0.00 | 0.64 |
| Animal Fluency | ||||||||
| Model 1: TSH(N=1,471; N’=2,527) | +17.16±0.65 | <0.001 | +0.03±0.14 | 0.80 | +0.00±0.15 | 0.99 | −0.03±0.03 | 0.34 |
| Model 2: fT4(N=1,518; N’=2,618) | +15.3±1.0 | <0.001 | +0.08±0.20 | 0.70 | +1.70±0.76 | 0.024 | −0.13±0.15 | 0.39 |
| Model 3: tT4(N=1,421; N’=2,452) | +15.4±1.0 | <0.001 | −0.18±0.20 | 0.38 | +0.27±0.10 | 0.007 | +0.02±0.02 | 0.27 |
| Model 4: T3pu (N=1,477; N’=2,545) | +18.2±1.4 | <0.001 | −0.22±0.29 | 0.45 | −0.02±0.04 | 0.61 | +0.01±0.01 | 0.43 |
| Digits Span, Forward | ||||||||
| Model 1: TSH(N=1,466; N’=2,416) | +6.98±0.26 | <0.001 | −0.02±0.06 | 0.69 | −0.07±0.06 | 0.21 | +0.02±0.01 | 0.10 |
| Model 2: fT4(N=1,513; N’=2,496) | +6.39±0.40 | <0.001 | −0.04±0.09 | 0.67 | +0.41±0.31 | 0.18 | +0.05±0.07 | 0.42 |
| Model 3: tT4(N=1,598; N’=2,632) | +6.74±0.30 | <0.001 | −0.02±0.07 | 0.80 | +0.01±0.03 | 0.68 | +0.00±0.01 | 0.43 |
| Model 4: T3pu (N=1,472; N’=2,431) | +6.45±0.56 | <0.001 | −0.03±0.12 | 0.80 | +0.02±0.02 | 0.36 | +0.00±0.00 | 0.80 |
| Digits Span, Backward | ||||||||
| Model 1: TSH(N=1,465; N’=2,404) | +1.08±4.82 | 0.82 | +0.96±1.13 | 0.40 | −0.12±0.06 | 0.030 | −0.01±0.01 | 0.64 |
| Model 2: fT4(N=1,512; N’=2,482) | +0.94±4.76 | 0.84 | +1.26±1.11 | 0.25 | +0.47±0.29 | 0.10 | −0.05±0.07 | 0.46 |
| Model 3: tT4(N=1,420; N’=2,344) | +0.09±4.91 | 0.99 | +1.48±1.13 | 0.19 | +0.05±0.04 | 0.21 | −0.00±0.01 | 0.87 |
| Model 4: T3pu (N=1,420; N’=2,344) | +0.03±4.86 | 1.00 | +1.53±1.13 | 0.17 | +0.02±0.02 | 0.22 | +0.00±0.00 | 0.92 |
| Clock, command | ||||||||
| Model 1: TSH(N=1,470; N’=2,533) | +8.78±0.16 | <0.001 | −0.04±0.04 | 0.39 | +0.00±0.04 | 0.93 | −0.01±0.01 | 0.19 |
| TSH×Time×male: γ±SEE:+0.05±0.02, p=0.009 | ||||||||
| Men | +8.97±0.24 | <0.001 | −0.04±0.06 | 0.38 | −0.05±0.06 | 0.38 | +0.02±0.02 | 0.20 |
| Women | +8.71±0.21 | <0.001 | −0.04±0.06 | 0.48 | +0.04±0.05 | 0.43 | −0.03±0.01 | 0.008 |
| Model 2: fT4(N=1,517; N’=2,614) | +9.10±0.24 | <0.001 | −0.20±0.06 | 0.002 | −0.25±0.18 | 0.18 | +0.09±0.05 | 0.05 |
| Model 3: tT4(N=1,423; N’=2,454) | +8.81±0.24 | <0.001 | −0.13±0.06 | 0.037 | +0.00±0.01 | 0.52 | +0.00±0.01 | 0.52 |
| Model 4: T3pu (N=1,478; N=2,546) | +9.12±0.33 | <0.001 | −0.16±0.09 | 0.09 | −0.01±0.01 | 0.26 | +0.00±0.00 | 0.44 |
| Trailmaking test, Part A | ||||||||
| Model 1: TSH(N=1,436; N’=2,426) | +32.3±4.7 | <0.001 | +2.59±1.37 | 0.06 | 1.26±1.06 | 0.23 | −0.21±0.31 | 0.48 |
| Model 2: fT4(N=1,484; N’=2,511) | +46.4±7.0 | <0.001 | +2.00±2.04 | 0.33 | −10.42±5.31 | 0.05 | +0.16±1.53 | 0.91 |
| Model 3: tT4(N=1,395; N’=2,364) | +33.2±6.8 | <0.001 | +3.94±1.93 | 0.041 | +0.10±0.20 | 0.36 | −0.18±0.10 | 0.36 |
| Model 4: T3pu (N=1,447; N’=2,447) | +52.2±9.9 | <0.001 | −0.12±2.93 | 0.97 | −0.60±0.31 | 0.05 | +0.09±0.09 | 0.34 |
| Trailmaking test, Part B | ||||||||
| Model 1: TSH(N=1,426; N’=2,342) | +178.8±55.5 | 0.001 | +7.90±13.19 | 0.55 | +7.12±4.14 | 0.09 | −1.11±0.86 | 0.20 |
| Model 2: fT4(N1,472; N’=1,472) | +228.4±60.1 | <0.001 | +0.08±14.07 | 1.00 | −15.2±21.1 | 0.47 | +3.44±4.20 | 0.47 |
| Model 3: tT4(N=1,472; N’=2,419) | +202.2±60.2 | 0.001 | +4.08±14.85 | 0.78 | −1.53±2.79 | 0.58 | −1.53±2.79 | 0.58 |
| Model 4: T3pu (N=1,435; N’=2,362) | +201.7±68.5 | 0.003 | +8.45±14.74 | 0.57 | −1.33±1.19 | 0.27 | −0.08±0.26 | 0.76 |
Abbreviation: BVRT=Benton Visual Retention Test; CES-D=Center for Epidemiologic Studies-Depression; CVLT=California Verbal Learning Test; HANDLS=Healthy Aging in Neighborhoods of Diversity across the Life Span; MMSE=Mini-Mental State Examination; N=number of participants; N’=number of visits; THWRR=Thyroid hormone within reference range; TSH=Thyroid Stimulating Hormone; T3pu= % uptake of triiodothyronine; fT4= free thyroxine; tT4= total thyroxine; WRAT=Wide Range Achievement Test.
Multiple mixed-effects linear regression models adjusted for baseline age, sex, race/ethnicity, marital status, education, WRAT total score, poverty income ratio, current smoking status, current use of illicit drugs, body mass index, and 2010-HEI.
Most cognitive test scores were in the direction of higher score=better performance, except for BVRT (total errors), and Trailmaking Test both parts (expressed in seconds).
In a sensitivity analysis, poverty status was removed from the main mixed-effects regression model, allowing it to be an instrumental variable to compute the inverse mills ratio. Key results were not altered. A second sensitivity analysis in which anti-depressant use was included as an additional covariate in the models indicated that anti-depressant use was not an important confounder in the relationship between thyroid hormones, particularly within normal ranges, and cognitive performance or decline (data not shown).
Discussion
The present study examined associations between thyroid hormones (within and outside normal ranges) and over-time longitudinal change in cognitive performance among middle-aged US adults, using several domains of cognition and stratifying by sex and race. Several key findings emerged. Whites performed consistently better than AA on all cognitive tests, with only tests of mental status (MMSE), verbal memory (CVLT-List A and DFR) and visuo-motor/visuo-constructional abilities (BVRT) declining significantly over-time. Importantly, when examining cognitive change (Type I error corrected to 0.009) in relation to below reference range vs. within reference range hormonal status, none of the associations survived multiple testing correction. However, when comparing participants above reference ranges to those within, above reference range TSH was linked to faster rates of decline on DS-B, a test of working memory (P=0.006) and Clock-command, at test of visuo-spatial and visuo-construction abilities (P=0.004). This finding was replicated when comparing normal thyroid function to “subclinical hypothyroidism”. Within reference ranges, the higher the TSH level, the faster was the rate of decline on the clock-command test scores in women.
Our previous cross-sectional analysis of HANDLS data (Beydoun, et al., 2013) uncovered stratum-specific associations between thyroid hormones within normal ranges and cognitive performance which were not thoroughly reported in our present study. Moreover, two cognitive test scores (card rotation and identical pictures) were not measured at follow-up visits, thus precluding longitudinal analyses. Although a similar trend was detected in cross-sectional results, most of these associations at baseline did not pass correction for multiple testing, given the slightly different samples selected between studies.(Beydoun, et al., 2013)
At least nine previous cohort studies examined longitudinal relationships between thyroid hormones and cognitive performance.(Booth, et al., 2013,de Jong, et al., 2006,de Jong, et al., 2009,de Jongh, et al., 2011,Forti, et al., 2012,Gussekloo, et al., 2004,Hogervorst, et al., 2008,Tan, et al., 2008,Volpato, et al., 2002) Of those selected studies, six indicated significant (de Jong, et al., 2006,de Jong, et al., 2009,Forti, et al., 2012,Hogervorst, et al., 2008,Tan, et al., 2008,Volpato, et al., 2002) and three indicated non-significant findings.(Booth, et al., 2013,de Jongh, et al., 2011,Gussekloo, et al., 2004) While many of those studies used a single cognitive test score or dementia/AD diagnosis as the outcome, a number of findings are notable. For instance, a large cohort study of older adults (age≥65y, n=1,047) observed that both higher TSH and fT4 within normal ranges were associated with poorer performance and decline on the MMSE.(Hogervorst, et al., 2008) The latter study suggested that thyroxine can generate oxidative stress and damage neurons, and concluded that treatment with thyroxine when thyroid disease is absent is not recommended and that optimal levels of thyroxine in the elderly is possibly lower than previously indicated. (Hogervorst, et al., 2008) Thus, further large cohort studies are needed to assess whether fT4 levels indeed have a curvilinear relationship with cognitive function or decline among euthyroid individuals, whereby normal high fT4 may result in worse cognitive outcomes.
In another study that failed to show an association between thyroid hormones and incident dementia (age:60-90y, n=1,077), higher fT4 was shown to be associated with greater atrophy in the hippocampus and amygdala regions of the brain.(de Jong, et al., 2006) Those findings are comparable to ours, particularly the cross-sectional inverse relationship between fT4 and performance in the domain of language/verbal fluency and the longitudinal association between higher TSH and faster rates of decline in the domains of working memory and visuo-spatial/visuo-construction abilities. Similarly, higher TSH was associated with increased risk of vascular dementia (VaD), but not AD or mild cognitive impairment in another large cohort study, (Forti, et al., 2012) whereas in the Framingham study (N=1,864 cognitively intact individuals), the risk of AD incidence among women was linked to both a high (>2.1 mIU/L:HR=2.15 (95%CI:1.31-3.52, P=0.003) and a low (<1.0 mIU/L: HR=2.39 (95%CI:1.47-3.87, P <0.001) TSH level.(Tan, et al., 2008)
Of seven surveyed experimental studies, (Bono, et al., 2004,Burmeister, et al., 2001,Correia, et al., 2009,Miller, et al., 2006,Munte, et al., 2001,Osterweil, et al., 1992,Parle, et al., 2010) three had positive findings (Bono, et al., 2004,Correia, et al., 2009,Munte, et al., 2001), whereas the others reported mixed or null findings.(Burmeister, et al., 2001,Miller, et al., 2006,Osterweil, et al., 1992,Parle, et al., 2010) Specifically, L-thyroxine replacement was shown to normalize verbal memory in one trial for both overt and sub-clinical hypothyroid groups, and for spatial memory among the sub-clinical hypothyroid group.(Correia, et al., 2009) In another trial of L-thyroxine replacement conducted among 36 women, slight improvements in verbal fluency and depression scores were noted that were accompanied by an increase in serum fT4 in parallel with TSH level reduction.(Bono, et al., 2004)
Moreover, a neuroanatomical basis for the link between subclinical hypothyroidism and a defect in verbal working memory and executive function in particular was provided by a recent study.(Zhu, et al., 2006) In fact, subjects with a mean TSH of 14.7 mU/L were shown to have an impaired verbal working memory and abnormal fMRI findings in the frontal areas of the brain which are responsible for executive function. Of those participants, a sub-set was treated with L-T4 for 6 months reducing TSH to a mean of 1.35 mU/L which normalized both verbal working memory and fMRI results, reflecting increased regional brain glucose metabolism with such treatment. (Zhu, et al., 2006) Similarly, a more recent study by the same group (Yin, et al., 2013) showed similar results. Individuals with a mean TSH of 19.4 mIU/l exhibited decreased performance on a spatial working memory task (2-back), compared with euthyroid controls. Additionally, diminished functional activity in the right dorsolateral prefrontal cortex, right parietal lobe, and the supplementary motor area and anterior cingulate cortex was observed for those with elevated TCH levels, compared with controls. Following treatment with L-T4, TSH levels, visual working memory, and BOLD responses were similar between controls and sub-clinical hypothyroid patients. (Yin, et al., 2013)
Several mechanisms may explain the associations between thyroid function and cognition. First, both T4 and its more potent metabolite T3 are regulated in such a way as to preserve narrow concentration ranges in the brain, independent of changes in their corresponding bloodstream levels. This indicates that minute changes in thyroid hormones within brain tissues can alter behavior significantly. Moreover, T3 levels in brain tissue is largely determined by circulating T4 through local enzymatic deiodination (5′D-II deiodinase), rather than through active transport of serum T3 into the brain. Importantly, thyroid hormones in several animal studies were shown to inhibit the expression of the β-amyloid precursor protein gene. (Volpato, et al., 2002) Other animal studies also show that adult-onset hypothyroidism in rats can reduce granule cells in the dentate gyrus and pyramidal cells of the hippocampal CA1 region, reduce apical dentritic spine density in the hippocampal CA1 pyramidal neurons, decrease synaptic plasticity within the hippocampus, and impair learning, particularly in spatial and memory domains.(Cao, et al., 2012) Other adverse effects of thyroid dysfunction include altered expression of hippocampal enzymes that regulate catecholamine, serotonin, and GABA systems.(Koromilas, et al., 2010)
Our study has several notable strengths. In addition to its large sample size allowing for stratified analyses by sex and race, and its longitudinal design which allows us to ascertain temporality of associations, our study also included cognitive tests that spanned many domains of cognition, controlled for key potentially confounding factors that were socio-demographic, lifestyle and health-related. It made use of advanced multivariable techniques, including mixed-effects regression models that took into account sample selectivity. In addition, the descriptive part of the analysis also accounted for sampling weights to obtain representative estimates of means and proportions.
Despite its strengths, our study findings should be interpreted in light of key limitations. First, although major potentially confounding variables were adjusted for, residual confounding cannot be ruled out. Specifically, although many CNS medications aside from anti-depressants may affect thyroid hormonal level, previous studies have shown that their key findings were not affected by excluding individuals who were on any type of CNS medication.(Prinz, et al., 1999) Moreover, T3 and TBG were not directly available in the first-visit of HANDLS, which prevented us from examining their association with longitudinal cognitive change over-time in this sample of US middle-aged urban adults. Although reference ranges are indicative of normal levels of thyroid hormones, they may vary according to populations and published evidence. Furthermore, only 2 time points were available for our longitudinal analyses, which though an improvement over cross-sectional analyses, may be limited compared to having 3 or more time points. Thus, our key finding of a significant relationship between higher baseline TSH and cognitive decline in domains of working memory and visuo-spatial/visuo-construction abilities can possibly be the result of random fluctuation in performance rather than true decline. This random fluctuation is a result of reliability in the instrument itself and may also differ across study groups. Until further studies are done with 3 or more assessment on a comparable population of urban adults, this finding needs to be interpreted with caution. Furthermore, the effect size of the association between elevated TSH and the rate of change in measures of working memory and visuo-spatial/visuo-construction abilities may have been large in relative terms compared to the “normal” group. However, in terms of absolute decline, the effect size was smaller than anticipated, possibly due to the young age at baseline of this study population. Finally, although a large battery of neuropsychological tests was available from which cognitive domains could be extracted using factor analysis, a prior attempt to group those individual tests into distinctive domains showed that there was a lack of factorial invariance across the major variables used in HANDLS sampling design, including sex, race, age and poverty status. For this reason, only individual test scores were used and interpreted in terms of their salient domain of cognitive performance.
In sum, our study findings indicated that thyroid hormones, particularly higher TSH, are linked to faster rate of cognitive decline over-time, particularly in domains of working memory and visuo-spatial/visuo-construction ability. Moreover subclinical hypothyroidism whereby higher TSH levels are coupled with normal fT4 levels was specifically linked to decline over-time, as well as higher TSH values within normal ranges among women in the case of visuo-spatial/visuo-construction ability. Further large cohort studies are needed to replicate those findings as well as hormone replacement interventions that examine both short-term and long-term effects of thyroid hormones on age-related cognitive decline in different domains of cognition.
Supplementary Material
Highlights.
Four cognitive test scores of 11 declined significantly; Whites performed better.
Abnormal TSH lead to faster decline in working memory and visuo/spatial abilities.
This was true for “subclinical hypothyroidism” as contrasted with normal function.
Normal high TSH was related to decline in visuo/spatial abilities in women.
ACKNOWLEDGEMENTS
This study was entirely supported by the National Institute on Aging, Intramural Research Program (NIA/NIH/IRP).
ABBREVIATIONS
- AD
Alzheimer’s Disease
- AF
Animal Fluency test
- BTA
Brief Test of Attention
- BVRT
Benton Visual Retention Test
- CDT
Clock Drawing Test
- CES-D
Center for Epidemiologic Studies-Depression.
- CR
Card Rotations
- CVLT-DFR
California Verbal Learning Test, Delayed Free Recal; (List A).
- CVLT-List A
California Verbal Learning Test, immediate recall (List A).
- DS-B
Digit Span Backwards
- DS-F
Digit Span Forward
- EDS
Elevated Depressive Symptoms
- HANDLS
Healthy Aging in Neighborhoods of Diversity Across the Life Span
- HS
High School
- ICMA
Immunochemiluminometric assays
- IP
Identical Pictures
- OLS
Ordinary Least Square
- PIR
Poverty Income Ratio
- T3
triiodothyronine
- T4
thyroxine
- TBG
Thyroxin binding globulin
- Trails A
Trailmaking test, Part A
- Trails B
Trailmaking test, Part B
- TSH
Thyroid Stimulating Hormone
- WRAT
Wide Range Achievement Test
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
MAB had full access to the data used in this manuscript and completed all the statistical analyses.
REFERENCES
- Almeida C, Vaisman M, Costa AJ, Reis FA, Reuters V, Teixeira P, Ferreira M, Teixeira LB, Araujo GR, Brasil MA. Are neuropsychological changes relevant in subclinical hypothyroidism? Arq Bras Endocrinol Metabol. 2007;51(4):606–11. doi: 10.1590/s0004-27302007000400016. doi:S0004-27302007000400016 [pii] [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association 2009 Alzheimer’s disease facts and figures. Alzheimers Dement. 2009;5(3):234–70. doi: 10.1016/j.jalz.2009.03.001. doi:S1552-5260(09)00074-0 [pii] 10.1016/j.jalz.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Belin RM, Clickner R, Jeffries R, Phillips L, Mahaffey KR. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999-2002) Thyroid. 2007;17(12):1211–23. doi: 10.1089/thy.2006.0235. doi:10.1089/thy.2006.0235. [DOI] [PubMed] [Google Scholar]
- Baskin HJ, Cobin RH, Duick DS, Gharib H, Guttler RB, Kaplan MM, Segal RL. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr Pract. 2002;8(6):457–69. [PubMed] [Google Scholar]
- Beydoun MA, Beydoun HA, Kitner-Triolo MH, Kaufman JS, Evans MK, Zonderman AB. Thyroid hormones are associated with cognitive function: moderation by sex, race, and depressive symptoms. J Clin Endocrinol Metab. 2013;98(8):3470–81. doi: 10.1210/jc.2013-1813. doi:10.1210/jc.2013-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun MA, Beydoun HA, Shroff MR, Kitner-Triolo MH, Zonderman AB. Serum leptin, thyroxine and thyroid-stimulating hormone levels interact to affect cognitive function among US adults: evidence from a large representative survey. Neurobiology of aging. 2012;33(8):1730–43. doi: 10.1016/j.neurobiolaging.2011.05.008. doi:10.1016/j.neurobiolaging.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun MA, Gamaldo AA, Beydoun HA, Tanaka T, Tucker KL, Talegawkar SA, Ferrucci L, Zonderman AB. Caffeine and alcohol intakes and overall nutrient adequacy are associated with longitudinal cognitive performance among U.S. adults. The Journal of nutrition. 2014;144(6):890–901. doi: 10.3945/jn.113.189027. doi:10.3945/jn.113.189027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bono G, Fancellu R, Blandini F, Santoro G, Mauri M. Cognitive and affective status in mild hypothyroidism and interactions with L-thyroxine treatment. Acta Neurol Scand. 2004;110(1):59–66. doi: 10.1111/j.1600-0404.2004.00262.x. doi:10.1111/j.1600-0404.2004.00262.x ANE262 [pii] [DOI] [PubMed] [Google Scholar]
- Booth T, Deary IJ, Starr JM. Thyroid stimulating hormone, free thyroxine and cognitive ability in old age: The Lothian birth cohort study 1936. Psychoneuroendocrinology. 2013;38(4):597–601. doi: 10.1016/j.psyneuen.2012.07.018. doi:10.1016/j.psyneuen.2012.07.018. [DOI] [PubMed] [Google Scholar]
- Burmeister LA, Ganguli M, Dodge HH, Toczek T, DeKosky ST, Nebes RD. Hypothyroidism and cognition: preliminary evidence for a specific defect in memory. Thyroid. 2001;11(12):1177–85. doi: 10.1089/10507250152741037. doi:10.1089/10507250152741037. [DOI] [PubMed] [Google Scholar]
- Cao L, Wang F, Yang QG, Jiang W, Wang C, Chen YP, Chen GH. Reduced thyroid hormones with increased hippocampal SNAP-25 and Munc18-1 might involve cognitive impairment during aging. Behavioural brain research. 2012;229(1):131–7. doi: 10.1016/j.bbr.2012.01.014. doi:10.1016/j.bbr.2012.01.014. [DOI] [PubMed] [Google Scholar]
- Ceballos A, Belinchon MM, Sanchez-Mendoza E, Grijota-Martinez C, Dumitrescu AM, Refetoff S, Morte B, Bernal J. Importance of monocarboxylate transporter 8 for the blood-brain barrier-dependent availability of 3,5,3′-triiodo-L-thyronine. Endocrinology. 2009;150(5):2491–6. doi: 10.1210/en.2008-1616. doi:10.1210/en.2008-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceresini G, Lauretani F, Maggio M, Ceda GP, Morganti S, Usberti E, Chezzi C, Valcavi R, Bandinelli S, Guralnik JM, Cappola AR, Valenti G, Ferrucci L. Thyroid function abnormalities and cognitive impairment in elderly people: results of the Invecchiare in Chianti study. Journal of the American Geriatrics Society. 2009;57(1):89–93. doi: 10.1111/j.1532-5415.2008.02080.x. doi:10.1111/j.1532-5415.2008.02080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia N, Mullally S, Cooke G, Tun TK, Phelan N, Feeney J, Fitzgibbon M, Boran G, O’Mara S, Gibney J. Evidence for a specific defect in hippocampal memory in overt and subclinical hypothyroidism. J Clin Endocrinol Metab. 2009;94(10):3789–97. doi: 10.1210/jc.2008-2702. doi:jc.2008-2702 [pii] 10.1210/jc.2008-2702. [DOI] [PubMed] [Google Scholar]
- de Jong FJ, den Heijer T, Visser TJ, de Rijke YB, Drexhage HA, Hofman A, Breteler MM. Thyroid hormones, dementia, and atrophy of the medial temporal lobe. The Journal of clinical endocrinology and metabolism. 2006;91(7):2569–73. doi: 10.1210/jc.2006-0449. doi:10.1210/jc.2006-0449. [DOI] [PubMed] [Google Scholar]
- de Jong FJ, Masaki K, Chen H, Remaley AT, Breteler MM, Petrovitch H, White LR, Launer LJ. Thyroid function, the risk of dementia and neuropathologic changes: the Honolulu-Asia aging study. Neurobiology of aging. 2009;30(4):600–6. doi: 10.1016/j.neurobiolaging.2007.07.019. doi:10.1016/j.neurobiolaging.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jongh RT, Lips P, van Schoor NM, Rijs KJ, Deeg DJ, Comijs HC, Kramer MH, Vandenbroucke JP, Dekkers OM. Endogenous subclinical thyroid disorders, physical and cognitive function, depression, and mortality in older individuals. European journal of endocrinology / European Federation of Endocrine Societies. 2011;165(4):545–54. doi: 10.1530/EJE-11-0430. doi:10.1530/EJE-11-0430. [DOI] [PubMed] [Google Scholar]
- Dugbartey AT. Neurocognitive aspects of hypothyroidism. Arch Intern Med. 1998;158(13):1413–8. doi: 10.1001/archinte.158.13.1413. [DOI] [PubMed] [Google Scholar]
- Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, Zonderman AB. Healthy aging in neighborhoods of diversity across the life span (HANDLS): overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethn Dis. 2010;20(3):267–75. [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Klein S, Holloszy JO, Premachandra BN. Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J Clin Endocrinol Metab. 2006;91(8):3232–5. doi: 10.1210/jc.2006-0328. doi:10.1210/jc.2006-0328. [DOI] [PubMed] [Google Scholar]
- Formiga F, Ferrer A, Padros G, Contra A, Corbella X, Pujol R, Octabaix Study G. Thyroid status and functional and cognitive status at baseline and survival after 3 years of follow-up: the OCTABAIX study. European journal of endocrinology / European Federation of Endocrine Societies. 2014;170(1):69–75. doi: 10.1530/EJE-13-0722. doi:10.1530/EJE-13-0722. [DOI] [PubMed] [Google Scholar]
- Forti P, Olivelli V, Rietti E, Maltoni B, Pirazzoli G, Gatti R, Gioia MG, Ravaglia G. Serum thyroid-stimulating hormone as a predictor of cognitive impairment in an elderly cohort. Gerontology. 2012;58(1):41–9. doi: 10.1159/000324522. doi:10.1159/000324522. [DOI] [PubMed] [Google Scholar]
- Grigorova M, Sherwin BB. Thyroid hormones and cognitive functioning in healthy, euthyroid women: a correlational study. Hormones and behavior. 2012;61(4):617–22. doi: 10.1016/j.yhbeh.2012.02.014. doi:10.1016/j.yhbeh.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frolich M, Westendorp RG. Thyroid status, disability and cognitive function, and survival in old age. JAMA : the journal of the American Medical Association. 2004;292(21):2591–9. doi: 10.1001/jama.292.21.2591. doi:10.1001/jama.292.21.2591. [DOI] [PubMed] [Google Scholar]
- Heckman JJ. Sample selection bias as a specification error. Econometrica. 1979;47:153–61. [Google Scholar]
- Hochberg Y, Tamhane AC. Multiple comparison procedures. Wiley; New York: 1987. [Google Scholar]
- Hogervorst E, Huppert F, Matthews FE, Brayne C. Thyroid function and cognitive decline in the MRC Cognitive Function and Ageing Study. Psychoneuroendocrinology. 2008;33(7):1013–22. doi: 10.1016/j.psyneuen.2008.05.008. doi:10.1016/j.psyneuen.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Joffe RT, Pearce EN, Hennessey JV, Ryan JJ, Stern RA. Subclinical hypothyroidism, mood, and cognition in older adults: a review. International journal of geriatric psychiatry. 2013;28(2):111–8. doi: 10.1002/gps.3796. doi:10.1002/gps.3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koromilas C, Liapi C, Schulpis KH, Kalafatakis K, Zarros A, Tsakiris S. Structural and functional alterations in the hippocampus due to hypothyroidism. Metabolic brain disease. 2010;25(3):339–54. doi: 10.1007/s11011-010-9208-8. doi:10.1007/s11011-010-9208-8. [DOI] [PubMed] [Google Scholar]
- Kramer CK, von Muhlen D, Kritz-Silverstein D, Barrett-Connor E. Treated hypothyroidism, cognitive function, and depressed mood in old age: the Rancho Bernardo Study. European journal of endocrinology / European Federation of Endocrine Societies. 2009;161(6):917–21. doi: 10.1530/EJE-09-0606. doi:10.1530/EJE-09-0606. [DOI] [PubMed] [Google Scholar]
- Lohr SL. Sampling: Design and Analysis. Duxbury-Press; 1999. [Google Scholar]
- Miller KJ, Parsons TD, Whybrow PC, van Herle K, Rasgon N, van Herle A, Martinez D, Silverman DH, Bauer M. Memory improvement with treatment of hypothyroidism. The International journal of neuroscience. 2006;116(8):895–906. doi: 10.1080/00207450600550154. doi:10.1080/00207450600550154. [DOI] [PubMed] [Google Scholar]
- Munte TF, Radamm C, Johannes S, Brabant G. Alterations of cognitive functions induced by exogenous application of thyroid hormones in healthy men: a double-blind cross-over study using event-related brain potentials. Thyroid. 2001;11(4):385–91. doi: 10.1089/10507250152039145. doi:10.1089/10507250152039145. [DOI] [PubMed] [Google Scholar]
- Osterweil D, Syndulko K, Cohen SN, Pettler-Jennings PD, Hershman JM, Cummings JL, Tourtellotte WW, Solomon DH. Cognitive function in non-demented older adults with hypothyroidism. J Am Geriatr Soc. 1992;40(4):325–35. doi: 10.1111/j.1532-5415.1992.tb02130.x. [DOI] [PubMed] [Google Scholar]
- Parle J, Roberts L, Wilson S, Pattison H, Roalfe A, Haque MS, Heath C, Sheppard M, Franklyn J, Hobbs FD. A randomized controlled trial of the effect of thyroxine replacement on cognitive function in community-living elderly subjects with subclinical hypothyroidism: the Birmingham Elderly Thyroid study. J Clin Endocrinol Metab. 2010;95(8):3623–32. doi: 10.1210/jc.2009-2571. doi:10.1210/jc.2009-2571. [DOI] [PubMed] [Google Scholar]
- Prinz PN, Scanlan JM, Vitaliano PP, Moe KE, Borson S, Toivola B, Merriam GR, Larsen LH, Reed HL. Thyroid hormones: positive relationships with cognition in healthy, euthyroid older men. J Gerontol A Biol Sci Med Sci. 1999;54(3):M111–6. doi: 10.1093/gerona/54.3.m111. [DOI] [PubMed] [Google Scholar]
- Ross DS. Subclinical hyperthyroidism: possible danger of overzealous thyroxine replacement therapy. Mayo Clin Proc. 1988;63(12):1223–9. doi: 10.1016/s0025-6196(12)65409-3. [DOI] [PubMed] [Google Scholar]
- Samuels MH. Thyroid disease and cognition. Endocrinology and metabolism clinics of North America. 2014;43(2):529–43. doi: 10.1016/j.ecl.2014.02.006. doi:10.1016/j.ecl.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Samuels MH, Schuff KG, Carlson NE, Carello P, Janowsky JS. Health status, psychological symptoms, mood, and cognition in L-thyroxine-treated hypothyroid subjects. Thyroid. 2007;17(3):249–58. doi: 10.1089/thy.2006.0252. doi:10.1089/thy.2006.0252. [DOI] [PubMed] [Google Scholar]
- Selvin S. Statistical Analysis of Epidemiologic Data. 3rd ed Oxford University Press; 2004. [Google Scholar]
- Tan ZS, Beiser A, Vasan RS, Au R, Auerbach S, Kiel DP, Wolf PA, Seshadri S. Thyroid function and the risk of Alzheimer disease: the Framingham Study. Archives of internal medicine. 2008;168(14):1514–20. doi: 10.1001/archinte.168.14.1514. doi:10.1001/archinte.168.14.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boxtel MP, Menheere PP, Bekers O, Hogervorst E, Jolles J. Thyroid function, depressed mood, and cognitive performance in older individuals: the Maastricht Aging Study. Psychoneuroendocrinology. 2004;29(7):891–8. doi: 10.1016/j.psyneuen.2003.08.002. doi:10.1016/j.psyneuen.2003.08.002. [DOI] [PubMed] [Google Scholar]
- van de Rest O, Berendsen AA, Haveman-Nies A, de Groot LC. Dietary patterns, cognitive decline, and dementia: a systematic review. Advances in nutrition. 2015;6(2):154–68. doi: 10.3945/an.114.007617. doi:10.3945/an.114.007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpato S, Guralnik JM, Fried LP, Remaley AT, Cappola AR, Launer LJ. Serum thyroxine level and cognitive decline in euthyroid older women. Neurology. 2002;58(7):1055–61. doi: 10.1212/wnl.58.7.1055. [DOI] [PubMed] [Google Scholar]
- Wijsman LW, de Craen AJ, Trompet S, Gussekloo J, Stott DJ, Rodondi N, Welsh P, Jukema JW, Westendorp RG, Mooijaart SP. Subclinical thyroid dysfunction and cognitive decline in old age. PloS one. 2013;8(3):e59199. doi: 10.1371/journal.pone.0059199. doi:10.1371/journal.pone.0059199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JJ, Liao LM, Luo DX, Xu K, Ma SH, Wang ZX, Le HB, Huang RR, Cai ZL, Zhang J. Spatial working memory impairment in subclinical hypothyroidism: an FMRI study. Neuroendocrinology. 2013;97(3):260–70. doi: 10.1159/000343201. doi:10.1159/000343201. [DOI] [PubMed] [Google Scholar]
- Zhu DF, Wang ZX, Zhang DR, Pan ZL, He S, Hu XP, Chen XC, Zhou JN. fMRI revealed neural substrate for reversible working memory dysfunction in subclinical hypothyroidism. Brain : a journal of neurology. 2006;129(Pt 11):2923–30. doi: 10.1093/brain/awl215. doi:10.1093/brain/awl215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.