Abstract
Melanocytic nevi are a benign clonal proliferation of cells expressing the melanocytic phenotype, with heterogeneous clinical and molecular characteristics. In this review, we discuss the genetics of nevi by salient nevi subtypes: congenital melanocytic nevi, acquired melanocytic nevi, blue nevi, and Spitz nevi. While the molecular etiology of nevi has been less thoroughly studied than melanoma, it is clear that nevi and melanoma share common driver mutations. Acquired melanocytic nevi harbor oncogenic mutations in BRAF, which is the predominant oncogene associated with melanoma. Congenital melanocytic nevi and blue nevi frequently harbor NRAS mutations and GNAQ mutations, respectively, while Spitz and atypical Spitz tumors often exhibit HRAS and kinase rearrangements. These initial “driver” mutations are thought to trigger the establishment of benign nevi. After this initial phase of cell proliferation, a senescence program is executed, causing termination of nevi growth. Only upon the emergence of additional tumorigenic alterations, which may provide an escape from oncogene-induced senescence, can malignant progression occur. Here, we review the current literature on the pathobiology and genetics of nevi in the hope that additional studies of nevi promise to inform our understanding of the transition from benign neoplasm to malignancy.
Keywords: congenital melanocytic nevi, acquired melanocytic nevi, Spitz nevi, blue nevi, genetics
Introduction
The term “melanocytic neoplasm” is used to describe the presence of melanocytic cells in epidermal nests, which are defined as three or more melanocytic cells in direct contact, within the dermis or other tissues. Melanocytic neoplasms represent a diverse group of tumors that can be either benign (nevi) or malignant (melanoma). Nevi are believed to be benign clonal proliferations of cells expressing the melanocytic phenotype (Magana-Garcia and Ackerman, 1990). The development of melanocytic nevi is a multifactorial and heterogeneous biologic process, and the molecular events resulting in melanocytic neoplasms are multifold and only beginning to be unraveled. In contrast to the extensive and genome-wide studies of melanomas, studies of benign melanocytic lesions have been restricted to mutational analyses targeting genes classically involved in melanomagenesis (Papp et al., 2003; Pollock et al., 2003). This lack of comprehensive molecular profiling is partly an artifact of limited nevi tissue availability after clinically biopsied samples are processed for histologic diagnoses. In melanoma, BRAF and NRAS gene mutations have been used as genetic markers in studies attempting to integrate genetic and morphologic features to improve classification (Cancer Genome Atlas Network. Electronic address and Cancer Genome Atlas, 2015; Pozzobon et al., 2014; Viros et al., 2008). BRAF is a serine-threonine kinase that is activated by the RAS family of proteins. Once activated, it triggers the MAPK signaling cascade that ultimately leads to cell cycle progression, upregulation of transcription, and differentiation. NRAS is one of the three major isoforms of the RAS family of GTPase proteins that are involved in cell growth, differentiation, and survival. A high frequency of oncogenic BRAF mutations at a V660E in the kinase domain of exon 15 has been reported in melanomas and melanocytic nevi, suggesting mutational activation of the RAS/RAF/MEK/ERK pathway is a critical step in the development of these melanocytic tumors (Davies et al., 2002; Pollock et al., 2003) (Figure 1).
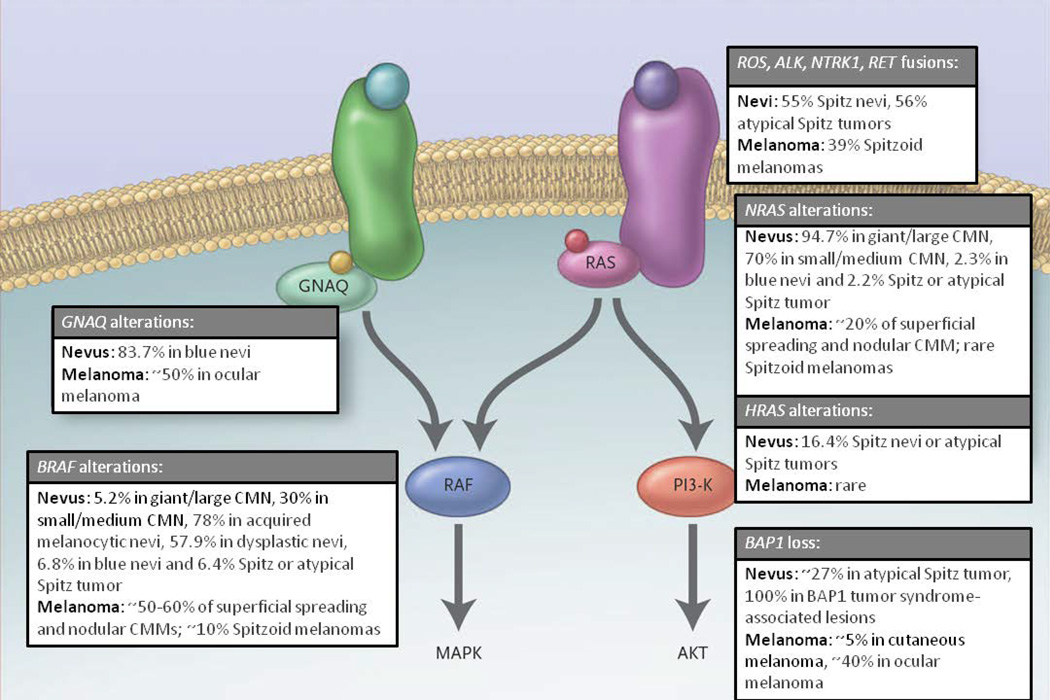
Figure 1. The RAS Signaling pathways and Nevi.
MAPK signaling promotes cell growth and survival and is constitutively active in nevi. RAS family members are activated by receptor tyrosine kinases and signal through effector proteins, including RAF kinases and PI3K. Acquired melanocytic nevi harbor oncogenic mutations in BRAF, which is the predominant oncogene associated with melanoma. Congenital melanocytic nevi, blue nevi, and Spitz nevi frequently harbor NRAS mutations, GNAQ mutations, and HRAS alterations, respectively (Modified from N Engl J Med. 2010 Sep 30;363(14):1352-60 Copyright © (2010) Massachusetts Medical Society. Reprinted with permission.)
In contrast to the various mouse models developed to investigate the molecular events underlying melanomagenesis, there are few models to investigate nevus development. The first model system employed targeted overexpression of BRAFV600E in the melanocyte compartment of transgenic zebrafish, which caused proliferation of melanocytes, producing fish nevi (Patton et al., 2005). Similarly, in mice, expression of BRAFV600E in the melanocytic lineage produced nevi with signs of senescence (Goel et al., 2009). Most recently, a mouse model has been developed that closely resembles nevi in humans. In this model, BRAFV600E was conditionally expressed from its endogenous promoter and restricted to melanocytes (Dankort et al., 2009) (Dhomen et al., 2009). Also, pigmented hairless mice with genetic deficiencies in Ink4a/Arf and Xpa genes can produce nevi (~1 to 2 per mouse), but none advance to melanoma (van Schanke et al., 2006). Also, a substantial proportion of these mice remain nevus-free, thus limiting their use as a model for nevus development. Nasti et al. developed a mouse model of nevus initiation and progression with C3H/HeN mice using a modified chemical carcinogenesis protocol (Nasti et al., 2015). In this model, dysplastic pigmented skin lesions appeared in 7–9 weeks with 100% penetrance and nests of melanocytic cells appeared in a subset of skin draining lymph nodes. This model may yield insight into acquired mutations at the early stages of nevus initiation and promotion of nevus cell transformation. However, additional nevus/melanoma models are needed to identify genetic underpinnings of nevus formation, novel oncogenic pathways, targets for immune-prevention, and mechanisms of nevus development.
Benign melanocytic neoplasms can be sub-categorized based on clinical and histological characteristics. Common subgroups include congenital melanocytic nevi (CMN), acquired melanocytic nevi, blue nevi, and Spitz nevi (Figure 2). We will discuss the genetics of nevi by type.
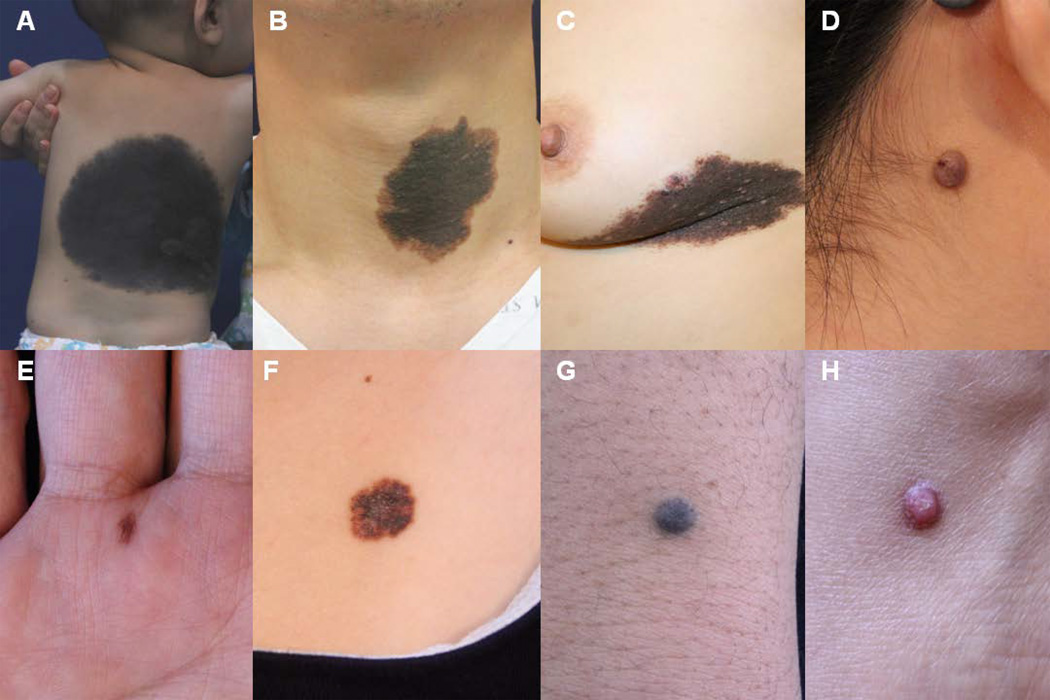
Figure 2.
Clinical images of nevi. Subtypes of nevi include large CMN (A), medium CMN (B), melanoma arising in CMN (C), intradermal nevus (D), acral junctional nevus (E), dysplastic nevus (F), blue nevus (G), and Spitz nevus (H).
CONGENITAL MELANOCYTIC NEVI
Congenital melanocytic nevi (CMN) overwhelmingly occur in a sporadic pattern, with rare reports of familial cases, and as such are believed to result from somatic mutations in utero (Charbel et al., 2014). There have been several studies seeking to identify genetic hits underlying CMN pathogenesis, and the aggregate results of these studies suggest NRAS mutations and, to a lesser extent, BRAF mutations, contribute to the development of CMN (Table 1). The literature on the molecular characterization of CMN often cites discrepancies between studies demonstrating a high prevalence of BRAF mutations (Ichii-Nakato et al., 2006; Pollock et al., 2003; Qi et al., 2011; J. Wu et al., 2007; Yazdi et al., 2003), and others that report NRAS mutations as more common (Bauer et al., 2007; Carr and Mackie, 1994; Charbel et al., 2014; Dessars et al., 2009; Kinsler et al., 2013; D. Wu et al., 2011). These inconsistencies may be related to differences in study methodology. For example, some studies only sequenced CMN for either BRAF or NRAS mutations, but not both; others examined one size of CMN, or failed to describe the size of studied lesions altogether. Many of these studies do not report how diagnoses were made, or if lesions diagnosed as CMN were documented as being present at birth (Table 1).
Table 1.
Studies evaluating the mutation status of BRAF and NRAS in congenital melanocytic nevi.
| Study | Size of CMN analyzed* |
BRAF mutations |
NRAS mutations |
Median age (years) at biopsy/excision |
Documented presence at birth |
|---|---|---|---|---|---|
| (Carr and Mackie, 1994) | Small | -- | 12/43(27.9%) | 28 | Yes |
| (Papp et al., 1999)† | Small | -- | 1/2(50%) | -- | |
| Medium | -- | 9/16(56.2%) | 12 | -- | |
| (Pollock et al., 2003) | -- | 6/7(85.7%) | 2/7(28.6%) | -- | -- |
| (Yazdi et al., 2003) | -- | 6/13(46.2%) | -- | -- | -- |
| (Papp et al., 2005)† | Small | 1/2(50%) | -- | -- | |
| Medium | 6/16(37.5%) | -- | 12 | -- | |
| (De Raeve et al., 2006) | Giant | 0/9 | -- | <1 | Yes |
| (Ichii-Nakato et al., 2006) | Small | 37/42(88.1%) | -- | 26 (mean) | Yes |
| Medium | 6/20(30%) | 9/20(45%) | 19 (mean) | Yes | |
| (Bauer et al., 2007) | Small# | 20/28(71.4%) | 7/28(25%) | 40 | -- |
| Medium/Large | 0/32 | 26/32(81.2%) | 1.33 | Yes | |
| (J. Wu et al., 2007) | Small# | 20/25(80%) | -- | -- | -- |
| Large | 6/9(66.7%) | -- | -- | Yes | |
| (Dessars et al., 2009) | Medium | 1/3(33.3%) | 1/3 (33.3%) | -- | -- |
| Large | 3/24(12.5%)▪ | 18/24(75%) | -- | -- | |
| (Phadke et al., 2011) | Small/Medium | 7/16(43.8%) | 1/16(6.2%) | 4 | -- |
| Giant | 2/27(7.4%) | 12/27(44.4%) | -- | ||
| (Qi et al., 2011) | -- | 61/104(58.7%) | 2/104(1.9%) | -- | Yes |
| (D. Wu et al., 2011) | Medium | 9/37(24.3%) | 10/37(27%) | 10 (mean) | Yes |
| Giant | 0/18 | 3/18 (16.7%) | 7.9 (mean) | Yes | |
| (Kinsler et al., 2013) | Medium/Large/Giant | -- | 10/13(76.9%) | 8.3 | -- |
| (Charbel et al., 2014) | Small/Medium | 6/20(30%) | 14/20(70%) | 4.17 | Yes |
| Large/Giant | 1/19(5.3%) | 18/19(94.7%) | 0.66 | Yes |
Studies used different classification schemes to define medium, large, and giant CMN
Same CMN samples used in both studies by Papp et al.
“congenital pattern nevi”
Two BRAF wild-type CMN showed chromosomal translocations affecting BRAF loci, with suspected oncogene activation.
More recent studies, which have included different CMN sizes, have refined the picture of this genetic landscape, revealing a consistent relationship between the size of lesion and mutation status (Table 1). BRAF or NRAS mutations are typically found in small CMN, but the prevalence of NRAS mutations is greater among medium to giant CMN. The increase in frequency of NRAS mutations is concomitant with a decrease in frequency of BRAF mutations. The most recent genetic study, conducted by Charbel et al. using multiple powerful sequencing techniques and comparable cohorts of small/medium and large/giant CMN documented at birth, affirms this trend. Of the 19 large/giant CMN examined, 18 (94.7%) were positive for an NRAS mutation and the remaining one harbored a BRAF mutation; 6 out of 20 (30%) small/medium CMN carried a BRAF mutation, and the remaining 70% were NRAS-mutants (Charbel et al., 2014) (Table 1). The inverse relationship between prevalence of BRAF mutations and size of CMN lesion is supported further by reports of zero BRAF mutations found in two series of giant CMN (De Raeve et al., 2006; D. Wu et al., 2011) and one series of medium/large CMN (Bauer et al., 2007) (Table 1).
It was aforementioned that many of these studies do not include statements regarding documentation confirming the presence of CMN lesions at birth, or arising shortly thereafter. This is an often overlooked weakness of these studies because, as Bauer et al. remarks, the diagnosis of CMN is not always made based on a documented history at birth. The distinctive histopathologic features of CMN are sometimes used to diagnose nevi with an unknown history as “congenital pattern nevi,” and nevi with this classification may be grouped together with documented congenital nevi. The accuracy of such diagnoses has been evaluated, and it has been repeatedly determined that the classic histopathologic features of CMN have limited specificity in predicting which nevi were truly present at birth (Bauer et al., 2007; Clemmensen and Kroon, 1988; Cribier et al., 1999; Rhodes et al., 1985). Misdiagnosing acquired nevi as congenital would presumably falsely increase the prevalence of BRAF mutations since acquired melanocytic nevi have high rates of BRAF mutations (Pollock et al., 2003; Poynter et al., 2006; Uribe et al., 2003). Furthermore, as CMN larger than a few centimeters are unlikely to be missed at birth, misdiagnoses based on histological features are more likely to occur with small lesions and may account, in part, for the high rates of BRAF mutations reported in small CMN. Another potential cause for the variation in rates of observed mutations may be due to sampling errors secondary to low proportions of mutated nevus cells relative to keratinocytes and stroma, or a clonal heterogeneity of nevus cells (Charbel et al., 2014; Ichii-Nakato et al., 2006).
Attempts at molecularly characterizing CMN have primarily focused on genetic aberrations in the BRAF and NRAS loci, in part because of the increased risk of melanoma inherent to larger CMN (Krengel et al., 2006; Watt et al., 2004) and the prominent role of BRAF and NRAS mutations in melanoma biology. It is becoming clearer that different classes of CMN likely have different genetic signatures, but our knowledge of these genetic signatures has only scratched the surface, with few studies interested in potential targets of mutation other than BRAF and NRAS. Papp et al. screened 18 small/medium CMN for mutations and deletions in TP53, CDKN2A, and CDK4 genes, and only found two silent mutations in TP53 (Papp et al., 1999). Kinsler et al. also performed mutational analyses for several genes, reporting one incident each of GNAQ and HRAS mutations and 3 KRAS mutations from a panel of over two-dozen CMN (Kinsler et al., 2013). Interested in identifying other somatic mutations that might be cooperating with NRAS mutations to foster CMN pathogenesis, Charbel et al. performed whole exome sequencing on 5 large/giant CMN. After eliminating several possible hits as false positives using confirmatory sequencing, the only recurrent somatic mutation identified was, unsurprisingly, in the NRAS gene (Charbel et al., 2014).Shakhova et al. described a mouse model for giant congenital nevi and show that nevi and melanoma prominently express Sox10. Sox10 haploinsufficiency counteracts NRASQ61K-driven congenital nevus and melanoma formation without affecting the physiological functions of neural crest derivatives in the skin. Furthermore, they showed that SOX10 silencing in human melanoma cells suppresses neural crest stem cell properties, counteracting proliferation and cell survival, and completely abolishes in vivo tumor formation (Shakhova et al., 2012). Although much remains to be learned about CMN genetics, as sequencing technology continues to advance in accuracy, ease, and cost, it is plausible that CMN will one day be classified by genotype rather than phenotype.
ACQUIRED MELANOCYTIC NEVI
The term nevus is derived from a Latin root meaning “birthmark,” implying that nevi are present at birth. However, despite the root meaning, the majority of nevi are acquired after birth. Interestingly, these acquired nevi share genetic and environmental risk factors with malignant melanoma. Individuals with fair skin, a tendency to sunburn, and poor tanning ability are at an increased risk for malignant melanoma (Bataille, 2003) and have increased number of melanocytic nevi (Bauer and Garbe, 2003). Additionally, several studies implicate a propensity to develop melanocytic nevi as an independent risk factor for cutaneous melanoma (Bauer and Garbe, 2003). As 20–30% of melanomas arise from preexisting melanocytic nevi (Rivers, 2004), it is not surprising that many of the genetic underpinnings of melanoma have also been found in nevi.
The role of the BRAF mutation in the current genetic basis of melanoma development and progression is based on the Clark model for melanoma progression (Bennett, 2003), corresponding to the proposed morphologic changes from benign nevus to dysplastic nevus to melanoma (W. E. Damsky et al., 2014). BRAF mutations were first described in 2003 (Pollock et al., 2003), with 81.5% (53 of 65) of acquired nevi studied harboring a BRAF mutation. Since this report, many benign acquired melanocytic nevi have been analyzed, and 78% (373/478) were found to have a BRAF mutation; an NRAS mutation was found in only 6.0% (8/134) of acquired melanocytic nevi (Table 2).
Table 2.
Studies evaluating the mutation status of BRAF and NRAS in acquired melanocytic nevi
| Study | Histology of nevi analyzed* |
BRAF mutations |
NRAS mutations |
|---|---|---|---|
| (Jafari et al., 1995) | Benign nevi | -- | 0/5 (0%) |
| (Pollock et al., 2003) | Intradermal | 37/42(88%) | -- |
| Compound | 16/23(70%) | -- | |
| (Dong et al., 2003) | Benign melanocytic nevi | 17/24(70.8%) | 0/24(0%) |
| (Saldanha et al., 2004) | Common acquired nevi | 14/16(87.5%) | 2/11(18.2%) |
| (Poynter et al., 2006) | Benign melanocytic nevi | 42/51(82.3%) | 3/51(5.9%) |
| (Ichii-Nakato et al., 2006) | Common acquired nevi | 105/120(87.5%) | -- |
| (Uribe et al., 2006) | Common acquired nevi | 16/24(66.7%) | -- |
| (Bloethner et al., 2007) | Benign melanocytic nevi | 18/30(60%) | -- |
| (J. Wu et al., 2007) | Common acquired nevi | 83/101(82.2%) | -- |
| (Venesio et al., 2008) | Compound nevi | 6/13(46.2%) | 0/13(0%) |
| Intradermal nevi | 3/6(50%) | 0/6(0%) | |
| Junctional nevi | 2/3(66.7%) | 0/3(0%) | |
| (Tschandl et al., 2013) | Control nevi | 14/25(56%) | 3/21(14.3%) |
Like melanoma, melanocytic nevi frequently harbor activating BRAF mutations, which are thought to be an initial step in melanocytic neoplasia; this idea is supported by the finding that inducible expression of BRAFV600E in melanocytes of mice yields melanocytic nevi and melanoma (Dhomen et al., 2009). However, the initial growth of a melanocytic nevus is followed by loss of proliferative activity and stabilization of size. Mirroring this clinical observation, sustained BRAFV600E expression in normal human melanocytes induces cell cycle arrest accompanied by the induction of both p16INK4a and acidic β-galactosidase activity, which are also demonstrated in lesions of melanoma in situ (Gray-Schopfer et al., 2006; Michaloglou et al., 2008). Thus, some melanocytic nevi are likely benign clonal tumors that temporarily undergo proliferation via oncogenic BRAF signaling followed by growth arrest due to oncogene-induced senescence (OIS), a concept discussed later (Michaloglou et al., 2008). It is clear that BRAF gene mutations are not sufficient to confer malignant change to melanocytes and other factors must play a role. Furthermore, the high concordance rate (80.4%) of BRAF mutation between melanoma and its nevus counterpart (Tschandl et al., 2013), as well as the occurrence of BRAF wild-type melanoma arising in BRAF-mutant nevi (Tan et al., 2015) suggest that other molecular signatures are involved in melanoma development.
Distinct dermoscopic structures have been identified that correspond to histopathological features of nevi (Yadav et al., 1993). For example, a pigment network (or reticulation) corresponds to a junctional, predominantly lentiginous melanocytic proliferation whereas globules correspond to large dermal or epidermal melanocytic nests (Argenziano et al., 2007). Recently, Marchetti et al. showed that the frequency of BRAFV600E mutations differs in nevi distinguished by unique dermoscopic structures and microanatomical growth patterns (Marchetti et al., 2014). Globular nevi correspond histopathologically to a predominantly dermal growth pattern or the presence of large junctional nests, and were significantly more likely to express BRAFV600E than reticular nevi(Marchetti et al., 2014).These finding were overall consistent with previous studies showing that intradermal nevi are significantly more likely to have BRAF mutations than juctional nevi(Hafner et al., 2009; Karram et al., 2013), and that BRAFV600E positive nevi are less likely to have a junctional component or show lentiginous growth characteristics compared with BRAF wild-type nevi (Tschandl et al., 2013).
DYSPLASTIC NEVI
Patients with multiple dysplastic nevi are known to be at higher risk of developing cutaneous melanoma. An individual with a single clinically dysplastic nevus has a 2-fold increased risk for melanoma, whereas having greater than 10 clinically dysplastic nevi is associated with a 12-fold increased risk (Bataille et al., 1996; Marghoob et al., 1994). Moreover, dysplastic nevi are more common in patients with melanoma; 30% of melanomas seem to arise in association with nevi, most commonly a dysplastic nevus, and a personal and family history of melanoma can significantly increase the incidence of finding dysplastic nevi in a patient (Bataille et al., 1996; Seykora and Elder, 1996; Sober and Burstein, 1995). When dysplastic nevi were separately analyzed, 57.9 % (84/145) of the nevi were positive for a BRAF mutation (Table 3). In the late 1970s, Clark et al. (Clark et al., 1978) proposed that dysplastic nevi are an evolutionary precursor to melanoma in patients with a family history of melanoma (Clark et al., 1984; Rhodes et al., 1983; Rhodes et al., 1982). However, the biologic and clinical implications of this observation remain unclear as benign, normal-appearing acquired melanocytic nevi can also be found in association with melanomas (Hastrup et al., 1991; Rhodes et al., 1982).
Table 3.
Studies evaluating the mutation status of BRAF in dysplastic nevi
| Study | Nevi included |
BRAF mutations |
NRAS mutations |
|---|---|---|---|
| (Pollock et al., 2003) | Dysplastic nevi | 4/5(80%) | -- |
| (Papp et al., 2005) | Dysplastic nevi | 4/18(22%) | -- |
| (Uribe et al., 2006) | Atypical nevi | 13/21(61.9%) | -- |
| (Saroufim et al., 2014) | Dysplastic nevi | 63/125(55.8%) | -- |
BLUE NEVI
Blue nevi are a group of acquired, pigmented, dermal dendritic melanocytic proliferations comprised of many described variants, the two major ones being common blue nevi and cellular blue nevi (Held et al., 2013; Zembowicz and Mihm, 2004; Zembowicz and Phadke, 2011). Common blue nevi may arise at any age, or be congenital in nature, but have a tendency to appear in adolescence, and most often present as a small (<1 cm) solitary, dark blue-to-black papule (Argenziano et al., 2007; Gonzalez-Campora et al., 1994; Murali et al., 2009; Zembowicz and Phadke, 2011). Like common blue nevi, cellular blue nevi occur at all ages, but are most common in adults younger than 40 years of age (Murali et al., 2009). Cellular blue nevi also have a deep bluish-black color, but present as larger plaques or nodules greater than 1 cm, and sometimes several centimeters, in diameter (Zembowicz and Phadke, 2011). The distinct color of these lesions is due to the dermal location of the melanin, which causes preferential reflection of short-wavelength blue light due to a scattering effect known as Tyndall scattering (Zembowicz and Mihm, 2004). Blue nevi have a predilection for certain body sites, including the scalp, dorsal surfaces of distal extremities, and sacral region; common blue nevi are most often located on extremities, and cellular blue nevi most often in the sacral region (Gonzalez-Campora et al., 1994; Temple-Camp et al., 1988; Zembowicz and Phadke, 2011). It is theorized that blue nevi consistently occur in these body regions because these are the sites with active dermal melanocytes at birth, whereas melanocytes migrate through and eventually disappear from the majority of the body’s dermis during fetal life (Leopold and Richards, 1968; Zembowicz and Phadke, 2011; Zimmermann and Cornbleet, 1948). Histologically, blue nevi are dermal lesions that rarely have a junctional component (may be present in the case of combined nevi), with the diagnostic cell type of common and cellular blue nevi being spindled dendritic and ovoid melanocytes, respectively (Held et al., 2013; Zembowicz and Phadke, 2011).
Clinically and histopathologically, blue nevi are distinguishable as an independent class of melanocytic proliferations, and genetic studies of blue nevi have provided further support that they are a distinct entity separate from acquired melanocytic nevi. Most acquired melanocytic nevi harbor somatic mutations in genes involved in the MAPK signaling pathway, particularly BRAF and NRAS (Davies et al., 2002; Pollock et al., 2003; Van Raamsdonk et al., 2009). Mutations in these genes are uncommonly identified in blue nevi; Saldanha et al. reported BRAF exon 15 mutations in 3 (12%) of 25 blue nevi and did not identify any NRAS mutations, while Emley et al. reported zero BRAF and 1 (5.3%) NRAS mutations in 19 common and cellular blue nevi, but found KRAS mutations among 16.7% (3/18) of these blue nevi (Emley et al., 2011; Saldanha et al., 2004).
The observation that mice with activating mutations in the G protein α-subunits, GNAQ and GNA11, acquire a phenotype of dermal hyperpigmentation similar in appearance to blue nevi in humans prompted the discovery that the majority of blue nevi harbor a somatic mutation of either GNAQ or GNA11 (Van Raamsdonk et al., 2004). In their first study, Van Raamsdonk et al. sequenced the entire coding regions of these genes in 29 blue nevi and reported GNAQ mutations in 24 (82.8%); no GNA11 mutations were reported (Van Raamsdonk et al., 2009). In a follow up study, out of a group of 96 common and cellular blue nevi, 65 (67.7%) and 8 (8.3%) harbored GNAQ and GNA11 mutations respectively, with all but one affecting glutamine at codon 209 (Q209) (Van Raamsdonk et al., 2010). More recently, a genetic analysis of 30 common and cellular blue nevi revealed that 86.7% (26/30) and 3.6% (1/28) had GNAQ and GNA11 mutations, respectively, all in codon 209 (Bender et al., 2013). The previously mentioned study by Emley et al. reported a lower prevalence of GNAQ mutations in common and cellular blue nevi, as only 42.1% (8/19) lesions carried mutations, but once again, all were in codon 209 (Emley et al., 2011). These mutations cause a loss of GTPase activity, leading to constitutive activation and subsequent upregulation of MAPK pathway signaling, a consequence also seen with constitutively active mutated BRAF and NRAS. Additionally, like mutated BRAF and NRAS, in vivo tumorigenicity studies have shown that, although mutated GNAQ acts as an oncogenic protein, it is not sufficient for melanomagenesis when acting alone (Van Raamsdonk et al., 2009). This is corroborated by the generally benign and stable nature of blue nevi, which rarely progress to malignancy.
Genetic analyses of rare blue nevi variants and blue nevi-related dermal melanocytoses (i.e. Nevus of Ito and Nevus of Ota) are sparse, with small sample sizes, but the findings tend to agree that GNAQ and GNA11 mutations are much less prevalent in these lesions than in common and cellular blue nevi (Bender et al., 2013; Emley et al., 2011; Held et al., 2013; Van Raamsdonk et al., 2009; Van Raamsdonk et al., 2010). Further genetic studies and functional validation investigations are needed to better elucidate how GNAQ and GNA11 mutations influence the biology and pathophysiology of blue nevi and related dermal melanocytic lesions.
SPITZ NEVI
Spitz tumors are a subgroup of melanocytic neoplasms, ranging from benign to malignant, with a predominance of large epithelioid or spindled melanocytes. Cases on the benign end of the spectrum, with no overlapping morphologic features of melanoma are designated as Spitz nevi, whereas cases with unequivocal features of melanoma are designated as spitzoid melanoma. Consistent with Spitz nevi’s lack of morphologic features of melanoma, the genetics of Spitz nevi also do not align well with melanoma. BRAF and NRAS mutations are prevalent in melanomas, but the majority of studies investigating the genetic status of Spitz nevi (Table 4) have demonstrated an absence of these mutations. In total, BRAF and NRAS mutations were detected in 6.4% (21/330) and 2.2% (4/178) of Spitz nevi respectively (Table 4). The most frequently observed genetic alterations in Spitz nevi involve the HRAS gene; an aggregate of data from multiple studies revealed 48 of 293 (16.4%) Spitz nevi harbored HRAS alterations (copy number gain or mutation) (Table 4). HRAS belongs to the family of RAS genes, which contains two additional members, KRAS and NRAS (Barbacid, 1987). In contrast to NRAS, HRAS is rarely mutated in melanoma (Jiveskog et al., 1998; van Elsas et al., 1996). HRAS mutation seems to occur almost exclusively in Spitz nevi. Bastian et al. first described HRAS copy number amplification in 23.5% (4/17) of Spitz nevi in 1999, and subsequently confirmed the presence of increased HRAS copy number in 11.8% (12/102) of Spitz nevi, while also showing HRAS mutations in 66.7% (8/12) of the Spitz nevi with increased copy number (Bastian et al., 2000; Bastian et al., 1999). HRAS is known to have higher affinity for the PI3K/ATK pathway when compared to other RAS isoforms (Yan et al., 1998), however, the significance of HRAS mutations in the nevogenesis of Spitz nevi remains unclear. It has been suggested that HRAS drives the symmetrical overgrowth of cells with epitheloid morphology via preferential PI3K/AKT activation, without marked increase of the melanin activation pathway (Ross et al., 2011). Clinically, the presence of an HRAS mutation seems to be associated with a favorable prognosis. Thus far, studies have found no metastases in patients with HRAS-mutated Spitz tumors, and no HRAS mutations have been reported in Spitzoid melanomas with distant metastasis or fatal outcome (Da Forno et al., 2009; Takata et al., 2007; van Dijk et al., 2005; van Engen-van Grunsven et al., 2010). Based on the frequency of HRAS mutations in Spitz nevi and the lack thereof in spitzoid melanomas, it seems unlikely that HRAS-mutated Spitz nevi progress to spitzoid melanomas.
Table 4.
Studies evaluating the genetic alteration status of BRAF, NRAS, and HRAS in Spitz nevi.
| Study | Histological Subtypes |
BRAF mutations |
NRAS mutations |
HRAS alterations |
|---|---|---|---|---|
| (Bastian et al., 1999) | Spitz Nevi | -- | -- | 4/17(23.5%)* |
| (Bastian et al., 2000) | Spitz Nevi | -- | -- | 12/102(12%)* |
| (Yazdi et al., 2003) | Spitz Nevi | 0/69(0%) | -- | -- |
| (Palmedo et al., 2004) | Spitz Nevi | 0/21(0%) | -- | -- |
| (Mihic-Probst et al., 2004) | Spitz Nevi | 0/20(0%) | -- | -- |
| (Saldanha et al., 2004) | Spitz Nevi | 0/26(0%) | 1/16(6.25%) | -- |
| (Gill et al., 2004) | Spitz Nevi | 0/30(0%) | 0/30(0%) | 0/30(0%) |
| (Turner et al., 2005) | Spitz Nevi | 0/24(0%) | -- | -- |
| (van Dijk et al., 2005) | Spitz Nevi and Atypical Spitz Nevi | 0/30(0%) | 0/30(0%) | 6/30(20%) |
| (Fullen et al., 2006) | Spitz Nevi | 10/48(20.8%) | 1/48(2.08%) | -- |
| (La Porta et al., 2006) | Spitz Nevi | 8/8(100%) | -- | -- |
| (Takata et al., 2007) | Spitz Nevi | 0/12(0%) | 0/12(0%) | 0/12(0%) |
| (Da Forno et al., 2009) | Spitz Nevi and Atypical Spitz Nevi | 2/22(9.1%) | 2/22(9.1%) | 2/19(10.5%) |
| (Emley et al., 2010) | Spitz Nevi and Atypical Spitz Nevi | 1/20(5%) | 0/20(0%) | -- |
| (van Engen-van Grunsven et al., 2010) | Spitz Nevi | -- | -- | 24/93(25.8%) |
These studies reported frequency of copy number gain, not mutation
Another subset of spitzoid neoplasms are characterized by BRAF mutations combined with bi-allelic BAP1 loss (Wiesner et al., 2012; Wiesner et al., 2011). BAP1 is a deubiquitinating enzyme whose gene is located on chromosome region 3p12 (Jensen et al., 1998). BAP1 has been suggested to be a tumor suppressor gene with a role in cell proliferation and growth inhibition (Jensen and Rauscher, 1999). This subset of melanocytic neoplasm described by Wiesner et al. histologically resembled so-called “atypical Spitz tumors” (ASTs), which are an ill-defined and heterogeneous group of melanocytic tumors that display histologic features seen in both Spitz nevi and melanoma. These BRAF mutant tumors had similar cytologic features to Spitzoid tumors characterized by HRAS mutations, however, the HRAS mutant neoplasms were associated with marked desmoplasia and did not show dense cellular aggregates compared to BRAFV600E/BAP1−/− tumors (Wiesner et al., 2012).
Recently, gene rearrangements of ROS1, NTRK1, ALK, BRAF, and RET, resulting in in-frame kinase fusions, were reported through genomic analysis of 140 Spitzoid neoplasms (Wiesner et al., 2014). These kinase fusions occurred across the entire biologic spectrum of spitzoid neoplasms, including Spitz nevi, ASTs, and spitzoid melanoma. All fusions occurred in a mutually exclusive pattern, and no fusions were detected in tumors with HRAS mutations (Wiesner et al., 2014). The results of the study suggest that these fusions occur early in the pathogenesis of spitzoid neoplasms and are therefore necessary, but not sufficient, for malignant transformation. In this respect, the oncogenic role of these gene arrangements seems to be similar to that of mutations in oncogenes (such as BRAF, NRAS, GNAQ, and GNA11) commonly found in melanocytic neoplasms (Wiesner et al., 2014).
NEVI AND MELANOMAGENESIS
To understand the molecular basis of melanomagenesis, it is imperative to identify the genetic changes that facilitate the different steps in progression from normal melanocyte to nevus and melanoma. Commonly, an initial “driver” mutation, either activation of an oncogene or the loss of a tumor suppressor gene, triggers the establishment of a benign growth. After this initial phase of proliferation, a senescence program is executed, causing termination of the tumor expansion. Cellular senescence is a physiologic process by which cells gradually lose their growth potential and eventually exit cell cycle progression. In normal human cells, senescence can occur due to either telomere shortening that takes place after a certain number of cell divisions (replicative senescence) (Dimri et al., 1996) or, paradoxically, because of the aberrant activation of oncogenes (oncogene-induced senescence) (Serrano et al., 1997). In humans, naturally occurring oncogene-induced senescence (OIS) is best exemplified by acquired melanocytic nevi and CMN, which represent benign aggregations of non-proliferative melanocytes that often harbor activating mutations in BRAF and NRAS genes, respectively. Michaloglou et al. showed that overexpression of BRAFV600 in cultured human melanocytes caused growth arrest and also observed hallmarks of OIS in human congenital melanocytic nevi: expression of an activated oncoprotein (BRAFV600), stable and total, or near-total, proliferative arrest, upregulation of a tumor suppressor (p16INK4A), and emergence of senescence-associated acidic β-galactosidase (Michaloglou et al., 2008). Additionally, there was no significant difference in telomere fluorescence between congenital nevi and surrounding tissues, which supports results from common acquired nevi and Spitz nevi (Miracco et al., 2002), and further suggests that nevi are growth arrested due to OIS rather than replicative senescence. In spite of the activation of the MAPK pathway (RAS/RAF/MEK/ERK) that mediates a potent proliferative signal, benign nevi eventually lose all proliferative activity, and their growth remains arrested for decades, until they gradually disappear; however, with additional genetic alterations, a few nevus cells can develop into malignant melanoma (Kuwata et al., 1993; Maldonado et al., 2004). In large CMN, Charbel et al. demonstrated that after birth, certain large CMN cell subtypes still display clonogenic potential and can expand into nevus-like structures when interacting with adjacent keratinocytes(Charbel et al., 2015). This explains nevus secondary resurgences, which have been described following nevus excisions, as well as the natural expansion of nevi during childhood.
It is widely believed that a substantial percentage of melanomas arise from melanocytic nevi (Mooi and Peeper, 2006) and several groups have provided genetic evidence that supports the progression model (Bogdan et al., 2003; Dadzie et al., 2009; Demunter et al., 2001). It seems that OIS, similar to apoptosis, is a fail-safe mechanism to prevent malignant transformation of normal cells. Therefore, overcoming OIS may act as a rate-limiting event for melanomagenesis (Bennett, 2003; Mooi and Peeper, 2006). That is, only upon the emergence of additional tumorigenic alterations can a malignant process occur. The molecular mechanisms underlying this malignant transformation from nevus to melanoma are not yet resolved. Loss of p16INK4A has long been suspected to play a critical role in the abrogation of OIS and collective evidence support a redundant role for p16INK4A in senescence induced by mutant BRAF or NRAS in vitro (Michaloglou et al., 2008) and in vivo (Dhomen et al., 2009). Another common genetic event in melanoma is activation of the PI3K pathway (Dhawan et al., 2002). Vredeveld et al. recently demonstrated that PI3K pathway activation serves as a critical event in melanomas arising from nevi, acting in part by abrogating OIS (Vredeveld et al., 2012). They showed that PTEN depletion and consequent activation of the PI3K pathway abrogates BRAFV600E-induced senescence. Similarly, Damsky et al. reported that simultaneous Cdnk2a and Lkb1 inactivation in BRAFV600E melanocytes results in activation of both mTORC1 and Akt/mTORC2, inducing rapid melanoma formation in mice (W. Damsky et al., 2015). Loss of Cdkn2a is insufficient to release melanocytes from OIS; however, this primes a subset of growth-arrested, BRAFV600E nevi for later stochastic progression to melanoma, which appears to occur through activation of Akt/mTORC2 and mTORC1 signaling (W. Damsky et al., 2015).
Melanomas associated with nevi have been studied thoroughly with the hope of better understanding melanoma biology. Tschandl et al. demonstrated that no significant difference in the distribution of BRAF or NRAS mutations is found between melanomas and associated nevi or between melanoma-associated nevi and control nevi (Tschandl et al., 2013). Notably, this study concludes that the presence of a BRAFV600E or NRASQ61 mutation within a nevus does not alter the risk of malignant transformation. A recent meta-analysis of 13 studies, with 4,346 cumulative patients, revealed that 32% of melanomas are associated with a nevus, however, there was no prognostic implication in sentinel lymph node status or overall survival for these nevus-associated melanomas compared to de novo melanomas (Lin et al., 2015).
Since the use of specific BRAFV600E inhibitors has become more commonplace in the treatment of melanoma, interesting findings have been reported regarding the effect of these drugs on benign nevi, which frequently harbor BRAFV600E mutations (Pollock et al., 2003); (Dalle et al., 2011; Haenssle et al., 2012). Dermoscopic changes to preexisting nevi in color, appearance and disappearance of globules, dermoscopic island pigmentation, and increases in size have been reported following BRAF inhibitor therapy (Perier-Muzet et al., 2014). Interestingly, the evolving nevi were BRAFV600E mutated while the stable nevi were BRAF wild-type. This phenomenon maybe related to decreased MAPK pathway activity due to BRAF inhibition (McClenahan et al., 2014). Others have reported that BRAFV600E inhibitor-treated patients show increased size and pigmentation in some nevi and development of new BRAF wild-type melanomas driven by paradoxical MAPK activation (Hatzivassiliou et al., 2010). Clearly, BRAF and its associated pathway are linked to nevogenesis and stability, but larger studies are required to better understand escape pathways and paradoxical MAPK pathway activation.
SUMMARY
Nevi are a heterogeneous group of benign melanocytic neoplasms, with varying clinical and molecular characteristics. While the molecular etiology of nevi have been less thoroughly studied than melanoma, it is clear that nevi and melanoma share common driver mutations. Acquired melanocytic nevi harbor oncogenic mutations in BRAF, which is the predominant oncogene associated with melanoma. While congenital melanocytic nevi, blue nevi, and acquired nevi predominantly harbor NRAS mutations, GNAQ mutations, and BRAF alterations, respectively, Spitz nevi and spitzoid tumors have now been linked to an assortment of oncogenic drivers including HRAS and BAP1 alterations and kinase fusions (Figure 1). Excepting HRAS, all of the “nevus” genes have also been described in melanoma development and progression. Thus, the distinction between melanoma and nevi seems to be a nevus cell’s ability to undergo senescence after sustaining pro-proliferative mutations. Melanoma’s ability to escape this senescence pathway, tipping the balance from benign neoplasm to malignancy, remains an area of active investigation. The genetics of nevi have not been as thoroughly studied as its malignant counterpart, despite the potential to provide insight into how melanomas undergo malignant transformation. Molecular studies have been limited by a lack of robust nevi mouse models and limited availability of excised tissue for analysis. Nevertheless, further genetic studies of nevi promise to shape our understanding of melanoma and the delicate tipping-point from benign neoplasm to malignancy.
Acknowledgements
This scholarly work was made possible by a grant from the NIH (K24 CA149202 to HT), a grant from the Basic Science Research Program through the National Research Foundation of Korea, which is funded by the Ministry of Education, Science, and Technology (2011-0022376 to MRR), a Melanoma Research Foundation Student Grant (to SG), and the generous donors to the MGH on behalf of melanoma research. We also would like to apologize to the numerous other authors whose contributions we could not cite in this short review because of space considerations.
Footnotes
Conflicts of Interest: The authors state no conflicts of interest.
References
- Argenziano G, Zalaudek I, Ferrara G, Hofmann-Wellenhof R, Soyer HP. Proposal of a new classification system for melanocytic naevi. The British journal of dermatology. 2007;157:217–227. doi: 10.1111/j.1365-2133.2007.07972.x. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annual review of biochemistry. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bastian BC, LeBoit PE, Pinkel D. Mutations and copy number increase of HRAS in Spitz nevi with distinctive histopathological features. The American journal of pathology. 2000;157:967–972. doi: 10.1016/S0002-9440(10)64609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian BC, Wesselmann U, Pinkel D, Leboit PE. Molecular cytogenetic analysis of Spitz nevi shows clear differences to melanoma. The Journal of investigative dermatology. 1999;113:1065–1069. doi: 10.1046/j.1523-1747.1999.00787.x. [DOI] [PubMed] [Google Scholar]
- Bataille V. Genetic epidemiology of melanoma. European journal of cancer. 2003;39:1341–1347. doi: 10.1016/s0959-8049(03)00313-7. [DOI] [PubMed] [Google Scholar]
- Bataille V, Bishop JA, Sasieni P, Swerdlow AJ, Pinney E, Griffiths K, Cuzick J. Risk of cutaneous melanoma in relation to the numbers, types and sites of naevi: a case-control study. British journal of cancer. 1996;73:1605–1611. doi: 10.1038/bjc.1996.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J, Curtin JA, Pinkel D, Bastian BC. Congenital melanocytic nevi frequently harbor NRAS mutations but no BRAF mutations. The Journal of investigative dermatology. 2007;127:179–182. doi: 10.1038/sj.jid.5700490. [DOI] [PubMed] [Google Scholar]
- Bauer J, Garbe C. Acquired melanocytic nevi as risk factor for melanoma development. A comprehensive review of epidemiological data. Pigment cell research / sponsored by the European Society for Pigment Cell Research and the International Pigment Cell Society. 2003;16:297–306. doi: 10.1034/j.1600-0749.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- Bender RP, McGinniss MJ, Esmay P, Velazquez EF, Reimann JD. Identification of HRAS mutations and absence of GNAQ or GNA11 mutations in deep penetrating nevi. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2013;26:1320–1328. doi: 10.1038/modpathol.2013.77. [DOI] [PubMed] [Google Scholar]
- Bennett DC. Human melanocyte senescence and melanoma susceptibility genes. Oncogene. 2003;22:3063–3069. doi: 10.1038/sj.onc.1206446. [DOI] [PubMed] [Google Scholar]
- Bloethner S, Snellman E, Bermejo JL, Hiripi E, Gast A, Thirumaran RK, Wellenreuther R, Hemminki K, Kumar R. Differential gene expression in melanocytic nevi with the V600E BRAF mutation. Genes, chromosomes & cancer. 2007;46:1019–1027. doi: 10.1002/gcc.20488. [DOI] [PubMed] [Google Scholar]
- Bogdan I, Smolle J, Kerl H, Burg G, Boni R. Melanoma ex naevo: a study of the associated naevus. Melanoma research. 2003;13:213–217. doi: 10.1097/01.cmr.0000056226.78713.99. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network. Electronic address, i.m.o., and Cancer Genome Atlas, N. Genomic Classification of Cutaneous Melanoma. Cell. 2015;161:1681–1696. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr J, Mackie RM. Point mutations in the N-ras oncogene in malignant melanoma and congenital naevi. The British journal of dermatology. 1994;131:72–77. doi: 10.1111/j.1365-2133.1994.tb08460.x. [DOI] [PubMed] [Google Scholar]
- Charbel C, Fontaine RH, Kadlub N, Coulomb-L'Hermine A, Rouille T, How-Kit A, Moguelet P, Tost J, Picard A, Aractingi S, et al. Clonogenic cell subpopulations maintain congenital melanocytic nevi. The Journal of investigative dermatology. 2015;135:824–833. doi: 10.1038/jid.2014.437. [DOI] [PubMed] [Google Scholar]
- Charbel C, Fontaine RH, Malouf GG, Picard A, Kadlub N, El-Murr N, How-Kit A, Su X, Coulomb-L'Hermine A, Tost J, et al. NRAS mutation is the sole recurrent somatic mutation in large congenital melanocytic nevi. The Journal of investigative dermatology. 2014;134:1067–1074. doi: 10.1038/jid.2013.429. [DOI] [PubMed] [Google Scholar]
- Clark WH, Jr, Elder DE, Guerry Dt, Epstein MN, Greene MH, Van Horn M. A study of tumor progression: the precursor lesions of superficial spreading and nodular melanoma. Human pathology. 1984;15:1147–1165. doi: 10.1016/s0046-8177(84)80310-x. [DOI] [PubMed] [Google Scholar]
- Clark WH, Jr, Reimer RR, Greene M, Ainsworth AM, Mastrangelo MJ. Origin of familial malignant melanomas from heritable melanocytic lesions. 'The B-K mole syndrome'. Archives of dermatology. 1978;114:732–738. [PubMed] [Google Scholar]
- Clemmensen OJ, Kroon S. The histology of "congenital features" in early acquired melanocytic nevi. Journal of the American Academy of Dermatology. 1988;19:742–746. doi: 10.1016/s0190-9622(88)70231-5. [DOI] [PubMed] [Google Scholar]
- Cribier BJ, Santinelli F, Grosshans E. Lack of clinical-pathological correlation in the diagnosis of congenital naevi. The British journal of dermatology. 1999;141:1004–1009. doi: 10.1046/j.1365-2133.1999.03197.x. [DOI] [PubMed] [Google Scholar]
- Da Forno PD, Pringle JH, Fletcher A, Bamford M, Su L, Potter L, Saldanha G. BRAF, NRAS and HRAS mutations in spitzoid tumours and their possible pathogenetic significance. The British journal of dermatology. 2009;161:364–372. doi: 10.1111/j.1365-2133.2009.09181.x. [DOI] [PubMed] [Google Scholar]
- Dadzie OE, Yang S, Emley A, Keady M, Bhawan J, Mahalingam M. RAS and RAF mutations in banal melanocytic aggregates contiguous with primary cutaneous melanoma: clues to melanomagenesis. The British journal of dermatology. 2009;160:368–375. doi: 10.1111/j.1365-2133.2008.08887.x. [DOI] [PubMed] [Google Scholar]
- Dalle S, Poulalhon N, Thomas L. Vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine. 2011;365:1448–1449. doi: 10.1056/NEJMc1108651. author reply 1450. [DOI] [PubMed] [Google Scholar]
- Damsky W, Micevic G, Meeth K, Muthusamy V, Curley DP, Santhanakrishnan M, Erdelyi I, Platt JT, Huang L, Theodosakis N, et al. mTORC1 activation blocks BrafV600E-induced growth arrest but is insufficient for melanoma formation. Cancer cell. 2015;27:41–56. doi: 10.1016/j.ccell.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky WE, Theodosakis N, Bosenberg M. Melanoma metastasis: new concepts and evolving paradigms. Oncogene. 2014;33:2413–2422. doi: 10.1038/onc.2013.194. [DOI] [PubMed] [Google Scholar]
- Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr, You MJ, DePinho RA, McMahon M, Bosenberg M. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nature genetics. 2009;41:544–552. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- De Raeve LE, Claes A, Ruiter DJ, van Muijen GN, Roseeuw D, van Kempen LC. Distinct phenotypic changes between the superficial and deep component of giant congenital melanocytic naevi: a rationale for curettage. The British journal of dermatology. 2006;154:485–492. doi: 10.1111/j.1365-2133.2005.07055.x. [DOI] [PubMed] [Google Scholar]
- Demunter A, Stas M, Degreef H, De Wolf-Peeters C, van den Oord JJ. Analysis of N- and K-ras mutations in the distinctive tumor progression phases of melanoma. The Journal of investigative dermatology. 2001;117:1483–1489. doi: 10.1046/j.0022-202x.2001.01601.x. [DOI] [PubMed] [Google Scholar]
- Dessars B, De Raeve LE, Morandini R, Lefort A, El Housni H, Ghanem GE, Van den Eynde BJ, Ma W, Roseeuw D, Vassart G, et al. Genotypic and gene expression studies in congenital melanocytic nevi: insight into initial steps of melanotumorigenesis. The Journal of investigative dermatology. 2009;129:139–147. doi: 10.1038/jid.2008.203. [DOI] [PubMed] [Google Scholar]
- Dhawan P, Singh AB, Ellis DL, Richmond A. Constitutive activation of Akt/protein kinase B in melanoma leads to up-regulation of nuclear factor-kappaB and tumor progression. Cancer research. 2002;62:7335–7342. [PubMed] [Google Scholar]
- Dhomen N, Reis-Filho JS, da Rocha Dias S, Hayward R, Savage K, Delmas V, Larue L, Pritchard C, Marais R. Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer cell. 2009;15:294–303. doi: 10.1016/j.ccr.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Testori A, Acosta M, Campisi J. Replicative senescence, aging and growth-regulatory transcription factors. Biological signals. 1996;5:154–162. doi: 10.1159/000109185. [DOI] [PubMed] [Google Scholar]
- Dong J, Phelps RG, Qiao R, Yao S, Benard O, Ronai Z, Aaronson SA. BRAF oncogenic mutations correlate with progression rather than initiation of human melanoma. Cancer research. 2003;63:3883–3885. [PubMed] [Google Scholar]
- Emley A, Nguyen LP, Yang S, Mahalingam M. Somatic mutations in GNAQ in amelanotic/hypomelanotic blue nevi. Human pathology. 2011;42:136–140. doi: 10.1016/j.humpath.2010.05.027. [DOI] [PubMed] [Google Scholar]
- Emley A, Yang S, Wajapeyee N, Green MR, Mahalingam M. Oncogenic BRAF and the tumor suppressor IGFBP7 in the genesis of atypical spitzoid nevomelanocytic proliferations. Journal of cutaneous pathology. 2010;37:344–349. doi: 10.1111/j.1600-0560.2009.01433.x. [DOI] [PubMed] [Google Scholar]
- Fullen DR, Poynter JN, Lowe L, Su LD, Elder JT, Nair RP, Johnson TM, Gruber SB. BRAF and NRAS mutations in spitzoid melanocytic lesions. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2006;19:1324–1332. doi: 10.1038/modpathol.3800653. [DOI] [PubMed] [Google Scholar]
- Gill M, Renwick N, Silvers DN, Celebi JT. Lack of BRAF mutations in Spitz nevi. The Journal of investigative dermatology. 2004;122:1325–1326. doi: 10.1111/j.0022-202X.2004.22530.x. [DOI] [PubMed] [Google Scholar]
- Goel VK, Ibrahim N, Jiang G, Singhal M, Fee S, Flotte T, Westmoreland S, Haluska FS, Hinds PW, Haluska FG. Melanocytic nevus-like hyperplasia and melanoma in transgenic BRAFV600E mice. Oncogene. 2009;28:2289–2298. doi: 10.1038/onc.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Campora R, Galera-Davidson H, Vazquez-Ramirez FJ, Diaz-Cano S. Blue nevus: classical types and new related entities. A differential diagnostic review. Pathology, research and practice. 1994;190:627–635. doi: 10.1016/S0344-0338(11)80402-4. [DOI] [PubMed] [Google Scholar]
- Gray-Schopfer VC, Cheong SC, Chong H, Chow J, Moss T, Abdel-Malek ZA, Marais R, Wynford-Thomas D, Bennett DC. Cellular senescence in naevi and immortalisation in melanoma: a role for p16? British journal of cancer. 2006;95:496–505. doi: 10.1038/sj.bjc.6603283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenssle HA, Kraus SL, Brehmer F, Kretschmer L, Volker B, Asper H, Kapp A, Gutzmer R. Dynamic changes in nevi of a patient with melanoma treated with vemurafenib: importance of sequential dermoscopy. Archives of dermatology. 2012;148:1183–1185. doi: 10.1001/archdermatol.2012.2649. [DOI] [PubMed] [Google Scholar]
- Hafner C, Stoehr R, van Oers JM, Zwarthoff EC, Hofstaedter F, Klein C, Landthaler M, Hartmann A, Vogt T. The absence of BRAF, FGFR3, and PIK3CA mutations differentiates lentigo simplex from melanocytic nevus and solar lentigo. The Journal of investigative dermatology. 2009;129:2730–2735. doi: 10.1038/jid.2009.146. [DOI] [PubMed] [Google Scholar]
- Hastrup N, Osterlind A, Drzewiecki KT, Hou-Jensen K. The presence of dysplastic nevus remnants in malignant melanomas. A population-based study of 551 malignant melanomas. The American Journal of dermatopathology. 1991;13:378–385. doi: 10.1097/00000372-199108000-00009. [DOI] [PubMed] [Google Scholar]
- Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, Ludlam MJ, Stokoe D, Gloor SL, Vigers G, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–435. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- Held L, Eigentler TK, Metzler G, Leiter U, Messina JL, Glass LF, Garbe C, Bauer J. Proliferative activity, chromosomal aberrations, and tumor-specific mutations in the differential diagnosis between blue nevi and melanoma. The American journal of pathology. 2013;182:640–645. doi: 10.1016/j.ajpath.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Ichii-Nakato N, Takata M, Takayanagi S, Takashima S, Lin J, Murata H, Fujimoto A, Hatta N, Saida T. High frequency of BRAFV600E mutation in acquired nevi and small congenital nevi, but low frequency of mutation in medium-sized congenital nevi. The Journal of investigative dermatology. 2006;126:2111–2118. doi: 10.1038/sj.jid.5700366. [DOI] [PubMed] [Google Scholar]
- Jafari M, Papp T, Kirchner S, Diener U, Henschler D, Burg G, Schiffmann D. Analysis of ras mutations in human melanocytic lesions: activation of the ras gene seems to be associated with the nodular type of human malignant melanoma. Journal of cancer research and clinical oncology. 1995;121:23–30. doi: 10.1007/BF01202725. [DOI] [PubMed] [Google Scholar]
- Jensen DE, Proctor M, Marquis ST, Gardner HP, Ha SI, Chodosh LA, Ishov AM, Tommerup N, Vissing H, Sekido Y, et al. BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene. 1998;16:1097–1112. doi: 10.1038/sj.onc.1201861. [DOI] [PubMed] [Google Scholar]
- Jensen DE, Rauscher FJ., 3rd BAP1, a candidate tumor suppressor protein that interacts with BRCA1. Annals of the New York Academy of Sciences. 1999;886:191–194. doi: 10.1111/j.1749-6632.1999.tb09414.x. [DOI] [PubMed] [Google Scholar]
- Jiveskog S, Ragnarsson-Olding B, Platz A, Ringborg U. N-ras mutations are common in melanomas from sun-exposed skin of humans but rare in mucosal membranes or unexposed skin. The Journal of investigative dermatology. 1998;111:757–761. doi: 10.1046/j.1523-1747.1998.00376.x. [DOI] [PubMed] [Google Scholar]
- Karram S, Novy M, Saroufim M, Loya A, Taraif S, Houreih MA, Rauscher B, Habib RH, Oberkanins C, Khalifeh I. Predictors of BRAF mutation in melanocytic nevi: analysis across regions with different UV radiation exposure. The American Journal of dermatopathology. 2013;35:412–418. doi: 10.1097/DAD.0b013e31826db181. [DOI] [PubMed] [Google Scholar]
- Kinsler VA, Thomas AC, Ishida M, Bulstrode NW, Loughlin S, Hing S, Chalker J, McKenzie K, Abu-Amero S, Slater O, et al. Multiple congenital melanocytic nevi and neurocutaneous melanosis are caused by postzygotic mutations in codon 61 of NRAS. The Journal of investigative dermatology. 2013;133:2229–2236. doi: 10.1038/jid.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krengel S, Hauschild A, Schafer T. Melanoma risk in congenital melanocytic naevi: a systematic review. The British journal of dermatology. 2006;155:1–8. doi: 10.1111/j.1365-2133.2006.07218.x. [DOI] [PubMed] [Google Scholar]
- Kuwata T, Kitagawa M, Kasuga T. Proliferative activity of primary cutaneous melanocytic tumours. Virchows Archiv. A, Pathological anatomy and histopathology. 1993;423:359–364. doi: 10.1007/BF01607148. [DOI] [PubMed] [Google Scholar]
- La Porta CA, Cardano R, Facchetti F, Presicce P, Rao S, Privitera E, Clemente C, Mihm MC., Jr BRAF V599E mutation occurs in Spitz and Reed naevi. Journal of the European Academy of Dermatology and Venereology : JEADV. 2006;20:1164–1165. doi: 10.1111/j.1468-3083.2006.01665.x. [DOI] [PubMed] [Google Scholar]
- Leopold JG, Richards DB. The interrelationship of blue and common naevi. The Journal of pathology and bacteriology. 1968;95:37–46. doi: 10.1002/path.1700950106. [DOI] [PubMed] [Google Scholar]
- Lin WM, Luo S, Muzikansky A, Lobo AZ, Tanabe KK, Sober AJ, Cosimi AB, Tsao H, Duncan LM. Outcome of patients with de novo versus nevus-associated melanoma. Journal of the American Academy of Dermatology. 2015;72:54–58. doi: 10.1016/j.jaad.2014.09.028. [DOI] [PubMed] [Google Scholar]
- Magana-Garcia M, Ackerman AB. What are nevus cells? The American Journal of dermatopathology. 1990;12:93–102. doi: 10.1097/00000372-199002000-00014. [DOI] [PubMed] [Google Scholar]
- Maldonado JL, Timmerman L, Fridlyand J, Bastian BC. Mechanisms of cell-cycle arrest in Spitz nevi with constitutive activation of the MAP-kinase pathway. The American journal of pathology. 2004;164:1783–1787. doi: 10.1016/S0002-9440(10)63736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti MA, Kiuru MH, Busam KJ, Marghoob AA, Scope A, Dusza SW, Cordova MA, Fonseca M, Wu X, Halpern AC. Melanocytic naevi with globular and reticular dermoscopic patterns display distinct BRAF V600E expression profiles and histopathological patterns. The British journal of dermatology. 2014;171:1060–1065. doi: 10.1111/bjd.13260. [DOI] [PubMed] [Google Scholar]
- Marghoob AA, Kopf AW, Rigel DS, Bart RS, Friedman RJ, Yadav S, Abadir M, Sanfilippo L, Silverman MK, Vossaert KA. Risk of cutaneous malignant melanoma in patients with 'classic' atypical-mole syndrome. A case-control study. Archives of dermatology. 1994;130:993–998. [PubMed] [Google Scholar]
- McClenahan P, Lin LL, Tan JM, Flewell-Smith R, Schaider H, Jagirdar K, Atkinson V, Lambie D, Prow TW, Sturm RA, et al. BRAFV600E mutation status of involuting and stable nevi in dabrafenib therapy with or without trametinib. JAMA dermatology. 2014;150:1079–1082. doi: 10.1001/jamadermatol.2014.436. [DOI] [PubMed] [Google Scholar]
- Michaloglou C, Vredeveld LC, Mooi WJ, Peeper DS. BRAF(E600) in benign and malignant human tumours. Oncogene. 2008;27:877–895. doi: 10.1038/sj.onc.1210704. [DOI] [PubMed] [Google Scholar]
- Mihic-Probst D, Perren A, Schmid S, Saremaslani P, Komminoth P, Heitz PU. Absence of BRAF gene mutations differentiates spitz nevi from malignant melanoma. Anticancer research. 2004;24:2415–2418. [PubMed] [Google Scholar]
- Miracco C, Margherita De Santi M, Schurfeld K, Santopietro R, Lalinga AV, Fimiani M, Biagioli M, Brogi M, De Felice C, Luzi P, et al. Quantitative in situ evaluation of telomeres in fluorescence in situ hybridization-processed sections of cutaneous melanocytic lesions and correlation with telomerase activity. The British journal of dermatology. 2002;146:399–408. doi: 10.1046/j.1365-2133.2002.04600.x. [DOI] [PubMed] [Google Scholar]
- Mooi WJ, Peeper DS. Oncogene-induced cell senescence--halting on the road to cancer. The New England journal of medicine. 2006;355:1037–1046. doi: 10.1056/NEJMra062285. [DOI] [PubMed] [Google Scholar]
- Murali R, McCarthy SW, Scolyer RA. Blue nevi and related lesions: a review highlighting atypical and newly described variants, distinguishing features and diagnostic pitfalls. Advances in anatomic pathology. 2009;16:365–382. doi: 10.1097/PAP.0b013e3181bb6b53. [DOI] [PubMed] [Google Scholar]
- Nasti TH, Cochran JB, Tsuruta Y, Yusuf N, McKay KM, Athar M, Timares L, Elmets CA. A murine model for the development of melanocytic nevi and their progression to melanoma. Molecular carcinogenesis. 2015 doi: 10.1002/mc.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmedo G, Hantschke M, Rutten A, Mentzel T, Hugel H, Flaig MJ, Yazdi AS, Sander CA, Kutzner H. The T1796A mutation of the BRAF gene is absent in Spitz nevi. Journal of cutaneous pathology. 2004;31:266–270. doi: 10.1111/j.0303-6987.2003.00179.x. [DOI] [PubMed] [Google Scholar]
- Papp T, Pemsel H, Rollwitz I, Schipper H, Weiss DG, Schiffmann D, Zimmermann R. Mutational analysis of N-ras, p53, CDKN2A (p16(INK4a)), p14(ARF), CDK4, and MC1R genes in human dysplastic melanocytic naevi. Journal of medical genetics. 2003;40:E14. doi: 10.1136/jmg.40.2.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp T, Pemsel H, Zimmermann R, Bastrop R, Weiss DG, Schiffmann D. Mutational analysis of the N-ras, p53, p16INK4a, CDK4, and MC1R genes in human congenital melanocytic naevi. Journal of medical genetics. 1999;36:610–614. [PMC free article] [PubMed] [Google Scholar]
- Papp T, Schipper H, Kumar K, Schiffmann D, Zimmermann R. Mutational analysis of the BRAF gene in human congenital and dysplastic melanocytic naevi. Melanoma research. 2005;15:401–407. doi: 10.1097/00008390-200510000-00008. [DOI] [PubMed] [Google Scholar]
- Patton EE, Widlund HR, Kutok JL, Kopani KR, Amatruda JF, Murphey RD, Berghmans S, Mayhall EA, Traver D, Fletcher CD, et al. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Current biology : CB. 2005;15:249–254. doi: 10.1016/j.cub.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Perier-Muzet M, Thomas L, Poulalhon N, Debarbieux S, Bringuier PP, Duru G, Depaepe L, Balme B, Dalle S. Melanoma patients under vemurafenib: prospective follow-up of melanocytic lesions by digital dermoscopy. The Journal of investigative dermatology. 2014;134:1351–1358. doi: 10.1038/jid.2013.462. [DOI] [PubMed] [Google Scholar]
- Phadke PA, Rakheja D, Le LP, Selim MA, Kapur P, Davis A, Mihm MC, Jr, Hoang MP. Proliferative nodules arising within congenital melanocytic nevi: a histologic, immunohistochemical, and molecular analyses of 43 cases. The American journal of surgical pathology. 2011;35:656–669. doi: 10.1097/PAS.0b013e31821375ea. [DOI] [PubMed] [Google Scholar]
- Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, Moses TY, Hostetter G, Wagner U, Kakareka J, et al. High frequency of BRAF mutations in nevi. Nature genetics. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- Poynter JN, Elder JT, Fullen DR, Nair RP, Soengas MS, Johnson TM, Redman B, Thomas NE, Gruber SB. BRAF and NRAS mutations in melanoma and melanocytic nevi. Melanoma research. 2006;16:267–273. doi: 10.1097/01.cmr.0000222600.73179.f3. [DOI] [PubMed] [Google Scholar]
- Pozzobon FC, Puig-Butille JA, Gonzalez-Alvarez T, Carrera C, Aguilera P, Alos L, Badenas C, Grichnik JM, Malvehy J, Puig S. Dermoscopic criteria associated with BRAF and NRAS mutation status in primary cutaneous melanoma. The British journal of dermatology. 2014;171:754–759. doi: 10.1111/bjd.13069. [DOI] [PubMed] [Google Scholar]
- Qi RQ, He L, Zheng S, Hong Y, Ma L, Zhang S, Zhao L, Guo X, Wang Y, Yu JY, et al. BRAF exon 15 T1799A mutation is common in melanocytic nevi, but less prevalent in cutaneous malignant melanoma, in Chinese Han. The Journal of investigative dermatology. 2011;131:1129–1138. doi: 10.1038/jid.2010.405. [DOI] [PubMed] [Google Scholar]
- Rhodes AR, Harrist TJ, Day CL, Mihm MC, Jr, Fitzpatrick TB, Sober AJ. Dysplastic melanocytic nevi in histologic association with 234 primary cutaneous melanomas. Journal of the American Academy of Dermatology. 1983;9:563–574. doi: 10.1016/s0190-9622(83)70171-4. [DOI] [PubMed] [Google Scholar]
- Rhodes AR, Silverman RA, Harrist TJ, Melski JW. A histologic comparison of congenital and acquired nevomelanocytic nevi. Archives of dermatology. 1985;121:1266–1273. [PubMed] [Google Scholar]
- Rhodes AR, Sober AJ, Day CL, Melski JW, Harrist TJ, Mihm MC, Jr, Fitzpatrick TB. The malignant potential of small congenital nevocellular nevi. An estimate of association based on a histologic study of 234 primary cutaneous melanomas. Journal of the American Academy of Dermatology. 1982;6:230–241. doi: 10.1016/s0190-9622(82)70016-7. [DOI] [PubMed] [Google Scholar]
- Rivers JK. Is there more than one road to melanoma? Lancet. 2004;363:728–730. doi: 10.1016/S0140-6736(04)15649-3. [DOI] [PubMed] [Google Scholar]
- Ross AL, Sanchez MI, Grichnik JM. Molecular nevogenesis. Dermatol Res Pract. 2011;2011:463184. doi: 10.1155/2011/463184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha G, Purnell D, Fletcher A, Potter L, Gillies A, Pringle JH. High BRAF mutation frequency does not characterize all melanocytic tumor types. International journal of cancer. Journal international du cancer. 2004;111:705–710. doi: 10.1002/ijc.20325. [DOI] [PubMed] [Google Scholar]
- Saroufim M, Novy M, Taraif S, Habib RH, Loya A, Rauscher B, Kriegshauser G, Oberkanins C, Khalifeh I. BRAF mutational epidemiology in dysplastic nevi: does different solar UV radiation exposure matter? Journal of the European Academy of Dermatology and Venereology : JEADV. 2014;28:615–625. doi: 10.1111/jdv.12148. [DOI] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Seykora J, Elder D. Dysplastic nevi and other risk markers for melanoma. Seminars in oncology. 1996;23:682–687. [PubMed] [Google Scholar]
- Shakhova O, Zingg D, Schaefer SM, Hari L, Civenni G, Blunschi J, Claudinot S, Okoniewski M, Beermann F, Mihic-Probst D, et al. Sox10 promotes the formation and maintenance of giant congenital naevi and melanoma. Nature cell biology. 2012;14:882–890. doi: 10.1038/ncb2535. [DOI] [PubMed] [Google Scholar]
- Sober AJ, Burstein JM. Precursors to skin cancer. Cancer. 1995;75:645–650. doi: 10.1002/1097-0142(19950115)75:2+<645::aid-cncr2820751405>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Takata M, Lin J, Takayanagi S, Suzuki T, Ansai S, Kimura T, Cerroni L, Saida T. Genetic and epigenetic alterations in the differential diagnosis of malignant melanoma and spitzoid lesion. The British journal of dermatology. 2007;156:1287–1294. doi: 10.1111/j.1365-2133.2007.07924.x. [DOI] [PubMed] [Google Scholar]
- Tan JM, Lin LL, Lambie D, Flewell-Smith R, Jagirdar K, Schaider H, Sturm RA, Prow TW, Soyer HP. BRAF Wild-Type Melanoma in Situ Arising In a BRAF V600E Mutant Dysplastic Nevus. JAMA dermatology. 2015;151:417–421. doi: 10.1001/jamadermatol.2014.3775. [DOI] [PubMed] [Google Scholar]
- Temple-Camp CR, Saxe N, King H. Benign and malignant cellular blue nevus. A clinicopathological study of 30 cases. The American Journal of dermatopathology. 1988;10:289–296. doi: 10.1097/00000372-198808000-00002. [DOI] [PubMed] [Google Scholar]
- Tschandl P, Berghoff AS, Preusser M, Burgstaller-Muehlbacher S, Pehamberger H, Okamoto I, Kittler H. NRAS and BRAF mutations in melanoma-associated nevi and uninvolved nevi. PloS one. 2013;8:e69639. doi: 10.1371/journal.pone.0069639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DJ, Zirvi MA, Barany F, Elenitsas R, Seykora J. Detection of the BRAF V600E mutation in melanocytic lesions using the ligase detection reaction. Journal of cutaneous pathology. 2005;32:334–339. doi: 10.1111/j.0303-6987.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- Uribe P, Andrade L, Gonzalez S. Lack of association between BRAF mutation and MAPK ERK activation in melanocytic nevi. The Journal of investigative dermatology. 2006;126:161–166. doi: 10.1038/sj.jid.5700011. [DOI] [PubMed] [Google Scholar]
- Uribe P, Wistuba II, Gonzalez S. BRAF mutation: a frequent event in benign, atypical, and malignant melanocytic lesions of the skin. The American Journal of dermatopathology. 2003;25:365–370. doi: 10.1097/00000372-200310000-00001. [DOI] [PubMed] [Google Scholar]
- van Dijk MC, Bernsen MR, Ruiter DJ. Analysis of mutations in B-RAF, N-RAS, and H-RAS genes in the differential diagnosis of Spitz nevus and spitzoid melanoma. The American journal of surgical pathology. 2005;29:1145–1151. doi: 10.1097/01.pas.0000157749.18591.9e. [DOI] [PubMed] [Google Scholar]
- van Elsas A, Zerp SF, van der Flier S, Kruse KM, Aarnoudse C, Hayward NK, Ruiter DJ, Schrier PI. Relevance of ultraviolet-induced N-ras oncogene point mutations in development of primary human cutaneous melanoma. The American journal of pathology. 1996;149:883–893. [PMC free article] [PubMed] [Google Scholar]
- van Engen-van Grunsven AC, van Dijk MC, Ruiter DJ, Klaasen A, Mooi WJ, Blokx WA. HRAS-mutated Spitz tumors: A subtype of Spitz tumors with distinct features. The American journal of surgical pathology. 2010;34:1436–1441. doi: 10.1097/PAS.0b013e3181f0a749. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O'Brien JM, Simpson EM, Barsh GS, Bastian BC. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457:599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk CD, Fitch KR, Fuchs H, de Angelis MH, Barsh GS. Effects of G-protein mutations on skin color. Nature genetics. 2004;36:961–968. doi: 10.1038/ng1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T, Obenauf AC, Wackernagel W, Green G, Bouvier N, et al. Mutations in GNA11 in uveal melanoma. The New England journal of medicine. 2010;363:2191–2199. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schanke A, van Venrooij GM, Jongsma MJ, Banus HA, Mullenders LH, van Kranen HJ, de Gruijl FR. Induction of nevi and skin tumors in Ink4a/Arf Xpa knockout mice by neonatal, intermittent, or chronic UVB exposures. Cancer research. 2006;66:2608–2615. doi: 10.1158/0008-5472.CAN-05-2476. [DOI] [PubMed] [Google Scholar]
- Venesio T, Chiorino G, Balsamo A, Zaccagna A, Petti C, Scatolini M, Pisacane A, Sarotto I, Picciotto F, Risio M. In melanocytic lesions the fraction of BRAF V600E alleles is associated with sun exposure but unrelated to ERK phosphorylation. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2008;21:716–726. doi: 10.1038/modpathol.2008.41. [DOI] [PubMed] [Google Scholar]
- Viros A, Fridlyand J, Bauer J, Lasithiotakis K, Garbe C, Pinkel D, Bastian BC. Improving melanoma classification by integrating genetic and morphologic features. PLoS medicine. 2008;5:e120. doi: 10.1371/journal.pmed.0050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vredeveld LC, Possik PA, Smit MA, Meissl K, Michaloglou C, Horlings HM, Ajouaou A, Kortman PC, Dankort D, McMahon M, et al. Abrogation of BRAFV600E-induced senescence by PI3K pathway activation contributes to melanomagenesis. Genes & development. 2012;26:1055–1069. doi: 10.1101/gad.187252.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt AJ, Kotsis SV, Chung KC. Risk of melanoma arising in large congenital melanocytic nevi: a systematic review. Plastic and reconstructive surgery. 2004;113:1968–1974. doi: 10.1097/01.prs.0000122209.10277.2a. [DOI] [PubMed] [Google Scholar]
- Wiesner T, He J, Yelensky R, Esteve-Puig R, Botton T, Yeh I, Lipson D, Otto G, Brennan K, Murali R, et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nature communications. 2014;5:3116. doi: 10.1038/ncomms4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner T, Murali R, Fried I, Cerroni L, Busam K, Kutzner H, Bastian BC. A distinct subset of atypical Spitz tumors is characterized by BRAF mutation and loss of BAP1 expression. The American journal of surgical pathology. 2012;36:818–830. doi: 10.1097/PAS.0b013e3182498be5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner T, Obenauf AC, Murali R, Fried I, Griewank KG, Ulz P, Windpassinger C, Wackernagel W, Loy S, Wolf I, et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nature genetics. 2011;43:1018–1021. doi: 10.1038/ng.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Wang M, Wang X, Yin N, Song T, Li H, Yu J, Wang DM, Zhao Z. Lack of BRAF(V600E) mutations in giant congenital melanocytic nevi in a Chinese population. The American Journal of dermatopathology. 2011;33:341–344. doi: 10.1097/DAD.0b013e3181fb5bc7. [DOI] [PubMed] [Google Scholar]
- Wu J, Rosenbaum E, Begum S, Westra WH. Distribution of BRAF T1799A(V600E) mutations across various types of benign nevi: implications for melanocytic tumorigenesis. The American Journal of dermatopathology. 2007;29:534–537. doi: 10.1097/DAD.0b013e3181584950. [DOI] [PubMed] [Google Scholar]
- Yadav S, Vossaert KA, Kopf AW, Silverman M, Grin-Jorgensen C. Histopathologic correlates of structures seen on dermoscopy (epiluminescence microscopy) The American Journal of dermatopathology. 1993;15:297–305. doi: 10.1097/00000372-199308000-00001. [DOI] [PubMed] [Google Scholar]
- Yan J, Roy S, Apolloni A, Lane A, Hancock JF. Ras isoforms vary in their ability to activate Raf-1 and phosphoinositide 3-kinase. The Journal of biological chemistry. 1998;273:24052–24056. doi: 10.1074/jbc.273.37.24052. [DOI] [PubMed] [Google Scholar]
- Yazdi AS, Palmedo G, Flaig MJ, Puchta U, Reckwerth A, Rutten A, Mentzel T, Hugel H, Hantschke M, Schmid-Wendtner MH, et al. Mutations of the BRAF gene in benign and malignant melanocytic lesions. The Journal of investigative dermatology. 2003;121:1160–1162. doi: 10.1046/j.1523-1747.2003.12559.x. [DOI] [PubMed] [Google Scholar]
- Zembowicz A, Mihm MC. Dermal dendritic melanocytic proliferations: an update. Histopathology. 2004;45:433–451. doi: 10.1111/j.1365-2559.2004.01975.x. [DOI] [PubMed] [Google Scholar]
- Zembowicz A, Phadke PA. Blue nevi and variants: an update. Archives of pathology & laboratory medicine. 2011;135:327–336. doi: 10.5858/2009-0733-RA.1. [DOI] [PubMed] [Google Scholar]
- Zimmermann AA, Cornbleet T. The development of epidermal pigmentation in the Negro fetus. The Journal of investigative dermatology. 1948;11:383–395. [PubMed] [Google Scholar]