Abstract
Background and aims
Fetuin-A has a plausible role in the inhibition of arterial calcification, but its association with risk of coronary heart disease (CHD) in the general population is unclear. We used two common genetic variants in the fetuin-A gene (AHSG) that are strongly associated with circulating fetuin-A levels to investigate the associations with risk of CHD and subclinical cardiovascular measures (intima- media thickness, ankle-arm index, and coronary artery calcification).
Methods
Genetic variation and fetuin-A levels were assessed in 3,299 community-living individuals (2,733 Caucasians and 566 African Americans) 65 years of age or older, free of previous cardiovascular disease, who participated in the Cardiovascular Health Study (CHS) in 1992– 1993.
Results
Among Caucasians, both rs2248690 and rs4917 were associated with 12% lower fetuin-A concentrations per minor allele (P<0.0001). The hazard ratios (HRs) per minor allele for incident CHD were 1.12 (95% CI: 1.00– 1.26) for rs2248690 and 1.02 (0.91- 1.14) for rs4917. Using both genotypes as an instrumental variable for measured fetuin-A, the HRs for one standard deviation increase in genetically determined fetuin-A levels on CHD risk were 0.84 (95% CI: 0.70– 1.00) for rs2248690 and 0.97 (95% CI: 0.82– 1.14) for rs4917, respectively. However, in CHS neither of the genotypes were associated with subclinical cardiovascular measures and when CHS data were meta-analyzed with data from six other prospective studies (totaling 26,702 Caucasian participants and 3,295 CHD cases), the meta-analyzed HRs for incident CHD were 1.12 (0.93– 1.34) and 1.06 (0.93– 1.20) for rs2248690 and rs4917, respectively (p heterogeneity 0.005 and 0.0048).
Conclusion
Common variants in the AHSG gene are strongly associated with fetuin-A levels, but their concurrent association with CHD risk in current prospective studies is inconsistent. Further investigation in studies with measured fetuin-A and AHSG variants is needed to clarify the potential causal association of fetuin-A with CHD risk.
Keywords: Fetuin-A, single nucleotide polymorphisms, coronary heart disease, Mendelian randomization, meta-analysis
INTRODUCTION
Fetuin-A, also known as α-Heremans- Schmid glycoprotein (AHSG), is synthesized and secreted from the liver and is found in the extracellular space throughout the body.1 Its main function is thought to be regulation of osseous and vascular calcification by transient formation of soluble colloidal spheres containing fetuin-A, calcium, and phosphate that prevent hydroxyapatite crystallization.2 In addition to its function as an inhibitor of arterial calcification, fetuin-A binds the insulin receptor tyrosine kinase in peripheral tissues, thereby inhibiting the insulin- induced intracellular signal cascade.3, 4 Consequently, higher levels of fetuin-A are associated with higher triglycerides, LDL cholesterol, body mass index, insulin resistance, and incident type 2 diabetes.5–9
The association of fetuin-A with cardiovascular disease (CVD) has not been well studied in general population samples. Only a few population-based studies have investigated the association of circulating fetuin-A levels and risk of CVD prospectively, and the results have been conflicting.10–13 Recently, we reported that higher fetuin-A levels were associated with lower risk of CVD among participants without type 2 diabetes, whereas the association of fetuin-A levels trended in opposite direction among participants with type 2 diabetes.10 Similar to these findings, in the Rancho-Bernardo Study, higher fetuin-A levels were associated with lower risk of CVD death in participants without type 2 diabetes.11 In the Nurses` Health Study, higher fetuin-A levels were only associated with lower risk of CHD among women with high C-reactive protein levels.12
However, contradictory to these previous findings, the EPIC-Potsdam Study reported a strong and direct association between higher fetuin-A levels and increased risk of myocardial infarction (MI) and stroke.13 They also assessed the association of common genetic variants in the AHSG gene with fetuin-A levels and risk of CVD and reported that single nucleotide polymorphisms (SNPs) associated with lower fetuin-A were concomitantly associated with lower risk of CVD.14 Since the EPIC- Potsdam study was limited in sample size and the reported an association was in opposite direction compared to previous studies, the main aim of the present study was to address the potential causal role of fetuin-A in relation to risk of CVD using data from the Cardiovascular Health Study (CHS) with supporting genetic information from six other cohort studies.
CHS has measured plasma fetuin-A levels, common genetic variants in the fetuin-A gene (AHSG), subclinical measures of CVD, and clinical CVD events during two decades of follow-up. We used two SNPs from the AHSG gene (rs2248690 and rs4917) that are known as uniquely strong instruments for circulating fetuin-A levels due to their direct regulatory role in AHSG protein transcription,6, 14–18 and resulting strong associations with fetuin-A levels. To maximize statistical power, we additionally meta-analyzed the association of these two common variants and risk of coronary heart disease (CHD) based on current genome-wide association studies in the prospective CHARGE (Cohorts for Heart and Aging Research in Genome Epidemiology) Consortium, the Nurses’ Health Study (NHS), and the Health Professionals Follow-Up Study (HPFS).
METHODS
Study population
The Cardiovascular Health Study (CHS) is a population-based longitudinal study of older adults designed to evaluate risk factors for development and progression of cardiovascular disease. A total of 5,201 men and women 65 years or older were recruited from 4 communities between 1989 and 1990 using Medicare eligibility lists in each area (Sacramento, California; Washington County, Maryland; Forsyth County, North Carolina; and Allegheny County, Pennsylvania). Eligibility was limited to persons who were not institutionalized and expected to remain in the area for at least 3 years after recruitment, received no active cancer treatment and had the ability to provide informed consent without a proxy. A second cohort of 687 participants (including mostly African Americans) was recruited between 1992 and 1993 by similar methods. Details about the study have been published elsewhere.19 This study was approved by the Investigational Review Boards of the four clinical sites, the University of California San Diego, and the Data Coordinating Center at the University of Washington.
We considered the visit in 1992–1993 as the baseline study visit for our analyses (concurrent with plasma fetuin-A measurement). Among the 5,265 participants who participated at this visit, we excluded individuals who had not given consent for genetic analyses (n=270), whose samples failed genotypic quality control (n=750), and those with insufficient blood specimen for fetuin-A measurement (n=390). In the prospective analysis of incident CHD, we also excluded participants with prevalent CVD (n=556), resulting in a study sample of 3,299 participants (2,733 Caucasians and 566 African Americans) for this analysis.
Fetuin-A
Plasma was collected at the 1992–1993 study visit after participants had fasted overnight and was stored at −70 Celsius until 2010, when it was thawed and measured for fetuin-A using an ELISA kit (Epitope Diagnostics, San Diego, CA). The assay uses a two-site “sandwich” technique, with polyclonal antibodies that bind different epitopes of human fetuin-A. Plasma samples were measured twice in each participant and results were averaged. We observed coefficients of variation between 3.3 and 9.1%.
Genotypes
CHS is part of the National Heart, Lung, and Blood Institute- funded Candidate Gene Association Resource (CARe) study.20 DNA was collected at baseline and genotyping was performed using a gene-centric 50K single nucleotide polymorphism (SNP) array.21 This genotyping array was designed to capture genetic variation at more than 2000 genetic loci of relevance to a range of cardiovascular, metabolic, and inflammatory syndromes. At the time of chip development, a multistage approach for SNP selection was taken for the selection of SNPs within the candidate loci for the ITMAT/Broad/ CARE (IBC) array. For a given locus, known or putative functional SNPs were included first and then additional tagging SNPs were added to capture the known variation at the locus (with minor allele frequency (MAF) > 0.02 and r2 ≥ 0.8). Priority was given to nonsynonymous and functional variants if possible. This chip included 13 variants at the AHSG locus. We chose two common SNPs (MAF > 25%) that are not in complete linkage disequilibrium (LD); rs4917 and rs2248690. We previously found these two SNPs satisfy the three main criteria for Mendelian randomization analysis; 1) they are strongly associated with fetuin-A levels, 2) they are not associated with potential confounding factors, and 3) to the best of our knowledge, they influence clinical outcome only through the specific intermediate biomarker (i.e. previous GWAS studies have not reported that these SNPs have pleiotropic effects and influence other pathways relevant for CVD).22 From the CARe project, principal components were generated based on ancestry-related SNPs.
Cardiovascular outcomes
Between enrollment in the CHS study and 1998–1999, participants were seen in the clinic annually, and contacted by phone at 6-month intervals to collect information about hospitalizations and potential cardiovascular events. Subsequently, telephone calls occurred every 6 months. In our study, we primarily evaluated incident coronary heart disease (CHD), defined as time to first myocardial infarction (MI) or CHD death. Secondarily, we also evaluated a composite cardiovascular disease (CVD) endpoint including MI, stroke, and CVD death. Hospital records of all potential events were obtained, and all events were adjudicated by a CHS Events Committee. MI was indicated by symptoms of coronary ischemia, elevated serum levels of troponin and cardiac enzymes, and specified electrocardiographic changes.23 Stroke was adjudicated by a committee of neurologists, neuroradiologists, and internists on the basis of interviews with the patients, medical records, and brain imaging studies.24 Deaths were identified by a review of obituaries, medical records, death certificates, and the Centers of Medicare and Medicaid Service health care utilization data base for hospitalization, and from household contacts; 100% complete follow-up for mortality status was achieved. Deaths from cardiovascular causes included deaths by coronary heart disease, heart failure, peripheral vascular disease, or cerebrovascular disease.25
Subclinical cardiovascular characteristics
At the 1992–1993 study visit (concurrent with plasma fetuin-A measurement), participants underwent measurements of the intima media thickness (IMT) in the carotid arteries and ankle-arm index (AAI). IMT was obtained by averaging multiple measurements of the maximum common carotid artery IMT and maximum internal carotid artery IMT on both the left and right sides after standardization (subtraction of the mean and division by the standard deviation for the measurement) using high-resolution B-mode ultrasonography (model SSA-270A; Toshiba, America Medical Systems).26, 27
Trained technicians measured duplicate resting measurements of blood pressure among participants according to standard protocol. The AAI was calculated as the lower of the ratio of the average of two blood pressure measurements in each lower extremity. Methods of measurements and quality control have been published previously.28
A total of 614 participants from Pittsburgh, Pennsylvania, also underwent electron beam computed tomography to assess coronary artery calcification (CAC) approximately 6 years after plasma fetuin-A measurement (between 1998 and 2000). CAC was assessed using an Imatron C-150 EBT scanner and the Agatston scoring method, as described previously.17
Other characteristics
Information on sociodemographics, lifestyle factors, medication use, and medical history was obtained by self-report. Responses to questions related to smoking were categorized as current, previous, or never smoking. Leisure physical activity was calculated as the weighted sum of kilocalories consumed in 15 physical activities.29 Education was dichotomized as less or more than high school education. Participants were classified according to alcohol consumption as abstainers or not.
The clinical examination was conducted by trained personnel and included standardized assessment of blood pressure, weight, and height. Height was measured to the nearest centimeter using a stadiometer, and weight was measured using a balance beam scale in pounds with participants wearing examination gowns and no shoes. Body mass index (BMI) was computed as weight (in kilograms) divided by the squared value of height (in meters). Hypertension was defined as systolic blood pressure > 140 mmHg, or diastolic blood pressure > 90 mmHg, or medical treatment for hypertension.
A fasting serum sample was drawn from each participant, and laboratory measurements included LDL and HDL cholesterol, triglycerides, cystatin-C, C-reactive protein, and glucose). Participants were classified as having type 2 diabetes mellitus if fasting glucose was ≥ 126 mg/dL, or if individuals used insulin or oral hypoglycemic agents. Insulin resistance was assessed by the homeostasis model assessment for insulin resistance (HOMA-IR) method in participants without a history of diabetes mellitus. Estimated glomerular filtration rate (eGFR) was calculated using the equation eGFR= 76.7 x cystatin C (mg/L)−1.19. Quality control procedures, laboratory methods, and procedures for blood pressure measurements have been published previously.30
Replication cohorts
Data on the association of rs4917 and rs2248690 with risk of incident CHD were available in current genome-wide association studies. From the CHARGE (Cohorts for Heart and Aging Research in Genome Epidemiology) Consortium, information was gathered from 4 population-based studies of Caucasians in addition to CHS: Atherosclerosis Risk in Communities Study (ARIC), the Age, Gene Environment Susceptibility Reykjavik Study (AGES), the Rotterdam Study (RS), and the Framingham Heart Study. We also had access to genome-wide data (Affymetrix 6.0) and risk of CHD in nested case-controls studies of the Nurses` Health Study (NHS) and the Health Professionals Follow-Up Study (HPFS).31 Local ethical committees at each institution approved the individual study protocols.
Statistical analysis
AHSG genetic variants and risk of CHD in the Cardiovascular Health Study
We used means and proportions of the demographics, cardiovascular risk factors and subclinical cardiovascular disease to describe the CHS population according to the two AHSG genetic variants. For the baseline characteristics, we also modelled the additive effects (per minor allele) and estimated the p for trend. All subsequent analyses were performed separately for Caucasians and African American participants. We assessed the genotype and MAF for the genetic variants and tested Hardy-Weinberg equilibrium. As reported,22 the pairwise linkage disequilibrium between rs2248690 and rs4917 was 0.91 in Caucasian and 0.53 in African Americans as measured by D`; r2 was 0.57 and 0.27, respectively.
Among participants free of CVD at baseline, we used Cox proportional hazard models adjusted for age, sex, and field center site to examine the prospective association between the genotypes and subsequent risk of CHD. We calculated hazard ratios (HRs) with 95 % confidence intervals. Follow- up was continued through 30 June 2009. We conducted several stratified analyses to assess whether the association of genotypes per minor allele copy and risk of CHD could be modified by other factors. Thus, we investigated the potential effect modification by sex, age (dichotomized at 75 years), BMI (dichotomized at 30 kg/m2), type 2 diabetes mellitus, and HOMA-IR (dichotomized at 2.2 units) among the subgroup of participants without type 2 diabetes. We also formally tested the homogeneity of stratum-specific relative risks. For these tests of interaction, we modeled genotype per additional minor allele. We tested the proportionality of hazards using Schoenfeld residuals, and there was no appreciable evidence of violations of the proportionality assumption.
Using AHSG variants as an instrumental variable in analysis of fetuin-A and CHD-risk in the Cardiovascular Health Study
Evidence for a causal role of plasma fetuin-A in the pathophysiology of CHD can be assessed by applying the concept of Mendelian randomization. The application of the Mendelian randomization approach relies on the appropriate selection of genotypes as instruments for the intermediate biomarker.32 The Mendelian randomization approach assumes that any factor that possibly confounds a true association between an intermediate phenotype (i.e. Fetuin-A) and a disease (i.e. CHD) is distributed evenly among those who carry and those who do not carry a predisposing genotype for the intermediate phenotype. Therefore, the disease risk among those carrying an allele that exposes individuals to long-term higher or lower circulating fetuin-A should correspond proportionally to the difference in circulating fetuin-A levels that can be attributed to the genetic variant.
Using CHS data, where we had information about plasma fetuin-A levels, common genetic variants in the AHSG gene, and CHD events registered during follow-up among the participants, we examined the estimated causative effect of fetuin-A on CHD risk using the genotypes as instrumental variables. As a first step, we explored if these two SNPs were associated with any of the potential confounders in Table1, and found no statistical evidence for strong associations. Next, we estimated mean fetuin-A concentrations across genotypes and per minor allele copy using linear regression models adjusted for age, sex, and field center site. In this step we also assessed the strength of our instrumental variables estimating the first stage F statistic. Finally, the predicted values and residuals from the previous step were modeled using Cox regression with CHD as the outcome.33 The strength of association per 1-SD higher genetically elevated fetuin-A level was estimated by using rs4917 and rs2248690 as instruments, respectively, adjusted for age, sex, race, and field center. We assumed an additive genetic model (i.e. fetuin-A concentration decreasing linearly with each additional minor allele of the genotypes).
AHSG variants and subclinical cardiovascular disease in the Cardiovascular Health Study
We also investigated the effects of genetically elevated fetuin-A levels on subclinical measures of CVD (IMT, AAI, and CAC) using the genotypes as instrumental variables. For these analyses, we used a two-stage least squares approach to estimate the mean difference in IMT, AAI, and CAC per 1 SD difference in genetically predicted fetuin-A concentrations, respectively.34
Sensitivity analyses
To address the robustness of our findings and to gain more statistical power we a) added incident cases of stroke to use the composite CVD endpoint and b) analyzed the association of genotypes with both prevalent cases and incident cases of CVD combined using logistic regression models.
In another set of sensitivity analysis, we repeated the analyses with adjustment for population stratification by including the top 10 principal components as covariates.
Meta-analysis of AHSG variants in relation to risk of CHD
Information on the association of the two SNPs with risk of CHD was pulled from genome wide association analyses of incident CHD that have already been conducted within the CHARGE consortium, which included the Caucasian participants of CHS, and the NHS and HPFS studies. We pooled effect estimates using random effects meta-analysis (using metan command in STATA), and examined the potential influence of age of the study participants on the size of the effect estimate using meta-regression analysis (using metareg command in STATA).
P values < 0.05 were considered statistically significant for all analyses including interaction terms. All statistical analyses were conducted using Stata (version 10.1 for windows, STATA Corp).
RESULTS
Baseline characteristic in the Cardiovascular Health Study
The mean age of the 3,299 CHS study participants was 74.7± 5.2 year, 60.9 % were female, 17.2 % were African American, and 15.5 % had prevalent diabetes. The mean plasma fetuin-A concentration was 0.47± 0.10 mg/L. For rs4917, the minor allele frequencies (MAF) were 32.5% and 25.1% among Caucasians and African Americans, respectively. For rs2248690, the MAFs were 24.8% and 26.2% among Caucasians and African Americans, respectively. For the analyzed SNPs, we observed no deviations from Hardy-Weinberg equilibrium among Caucasians or African Americans (p> 0.05).
Table 1 displays the characteristics of the study population according to the AHSG genotypes rs4917 and rs2248690, respectively. Genotypes were not strongly associated with any of the potential confounders in Table 1 (p> 0.05). Furthermore, the SNPs were not associated with any of the subclinical cardiovascular measurements in unadjusted analysis (p> 0.05).
Table 1.
Baseline characteristics according to AHSG genotypes in CHS
| SNP | rs4917 | rs2248690 | ||||||
|---|---|---|---|---|---|---|---|---|
| Alleles | CC | Ct | tt |
P for trend |
AA | At | tt |
P for trend |
| N participants (%) | 1583 (48) | 1372 (42) | 344 (10) | 1855 (56) | 1236(37) | 208 (6) | ||
| Fetuin-A levels, g/L (SD) | 0.51 (0.002) | 0.45 (0.002) | 0.39 (0.005) | <0.0001 | 0.50 (0.002) | 0.44 (0.002) | 0.38 (0.006) | <0.0001 |
| Mean age, years (SD) | 74.5 (5.2) | 74.7 (5.2) | 75.2 (5.6) | 0.02 | 75 (5) | 75 (5) | 75 (5) | 0.51 |
| Male, N (%) | 617 (40) | 534 (40) | 138 (40) | 0.78 | 718 (39) | 484 (39) | 87 (41) | 0.47 |
| African American, N (%) | 318 (20) | 212 (15) | 36 (10) | <0.001 | 304 (16) | 227 (18) | 35 (17) | 0.31 |
| Less than high school education, N (%) | 413 (26) | 341 (25) | 93 (27) | 0.06 | 472 (25) | 311 (25) | 64 (30) | 0.34 |
| Mean BMI, kg/m2 (SD) | 27 (5) | 27 (5) | 27 (5) | 0.58 | 27 (5) | 27 (5) | 27 (5) | 0.42 |
| Mean physical activity, kcal/day (IQR) | 893 (1705) | 855 (1695) | 750 (1395) | 0.06 | 903 (1730) | 825 (1680) | 696 (1300) | 0.07 |
| Current smoker, N (%) | 180 (12) | 128 (9) | 32 (9) | 0.24 | 194 (11) | 127 (10) | 19 (9) | 0.98 |
| No alcohol intake, N (%) | 872 (55) | 714 (52) | 192 (56) | 0.56 | 55 (1011) | 52 (649) | 57 (118) | 0.81 |
| Prevalent type 2 diabetes, N (%) | 240 (15) | 220 (16) | 49 (14) | 0.99 | 277 (15) | 199 (16) | 33 (16) | 0.43 |
| Prevalent hypertension, N (%) | 1020 (64) | 860 (62) | 225 (65) | 0.81 | 1190 (64) | 776 (63) | 139 (66) | 1.00 |
| Systolic blood pressure, mmHg (SD) | 137 (22) | 136 (21) | 136 (21) | 0.39 | 137 (22) | 136 (21) | 137 (21) | 0.98 |
| Diastolic blood pressure, mmHg (SD) | 72 (11) | 72 (11) | 72 (11) | 0.60 | 71 (11) | 72 (11) | 73 (11) | 0.15 |
| Mean eGFR, ml/min/1.73m2 (SD) | 74 (18) | 74 (18) | 74 (18) | 0.75 | 73 (18) | 75 (19) | 74 (19) | 0.02 |
| Median C-reactive protein, g/L (IQR) | 2.5 (4.4) | 2.6 (4.8) | 2.6 (4.9) | 0.34 | 2.5 (4.4) | 2.6 (4.6) | 3.2 (5.4) | 0.14 |
| Mean LDL cholesterol, mg/dL (SD) | 128 (34) | 127 (35) | 123 (31) | 0.02 | 128 (34) | 126 (33) | 125 (32) | 0.12 |
| Mean HDL cholesterol, mg/dL (SD) | 54 (15) | 54 (14) | 54 (15) | 0.55 | 54 (14) | 54 (15) | 54 (15) | 0.80 |
| Median triglycerides, mg/dL (IQR) | 121 (83) | 123 (83) | 121 (81) | 0.36 | 123 (82) | 123 (82) | 122 (87) | 0.85 |
| Use of oral hypoglycemic, N (%) | 94 (6) | 90 (7) | 20 (6) | 0.78 | 113 (6) | 75 (6) | 16 (8) | 0.57 |
| Estrogen use women, N (%) | 135 (9) | 126 (9) | 23 (7) | 0.61 | 175 (9) | 95 (8) | 14 (7) | 0.05 |
| Mean internal carotid IMT, mm (SD) | 1.41 (0.55) | 1.39 (0.54) | 1.40 (0.57) | 0.81 | 1.40 (0.54) | 1.41 (0.56) | 1.38 (0.54) | 0.74 |
| Mean common carotid IMT, mm (SD) | 1.08 (0.23) | 1.05 (0.21) | 1.08 (0.24) | 0.22 | 1.07 (0.23) | 1.06 (0.21) | 1.08 (0.26) | 0.45 |
| Mean ankle-arm index, (SD) | 1.08 (0.18) | 1.09 (0.16) | 1.09 (0.17) | 0.22 | 1.08 (0.17) | 1.09 (0.17) | 1.08 (0.17) | 0.78 |
| Coronary calcium score, (IQR) | 277 (809) | 332 (802) | 258 (481) | 0.49 | 321 (864) | 251 (731) | 367 (429) | 0.61 |
Both common genetic variants of AHSG genotype were strongly associated with lower mean fetuin-A concentrations among Caucasians and African Americans. Carriers of the minor alleles for rs4917 (T) had lower fetuin-A concentrations (−0.06 g/L [12%] ± 0.002 per minor allele in Caucasians, P additive=6.4E-162, and −0.04 g/L [8%] ± 0.006 in African Americans, P additive= 3.5E-16). Carriers of the rs2248690 T allele also had lower fetuin-A concentrations (−0.06 g/L ± 0.003 in Caucasians, P=3.2E-121, and −0.03 g/L ± 0.006 in African Americans, P=3.3E-10).
AHSG and risk of CHD in the Cardiovascular Health Study
Among the 3,299 CHS participants free of CVD at baseline, a total of 862 developed incident CHD during a median follow-up of 10.8 years (interquartile range, 5.6–16.2 years). As shown in Table 2, we found borderline associations between the AHSG genotypes and risk of CHD after adjustment for age, sex, race, and field center. For rs2248690, each additional copy of the minor allele was associated with a slightly elevated risk of CHD among Caucasians (HR 1.12, 95% CI: 1.00–1.26, p=0.05), but not in the smaller African American sample (HR 0.98, 95% CI: 0.74–1.29, p=0.87). The risk of CHD per additional copy of the rs4917 minor allele was HR 1.02 (95% CI: 0.91– 1.14, p=0.73) in Caucasians and HR 0.88 (95% CI: 0.67– 1.17, p=0.39) in African Americans.
Table 2.
Risk of CHD (MI/ CHD deaths) (HR with 95 % CIs) per minor allele of AHSG genotypes and per 1 SD difference in genetically predicted fetuin-A in CHS cohort.
| CHS cohort | Caucasians | African Americans | ||
|---|---|---|---|---|
| P | P | |||
| Genotype | n= 2,733 | n= 566 | ||
| rs2248690 | 1.12 (1.00– 1.26) | 0.05 | 0.98 (0.74– 1.29) | 0.87 |
| rs4917 | 1.02 (0.91– 1.14) | 0.73 | 0.88 (0.67–1.17) | 0.39 |
| Genetically predicted fetuin-A | n= 2,733 | n= 566 | ||
| rs2248690 | 0.84 (0.70– 1.00) | 0.05 | 1.06 (0.47– 2.42) | 0.89 |
| rs4917 | 0.97 (0.82– 1.14) | 0.72 | 1.34 (0.72– 2.48) | 0.36 |
| Both variants | 0.93 (0.79– 1.09) | 0.35 | 1.26 (0.70– 2.28) | 0.44 |
Association of AHSG genotypes and risk of CHD was assessed by ordinary Cox– regression and association of genetically predicted fetuin-A and risk of CHD was assessed by two-stage Cox-regression. All analyses were adjusted for age, sex, and clinic. Participants reporting previous CVD were excluded from the analyses.
Previous reports have observed that the direction of association of plasma fetuin-A levels with risk of CHD differed by diabetes, however we did not observe that the association between AHSG SNPs with risk of CHD differed by diabetes status. The risks of CHD per additional copy of rs2248690 minor allele were HR 1.10 (95% CI: 0.98– 1.24, p=0.11) in participants without type 2 diabetes and HR 1.06 (95% CI: 0.83– 1.36, p=0.64) in participants with type 2 diabetes, respectively (P for interaction 0.74). Similarly, the estimates per additional copy of rs4917 were HR 0.99 (95% CI: 0.88– 1.11, p=0.84) in non-diabetic individuals and HR 0.99 (95% CI: 0.78– 1.26, p=0.96) in diabetic individuals (p for interaction 0.88). Also, we found no statistical evidence for any effect modification when stratifying on sex, age, HOMA-IR, or BMI (p values all > 0.05).
Mendelian Randomization analyses in the Cardiovascular Health Study
We used rs4917 and rs2248690 as instrumental variables to estimate the causal effect of fetuin-A levels with CHD risk (Table 2). Among Caucasians, a standard deviation increment in genetically predicted fetuin-A concentration was associated with a HR of 0.84 (95% CI: 0.70–1.00, p=0.05) for rs2248690 and HR of 0.97 (95% CI: 0.82–1.14, p=0.72) for rs4917, respectively. The AHSG SNPs, rs4917 and rs2248690 variation appeared to be have more than sufficient instrumental strength with first stage F statistic >100 among Caucasians to avoid the problem of so-called weak instruments. However, the instrumental strength among African Americans was far weaker with first stage F statistic between 6 and 10.
We did not find any evidence for an association of fetuin-A and subclinical cardiovascular measures or an effect of genetically elevated fetuin-A on subclinical cardiovascular measures using the genotypes as instrumental variables (p values all > 0.05) (Table 3).
Table 3.
Differences in subclinical cardiovascular risk measures per 1SD difference in fetuin-A and per 1 SD difference in genetically predicted fetuin-A in CHS cohort.
| CHS cohort | Caucasians | African Americans | |||
|---|---|---|---|---|---|
| P | P | ||||
| IMT1 | Measured fetuin-A | n= 3,177 | n= 658 | ||
| Adjusted for age, sex, clinic | 0.025 (− 0.003– 0.054) | 0.08 | 0.011 (− 0.054– 0.076) | 0.74 | |
| Genetically predicted fetuin-A | n= 3,177 | n= 658 | |||
| rs2248690 | − 0.017 (− 0.089– 0.055) | 0.64 | 0.062 (− 0.215– 0.339) | 0.66 | |
| rs4917 | − 0.004 (− 0.069– 0.061) | 0.90 | 0.118 (− 0.093– 0.328) | 0.27 | |
| Both variants | − 0.008 (− 0.072– 0.055) | 0.80 | 0.106 (− 0.095– 0.307) | 0.30 | |
| AAI2 | Measured fetuin-A | n= 2,568 | n= 545 | ||
| Adjusted for age, sex, clinic | − 0.001 (− 0.007– 0.005) | 0.71 | 0.004 (− 0.015– 0.023) | 0.66 | |
| Genetically predicted fetuin-A | n= 2,568 | n= 545 | |||
| rs2248690 | − 0.002 (− 0.017– 0.012) | 0.74 | − 0.053 (− 0.110– 0.003) | 0.07 | |
| rs4917 | − 0.006 (− 0.002– 0.008) | 0.44 | − 0.053 (− 0.131– 0.025) | 0.19 | |
| Both variants | − 0.005 (− 0.018–0.009) | 0.50 | − 0.053 (− 0.108–0.002) | 0.06 | |
| CAC3 | Measured fetuin-A | n= 347 | n= 113 | ||
| Adjusted for age, sex, clinic | 29.3 (– 71.0– 129.6) | 0.57 | 31.3 (− 87.1– 149.7) | 0.60 | |
| Genetically predicted fetuin-A | n= 347 | n= 113 | |||
| rs2248690 | 99.3 (− 120.6– 319.3) | 0.38 | 128.7 (− 239.2– 496.6) | 0.49 | |
| rs4917 | 155.1 (− 45.5– 355.7) | 0.13 | − 3.0 (− 424.1– 418.1) | 0.99 | |
| Both variants | 136.8 (− 57.2– 330.8) | 0.17 | 59.7 (− 311.2– 430.6) | 0.75 | |
Intima media thickness in mm
Ankle-arm index
Coronary artery calcium score.
Sensitivity analyses in the Cardiovascular Health Study
The observed associations yielded results that were essentially the same when 388 additional stroke cases were added to the composite CVD endpoint (e-table 1). Including prevalent CVD cases in addition to incident CHD as the outcome of interest the associations remained unchanged (data not shown). Inclusion of the top 10 principal components as covariates in our analyses, capturing potential confounding by population stratification, also did not change the results (data not shown).
Meta-analyses of AHSG variants and CHD
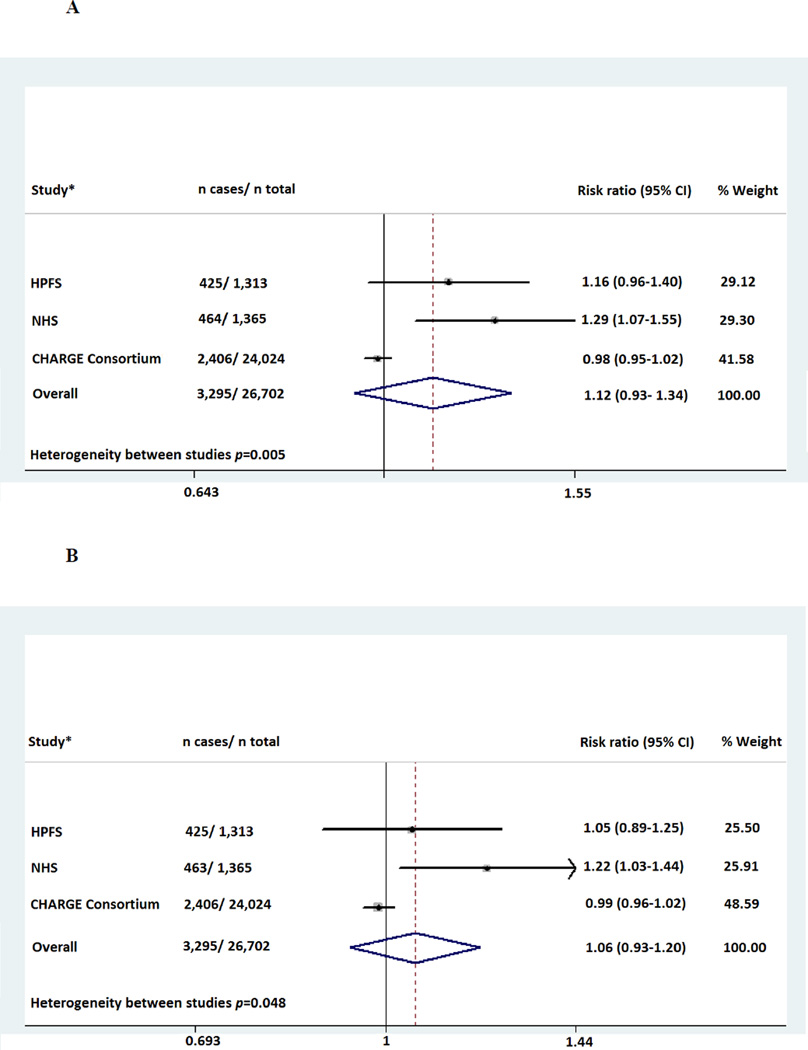
In a cumulative meta-analysis that included 26,702 Caucasians participants from the CHARGE Consortium, NHS, and HPFS, with a total of 3,295 incident cases, rs2248690 and rs4917 were not statistically significantly associated with the risk of CHD (figure 1A and 1B). We observed between-study heterogeneity for both SNPs (p=0.005 for rs2248690 and p=0.048 for rs4917) and the pooled random effects estimates per additional copy of the minor alleles were 1.12 (95% CI: 0.93– 1.34, p=0.23) for rs2248690 and 1.06 (95% CI: 0.93– 1.20, p=0.37) for rs4917. The effect-sizes were generally largest in the youngest cohorts and tended to diminish with age, however in meta-regression analysis, mean age of study population was not a statistically significant moderator of the study effect sizes (p=0.40).
Figure 1.
A- Meta-analysis for the association of rs2248690 with risk of CHD among Caucasians
B- Meta-analysis for the association of rs4917 with risk of CHD among Caucasians
HPFS; Health Professionals Follow-Up Study, NHS; Nurses ‘Health Study, CHARGE; Cohorts for Heart and Aging Research in Genome Epidemiology including Cardiovascular Health Study (CHS), Atherosclerosis Risk in Communities Study (ARIC), the Age, Gene Environment Susceptibility Reykjavik Study (Ages), the Rotterdam Study (RS), and the Framingham Heart study. *The estimated hazard ratios are derived from random effects meta-analyses in 7 prospective studies.
DISCUSSION
In this large prospective cohort study of community- living older people, common genetic variants in the AHSG gene were strongly associated with lower fetuin-A levels and a formal instrumental variable analysis supported a borderline inverse association between plasma fetuin-A levels and CHD. However, in a meta-analysis, using data from the CHARGE consortium (which included the CHS study) in combination with data from the NHS and HPFS, these common genetic variants in the AHSG gene were not statistically associated with risk of CHD, although the hazard ratios were similar in both analyses. Overall, these findings suggest that the association of fetuin-A with CHD-risk may require even larger sample sizes to characterize accurately and leave open the possibility that the association of fetuin-A with CHS may not be causal.
Atherosclerosis is characterized by accumulation of lipids, connective tissue and inflammatory cells in the intima-media layer of the arterial wall. It is known to develop in childhood and adolescence, and the progression from an early to an advanced stage is modified by various factors.35 Emerging evidence suggest involvement of the liver-secreted glycoprotein fetuin-A in the development of vascular disease via two distinct effects.36, 37 First, fetuin-A reversibly binds the insulin receptor tyrosine kinase in peripheral tissues leading to inhibition of the insulin-induced intracellular signal cascade.3, 4 Consequently, in humans, higher levels of fetuin-A have been associated with dyslipidemia and insulin resistance, in addition to incident diabetes mellitus,5–9 which are strong risk factors for cardiovascular disease. Secondly, fetuin-A also solubilizes the calcium phosphate salt, and thereby inhibits ectopic arterial calcification.2 Thus, naturally fetuin-A is expected to be a protective factor against vascular calcification. Based on these findings of a dual role of action, it has been hypothesized that fetuin-A is a harmful factor in early atherosclerosis due to the adverse metabolic effects and insulin resistance, while fetuin-A might be a protective factor in more advanced atherosclerosis with ectopic calcification.36 Furthermore, various potential confounding and/or modifying factors such as the presence of diabetes mellitus, renal dysfunction, drug interventions, and inflammation might also influence the association of fetuin-A and vascular disease.36
The association of fetuin-A with risk of CVD has not been well-studied in general population based studies, and existing reports have shown mixed results.10–13 In a recent nested case-control study from the Nurses` Health Study, fetuin-A levels were not associated with increased risk of CHD during 16 years of follow-up.12 However, fetuin-A levels were associated with decreased risk of CHD among women with high C-reactive protein levels, whereas no association was observed among the remainder of the women. In the Rancho-Bernardo Study, among 1,700 community-living Caucasians with long-term follow-up, low fetuin-A levels were associated with higher risk of CVD death in participants without type 2 diabetes, whereas high fetuin-A levels were associated with higher risk of CVD death in those with type 2 diabetes.11 Similar to these findings, we recently reported that the association of fetuin-A with CVD risk in CHS was modified by type 2 diabetes.10 Higher fetuin-A levels was associated with lower CVD risk among participants without type 2 diabetes (HR 0.93 (95% CI: 0.88– 0.99)), whereas a trend in the opposite direction was observed among participants with type 2 diabetes (HR 1.07 (95% CI: 0.93– 1.22). Among individuals without type 2 diabetes, similar effect modification was observed by obesity and insulin resistance. Although there are several potential mechanisms, the nature of the different directions of association of fetuin-A with CVD among individuals with or without diabetes 2 diabetes or insulin resistance is not known. First, long duration of diabetes mellitus is linked both to increased insulin resistance and accelerated vascular calcification.36 Thus, it has been suggested that diabetes mellitus may mask the beneficial effects of fetuin-A on vascular calcification. Moreover, the diabetes disease itself, diabetic medication, and other metabolic abnormalities such as dyslipidemia, hypertension, and obesity may modify the function or levels of fetuin-A.38 In the current study, we found no evidence for effect modification by diabetes or insulin resistance measured as HOMA-IR among participants without type 2 diabetes on the association of AHSG genotypes and risk of CHD, but a larger sample size may be needed to address this issue definitively.
To the best of our knowledge, only the EPIC-Potsdam study investigated the potential causal nature of the association of fetuin-A and CVD risk using the AHSG genotypes as instrumental variables14 In contrast to our findings, the smaller EPIC-Potsdam nested case-cohort study including 2,520 participants and 369 CVD cases, reported that elevated plasma fetuin-A levels were associated with greater risk of MI and ischemic stroke.13 Further, in contrast to our findings they found that the rs4917 minor allele was associated with lower risk of MI. The reasons for the discrepancy between the EPIC-Potsdam study and our study are uncertain. Besides a considerably older age of CHS participants, other differences between the studies are not obvious. One might speculate that the hypothesized dual roles of fetuin-A may explain these seemingly contradictory findings. In the younger EPIC Potsdam population, the influence of fetuin-A in promoting insulin resistance may predominate, contributing to a greater risk of CVD. In the elderly CHS population which is more susceptible to arterial calcification, fetuin-A might confer protection against CVD by preferentially inhibiting calcification. In contrast, the meta-regression analysis did show a tendency for decreasing study effect sizes of the rs2248690 variant with CHD risk with greater mean age of the study population, and this does not explain why the observed association in EPIC Potsdam is opposite in direction. Nevertheless, when combining our results with 6 other prospective cohorts, the cumulative body of evidence appears to suggest no consistent relationship of these AHSG SNPs (and by proxy, fetuin-A levels themselves) with CHD. Moreover, the cross-sectional CARDIoGRAM (coronary artery disease genome wide replication and meta-analysis), which represents a collaboration including data from multiple large genetic studies to identify risk loci for CHD, reports no significant associations of the AHSG SNPs rs4917 and rs2248690 and risk of CHD (http://www.cardiogramplusc4d.org/). In the elderly CHS cohort, we also did not observe any association between the AHSG SNPs and subclinical cardiovascular measures. However, given that the majority of the CHS participants had documented prevalent coronary calcification; further investigations of these relationships might be more appropriately conducted in younger populations.
Strengths of the present study included a large sample size, prospective design, long and complete follow- up, and large number of CVD events. However, our work also has important limitations. CHS participants were older adults (mean age 74), and the risk of CHD was relatively high among the participants. Thus, the CHS study might not be easily comparable to other population-based studies with younger participants. Moreover, thus, our study is not suited for assessing the role of fetuin-A in the earlier stage of atherosclerosis and the absence of an association among older people in our study does not exclude a causal association among younger people. One might speculate that individuals who carry the major AHSG allelic variants, and thus have lived with elevated fetuin-A levels into older age, may have developed compensatory mechanisms that buffer against the CHD-risk associated with higher plasma fetuin-A concentrations. It is possible that it would take years to develop feedback mechanisms that dampen the effect of continuously elevated fetuin-A concentrations blunting an association between the studied genetic variants and CHD risk in the elderly population in the CHS. We did not find any evidence of effect modification when we stratified by younger than 75 years of age compared with older than 75 years of age to explore the associations of the genetic variants with CHD risk. However, to test this hypothesis formally, a study population with a larger distribution in age would be necessary. Furthermore, fetuin-A level was only evaluated at the beginning of the follow-up, and measured values may change during follow-up. Thus, we could not examine the possible effects of time-dependent changes in fetuin-A level. These factors, however, should not interfere with our analyses of genotype and risk of CVD. Finally, the analyses on African Americans had less statistical power than the analyses on Caucasians.
CONCLUSION
Common genetic variants in the AHSG gene were strongly associated with lower fetuin-A levels, but not consistently with risk of CHD in a pooled analysis of 7 large prospective cohorts. Nevertheless, formal instrumental variable analysis from CHS, the study with the largest number of incident CHD events with both measures of plasma fetuin-A and AHSG genotypes, was suggestive of an inverse association between plasma fetuin-A and risk of CHD. Thus, more investigations are warranted to further examine the potential causal association of fetuin-A with CHD risk.
Supplementary Material
Highlights.
High levels of the liver-secreted glycoprotein fetuin-A are associated with less vascular calcification as well as insulin resistance and metabolic dysregulation.
Common variants in the AHSG gene are strongly associated with fetuin-A levels, but their concurrent associations with risk of coronary heart disease (CHD) are inconsistent.
Formal instrumental variable analysis from Cardiovascular Health Study (CHS) suggested an inverse association between plasma fetuin-A and risk of CHD.
More investigations are warranted to further examine the potential causal association of fetuin-A with CHD.
ACKNOWLEDGEMENT
The manuscript was supported by a grant from the National Heart, Lung, and Blood Institute (NHLBI) (R01 HL094555 to LD, J.R.K. J.H.I, and K.J.M) and genotyping was funded by the NHLBI CARe project (Broad Institute of Massachusetts Institute of Technology and Harvard, N01HC65226). The Cardiovascular Health Study was supported by contracts HHSN268201200036C, HHSN268200800007C, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, and NO1HC85086, and by grant HL080295 from NHLBI, with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A complete list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/PI.htm. Lars E. Laugsand received a research fellowship grant from the Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology.
Replication studies:
The HPFS CHD case-control study was supported by HL35464, and CA55075 from the National Institutes of Health, Bethesda, MD, with additional support for genotyping from Merck/Rosetta Research Laboratories, North Wales, PA. NHS: The NHS CHD case-control study was supported by CA87969 and HL34594 from the National Institutes of Health, Bethesda, MD, with additional support for genotyping from Merck/Rosetta Research Laboratories, North Wales, PA.
CHARGE cohorts: AGES study was funded by NIH contract N01-AG-12100, the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). ARIC is supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C), R01HL087641, R01HL59367 and R01HL086694; National Human Genome Research Institute contract U01HG004402; and National Institutes of Health contract HHSN268200625226C. FHS was partially supported by the National Heart, Lung and Blood Institute’s Framingham Heart Study (N01-HC-25195) and its contract with Affymetrix, Inc for genotyping services (N02-HL-6-4278). Abbas Dehghan is supported by Netherlands Organization for Scientific Research (NWO) grant (veni, 916.12.154) and the EUR Fellowship. Rotterdam Study is supported by the Erasmus Medical Center and Erasmus University Rotterdam; the Netherlands Organization for Scientific Research (NWO); the Netherlands Organization for Health Research and Development (ZonMw); the Research Institute for Diseases in the Elderly (RIDE); the Netherlands Heart Foundation; the Ministry of Education, Culture and Science; the Ministry of Health Welfare and Sports; the European Commission; and the Municipality of Rotterdam. Support for genotyping was provided by the Netherlands Organisation of Scientific Research NWO Investments (nr. 175.010.2005.011, 911-03-012), the Research Institute for Diseases in the Elderly (014-93-015; RIDE2), the Netherlands Genomics Initiative (NGI)/Netherlands Consortium for Healthy Aging (NCHA) project nr. 050-060-810. Health ABC was funded by the National Institutes of Aging. This research was supported by NIA contracts N01AG62101, N01AG62103, and N01AG62106. The genome-wide association study was funded by NIA grant 1R01AG032098-01A1 to Wake Forest University Health Sciences and genotyping services were provided by the Center for Inherited Disease Research (CIDR)
L.E.L conducted statistical analyses, assisted in interpretation of the data, wrote all versions of the manuscript. J.H.I conceived the idea for this study, designed the study, assisted in obtaining funding for the study, assisted in directing the data analyses, interpreted the results, and critically edited all versions of the manuscript. T.M.B assisted in conducting statistical analyses, assisted in interpretation of the data, and critically edited the manuscript. L.D and J.R.K assisted in obtaining funding for the project, assisted in interpretation of the data, and critically edited the manuscript. D.S.S directed the study`s implementation, including quality control procedures, assisted in interpretation of the data, and critically edited the manuscript. A.D. analyzed the CHARGE data and reviewed the final manuscript. K.J.M assisted in obtaining funding for the project, assisted in interpretation of the data, critically edited the manuscript, conceived the idea for this study, designed the study, and assisted in directing the data analysis. M.K.J assisted in study design, assisted in interpretation of the data, assisted in directing the data analysis, critically edited all versions of the manuscript. E.B.R, R.P.T, K.R, O.L.L, C.J.O, A.N all contributed data to the study, obtained funding of main studies, edited and approved the final manuscript.
M.K.J is the guarantor of this work and, as such, had full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No potential conflicts of interests relevant to the article were reported.
References
- 1.Denecke B, Gräber S, Schäfer C, et al. Tissue distribution and activity testing suggest a similar but not identical function of fetuin-B and fetuin-A. Biochem. J. 2003;376:135–145. doi: 10.1042/BJ20030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schafer C, Heiss A, Schwarz A, et al. The serum protein α2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. The Journal of Clinical Investigation. 2003;112:357–366. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srinivas PR, Wagner AS, Reddy LV, et al. Serum alpha 2-HS-glycoprotein is an inhibitor of the human insulin receptor at the tyrosine kinase level. Molecular Endocrinology. 1993;7:1445–1455. doi: 10.1210/mend.7.11.7906861. [DOI] [PubMed] [Google Scholar]
- 4.Mathews ST, Srinivas PR, Leon MA, et al. Bovine fetuin is an inhibitor of insulin receptor tyrosine kinase. Life Sciences. 1997;61:1583–1592. doi: 10.1016/s0024-3205(97)00737-6. [DOI] [PubMed] [Google Scholar]
- 5.Stefan N, Hennige AM, Staiger H, et al. α2-Heremans-Schmid Glycoprotein/ Fetuin-A Is Associated With Insulin Resistance and Fat Accumulation in the Liver in Humans. Diabetes Care. 2006;29:853–857. doi: 10.2337/diacare.29.04.06.dc05-1938. [DOI] [PubMed] [Google Scholar]
- 6.Ix JH, Shlipak MG, Brandenburg VM, et al. Association Between Human Fetuin-A and the Metabolic Syndrome: Data From the Heart and Soul Study. Circulation. 2006;113:1760–1767. doi: 10.1161/CIRCULATIONAHA.105.588723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stefan N, Fritsche A, Weikert C, et al. Plasma Fetuin-A Levels and the Risk of Type 2 Diabetes. Diabetes. 2008;57:2762–2767. doi: 10.2337/db08-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ix JH, Wassel CL, Kanaya AM, et al. Fetuin-a and incident diabetes mellitus in older persons. JAMA. 2008;300:182–188. doi: 10.1001/jama.300.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ix JH, Biggs ML, Mukamal KJ, et al. Association of Fetuin-A With Incident Diabetes Mellitus in Community-Living Older Adults: The Cardiovascular Health Study. Circulation. 2012;125:2316–2322. doi: 10.1161/CIRCULATIONAHA.111.072751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen MK, Bartz TM, Mukamal KJ, et al. Fetuin-A, Type 2 Diabetes, and Risk of Cardiovascular Disease in Older Adults: The Cardiovascular Health Study. Diabetes Care. 2013;36:1222–1228. doi: 10.2337/dc12-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laughlin GA, Cummins KM, Wassel CL, et al. The association of fetuin-A with cardiovascular disease mortality in older community-dwelling adults: the Rancho Bernardo study. J Am Coll Cardiol. 2012;59:1688–1696. doi: 10.1016/j.jacc.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Q, Jiménez MC, Townsend MK, et al. Plasma Levels of Fetuin-A and Risk of Coronary Heart Disease in US Women: The Nurses’ Health Study. Journal of the American Heart Association. 2014:3. doi: 10.1161/JAHA.114.000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weikert C, Stefan N, Schulze MB, et al. Plasma Fetuin-A Levels and the Risk of Myocardial Infarction and Ischemic Stroke. Circulation. 2008;118:2555–2562. doi: 10.1161/CIRCULATIONAHA.108.814418. [DOI] [PubMed] [Google Scholar]
- 14.Fisher E, Stefan N, Saar K, et al. Association of AHSG Gene Polymorphisms With Fetuin-A Plasma Levels and Cardiovascular Diseases in the EPIC-Potsdam Study. Circulation: Cardiovascular Genetics. 2009;2:607–613. doi: 10.1161/CIRCGENETICS.109.870410. [DOI] [PubMed] [Google Scholar]
- 15.Osawa M, Tian W, Horiuchi H, et al. Association of α2-HS glycoprotein (AHSG, fetuin-A) polymorphism with AHSG and phosphate serum levels. Hum Genet. 2005;116:146–151. doi: 10.1007/s00439-004-1222-7. [DOI] [PubMed] [Google Scholar]
- 16.Detrano R, Guerci AD, Carr JJ, et al. Coronary Calcium as a Predictor of Coronary Events in Four Racial or Ethnic Groups. New England Journal of Medicine. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 17.Newman AB, Naydeck B, Sutton-Tyrrell K, et al. Coronary artery calcification in older adults with minimal clinical or subclinical cardiovascular disease. Journal of the American Geriatrics Society. 2000;48:256–263. doi: 10.1111/j.1532-5415.2000.tb02643.x. [DOI] [PubMed] [Google Scholar]
- 18.Inoue M, Takata H, Ikeda Y, et al. A promoter polymorphism of the α2-HS glycoprotein gene is associated with its transcriptional activity. Diabetes Research and Clinical Practice. 2008;79:164–170. doi: 10.1016/j.diabres.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Fried LP, Borhani NO, Enright P, et al. The cardiovascular health study: Design and rationale. Annals of Epidemiology. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 20.Musunuru K, Lettre G, Young T, et al. Candidate Gene Association Resource (CARe): Design, Methods, and Proof of Concept. Circulation: Cardiovascular Genetics. 2010;3:267–275. doi: 10.1161/CIRCGENETICS.109.882696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keating BJ, Tischfield S, Murray SS, et al. Concept, design and implementation of a cardiovascular gene-centric 50 k SNP array for large-scale genomic association studies. PloS one. 2008;3:1. doi: 10.1371/journal.pone.0003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen MK, Bartz TM, Djousse L, et al. Genetically elevated fetuin-A levels, fasting glucose levels, and risk of type 2 diabetes: the cardiovascular health study. Diabetes Care. 2013;36:3121–3127. doi: 10.2337/dc12-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events: The Cardiovascular Health Study. Annals of Epidemiology. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 24.Price TR, Psaty B, O’Leary D, et al. Assessment of cerebrovascular disease in the cardiovascular health study. Annals of Epidemiology. 1993;3:504–507. doi: 10.1016/1047-2797(93)90105-d. [DOI] [PubMed] [Google Scholar]
- 25.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Annals of Epidemiology. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 26.O’Leary DH, Polak JF, Wolfson SK, et al. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1991;22:1155–1163. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 27.O’Leary DH, Polak JF, Kronmal RA, et al. Thickening of the Carotid Wall: A Marker for Atherosclerosis in the Elderly? Stroke. 1996;27:224–231. doi: 10.1161/01.str.27.2.224. [DOI] [PubMed] [Google Scholar]
- 28.Newman AB, Siscovick DS, Manolio TA, et al. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993;88:837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 29.Taylor HL, Jacobs DR, Jr, Schucker B, et al. A questionnaire for the assessment of leisure time physical activities. Journal of Chronic Diseases. 1978;31:741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 30.Cushman M, Cornell ES, Howard PR, et al. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clinical Chemistry. 1995;41:264–270. [PubMed] [Google Scholar]
- 31.Jensen MK, Pers TH, Dworzynski P, et al. Protein interaction-based genome-wide analysis of incident coronary heart disease. Circ Cardiovasc Genet. 2011;4:549–556. doi: 10.1161/CIRCGENETICS.111.960393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 33.Ding EL, Song Y, Manson JE, et al. Sex Hormone-Binding Globulin and Risk of Type 2 Diabetes in Women and Men. New England Journal of Medicine. 2009;361:1152–1163. doi: 10.1056/NEJMoa0804381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stock JH, Wright JH, Yogo M. A Survey of Weak Instruments and Weak Identification in Generalized Method of Moments. Journal of Business & Economic Statistics. 2002;20:518–529. [Google Scholar]
- 35.Santos-Gallego CG, Picatoste B, Badimón JJ. Pathophysiology of acute coronary syndrome. Current atherosclerosis reports. 2014;16:1–9. doi: 10.1007/s11883-014-0401-9. [DOI] [PubMed] [Google Scholar]
- 36.Mori K, Emoto M, Inaba M. Fetuin-A and the cardiovascular system. Advances in clinical chemistry. 2012;56:176. doi: 10.1016/b978-0-12-394317-0.00010-8. [DOI] [PubMed] [Google Scholar]
- 37.Singh M, Sharma PK, Garg VK, et al. Role of fetuin-A in atherosclerosis associated with diabetic patients. Journal of Pharmacy and Pharmacology. 2012;64:1703–1708. doi: 10.1111/j.2042-7158.2012.01561.x. [DOI] [PubMed] [Google Scholar]
- 38.Mori K, Emoto M, Araki T, et al. Effects of pioglitazone on serum fetuin-A levels in patients with type 2 diabetes mellitus. Metabolism. 2008;57:1248–1252. doi: 10.1016/j.metabol.2008.04.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.