Abstract
Background and Aims
Coronary artery disease is a growing clinical problem in HIV-infected subjects. The increased risk of coronary events in this population has been linked to low levels of HDL, but the effects of HIV infection and anti-retroviral treatment (ART) on HDL structure and function remain unknown. Here, we aimed to determine the composition and function of HDL particles isolated from ART-naive and ART-positive HIV-infected patients.
Methods and Results
Proteomic profiling revealed decreased levels of paraoxonase (PON) 1 and PON 3 in HDL from HIV patients relative to HDL from uninfected controls (p<0.0001), and PON activity of HDL from control group (0.13±0.01 U/μl) was significantly higher than PON activity of HDL from HIV-infected untreated subjects (0.12±0.01U/μl, p=0.0035), subjects treated with non-nucleoside reverse transcriptase inhibitor (NNRTI)-based therapy (0.11±0.01 U/μl, p<0.0001), subjects treated with protease inhibitor (PI)-based therapy with detectable viral load (0.11±0.01 U/μl, p<0.0001), and PI-treated patients with undetectable viral load (0.12±0.01 U/μl, p=0.0164). Lipidomic profiling uncovered a negative correlation between CD4 T cell counts and particle sphingomyelin, lyso-phosphatidylcholine and ether-linked phosphatidylserine content in the ART-naive (R2=0.2611, p<0.05; R2=0.2722, p<0.05; and R2=0.3977, p<0.05, respectively) but not treated HIV-infected subjects. Functional analysis demonstrated a negative correlation between cholesterol efflux capacity of HDL and viral load in the ART-naive HIV-infected group (R2=0.26, p=0.026).
Conclusions
Taken together, these results indicate that HIV infection associates with a number of both protein and lipid compositional changes in HDL particles. Moreover, HIV infection affects cholesterol efflux function of HDL, thus contributing to an increased risk of atherosclerosis in this patient population.
Keywords: HIV; anti-retroviral treatment; HDL; proteomics; lipidomics; cholesterol efflux; PON, atherosclerosis
Introduction
Accelerated atherosclerosis and the resultant coronary artery disease have been recognized as a serious and growing problem in HIV-infected subjects 1-3. Despite the fact that anti-retroviral treatment (ART) has resulted in a dramatic improvement in the morbidity and mortality of HIV-infected patients, conflicting data have been reported regarding the effects of ART on the risk of cardiovascular disease (CVD). Early publications suggested that ART reduces the risk of atherosclerosis 4, although some drugs, in particular abacavir (nucleoside reverse transcriptase inhibitor or NRTI) and ritonavir (protease inhibitor or PI), were associated with increased risk of myocardial infarction and metabolic syndrome, respectively 5-7. Later studies established that ART may reduce but does not eliminate the risk for developing CVD in HIV-infected individuals 8. Our recent study comparing groups of HIV-infected subjects treated with PI, non-nucleoside reverse transcriptase inhibitors (NNRTI) or untreated demonstrated that drugs increase cholesterol efflux to apoB-depleted plasma, most likely due to increased abundance of high density lipoprotein (HDL) particles 9.
The reason for the increased risk of CVD in HIV-infected patients is not clearly understood. Recent reports have demonstrated that atherosclerotic changes occur in drug-naïve patients and in patients undergoing treatment interruption 10-13, indicating a direct contribution of HIV to CVD. Given that low level viral replication may occur even in well-controlled patients 14, it appears likely that some risk of atherosclerosis in drug-treated subjects may be attributed to direct effects of the virus. Chronic low-level inflammation associated with HIV infection, and in particular monocyte activation, is also a likely contributing factor 15.
A characteristic feature in both treated and untreated HIV-infected subjects is a disturbance in plasma lipoprotein metabolism characterized by low levels of HDL 16, 17. HDL has been established as a major anti-atherogenic lipoprotein, both because of its role in reverse cholesterol transport and its anti-inflammatory properties 18. Low HDL levels are associated with a high risk of atherosclerosis in animal models and in humans 19. A defect in HDL maturation has been demonstrated in macaques infected with simian immunodeficiency virus 20, suggesting that HDL particles in virally-infected subjects may be structurally and functionally altered compared to uninfected controls. This study was designed to test the hypothesis that HIV infection and/or HIV therapy induce qualitative and quantitative changes in HDL protein and lipid composition thereby impairing HDL functionality, which could account for an increased risk of atherosclerosis in infected subjects.
Methods
Study Participants
Samples from three distinct groups of HIV-infected subjects, stored in GW HIV Institute Biorepository or the MFA's HIV Specimen Bank, were used in this retrospective study (IRB approval #091034): HIV-infected subjects who were naïve to ART or had been off ART for over 1 year, HIV-infected subjects on PI-based antiretroviral therapy for over 1 year, and HIV-infected subjects on NNRTI-based ART for over 1 year. None of these subjects were on any lipid lowering medications including statins, which was the only exclusion criterion. Samples from these three groups of subjects were compared with HDL samples collected from HIV-negative subjects not treated with lipid lowering medications, who were used as the control group. The control group was a group of convenience composed of adults recruited at GWU MFA. Post-prandial plasma samples were collected from all groups under the George Washington University HIV Institute Biorepository protocol, and the study was approved by the George Washington University IRB. The samples were aliquoted and stored at −80°C. We used plasma samples collected from 21 HIV-infected subjects not on ART therapy, 27 HIV-infected subjects on PI-based ART, 27 HIV-infected subjects on NNRTI-based ART, and 19 control uninfected subjects. The exact regimens were as follows: in the PI-treated group 10 subjects received emtricitabine/tenofovir with ritonavir-boosted atazanavir, 2 - emtricitabine/tenofovir with unboosted atazanavir, 2 – emtricitabine/tenofovir with ritonavir-boosted darunavir, 2 – abacavir/lamivudine with unboosted atazanavir, 2 – emtricitabine/tenofovir with ritonavir-boosted fosamprenavir, 1 – emtricitabine/tenofovir with lopinavir/ritonavir, 1 – lopinavir/lamivudine with lamivudine and raltegravir, 1 – abacavir/lamivudine with ritonavir-boosted fosamprenavir, 1 - abacavir/lamivudine with tenofovir and ritonavir-boosted atazanavir, 1 - abacavir/lamivudine with ritonavir-boosted atazanavir, 1 - emtricitabine/tenofovir with unboosted fosamprenavir, 1 - abacavir/lamivudine with unboosted fosamprenavir, 1 - abacavir/lamivudine with lopinavir/ritonavir and didanosine, and 1 – tenofovir and lamivudine and ritonavir-boosted darunavir (ritonavir was part of the treatment of 21 of 27 patients in this group); in the NNRTI-treated group 23 subjects received efavirenz/emtricitabine/tenofovir, 1 etravirine with raltegravir and ritonavir-boosted darunavir, 1 – abacavir/lamivudine with efavirenz, 1 – tenofovir with didanosine and efavirenz, and 1 - emtricitabine/tenofovir with nevirapine (efavirenz was part of the treatment of 25 of 27 patients in this group). The age, sex and BMI distribution for the groups are presented in Table 1. BMI data were not collected from control group, but all subjects in this group were non-obese. It should be noted that some subjects in treated groups exhibited elevated viral load at the time of testing (6 subjects on PI-based therapy and one on NNRTI-based therapy had viral load above 1,000 copies/ml, and 9 on PI therapy and one on NNRTI therapy had viral loads between 50 and 1,000 copies/ml). In all cases the reason for high viral load was non-compliance, as viral loads were undetectable in follow-up measurements. Nevertheless, given that 15 out of 27 PI-treated patients had detectable viral load on the day of testing (only 1 such patient was in the NNRTI-treated group), we analyzed virus-positive and virus-negative PI-treated patients separately.
Table 1.
Clinical characteristics of study participants1
| Control (n=19) |
No ART (n=21) |
PI VL+ (n=15) |
PI VL− (n=12) |
NNRTI (n=27) |
|
|---|---|---|---|---|---|
| Age (years) | 38.4±10.2 | 45.0±12.9a | 47.7±7.2a | 47.9±9.7a | 51.4±9.7a,b |
| Gender (% male) | 42% | 75% | 73% | 75% | 85%c |
| BMI | ND2 | 25.2±2.6 (n=15) |
28.6±9.2 (n=14) |
29.7±6.5 (n=10) |
25.6±5.8 (n=24) |
| Log VL3 | ND2 | 4.4±1.0 | 2.9±1.1d | Neg4 | 1.5±0.6e |
| CD4 (cells/mm3) | ND2 | 357±257 | 337±250 | 555±354 | 537±365 |
| LDL (mg/dl) | 74.8±23.2 (n=8) |
110.3±30.0a (n=20) |
84.1±28.2b (n=14) |
101.3±38.3a | 85.6±24.6b |
| TG (mg/dl) | 143±70.5 (n=8) |
118.4±62.3 | 88.0±37.2 (n=14) |
125.3±41.4 | 126.8±82.9 |
| HDL (mg/dl) | 56.0±7.5 | 45.1±11.1f | 54.4±21.1 | 56.2±26.8 | 54.1±22.6 |
Means±SD are shown; table cell n shown when there are missing values
ND – not determined
base 10, below limit of detection imputed as 24 copies/ml
Neg - negative.
p<0.05 relative to Control
p<0.05 relative to No ART
p<0.004 relative to Control
p<0.001 relative to No ART
p<0.0001 relative to No ART and PI VL+
p<0.001 relative to Control
Laboratory Assays
HDL isolation
HDL was isolated from plasma samples using gradient purification as previously described 21. Briefly, plasma density was adjusted to 1.085 g/ml by adding KBr. Samples were centrifuged at 100,000 rpm/18 h/4°C in Ti70 rotor, the bottom HDL fraction was collected, the density was adjusted to 1.21 g/ml by adding KBr, and samples were centrifuged again at 100,000 rpm/18 h/4°C in Ti70 rotor. The upper fraction of HDL was collected and analyzed on a 12% SDS gel to verify albumin depletion. HDL samples were dialyzed against PBS to remove KBr. Total protein was measured by Bradford assay, and apoA-I was measured by ELISA (Mabtech, Cincinnati, OH).
Cholesterol efflux
Cholesterol efflux (CE) was measured as described recently 22. Briefly, human monocyte cell line THP-1 was differentiated by PMA and labeled by incubation with [3H]cholesterol for 48 h. Expression of ABC transporters was induced by incubation with LXR agonist TO-901317 (1 μmol/L, 18 h) and cells were incubated for 6 h with apoB-depleted plasma (2%) or isolated HDL (5 μg/ml). Cholesterol efflux was calculated as percentage of released cholesterol.
PON activity
PON activity of 5 μg isolated HDL was measured using EnzCheck Paraoxonase Assay kit (Invitrogen) following manufacturer's instructions.
Mass spectrometry (MS) proteomic and lipidomic profiling
MS was used to profile protein and lipid content of the purified HDL as follows. To determine the particle protein complement, 50 μg of total HDL protein (determined by BCA assay) was precipitated with 100 L of 100% trichloroacetic acid (w/v) (Sigma, biotech grade, redistilled, ≥99%) dissolved in H O (Fluka, LC-MS grade), after mixing and a 15 minute 4°C incubation, samples were spun (14,000 rpm, 4°C, 30 min) and the resulting pellets were washed progressively with 1 mL 10% TCA (w/v) followed by 1 mL acetone (Sigma, HPLC grade) and solubilized in 50 mM NH4HCO3 (Sigma, 99.5% purity) and run approximately 4 centimeters on a 12% SDS-PAGE gel. After staining with Coomassie Blue, each gel lane was separated into two individual samples: one containing apoA-I, which comprises approximately 75% of the protein mass of HDL, while the second sample contained the remainder of the gel lane containing the separated lower abundant proteins. After trypsin digestion of the gel bands the resulting peptides were identified using an LTQ Orbitrap Velos system mass spectrometer equipped with a Famos Autosampler, and Surveyor MS Pump (Thermo Electron) with split flow to deliver 200 nL/min to the in-house packed C18 column (75 cm, inner diameter - 18 cm). For each run, 10 μl of reconstituted sample was injected and separated using a gradient of 5–60% buffer B (acetonitrile with 0.1% formic acid) over the course of 90 min. In between each set of samples, standards (a mix of five angiotensin peptides, Michrom BioResources) were analyzed to ascertain column performance and to determine any potential carryover. The LTQ was operated in the data-dependent mode with a top five configuration (one full MS scan followed by five MS/MS scans). Dynamic exclusion was set to 1 with a limit of 30 s. Peptides were identified using the Sequest software package through the Bioworks Browser 3.3.1. Sequential database searches were made using the NCBI RefSeqHuman Data base using differential carbamidomethyl-modified cysteines and oxidized methionines. Peptide score cutoff values were chosen at a cross-correlation of 1.8 for singly charged ions, 2.5 for doubly charged ions, and 3.0 for triply charged ions, along with CN values of 0.1 or greater, and RSP values of 1. The cross-correlation values chosen for each peptide assure a high confidence match for the different charge states, whereas the CN cutoff insures the uniqueness of the peptide hit. The rank score preliminary (RSP) value of 1 insures that the peptide matched the top hit in the preliminary scoring.
To profile the lipid species of the HDL particles, an electrospray ionization-MS/MS approach was used as reported previously 23, 24. In brief, 50 μg of total HDL protein was extracted with 2.5 ml of chloroform/methanol/distilled water (1:1:0.5, v/v), and two 0.5-ml chloroform extractions. Combined organic phases were washed with 0.5 ml of KCl (1 M, 1 volume) and with 0.5 ml of dH20 (2 volumes) and dried with N2 gas. Samples were resuspended in 1 ml of chloroform, mass of extracted lipids was determined by drying 10 ml of the extracted lipids and measuring the resulting mass in an electro-microbalance (Sartorius Cubis), and phospholipid scans were performed as described 23, 24. To analyze ceramides (Cers) and glucosylceramides (GlcCers), additional scans were performed for precursors of m/z 264 in the positive mode (collision energy, 50 V) for GlcCers and high mass Cers and neutral loss of m/z 316 in the negative mode (collision energy, 50 V) for low mass Cers. Internal standards for quantitating these sphingolipids were d18:1/14:0Cer and d18:1/12:0GlcCer. To measure oxidized phospholipids within the HDL particles, we focused on phosphatidylcholine, the most abundant species and quantitated levels of the following oxidized acyl side chains: oxovaleric, oxo-nonanoic, hydroxy-octadecadienoic, hydroxy-octadecenoic, hydroperoxy-octadecadienoic, unknown structure). The amounts of the analyte lipids are indicated in units of normalized mass spectral signal with 1 unit representing the amount of lipid producing the same amount of signal as 1 nmol of the internal standard. These amounts are then expressed as nanomole lipid species per μg of total HDL lipid extract.
Principal Components analysis
Multivariate transformations, normality transformations, data imputation and principal components analysis (PCA) were performed using the Microsoft Excel implementation of the R statistical program, imDEV v1.4.2 25. Data was transformed to normality prior to statistical analyses using the procedures of Box and Cox 26. Imputation of missing values was performed using probabilistic PCA, if greater than 80% of the dataset was available. The PCA was calculated using the NIPALS model, univariate scaling, and mean centering.
Statistical Analysis
HIV viral load below the limit of detection (48 copies/ml) was imputed at 24. For group comparisons, variables were examined for evidence of non-normality and tested with parametric or non-parametric tests, accordingly. Parametric testing of group differences was by one-way ANOVA, followed, when statistically significant (p<0.05), by t-tests of all group pairs. Non-parametric testing followed the same procedure using the Kruskal-Wallis and Wilcoxon Sum Rank tests. This two-step procedure was designed to balance type 1 error rates and statistical power.
Results
Table 1 shows clinical characteristics of the study participants. Control (uninfected) subjects were younger and this group contained more females; however, the four HIV-infected groups were similar in terms of age, gender and BMI. The viral load in the untreated group was higher than in the PI-treated viral load positive (p<0.001) and the NNRTI-treated (p<0.0001) groups, but differences in CD4+ T cell counts were not statistically significant. Unexpectedly, LDL-C levels were higher and triglyceride (TG) levels were lower in subjects in HIV-infected groups compared to controls, although TG differences were not statistically significant. The PI subgroup with detectable viral load had lower LDL-C and higher TG levels than the subgroup with undetectable virus (Table 1), but not at a statistically significant level. In comparison to uninfected controls, a statistically significant decrease in HDL levels was observed in the untreated group of HIV-infected subjects (p<0.001), but, in contrast to previous reports 16, 17, no statistically significant differences in HDL levels were seen between the control group and groups of HIV-infected subjects treated with PI- or NNRTI-based HAART (Table 1). As has been previously reported 27, 28, HDL levels in both subgroups of PI-treated patients (56.2±26.8 mg/dl for virus-negative patients and 54.4±21.1 mg/dl for virus-positive patients) and NNRTI group (54.1±22.6 mg/dl) were higher than in untreated group (45.1±11.1 mg/dl), but the differences did not reach statistical significance. When cholesterol efflux to apoB-depleted plasma was analyzed, efflux to plasma from each group of HIV-infected subjects was significantly lower than efflux to plasma from control subjects (Table 2). Both subgroups of PI-treated subjects, one with undetectable viral load (cholesterol efflux of 6.9±1.9%), and another with detectable viral load (cholesterol efflux of 7.3±2.7%), showed a trend towards increased cholesterol efflux relative to untreated HIV-infected group (cholesterol efflux 6.4±1.4%). Lack of significant differences between the subgroups of PI-treated subjects in HDL content and cholesterol efflux is likely due to transitory nature of viral load increase. Results of this analysis are consistent with our previous report, which suggested that increase of cholesterol efflux in patients treated with anti-retroviral drugs is likely due to an increased abundance of HDL particles 9. However, these results indicate that ART, while reversing the reduction of HDL in HIV patients, does not fully restore HDL functionality.
Table 2.
Cholesterol efflux to apoB-depleted plasma1
| Control (n=8) |
No ART (n=19) |
PI VL+ (n=15) |
PI VL− (n=10) |
NNRTI (n=25) |
|
|---|---|---|---|---|---|
| CE (%) | 10.5±0.9 | 6.4±1.4a | 7.3±2.7a | 6.9±1.9a | 7.5±3.0a |
Only a subset of samples was used for this assay due to limited sample availability. Means ± SD are shown.
p<0.05 relative to Control.
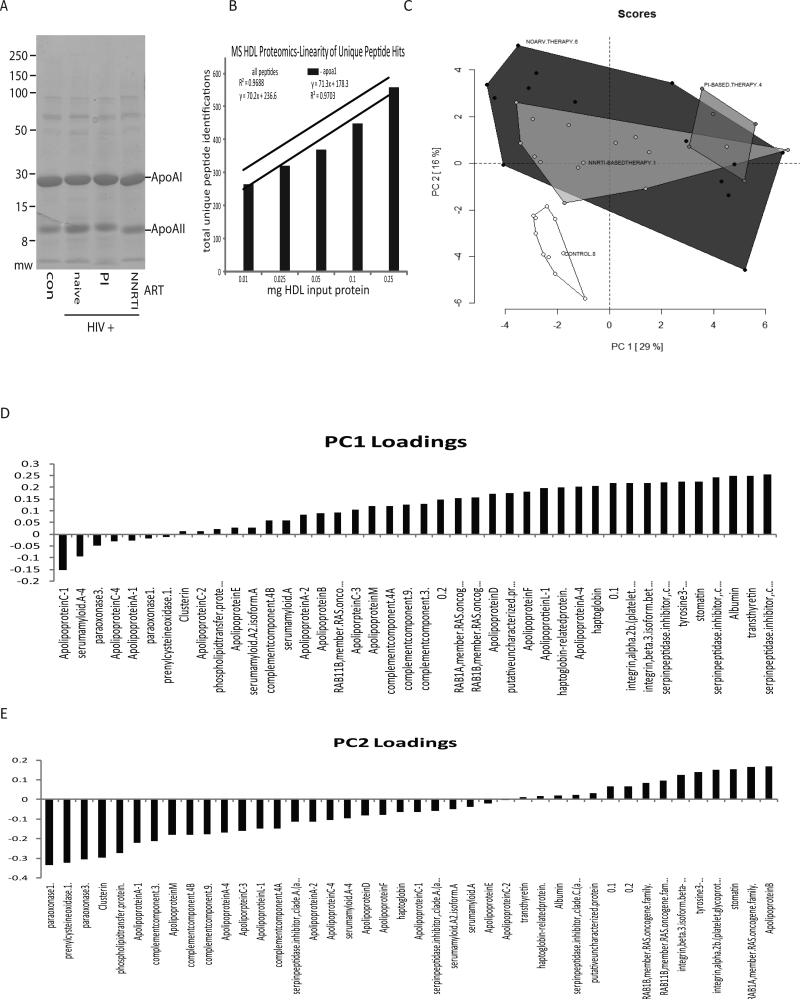
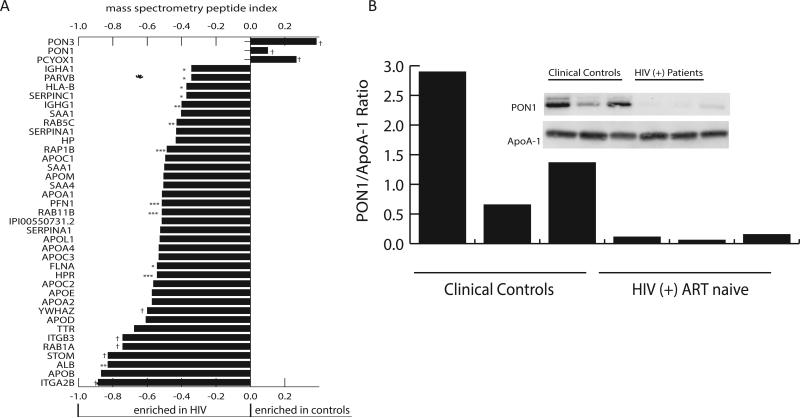
To characterize the effects of HIV infection and ART on HDL composition, we purified HDL particles from a subset of samples (10 control, 14 untreated, 15 NNRTI-treated, and 5 PI-treated using gradient centrifugation (Fig. 1A) and subjected them to proteomics analysis. Of note, a subset of samples for this analysis that came from ART-treated patients included four subjects with detectable viral load: two from NNRTI-treated group (viral load 120 and 35,020 copies/ml) and two from PI-treated group (viral load 389 and 2,070 copies/ml); other ART-treated patients had undetectable virus in the blood. Samples with detectable viral load did not stand out from other samples in the analysis. To profile the protein complement of the particles we used an approach described by Vaisar et al. 29. During implementation of this method we found that direct analysis of the crude trichloroacetic acid (TCA) precipitated protein derived from the particles did not show a strong positive linear correlation between the input HDL protein mass and the number of total or unique identified peptides. Hence, we added a one dimensional SDS-PAGE separation of the TCA precipitated and solubilized material into two fractions - a gel slice containing apoA-I, which constituted approximately 75% of the protein, and the remainder of sample lane, containing lower abundant proteins. With this step added, we obtained an acceptable dose response relation between input HDL protein mass and the total number of identified peptides (Fig. 1B). To characterize this data set, we initially used principal component analysis (PCA) to test if the various groups contained distinct protein cargos (Fig. 1C). This analysis showed a clear separation of the control HDLs from the HDLs of HIV-infected subjects, but did not further segregate the HIV treatment arms. The PCA loading values that were separating out the controls included a number of apolipoproteins, but interestingly also included paraoxonase (PON) 1 and 3 (Figs. 1D and E). Likewise, the computed peptide index for the data set derived as described by Vaisar et al. 29 showed significantly lower PON1 and 3 associated peptides in the HIV-positive samples (p<1×1010 and p<1×106, respectively, with Bonferroni correction for multiple tests, Fig. 2A). Another peptide underrepresented in HDL from HIV-infected subjects was prenylcysteine oxidase 1 (PCYOX1). To verify the correlation between the abundance of peptides identified by our MS method for PON1 and protein levels, we conducted an immunoblot analysis of PON1 in a subset of the HDLs from control and ART-naïve HIV-positive subjects, which confirmed the lower level of this protein in the HIV-positive samples (Fig. 2B). We further measured PON activity in HDL samples, using 5 μg of protein per assay. Again, PON activity of HDL from HIV-infected groups was significantly lower than the activity of control HDL (Table 3). It should be acknowledged that the differences in PON activity were not as dramatic as differences in protein levels. A possible reason for this discrepancy is an excess of PON substrates in HDL from all groups, resulting in close to saturation conditions of the assay 30. It may also be related to differences in PON distribution between large and small HDL particles in HIV-infected and control samples, as PON activity depends very much on the HDL particles it is located on 31.
Figure 1. Proteomic profiling of HDL particles.
A - Purified HDLs from the indicated groups were acetone precipitated and analyzed by SDS-PAGE. Panel shows representative lanes from the sample run. B - Graph shows a linear response of total and ApoA-I peptide identifications with increasing amounts of input total HDL protein. C - Principal component analysis of all analyzed samples (n=45) indicates a significant separation in the proteomic content of the control HDL particles versus the HDL particles isolated from HIV-positive individuals. Scores from PC1 and PC2 explained 45% of the total variance within the data set. Loading plots from both PC1 and PC2 are also presented to illustrate variables driving separation of experimental groups. The PCA plot shown was used as a tool for data exploration. D and E - Proteins associated with the two principal components affecting the separation of the groups.
Figure 2. Analysis of PON in HDL particles.
A - Peptide indices for HDL associated proteins that showed marked differences between control and HIV infected samples. Positive scores indicate a greater abundance of the protein in the HDL from control samples, while negative scores indicate a greater abundance of the protein in the HDL from HIV-positive subjects. Peptide indices of the uninfected controls and HIV-positive samples were compared via a Student's t-test. * indicates significance at the p=0.05 level, ** indicates significance at the p=0.01 level, *** indicates significance at the p=0.001 level, and † indicates significance with p<0.0001. All p values were Bonferroni corrected for multiple tests (n=163 comparisons). B - Analysis of PON1 and apoA-1 levels by Western blotting in HDL from 3 controls and 3 HIV-positive ART-naïve subjects.
Table 3.
HDL PON activity1
| Control (n=19) |
No ART (n=20) |
PI VL+ (n=9) |
PI VL− (n=10) |
NNRTI (n=21) |
|
|---|---|---|---|---|---|
| PON activity (U/μl)2 | 0.13±0.01 | 0.12±0.01a | 0.11±0.01b | 0.12±0.01c | 0.11±0.01b |
Only a subset of samples was used for this assay due to limited sample availability.
Shown are means ± SD measured on 5 μg of isolated HDL.
p=0.0035 relative to control group
p<0.0001 relative to control group
p=0.0164 relative to control group.
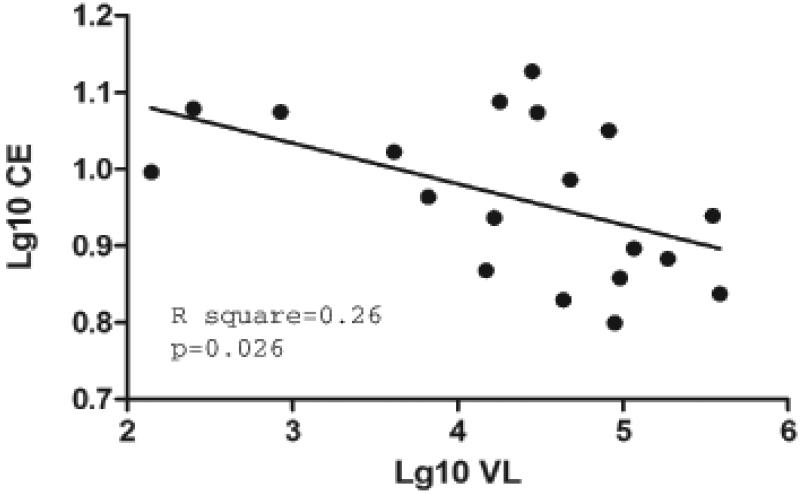
To further characterize the effect of HIV infection on functional activity of HDL particles, we analyzed cholesterol efflux to isolated HDL. Surprisingly, and in seeming conflict with the efflux to apoB-depleted sera, cholesterol efflux to HDL from HIV-infected subjects trended to be higher than to HDL from control group, although the difference was not statistically significant (Table 4). However, a strong and statistically significant negative correlation was revealed between the viral load and cholesterol efflux to HDL from untreated group (Fig. 3). No such correlation was observed within ARV-treated groups, likely because of transient nature of virus replication in these subjects.
Table 4.
Cholesterol efflux to HDL1
| Control (n=19) |
No ART (n=18) |
PI VL+ (n=10) |
PI VL− (n=11) |
NNRTI (n=25) |
|
|---|---|---|---|---|---|
| CE (%) | 5.3±1.04 | 6.43±1.8 | 6.24±2.12 | 6.07±1.68 | 6.28±1.65 |
Only a subset of samples was used for this assay due to limited sample availability. Means ± SD are shown. The differences between the groups were not statistically significant.
Figure 3. Analysis of colesterol efflux to isolated HDL.
Spearman correlation analysis between viral load and cholesterol eflux to isolated HDL was performed on samples from ART-naïve group. Cholesterol efflux was measured in THP-1 cells incubated with HDL (20 μg/ml) as described in Materials and Methods.
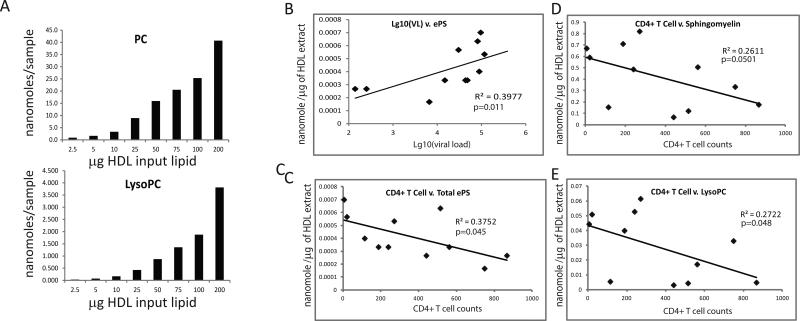
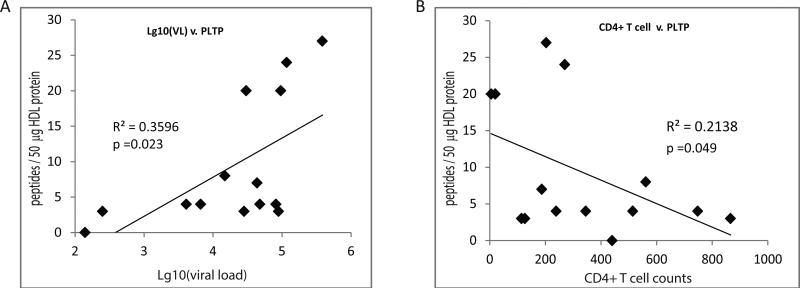
We next profiled particle lipid composition using an electrospray ionization-MS/MS approach, which measures over 400 individual lipid species. Using increasing amounts of input total HDL, we confirmed detection of both highly prevalent species (e.g., PC) as well as low abundant species (e.g., lysoPC) in a linear manner across an acceptable dynamic range (Fig. 4A). When the samples from different groups were analyzed, we found that the particles from ART-naïve HIV-positive subjects trended to have lower total PC levels, while showing an increase in less abundant species, including the sphingomyelins and other potentially pro-inflammatory ether-linked glycerophospholipid species; significant differences were found in the levels of phosphatidic acid, which was increased in HIV-infected untreated group relative to controls, and returned to levels not significantly different from controls in NNRTI-treated group (Table 5). Moreover, a number of significant correlations were observed between the lipid levels in the ART naïve HIV-positive samples and viral load (Fig. 4B) and CD4+ T cell counts (Figs. 4C-E). Here, sphingomyelin and ether-linked glycerophospholipid species in the HDL particles correlated positively or negatively with viral load and CD4+ T cell counts, respectively. Given these correlations of lipid levels, we tested for correlations of phospholipid transfer protein (PLTP) to viral load and CD4+ T cell counts. Consistent with analysis of lipids, the level of PLTP showed a significant positive correlation with viral load and was negatively correlated with CD4+ T cell counts in ART-naïve HIV-positive samples (Fig. 5).
Figure 4. Analysis of lipids in HDL particles.
A - Graphs show that both PC and lysoPC levels are detected in a linear manner with increasing amount of input total HDL lipid. B-E - Spearman correlation analyses of the indicated lipid species relative to viral load and CD4+ T cell counts in the HIV-positive ART-naïve samples.
Table 5.
Lipidomic changes in HDL particles as assessed by mass spectrometry1
| Lipids (nanomole/μl) |
Controls (n=10) | HIV+, ART naive (n=11) |
HIV+, NNRTI (n=15) |
|---|---|---|---|
| lysoPC | 0.025±0.13 [0.020] |
0.029±0.022 [0.033] |
0.022±0.12 [0.022] |
| PC | 3.36±0.95 | 2.78±1.34 | 2.56±1.053 |
| SM & DSM | 0.34±0.16 | 0.42±0.26 | 0.33±0.18 |
| ePC | 0.31±0.12 | 0.30±0.15 | 0.25±0.11 |
| lysoPE | 0.00058±0.00035 | 0.00094±0.00076 | 0.00084±0.0010 |
| PE | 0.0079±0.0063 [0.0059] |
0.019±0.028 [0.0098] |
0.014±0.019 [0.0035] |
| ePE | 0.0013±0.0017 [0.00097] |
0.0031±0.0039 [0.0016] |
0.0032±0.0051 [0.00063] |
| PI | 0.073±0.036 | 0.086±0.061 | 0.062±0.034 |
| PS | 0.0032±0.0028 [0.0028] |
0.0072±0.0072 [0.0046] |
0.0036±0.0023 [0.0035] |
| ePS | 0.00028±0.00017 | 0.00041±0.00017 | 0.00031±0.00021 |
| PA | 0.00049±0.00028 [0.00048] |
0.0011±0.0053a [0.0011] |
0.00074±0.00019b [0.00070] |
| CE | 18.65±8.00 | 19.41±12.70 | 17.29±8.17 |
| Cer | 0.016±0.0048 | 0.017±0.0088 | 0.013±0.013±0.0045 |
| Hex-Cer | 0.010±0.0077 | 0.011±0.0094 | 0.0069±0.0032 |
Only a subset of samples was used for this assay due to limited sample availability. Means ± SD are shown and also medians (in square brackets) for lipid classes with skewed distributions.
p=0.006 relative to control group.
p=0.03 relative to control group; p=0.01 relative to no ART group.
Figure 5. Spearman correlation analysis of HDL PLTP.
Graphs show significant correlations between the level of HDL-associated PLTP peptides and viral load (A) or CD4+ T cell counts (B) in the HIV-positive ART-naïve subjects.
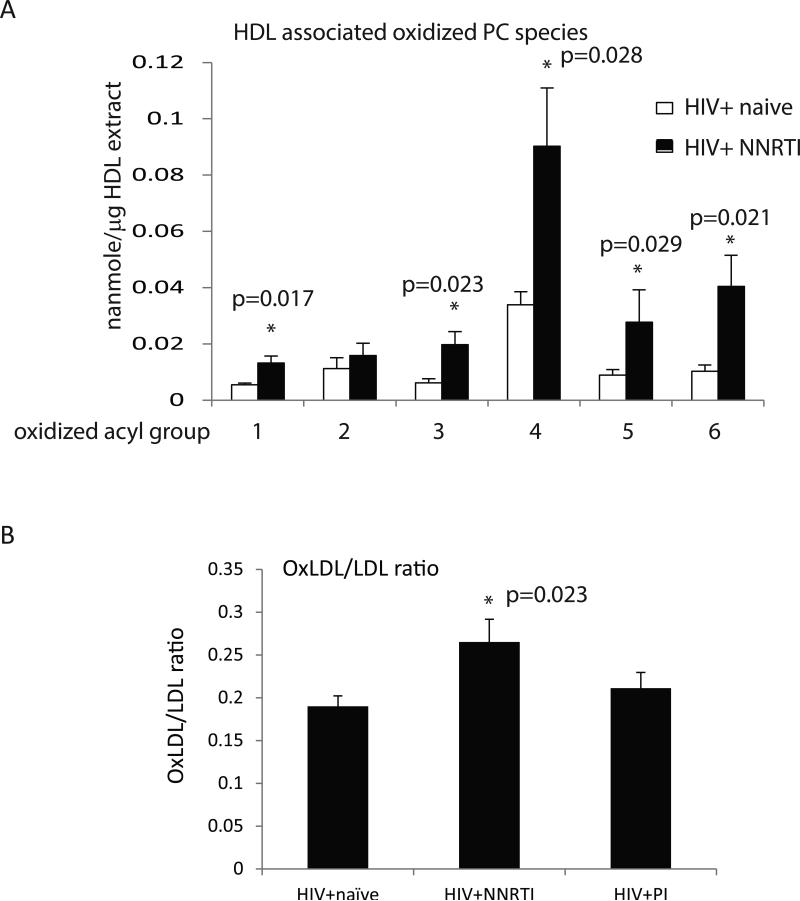
Given the low PON1 and PON3 levels in HDL from HIV-infected subjects, we tested for the level of oxidized phospholipids in the HDL particles from a subset of HIV+ ART-naïve and HIV+ NNRTI-treated subjects with completely suppressed viremia; these groups were well matched for age and gender. Interestingly, a significant increase in the level of 5 out of the 6 oxidized lipid species measured was detected in the NNRTI-treated samples (Fig. 6A). No significant differences in oxidized lipids were found between HDL from control and untreated or PI-treated subjects (not shown). To test whether the increased level of oxidized lipid seen in the HDL particles was potentially a more general effect of the NNRTI drug therapy, we also measured the level of oxidized LDL using an ELISA approach. Although the absolute level of oxLDL detected in this assay did not significantly differ between samples from HIV+ ART-naïve subjects and those from NNRTI-treated patients (n=10, 22.7±4.5 U/L versus n=11, 23.6±6.4 U/L, respectively), when these values were normalized to total LDL levels the ratio of oxLDL to LDL was significantly increased in the NNRTI-treated samples (Fig. 6B). These results suggest that reduced PON levels in HDL of HIV-infected subjects do not translate to increased levels of oxidized lipids, however, low PON may contribute to increased oxidation in NNRTI-treated patients.
Figure 6. Analysis of oxidized lipid in HDL and LDL.
A – Graph shows levels of oxidized PC species in HIV+ ART-naïve (n=5) and HIV+ NNRTI-treated subjects with completely suppressed viremia (n=5). Data are presented as mean ± SEM, * - significant p values are shown above the bars. Lipid acyl side chains: 1 - oxovaleric, 2 - oxo-nonanoic, 3 - hydroxy-octadecadienoic, 4 - hydroxy-octadecenoic, 5 - hydroperoxy-octadecadienoic, 6 - unknown. B - Graph shows the ratio of oxLDL to LDL in samples from HIV+ ART-naïve (n=10), HIV+ NNRTI-treated (n=11), and HIV+ PI-treated (10). Data are presented as mean ± SEM, the difference between untreated and NNRTI-treated groups is significant (p=0.023).
Discussion
In this study, we characterized functional and structural features of HDL from a cross-sectional group of HIV-infected subjects treated or not with anti-retroviral drugs. Cholesterol efflux to apoB-depleted plasma from all HIV-infected patients was reduced relative to efflux to plasma from control individuals. Interestingly, cholesterol efflux did not fully correlate with HDL concentration in the plasma, as HDL levels in PI- and NNRTI-treated groups were not reduced relative to control group in our study subjects, whereas cholesterol efflux was significantly reduced. This result indicates a functional defect in HDL from HIV-infected subjects, and suggests that HAART treatment does not fully reverse this defect. It also minimizes the bias introduced by a higher number of women in the control group: higher HDL levels in pre-menopause women would have increased the differences in cholesterol efflux to plasma. The HIV-induced defect in HDL functionality was supported by a strong negative correlation between cholesterol efflux to HDL isolated from plasma of ART-naïve HIV-infected subjects and viral load, indicating that HIV infection contributes to the impairment of this function of HDL. However, no significant differences were observed between the groups in cholesterol efflux to isolated HDL; if anything, cholesterol efflux trended to be higher in HIV-infected groups. This observation suggests a compensatory reaction of the organism to infection that compensates for virus-induced impairment (see below). Such interpretation is supported by a slightly higher efflux to HDL from PI-treated subjects that had detectable viral load (6.24% ± 2.12%) compared to subjects in this group with undetectable viral load (6.07% ± 1.68%), although this difference did not reach statistical significance. Also, no correlation between cholesterol efflux to HDL and viral load was detected in ART-treated groups, despite detectable viral load in a number of subjects. A likely explanation to this inconsistency is a transient nature of viral replication in ART-treated subjects, due to non-compliance.
Decreased levels of PON1 and PON3 in HDL from HIV-infected subjects compared to the control samples is an intriguing finding of this study, and supports previous report of decreased PON1 activity in HIV-infected subjects, both treated and untreated 32. While we cannot fully exclude the possibility that the observed result was affected by the gender and age differences between HIV-infected and control samples, sub-analysis of 6 samples from control females and 4 samples from HIV-infected females (3 from untreated group and 1 from NNRTI-treated group) matched by age (control: 42.7 ± 3.8; HIV+: 45.5 ± 4.7; p=0.65) confirmed significant differences in PON1 (control: 37.3 ± 4.5; HIV+: 8.0 ± 4.1; p=0.0019) and PON3 (control: 22.0 ± 2.0; HIV+: 3.3 ± 2.5; p=0.0006). PON1 and PON3 are enzymatic proteins located on the surface of HDL particles that have the ability to prevent the oxidation of phospholipids in low density lipoproteins (LDL), as well as alter the oxidative state of macrophages 33. In addition, these proteins have been shown to influence the cholesterol efflux activity of HDL, in part by catalyzing lysophosphatidylcholine (lysoPC) formation on target cells thus increasing HDL binding 34. PON1 has been shown to be an important anti-atherosclerotic component of plasma HDL 35, and low PON1 levels have been prospectively shown to predict new coronary events 36. There have only been a few studies published to date looking at the association between HIV infection and PON levels, and these have produced conflicting results 32, 37-39. Daminelli et al. demonstrated significantly decreased activity of PON1 in HIV-infected subjects compared to uninfected controls, and ART treatment did not reverse this deficiency 32. Parra et al. also found significantly decreased PON1 activity, but saw increased PON1 concentrations in HIV-infected subjects compared to controls 39. We measured both the activity and concentration of PON in HDL particles, and found decreased levels of both. In view of the findings by Parra et al., a potential explanation of our results is that a defect in incorporation of PON1 into HDL, rather than low PON abundance in these patients, was responsible for low PON levels we observed. PON1 activity measured in isolated HDL was also found to inversely correlate with age 40, which, given that our control group was younger than HIV-infected subjects, could contribute to lower observed PON1 activity. Similar to PON1, the study by Aragonès et al. reported a 3-fold increase in PON3 concentrations in HIV-infected subjects relative to control subjects, and this difference was partially reversed in patients treated with NNRTI-based ART 38. Again, incorporation of PON3 into HDL particles may be impaired in HIV-infected subjects. Interestingly, we also found decreased levels of prenylcysteine oxidase 1 (PCYOX1) corresponding peptides in HDL samples from HIV-infected subjects. This enzyme cleaves the thioether bond of prenylcysteines in lipid-modified proteins to generate H2O2, and may contribute to pro-atherogenic activity of LDL 41, however, its role in HDL has not been established.
Comparing HDL from HIV-infected patients to control samples, we found increased levels of oxidized phospholipids only in HDL from NNRTI-treated subjects, but not in untreated or PI-treated groups. This finding suggests that PON levels are not the bottleneck for lipid oxidation, but low PON may contribute to NNRTI-induced oxidation. Increased lipid oxidation in NNRTI-treated subjects is supported by our finding of a significant increase in the oxLDL to LDL ratio in this group relative to either HIV+ ART-naïve subjects or subjects in the PI-treated group. Thus, although a number of our findings indicated that viremia may be a significant factor in modifying the HDL particle composition and function, viremia is not correlated with an increase in oxidized lipid associated with either HDL or LDL. That a NNRTI regimen may cause a general increase in the level of oxidized lipids carried by HDL and LDL is suggested by these results but will need confirmation in larger longitudinal studies.
Another interesting finding of this study is a positive correlation between etherPS levels in the HDL particles from the ART-naïve subjects and the viral load. Moreover, when individuals on ART with detectable viral load were included in the linear regression analyses, the positive association between viral load and ePS was not changed (not shown). Phosphatidylserine is the key HDL phospholipid that determines the magnitude of efflux and other functional activities of HDL 42, and an increase in ePS levels may be a compensatory reaction to HIV infection. Therefore, it appears that the functional status of cholesterol efflux activity of HDL depends on the balance between virus-induced damage to HDL, such as depletion of the PON factors, and compensatory reactions of the organism, such as increase in HDL phosphatidylserine. However, PON1 depletion is unlikely to be a major cause of changes in cholesterol efflux capacity of HDL in our subjects, as we did not observe correlation between cholesterol efflux and PON1 content in HDL, although previous study in diabetic patients demonstrated that enriching HDL with PON1 increased cholesterol efflux in vitro 43. Additionally, and consistent with viral load correlations, in the ART-naïve subjects there was a negative correlation between CD4+ T cell counts and the levels of HDL sphingomyelins, etherPS, and lysoPC. When samples from ART patients with detectable viral load were included in this analysis, the negative correlation between CD4+ T cell levels and ePS or PLTP was preserved but not strengthened (not shown), likely because of a transient nature of viral load rise which does not significantly affect CD4+ T cells. Thus, our results indicate remodeling of lipid and protein content of the HDL particles in HIV-infected subjects. The increased level of sphingomyelin in the HDL of ART-naïve patients with low CD4+ T cell counts is of interest since HDL with excess sphingomyelin has been previously associated with impaired HDL biogenesis and maturation in Niemann-Pick disease 44. Interestingly, in the ART-treated groups we did not find a correlation between sphingomyelin levels and CD4 counts. This difference may, in part, explain why we saw an efflux defect only in HDL particles from the ART-naïve samples and not the ART-treated samples.
There are several limitations of our study. The number of subjects in groups was small, in particular in subsets used for proteomics and lipidomics analyses, which could increase the possibility of false positive results. Our control group was a convenience group of adults recruited from clinical personnel and had a different gender ratio (more females) than HIV-infected groups, which may have impacted our comparisons. However, when male and female samples were compared separately the results did not change: cholesterol efflux to apoB-depleted plasma and PON activity were significantly lower in HIV-infected subjects than in controls, whereas cholesterol efflux to HDL did not significantly differ (Table 6). There was also a difference in age between control and infected groups. While the age of control and untreated groups was not significantly different by statistical analysis, there was statistically significant difference between control group, which was younger, and two drug-treated groups. However, correlation analyses performed in HIV-infected groups do not suffer from these limitations. In addition, most of our analyses were performed on isolated HDL, thus negating the bias in HDL levels introduced by age and gender differences, which, to published reports, mainly influence HDL levels 45-47. Finally, PON1 haplotypes were shown to segregate with HIV infection, and certain haplotypes associated with higher HDL levels and better CD4+ T cell recovery after treatment 48, whereas we did not consider the PON gene polymorphisms in our study.
Table 6.
Separate analysis of male and female samples.
| Cont. male |
Cont. female |
no ART male |
no ART female |
PI, VL+ male |
PI, VL+ female |
PI, VL− male |
PI, VL− female |
NNRTI male |
NNRTI female |
|
|---|---|---|---|---|---|---|---|---|---|---|
| CE to apoB-depleted plasma (%) | 10.2±0.8 (n=3) | 10.6±1.1 (n=5) | 6.1±1.11 (n=13) | 7.1±1.82 (n=6) | 7.0±2.6 (n=11) | 8.5±2.8 (n=5) | 6.1±1.73 (n=7) | 8.9±0.9 (n=3) | 7.7±3.2 (n=21) | 6.8±1.54 (n=4) |
| CE to HDL (%) | 5.5±1.1 (n=8) | 5.2±1.0 (n=11) | 6.5±2.0 (n=14) | 6.3±1.2 (n=4) | 6.3±2.4 (n=7) | 6.1±1.9 (n=3) | 6.2±1.8 (n=9) | 5.6±1.1 (n=2) | 6.5±1.7 (n=22) | 5.0±1.1 (n=3) |
| PON activity of HDL (U/μl) | 0.13±0.001 (n=8) | 0.13±0.013 (n=11) | 0.121±0.0075 (n=15) | 0.117±0.007 (n=5) | 0.112±0.0096 (n=6) | 0.116±0.007 (n=3) | 0.114±0.017 (n=8) | 0.124±0.003 (n=2) | 0.113±0.0128 (n=19) | 0.123±0.006 (n=2) |
p<0.0001 relative to control male
p=0.0036 relative to control male
p=0.0033 relative to control female
p=0.0029 relative to control female
p=0.0063 relative to control male
p=0.0019 relative to control male
p=0.0024 relative to control male
p=0.0008 relative to control male.
Despite these limitations, the findings of our study are provocative and warrant further investigation. A particularly important question to be addressed in future studies is the mechanism of PON reduction in HDL. We did not detect a significant correlation between PON levels and viral load or CD4 counts, and there was no significant difference in PON levels between ART-treated and untreated groups, suggesting that some features of chronic infection, such as persistent inflammation, rather than HIV itself, may be responsible for PON disbalance in HDL. The functional effect of PON reduction remains unclear, as the only group with increased oxidation levels was NNRTI-treated group. The oxidative effect of NNRTI treatment and the role of reduced PON in this phenomenon remain to be confirmed in larger studies. We also did not find a correlation between PON levels and cholesterol efflux, suggesting that PON deficiency may not be the major contributor to virus-induced impairment of this HDL function. In summary, our results indicate substantial remodeling and functional impairment of HDL in HIV-infected subjects, which are likely responsible for increased risk of atherosclerosis in HIV infection, even in ART-treated individuals with higher levels of HDL.
Supplementary Material
Highlights.
HDL particles from HIV-infected subjects have reduced PON levels and PON activity
EtherPS levels in HDL from ART-naïve HIV patients correlated with viral load
Cholesterol efflux to HDL from ART-naïve subjects negatively correlated with HIV load
Oxidized LDL were increased in NNRTI-treated paetients
Our results indicate remodeling of lipid and protein HDL content in HIV patients
Acknowledgements
We wish to acknowledge the assistance of the clinical research coordinators in the Division of Infectious Diseases for their effort in the collection of these serum samples. We also wish to acknowledge Dr. Sylvia Silver at the George Washington University whose laboratory is responsible for maintaining the patient samples in the HIV Institute Biorepository. This study was supported by NIH grants HL093818, HL101274 and HL112661, and by the District of Columbia Developmental Center for AIDS Research (DC D-CFAR), an NIH-funded program (5P30AI087714). DS is a fellow of the National Health and Medical Research Council of Australia. The Kansas Lipidomics Research Center was supported by National Science Foundation Grants MCB 0455318 and DBI 0521587, by EPSCoR Grant EPS-0236913 with matching support from the State of Kansas through Kansas Technology Enterprise Corporation and Kansas State University, and by National Institutes of Health Grant P20 RR16475.
Abbreviations
- ART
anti-retroviral treatment
- CE
cholesterol ester
- Cer
ceramide
- DSM
dihydrosphingomyelin
- ePC
etherphosphatidylcholine
- ePS
etherphosphatidylserine
- ePE
ehterphosphatidylethanolamine
- Hex-Cer
hexylceramide
- HIV
human immunodeficiency virus
- LPE
lysophosphatidylethanolamine
- LPC
lysophosphatidylcholine
- LXR
liver X receptor
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- PA
phosphatidic acid
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PCYOX1
Prenylcysteine oxidase 1
- PI
protease inhibitor
- PIn
phosphatidylinositol
- PLTP
phospholipid transfer protein
- PON
paraoxonase
- PS
phosphatidylserine
- VCAM
vascular cell adhesion molecule
- VL
viral load
- TCA
trichloroacetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest in this study.
References
- 1.Escaut L, Monsuez JJ, Chironi G, et al. Coronary artery disease in HIV infected patients. Intensive Care Med. 2003;29:969–973. doi: 10.1007/s00134-003-1740-0. [DOI] [PubMed] [Google Scholar]
- 2.Lang S, Mary-Krause M, Cotte L, et al. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS. 2010;24:1228–1230. doi: 10.1097/QAD.0b013e328339192f. [DOI] [PubMed] [Google Scholar]
- 3.Triant VA, Lee H, Hadigan C, et al. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwong GP, Ghani AC, Rode RA, et al. Comparison of the risks of atherosclerotic events versus death from other causes associated with antiretroviral use. AIDS. 2006;20:1941–1950. doi: 10.1097/01.aids.0000247115.81832.a1. [DOI] [PubMed] [Google Scholar]
- 5.Strategies for Management of Anti-Retroviral Therapy I and Groups DADS. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients. AIDS. 2008;22:F17–24. doi: 10.1097/QAD.0b013e32830fe35e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Purnell JQ, Zambon A, Knopp RH, et al. Effect of ritonavir on lipids and post-heparin lipase activities in normal subjects. AIDS. 2000;14:51–57. doi: 10.1097/00002030-200001070-00006. [DOI] [PubMed] [Google Scholar]
- 7.Khunnawat C, Mukerji S, Havlichek D, Jr., et al. Cardiovascular manifestations in human immunodeficiency virus-infected patients. Am J Cardiol. 2008;102:635–642. doi: 10.1016/j.amjcard.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 8.Longenecker CT, Triant VA. Initiation of antiretroviral therapy at high CD4 cell counts: does it reduce the risk of cardiovascular disease? Current opinion in HIV and AIDS. 2014;9:54–62. doi: 10.1097/COH.0000000000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rose H, Low H, Dewar E, et al. The effect of HIV infection on atherosclerosis and lipoprotein metabolism: a one year prospective study. Atherosclerosis. 2013;229:206–211. doi: 10.1016/j.atherosclerosis.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varriale P, Saravi G, Hernandez E, et al. Acute myocardial infarction in patients infected with human immunodeficiency virus. Am Heart J. 2004;147:55–59. doi: 10.1016/j.ahj.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Neumann T, Woiwoid T, Neumann A, et al. Cardiovascular risk factors and probability for cardiovascular events in HIV-infected patients: part I. Differences due to the acquisition of HIV-infection. Eur J Med Res. 2003;8:229–235. [PubMed] [Google Scholar]
- 12.Baker JV, Lundgren JD. Cardiovascular implications from untreated human immunodeficiency virus infection. Eur Heart J. 2011;32:945–951. doi: 10.1093/eurheartj/ehq483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 14.Hatano H, Strain MC, Scherzer R, et al. Increase in 2-long terminal repeat circles and decrease in D-dimer after raltegravir intensification in patients with treated HIV infection: a randomized, placebo-controlled trial. J Infect Dis. 2013;208:1436–1442. doi: 10.1093/infdis/jit453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westhorpe CL, Maisa A, Spelman T, et al. Associations between surface markers on blood monocytes and carotid atherosclerosis in HIV-positive individuals. Immunol Cell Biol. 2014;92:133–138. doi: 10.1038/icb.2013.84. [DOI] [PubMed] [Google Scholar]
- 16.Saves M, Chene G, Ducimetiere P, et al. Risk factors for coronary heart disease in patients treated for human immunodeficiency virus infection compared with the general population. Clin Infect Dis. 2003;37:292–298. doi: 10.1086/375844. [DOI] [PubMed] [Google Scholar]
- 17.Rose H, Hoy J, Woolley I, et al. HIV infection and high density lipoprotein metabolism. Atherosclerosis. 2008;199:79–86. doi: 10.1016/j.atherosclerosis.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Annema W, von Eckardstein A. High-density lipoproteins. Multifunctional but vulnerable protections from atherosclerosis. Circ J. 2013;77:2432–2448. doi: 10.1253/circj.cj-13-1025. [DOI] [PubMed] [Google Scholar]
- 19.Boekholdt SM, Arsenault BJ, Hovingh GK, et al. Levels and changes of HDL cholesterol and apolipoprotein A-I in relation to risk of cardiovascular events among statin-treated patients: a meta-analysis. Circulation. 2013;128:1504–1512. doi: 10.1161/CIRCULATIONAHA.113.002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asztalos BF, Mujawar Z, Morrow MP, et al. Circulating Nef induces dyslipidemia in simian immunodeficiency virus-infected macaques by suppressing cholesterol efflux. J Infect Dis. 2010;202:614–623. doi: 10.1086/654817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapman MJ, Goldstein S, Lagrange D, et al. A density gradient ultracentrifugal procedure for the isolation of the major lipoprotein classes from human serum. J Lipid Res. 1981;22:339–358. [PubMed] [Google Scholar]
- 22.Low H, Hoang A, Sviridov D. Cholesterol efflux assay. J Vis Exp. 2012;61:e3810. doi: 10.3791/3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuo Y, Zhuang DZ, Han R, et al. ABCA12 maintains the epidermal lipid permeability barrier by facilitating formation of ceramide linoleic esters. J Biol Chem. 2008;283:36624–36635. doi: 10.1074/jbc.M807377200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitzgerald ML, Xavier R, Haley KJ, et al. ABCA3 inactivation in mice causes respiratory failure, loss of pulmonary surfactant, and depletion of lung phosphatidylglycerol. J Lipid Res. 2007;48:621–632. doi: 10.1194/jlr.M600449-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Grapov D, Newman JW. imDEV: a graphical user interface to R multivariate analysis tools in Microsoft Excel. Bioinformatics. 2012;28:2288–2290. doi: 10.1093/bioinformatics/bts439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Box GEPaDRC An analysis of transformations. J Royal Stat Soc. 1964;26:211–252. [Google Scholar]
- 27.Young J, Weber R, Rickenbach M, et al. Lipid profiles for antiretroviral-naive patients starting PI- and NNRTI-based therapy in the Swiss HIV cohort study. Antivir Ther. 2005;10:585–591. [PubMed] [Google Scholar]
- 28.Bernal E, Masia M, Padilla S, et al. High-density lipoprotein cholesterol in HIV-infected patients: evidence for an association with HIV-1 viral load, antiretroviral therapy status, and regimen composition. AIDS Patient Care STDS. 2008;22:569–575. doi: 10.1089/apc.2007.0186. [DOI] [PubMed] [Google Scholar]
- 29.Vaisar T, Pennathur S, Green PS, et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forte TM, Subbanagounder G, Berliner JA, et al. Altered activities of anti-atherogenic enzymes LCAT, paraoxonase, and platelet-activating factor acetylhydrolase in atherosclerosis-susceptible mice. J Lipid Res. 2002;43:477–485. [PubMed] [Google Scholar]
- 31.Dullaart RP, Otvos JD, James RW. Serum paraoxonase-1 activity is more closely related to HDL particle concentration and large HDL particles than to HDL cholesterol in Type 2 diabetic and non-diabetic subjects. Clin Biochem. 2014;47:1022–1027. doi: 10.1016/j.clinbiochem.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Daminelli EN, Spada C, Treitinger A, et al. Alterations in lipid transfer to high-density lipoprotein (HDL) and activity of paraoxonase-1 in HIV+ patients. Rev Inst Med Trop Sao Paulo. 2008;50:223–227. doi: 10.1590/s0036-46652008000400007. [DOI] [PubMed] [Google Scholar]
- 33.Precourt LP, Amre D, Denis MC, et al. The three-gene paraoxonase family: physiologic roles, actions and regulation. Atherosclerosis. 2011;214:20–36. doi: 10.1016/j.atherosclerosis.2010.08.076. [DOI] [PubMed] [Google Scholar]
- 34.Rosenblat M, Vaya J, Shih D, et al. Paraoxonase 1 (PON1) enhances HDL-mediated macrophage cholesterol efflux via the ABCA1 transporter in association with increased HDL binding to the cells: a possible role for lysophosphatidylcholine. Atherosclerosis. 2005;179:69–77. doi: 10.1016/j.atherosclerosis.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 35.Mackness MI, Arrol S, Durrington PN. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991;286:152–154. doi: 10.1016/0014-5793(91)80962-3. [DOI] [PubMed] [Google Scholar]
- 36.Mackness B, Durrington P, McElduff P, et al. Low paraoxonase activity predicts coronary events in the Caerphilly Prospective Study. Circulation. 2003;107:2775–2779. doi: 10.1161/01.CIR.0000070954.00271.13. [DOI] [PubMed] [Google Scholar]
- 37.Bobin-Dubigeon C, Biron C, Volteau C, et al. Short communication: Paraoxonase 1 (PON1) in French HIV-infected patients under antiretroviral therapy: relationship with the metabolic syndrome and inflammation. AIDS Res Hum Retroviruses. 2013;29:1571–1574. doi: 10.1089/AID.2013.0010. [DOI] [PubMed] [Google Scholar]
- 38.Aragones G, Garcia-Heredia A, Guardiola M, et al. Serum paraoxonase-3 concentration in HIV-infected patients. Evidence for a protective role against oxidation. J Lipid Res. 2012;53:168–174. doi: 10.1194/jlr.P018457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parra S, onso-Villaverde C, Coll B, et al. Serum paraoxonase-1 activity and concentration are influenced by human immunodeficiency virus infection. Atherosclerosis. 2007;194:175–181. doi: 10.1016/j.atherosclerosis.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 40.Cakatay U, Kayali R, Uzun H. Relation of plasma protein oxidation parameters and paraoxonase activity in the ageing population. Clin Exp Med. 2008;8:51–57. doi: 10.1007/s10238-008-0156-0. [DOI] [PubMed] [Google Scholar]
- 41.Banfi C, Brioschi M, Barcella S, et al. Proteomic analysis of human low-density lipoprotein reveals the presence of prenylcysteine lyase, a hydrogen peroxide-generating enzyme. Proteomics. 2009;9:1344–1352. doi: 10.1002/pmic.200800566. [DOI] [PubMed] [Google Scholar]
- 42.Camont L, Lhomme M, Rached F, et al. Small, dense high-density lipoprotein-3 particles are enriched in negatively charged phospholipids: relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti-inflammatory, and antiapoptotic functionalities. Arterioscler Thromb Vasc Biol. 2013;33:2715–2723. doi: 10.1161/ATVBAHA.113.301468. [DOI] [PubMed] [Google Scholar]
- 43.Rosenblat M, Karry R, Aviram M. Paraoxonase 1 (PON1) is a more potent antioxidant and stimulant of macrophage cholesterol efflux, when present in HDL than in lipoprotein-deficient serum: relevance to diabetes. Atherosclerosis. 2006;187:74–81. doi: 10.1016/j.atherosclerosis.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 44.Lee CY, Lesimple A, Denis M, et al. Increased sphingomyelin content impairs HDL biogenesis and maturation in human Niemann-Pick disease type B. J Lipid Res. 2006;47:622–632. doi: 10.1194/jlr.M500487-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Wakabayashi I. Influence of age and gender on triglycerides-to-HDL-cholesterol ratio (TG/HDL ratio) and its association with adiposity index. Arch Gerontol Geriatr. 2012;55:729–734. doi: 10.1016/j.archger.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Ferrara A, Barrett-Connor E, Shan J. Total, LDL, and HDL cholesterol decrease with age in older men and women. The Rancho Bernardo Study 1984-1994. Circulation. 1997;96:37–43. doi: 10.1161/01.cir.96.1.37. [DOI] [PubMed] [Google Scholar]
- 47.Barton M. Cholesterol and atherosclerosis: modulation by oestrogen. Curr Opin Lipidol. 2013;24:214–220. doi: 10.1097/MOL.0b013e3283613a94. [DOI] [PubMed] [Google Scholar]
- 48.Parra S, Marsillach J, Aragones G, et al. Paraoxonase-1 gene haplotypes are associated with metabolic disturbances, atherosclerosis, and immunologic outcome in HIV-infected patients. J Infect Dis. 2010;201:627–634. doi: 10.1086/650312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.