Abstract
Naming and word-retrieval deficits, which are common characteristics of primary progressive aphasia (PPA), differentially affect production across word classes (e.g., nouns, verbs) in some patients. Individuals with the agrammatic variant (PPA-G) often show greater difficulty producing verbs whereas those with the semantic variant (PPA-S) show greater noun deficits and those with logopenic PPA (PPA-L) evince no clear-cut differences in production of the two word classes. To determine the source of these production patterns, the present study examined word-finding pauses as conditioned by lexical variables (i.e., word class, frequency, length) in narrative speech samples of individuals with PPA-S (n=12), PPA-G (n=12), PPA-L (n=11), and cognitively healthy controls (n=12). We also examined the relation between pause distribution and cortical atrophy (i.e., cortical thickness) in nine left hemisphere regions of interest (ROIs) linked to word production. Results showed higher overall pause rates for PPA compared to unimpaired controls; however, greater naming severity was not associated with increased pause rate. Across all groups, more pauses were produced before lower vs. higher frequency words, with no independent effects of word length after controlling for frequency. With regard to word class, the PPA-L group showed a higher rate of pauses prior to production of nouns compared to verbs, consistent with noun-retrieval deficits arising at the lemma level of word production. Those with PPA-G and PPA-S, like controls, produced similar pause rates across word classes; however, lexical simplification (i.e., production of higher-frequency and/or shorter words) was evident in the more-impaired word class: nouns for PPA-S and verbs for PPA-G. These patterns are consistent with conceptual and/or lemma-level impairments for PPA-S, predominantly affecting objects/nouns, and a lemma-level verb-retrieval deficit for PPA-G, with a concomitant impairment in phonological encoding and articulation affecting overall pause rates. The greater tendency to pause before nouns was correlated with atrophy in the left precentral gyrus, inferior frontal gyrus and inferior parietal lobule, whereas the greater tendency to pause before less frequent and longer words was associated with atrophy in left precentral and inferior parietal regions.
Keywords: primary progressive aphasia, narrative analysis, word retrieval deficits, word class effects, brain-behavior relationship
Introduction
Naming and word-finding deficits are commonly seen in patients with primary progressive aphasia (PPA), even though the nature of these and other deficits differs across PPA variants. Models of word production address multiple stages of naming, including conceptual preparation, lemma retrieval (i.e., access and selection of an abstract lexical unit specified for semantic and syntactic information), retrieval of the phonological word-form, phonological encoding (i.e., syllabification and segmentation), and motor speech planning and articulation (Caramazza, 1997; Dell & O'Seaghdha, 1992; Levelt, Roelofs & Meyer, 1999; Roelofs, 1992). Accordingly, naming impairments in PPA may reflect breakdown at one or more of these stages. For example, the semantic variant of PPA (PPA-S) has been linked to difficulty at the conceptual and/or lemma levels. Noun naming is more impaired than verb naming in PPA-S, stemming from impaired lexical-semantic representations (e.g., a blurring of semantic distinctions within taxonomic categories) as well as impaired mapping from the semantic features of objects to the retrieval of noun lemmas (Hillis et al., 2006; Hillis, Oh, & Ken, 2004; Hurley, Paller, Rogalski, & Mesulam, 2012; Mesulam et al., 2009a, 2013; Thompson et al., 2012a; Thompson, Lukic, King, Mesulam, & Weintraub, 2012c; Wilson et al., 2010). Noun naming deficits lead to semantically impoverished language, with production of primarily high-frequency, abstract, and/or vague words (Bird, Lambon Ralph, Patterson, & Hodges, 2000; Hoffman, Meteyard, & Patterson, 2014; Meteyard & Patterson, 2009; Wilson et al., 2010).
The source of naming deficits in the logopenic (PPA-L) and agrammatic (PPA-G) variants is less clear. Both subtypes have been associated with impairments at the phonological level, with some studies pointing to impaired phonological word-form retrieval in PPA-L and deficits in phonological encoding, as well as motor speech planning and articulation, in PPA-G (Ash et al., 2006; Ballard et al., 2014; Bird et al., 2000; Hoffman et al., 2014; Hurley et al., 2012; Mack et al., 2013a; Meteyard & Patterson, 2009; Mesulam et al., 2009a, 2013; Mesulam, Wieneke, Thompson, Rogalski, & Weintraub, 2012; Wilson et al., 2010). However, studies of online word production, showing abnormal semantic interference effects in individuals with PPA-L and PPA-G, suggest possible lemma-level deficits as well (Thompson et al., 2012b). In PPA-G, verbs are more impaired than nouns and these effects are more pronounced in confrontation naming (Hillis et al., 2006; Hillis, Oh, & Ken, 2004; Thompson et al., 2012c) than in narrative speech, although noun to verb (N:V) ratios are numerically (but not significantly) elevated compared to controls (Thompson et al., 2012a; Wilson et al., 2010). In addition, individuals with PPA-G exhibit more difficulty retrieving verbs with complex (two-argument) vs. simple (one-argument) lemmas, despite intact comprehension of both verb types (Thompson et al., 2012c), suggesting that verb production deficits may arise at the lemma level. Differences in verb production between naming and narrative tasks have not yet been explained. One possibility is that verb deficits in narratives are manifest in part through lexical simplification, e.g., selection of higher-frequency and/or phonologically less complex word forms to lessen retrieval demands, which may reduce the degree of impairment as measured by overall word class production patterns (i.e., N:V ratios).
In PPA-L, word class effects have not been found in naming (Thompson et al., 2012c), but trends towards impaired noun production have been noted in narrative speech (Thompson et al., 2012a; Wilson et al., 2010). One possible reason for this is that PPA-L may be associated with lemma-level noun retrieval deficits that emerge due to the demands of narrative speech, i.e., tracking a set of referents (e.g., Cinderella, prince, fairy godmother, glass slipper) and repeatedly selecting from this set throughout the construction of the narrative. Alternatively, trends towards reduced noun production in narrative speech may result from impaired phonological word-form retrieval (Mack et al., 2013a). Even in unimpaired speakers, nouns tend to be longer and of lower frequency than verbs in Cinderella story narratives (Bird and Franklin, 1996; MacWhinney, Fromm, Holland, Forbes, & Wright, 2010). Because word length and frequency may affect the ease of phonological word-form retrieval (Garrett, 1982; Jescheniak & Levelt, 1994; Wilson, Isenberg, & Hickok, 2009), these variables may particularly affect noun production in PPA-L.
One variable in spoken language that reflects word retrieval processes is the distribution of pauses. In unimpaired individuals, pauses have been shown to reflect word production difficulty both at the level of lemma access and selection (pauses tend to be produced before less semantically-predictable words; Beattie & Butterworth, 1979; Goldman-Eisler, 1958; Griffin & Bock, 1998) and at the level of phonological word-form retrieval and encoding (pauses tend to occur before less-frequent words; Beattie & Butterworth, 1979; Griffin & Bock, 1998; Jescheniak & Levelt, 1994). Similarly, aphasic individuals may produce pauses due to processing difficulty either at the lemma or phonological level (Garrett, 1982). In addition, pauses may result from deficits in motor speech planning and articulatory processes (Ballard et al., 2014; Duffy, 2006). Thus, the distribution of pauses can potentially inform accounts of word retrieval deficits in PPA across multiple levels of representation.
Pauses also are of interest due to their putative prevalence in PPA-L. Indeed, Mesulam and Weintraub (1992) initially defined “logopenic” speech as a paucity of output due to long word-finding pauses (p. 587), and more recently, we (Mesulam et al., 2012, p. 1550) noted that word-finding pauses are the “most conspicuous clinical feature” of PPA-L, given that grammar and word comprehension are generally intact in this subtype. Consistent with this observation, previous studies have found a high rate of pauses in PPA-L (Ash et al., 2013; Teichmann et al., 2013; Wilson et al., 2010). However, PPA-G has also been associated with abnormally frequent and/or long pauses (Ballard et al., 2014; Ash et al., 2013; Rohrer et al., 2010; Wilson et al., 2010). This raises the question of whether the distribution of pauses differs between the two subtypes, potentially reflecting distinct language processing impairments.
The present study examined the distribution of pauses across nouns and verbs produced in the narrative speech of individuals with PPA and age-matched cognitively healthy control speakers. Word class effects on pause distribution may reflect lemma-level retrieval deficits, whereas word frequency effects may relate to impaired phonological word-form retrieval (Jescheniak & Levelt, 1994) and word length effects may reflect impaired phonological word-form retrieval or subsequent phonological encoding and articulatory processes (Wilson et al., 2009), neither of which would affect production by word class. On the hypothesis that noun-retrieval deficits in PPA-S emerge at the lemma and/or conceptual levels (Hurley et al., 2012; Mesulam et al., 2009a, 2013), we predicted that individuals with PPA-S would pause more frequently before nouns compared to verbs. In contrast, given that verb production is more impaired than noun production in PPA-G (Thompson et al., 2012c), we expected more frequent pausing before verbs, reflecting lemma level impairment. However, if impaired phonological encoding and articulatory processes also are impaired in PPA-G (Ash et al., 2010; Mack et al., 2013a; Ballard et al., 2014), high pause rates overall, influencing both noun and verb production, were expected. For PPA-L, we tested two possible accounts: that word-retrieval impairments (1) are lemma-based (cf. Hurley et al., 2012; Thompson et al., 2012b) and would, therefore, result in increased pausing before nouns, and/or (2) result from impaired phonological word-form retrieval (Mack et al., 2013a), in which case increased pausing before low-frequency and longer words, with no independent effects of word class, was predicted.
The present study also aimed to identify the neural correlates of pauses in PPA. Only three studies, to our knowledge, have investigated this relationship, finding atrophy in the left superior temporal gyrus (STG) (Ash et al., 2013; Wilson et al., 2010), the inferior parietal region (Ash et al. 2013), and/or frontal regions (Ballard et al., 2014). Differences across studies may relate to pause coding procedures and the specific pause measures chosen (e.g., filled pauses vs. unfilled pauses, pause rate vs. pause duration, etc.). In addition, the neural substrates of pauses may differ depending on their source (e.g., lemma retrieval vs. phonological word-form retrieval vs. phonological encoding and articulation), which has not been addressed in previous studies. We examined cortical atrophy in nine left hemisphere ROIs that have been linked to fluency and word production in healthy individuals as well as those with stroke aphasia and PPA: the pars opercularis, pars triangularis, and pars orbitalis of the inferior frontal gyrus (IFG), the caudal middle frontal gyrus (cMFG), the precentral gyrus, the middle temporal gyrus (MTG), the superior temporal gyrus (STG), the inferior parietal cortex (IPC), and the supramarginal gyrus (SMG) (Ash et al., 2013; Gunwardena et al., 2010; Indefrey, 2011; Indefrey & Levelt, 2004; Mirman et al., 2015; Rogalski et al., 2011; Schnur, Schwartz, Brecher, & Hodgson, 2006; Schnur et al., 2009; Schwartz et al., 2009; Wilson et al., 2010; de Zubicaray, McMahon, Eastburn, & Wilson, 2002). We hypothesized that pause distribution by word class would predict atrophy in regions supporting lemma retrieval, such as the MTG (Indefrey, 2011; Indefrey & Levelt, 2004; Schwartz et al., 2009; de Zubicaray et al., 2002) and/or the IFG, which has been claimed to exert top-down control over lemma access and selection during word production (Schnur et al., 2006, 2009; Schwartz et al., 2009). However, we did not make specific predictions about the direction of word class effects due to mixed results in the neuroimaging literature (Crepaldi, Berlingeri, Paulesu, & Luzzatti, 2011; Vigliocco, Vinson, Druks, Barber, & Cappa, 2011). Pause distribution by frequency was expected to predict atrophy in temporoparietal regions that support phonological word-form retrieval (Abel et al., 2009; Indefrey, 2011; Indefrey & Levelt, 2004; Prabhakaran, Blumstein, Myers, Hutchison, & Britton, 2006; Wilson et al., 2009; de Zubicaray et al., 2002), whereas pause distribution by word length was expected to predict atrophy in these regions as well as regions that support phonological encoding and articulation, such as the precentral gyrus, IFG, and cMFG (Ballard et al., 2014; Graves, Grabowski, Mehta, & Gordon, 2007; Gunawardena et al., 2010; Mirman et al., 2015; Rogalski et al., 2011; Wilson et al., 2009; 2010).
Method
Participants
Participants in the experiment included three groups of patients with PPA and a group of 12 healthy controls matched for age and education (see Table 11. The PPA patients presented with progressive language deficits and no evidence of other neurological impairments. The PPA groups consisted of 12 patients with PPA-G, 11 with PPA-L, and 12 with PPA-S. The three patient groups did not differ in symptom duration (1.5 to 7 years). All participants were monolingual English speakers with no prior history of neurological or psychiatric disorders, and all but two were right handed. In order to meet the study's aim of investigating the distribution of pauses across word classes in narrative speech, only participants who produced at least 10 nouns and 10 verbs in their narrative samples were included. All participants were part of the PPA Research program at Northwestern University, tested in the Cognitive Neurology and Alzheimer's Disease Center (CNADC) (Chicago, IL) and the Aphasia and Neurolinguistics Research Laboratory at Northwestern University, Center for the Neurobiology of Language Recovery (CNLR) (Evanston, IL). Prior to the study, informed consent was obtained. The study was approved by the Institutional Review Board at Northwestern University.
Table 1.
Demographic and Neuropsychological Measures for Participants with PPA and Control Participants.
| PPA-G N = 12 |
PPA-L N = 11 |
PPA-S N = 12 |
Controls N = 12 |
Omnibus Significance |
|
|---|---|---|---|---|---|
| Demographic Variables | |||||
| Age | 62.33 (7.34) |
66.18 (6.24) |
60.58 (5.65) |
64.00 (6.76) |
n.s. |
| Gender | 5 Males 7 Females |
3 Males 8 Females |
7 Males 5 Females |
6 Males 6 Females |
|
| Education | 16.58 (2.47) |
16.64 (1.43) |
16.08 (3.15) |
16.00 (2.52) |
n.s. |
| Handedness | 12 Right | 10 Right 1 Left |
11 Right 1 Ambidextrous |
12 Right | |
| Symptom Duration | 3.83 (1.83) |
3.55 (1.98) |
3.79 (1.23) |
-- | n.s. |
| Non-language Neuropsychological Measures | |||||
| MMSE Total (max 30) |
26.83C (3.04) |
25.27C (4.03) |
25.17C (2.62) |
29.58 (0.67) |
*** |
| CDRSOB (max 18) |
1.08C (1.64) |
0.50 (0.63) |
1.73C (1.78) |
0.05 (0.16) |
** |
| Digit Span Forward (max 16) |
4.00C,S (2.04) |
4.64C,S (0.81) |
6.33 (1.30) |
7.25 (0.97) |
*** |
| Digit Span Backward (max 14) |
3.67C,S (1.61) |
3.73C,S (0.79) |
5.25 (0.87) |
5.42 (1.16) |
*** |
Values provided are means (standard deviations). Max = maximum score; MMSE = Mini Mental State Examination; CDR SOB = Clinical Dementia Rating Scale Sum of Boxes
= significantly impaired relative to control group (p < .05)
G = significantly impaired relative to PPA-G group (p < .05)
L = significantly impiared relative to PPA-L group (p < .05)
= significantly impaired relative to PPA-S group (p < .05); omnibus significance levels
* = p < .05
= p < .01
= p < .001, n.s. = not significant
A battery of non-language neuropsychological tests was administered (see Table 1 for a summary of scores), which included the Mini-Mental State Examination (MMSE: Folstein, Folstein, & McHugh, 1975), the Clinical Dementia Rating (CDR: Morris, 1993), and the Digit Span (forward and backward spans) subtest from the Wechsler Memory Scale-III (WMS-III: Wechsler, 1997). All PPA groups performed significantly lower than controls on the MMSE, likely reflecting compromised language abilities (see Golper, Rau, Erskine, Langhaus, & Houlihan, 1987; Osher, Wicklund, Rademaker, Johnson, & Weintraub, 2008). The CDR Sums of Boxes scores of PPA-G and PPA-S groups, indicative of the impact of cognitive deficits on daily living activities, were also significantly higher than controls. However, the mean total scores obtained by the PPA groups were in the very mildly impaired range. The PPA-G and PPA-L groups performed significantly worse than controls on the Digit Span Forward and Backward tests, possibly reflecting working memory deficits, although we point out that digit span scores also may be compromised by language impairments.
PPA subtyping was based on criteria presented in Mesulam et al. (2012). As demonstrated through performance on a language test battery (see Table 2 for a summary of scores), individuals with PPA-G exhibited grammatical deficits with relatively intact word comprehension, and those with PPA-S exhibited impaired word comprehension with relatively intact grammatical processing. Participants with PPA-L evinced impaired phrase and sentence repetition, with relatively intact word comprehension and grammar. Single word comprehension was measured using a 36-item subset of the Peabody Picture Vocabulary Test (PPVT, i.e. moderately difficult items, 157-192; Dunn & Dunn, 2007), and PPA-S participants performed significantly lower than all other groups. Grammatical sentence processing abilities were assessed with the Sentence Production Priming Test (SPPT) and Sentence Comprehension Test (SCT) of the Northwestern Assessment of Verbs and Sentences (NAVS; Thompson, 2011) and the Northwestern Anagram Test (NAT: Thompson, Weintraub, & Mesulam, 2012; also see Weintraub et al., 2009). The PPA-G group exhibited poorer noncanonical sentence production and comprehension as compared to all three other groups. Phrase/sentence repetition was measured using a subset of items (10-15; Rep6) from the Repetition subtest of the Western Aphasia Battery-Revised (WAB-R: Kertesz, 2006). All PPA groups showed impaired performance on this measure relative to controls.
Table 2.
Language Test Scores for Participants with PPA and Control Participants.
| PPA-G (n = 12) |
PPA-L (n = 11) |
PPA-S (n = 12) |
Controls (n = 12) |
Omnibus Significance |
|
|---|---|---|---|---|---|
| Grammatical Processing, Word Comprehension, and Repetition | |||||
| NAVS SPPT Canonical (% correct) |
78.67C,S (21.27) |
87.88 (22.07) |
97.04 (8.89) |
100.00 (0.00) |
** |
| NAVS SPPT Noncanonical (% correct) |
50.00C,L,S (27.62) |
82.42C (20.93) |
93.33 (15.63) |
100.00 (0.00) |
*** |
| NAT Canonical (% correct) |
94.55 (10.25) |
95.56 (5.77) |
99.44 (1.92) |
100.00 (0.00) |
* |
| NAT Noncanonical (% correct) |
50.27C,L,S (16.43) |
83.67C (13.75) |
86.67 (19.48) |
100.00 (0.00) |
*** |
| NAVS SCT Canonical (% correct) |
87.22C,S (11.53) |
96.97 (8.09) |
99.33 (2.11) |
100.00 (0.00) |
*** |
| NAVS SCT Noncanonical (% correct) |
77.78C,L,S (13.731) |
90.31C (11.30) |
94.67C (6.13) |
100.00 (0.00) |
*** |
| PPVT (max 36) |
34.58C (1.16) |
34.30C (1.57) |
19.75C,G,L (8.20) |
35.55 (0.69) |
*** |
| WAB Rep6 (% correct) |
67.47C,S (18.34) |
78.86C (12.67) |
87.29C (11.23) |
100.00 (0.00) |
*** |
| Naming | |||||
| BNT (max 60) |
46.92C (12.27) |
49.45C (15.19) |
9.83C,G,L (3.74) |
58.50 (1.51) |
*** |
| NNB Noun: Verb Ratio | 1.13C (0.16) |
0.92 (0.31) |
0.65C,G,L (0.35) |
1.00 (0.00) |
*** |
| Other Language Measures | |||||
| WAB Aphasia Quotient (max 100) |
84.08C (7.51) |
90.45C (6.84) |
84.62C,L (8.02) |
99.64 (0.73) |
*** |
| PPT (pictures) (max 52) |
50.08C (1.08) |
49.00 (3.49) |
43.67C,G,L (6.15) |
51.08 (0.67) |
*** |
| PPT (words) (max 52) |
49.92C (1.83) |
50.78 (1.72) |
43.82C,G,L (3.63) |
51.25 (1.06) |
*** |
| 1-syllable Repetition (max 2) |
1.98 (0.06) |
2.00 (0.00) |
2.00 (0.00) |
2.00 (0.00) |
n.s. |
| 2-syllable Repetition (max 2) |
1.96 (0.08) |
2.00 (0.00) |
2.00 (0.00) |
2.00 (0.00) |
n.s. |
| 3-syllable Repetition (max 2) |
1.58C,L,S (0.40) |
1.96 (0.08) |
1.98 (0.06) |
2.00 (0.00) |
*** |
Values provided are means (standard deviations). Max = maximum score; NAVS = Northwestern Assessment of Verbs and Sentences; SPPT = Sentence Priming Production Test; NAT = Northwestern Anagram Test; SCT = Sentence Comprehension Test; PPVT = Peabody Picture Vocabulary Test; WAB = Western Aphasia Battery; Rep6 = WAB Repetition subtest, items 10-15; BNT = Boston Naming Test; NNB = Northwestern Naming Battery; PPT = Pyramids and Palm Trees Test
= significantly impaired relative to control group (p < .05)
= significantly impaired relative to PPA-G group (p < .05)
= significantly impaired relative to PPA-L group (p < .05)
= significantly impaired relative to PPA-S group (p < .05); omnibus significance levels
= p < .05
= p < .01
= p < .001, n.s. = not significant
Verb and noun naming ability was characterized using the Boston Naming Test (BNT: Kaplan, Goodglass, & Weintraub, 1983) and the Northwestern Naming Battery (NNB: Thompson & Weintraub, 2014). Noun naming ability as measured by the BNT was significantly impaired for the PPA-S group compared to all other groups. The PPA-G and PPA-L groups also showed significant noun naming impairments relative to controls. On the NNB, participants with PPA-G showed greater difficulty naming verbs compared to nouns, with higher Noun to Verb (N:V) ratios than the controls, whereas individuals with PPA-S exhibited differentially impaired noun naming, as reflected by lower N:V ratios than all other groups.
Additional tests were also administered to detail patient language deficit patterns, including the Western Aphasia Battery-Revised (WAB-R; Kertesz, 2006), the Pyramids and Palm Trees Test (PPT: Howard & Patterson, 1992), and a motor speech screening (i.e., an oral apraxia screen and repetition of one-, two-, and three-syllable words; after Dabul (2000) and Wertz, LaPointe, & Rosenbek (1984)) (see Table 2 for a summary of scores). All PPA groups showed impaired performance on the WAB, with Aphasia Quotients differing significantly from controls. In addition, semantic knowledge was evaluated using both the picture and word versions of the PPT test. On both measures, the PPA-S group showed significant impairment relative to all other groups. On the single-word repetition task used to screen for motor speech deficits, the PPA-G group showed impaired performance relative to all groups on three-syllable words only.
Procedure
Collection and Coding of Narrative Speech Samples
Participants sat in a quiet room with the examiner and were asked to tell the story of Cinderella. A wordless picture book was provided to help the participant remember the story, but this was removed before narrative production. The experimenter provided minimal cueing when necessary (e.g., “Can you tell me more?” or “What happened next?”). Narrative samples were recorded using Praat or Audacity, transcribed, and coded using the Northwestern Narrative Language Analysis system (NNLA; Thompson, Ballard, Tait, Weintraub, & Mesulam, 1997; Thompson et al., 1995, 2012a). All language samples were transcribed and coded in their entirety by two researchers, with disagreements resolved through discussion. The participant's speech was transcribed verbatim and then segmented into utterances using prosodic, syntactic, and semantic criteria. The utterances were then coded for a variety of lexical and morphosyntactic properties, including morphosyntactic and semantic well-formedness, the number and type of syntactically complex utterances, the word class and morphological structure of all words produced, and the argument structure of each verb produced (see e.g., Thompson et al., 2012a, for details). These analyses yielded the following measures: Words Per Minute (WPM), Mean Length of Utterance (MLU), % Grammatical Sentences, Open: Closed class ratio, Noun: Verb ratio, and the Total Numbers of Nouns and Verbs produced. Copula and auxiliary verbs were not included in the verb totals.
To determine the distribution of pauses across word classes, we tallied and timed pauses preceding nouns and verbs (again excluding copula and auxiliary verbs) produced in the Cinderella narrative sample. For the PPA participants we coded all nouns and verbs produced up to a maximum of 120 tokens (nouns and verbs combined); whereas for control participants, we coded only the first 60 tokens produced, approximately matching the number of tokens coded for the PPA participants. There were no significant group differences in the number of tokens coded for nouns (Mean (Standard Deviation) for PPA-G: 48.5 (22.6); PPA-L: 44.5 (17.7); PPA-S: 29.9 (18.1); Controls: 35.4 (3.1); Kruskal-Wallis test, p > .05) or for verbs (PPA-G: 32.8 (12.2); PPA-L: 38.9 (16.7); PPA-S: 31.1 (17.1); Controls: 24.6 (3.1); Kruskal-Wallis test, p > .05).
Nouns and verbs (the same tokens included in the pause analyses) were coded for lexical frequency, using the Corpus of Contemporary American English (Davies, 2008-) and for length in phonemes. Both unfilled and filled pauses were included in the pause coding. A period of silence of greater than 300 ms between the word preceding the target word and production of the target word constituted an unfilled pause, based on research indicating that pauses greater than 200 ms reflect disfluency in neurotypical adult English speakers (Goldman-Eisler, 1968). Filled pauses contained a filler word (e.g., um, er) between the word preceding the target and the target word. When a word was preceded by a filled and unfilled pause, only one pause was counted in the overall pause rate. The overall rate of pauses was calculated as the percent of words preceded by a pause. The rate of pauses for each word class (noun, verb) was calculated in the same way.
Structural MRI Data Acquisition
Structural T1-weighted images (MP-RAGE sequences; repetition time (TR) = 2300 ms, echo time (TE) = 2.86 ms, flip angle = 9°, field of view (FOV) = 256 mm; 160 slices with a slice thickness of 1.0 mm) were acquired using a 3T Siemens Trio system with a 12-channel birdcage head coil. Scanning took place at the Center for Translational Imaging at Northwestern University.
Data Analysis
Narrative Speech Analysis
We used nonparametric statistics to test for group differences in narrative variables. For variables with significant omnibus group differences (Kruskal-Wallis test), pairwise Mann-Whitney U tests, with p-values FDR-adjusted to correct for multiple comparisons, were used to test differences between groups.
In addition, we used mixed-effects logistic regression to investigate the distribution of pauses as conditioned by lexical variables. The dependent variable for this analysis was the presence/absence of a pause. The independent variables tested were group (PPA-G, PPA-L, PPA-S, Control), word class (noun, verb), word length (phonemes), (log) lexical frequency, the interactions between group and each lexical variable, and words per minute (WPM). WPM was included to determine the nature of the relationship between pause rate and speech rate, and to control for this relationship to the extent possible. A “simple coding” system was used for the categorical variables and continuous variables were centered in order to reduce co-linearity. A stepwise model comparison procedure using the ANOVA test was used to determine which fixed effects significantly contributed to the model fit. All models tested contained random by-participant intercepts and slopes.
In addition, we investigated the sensitivity and specificity of the pause rate by word class for discriminating participant groups. Pause rate by word class was calculated using the following formula: [((Noun pause rate – Verb pause rate)/((Noun pause rate + Verb pause rate)/2)) * 100]. This number reflects the percent difference in pause rate for nouns vs. verbs, divided by the mean pause rate across categories; thus, a positive number indicates a greater tendency to pause before nouns, while a negative number indicates a tendency to pause before verbs. Because we had nearly equal numbers of participants in each group, we used the third quartile value as the cutoff value for calculating sensitivity and specificity.
To investigate the relationship between naming ability and pauses in narrative speech, we computed correlations between overall naming ability (combined score on the 16 noun and 16 verb items that contribute to the Northwestern Naming Battery Noun: Verb ratio) and overall pause rate, as well as between word class effects in naming (Noun: Verb ratio from the NNB) and the pause rate by word class in narrative speech. Control participants were excluded from these analyses due to ceiling performance on naming measures, and NNB scores were unavailable for four participants with PPA-S. Correlations were computed for the full group of participants with PPA and for each PPA subtype separately, with FDR-correction for multiple comparisons.
Statistical Analyses: Structural MRI Data
Structural MR images were processed using FreeSurfer (version 5.1.0; http://surfer.nmr.mgh.harvard.edu/) using procedures described previously (Mesulam et al., 2009b, 2012; Rogalski et al., 2011). In order to identify regions of significant cortical atrophy, cortical thickness maps for each participant group were contrasted with those from a cohort of 35 right-handed, age- and education-matched controls (18 females), which included 11 of 12 control participants from the narrative study. The control cohort was matched to the PPA participants for whom MRI scans were available (n=30: 9 with PPA-G, 10 with PPA-L, and 11 with PPA-S) with respect to age and education (age: χ2 (3, N = 65) = 5.216, p = .157; education: χ2(3, N = 65) = 1.134; p = .769, Kruskal-Wallis Test). The analyses employed a general linear model (GLM) for each vertex along the cortical surface. False Discovery Rate (FDR; threshold of 0.01) was used to correct for multiple comparisons (Genovese, Lazar, & Nichols, 2002).
In addition, we used multiple regression to investigate the relationship between pause measures and cortical thickness in a set of 9 left-hemisphere regions of interest (ROIs) using the Desikan parcellations provided in FreeSurfer: pars opercularis, pars triangularis, pars orbitalis, caudal middle frontal, precentral gyrus, middle temporal gyrus, superior temporal gyrus, inferior parietal, and supramarginal gyrus (Desikan et al., 2006). All participants for whom structural MRI images were available (n=30 PPA and n=11 controls) were included in these analyses. Control participants were included in order to examine the relationship between pause variables and cortical thickness in healthy older adults, as well as in those with PPA. Using multiple linear regression, the mean thickness of each ROI was predicted using a) pause measures derived from the logistic regression analysis of pause distribution, i.e., participant-specific pause rate as conditioned by word class, lexical frequency, and length2; b) group (PPA-G, PPA-L, PPA-S, control), and the interaction between group and each pause variable; c) WAB Aphasia Quotient (AQ) and age, included as nuisance variables to control for overall aphasia severity and effects of aging on cortical thickness; d) speech rate (WPM), parallel with the behavioral analyses of pause distribution. Categorical variables were simple-coded and continuous variables were centered. A stepwise model comparison procedure was used to identify the predictors that significantly contributed to model fit.
Results
Narrative results
Table 3 summarizes the narrative language profiles and lexical properties of nouns and verbs produced, for each participant group. The PPA-G group exhibited reduced speech rate (words per minute; WPM) as compared to the three other groups (PPA-G vs. controls: Z = −3.926, p < .001; PPA-G vs. PPA-L: Z = −3.200, p < .01; PPA-G vs. PPA-S: Z = −3.291, p < .001), as well as decreased mean length of utterance (MLU) as compared to controls (Z = −3.406, p < .001). The PPA-G group also produced the lowest proportion of grammatical sentences, which differed significantly from the control and PPA-L groups (PPA-G vs. controls: Z = −2.599, p < .05; PPA-G vs. PPA-L: Z = −2.277, p < .05); the participants with PPA-S also produced fewer grammatical sentences than controls (Z = −2.484, p < .05). Open: Closed (O:C) class and Noun: Verb (N:V) ratios indicated reduced production of open-class words, especially nouns, in the PPA-S group as compared to controls and participants with PPA-G (O:C Ratio: PPA-S vs. controls: Z = −2.944, p < .01; PPA-S vs. PPA-G: Z = −2.944, p < .01; N:V Ratio: PPA-S vs. controls: Z = −3.44, p < .001; PPA-S vs. PPA-G: Z = −3.09, p < .001). Participants with PPA-L also produced fewer nouns than controls (Z = −2.682; p < .05), but no significant group differences were observed in the total number of verbs produced.
Table 3.
Narrative Language Profiles and Lexical Properties of Nouns and Verbs.
| PPA-G (n=12) |
PPA-L (n=11) |
PPA-S (n=12) |
Controls (n=12) |
Omnibus Significance |
|
|---|---|---|---|---|---|
| Narrative Language Profiles (Northwestern Narrative Language Analysis) | |||||
| Words Per Minute | 73.27C,L,S (22.62) |
112.00 (23.74) |
118.13 (28.66) |
131.70 (17.66) |
*** |
| Mean Length of Utterance | 8.02C (1.72) |
9.23 (2.34) |
9.70 (2.85) |
11.53 (2.10) |
** |
| % Grammatical Sentences | 62.61C,L (27.17) |
84.68 (10.18) |
75.77C (16.00) |
90.18 (6.70) |
* |
| Open: Closed Ratio | 0.94 (0.10) |
0.87 (0.07) |
0.80C,G (0.11) |
0.96 (0.10) |
** |
| Noun: Verb Ratio | 1.48 (0.49) |
1.25 (0.35) |
1.00C,G (0.46) |
1.46 (0.24) |
** |
| Total Nouns Produced | 56.75 (37.17) |
48.09C (20.00) |
33.08C (22.32) |
73.67 (18.65) |
** |
| Total Verbs Produced | 38.42 (20.40) |
41.18 (20.07 |
35.42 (25.79) |
51.58 (14.70) |
n.s. |
| Lexical Properties of Nouns and Verbs | |||||
| Log Lexical Frequency: Nouns | 7.80 (0.76) |
7.75 (1.17) |
9.57C,G,L (0.45) |
7.36 (0.47) |
*** |
| Log Lexical Frequency: Verbs | 11.35 (0.68) |
11.14 (0.68) |
11.07 (0.46) |
10.92 (0.68) |
n.s. |
| Mean Length in Phonemes: Nouns | 4.86C (0.51) |
5.27 (0.70) |
4.08C,G,L (0.33) |
5.57 0.42 |
*** |
| Mean Length in Phonemes: Verbs | 3.72S (0.30) |
3.84 (0.34) |
4.01 (0.17) |
4.02 (0.39) |
* |
The ‘Narrative Language Profile’ measures, calculated using the Northwestern Narrative Language Analysis system, were derived from the full narrative samples. The measures of ‘Lexical Properties of Nouns and Verbs’ were derived from a subset of the data in some participants; see Methods for details. Values provided are means (standard deviations).
= significantly impaired relative to control group (p <.05)
= significantly impaired relative to PPA-G group (p <.05)
= significantly impaired relative to PPA-L group
= significantly impaired relative to PPA-S group (p < .05); omnibus significance levels
= p < .05
= p < .01
= p < .001, n.s. = not significant
As for the lexical properties of nouns and verbs produced, the mean word frequency for nouns was higher in PPA-S than in all other groups (PPA-S vs. controls: z = −4.157, p < .001; PPA-S vs. PPA-G: z = −3.984; p < .001; PPA-S vs. PPA-L: z = −3.323, p < .001). No significant group differences were observed for verb frequency; however, individuals with PPA-G exhibited a trend towards production of higher-frequency verbs than controls (uncorrected p = .06). The analysis of word length indicated that the PPA-S group produced shorter nouns as compared to all other groups (PPA-S vs. controls: z = 4.13, p < .001; PPA-S vs. PPA-G: z = 3.26; p < .001; PPA-S vs. PPA-L: z = 3.35, p < .001). The PPA-G group also produced shorter nouns than controls (z = 2.97, p < .01) and shorter verbs than individuals with PPA-S (z = 2.71, p<.05). Across all groups, the nouns produced were longer and of lower frequency than verbs (paired t-tests, p's < .001) and word length and frequency were strongly inversely correlated (r = −.672, p < .001).
Table 4 provides summary statistics for the overall pause rate and pause rate by word class by participant group, as well as the parameters of the mixed-effects model of pause distribution. The following factors significantly contributed to model fit and were thus retained: group, WPM, lexical frequency, word class, and the interaction between group and word class. Word length and the interactions between group, word length, and lexical frequency did not improve model fit and were thus excluded from the model. Starting with the main effect of group, individuals from all PPA groups produced more pauses than controls (PPA-G: Z = 4.766, p < .001; PPA-L: Z= 3.930, p < .001; PPA-S: Z = 2.394, p < .05), and those with PPA-G produced significantly more pauses than those with PPA-S (Z = 3.152, p < .001) and marginally more pauses than those with PPA-L (Z = 1.926, p = .054). Speech rate also affected pause distribution, such that individuals with slower speech rates produced more pauses (Z = −4.044, p < .001). Across groups, higher pause rates were found for low-frequency vs. high-frequency words (Z = −10.109, p < .001) and for verbs vs. nouns (Z = −4.117, p < .001). There was a significant interaction between group and word class, indicating that individuals with PPA-L paused more often before nouns (vs. verbs) as compared to controls (Z = 3.009, p < 0.01) and participants with PPA-G (Z = 1.966, p=.049), though they did not differ statistically from individuals with PPA-S (Z = 1.634, p =.102), The distribution of pauses across word classes did not differ between individuals with PPA-G, PPA-S, and controls (p's > .1). Post-hoc paired t-tests comparing the pause rates for nouns vs. verbs indicated that the PPA-L group exhibited a significantly higher pause rate before nouns than verbs (p < .05), whereas no word class effects on pause rate were observed within the other participant groups (p's > .1).
Table 4.
Results of Analyses of Pause Distribution
| Pause Distribution | ||||
|---|---|---|---|---|
| PPA-G (n=12) |
PPA-L (n=11) |
PPA-S (n=12) |
Controls (n=12) |
|
| Overall Pause Rate (% Words with Pauses) |
40.30 (12.29) |
24.74 (7.28) |
16.71 (9.69) |
11.89 (5.54) |
| Noun Pause Rate (% Words with Pauses) |
40.25 (10.42) |
30.94 (10.29) |
14.42 (9.37) |
10.69 (5.68) |
| Verb Pause Rate (% Words with Pauses) |
39.62 (18.32) |
18.47 (9.50) |
18.80 (11.01) |
13.84 (6.77) |
| Parameters of the Mixed-Effects Regression Model of Pause Distribution | ||
|---|---|---|
| Variable | Z | p |
| Intercept | −21.304 | < 0.001 |
| Group: PPA-G vs. Control | 4.766 | < 0.001 |
| Group: PPA-L vs. Control | 3.930 | < 0.001 |
| Group: PPA-S vs. Control | 2.394 | 0.017 |
| Group: PPA-G vs. PPA-L | 1.926 | 0.054 |
| Group: PPA-G vs. PPA-S | 3.152 | 0.002 |
| Group: PPA-L vs. PPA-S | 1.578 | 0.115 |
| WPM | −4.044 | < 0.001 |
| Log Lexical Frequency | −10.109 | < 0.001 |
| Word Class: Noun vs. Verb | −4.117 | < 0.001 |
| Group (PPA-G vs. Control) by Word Class (Noun vs. Verb) | 1.487 | 0.137 |
| Group (PPA-L vs. Control) by Word Class (Noun vs. Verb) | 3.009 | 0.003 |
| Group (PPA-S vs. Control) by Word Class (Noun vs. Verb) | 1.402 | 0.161 |
| Group (PPA-G vs. PPA-L) by Word Class (Noun vs. Verb) | −1.966 | 0.049 |
| Group (PPA-G vs. PPA-S) by Word Class (Noun vs. Verb) | 0.078 | 0.938 |
| Group (PPA-L vs. PPA-S) by Word Class (Noun vs. Verb) | 1.634 | 0.102 |
Values provided for measures of ‘Pause Distribution’ are means (standard deviations). Under ‘Parameters of the Mixed Effects Model of Pause Distribution’, the effect of each variable on the likelihood of pause production is reported. Positive z-values indicate a higher rate of pause production. The results indicate higher pause rates for 1) all three PPA groups vs. controls; 2) individuals with lower vs. higher WPM; 3) words with lower vs. higher lexical frequency; 4) verbs vs. nouns; 5) individuals with PPA-L for nouns vs. verbs, as compared to controls and PPA-G. WPM = words per minute.
Pause rate by word class was found to have relatively low sensitivity but high specificity in identifying PPA-L. Seven of 11 participants with PPA-L scored above the third quartile value for this measure of 35% (i.e., a 35% higher pause rate for nouns than verbs), resulting in 64% sensitivity for this measure. Overall, 31 of 36 individuals who did not have PPA-L scored below this cutoff, resulting in 86% specificity. The proportion of participants who scored below the cutoff was nine of 12 with PPA-G (75%), 11 of 12 with PPA-S (92%) and 11 of 12 controls (92%).
Correlations between naming impairments and pauses in narrative speech
Figure 1 illustrates the relationships between 1) naming ability (combined verb and noun naming score on the Northwestern Naming Battery) and overall pause rate in narrative speech; 2) word class effects in naming (Noun: Verb ratio on the NNB) and the distribution of pauses by word class in narrative speech. In the full PPA group, a positive correlation between severity and pause rate was found (r = .38, p= .03), with greater naming severity (i.e., poorer NNB naming score) associated with fewer pauses. N:V ratios on the NNB were positively correlated with the pause rate by word class, i.e., patients who named nouns better than verbs tended to pause more frequently before nouns than verbs (r = .38, p= .03). This correlation was driven predominantly by individuals with PPA-S, who exhibited poor noun naming but paused relatively infrequently before nouns. Within each PPA subtype, no significant correlations between naming ability and pause rate were found.
Figure 1.
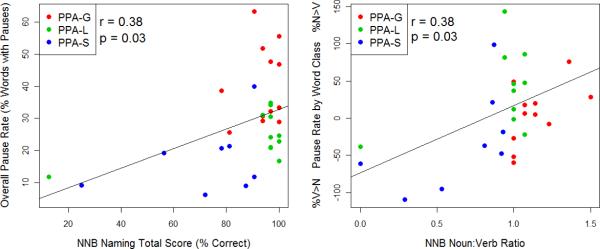
Correlations between Naming Measures and Pauses in Narrative Speech.
The left figure illustrates the positive correlation between overall naming performance and overall pause rate in PPA individuals. The right figure shows the positive correlation between Noun: Verb ratios in naming and the distribution of pauses by word class in PPA narrative speech. NNB = Northwestern Naming Battery; N = noun; V = verb.
Neuroimaging results
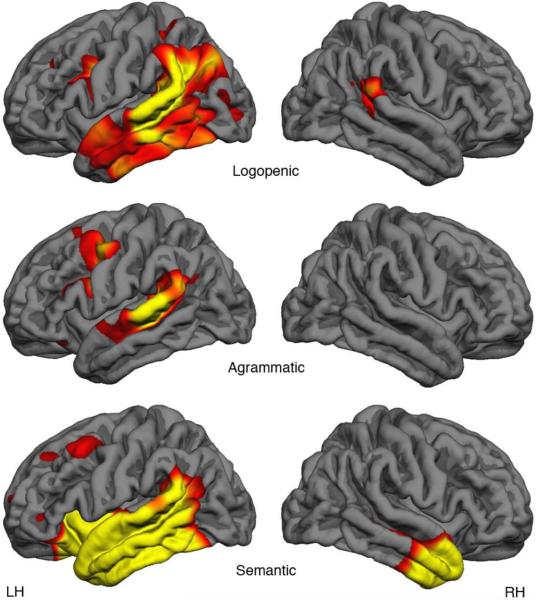
All of the PPA subtypes displayed asymmetrically greater atrophy in the left hemisphere (Figure 2), with no significant right hemisphere atrophy noted for the PPA-G group, minimal atrophy in the right posterior superior temporal and supramarginal gyri for the PPA-L participants, and atrophy restricted to the anterior third portion of the temporal lobe region in the right hemisphere for the PPA-S group. In the left hemisphere, the PPA-G group (n=9) showed peak atrophy in the posterior portion of the inferior frontal gyrus and the caudal middle frontal gyrus of the left hemisphere, with concomitant significant thinning extending from the middle to posterior aspects of the superior temporal gyrus including part of the supramarginal gyrus. Peak atrophy in PPA-L (n=10) included the left temporoparietal junction as well as the superior, middle and inferior temporal gyri with relative sparing of the anterior aspects of the temporal pole. Additionally, left hemisphere atrophy was seen in the sulci of the posterior aspects of the inferior frontal gyrus. The PPA-S group (n=11) showed atrophy largely confined to the left temporal lobe, including the superior, middle and inferior temporal gyri and the fusiform gyrus. These atrophy patterns are consistent with previous reports (Mesulam et al., 2009b, 2012; Rogalski et al., 2011).
Figure 2.
Regions of Significant Atrophy for each PPA Group.
Cortical thickness in each PPA group is compared to an age- and education matched cohort (n=35) of cognitively healthy control participants. Results are corrected for multiple comparisons (FDR.01).
Multiple regression analyses identified several brain regions in which pause variables were significant predictors of cortical thickness, after controlling for aphasia severity (WAB AQ), age, and speech rate (WPM) (see Table 5 and Figure 3). We report the ROIs in which pause variables were significant or marginally significant (p < .1) predictors of cortical thickness, as well as any significant modulation of these effects by participant group. Pause rate by word class (i.e., the tendency to pause more frequently before nouns vs. verbs) predicted cortical thinning in the left pars triangularis, pars opercularis, precentral gyrus, supramarginal gyrus, and inferior parietal cortex. In the inferior parietal cortex only, these effects were stronger in controls than in participants with PPA-G (Z = 2.097; p = 0.044) and PPA-L (Z = 2.466; p= 0.019). Pause rate by lexical frequency (i.e., the tendency to pause more frequently before low- vs. high-frequency words) was also associated with cortical thinning in the inferior parietal cortex, with no modulation of this effect by participant group. Pause rate by length (i.e., the tendency to pause more frequently before long vs. short words) significantly predicted cortical thinning in the supramarginal gyrus and inferior parietal cortex, and marginally so in the precentral gyrus (p = 0.051), with no modulation of these effects by participant group.
Table 5.
Multiple Regression Results: Pause Variables as Predictors of Cortical Thickness.
| Pause Rate by Word Class (Noun > Verb) | Pause Rate by Frequency (Low > High) | Pause Rate by Length (Long > Short) | ||||
|---|---|---|---|---|---|---|
| Region | Z | p | Z | p | Z | p |
| Pars orbitalis | n.s. | n.s. | n.s. | |||
| Pars triangularis | −2.744 | 0.009 | n.s. | n.s. | ||
| Pars opercularis | −2.140 | 0.039 | n.s. | n.s. | ||
| Caudal middle frontal gyrus | n.s. | n.s. | n.s. | |||
| Precentral gyrus | −2.263 | 0.029 | n.s. | −2.012 | 0.051 | |
| Middle temporal gyrus | n.s. | n.s. | n.s. | |||
| Superior temporal gyrus | n.s. | n.s. | n.s. | |||
| Supramarginal gyrus | −2.352 | 0.024 | n.s. | −2.891 | 0.006 | |
| Inferior parietal cortex | −2.724 | 0.010 | −2.088 | 0.044 | −2.537 | 0.015 |
Regions in which pause variables predicted cortical thickness (p < .1) are summarized.
Figure 3.
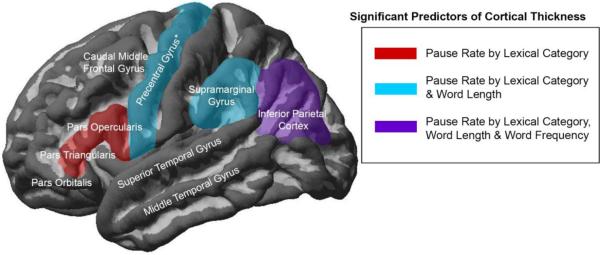
Pause Variables that Significantly Predict Cortical Thickness.
Note: Cortical atrophy in the highlighted regions was predicted by 1) pause rate by lexical category (a tendency to pause before nouns vs. verbs), 2) pause rate by word length (a tendency to pause before longer vs. shorter words), and/or 3) pause length by word frequency (a tendency to pause before lower vs. higher frequency words). In the non-highlighted regions, pause variables did not predict cortical thickness. *The effect of word length in the precentral gyrus is marginally significant (p =.051).
Discussion
The goal of the present study was to contribute to the understanding of the neurolinguistic basis of word retrieval impairments in PPA. Pauses have been shown to reflect word production difficulty at multiple stages, including lemma retrieval (access and selection) (Beattie & Butterworth, 1979; Goldman-Eisler, 1958; Griffin & Bock, 1998), phonological word-form retrieval and encoding (Beattie & Butterworth, 1979; Griffin & Bock, 1998; Jescheniak & Levelt, 1994), and motor speech planning and articulation (Ballard et al., 2014; Duffy, 2006), and thus we examined pauses in narrative speech samples. In order to tease out the source of word retrieval impairments across PPA variants, we detailed the distribution of pauses as conditioned by lexical variables (i.e., word class, lexical frequency, and word length) in narratives produced by individuals with agrammatic, logopenic, and semantic PPA and unimpaired controls. We also investigated the neural correlates of pause production in PPA, with the goal of identifying the neural basis of word-finding pauses.
The three PPA groups exhibited word class deficits which were largely consistent with previous studies. For PPA-S, noun retrieval deficits were observed in confrontation naming and narrative speech (cf. Hillis et al., 2004, 2006; Thompson et al., 2012a, 2012c; Wilson et al., 2010). Also consistent with previous studies (Hillis et al., 2004, 2006; Thompson et al., 2012c), individuals with PPA-G exhibited impaired naming of verbs. However, they did not exhibit reduced verb production in narrative speech, in contrast with trends reported in previous studies (Thompson et al., 2012a; Wilson et al., 2010). The latter finding may in part be due to the inclusion of participants with relatively mild agrammatism, given the requirement that all participants produce at least ten verbs (and ten nouns) in the narrative sample. However, the PPA-G group did exhibit typical agrammatic sentence production both in narrative speech and in structured tasks (i.e., fewer grammatical sentences, greater impairments for noncanonical vs. canonical sentence production). For PPA-L, no word class effects were observed in confrontation naming (cf. Thompson et al., 2012c) or in narrative speech based on Noun: Verb ratios, which did not significantly differ from controls. However, these ratios were numerically reduced (cf. Thompson et al., 2012a; Wilson et al., 2010) and individuals with PPA-L produced significantly fewer total nouns than controls, suggesting possible noun production impairments. Correlation analyses investigating the relationship between naming ability and pause production revealed that greater severity of naming impairments was not associated with increased pause rate. PPA individuals with more severe naming impairments actually produced fewer pauses, largely due to the inclusion of individuals with PPA-S who had relatively severe anomia yet produced fluent speech with few pauses. Similarly, individuals with more severe noun production deficits tended to pause less often before nouns. These findings point to differences in the processes supporting noun and verb production in naming and in narrative speech.
The analysis of pause distribution quantified patterns of pauses across lexical variables known to affect different stages of word production: word class, which is represented at the lemma level, lexical frequency, which has been argued to affect phonological word-form retrieval (Beattie & Butterworth, 1979; Griffin & Bock, 1998; Jescheniak & Levelt, 1994; Wilson et al., 2009), and word length, claimed to affect both phonological word-form retrieval and subsequent phonological encoding and articulatory processes (Wilson et al., 2009). Overall, individuals with PPA-G produced the highest rate of pauses, an effect that persisted even after controlling for speech rate (words per minute) (cf. Ballard et al., 2014; Rohrer et al., 2010b). Notably, a word class effect was not found for the PPA-G group in that similar pause rates were found for both nouns and verbs. Individuals with PPA-S and PPA-L also produced more pauses than unimpaired individuals. However, like PPA-G, no word class effect was found for the PPA-S group. Only the PPA-L group showed a unique distribution of pauses across word classes. Specifically, individuals with PPA-L produced a higher rate of pauses before nouns than verbs, whereas no significant word class effects were found in other groups. Critically, these effects persisted even though lexical frequency was included in the model of pause distribution, suggesting that these effects are not merely due to frequency differences between nouns and verbs in the Cinderella narrative. Across groups, lexical frequency was a significant predictor of pause rate, with more pauses produced before low-frequency words, but this variable did not differentially affect pause rate between participant groups. Word length also did not significantly predict pause rate after controlling for lexical frequency and word class.
What do these patterns suggest about the source of word retrieval deficits in PPA? First, with regard to PPA-S, noun naming deficits in this population have been linked to deficits at the lemma and/or the conceptual level of word retrieval (Hurley et al., 2012; Mesulam et al. 2009a; 2013), and thus we predicted elevated pause rates before nouns (but not verbs) in this group. However, we found a similar distribution of pauses across word classes in PPA-S and control participants. But, we also found that the nouns (but not verbs) produced by the PPA-S individuals were more frequently-occurring than those produced by unimpaired individuals and by those with PPA-G and PPA-L. This lexical simplification (i.e., selection of higher-frequency nouns that are easier to retrieve and/or represent more familiar concepts) supports patterns reported in previous studies and is consistent with conceptual and/or lemma level impairments (Bird et al., 2000; Hoffman et al., 2014; Meteyard & Patterson, 2009; Wilson et al., 2010). However, this phenomenon may have diminished noun-retrieval difficulty at least to some extent in the narrative task, precluding clear word-class pause patterns.
For PPA-G, because of the prevalence of verb naming deficits in these patients and verb-lemma complexity effects in naming (Thompson et al., 2012c), we again predicted that pauses would occur with relatively high frequency prior to the impaired word class: verbs. We also entertained the idea that PPA-G individuals are impaired at subsequent stages of word production, i.e., phonological and articulatory processes (e.g., Ballard et al., 2014; Mack et al., 2013a), resulting in a high overall pause rate. The results were consistent with the latter interpretation, as individuals with PPA-G produced a high rate of pauses overall; however, pauses were equally distributed across word classes. Two factors may contribute to the lack of word class effects in pause distribution. First, as mentioned above, the PPA-G participants in the present study were somewhat atypical in that they did not exhibit a trend towards reduced verb production in narratives (in contrast with e.g., Thompson et al., 2012a; Wilson et al., 2010). Thus, the absence of word class effects in pause distribution may relate to relatively mild verb production deficits in these participants. Second, lexical simplification may have eased verb retrieval demands, precluding the need to use pauses; hence, verb deficits were not reflected in the distribution of pauses. Some trends in the data are consistent with this possibility, specifically that individuals with PPA-G produced numerically shorter and higher-frequency verbs as compared to the other groups; however, the only significant difference observed on these measures was between PPA-G and PPA-S on verb length.
For PPA-L, we aimed to determine whether the distribution of pauses reflects lemma-level noun-retrieval impairments (resulting in more pauses before nouns than verbs) or impaired phonological word-form retrieval (resulting in more pauses before low-frequency and longer words, with no independent word class effects). The pause distribution results were consistent with lemma-level noun retrieval deficits, as also suggested by previous studies (Hurley et al., 2012; Thompson et al., 2012b). However, it should be noted that the pattern of results is also consistent with deficits in earlier stages of word production (i.e., impaired conceptualization of objects), although this possibility seems less likely given that PPA-L is not typically associated with conceptual semantic deficits. The distribution of pauses did not provide clear evidence for phonological word-form deficits in PPA-L (Mack et al., 2013a), as these individuals did not differ significantly from other groups with respect to effects of word frequency and length on pause distribution.
These findings also support previous qualitative (Mesulam & Weintraub, 1992; Mesulam et al., 2012) and quantitative (Teichmann et al., 2013) observations that PPA-L is associated with a unique pattern of word-finding pauses. Indeed, including a measure of word-finding pauses in the classification criteria for PPA-L, perhaps using automated or semi-automated methods for pause coding that would be more efficient and clinically feasible (e.g., Ballard et al., 2014), may improve the classification accuracy for this subtype. While some studies have reported success in using the 2011 criteria (Gorno-Tempini et al., 2011) to classify participants with PPA-L (Leyton et al., 2011), others have reported challenges (Sajjadi et al., 2012; Wicklund et al., 2014). Sajjadi and colleagues (2012) reported that only 4.3% of PPA patients met the diagnostic criteria for PPA-L (as compared to 28.3% for PPA-S and 26.1% for PPA-G), and on the basis of these results questioned whether PPA-L is a discrete subtype of PPA. Wicklund et al. (2014) reported a higher rate of PPA-L classification (35%), but also noted a group of “unclassifiable” patients who lacked repetition impairments but otherwise met the criteria for PPA-L. Addressing these issues, Mesulam and Weintraub (2014) recommended that word retrieval deficits should remain a core feature of PPA-L, whereas repetition impairments should be treated as an ancillary feature. In order to improve classification accuracy under such a system, it would be necessary to identify quantitative measures of word retrieval that distinguish PPA-L from the other subtypes. In the present study, we tested the sensitivity and specificity of pause rate by word class (using a third-quartile cutoff of 35% greater pauses for nouns than verbs) for distinguishing PPA-L from other PPA subtypes and controls. This measure exhibited modest sensitivity (correctly identifying 64% of patients with PPA-L) but good specificity (75% for PPA-G, 92% for PPA-S and controls). In future research, it may be possible to identify pause coding procedures or measures that result in improved sensitivity to PPA-L, while retaining the specificity of the pause rate by word class measure.
The neuroimaging results revealed regions of cortical atrophy that are associated with the distribution of pauses in PPA. Increased pause rate by word class (a relatively strong tendency to pause before nouns vs. verbs) was associated with increased atrophy in left frontal and inferior parietal regions, specifically the pars triangularis and opercularis of the inferior frontal gyrus (IFG), the precentral gyrus, the supramarginal gyrus (SMG) and the inferior parietal cortex (IPC). These brain regions partially overlapped with regions sensitive to the distribution of pauses as conditioned by frequency and word length. Frequency (a relatively strong tendency to pause before low-frequency vs. high-frequency words) was associated with greater atrophy in the IPC, whereas word length (a relatively strong tendency to pause before longer vs. shorter words) was significantly associated with increased atrophy in the IPC and SMG and a marginal predictor of atrophy in the precentral gyrus.
The observed relationships between pause variables and atrophy in the precentral gyrus and inferior parietal regions (SMG and IPC) may be due to the role of these regions in supporting phonological and articulatory processes. Temporoparietal regions including the posterior STG and inferior parietal regions have been associated with the retrieval of phonological forms in neuroimaging studies of healthy individuals (e.g., Abel et al., 2009; Graves et al., 2007; Indefrey & Levelt, 2004; Indefrey, 2011; Prabhakaran et al., 2006; Righi, Blumstein, Mertus, & Worden, 2010; Wilson et al., 2009), and left fronto-parietal regions including the SMG, pre- and post-central gyri, and premotor cortex have been linked to the production of phonological errors in individuals with stroke aphasia (Mirman et al., 2015; Schwartz et al., 2009). Furthermore, regions including the precentral gyrus and inferior and middle frontal gyri have been shown to support motor speech processing in healthy individuals as well as individuals with PPA (Ballard et al., 2014; Indefrey & Levelt, 2004; Indefrey, 2011). The fact that multiple pause variables were associated with increased atrophy in the precentral gyrus, SMG, and IPC likely relates to the close relationships between word class, frequency, and length in the narrative samples: nouns tended to be longer and less frequent than verbs. All three pause variables predicted atrophy in inferior parietal regions (word class and length in the SMG; word class, length, and frequency in the IPC), possibly reflecting difficulty associated with retrieving the phonological form of less-frequent and longer words (which also happened to be nouns). In contrast, articulatory demands (i.e., the difficulty of producing longer vs. shorter words) may account for the effects of word length in the precentral gyrus; the effects of pause rate by word class in this region may be attributable to the length difference between nouns and verbs.
In contrast, the left posterior IFG was associated with pause rate by word class but not with pause distribution as conditioned by frequency and length. The left IFG has been argued to support top-down control of lexical access and selection processes in unimpaired adults, both in word production (Schnur et al., 2006; 2009) and comprehension (Bedny, McGill, & Thompson-Schill, 2008; Righi, 2010). Furthermore, damage to the left IFG in stroke aphasia has been argued to contribute to lexical selection errors in word production (Schwartz et al., 2009) as well as impairment of higher-level lexical processes during word and sentence comprehension (e.g., slowed lexical rise time, slowed prediction, abnormal activation of lexical competitors; Love, Swinney, Walenski, & Zurif, 2008; Mack, Ji, & Thompson, 2013b; Mirman, Yee, Blumstein, & Magnuson, 2011). The results thus suggest that impaired top-down control of lexical processes, caused by atrophy in the left pars triangularis and pars opercularis, may contribute to noun retrieval deficits. Specifically, atrophy in these regions may impair the ability to select between multiple referents, which must be tracked throughout the discourse. Although individuals with PPA-L exhibited the strongest tendency to pause before nouns vs. verbs, the relationship between pause rate by word class and atrophy in the left IFG was of comparable strength across participant groups. This suggests that atrophy in the posterior IFG may contribute to impaired noun retrieval across PPA subtypes as well as in healthy aging.
Conclusion
Results of the present study, examining pause distribution in the narrative speech in PPA, contributes to our understanding of the source of word retrieval deficits in this patient group. Individuals with PPA produced more pauses than control speakers, but those with more severe confrontation naming deficits did not produce a higher rate of pauses in narratives, suggesting that distinct processes support word retrieval in naming and narrative tasks. Individuals with PPA-L produced a unique distribution of pauses across word classes, suggesting that word-finding pauses in PPA-L may stem from lemma-level noun retrieval deficits. These results support previous observations that word-finding pauses are one of the most prominent features of connected speech in PPA-L and accordingly, it may be useful to incorporate a quantitative measure of the distribution of pauses into procedures for PPA subtype classification, in order to aid in the classification of patients with PPA-L. No word class effects were evident in PPA-G, PPA-S, or in unimpaired controls. However, trends toward lexical simplification were evident for verbs in PPA-G, suggesting verb-lemma retrieval impairments, and high overall pause rates in this group were consistent with impaired phonological encoding and articulation. For PPA-S, lexical simplification was evident for nouns, consistent with conceptual-level deficits in object representations and/or lemma-level noun-retrieval deficits. The neuroimaging results also indicated the importance of investigating the distribution of pauses for understanding the neural basis of word retrieval deficits. In particular, pause rate by word class (i.e., the tendency to pause before nouns vs. verbs) was associated with greater atrophy in left fronto-parietal regions, overlapping with other lexical variables (length, frequency) in predicting atrophy in the precentral gyrus and inferior parietal regions, but uniquely predicting atrophy in the posterior IFG. The latter finding suggests that the posterior IFG, which has been argued to support top-down control of word retrieval processes, may play an important role in selecting noun lemmas during narrative speech production.
Highlights.
Pauses in narrative speech were examined in individuals with PPA and controls.
Word class affected pause rate only in PPA-L (nouns > verbs).
Increased pause rate for nouns was associated with left fronto-parietal atrophy.
Increased pause rate for low-frequency words correlated with left parietal atrophy.
Increased pause rate for longer words correlated with left fronto-parietal atrophy.
Acknowledgments
This research was supported in part by DC008552 from the National Institute on Deafness and Other Communication Disorders; AG13854 (Alzheimer Disease Core Center) from the National Institute on Aging (Northwestern University); NS075075 from the National Institute of Neurological Disorders and Stroke (Mesulam) and NIH R01-DC001948 (Thompson). The authors wish to thank Christina Wieneke, Kristen Whitney and Amanda Rezutek for assistance with data collection, Adam Martersteck and Derin Cobia for MR processing and Melanie Annis for assistance with narrative data analysis. We thank all the research participants and their families for their commitment to the research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Participant groups were compared using nonparametric statistical tests (Kruskal-Wallis tests, with follow-up pairwise Mann-Whitney U tests with FDR adjustment of p-values to correct for multiple comparisons).
Separate mixed-effects logistic regression models were computed for each lexical variable (word class, frequency, length), in which the lexical variable was used to predict the likelihood of a pause. Participant-specific random slopes for that variable were extracted and entered into the cortical thickness analyses described above.
References
- Abel S, Dressel K, Bitzer R, Kummerer D, Mader I, Weiller C, Huber W. The separation of processing stages in a lexical interference fMRI-paradigm. Neuroimage. 2009;44:1113–1124. doi: 10.1016/j.neuroimage.2008.10.018. doi:10.1016/j.neuroimage.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Ash S, Evans E, O'Shea J, Powers J, Boller A, Weinberg D, Grossman M. Differentiating primary progressive aphasias in a brief sample of connected speech. Neurology. 2013;81(4):329–336. doi: 10.1212/WNL.0b013e31829c5d0e. doi: 10.1212/WNL.0b013e31829c5d0e WNL.0b013e31829c5d0e [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash S, McMillan C, Gunawardena D, Avants B, Morgan B, Khan A, Grossman M. Speech errors in progressive non-fluent aphasia. Brain and Language. 2010;113(1):13–20. doi: 10.1016/j.bandl.2009.12.001. doi: 10.1016/j.bandl.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash S, Moore P, Antani S, McCawley G, Work M, Grossman M. Trying to tell a tale: discourse impairments in progressive aphasia and frontotemporal dementia. Neurology. 2006;66(9):1405–1413. doi: 10.1212/01.wnl.0000210435.72614.38. doi: 10.1212/01.wnl.0000210435.72614.38. [DOI] [PubMed] [Google Scholar]
- Ballard KJ, Savage S, Leyton CE, Vogel AP, Hornberger M, Hodges JR. Logopenic and nonfluent variants of primary progressive aphasia are differentiated by acoustic measures of speech production. PLoS One. 2014;9(2):e89864. doi: 10.1371/journal.pone.0089864. doi: 10.1371/journal.pone.0089864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie GW, Butterworth BL. Contextual probability and word frequency as determinants of pauses and errors in spontaneous speech. Language and Speech. 1979;22(3):201–211. [Google Scholar]
- Bedny M, McGill M, Thompson-Schill SL. Semantic adaptation and competition during word comprehension. Cerebral Cortex. 2008;18(11):2574–2585. doi: 10.1093/cercor/bhn018. doi: 10.1093/cercor/bhn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird H, Franklin S. Cinderella revisited: A comparison of fluent and non-fluent aphasic speech. Journal of Neurolinguistics. 1996;9(3):187–206. [Google Scholar]
- Bird H, Lambon Ralph MA, Patterson K, Hodges JR. The rise and fall of frequency and imageability: noun and verb production in semantic dementia. Brain and Language. 2000;73(1):17–49. doi: 10.1006/brln.2000.2293. doi: 10.1006/brln.2000.2293. [DOI] [PubMed] [Google Scholar]
- Caramazza A. How many levels of processing are there in lexical access? Cognitive Neuropsychology. 1997;14(1):177–208. [Google Scholar]
- Clark DG, Charuvastra A, Miller BL, Shapira JS, Mendez MF. Fluent versus nonfluent primary progressive aphasia: A comparison of clinical and functional neuroimaging features. Brain and Language. 2005;94:54–60. doi: 10.1016/j.bandl.2004.11.007. DOI: 10.1016/j.bandl.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Crepaldi D, Berlingeri M, Paulesu E, Luzzatti C. A place for nouns and a place for verbs? A critical review of neurocognitive data on grammatical-class effects. Brain and Language. 2011;116(1):33–49. doi: 10.1016/j.bandl.2010.09.005. doi:10.1016/j.bandl.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Dabul BL. Apraxia Battery for Adults. Pro-Ed; Austin, TX: 2000. [Google Scholar]
- Davies M. The Corpus of Contemporary American English: 450 million words, 1990-present. 2008 Retrieved from http://corpus.byu.edu/coca/
- Dell GS, O'Seaghdha PG. Stages of lexical access in language production. Cognition. 1992;42:287–314. doi: 10.1016/0010-0277(92)90046-k. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. doi: S1053-8119(06)00043-7 [pii] 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Duffy JR. Apraxia of speech in degenerative neurologic disease. Aphasiology. 2006;20:511–527. [Google Scholar]
- Dunn LM, Dunn DM. PPVT-4 Manual. NCS Pearson, Inc.; Bloomington, MN: 2007. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Garrett MF. Production of speech: Observations from normal and pathological use. In: Ellis AW, editor. Normality and Pathology in Cognitive Functions. Academic Press; San Diego, CA: 1982. [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. doi: 10.1006/nimg.2001.1037 S1053811901910377 [pii] [DOI] [PubMed] [Google Scholar]
- Goldman-Eisler F. Speech production and the predictability of words in context. Quarterly Journal of Experimental Psychology. 1958;10:96–106. [Google Scholar]
- Goldman-Eisler F. Psycholinguistics: Experiments in Spontaneous Speech. Academic Press; New York: 1968. [Google Scholar]
- Golper LAC, Rau MT, Erskine B, Langhaus JJ, Houlihan J. Aphasic Patients' Performance on Mental Status Examination.. Paper presented at the Clinical Aphasiology Conference; Lake of the Ozarks, MO.. 1987. [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Miller BL. Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology. 2004;55(3):335–346. doi: 10.1002/ana.10825. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves WW, Grabowski TJ, Mehta S, Gordon JK. A neurological signature of phonological access: Distinguishing the effects of word frequency from familiarity and length in overt picture naming. Journal of Cognitive Neuroscience. 2007;19(4):617–631. doi: 10.1162/jocn.2007.19.4.617. doi:10.1162/jocn.2007.19.4.617. [DOI] [PubMed] [Google Scholar]
- Griffin ZM, Bock K. Constraint, word frequency, and the relationship between lexical processing levels in spoken word production. Journal of Memory and Language. 1998;38:313–338. [Google Scholar]
- Gunawardena D, Ash S, McMillan C, Avants B, Gee J, Grossman M. Why are patients with progressive nonfluent aphasia nonfluent? Neurology. 2010;75(7):588–594. doi: 10.1212/WNL.0b013e3181ed9c7d. doi: 10.1212/WNL.0b013e3181ed9c7d 5/7/588 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Heidler-Gary J, Newhart M, Chang S, Ken L, Bak TH. Naming and comprehension in primary progressive aphasia: The influence of grammatical word class. Aphasiology. 2006;20:246–256. [Google Scholar]
- Hillis AE, Oh S, Ken L. Deterioration of naming nouns versus verbs in primary progressive aphasia. Annals of Neurology. 2004;55(2):268–275. doi: 10.1002/ana.10812. doi: 10.1002/ana.10812. [DOI] [PubMed] [Google Scholar]
- Hoffman P, Meteyard L, Patterson K. Broadly speaking: vocabulary in semantic dementia shifts towards general, semantically diverse words. Cortex. 2014;55:30–42. doi: 10.1016/j.cortex.2012.11.004. doi: 10.1016/j.cortex.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson KE. The Pyramids and Palm Trees Test: A test of semantic access from words and pictures. Thames Valley Test Company; 1992. [Google Scholar]
- Hurley RS, Paller KA, Rogalski EJ, Mesulam MM. Neural mechanisms of object naming and word comprehension in primary progressive aphasia. Journal of Neuroscience. 2012;32(14):4848–4855. doi: 10.1523/JNEUROSCI.5984-11.2012. doi: 10.1523/JNEUROSCI.5984-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P. The spatial and temporal signatures of word production components: a critical update. Front Psychol. 2011;2:255. doi: 10.3389/fpsyg.2011.00255. doi: 10.3389/fpsyg.2011.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJ. The spatial and temporal signatures of word production components. Cognition. 2004;92(1-2):101–144. doi: 10.1016/j.cognition.2002.06.001. doi: 10.1016/j.cognition.2002.06.001 S0010027703002294 [pii] [DOI] [PubMed] [Google Scholar]
- Jescheniak JD, Levelt WJM. Word frequncy effects in speech production: Retrieval of syntactic information and of phonological form. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994;20(4):824–843. [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, Petersen RC. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129(Pt 6):1385–1398. doi: 10.1093/brain/awl078. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test (BNT) Lea and Febiger; Philadelphia, PA: 1983. [Google Scholar]
- Kertesz A. Western Aphasia Battery-Revised (WAB-R) Pro-Ed; Austin, TX: 2006. [Google Scholar]
- Levelt WJ, Roelofs A, Meyer AS. A theory of lexical access in speech production. Behavioral and Brain Sciences. 1999;22(1):1–38. doi: 10.1017/s0140525x99001776. discussion 38-75. [DOI] [PubMed] [Google Scholar]
- Leyton CE, Villemagne VL, Savage S, Pike KE, Ballard KJ, Piguet O, Hodges JR. Subtypes of progressive aphasia: application of the International Consensus Criteria and validation using beta-amyloid imaging. Brain. 2011;134(Pt 10):3030–3043. doi: 10.1093/brain/awr216. doi: 10.1093/brain/awr216 awr216 [pii] [DOI] [PubMed] [Google Scholar]
- Love T, Swinney D, Walenski M, Zurif E. How left inferior frontal cortex participates in syntactic processing: Evidence from aphasia. Brain and Language. 2008;107(3):203–219. doi: 10.1016/j.bandl.2007.11.004. doi: S0093-934X(07)00294-5 [pii] 10.1016/j.bandl.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack JE, Cho-Reyes S, Kloet JD, Weintraub S, Mesulam M-M, Thompson CK. Phonological facilitation of naming in agrammatic and logopenic primary progressive aphasia (PPA). Cognitive Neuropsychology. 2013a;30(3):172–193. doi: 10.1080/02643294.2013.835717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack JE, Ji W, Thompson CK. Effects of verb meaning on lexical integration in agrammatic aphasia: Evidence from eyetracking. Journal of Neurolinguistics. 2013b;26(6):619–636. doi: 10.1016/j.jneuroling.2013.04.002. doi: 10.1016/j.jneuroling.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacWhinney B, Fromm D, Holland A, Forbes M, Wright H. Automated analysis of the Cinderella story. Aphasiology. 2010;24(6-8):856–868. doi: 10.1080/02687030903452632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Rogalski E, Wieneke C, Cobia D, Rademaker A, Thompson C, Weintraub S. Neurology of anomia in the semantic variant of primary progressive aphasia. Brain. 2009a;132(Pt 9):2553–2565. doi: 10.1093/brain/awp138. doi: 10.1093/brain/awp138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M, Weintraub SW. Spectrum of primary progressive aphasia. In: Rossor MN, editor. Unusual Dementias. Bailliere Tindal; London: 1992. pp. 583–608. [PubMed] [Google Scholar]
- Mesulam MM, Weintraub S. Is it time to revisit the classification guidelines for primary progressive aphasia? Neurology. 2014;82(13):1108–1109. doi: 10.1212/WNL.0000000000000272. doi: 10.1212/WNL.0000000000000272. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Wieneke C, Hurley R, Rademaker A, Thompson CK, Weintraub S, Rogalski EJ. Words and objects at the tip of the left temporal lobe in primary progressive aphasia. Brain. 2013;136(Pt 2):601–618. doi: 10.1093/brain/aws336. doi: 10.1093/brain/aws336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Wieneke C, Rogalski E, Cobia D, Thompson C, Weintraub S. Quantitative template for subtyping primary progressive aphasia. Archives of Neurology. 2009b;66(12):1545–1551. doi: 10.1001/archneurol.2009.288. doi: 10.1001/archneurol.2009.288 66/12/1545 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Wieneke C, Thompson C, Rogalski E, Weintraub S. Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain. 2012;135(Pt 5):1537–1553. doi: 10.1093/brain/aws080. doi: 10.1093/brain/aws080 aws080 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meteyard L, Patterson K. The relation between content and structure in language production: an analysis of speech errors in semantic dementia. Brain and Language. 2009;110(3):121–134. doi: 10.1016/j.bandl.2009.03.007. doi: 10.1016/j.bandl.2009.03.007 S0093-934X(09)00050-9 [pii] [DOI] [PubMed] [Google Scholar]
- Mirman D, Chen Q, Zhang Y, Wang Z, Faseyitan O, Coslett HB, Schwartz MF. Neural organization of spoken language revealed by lesion-symptom mapping. Nature Communications. 2015;6 doi: 10.1038/ncomms7762. doi:10.1038/ncomms7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, Yee E, Blumstein SE, Magnuson JS. Theories of spoken word recognition deficits in aphasia: evidence from eye-tracking and computational modeling. Brain and Language. 2011;117(2):53–68. doi: 10.1016/j.bandl.2011.01.004. doi: 10.1016/j.bandl.2011.01.004 S0093-934X(11)00016-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Osher JE, Wicklund AH, Rademaker A, Johnson N, Weintraub S. The mini-mental state examination in behavioral variant frontotemporal dementia and primary progressive aphasia. American Journal of Alzheimer's Disease and Other Dementias. 2008;22(6):468–473. doi: 10.1177/1533317507307173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran R, Blumstein SE, Myers EB, Hutchison E, Britton B. An event-related fMRI investigation of phonological-lexical competition. Neuropsychologia. 2006;44:2209–2221. doi: 10.1016/j.neuropsychologia.2006.05.025. doi:10.1016/j.neuropsychologia.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, Gorno-Tempini ML. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Annals of Neurology. 2008;64(4):388–401. doi: 10.1002/ana.21451. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8(3):271–276. [Google Scholar]
- Roelofs A. A spreading-activation theory of lemma retrieval in speaking. Cognition. 1992;42:107–142. doi: 10.1016/0010-0277(92)90041-f. [DOI] [PubMed] [Google Scholar]
- Righi G, Blumstein SE, Mertus J, Worden MS. Neural systems underlying lexical competition: an eye tracking and fMRI study. Journal of Cognitive Neuroscience. 2010;22(2):213–224. doi: 10.1162/jocn.2009.21200. doi: 10.1162/jocn.2009.21200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Thompson CK, Weintraub S, Mesulam MM. Anatomy of language impairments in primary progressive aphasia. Journal of Neuroscience. 2011;31(9):3344–3350. doi: 10.1523/JNEUROSCI.5544-10.2011. doi: 10.1523/JNEUROSCI.5544-10.2011 31/9/3344 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Rossor MN, Warren JD. Syndromes of nonfluent primary progressive aphasia: a clinical and neurolinguistic analysis. Neurology. 2010;75(7):603–610. doi: 10.1212/WNL.0b013e3181ed9c6b. doi: 10.1212/WNL.0b013e3181ed9c6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Rossor MN, Warren JD. Alzheimer's pathology in primary progressive aphasia. Neurobiology of Aging. 2012;33(4):744–752. doi: 10.1016/j.neurobiolaging.2010.05.020. doi: 10.1016/j.neurobiolaging.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjadi SA, Patterson K, Arnold RJ, Watson PC, Nestor PJ. Primary progressive aphasia: a tale of two syndromes and the rest. Neurology. 2012;78(21):1670–1677. doi: 10.1212/WNL.0b013e3182574f79. doi: 10.1212/WNL.0b013e3182574f79 WNL.0b013e3182574f79 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnur TT, Schwartz MF, Brecher A, Hodgson C. Semantic interference during blocked-cyclic naming: Evidence from aphasia. Journal of Memory and Language. 2006;54:199–227. [Google Scholar]
- Schnur TT, Schwartz MF, Kimberg DY, Hirshorn E, Coslett HB, Thompson-Schill SL. Localizing interference during naming: convergent neuroimaging and neuropsychological evidence for the function of Broca's area. Proceedings of the National Academy of Sciences USA. 2009;106(1):322–327. doi: 10.1073/pnas.0805874106. doi: 10.1073/pnas.0805874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher A, Dell GS, Coslett HB. Anterior temporal involvement in semantic word retrieval: voxel-based lesion-symptom mapping evidence from aphasia. Brain. 2009;132(Pt 12):3411–3427. doi: 10.1093/brain/awp284. doi: 10.1093/brain/awp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichmann M, Kas A, Boutet C, Ferrieux S, Nogues M, Samri D, Migliaccio R. Deciphering logopenic primary progressive aphasia: a clinical, imaging and biomarker investigation. Brain. 2013;136(Pt 11):3474–3488. doi: 10.1093/brain/awt266. doi: 10.1093/brain/awt266. [DOI] [PubMed] [Google Scholar]
- Thompson CK. Northwestern Assessment of Verbs and Sentences (NAVS) Northwestern University; Evanston, IL: 2011. [Google Scholar]
- Thompson CK, Meltzer-Asscher A, Cho S, Lee S, Wieneke C, Weintraub S, Mesulam M-M. Syntactic and morphosyntactic processing in stroke-induced and primary progressive aphasia. Behavioral Neurology. 2013;26(1-2):35–54. doi: 10.3233/BEN-2012-110220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Ballard KJ, Tait ME, Weintraub S, Mesulam M. Patterns of language decline in non-fluent primary progressive aphasia. Aphasiology. 1997;4/5:297–321. [Google Scholar]
- Thompson CK, Cho S, Hsu C-J, Wieneke C, Rademaker A, Weitner BB, Weintraub S. Dissociations between fluency and agrammatism in primary progressive aphasia. Aphasiology. 2012a;26(1):20–43. doi: 10.1080/02687038.2011.584691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Cho S, Price C, Wieneke C, Bonakdarpour B, Rogalski E, Mesulam MM. Semantic interference during object naming in agrammatic and logopenic primary progressive aphasia (PPA). Brain and Language. 2012b;120(3):237–250. doi: 10.1016/j.bandl.2011.11.003. doi: 10.1016/j.bandl.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Lukic S, King MC, Mesulam MM, Weintraub S. Verb and noun deficits in stroke-induced and primary progressive aphasia: The Northwestern Naming Battery. Aphasiology. 2012c;26(5):632–655. doi: 10.1080/02687038.2012.676852. doi: 10.1080/02687038.2012.676852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Meltzer-Asscher A, Cho S, Lee J, Wieneke C, Weintraub S, Mesulam MM. Syntactic and morphosyntactic processing in stroke-induced and primary progressive aphasia. Behavioral Neurology. 2013;26(1-2):35–54. doi: 10.3233/BEN-2012-110220. Doi: 10.3233/BEN-2012-110220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Shapiro LP, Tait ME, Jacobs BJ, Schneider SS, Ballard K. A system for the linguistic analysis of agrammatic language production. Brain and Language. 1995;51:124–129. [Google Scholar]
- Thompson CK, Weintraub S. Northwestern Naming Battery (NNB) Northwestern University; Evanston, IL: 2014. [Google Scholar]
- Thompson CK, Weintraub S, Mesulam M. Northwestern Anagram Test (NAT) Northwestern University; Evanston, IL: 2012. [Google Scholar]
- Vigliocco G, Vinson DP, Druks J, Barber H, Cappa SF. Nouns and verbs in the brain: A review of behavioural, electrophysiological, neuropsychological, and imaging studies. Neuroscience & Biobehavioral Reviews. 2011;35(3):407–426. doi: 10.1016/j.neubiorev.2010.04.007. doi:10.1016/j.neubiorev.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler memory scale (WMS-III) Psychological Corporation; 1997. [Google Scholar]
- Wertz RT, LaPointe LL, Rosenbek JC. Apraxia of Speech in Adults: The Disorder and its Management. Grune & Stratton; Orlando, FL: 1984. [Google Scholar]
- Wicklund MR, Duffy JR, Strand EA, Machulda MM, Whitwell JL, Josephs KA. Quantitative application of the primary progressive aphasia consensus criteria. Neurology. 2014;82(13):1119–1126. doi: 10.1212/WNL.0000000000000261. doi: 10.1212/WNL.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Henry ML, Besbris M, Ogar JM, Dronkers NF, Jarrold W, Gorno-Tempini ML. Connected speech production in three variants of primary progressive aphasia. Brain. 2010;133(Pt 7):2069–2088. doi: 10.1093/brain/awq129. doi: 10.1093/brain/awq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Isenberg AI, Hickok G. Neural correlates of word production stages delineated by parametric modulation of psycholinguistic variables. Human Brain Mapping. 2009;30:3596–3608. doi: 10.1002/hbm.20782. doi: 10.1002/hbm.20782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zubicaray GI, McMahon KL, Eastburn MM, Wilson SJ. Orthographic/phonological facilitation of naming responses in the picture-word task: an event-related fMRI study using overt vocal responding. Neuroimage. 2002;16(4):1084–1093. doi: 10.1006/nimg.2002.1135. [DOI] [PubMed] [Google Scholar]