Abstract
Recent clinical trials have demonstrated that pre-exposure prophylaxis (PrEP) may prevent HIV infection in a significant number of HIV-1 negative individuals in venerable populations; however, trial efficacy has been highly variable, with notable successes and failures. Poor adherence to PrEP regimens has been implicated as a primary factor in determining efficacy of these trials. With the exception of CAPRISA 004 where use of a pericoital tenofovir gel led to a 39% reduction in HIV infection, all successful PrEP regimens to date have used the disoproxil fumarate ester prodrug of tenofovir (TDF) alone or in combination with emtricitabine (FTC). A sustained-release, intravaginal ring (IVR) formulation of TDF holds promise for improving adherence and, thus, increasing the effectiveness of PrEP. Here, a novel IVR delivering TDF with sustained zero-order release characteristics that may be controlled over nearly two orders of magnitude is described. Pod-IVRs containing 1-10 pods delivering TDF at 0.01 – 10 mg d−1 were fabricated and their release characteristics evaluated in vitro. The pod-IVRs stabilized TDF against hydrolytic degradation both in storage and during in vitro release experiments. Successful translation of the TDF pod-IVR from laboratory evaluation to large-scale clinical trials requires the ability to manufacture the devices at low cost and in high quantity. Methods for manufacturing and scale-up were developed and applied to pilot-scale production of TDF pod-IVRs that maintained the IVR’s release characteristics while significantly decreasing the variability in release rate observed between pod-IVRs. This pod-IVR enables for the first time the dose-ranging clinical studies that are required to optimize topical TDF PrEP in terms of efficacy and safety.
Keywords: Pre-exposure prophylaxis, intravaginal ring, sustained release, HIV/AIDS, tenofovir, antiretroviral
1. Introduction
The global human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS) epidemic is now in its fourth decade, with over 6300 new HIV infections occurring daily (Hecht et al., 2010; Shattock et al., 2011; UNAIDS, 2013). In the absence of an effective vaccine, pre-exposure prophylaxis (PrEP) is a promising strategy for preventing HIV transmission. Tenofovir disoproxil fumarate (TDF) is a prodrug of the nucleoside analog reverse transcriptase inhibitor (NRTI) tenofovir (TFV), developed to improve bioavailability following oral dosing. Four recent clinical trials have demonstrated that PrEP regimens based on TDF or TFV, alone or in combination with the NRTI emtricitabine (FTC), can be effective in preventing of HIV infection in a significant proportion of individuals. The relative risk reduction, however, varied from 39-75% among studies (Baeten et al., 2012; Grant et al., 2010; Karim et al., 2010; Thigpen et al., 2012). In contrast, trials where women used a once daily vaginal TFV gel or daily oral TDF or TDF-FTC pill, PrEP did not show efficacy in preventing HIV acquisition (MTN, 2013; Van Damme et al., 2012). Currently, all HIV PrEP regimens with demonstrated clinical efficacy include TFV or TDF, and daily oral TDF in combination with FTC was approved in 2012 by the U.S. Food and Drug Administration to reduce the risk of HIV infection in uninfected individuals who are at high risk for HIV infection.
A critical factor driving success or failure in these clinical trials was adherence to frequent dosing: study participants who followed the prescribed antiretroviral (ARV) dosing regimens were significantly protected from HIV infection compared to those who did not (Amico et al., 2013). These results underscore the need for successful non-vaccine prevention methods against HIV infection that not only exhibit high pharmacologic efficacy, but also encourage higher compliance. It is well established across different delivery methods that adherence to therapy is inversely related to dosing period (Bhanji et al., 2004; Haycox, 2005; Kruse et al., 1991; Kutilek et al., 2003; Quraishi and David, 2000; Sershen and West, 2002; Small and Dubois, 2007; Yeaw et al., 2009), suggesting that sustained-release delivery methods can improve the poor adherence observed in many of the previous trials. Topical delivery of ARV drugs using intravaginal rings (IVRs) has the potential to provide the sustained mucosal levels required for protection against HIV infection (Malcolm et al., 2010). and IVR methods are believed to improve adherence compared to coitally dependent and daily dosing methods (Montgomery et al., 2012).
We demonstrated in an in vivo sheep model that IVRs delivering the prodrug TDF topically to the vagina resulted in significantly higher drug levels (86× higher) in vaginal tissues than IVRs delivering an equivalent vaginal fluid concentration of TFV, suggesting that local delivery of TDF to the vagina will more effectively prevent HIV infection (Moss et al., 2012). Subsequently, Smith et al. reported an IVR releasing 0.4 – 4 mg d−1 TDF that provided complete protection of pigtail macaques against multiple simian-HIV (SHIV) challenges in a low-dose model (Smith et al., 2013). Although these studies demonstrate proof of concept that IVRs delivering TDF may be an effective approach for preventing sexual vaginal HIV infection, multiple technologies will likely be required to advance candidate products through the development pipeline. Successful IVR platforms must meet a number of important characteristics, including: consistent daily release at a target rate, the ability to control and modify the release rate simply and precisely, and the capability to be rapidly transitioned to scalable production in preparation for large-scale clinical trials. Here we describe the in vitro characterization and pilot-scale manufacturing of an IVR platform that meets these important criteria.
2. Material and Methods
2.1. Material
Tenofovir disoproxil fumarate (TDF) was kindly provided by Gilead Sciences, Inc. (Foster City, CA). Liquid silicone resin (LSR, MED-4940 and MED-4840) and silicone adhesive (MED3-4213) was obtained from Nusil, Inc. (Carpenteria, CA). Polyvinyl alcohol, USP (PVA, viscosity = 23.6 mPa s, 85-89% hydrolyzed) was obtained from Spectrum Chemical (Gardena, CA). D,L-Polylactic acid (PLA, Resomer R 202 S) was obtained from Evonik Industries AG (Darmstadt, Germany). All other chemicals were NF grade or equivalent and used as received.
2.2. Manufacture of silicone pod-intravaginal rings
Intravaginal rings of the pod-IVR design containing TDF were prepared using methods previously reported in detail (Baum et al., 2012). Briefly, cylindrical cores (3.2 mm diam.) of 20-40 mg TDF admixed with 0.5% magnesium stearate were formed using compaction with a pellet press (Globe Pharma MTCM-I, North Brunswick, NJ). The compressed TDF cores were coated with either PVA or PLA. Polymers were applied by drop coating from a 5% (w/v) aqueous PVA solution or 5% (w/v) PLA in 2:1 dichloromethane:ethyl acetate (v/v). A 6 μL aliquot of polymer solution was applied to one flat side of the core and allowed to dry. The core was inverted and a second 6 μL aliquot applied. After drying for ~4h, a second layer was applied using the same technique. As described previously (Baum et al., 2012), the polymer-coated TDF pods were embedded in silicone rings or ring segments fabricated by injection molding from LSR, with one to three delivery channels per pod (channel diameter 0.75 – 2.0 mm) formed by mechanical punching.
2.3. Pod-IVR production scale-up
For TDF core manufacture on commercial tableting equipment, a formulation consisting of 69.3% (w/w) TDF, 23.7% microcrystalline cellulose (Ceolus KG-1000, Asahi Kasei Pharma Corp., Tokyo, Japan), 3.0% cellulose ether (Methocel E5 Premium LV, Dow Chemical, Midland, MI), and 4.0% sodium stearyl fumarate (Pruv, JRS Pharma, Patterson, NY) was used. The TDF, Ceolus KG-1000, and Methocel were wet-granulated in a 4 L high shear granulator bowl using 40 g water per 100 g solids. The granulate was dried in a fluid bed dryer with inlet air at 50° C and the dried granulate milled in a Model 197 Quadro Comil (Quadro Engineering, Waterloo, ON, Canada) using a 0.055 in. round-hole screen and #1601 impeller. The milled granulate was blended with the sodium stearyl fumarate lubricant in a V-shell blender and compressed into solid cores (3.2 mm diam. × 4 mm ht.) using a FlexiTab single-station press (Robert Bosch GmbH Packaging Technology, Waiblingen, Germany) with custom 12-tip tooling. Cores were coated with PVA in a fluid-bed spray coating system (MFL.01 Micro Fluid Bed, Freund-Vector, Marion IA) from a 2% (w/v) solution of PVA in 1:3 (v/v) isopropanol:deionized water solution with fluidizing air at 25 L min−1 and 100°C. The PVA solution was applied at 0.5 mL PVA solution per 1 g cores using a 0.5 s spray cycle with a 2:3 on:off ratio. Silicone IVR scaffolds were manufactured by Specialty Silicone Fabricators (Paso Robles, CA) using a custom, production quality four-cavity mold. IVR scaffolds were of identical dimensions those described previously (56 mm O.D., 40 mm I.D., 8.0 mm cross-sectional diameter) (Baum et al., 2012) and contained four empty pod cavities (3.1 mm diameter, 6.5 mm depth) with delivery channels formed during the molding process (1.0 mm diameter, 1.2 mm length). Pod-IVRs using these production-quality pods and IVR scaffolds were assembled using an identical method (Baum et al., 2012) to that used for the ring segments described above.
2.4. In vitro studies
Studies to measure in vitro release of TDF into a simplified vaginal fluid simulant (VFS) were carried out on IVRs containing either one PLA-coated TDF pod or four PVA coated TDF pods. The VFS was adapted from Owen and Katz (Owen and Katz, 1999) and consisted of 25 mM acetate buffer (pH 4.2) with NaCl added to yield a 200 mOs solution. For in vitro release studies, the IVRs were placed in glass jars containing 100 mL VFS at 25 ± 2°C with shaking at 60 rpm on an orbital shaker. For in vitro release from the ten-pod IVR (~10 mg d−1), 700 mL deionized water was used as the release medium. Aliquots of the release medium were removed at specified time intervals and were replaced with an equal volume of fresh dissolution media, with compensation for dilution in the concentration determinations. The concentration of TDF in the aliquots was measured using UV absorption spectroscopy (λmax = 260 nm,) with a UV-2401PC dual-beam spectrophotometer (Shimadzu, Columbia, MD). Complete media changes were carried out as needed to maintain sink conditions.
2.5. Hydrolytic stability of TDF in pod-IVRs
Drug pods were extracted from IVRs by cutting away excess silicone between pods to isolate a single pod taking care not to cut into the pod itself, excising the pod from the silicone, and placing the pod and surrounding silicone in a vial containing 10 mL methanol. The vial was sonicated 5 min and placed on an orbital shaker for 1 h. A 25 μL aliquot of this solution was diluted to 1 mL with deionized water containing 0.1% formic acid. The concentration of TDF and TFV in the diluted solution was determined using an Agilent 1100 Series HPLC with diode-array (DAD) and single-quadrupole mass-spectrometric (MSD) detection. (Agilent Technologies, Santa Clara, CA). Separation was carried out on a Phenomenex (Torrance, CA) Kinetex XB-C18 column (2.1 × 100 mm; 2.6 μm) column using a gradient elution [A: 0.1% formic acid in water; B: acetonitrile; 0.2 mL min−1; 2 min at 5% B, gradient to 80% B over 4 min, 8 min at 65% B]. The retention times were 10.05 min for TFV disoproxil [bis(POC)TFV], 9.15 min for TFV isoproxil [mono(POC)TFV], and 1.65 min for TFV. The MSD used electrospray ionization (API- ES+) with simultaneous scanning and single-ion mode (SIM) detection of the following ions: [bis(POC)TFV], m/z 520; [mono(POC)TFV], m/z 404; TFV, m/z 288. For analysis of TDF hydrolysis in solution, 25 μL of a 2.25 μg mL−1 solution of TDF in methanol was added to 1.0 mL of a 25 mM pH 10 phosphate buffer. The stepwise loss of isoproxil groups was quantified as a function of time using the gradient HPLC method described above.
3. Results
3.1. Pod-IVR design and in vitro release
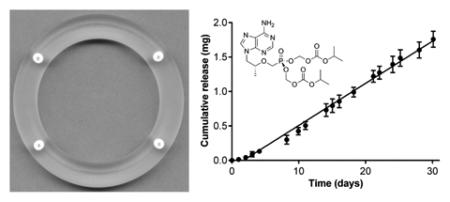
Pod-IVRs containing TDF were fabricated in the configurations indicated in Table 1. A photograph of a pod-IVR containing four TDF pods is shown in Fig. 1A and a cross-sectional drawing of the pod-IVR showing the pod and delivery channel location(s) is presented in Fig. 1B. The pod-IVRs exhibited sustained release of TDF for up to 30 days in vitro, with linear, zero order kinetics as demonstrated in Fig. 2a for a typical single-pod IVR. Release rate was controlled by a combination of the polymer coating encapsulating the drug core and by the size and number of delivery channels exposing the pod surface to the release fluid as shown in Table 1 and Fig. 3. Release rates of 0.014 – 0.20 mg d−1 pod−1 were obtained for PLA-coated, single-pod IVRs by varying the delivery channel diameter from 0.5 – 1.5 mm. A release rate range of 0.54 – 3.24 mg d−1 pod−1 (2.2 – 13.0 mg d−1 IVR−1) was obtained for PVA-coated pod-IVRs by varying both the delivery channel diameter (1.5 or 2.0 mm) and the number of channels per pod (1-3). For macaque-sized IVRs with four PLA-coated pods, a per-pod release rate was calculated by dividing the total TDF release rate for the pod-IVR by the number of pods. Fig. 2b illustrates a high TDF dose from the upper end of the accessible release rate range. Sustained release into deionized water over 28 days from a pod-IVR with ten PVA-coated TDF pods and three 1.5 mm diameter delivery channels per pod was maintained at a mean rate of 10.1 ± 0.3 mg d−1 delivery for the first 20 days and 9.0 ± 0.3 mg d−1 delivery over the 28 days (N=4). The deviation from linearity in the release profile occurs in pod-IVRs with high release rates as the amount of TDF released from each pod approaches the total pod load (~24 mg pod−1 in this configuration). During in vitro dissolution studies, erosion of the TDF drug core at the location of the delivery channel(s) could be observed. In the case of high-releasing pod configurations such as that shown in Fig. 2b, a majority of the TDF was released and the pod cavity filled with dissolution medium. Following removal of pod-IVRs from the dissolution medium, the PLA membranes could be observed as an intact “balloon” surrounding the remaining solid TDF inside the pod cavity. Polymer membranes of PVA were less intact less intact, but not dissolved completely, forming a hydrogel-like layer between the TDF and delivery channel.
Table 1.
In vitro release rate as a function of pod-IVR delivery channel configuration for a series of IVRs formulated using identical cores of 99.5% TDF and 0.5% magnesium stearate.
| Delivery Channel Size | Release Rate | |||||
|---|---|---|---|---|---|---|
| Polymer | Channels per pod |
Pods per ring |
Diameter (d) (mm) |
Area (A)a
(mm2) |
Total (mg d−1) |
per podb
(mg d−1) |
| PLA | 1 | 1 | 0.50 | 0.20 | 0.014 | 0.014 |
| PLA | 1 | 1 | 0.75 | 0.44 | 0.045 | 0.045 |
| PLA | 1 | 1 | 1.00 | 0.79 | 0.11 | 0.11 |
| PLA | 1 | 1 | 1.20 | 1.13 | 0.16 | 0.16 |
| PLA | 1 | 1 | 1.50 | 1.77 | 0.20 | 0.20 |
| PVA | 1 | 4 | 1.50 | 1.77 | 2.15 | 0.54 |
| PVA | 2 | 4 | 1.50 | 3.53 | 6.21 | 1.55 |
| PVA | 1 | 4 | 2.00 | 3.14 | 6.86 | 1.72 |
| PVA | 3 | 4 | 1.50 | 5.30 | 13.0 | 3.24 |
Calculated as A = π·(d/2)2
Calculated as total release rate divided by number of pods per ring.
Figure 1.
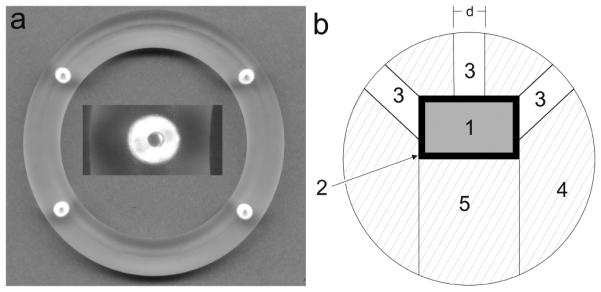
(A) Photograph of pod-IVR containing four pods of TDF and one 1 mm diameter delivery channel per pod. Inset shows a close-up of the embedded TDF pod and delivery channel formed during the ring scaffold injection molding process. (B) Cross-sectional diagram of pod-IVR sectioned through the pod showing TDF pod (1) with PVA or PLA coating (2) inserted into a pod cavity in the ring scaffold (4) and sealed in the ring by backfilling the pod cavity with silicone (5). Three delivery channels of diameter d (3) expose the TDF pod to vaginal fluids.
Figure 2.
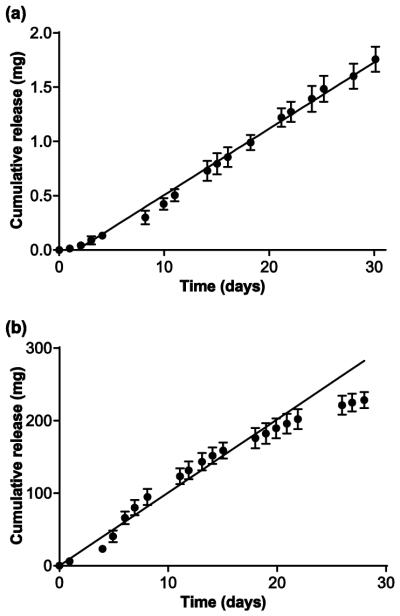
In vitro release of TDF from pod-IVRs in low and high release configurations. Data points are means and error bars represent standard deviation. (a) Low-releasing (61 ± 0.8 μg d−1) single-pod IVR (N=6). (b) High-releasing 10-pod IVR (10.1 ± 0.3 mg d−1). Average daily release rates were determined by linear regression of cumulative release data as indicated by the slope of the lines shown on each plot. For the high release configuration shown in 2b, release deviated from zero-order at later times as described in the text and the regression included only data from days 1-20.
Figure 3.

Plot of in vitro release rate versus delivery channel cross-sectional area [A = π·(d/2)2, where d is the delivery channel diameter] for single-pod IVRs with PLA-coated (open symbols) and PVA-coated (filled symbols) TDF pods. A single delivery channel per pod is denoted by circles and multiple delivery channels per pod is denoted by squares. Slopes were obtained by linear regression.
3.2. API formulation stability
The prodrug TDF is formulated as a fumarate salt of the phosphonate diester [bis(POC)TFV] of tenofovir. Stability of bis(POC)TFV toward hydrolysis of the methylene isopropyl carbonate protecting groups during pod-IVR fabrication and storage was measured by HPLC analysis. The extent of hydrolysis was characterized by the mole fraction of [bis(POC)TFV], [mono(POC)TFV], and [TFV] in the mixture. Recovered drug from pod-IVRs immediately following the fabrication process showed a large peak for bis(POC)TFV (tR = 10.05 min), no mono(POC)TFV (tR = 9.15 min), and trace TFV (tR = 1.65 min) corresponding to 0.17% hydrolysis of bis(POC)TFV. The TDF supplied by Gilead Sciences was equivalent [99.8% bis(POC)TFV, 0% mono(POC)TFV, and 0.16% TFV]. Pod-IVRs packaged in autoclave pouches (Duo-Check, Crosstex, Hauppauge, NY; i.e., not water-tight packaging) protected TDF from hydrolysis under accelerated stability conditions. Following 26 months storage at 40°C ± 2°C and 75% ± 5% RH, pods excised from rings contained >96% bis(POC)TFV, with 2.4-3.6% hydrolyzed to TFV. No mono(POC)TFV was observed. For TDF powder stored in a screw-cap vial under the same conditions, only 4% of the bis(POC)TFV remained after 26 months. For the pilot scale TDF formulation containing excipients, no degradation was observed during granulation, tableting, or coating steps, and >99.5% of the initial bis(POC)TFV was recovered following 8 months storage at 25°C ± 2°C. For TDF pods excised from pod-IVRs at the end of a 28 day in vitro release study in pH 4.2 VFS, <0.5% of the residual drug was hydrolyzed to TFV. Hydrolytic stability of TDF in pod-IVRs was also maintained in vivo in both macaques [>98% bis(POC)TFV, 14 days] and sheep [>90% bis(POC)TFV, 28 days].
3.3 Pilot-scale pod-IVR manufacture and evaluation
A production run of 5000 TDF cores (i.e., uncoated pods) utilized wet granulation and milling followed by drying in a fluid bed to obtain a solid formulation suitable for compression on a tablet press using multi-tip tooling. The cores then were PVA-coated in a fluidized bed system to afford 5000 cylindrical pods of consistent size (3.2 mm diameter × 4 mm height). The mean pod mass for the batch was 45.9 ± 2.0 mg (min 42.5, max 48.8 mg, N=20 pod random sample).
The impact of manufacturing method development and scale-up in TDF in vitro release characteristics was investigated. Pod-IVRs were fabricated using three methods: (1) IVRs using pods produced using a manual tablet press and dip-coating with PVA with delivery channels punched after ring molding; (2) IVRs using pods from the 5000 pod production run with delivery channels punched after ring molding; and (3) IVRs from the 100 ring production build (production pods and molded delivery channels). For all three pod-IVR groups, TDF formulations consisted of 64.5% TDF with excipients as described above, and all IVR configurations were four pods with one 1 mm diameter delivery channel per pod. The in vitro release of TDF into VFS over 17 days for the three pod-IVR groups is shown in Fig. 4. The release rates were 0.121 ± 0.002 mg d−1 (N=5) for production-build pod-IVRs with molded delivery channels and pilot-scale pods, 0.104 ± 0.015 mg d−1 (N=5) for pod-IVRs with pilot-scale pods and punched delivery channels, and 0.125 ± 0.027 mg d−1 (N=5) for pod-IVRs with manually fabricated pods and punched delivery channels. The magnitude of the release rates for the three groups are indistinguishable (P = 0.084, Kruskal-Wallis test); however, the variability in release rate for IVRs within each group as determined by the standard deviation decreases with increasing application of the manufacturing methods described above. The release rate of TDF from single-pod IVRs fabricated using the production pods varies linearly with the total cross-sectional area of the delivery channels over the range 0.1 - 1.69 mg d−1 pod−1 as shown in Fig. 5 and Table 2.
Figure 4.
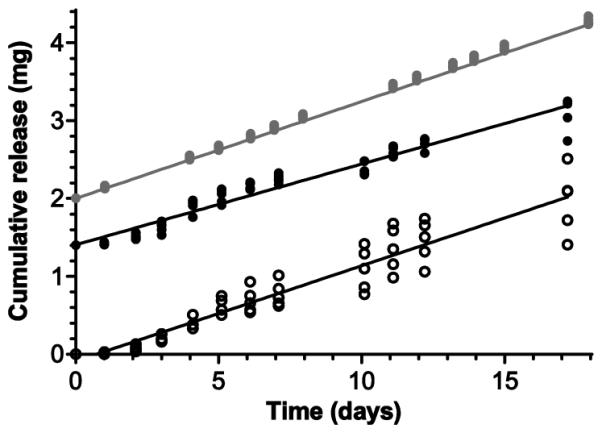
Cumulative release curves for three groups of identical TDF pod-IVRs fabricated at different stages of manufacturing development. The mean (N=5 per group) in vitro release rates were indistinguishable between groups (P = 0.084, Kruskal-Wallis test) while the intra-group release rate variability decreased with increasing manufacturing development. Symbols represent data for each individual pod-IVR and lines represent the mean release for each of the three groups: (a) manually fabricated pods and punched delivery channels (125 ± 27 μg d−1, open circles), (b) pilot-scale pods and punched delivery channels (104 ± 15 μg d−1, black circles), (c) pilot-scale pods and molded delivery channels (121 ± 2 μg d−1, gray circles). Release rates were calculated from the slope of the cumulative release for each pod-IVR and are reported as mean ± standard deviation (N=5). For clarity, Y-axis values are offset by 1.4 mg for (b) and 2.0 mg for (c).
Figure 5.
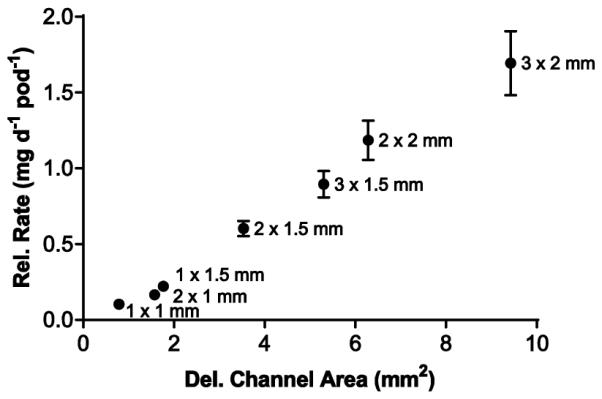
Plot of daily release rate versus delivery channel area for single-pod IVRs using PVA-coated TDF pods from the pilot-scale production lot (N=4 each delivery channel configuration).
Table 2.
In vitro release rate as a function of delivery channel area for single-pod IVRs using production-scale TDF pod formulation and coating, and delivery channels created by mechanical punching.
| Delivery Channel Size | Release Rateb | ||||
|---|---|---|---|---|---|
| Polymer | Channels per pod |
Diameter (d) (mm) |
Area (A)a
(mm2) |
Mean (mg d−1) |
Std. Dev. (mg d−1) |
| PVA | 1 | 1.00 | 0.79 | 0.10 | 0.01 |
| PVA | 2 | 1.00 | 1.57 | 0.17 | 0.03 |
| PVA | 1 | 1.50 | 1.77 | 0.22 | 0.02 |
| PVA | 2 | 1.50 | 3.53 | 0.61 | 0.05 |
| PVA | 3 | 1.50 | 5.30 | 0.90 | 0.09 |
| PVA | 2 | 2.00 | 6.28 | 1.19 | 0.13 |
| PVA | 3 | 2.00 | 9.42 | 1.69 | 0.21 |
Calculated as A = π·(d/2)2
N=4
4. Discussion
4.1. TDF for topical HIV PrEP
For TFV and its prodrug TDF, the target sustained release rates and resulting concentrations in the relevant compartments (vaginal fluids, tissues, blood, and associated CD4+ cells) that are clinically safe and effective in preventing HIV infection are currently not known. The site of action for both is believed to be the CD4+ cells in vaginal tissues (Hendrix et al., 2009), where diphosphorylation to the active moiety (TFV diphosphate, TFVpp) is followed by incorporation into viral cDNA and subsequent inhibition of viral reverse transcriptase (Anderson et al., 2011; Stein and Moore, 2001). Consequently, the concentration of TFV-DP in HIV target cells residing in the vaginal tissues should be the primary determinant of seroconversion outcomes in prophylaxis regimens based on TDF or TFV. The prodrug TDF has a number of advantages that make it a better candidate than TFV for topical administration to prevent HIV infection, but the hydrolytic instability of the prodrug has thus far limited the ability to formulate and clinically evaluate TDF in common topical dosage forms (i.e. gels, IVRs). First, orally delivered TDF is FDA approved for treatment of HIV infection and, in combination with the NRTI FTC, for prevention of infection in members of high-risk populations. Even though the approval is for oral and not topical administration, the safety profile established during the clinical trial process and subsequent clinical use significantly reduces the regulatory burden for TDF approval compared to the unapproved TFV. The prodrug TDF was developed to overcome the low oral bioavailability of TFV; however, recent research suggests that TDF may be superior to TFV for topical delivery as well. Because TFV exhibits a low intrinsic inhibitory potential (McMahon et al., 2009), it is thought that higher TFV concentrations in vaginal tissues will lead to greater protection from HIV infection in the absence of dose-related toxicity (Anderson et al., 2011). The use of TDF, which is hydrolyzed to TFV by esterases in vaginal tissues (Scheme 1), has been shown to increase cellular penetration, with concomitantly higher intracellular TFV-DP concentrations, as demonstrated in vitro (Durand-Gasselin et al., 2009; Naesens et al., 1998; Robbins et al., 1998) and in vivo following oral administration (Durand-Gasselin et al., 2009; Naesens et al., 1998). In an experiment specifically designed to compare the vaginal bioavailability of TDF and TFV in sheep, the prodrug led to significantly higher (86x) vaginal TFV tissue levels (Moss et al., 2012). The increased in vivo tissue penetration leads to TDF exhibiting greater HIV inhibition than TFV, as subsequently observed in cell culture and explant tissue-based ex vivo models (Mesquita et al., 2012).
Scheme 1.
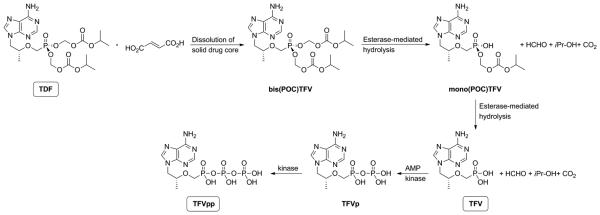
Hydrolysis and phosphorylation reactions of TDF following release from a pod-IVR in vivo. The solid pod-IVR core containing TDF, the fumarate salt of the tenofovir prodrug, dissolves in vaginal fluid and partitions into the vaginal lumen as bis(POC)TFV. The bis(POC)TFV in the lumen diffuses into vaginal tissue and accumulates, where it undergoes a two-step esterase-mediated hydrolysis to yield mono(POC)TFV and TFV. TFV is subsequently phosphorylated twice in kinase-mediated steps to form TFVp and TFVpp. In vitro, stepwise hydrolysis of bis(POC)TFV to mono(POC)TFV and TFV may also occur. The hydrolysis rate is increased in alkaline solution.
4.2. Pharmacokinetics and pharmacodynamics following topical application
Although several clinical trials have demonstrated that PrEP regimens based on TDF can prevent HIV infection, the drug concentrations in relevant compartments required for protection are still unknown. In these trials, vaginal tissue concentrations of TFV or TFV-DP in participants who remained uninfected were not measured directly; however, results of these and subsequent studies have provided insight into putative protective levels. Pharmacokinetic-pharmacodynamic (PK-PD) analysis of infection data from the CAPRISA 004 clinical trial of a topical 1% TFV gel indicate that TFV concentrations greater than 1000 ng mL−1 in cervicovaginal fluid were associated with significant protection from HIV infection. In a separate PK study, the measured median C24 values for TFV in vaginal tissues were 6-7 μg g-1 following single and multi-dose application of 1% TFV gel (Schwartz et al., 2011), and a subsequent bridging study of vaginally-applied 1% TFV gel measured median vaginal TFV levels of 113 μg g−1 (range 27-265 μg g−1) for samples collected at 2, 4, or 6 hours following gel application (Hendrix et al., 2013).
4.3. Delivery of TDF from IVRs
Three different IVR designs delivering TDF have been reported: a matrix IVR with TDF dispersed in a hydrophilic polyether urethane (PEU) elastomer (Mesquita et al., 2012), a reservoir IVR consisting of a hollow tube of hydrophilic PEU filled with a solid TDF formulation (Smith et al., 2013), and the pod-IVR (Moss et al., 2012) described here. There are a number of limitations associated with traditional matrix and reservoir IVR designs for delivering TDF and other relatively water soluble molecules. The hydrophobic nature of silicone and ethylene-co-vinyl acetate (EVA) elastomers used in all approved vaginal ring products prevents diffusion of TDF through the matrix. Consequently, novel hydrophilic elastomers are required for IVRs that utilize diffusion of TDF through the elastomer matrix. Drug release from matrix IVRs is not linear over time. There is typically a large initial burst release followed by a decrease in daily release rate over time. As a result, the dose changes throughout the period of use, making it difficult to maintain a target vaginal fluid or tissue concentration during PK-PD evaluations. A reservoir IVR consisting of a tubular silicone shell filled with one or more contraceptive hormones in silicone oil and/or other excipients was first described by de Leede (de Leede et al., 1986). A similar approach was applied to a TDF IVR, whereby a hydrophilic PEU shell was filled with a dry TDF formulation including an osmotic agent to draw vaginal fluid into the core and solubilize the drug (Smith et al., 2013). In pigtail macaques, these IVRs delivered TDF at release rates varying from 0.4 to 4 mg d−1 over 28 days, resulting in median concentrations of 7.2 × 104 ng mL−1 TDF in vaginal fluid and >2.9 × 103 ng mL−1 TFV in vaginal tissue, and providing complete protection of pigtail macaques from SHIV162p3 infection in a low-dose, repeat vaginal challenge model (Smith et al., 2013). The TDF release was highly variable over 28 days, with an in vitro release rate of 0.4-4 mg d−1 and cumulative release in vivo of 64±11 mg (high 90, low 44, N=36); however, the TVF levels observed in macaques over the first 15 days of release when the in vitro release rate varied from ca. 0.4 to 2.5 mg d−1 were all above concentrations that correlated with protection from HIV infection in women (Karim et al., 2011). The reproducible TFV concentrations in vaginal fluid and tissue observed despite large variations in release rate were attributed to either saturation in vivo or variation between in vivo and in vitro release. These interpretations are consistent with the four-compartment model proposed previously for vaginal TDF delivery (Moss et al., 2012), where TDF partitions from the IVR into the cervicovaginal lumen and diffuses into vaginal tissue where it accumulates, hydrolyzes to TFV, and forms a depot from which TFV diffuses back into the fluids of the vaginal lumen.
It is likely that studies to elucidate the PK-PD relationships for vaginally applied TDF and the subsequent clinical evaluation of TDF-based topical delivery devices will involve dose ranging studies and require the ability to titrate the vaginal TDF dose precisely over a large range to optimize for high mucosal TFV levels with minimal local toxicity. Unlike other IVR designs, the pod-IVR described here provides consistent daily release of TDF and the ability to vary the release rate in a controlled fashion over more than three orders of magnitude in a human-sized ring. The release rate of TDF from the pod-IVR is controlled by three independently variable parameters: the polymer membrane pod coating, the number and size of delivery channels per pod, and the number of pods per IVR. The pod’s polymer coating serves as the “coarse” rate control. Hydrophobic polymers decrease fluid penetration into the pod compared to hydrophilic polymers, slowing the drug dissolution and diffusive transport across the polymer membrane to the delivery channel. For TDF pod-IVRs, a hydrophobic PLA polymer selects a “low” release rate range and a hydrophilic PVA polymer selects a “high” range. The thickness of the membrane may provide additional control of the release properties. Specific release targets within the range determined by the polymer membrane are accessible by varying the number and size of the delivery channels for each pod, effectively determining the surface area of the pod that is exposed to the vaginal fluids. Human-sized pod-IVRs can accommodate up to ten pods per ring, with each pod/delivery channel combination acting an independent delivery device. The total pod-IVR dose scales linearly from the per pod release rate and the number of pods (Fig. 3) (Baum et al., 2012). This level of release rate control has not been achievable using other IVR designs.
Because delivery from each pod occurs simultaneously and is independent of the other pods in the ring, the total TDF loading per pod and the daily release rate determines the length of time release from the IVR may be sustained. In this study, each 3.2 mm diameter pod contained 20-40 mg TDF in the research IVRs and ca. 30 mg TDF in the pilot-scale production IVRs. A human-sized pod-IVR accommodates a maximum pod size of 4.8 mm diameter by 4 mm height. The packing density of a pod is dependent on the properties of the drug substance, any excipients in the formulation, and the tableting conditions. For TDF, scaling the pod mass from that obtained here for the 3.2 mm pods to a 4.8 mm diameter × 4 mm height pod results in 100 mg TDF per pod for pure TDF and 66 mg TDF per pod for the pilot-scale formulation with excipients; thus, a pod-IVR could sustain a maximum TDF release rate of ca. 20 mg d−1 (2 mg d−1 pod−1 for a 10 pod IVR) for 30 days, a much larger daily dose than would likely be evaluated in clinical trial. This is nearly an order of magnitude greater than the 2.3 mg d−1 mean dose that completely protected macaques from SHIV infection and provided vaginal fluid levels significantly higher than those correlated with protection in women receiving 1% TFV gel (Smith et al., 2013). At 5 mg d−1, a human-sized pod-IVR could deliver TDF for 90 days, or longer, thereby reducing the per day cost of the device significantly, an important consideration for resource-poor regions.
4.4. Formulation and stability in pod-IVRs
The hydrolytic instability of the two isopropoxycarbonyl-oxy-methyl moieties (Scheme 1) has precluded formulation of TDF in aqueous topical dosage forms such as gels, and complicates formulation in traditional IVR designs. In the pod-IVR, TDF is not dispersed in an elastomer matrix; rather, it is compressed into a core and coated with the release polymer prior to embedding in a pre-made, empty silicone ring. Recovered drug from pod-IVRs immediately after fabrication showed no measurable hydrolysis to either mono(POC)TFV or TFV. This observation is similar to the tablet formulation of the oral solid dosage form. The TDF pod-IVRs exhibit excellent good long-term stability: >99% of the initial bis(POC)TFV prodrug was recovered following 8 months storage at 25°C, and >96% remained in the bis(POC)TFV form following 28 months under accelerated stability conditions of 40°C and 75% RH. For the TDF formulation containing excipients used in the pilot scale pod-IVR production, no degradation was observed during granulation, tableting, or coating steps, and >99% of the initial TDF was recovered following 8 months storage at 25°C. In matrix IVRs with TDF dispersed in PEU elastomer, TDF was hydrolyzed to mono(POC)TFV (Mesquita et al., 2012). The addition of 0.5% w/w poly(acrylic acid) to the matrix formulation improved stability, resulting in 96.7%±3.9% remaining as bis(POC)TFV after 6 months under accelerated stability conditions. For reservoir IVRs with a dry, packed formulation of TDF and NaCl, no stability during long-term storage was reported (Smith et al., 2013).
Hydrolysis of TDF may be increased during in vitro and in vivo studies as the dry, solid drug core is wetted (Scheme 1). For pod-IVRs, negligible hydrolysis (<0.5%) of TDF was observed in the residual drug recovered from the device following a 28 day in vitro release study in VFS at pH ~ 4. For the PEU matrix rings described above, hydrolysis of TDF within the ring during in vitro release studies was significant: 13.6% of the bis(POC)TFV was converted to mono(POC)TFV in 10 days, and addition of poly(acrylic acid) to the formulation decreased the hydrolysis to 5.6% in 20 days (Mesquita et al., 2012). Hydrolysis of TDF prior to release from the device in vivo may dramatically impact the PK of TDF delivery and resulting PD outcomes. Pod-IVRs delivering the TDF prodrug resulted in 86-fold higher TFV vaginal tissue levels than those releasing TFV directly (Moss et al., 2012) due to the greater tissue penetration of bis(POC)TFV compared to free TVF. This advantage is only maintained if the drug remains as (un-hydrolyzed) bis(POC)TFV for the 30 days or more that the ring is wetted with vaginal fluid while being used. Pod-IVRs stabilize TDF in the ring toward hydrolysis in vivo, where enzymes in vaginal fluids may increase hydrolysis rates compared to in vitro conditions. Residual drug recovered from pod IVRs was >98% bis(POC)TFV following 14 days use in pigtail macaques (Moss et al., 2014) and >90% bis(POC)TFV following 28 days in sheep (Moss et al., 2012). This is highly significant because the vaginal pH of these species is 7.5-8.5 (sheep) (Vincent et al., 2009) and 5.5-8.5 (pig-tail macaques) (Cole et al., 2010), and alkaline relative to that of women (2.8-5.0) (O’Hanlon et al., 2013; Valore et al.), significantly increasing the rate of phosphonate ester hydrolysis of the prodrug (Arimilli et al., 1997). Smith et al. determined the amount of TDF released in vivo in macaques by measuring residual drug content in the IVRs following removal after 28 days, but did not report the extent of hydrolysis of the recovered drug. In their reservoir IVR design, hydrolysis of TDF to TFV in the IVR would result in delivery of TFV as well as TDF directly to the vaginal fluids and would decrease the observed tissue TFV concentration, as TFV uptake from vaginal fluids into tissue is nearly two orders of magnitude lower than TDF uptake followed by hydrolysis to TFV in the tissues. An increasing TFV:TDF ratio in the IVR reservoir over 28 days due to hydrolysis would explain their observation that TFV concentrations in vaginal tissue remained fairly constant throughout the study while the TDF release rate likely increased ten-fold.
4.5. Pod-IVR Manufacturability and Implications for Regulatory Approval
Successful development of IVRs for HIV PrEP will need to be safe, effective, well-tolerated, user-friendly, and affordable (Hendrix et al., 2009; Klasse et al., 2006). Several aspects of the TDF pod-IVR design facilitate overcoming the challenges of manufacturing the devices in suitable quantity and meeting the considerable regulatory requirements for product approval and eventual clinical application. The ability to cost-effectively produce pod-IVRs at clinical trial lot scale and beyond is enabled by the ring’s modular design. Manufacture is carried out in three distinct steps. (1) Empty elastomer rings are fabricated by a standard injection-molding process with no API present. (2) Drug cores containing TDF and excipients as binders, compression aids, and lubricants are prepared using standard tableting machinery and methods widely employed in pharmaceutical manufacturing to produce solid dosage forms. Cores are coated with a release-controlling polymer, using fluidized bed or pan-coating methods that are established methods for tablet coating. (3) Rings are assembled by punching appropriate delivery channels for each pod cavity (if channels are not formed during injection molding), placing pods, and backfilling with a room-temperature curing silicone to seal the pods in the ring. Assembly can be carried out manually for low production volumes, with automation of the punching, placement, and sealing operations integrated as needed to scale production capacity. A large number of contract manufacturing organizations (CMOs) are available for both pod fabrication and injection molding. The tableting and PVA coating described above were completed at one CMO, and the capacity for production of lots of >100,000 TDF pods under cGMP have been developed. Injection molding and assembly capacity have been developed with a second CMO. A production mold that is configurable with respect to number of pod cavities per IVR and with the capacity to mold in-place delivery channels of controlled size was designed and fabricated. Subsequently, a pilot-scale lot of 500 ring blanks with four pod cavities and a single 1 mm diameter delivery channel per cavity meeting all dimensional inspection criteria was produced. Manual assembly procedures for annual production volumes <5000 were developed and evaluated in an exploratory cGMP1 environment to produce a lot of 100 TDF pod-IVRs. A number of technologies common in medical device manufacture may be applied to the assembly step for scaling production capacity to enable Phase III clinical trials and eventual product rollout, including robotic pick-and-place assembly for pod insertion, automated fluid dispensing for backfilling pod cavities with silicone adhesive, automated packaging, and machine vision and inspection for assembly and packaging quality control. This approach was developed to provide flexibility to modify release rates and even drug choice with only minimal modification of the manufacturing processes. The only manufacturing step that is API dependent is core production by tableting, and the initial ring blank molding and all core coating and assembly steps are independent of the drug substance. Delivery rate is modified by changing the pod coating material or thickness and the size and/or number of delivery channels. The application of manufacturing methods described here to the fabrication of TDF pod-IVRs results in a dramatic improvement in the release rate variability within lots while maintaining identical release characteristics (dosing rates) across lots. As shown in Fig. 4, the daily release rate variability as measured by standard deviation of N=5 pod-IVRs decreases from 125 ± 27 μg d−1 to 104 ± 15 μg d−1 for manually compressed and PVA-coated pods compared to pods produced on pilot-scale equipment, and to 121 ± 2 μg d−1 (mean ± S.D.) when delivery channels formed by biopsy punch are replaced by channels molded in place during the injection molding step. The mean release rate for each set of 5 TDF pod-IVRs is indistinguishable between groups with p = 0.084 (Kruskal-Wallis test). The decrease in release rate variability observed may be attributed to two physicomechanical properties affected by the manufacturing scale-up: (1) an improvement in consistency of TDF core compression during tableting upon shifting from a dry blend process and manual tablet press to wet-granulation and multi-tip pilot-scale tablet press, and (2) the ability to fabricate delivery channels more reproducibly by molding in place during the injection molding process rather than mechanically punching after injection molding. The release rate range accessible for manufactured pod IVRs is 0.1 – 1.69 mg d−1 pod−1; thus, controlled delivery rate targets of 0.1 to 16.9 mg d−1 are achievable for IVRs with up to 10 pods per ring. This more than spans the delivery rate range needed for effective dose-ranging clinical studies of vaginal TDF delivery for HIV prophylaxis. Maintaining consistency in IVR release characteristics throughout the stages of manufacturing development and scale-up is important for ensuring that results from early, small-scale clinical trials may be compared directly to those from later Phase 3 studies, particularly regarding IVR PK and efficacy.
Unlike in matrix and reservoir IVR designs where drug release is controlled by diffusion of the drug substance through the elastomer material, the delivery characteristics of the pod-IVR are not dependent on the elastomer used to make the blank rings, and any biocompatible polymer can be used in its manufacture. This reduces cost and avoids setbacks due to possible material shortages in the marketplace, such as was encountered when Dow Corning discontinued five implant grade silicone products in the 1990s. The TDF pod-IVRs described here use an unrestricted medical-grade silicone thermoset elastomer, but could also be fabricated from thermoplastics such as poly(ethylene-co-vinyl acetate) (EVA) and polyether urethane (PEU), or other elastomers suitable for injection molding. Silicone and EVA are both widely used in FDA-approved vaginal ring products: EVA in the Nuvaring® contraceptive IVR and silicone in Estring® and Femring® estradiol IVRs for hormone replacement therapy. Release of TDF from matrix or reservoir IVR designs requires the use of hydrophilic PEU (Mesquita et al., 2012; Smith et al., 2013) or other hydrophilic elastomer materials that have not been used in FDA-approved delivery devices, and IVRs based on these polymers face a more difficult and expensive approval process without an established safety profile in other vaginal products. Because these hydrophilic elastomers are not widely used in medical device and drug delivery applications, they are available in limited supply and from a small number of sources compared to medical-grade silicone elastomers.
The success of IVR-delivered topical ARV agents for HIV and HSV prevention will depend on the ability to manufacture the devices in large quantities at a cost appropriate for the developing world. Estimates of manufacturing cost at several points during the scale-up process were developed with the CMOs. For pod production, the cost escalation as a function of batch size is minimal. For a single API, the cost increases <10% for a ten-fold increase in volume ($56,000 for 100,000 pods compared to $60,000 for 1,000,000 pods). For a pod-IVR with three TDF pods, this is $0.56 per IVR in 25,000 quantities and decreases to $0.06 per IVR in 250,000 quantities. For pod-IVR production (molding and assembly), the 100 ring exploratory cGMP build was completed at ~$20 per IVR. The estimated per IVR molding and assembly cost upon scale up are: $13-17 for quantities 500 - 5000; $6 - 8 for quantities 20,000 - 100,000; and $2.50 - 3.50 ($0.08 to $0.12 per day for a 30 day pod-IVR) for quantities 100,000 - 1,000,000. The initial cost reduction to $6-8 is driven primarily by an increase in the number of mold cavities and partial automation in pod dispensing during assembly. Additional reduction is made by sequential incorporation of improvements that are driven primarily by elimination of material handling by operators, including packaging. These costs are based on production at facilities in the USA and very much represent the upper limit of production costs on a worldwide basis.
5. Conclusions
Pod-IVRs delivering TDF were developed and evaluated in vitro. Linear TDF release at controlled, sustained delivery rates spanning three orders of magnitude (14 μg d−1 to 13 mg d−1) was obtained by simple modification of three ring parameters: size and number of delivery channels per TDF pod, number of pods per ring, and pod coating polymer. The pod-IVR stabilizes the TDF prodrug toward hydrolytic degradation, and significant hydrolysis to TFV during pod-IVR production, storage, or in vitro release studies was not observed. The pod formulation has been optimized for manufacture on commercial tableting and coating equipment, and the ability and capacity to scale pod-IVR production to volumes required for Phase 2 and Phase 3 clinical trials while maintaining identical in vitro release characteristics demonstrated. The TDF pod-IVR’s consistent daily release, capability for simple and precise modification of release rate, and the capacity for large-scale production are required for successful completion of the regulatory path to product approval and eventual clinical application.
Acknowledgements
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Numbers R33AI079791 and U19AI113048 (development of TDF pod-IVR), and R44AI081552 (manufacturing development and scale-up). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Bill Reid and Matthew Green at CoreRx Pharma (Tampa, FL) for carrying out manufacture of TDF pods at production scale in a pre-cGMP environment, and Don Hannula and Heather Wong of Specialty Silicone Fabricators (Paso Robles, CA) for development of the four-cavity production ring mold, production ring blank manufacture, and pre-cGMP pod-IVR assembly.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Chemical compounds studied in this article
Tenofovir disoproxil fumarate (PubChem CID: 6398764); tenofovir disoproxil (PubChem CID: 5481350); tenofovir monoisoproxil (PubChem CID: 56925685); tenofovir (PubChem CID: 464205)
The term “exploratory cGMP” refers to procedures carried out in a cGMP manufacturing environment using identical methods and controls as under cGMP, but without full validation. Exploratory cGMP is used to build the capacity for manufacture under cGMP.
REFERENCES
- Amico K, Mansoor L, Corneli A, Torjesen K, van der Straten A. Adherence support approaches in biomedical HIV prevention trials: experiences, insights and future directions from four multisite prevention trials. AIDS Behav. 2013;17:2143–2155. doi: 10.1007/s10461-013-0429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P, Kiser J, Gardner E, Rower J, Meditz A, Grant R. Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection. J. Antimicrob. Chemother. 2011;66:240–250. doi: 10.1093/jac/dkq447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimilli M, Kim C, Dougherty J, Mulato A, Oliyai R, Shaw J, Cundy K, Bischofberger N. Synthesis, in vitro biological evaluation and oral bioavailability of 9-[2-(phosphonomethoxy) propyl] adenine (PMPA) prodrugs. Antivir. Chem. Chemother. 1997;8:557–564. [Google Scholar]
- Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, Ronald A, Tumwesigye E, Were E, Fife KH, Kiarie J, Farquhar C, John-Stewart G, Kakia A, Odoyo J, Mucunguzi A, Nakku-Joloba E, Twesigye R, Ngure K, Apaka C, Tamooh H, Gabona F, Mujugira A, Panteleeff D, Thomas KK, Kidoguchi L, Krows M, Revall J, Morrison S, Haugen H, Emmanuel-Ogier M, Ondrejcek L, Coombs RW, Frenkel L, Hendrix C, Bumpus NN, Bangsberg D, Haberer JE, Stevens WS, Lingappa JR, Celum C. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. New Engl. J. Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum MM, Butkyavichene I, Gilman J, Kennedy S, Kopin E, Malone AM, Nguyen C, Smith TJ, Friend DR, Clark MR, Moss JA. An intravaginal ring for the simultaneous delivery of multiple drugs. J. Pharm. Sci. 2012;101:2833–2843. doi: 10.1002/jps.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanji N, Chouinard G, Margolese H. A review of compliance, depot intramuscular antipsychotics and the new long-acting injectable atypical antipsychotic risperidone in schizophrenia. Eur. Neuropsychopharmacol. 2004;14:87–92. doi: 10.1016/S0924-977X(03)00109-3. [DOI] [PubMed] [Google Scholar]
- Cole AM, Patton DL, Rohan LC, Cole AL, Cosgrove-Sweeney Y, Rogers NA, Ratner D, Sassi AB, Lackman-Smith C, Tarwater P, Ramratnam B, Ruchala P, Lehrer RI, Waring AJ, Gupta P. The formulated microbicide RC-101 was safe and antivirally active following intravaginal application in pigtailed macaques. PLoS ONE. 2010;5:e15111. doi: 10.1371/journal.pone.0015111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leede LGJ, Govers CPM, Denijs H. A multicompartment vaginal ring-system for independently adjustable release of contraceptive steroids. Contraception. 1986;34:589–602. doi: 10.1016/s0010-7824(86)80015-4. [DOI] [PubMed] [Google Scholar]
- Durand-Gasselin L, Rompay KKAV, Vela JE, Henne1 IN, Lee WA, Rhodes GR, Ray AS. Nucleotide analogue prodrug tenofovir disoproxil enhances lymphoid cell loading following oral administration in monkeys. Mol. Pharm. 2009;6:1145–1151. doi: 10.1021/mp900036s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapia M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernandez T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallas EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang FR, McConnell JJ, Zheng JH, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. New Engl. J. Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycox A. Pharmacoeconomics of long-acting risperidone: Results and validity of cost-effectiveness models. Pharmacoecon. 2005:23. doi: 10.2165/00019053-200523001-00002. [DOI] [PubMed] [Google Scholar]
- Hecht R, Stover J, Bollinger L, Muhib F, Case K, de Ferranti D. Financing of HIV/AIDS programme scale-up in low-income and middle-income countries, 2009-31. Lancet. 2010;376:1254–1260. doi: 10.1016/S0140-6736(10)61255-X. [DOI] [PubMed] [Google Scholar]
- Hendrix C, Cao Y, Fuchs E. Topical microbicides to prevent HIV: clinical drug development challenges. Annu. Rev. Pharmacol. Toxicol. 2009;49:349–375. doi: 10.1146/annurev.pharmtox.48.113006.094906. [DOI] [PubMed] [Google Scholar]
- Hendrix CW, Chen BA, Guddera V, Hoesley C, Justman J, Nakabiito C, Salata R, Soto-Torres L, Patterson K, Minnis AM, Gandham S, Gomez K, Richardson BA, Bumpus NN. MTN-001: Randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLoS ONE. 2013;8:e55013. doi: 10.1371/journal.pone.0055013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim QA, Karim SSA, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany ABM, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim S, Kashuba A, Werner L, Karim Q. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: Implications for HIV prevention in women. Lancet. 2011;378:279–281. doi: 10.1016/S0140-6736(11)60878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasse PJ, Shattock RJ, Moore JP. Which topical microbicides for blocking HIV-1 transmission will work in the real world? PLoS Med. 2006;3:e351. doi: 10.1371/journal.pmed.0030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse W, Eggertkruse W, Rampmaier J, Runnebaum B, Weber E. Dosage frequency and drug compliance behavior - a comparative study on compliance with a medication to be taken twice or 4 times daily. Eur. J. Clin. Pharmacol. 1991;41:589–592. doi: 10.1007/BF00314990. [DOI] [PubMed] [Google Scholar]
- Kutilek V, Sheeter D, Elder J, Torbett B. Is resistance futile? Curr. Drug Targets Infect. Disord. 2003;3:295–309. doi: 10.2174/1568005033481079. [DOI] [PubMed] [Google Scholar]
- Malcolm R, Edwards K, Kiser P, Romano J, Smith T. Advances in microbicide vaginal rings. Antivir. Res. 2010;88(Suppl. 1):S30–39. doi: 10.1016/j.antiviral.2010.09.003. [DOI] [PubMed] [Google Scholar]
- McMahon M, Shen L, Siliciano R. New approaches for quantitating the inhibition of HIV-1 replication by antiviral drugs in vitro and in vivo. Curr. Opin. Infect. Dis. 2009;22:574–582. doi: 10.1097/QCO.0b013e328332c54d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita PMM, Rastogi R, Segarra TJ, Teller RS, Torres NM, Huber AM, Kiser PF, Herold BC. Intravaginal ring delivery of tenofovir disoproxil fumarate for prevention of HIV and herpes simplex virus infection. J. Antimicrob. Chemothe. 2012;67:1730–1738. doi: 10.1093/jac/dks097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery E, van der Straten A, Cheng H, Wegne r.L., Masenga G, von Mollendorf C, Bekker L, Ganesh S, Young K, Romano J, Nel A, Woodsong C. Vaginal ring adherence in sub-Saharan Africa: expulsion, removal, and perfect use. AIDS Behav. 2012;16:1787–1798. doi: 10.1007/s10461-012-0248-4. [DOI] [PubMed] [Google Scholar]
- Moss JA, Baum MM, Malone AM, Kennedy S, Kopin E, Nguyen C, Gilman J, Butkyavichene I, Willis RA, Vincent KL, Motamedi M, Smith TJ. Tenofovir and tenofovir disoproxil fumarate pharmacokinetics from intravaginal rings. AIDS. 2012;26:707–710. doi: 10.1097/QAD.0b013e3283509abb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss JA, Srinivasan P, Smith TJ, Butkyavichene I, Lopez G, Brooks AA, Martin A, Dinh CT, Smith JM, Baum MM. Pharmacokinetics and preliminary safety study of pod-intravaginal rings delivering antiretroviral combinations for HIV prophylaxis in a macaque model. Antimicrob. Agents Chemother. 2014;58:5125–5135. doi: 10.1128/AAC.02871-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MTN . Daily HIV Prevention Approaches Didn’t Work for African Women in the VOICE Study. Microbicide Trials Network; Pittsburgh, PA: 2013. Website article: http://www.mtnstopshiv.org/node/4877 (Accessed Jun. 16, 2015) [Google Scholar]
- Naesens L, Bischofberger N, Augustijns P, Annaert P, Mooter G.V.d., Arimilli MN, Kim CU, Clercq ED. Antiretroviral efficacy and pharmacokinetics of oral bis(isopropyloxycarbonyloxymethyl)9-(2-phosphonylmethoxypropyl)adenine in mice. Antimicrob. Agents Chemother. 1998;42:1568–1573. doi: 10.1128/aac.42.7.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hanlon DE, Moench TR, Cone RA. Vaginal ph and microbicidal lactic acid when Lactobacilli dominate the microbiota. PLoS ONE. 2013;8:e80074. doi: 10.1371/journal.pone.0080074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999;59:91–95. doi: 10.1016/s0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- Quraishi S, David A. Depot haloperidol decanoate for schizophrenia. Cochrane DB Syst. Rev. 2000;2:CD001361. doi: 10.1002/14651858.CD001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins BL, Srinivas RV, Kim C, Bischofberger N, Fridland A. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob. Agents Chemother. 1998;42:612–617. doi: 10.1128/aac.42.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, Rountree W, Kashuba A, Brache V, Creinin M, Poindexter A, Kearney B. A multi-compartment, single and multiple dose pharmacokinetic study of the vaginal candidate microbicide 1% tenofovir gel. PLoS ONE. 2011;6:e25974. doi: 10.1371/journal.pone.0025974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sershen S, West J. Implantable polymeric systems for modulated drug delivery. Adv. Drug Deliv. Rev. 2002;54:1225–1235. doi: 10.1016/s0169-409x(02)00090-x. [DOI] [PubMed] [Google Scholar]
- Shattock RJ, Warren M, McCormack S, Hankins CA. Turning the tide against HIV. Science. 2011;333:42–43. doi: 10.1126/science.1206399. [DOI] [PubMed] [Google Scholar]
- Small G, Dubois B. A review of compliance to treatment in alzheimer's disease: Potential benefits of a transdermal patch. Curr. Med. Res. Opin. 2007;23:2705–2713. doi: 10.1185/030079907x233403. [DOI] [PubMed] [Google Scholar]
- Smith JM, Rastogi R, Teller RS, Srinivasan P, Mesquita PM, Nagaraja U, McNicholl JM, Hendry RM, Dinh CT, Martin A, Herold BC, Kiser PF. Intravaginal ring eluting tenofovir disoproxil fumarate completely protects macaques from multiple vaginal simian-HIV challenges. P. Natl. Acad. Sc.i USA. 2013;110:16145–16150. doi: 10.1073/pnas.1311355110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D, Moore K. Phosphorylation of nucleoside analog antiretrovirals: A review for clinicians. Pharmacother. 2001;21:11–34. doi: 10.1592/phco.21.1.11.34439. [DOI] [PubMed] [Google Scholar]
- Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, Henderson FL, Pathak SR, Soud FA, Chillag KL, Mutanhaurwa R, Chirwa LI, Kasonde M, Abebe D, Buliva E, Gvetadze RJ, Johnson S, Sukalac T, Thomas VT, Hart C, Johnson JA, Malotte CK, Hendrix CW, Brooks JT. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in botswana. New Engl. J. Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- UNAIDS Report on the Global AIDS Epidemic. Joint United Nations Programme on HIV/AIDS. 2013 http://www.unaids.org/en/resources/publications/2013 (Accessed June 16, 2015)
- Valore EV, Park CH, Igreti SL, Ganz T. Antimicrobial components of vaginal fluid. Am. J. Obstet. Gynecol. 2002;187:561–568. doi: 10.1067/mob.2002.125280. [DOI] [PubMed] [Google Scholar]
- Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, Malahleha M, Owino F, Manongi R, Onyango J, Monedi MC, Mak’Oketch P, Makanda M, Reblin I, Makatu SE, Saylor L, Kiernan H, Kirkendale S, Wong C, Grant R, Kashuba A, Nanda K, Mandala J, Fransen K, Deese J, Crucitti T, Mastro TD, Taylor D. Preexposure prophylaxis for HIV infection among african women. N. Engl. J. Med. 2012;367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent KL, Bourne N, Bell BA, Vargas G, Tan A, Cowan D, Stanberry LR, Rosenthal SL, Motamedi M. High resolution imaging of epithelial injury in the sheep cervicovaginal tract: A promising model for testing safety of candidate microbicides. Sex. Transm. Dis. 2009;36:312–318. doi: 10.1097/OLQ.0b013e31819496e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaw J, Benner J, Walt J, Sian S, Smith D. Comparing adherence and persistence across 6 chronic medication classes. J. Manag. Care Pharm. 2009;15:728–740. doi: 10.18553/jmcp.2009.15.9.728. [DOI] [PMC free article] [PubMed] [Google Scholar]


