Abstract
Recent advances in neuroimaging have identified a large number of neural measures that could be involved in age-related declines in cognitive functioning. A popular method of investigating neural-cognition relations has been to determine the brain regions in which a particular neural measure is associated with the level of specific cognitive measures. Although this procedure has been informative, it ignores the strong interrelations that typically exist among the measures in each modality. An alternative approach involves investigating the number and identity of distinct dimensions within the set of neural measures and within the set of cognitive measures prior to examining relations between the two types of measures. The procedure is illustrated with data from 297 adults between 20 and 79 years of age with cortical thickness in different brain regions as the neural measures, and performance on 12 cognitive tests as the cognitive measures. The results revealed that most of the relations between cortical thickness and cognition occurred at a general level corresponding to variance shared among different brain regions and among different cognitive measures. In addition, the strength of the thickness-cognition relation was substantially reduced after controlling the variation in age, which suggests that at least some of the thickness-cognition relations in age-heterogeneous samples may be attributable to the influence of age on each type of measure.
A large number of measures of brain structure and brain function have been found to be negatively related to age, and many of these measures have also been found to be related to measures of cognitive functioning. Consider measures of cortical thickness, as assessed by the distance between the gray matter – cerebral spinal fluid (CSF) boundary and the gray matter – white matter boundary. Because it is postulated to reflect the density of neurons, dendrites, spines, synapses, and glial cells, cortical thickness is a potentially important neural substrate of cognition.
Negative relations between adult age and measures of cortical thickness have been reported in numerous studies (e.g., Ecker et al., 2009; Fjell, et al., 2006; 2009; 2014; Hogstrom et al., 2013; Hutton et al., 2009; McGinnis et al., 2011; Salat, et al., 2004; 2009; Tustison, et al., 2014; Westlye, et al., 2011), and many studies have also reported positive relations between measures of cortical thickness and cognitive functioning (e.g., Choi et al., 2008; Desrivieres et al., 2014; Ehrlich, et al., 2012; Engvig et al., 1010; Fjell, et al., 2006; Haier et al., 2009; Karama et al., 2009; 2011; Narr, et al., 2007; Schilling et al., 2013; Walhovd et al., 2006; Westlye, et al., 2009; 2011; but see Colom et al., 2013). Based on these two sets of findings, it is tempting to postulate that age-related reductions in cortical thickness in specific neuroanatomical regions are involved in age-related reductions in particular types of cognitive functioning. However, we suggest that it is important to consider two issues when making these types of inferences; level of analysis, and the degree to which the relation between the two types of measures might be dependent on the relation of each measure with age.
Level of Analysis
Although sometimes considered individually, most neuroanatomical measures derived from different brain regions are highly related with one another, and most cognitive measures are highly related with one another. This lack of independence implies that some of the relations observed with an individual measure could be shared with influences that affect many measures, and are not unique to the target measure. However, shared and unique influences cannot be distinguished unless multiple measures are examined in some type of organizational structure.
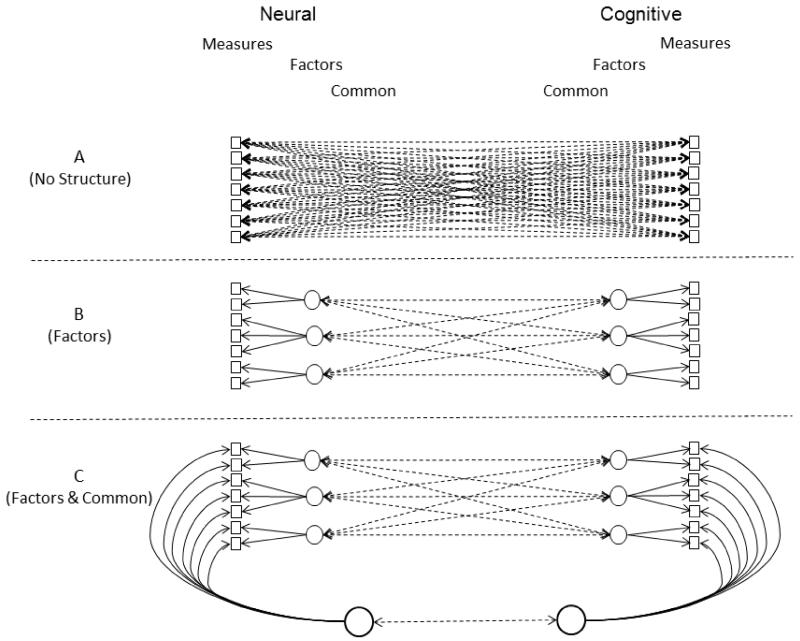
Consider Figure 1 which portrays three possible organizations with sets of neural measures and cognitive measures. Panel A illustrates a situation with no structure in either the neural or cognitive measures. Neural-cognition relations could be investigated within a framework such as this by examining all possible combinations of neural measures and cognitive measures. However, this is almost never done because of the extremely large number of possible neural measures that could be obtained across different regions of the brain. Instead analyses are often conducted to determine which clusters of neural measures are significantly related to particular cognitive measures. Any structure that emerges with this approach is therefore based on relations the neural measures have with that set of cognitive measures, and does not necessarily reflect the intrinsic dimensionality of the neural measures, independent of their relations with other types of measures.
Figure 1.
Alternative structural models of sets of neural measures and cognitive measures with (A) no structure among either set of measures, (B) organization of the measures into multiple specific factors, and (C) organization of the measures into specific factors and a common factor.
An alternative approach to investigate neural-cognition relations is portrayed in Panel B in which the two types of measures are first grouped into factors representing shared individual difference variance, and then neural-cognition relations are examined at the level of factors instead of individual measures. Unlike the situation portrayed in Panel A, interrelations among each type of measure are examined to determine a set of dimensions that parsimoniously represents the structure of individual differences within each type of measure before examining relations between the two types of measures.
A third possible structure is illustrated in Panel C in which the measures are organized into both specific factors and a common factor. In this latter structure the common factor represents influences shared among all measures, and the specific factors represent influences shared among subsets of measures that are independent of what is common across all measures. In the psychometric literature this type of model is known as a bi-factor (or nested-factors or orthogonal common factor) model, and the common factor is often designated g.
Bi-factor and related hierarchical models of cognitive functioning have been investigated in a large number of studies, including several examining influences associated with adult age (e.g., Hildebrandt et al., 2011; Salthouse, 2009; Salthouse & Davis, 2006; Salthouse & Ferrer-Caja, 2003; Schmiedek & Li, 2004). A consistent finding in these studies has been a moderately large influence of age on the common factor, with additional influences on one or more cognitive ability factors. Several studies have also examined relations of neural measures with structural models of cognitive ability. For example, Karama et al. (2011) and Menary et al. (2013) found that most of the relations between cortical thickness and cognition in samples of adolescents were evident at the level of the common cognitive factor, and Booth et al. (2013) and Penke et al. (2010) found that most of the relations between measures of white matter integrity and cognitive measures in older adults were at the level of the common cognitive factor.
Although cortical thickness is often measured across very small cortical regions, broad areas of significant thickness-cognition relations have been reported in many studies (e.g., Choi, et al., 2008; Desrivieres et al., 2014; Karama et al., 2011; Menary et al., 2013; Narr et al., 2007; Walhovd et al., 2006), and some researchers have used a measure of the average thickness across multiple brain regions when examining relations with cognition (e.g., Hedden et al., 2014; Hutton et al., 2009; Schnack et al., 2015). We are aware of only one study in which interrelations of cortical thickness measures were examined. In that study, Ecker et al. (2009) found that a hierarchical model with 1st-order factors corresponding to different lobes provided a good fit to cortical thickness data based on 28 gyrus-defined brain regions. Significant interrelations of measures of regional volume (e.g., Kennedy et al., 2009; Raz et al., 2005), and white matter integrity (e.g., Li et al., 2012; Lovden et al. 2013; Penke et al. 2010; 2012; Wahl et al., 2010) have also been found in several recent studies, which implies that the measures could be organized into a relatively small number of factors.
Because very few measures within a given modality are unrelated to other measures in that modality, it is important to consider relations among the measures within a given modality when investigating relations between neural measures and cognitive measures. The method advocated here is to first determine the organizational structure with each type of measure, and then examine relations at the broadest or most general levels in the structures before considering any relations that might exist at more specific levels.
Nature of the Relation with Age
As noted earlier, reductions in cortical thickness with increased age could be postulated to contribute to age-related differences in cognitive performance. However, because many neural and cognitive measures are related to age, at least some of the relations between neural and cognitive measures could be attributable to the relations each type of measure has with age. This possibility can be investigated by comparing the cortical thickness – cognition relations before and after statistical control of the variability in age. The reasoning is that if the neural-cognition relations are substantially reduced when there is little variation in age, at least some of the relations could be inferred to be attributable to the associations of both the neural and cognitive measures with age.
Current Study
To summarize, the primary goal of the current study was to demonstrate the usefulness of a proposed analytical procedure for investigating neural substrates of age-cognition relations involving examination of the structure of cortical thickness measures and of cognitive measures, and investigating the levels in the respective structures at which the two types of measures are related to one another. In addition, the thickness-cognition relations were examined before and after control of the variation in age to investigate the role of age on those relations.
Method
Participants
Participants were recruited using market-mailing procedures, flyers and by word of mouth. Potential participants were initially screened by telephone to ensure that they met basic inclusion criteria (i.e., right handed, English speaking, no psychiatric or neurological disorders, and normal or corrected-to-normal vision). All participants found eligible via the initial telephone screen were further screened in person with structured medical, neurological, psychiatric, and neuropsychological evaluations to ensure that they had no neurological or psychiatric disease or cognitive impairment. The screening procedure included a detailed interview that excluded individuals with a self-reported history of major or unstable medical illness, significant neurological history (e.g. epilepsy, brain tumor, stroke), history of head trauma with loss of consciousness for greater than 5 minutes, or history of Axis I psychiatric disorder. Individuals taking psychotropic medications were also excluded. Global cognitive functioning was assessed with the Mattis Dementia Rating Scale (Mattis, 1988), on which a score of at least 135 was required for retention in the study. In addition, any performance on tests in the cognitive test battery that was indicative of mild cognitive impairment was grounds for exclusion.
The study was approved by the Internal Review Board of the College of Physicians and Surgeons of Columbia University, and after the nature and risks of the study were explained, written informed consent was obtained from all participants prior to study participation. Participants were compensated for their participation in the study.
Demographic characteristics of the participants are summarized in Table 1. The participants ranged from 20 to 79 years of age, with some originally recruited to compare young and old adults and others recruited to provide a continuous comparison across adulthood. Although there was variability in the number of individuals in each age decade, this had little effect on the results because the analyses treated age as a continuous rather than categorical variable. Increased age was associated with lower levels of cognition (r = −.46, p<.001) and lower levels of cortical thickness (r = −.64, p < .001). Years of education ranged from 9 to 24, and more education was associated with higher levels of cognition (r = .27, p < .001), but was not related to cortical thickness (r = −.05, p > .35).
Table 1.
Demographic variables by age decade
| 20s | 30s | 40s | 50s | 60s | 70s | |
|---|---|---|---|---|---|---|
| N | 84 | 36 | 23 | 37 | 77 | 40 |
| Age | 25.3 (2.6) | 33.5 (2.9) | 44.9 (2.7) | 53.9 (3.1) | 64.7 (2.6) | 73.4 (2.6) |
| Prop. Female | .58 | .61 | .43 | .54 | .53 | .55 |
| Yrs. Educ. | 15.7 (2.1) | 16.5 (2.5) | 16.0 (2.6) | 16.2 (2.0) | 15.9 (2.6) | 17.5 (2.5) |
| Cognition | .56 (0.9) | .29 (0.9) | .27 (0.7) | −.22 (1.1) | −.36 (0.9) | −.69 (0.7) |
| Thickness | .75 (0.8) | .44 (0.6) | .52 (0.7) | −.13 (0.7) | −.68 (0.8) | −.79 (0.7) |
Note: Values in parentheses are standard deviations. The cognition and thickness values correspond to the first principal factors across the 12 cognitive measures and the 33 cortical thickness measures.
Cognitive Measures
Twelve measures were selected from a battery of neuropsychological tests to assess cognitive functioning. Three memory measures were based on sub-scores of the Selective Reminding Task (SRT; Buschke & Fuld, 1974). Participants in this task were initially read a list of 12 words and asked to recall as many as they could. For the following five trials they were reminded of the words that they did not report and were asked to again recall all of the words in the list. Words are considered to enter long term storage from the point when they are recalled twice in a row without reminders. The long-term storage sub-score (SRT_LTS) is the sum over all words of the number of trials when each word was in long-term storage. Continuous long-term retrieval (SRT_CLRT) is the sum over all words of the number of trials for which the word was continuously recalled. The third memory measure was the number of words recalled on the last trial (SRT_Last).
Three measures were selected to assess perceptual speed. One was the score on the Digit Symbol subtest from the Wechsler Adult Intelligence Scale (WAIS III; Wechsler, 1997). Participants in this test were instructed to write the symbol corresponding to specific digits as quickly as possible based on a key specifying the appropriate symbol for each digit. The score is the number of correctly produced symbols in 120 seconds. A second measure was the score on Part A of the Trail Making Test (Reitan & Wolfson, 1987), in which participants are instructed to connect circles numbered from 1 to 24 as rapidly as possible and performance is assessed as the time to connect all 24 circles. The third speed measure was the number of colored ink patches named in 90 seconds in the control condition of the Stroop Color Naming test.
Reasoning ability was assessed with scores on three different tests. One test was the WAIS III (Wechsler, 1997) Block Design test, in which participants are asked to reproduce a series of increasingly complex geometrical shapes using 4 or 9 identical blocks with red, white, or split red and white sides. A second test was the WAIS III (Wechsler, 1997) Letter-Number Sequencing test in which participants are asked to recall progressively longer lists of intermixed letters and numbers in alphabetical and then numerical order. The third reasoning test was the WAIS III (Wechsler, 1997) Matrix Reasoning test in which participants are asked to select which pattern in a set of eight possible patterns best completes a missing cell in a matrix.
Vocabulary was assessed with scores on the Vocabulary subtest from the WAIS III (Wechsler, 1997), the Wechsler Test of Adult Reading (WTAR; Wechsler, 2001), and the American National Adult Reading Test (AMNART; Grober & Sliwinski, 1991). The Vocabulary subtest asks participants to provide definitions for a series of increasingly advanced words, and the WTAR and AMNART both involve participants correctly pronouncing irregularly spelled English words.
Cortical Thickness Measures
MRI images were acquired in a 3.0T Philips Achieva Magnet using a standard quadrature head coil. A T1-weighted scout image was acquired to determine subject position. T1-weighted images of the whole brain were acquired for each subject with an MPRAGE sequence with 180 contiguous 1 mm thick axial slices using the following parameters: TR 6.5 ms, TE 3 ms; flip angle 8°, acquisition matrix 256×256 mm field of view. A neuroradiologist reviewed anatomical scans and data from one participant with clinically significant findings were removed from the sample prior to data analysis.
Each subject’s structural T1 scan was reconstructed using the FreeSurfer 5.1 analysis package (http://surfer.nmr.mgh.harvard.edu/), which parcellates cortical regions based on the morphology of the gyri and sulci in each individual participant. The accuracy of FreeSurfer’s cortical parcellation has been reported to be comparable to manual labeling (Fischl et al., 2002; 2004). Each subject’s white and gray matter boundaries as well as gray matter and CSF boundaries were visually inspected slice by slice by an experienced technician. Manual control points were added in the case of any visible discrepancy, and reconstruction was repeated until satisfactory results were reached for every subject (Fjell et al., 2009). The subcortical structure borders were overlaid on the T1 image by Freeview visualization tools and compared against the actual brain regions, and in case of a discrepancy, were corrected manually.
Cortical thickness was measured as the distance between the gray/white matter surface and the gray/CSF surface at each point across the cortical mesh. The points on each subject’s surface mesh were resampled into the standard surface mesh “fsaverage” given in the FreeSurfer package.
Using a validated automated labeling system (Fischl et al., 2004), the cortex was parcellated into 33 different gyrus-based areas in each hemisphere, and mean thickness was averaged in each area. This resulted in mean cortical thickness calculations for 66 regions divided among the two hemispheres. For the primary analyses the corresponding values in the two hemispheres were averaged to yield 33 cortical thickness measures, but very similar results were obtained with analyses on all 66 regions.
Analyses
A procedure described in Karama et al. (2011) for assessing common and domain-specific cognitive factors was used with both the cognitive and cortical thickness measures. The procedure can be considered equivalent to the bi-factor structural model portrayed in the bottom of Figure 1, and yields values of the common factor and of specific (i.e., independent of the common factor) group factors for each participant. The first step in the procedure involves derivation of a common factor, often designated g with cognitive measures, by saving scores from the first unrotated factor in a Principal Axis Factor analysis1. The second step consists of the derivation of factor scores by saving scores from a Principal Axis Factor analysis with promax (oblique) rotation. The third step consists of regressing the common factor score obtained in Step 1 from the factor scores obtained in Step 2 to derive specific factor scores that are independent of what is shared among all measures. The outcomes of this procedure are separate estimates of the common factor and of each specific factor for every participant.
Because of the large number of statistical comparisons, use of a conventional significance level of .05 would likely lead to some significant results because of chance. In order to minimize this possibility, significance levels of .01 and .001 are reported for all statistical tests, with the latter value considered a more conservative significance level.
Results
The initial factor analysis on the 12 cognitive measures yielded three factors with eigenvalues greater than 1. However, prior research with similar measures (e.g., Salthouse, 2009; Salthouse & Ferrer-Caja, 2003) suggested the existence of four factors, and therefore the analysis was repeated after specifying four factors. This analysis resulted in the expected pattern of loadings of the measures on factors corresponding to vocabulary, memory, speed and reasoning, as indicated in Table 2. Correlations among the factors ranged from .18 to .67, with a median of .49. The first unrotated principal factor was associated with 40.4% of the variance in the 12 cognitive measures, and the four factors together were associated with 80.1% of the variance. Entries in the right-most column in Table 2 are loadings on the common, first unrotated principal axis, factor.
Table 2.
Loadings of 12 cognitive measures on factors in a Principal Axis Factor Analysis with Promax Rotation
| Measure | F1 (Vocab) |
F2 (Memory) |
F3 (Speed) |
F4 (Reasoning) |
Common |
|---|---|---|---|---|---|
| AMNART | .968 | .109 | .143 | .349 | .457 |
| WTAR | .943 | .206 | .279 | .468 | .569 |
| WAISR_VOC | .814 | .123 | .151 | .342 | .422 |
| SRT_CLRT | .156 | .969 | .616 | .555 | .819 |
| SRT_LTS | .182 | .917 | .560 | .481 | .767 |
| SRT_LAST | .110 | .865 | .515 | .419 | .693 |
| WAISR_DIGSYM | .127 | .527 | .828 | .543 | .673 |
| STROOP_COLOR | .247 | .463 | .769 | .473 | .635 |
| TRAILS_A | .055 | .412 | .683 | .484 | .546 |
| WAISR_BLKDES | .368 | .430 | .582 | .890 | .664 |
| WAIS3_MATREAS | .348 | .438 | .486 | .690 | .611 |
| WAIS3_LETNUM | .401 | .474 | .568 | .576 | .656 |
Note: Values in bold indicate the factor on which each cognitive measure loads most highly. Entries in the column labeled Common are loadings on the unrotated first principal axis factor based on a separate analysis.
The initial factor analysis on the 33 cortical thickness measures yielded six factors with eigenvalues greater than 1, but two of the factors were dominated by measures from the temporal lobe. The analysis was therefore repeated after specifying five factors, and the loadings of the thickness measures on the five factors are reported in Table 3. Inspection of the measures with the highest entries in each column suggests that the factors approximately correspond to the parietal, temporal, cingulate, frontal, and occipital regions. Correlations among the factors ranged from .31 to .76, with a median of .64. The first unrotated principal factor was associated with 49.8% of the variance across the 33 cortical thickness measures, and the five factors together were associated with 70.4% of the variance. Loadings on the common, first unrotated principal axis, factor are reported in the right-most column in Table 3.
Table 3.
Loadings of 33 cortical thickness measures on factors in a Principal Axis Factor Analysis with Promax Rotation
| Measure | F1 (Parietal) |
F2 (Temporal) |
F3 (Cingulate) |
F4 (Frontal) |
F5 (Occipital) |
Common |
|---|---|---|---|---|---|---|
| Supramargil | .894 | .643 | .793 | .642 | .429 | .873 |
| Precuneus | .872 | .613 | .669 | .604 | .467 | .828 |
| Inferiorparietal | .868 | .572 | .713 | .606 | .433 | .821 |
| Superiorparietal | .864 | .466 | .519 | .520 | .499 | .745 |
| Precentral | .842 | .592 | .670 | .668 | .448 | .823 |
| Paracentral | .830 | .501 | .599 | .538 | .480 | .754 |
| Postcentral | .786 | .396 | .457 | .449 | .516 | .664 |
| Lateraloccipital | .775 | .567 | .664 | .484 | .552 | .752 |
| Transversetemporal | .580 | .515 | .551 | .390 | .395 | .602 |
| Fusiform | .716 | .857 | .721 | .611 | .329 | .826 |
| Lateralorbitofrontal | .546 | .851 | .554 | .779 | .303 | .758 |
| Inferiortemporal | .638 | .834 | .682 | .619 | .197 | .773 |
| Superiortemporal | .734 | .829 | .750 | .628 | .387 | .844 |
| Middletemporal | .753 | .811 | .792 | .670 | .299 | .854 |
| Temporalpole | .329 | .735 | .340 | .425 | .139 | .505 |
| Parsorbitalis | .579 | .732 | .584 | .687 | .278 | .725 |
| Medialorbitofrontal | .533 | .719 | .544 | .713 | .378 | .706 |
| Entorhil | .246 | .624 | .243 | .274 | .007 | .379 |
| Parahippocampal | .358 | .434 | .405 | .250 | .260 | .416 |
| Posteriorcingulate | .562 | .432 | .760 | .487 | .243 | .620 |
| Bankssts | .711 | .662 | .716 | .544 | .312 | .757 |
| Isthmuscingulate | .429 | .416 | .701 | .361 | .257 | .518 |
| Rostralanteriorcingulate | .477 | .451 | .640 | .522 | .283 | .577 |
| Caudalanteriorcingulate | .275 | .173 | .446 | .279 | .161 | .321 |
| Rostralmiddlefrontal | .699 | .612 | .652 | .893 | .395 | .811 |
| Superiorfrontal | .767 | .639 | .752 | .870 | .343 | .856 |
| Caudalmiddlefrontal | .767 | .497 | .630 | .794 | .354 | .780 |
| Parstriangularis | .732 | .721 | .718 | .771 | .440 | .843 |
| Parsopercularis | .711 | .657 | .686 | .767 | .379 | .725 |
| Frontalpole | .350 | .514 | .416 | .596 | .204 | .511 |
| Cuneus | .660 | .389 | .483 | .450 | .784 | .632 |
| Pericalcarine | .425 | .135 | .275 | .280 | .736 | .383 |
| Lingual | .621 | .514 | .615 | .420 | .673 | .656 |
Note: Measures are labeled with the FreeSurfer terminology. Values in bold indicate the factor on which each region loads most highly. Entries in the column labeled Common are loadings on the unrotated first principal axis factor based on a separate analysis.
Four sets of results suggest that, at least in terms of individual differences, the cortical thickness measures were less distinct from one another than the cognitive measures. That is, relative to the cognitive measures the thickness measures had a higher percentage of variance associated with the first factor (i.e., 49.8% vs. 40.4%), a higher median correlation between factors (i.e., .64 vs. .49), a smaller difference between the loadings of the variables on the designated factor and the loadings on the other factors (i.e., the median difference in loadings between the bold and non-bold values in each row, excluding the common factor, was .25 for the cortical thickness measures and .44 for the cognitive measures), and a higher median loading of the measures on the common factor (i.e., .75 vs. .65). Taken together, these findings indicate that there is less differentiation across people in the cortical thickness measures than in the cognitive measures. The justification for considering measures in terms of an organizational structure instead of independently is therefore at least as strong for the cortical thickness measures as for the cognitive measures.
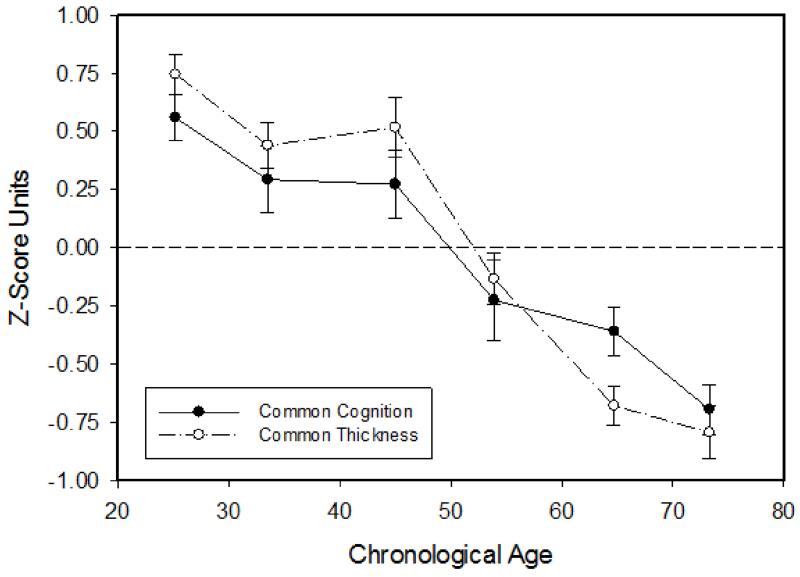
Figure 2 portrays the means (and standard errors) of the common (first principal factor) cognition and cortical thickness factors by age decade. Potential quadratic age relations on the common cognition and common cortical thickness factor scores were also examined, but they were not significant (p > .5).
Figure 2.
Mean (and standard error) of the common cognitive and common cortical thickness measures as a function of age decade.
A regression analysis was conducted to investigate whether the relation between the common cortical thickness factor and the common cognitive factor varied as a function of age. The outcome variable in the analysis was the common cognitive factor score and the predictor variables were age, the common cortical thickness factor, and the interaction of age and the cortical thickness factor. In order to minimize collinearity of the predictors, the age and common cortical thickness factors were centered before multiplying them with one another to form the interaction term. Standardized coefficients from this analysis were −.34 for age, .19 for cortical thickness, and .02 for the interaction. The two main effects were significant (p<.005), indicating that the common cognitive factor was smaller with increased age, but larger with higher values of cortical thickness. However, the interaction was not significant (p > .6), and therefore there was no evidence in these data that the relation between cortical thickness and cognition varied as a function of age.
Table 4 contains correlations between the cortical thickness factors and the cognitive factors before (column 1) and after (column 2) control of the relations at the level of common factors, and before (columns 1 and 2) and after (columns 3 and 4) control of the variation in age. The entries in the first column correspond to the conventional analyses in which the thickness factors and cognitive factors are each treated independently. It can be seen that the cortical thickness correlations were largest with the speed cognitive factor, intermediate for the reasoning and memory factors, and small with the vocabulary factor. However, the common cortical thickness and common cognition factors were correlated .41 with one another, and when a relation between these factors was specified, many of the previously significant correlations among the specific factors were substantially reduced, as indicated by the entries in the second column.
Table 4.
Correlations between cognitive factors and cortical thickness factors before and after control of relations between the common factors and control of age
| Ignore Age | Control Age | |||
|---|---|---|---|---|
| Alone | With Common |
Alone | With Common |
|
| Cognition – Thickness | .41** | .16* | ||
| Memory | ||||
| F1(Parietal) | .35** | .00 | .10 | .05 |
| F2(Temporal) | .33** | −.05 | .05 | −.07 |
| F3(Cingulate) | .38** | .10 | .07 | .06 |
| F4(Frontal) | .30** | .01 | −.00 | −.01 |
| F5(Occipital) | .23** | −.05 | .07 | −.04 |
| Reasoning | ||||
| F1(Parietal) | .38** | −.02 | .17* | .01 |
| F2(Temporal) | .39** | .05 | .16* | .04 |
| F3(Cingulate) | .40** | −.01 | .13 | −.04 |
| F4(Frontal) | .33** | −.03 | .06 | −.05 |
| F5(Occipital) | .27** | −.01 | .13 | −.00 |
| Speed | ||||
| F1(Parietal) | .42** | −.20** | .13 | −.11 |
| F2(Temporal) | .48** | .16* | .19** | .14 |
| F3(Cingulate) | .47** | .00 | .08 | −.09 |
| F4(Frontal) | .44** | .15* | .10 | .12 |
| F5(Occipital) | .24** | −.17* | .03 | −.18* |
| Vocabulary | ||||
| F1(Parietal) | .03 | .14 | .16* | .03 |
| F2(Temporal) | −.02 | −.08 | .11 | −.04 |
| F3(Cingulate) | −.02 | −.08 | .15 | .02 |
| F4(Frontal) | −.07 | −.11 | .06 | −.07 |
| F5(Occipital) | .12 | .15* | .21** | .17* |
p<.01;
p<.001.
The entries in the third column of Table 4 indicate that after control of the variation in age only a few of the correlations between cortical thickness factors and cognitive factors were significant. Furthermore, when relations between the common factors were also specified, as in the fourth column, the correlation of the common factors was .16 (compared to .41 when age was not controlled), and none of the correlations between specific factors were significant at the conservative p<.001 level.
Comparison of the correlations in columns 1 and 2 of Table 4 indicates that many of cortical thickness-cognition relations can be inferred to operate at the level of the common factors because the correlations between the cortical thickness and cognition factors were substantially reduced when common factors were included in the analysis. In addition, comparison of the correlations in columns 1 and 3 (and those in columns 2 and 4) indicates that many of the cortical thickness-cognition relations are attributable to the relations of the measures with age because the factor correlations were much smaller when the variability in age was statistically controlled.
Discussion
A popular approach to investigating neural-cognition relations involves examining the association between a set of neural measures and a set of cognitive measures, with each neural-cognitive pair treated independently. For example, a bivariate analysis in the data from this study might focus on the relation between the score on the block design test and cortical thickness in the superior temporal region. The correlation between these measures in the current study was .34, which could be interpreted as evidence that cortical thickness in the superior temporal region is one of the neural substrates of block design performance.
However, there is a long history of research with cognitive measures indicating that individuals who have high scores on one cognitive measure tend to have high scores on other cognitive measures. Moreover, organizational structures have been postulated in which the measures are grouped according to the strength of the correlations with one another. Most of the structures have several factors corresponding to distinct cognitive abilities, and an additional factor representing variance common to all cognitive measures (i.e., the general, or g, factor).
Many recent studies investigating neural substrates of cognition have relied on these types of organizational structures to examine associations with cognition at different levels of abstraction. For example, instead of linking neural measures to specific cognitive measures, researchers have examined associations of both general and specific cognitive factors with lesion location (e.g., Barbey, et al., 2013; Glascher, et al., 2010), regional volume (e.g., Colom, et al., 2009; Roman et al., 2014), white matter integrity (e.g., Booth, et al., 2013; Penke, et al., 2010; 2012), cortical surface area (e.g., Roman et al., 2014), and cortical thickness (e.g., Choi et al., 2008; Karama, et al., 2011; Menary, et al., 2013; Roman et al., 2014).
Perhaps because of the interest in regional specialization, less research has been conducted examining the organization of neural measures across different brain regions. However, several recent studies have reported significant correlations among neural measures in different regions, and in some of these studies the measures have been organized into correlation-based structures (e.g., Ecker, et al., 2009; Li, et al., 2012; Lovden, et al., 2013; 2014; Penke, 2010; 2012; Wahl et al., 2010).
At least two prior studies have relied on an organizational structure of neural measures when examining neural-cognitive relations. In the first, Penke et al. (2010) found that measures of white matter integrity in different tracts could be organized into a general factor which had significant associations with a general factor of speed. The second study, Salthouse (2011), was a re-analysis of measures of regional volumes and cognitive functioning originally reported in Kennedy et al. (2009). Although a significant relation was evident between a specific neural measure (i.e., lateral prefrontal volume) and a specific cognitive measure (i.e., fluid intelligence) in the bivariate analyses, no specific relations were evident when the associations were also examined between common factors of regional volume and cognitive measures.
The current study extended this type of multi-level neural-cognitive analysis with 33 cortical thickness measures and 12 cognitive measures. The results confirmed that neither cognitive nor neural measures exist in isolation. For example, the entries in Table 2 indicate that the block design measure had a loading of .890 on a first-order reasoning factor, and a loading of .664 on a common cognitive factor. In addition, the entries in Table 3 indicate that measure of cortical thickness in the superior temporal region had a loading of .829 on a temporal lobe factor, and a loading of .844 on a common cortical thickness factor. These strong loadings indicate that there are substantial interrelations among the cognitive measures on one hand, and among the neural measures on the other hand.
The lack of independence among the measures raises the possibility that relations between neural measures and cognitive measures could exist at different levels. That is, the relation could be between what is unique to each measure, between what is shared with similar measures reflecting the same constructs, or between what is common to different measures. However, influences at different levels cannot be distinguished unless the measures are considered in the context of an organizational structure.
The structure portrayed in Figure 1b represents neural-cognition relations at the level of specific factors, and the entries in the first column of Table 4 indicate that the cortical thickness factors had significant positive correlations with all cognitive factors except for vocabulary. Of particular interest is that the correlation of the reasoning factor with the temporal lobe factor was .39, but the correlation between block design score and superior temporal thickness was only .09 after partialling the variance in the reasoning and temporal lobe factors.
The structure portrayed in Figure 1c represents relations at both the level of specific factors and general factors, and the results in second column of Table 4 indicate that most of the thickness-cognition relations were considerably reduced after controlling the relation between the common factors for each type of measure. For example, the reasoning-temporal lobe thickness correlation was reduced from .39 to .05. Furthermore, after partialling the variance in both the specific and general factors, the correlation between the block design score and cortical thickness in the superior temporal region was only .08.
An important implication of these results is that neural measures in particular brain regions and scores on particular cognitive tests each have multiple influences, and if the influences are not distinguished, interpretations of the associations may be misleading. In the case of the relation between block design score and cortical thickness in the superior temporal region, the correlation was .34 when the measures were considered in isolation, but it was only .08 when the measures were considered in the context of other related measures. Merely because a single relation between two measures is being analyzed therefore does not mean that influences those measures share with other measures are not operating. Only by simultaneously considering shared and unique influences is it possible to accurately identify the contribution of unique influences. In this particular example, it would have been misleading to conclude that there was a specific relation between block design score and cortical thickness in the superior temporal region because the relation was greatly reduced when it was examined in the context of relations at broader levels of aggregation.
The results of this study suggest that many of the relations between measures of cortical thickness and measures of cognition appear to operate at a broad level representing what is common among many measures, and not exclusively at the level of factors or specific measures. In this respect, the results are consistent with previous studies involving measures of white matter integrity (e.g., Booth et al., 2013; Penke, et al., 2010) and regional volume (Salthouse, 2011). That is, each of these studies found that most of the associations between the neural measures and the cognitive measures were at the most general level corresponding to variance common across different brain regions and different cognitive measures.
Inspection of the entries in Table 4 reveals that most of the cortical thickness – cognition relations were eliminated after control of the relation between the common factors and/or control of the variation in age. However, some relations were significant at the .01 level, but not with the more conservative .001 level. These specific relations could be genuine, but because they are apparently not as robust as the other results in the study, it may be prudent to wait for them to be replicated before speculating about their meaning.
The empirical evidence supporting the existence of a common cognitive factor is extensive, but there is still no consensus on whether the g factor in cognitive abilities is merely a statistical abstraction, or whether it has a substantive existence in the form of a fundamental process or capacity such as working memory. Because research on common neural factors is much more recent, it may be some time before their nature is fully understood. Although the neurobiological bases of common cortical thickness factors and common cognitive factors are not yet known, they can be postulated to reflect systemic characteristics within an individual, such as those responsible for the sizes of different bones, or for the strengths of different limbs. Relations between bone size and limb strength could be examined in analyses of the size of individual bones and the strengths of particular limbs, but because neither bone size nor strength in different parts of the body are likely to be independent, some of those relations may reflect more general influences such as those between general stature and overall strength. Our suggestion is that an analogous situation applies when considering relations between neural measures and cognitive measures, and that specific relations are best interpreted in the context of relations that may be operating at more general levels. That is, rather than interpreting relations between particular neural measures and particular cognitive measures in isolation, we propose that it will often be more meaningful to interpret them relative to relations that exist both within, and between, other neural and cognitive measures.
The second major issue addressed in this study was whether at least some of the relation between a neural measure and a cognitive measure could be attributable to the relation of each measure to age. This possibility was investigated by using statistical procedures to control the variation in age, which had the effect of conducting the comparisons at the average age in the sample. The results in the third and fourth columns of Table 4 compared to those in the first and second columns indicate that the associations between the cortical thickness measures and the cognitive measures were substantially reduced when the variability in age was statistically controlled. These findings are therefore consistent with the hypothesis that at least some of the associations evident in age-heterogeneous samples are attributable to the influence of age on both cortical thickness and cognition. A similar finding of reduced cortical thickness-cognition relations after control of the variation in age was reported by Menary et al. (2013) in a sample of participants between 9 and 24 years of age.
It is sometimes assumed that a neural–cognition association evident in late adulthood reflects causal processes that have gradually become more salient with increasing age. Evidence relevant to this assumption is available from the regression analysis predicting the general cognitive factor from age, the general cortical thickness factor, and their interaction. Of particular interest in this analysis was the finding that the interaction of age and cortical thickness was very small, and not significantly different from zero. There was therefore no evidence in these results that the neural-cognition relations were stronger at older ages, as one might expect if diminished values of cortical thickness impose progressively more constraints on cognitive functioning at older ages. Another result consistent with this interpretation was the recent finding by Karama et al. (2013) of a significant association between cortical thickness measured in old age and cognitive functioning assessed in childhood, because this implies that the thickness-cognition association is apparent at all ages, and is not unique to the period of late life.
Several possible limitations of the study can be identified. For example, the measures of cortical thickness were relatively crude because they were based on aggregation of cortical thickness measures across 33 gyrus-defined cortical regions. It is therefore possible that there could be more evidence of selective relations with specific cognitive factors, and weaker relations with a common cortical thickness factor, with a finer level of resolution, and potentially higher dimensionality, of the cortical thickness measures. Another potential limitation is that results were based on cross-sectional comparisons of people across a 60-year age range. Smaller thickness-cognition relations might be evident among the changes in thickness and in the changes in cognition in longitudinal comparisons across shorter intervals. It is also possible that some of the older adults in the study may have been in preclinical stages of dementia, and the screening of physical and mental status described in the method section may not have detected all of these individuals, in which case the estimates of the common factors may have been inflated.
In conclusion, an analytical strategy was proposed in which the correlational structures among measures in neural and cognitive modalities are determined before examining relations between the two types of measures. The strategy was illustrated with cortical thickness in 33 different brain regions as the neural measures, and scores on 12 cognitive tests as the cognitive measures. The major findings were that there were moderately strong relations between measures of cortical thickness and measures of cognitive functioning, but that those relations were greatly reduced after adjusting for the relation between the common variance in each set of measures. These results imply that many of the relations between cortical thickness and cognition operate at a relatively broad level corresponding to the variance common across cortical thickness in different brain regions and corresponding to the variance common across different cognitive measures. In addition, because the thickness-cognition relations were substantially reduced after controlling the variability in age, at least some of those relations in age-heterogeneous samples can be inferred to be attributable to the associations of both cortical thickness and cognition with age.
Highlights.
Most neural and cognitive measures are positively correlated with one another
Based on the correlations, each type of measure can be organized into a structure
Many cortical thickness-cognition relations occur at high levels in the structures
Controlling the variation in age reduced many cortical thickness-cognition relations
Acknowledgments
This research was supported by grants from the National Institute on Aging (AG038465, PI Dr. Stern; R37AG024270, PI Dr. Salthouse). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The sponsors had no role in the study design, data collection, analysis or interpretation, writing of the report, or decision to submit the article for publication.
Footnotes
A principal axis factor analysis was used rather than a principal components analysis because the goal was to determine the structure among the shared variance in the measures, and not to identify mathematically independent components from the total variance in the measures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barbey AK, Colom R, Grafman J. Dorsolateral prefrontal contributions to human intelligence. Neuropsychologia. 2013;51:1361–1369. doi: 10.1016/j.neuropsychologia.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth T, Bastin ME, Penke L, Maniega SM, Murray C, Royle NA, et al. Brain white matter tract integrity and cognitive abilities in community-dwelling older people: The Lothian Birth Cohort, 1936. Neuropsychology. 2013;27:595–607. doi: 10.1037/a0033354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- Choi YY, Shamosh NA, Cho SH, DeYoung CG, Lee MJ, Lee JM, et al. Multiple bases of human intelligence revealed by cortical thickness and neural activation. Journal of Neuroscience. 2008;28:10323–10329. doi: 10.1523/JNEUROSCI.3259-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom R, Burgaleta M, Roman FJ, Karama S, Alvarez-Linera J, Abad FJ, et al. Neuroanatomic overlap between intelligence and cognitive factors: Morphometry methods provide support for the key role of the frontal lobes. NeuroImage. 2013;72:143–152. doi: 10.1016/j.neuroimage.2013.01.032. [DOI] [PubMed] [Google Scholar]
- Colom R, Haier RJ, Head K, Alvarez-Linera J, Quiroga MA, Shih PC, et al. Gray matter correlates of fluid, crystallized, and spatial intelligence: Testing the P-FIT model. Intelligence. 2009;37:124–135. [Google Scholar]
- Desrivieres S, Lourduscamy A, Tao C, Toro R, Jia T, Loth E, et al. Single nucleotide polymorphism in the neuroplastin locus associates with cortical thickness and intellectual ability in adolescents. Molecular Psychiatry. 2014:1–12. doi: 10.1038/mp.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker C, Stahl D, Daly E, Johnston P, Thomson A, Murphy DGM. Is there a common underlying mechanism for age-related decline in cortical thickness? NeuroReport. 2009;20:1155–1160. doi: 10.1097/WNR.0b013e32832ec181. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically Parcellating the Human Cerebral Cortex. Cerebral Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Reinvang I, Lundervold A, Salat D, Quinn BT, et al. Selective increase of cortical thickness in high-performing elderly – structural indices of optimal cognitive aging. NeuroImage. 2006;29:984–994. doi: 10.1016/j.neuroimage.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, et al. High consistency of regional cortical thinning across multiple samples. Cerebral Cortex. 2009;19:2001–12. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Grydeland H, Amlien I, Espeseth T, Reinvang I, et al. Accelerating cortical thinning: Unique to dementia or universal in aging? Cerebral Cortex. 2014;24:919–934. doi: 10.1093/cercor/bhs379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Glascher J, Rudrauf D, Colom R, Paul LK, Tranel D, Damasio H, et al. Distributed neural system for general intelligence revealed by lesion mapping. Proceedings of the National Academy of Sciences. 2010;107:4705–4709. doi: 10.1073/pnas.0910397107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. Journal of Clinical and Experimental Neuropsychology. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Hedden T, Schultz AP, Rieckmann A, Mormino EC, Johnson KA, Sperling RA, et al. Multiple brain markers are linked to age-related variation in cognition. Cerebral Cortex. doi: 10.1093/cercor/bhu238. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt A, Wilhelm O, Schmiedek F, Herzmann G, Sommer W. On the specificity of face cognition compared with general cognitive functioning across adult age. Psychology and Aging. 2011;26:701–715. doi: 10.1037/a0023056. [DOI] [PubMed] [Google Scholar]
- Hogstrom LJ, Westlye LT, Walhovd KB, Fjell AM. The structure of the cerebral cortex across adult life: Age-related patterns of surface area, thickness, and gyrification. Cerebral Cortex. 2013;23:2521–2530. doi: 10.1093/cercor/bhs231. [DOI] [PubMed] [Google Scholar]
- Hutton C, Draganski B, Ashburner J, Weiskopf N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. NeuroImage. 2009;48:371–380. doi: 10.1016/j.neuroimage.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karama S, Ad-Dab’bagh Y, Haier RJ, Deary IJ, Lyttelton OC, Lepage C, et al. Positive association between cognitive ability and cortical thickness in a representative US sample of healthy 6 to 18 year-olds. Intelligence. 2009;37:145–155. doi: 10.1016/j.intell.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karama S, Bastin ME, Murray C, Royle NA, Penke L, Maniega SM, et al. Childhood cognitive ability accounts for associations between cognitive ability and brain cortical thickness in old age. Molecular Psychiatry. 2013:1–5. doi: 10.1038/mp.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karama S, Colom R, Johnson IJ, Haier R, Waber DP, Lepage C, et al. Cortical thickness correlates of specific cognitive performance accounted for by the general factor of intelligence in healthy children aged 6 to 18. NeuroImage. 2011;55:1443–1453. doi: 10.1016/j.neuroimage.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Erickson KI, Rodrigue KM, Voss MW, Colcombe SJ, Kramer AF, et al. Age-related differences in regional brain volumes: A comparison of optimized voxel-based morphometry to manual volumetry. Neurobiology of Aging. 2009;30:1657–1676. doi: 10.1016/j.neurobiolaging.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y-O, Yang FG, Nguyen CT, Cooper SR, LaHue SC, Venugopal S, et al. Independent component analysis of DTI reveals multivariate microstructural correlations of white matter in the human brain. Human Brain Mapping. 2012;33:1431–1451. doi: 10.1002/hbm.21292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovden M, Kohncke Y, Laukka EJ, Kalpouzos G, Salami TQ, Fratiglioni L, et al. Changes in perceptual speed and white matter microstructure in the corticospinal tract are associated in very old age. NeuroImage. 2014;102:520–530. doi: 10.1016/j.neuroimage.2014.08.020. [DOI] [PubMed] [Google Scholar]
- Lovden M, Laukka EJ, Rieckmann A, Kalpouzos G, Jonsson T, Wahlund L-O, et al. The dimensionality of between-person differences in white matter microstructure in old age. Human Brain Mapping. 2013;34:1386–1398. doi: 10.1002/hbm.21518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale (DRS) Psychological Assessment Resources; Odessa, FL: 1988. [Google Scholar]
- McGinnis SM, Brickhouse M, Pascual B, Dickerson BC. Age-related changes in the thickness of cortical zones in humans. Brain Topogr. 2011;24:279–291. doi: 10.1007/s10548-011-0198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menary K, Collins PF, Porter JN, Muetzel R, Olson EA, Kuma V, et al. Associations between cortical thickness and general intelligence in children, adolescents and young adults. Intelligence. 2013;41:597–606. doi: 10.1016/j.intell.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KI, Woods RP, Thompson PM, Szeszko P, Robinson D, Dimtcheva T, et al. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cerebral Cortex. 2007;17:2163–2171. doi: 10.1093/cercor/bhl125. [DOI] [PubMed] [Google Scholar]
- Penke L, Maniega SM, Bastin ME, Hernandez MCV, Murray C, Royle NA, et al. Brain white matter tract integrity as a neural foundation for general intelligence. Molecular Psychiatry. 2012;17:1026–1030. doi: 10.1038/mp.2012.66. [DOI] [PubMed] [Google Scholar]
- Penke L, Maniega SM, Murray C, Gow AJ, Hernandez MCV, Clayden JD, et al. A general factor of white matter integrity predicts information processing speed in healthy older people. Journal of Neuroscience. 2010;30:7569–7574. doi: 10.1523/JNEUROSCI.1553-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: General trends, individual differences, and modifiers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. Neuropsychological Press; Tucson, AZ: 1987. [Google Scholar]
- Roman FJ, Abad FJ, Escorial S, Burgaleta M, Martinez K, Alvarez-Lineara J, et al. Reversed hierarchy in the brain for general and specific cognitive abilities: A morphometric analysis. Human Brain Mapping. 2014;35:3805–3818. doi: 10.1002/hbm.22438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, et al. Thinning of the cerebral cortex in aging. Cerebral Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Salat DH, Lee SY, van der Kouwe AJ, Greve DN, Fischl B, Rosas HD. Age-associated alterations in cortical gray and white matter signal intensity and gray to white matter contrast. NeuroImage. 2009;48:21–28. doi: 10.1016/j.neuroimage.2009.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Decomposing age correlations on neuropsychological and cognitive variables. Journal of the International Neuropsychological Society. 2009;15:650–661. doi: 10.1017/S1355617709990385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Neuroanatomical substrates of age-related cognitive decline. Psychological Bulletin. 2011;137:753–784. doi: 10.1037/a0023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Davis HP. Organization of cognitive abilities and neuropsychological variables across the lifespan. Developmental Review. 2006;26:31–54. [Google Scholar]
- Salthouse TA, Ferrer-Caja E. What needs to be explained to account for age-related effects on multiple cognitive variables? Psychology and Aging. 2003;18:91–110. doi: 10.1037/0882-7974.18.1.91. [DOI] [PubMed] [Google Scholar]
- Schmiedek F, Li SC. Toward an alternative representation for disentangling age-associated differences in general and specific cognitive abilities. Psychology and Aging. 2004;19:40–56. doi: 10.1037/0882-7974.19.1.40. [DOI] [PubMed] [Google Scholar]
- Schnack HG, van Haren NEM, Brouwer RM, Evans A, Durston S, Boomsma DI, et al. Changes in thickness and surface area of the human cortex and their relationship with intelligence. Cerebral Cortex. 2015;24:1608–1617. doi: 10.1093/cercor/bht357. [DOI] [PubMed] [Google Scholar]
- Tustison NJ, Cook PA, Klein A, Song G, Das SR, Duda JT, et al. Large-scale evaluation of ANTs and FreeSurfer cortical thickness measurements. NeuroImage. 2014;99:166–179. doi: 10.1016/j.neuroimage.2014.05.044. [DOI] [PubMed] [Google Scholar]
- Wahl M, Li YO, Ng J, LaHue SC, Cooper SR, Sherr EH, et al. NeuroImage. 2010;51:531–541. doi: 10.1016/j.neuroimage.2010.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Dale AM, Fischl B, Quinn BT, Makris N, et al. Regional cortical thickness matters in recall after months more than minutes. NeuroImage. 2006;31:1343–1351. doi: 10.1016/j.neuroimage.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale: Third edition. Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading. The Psychological Corporation; San Antonio, Texas: 1997. [Google Scholar]
- Westlye LT, Grydeland H, Walhovd KB, Fjell AM. Associations between regional cortical thickness and attentional networks as measured by the Attentional Network Test. Cerebral Cortex. 2011;21:345–356. doi: 10.1093/cercor/bhq101. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Espeseth T, Reinvang I, Raz N, et al. Increased sensitivity to effects of normal aging and Alzheimer’s disease on cortical thickness by adjustment for local variability in gray/white contrast: A multi-sample MRI study. NeuroImage. 2009;47:1545–1557. doi: 10.1016/j.neuroimage.2009.05.084. [DOI] [PMC free article] [PubMed] [Google Scholar]