Abstract
OBJECTIVE
To determine whether there is a threshold 3-hour OGTT value associated with accelerated risk of adverse pregnancy outcomes.
STUDY DESIGN
In a secondary analysis of a cohort of women with untreated mild gestational glucose intolerance, defined as 50 gram glucose loading test between 135 and 199 mg/dL and fasting glucose <95 mg/dL, we used generalized additive models with smoothing splines to explore non-linear associations between each of the 3-hour OGTT values (fasting, 1-h, 2-h, and 3-h) and adverse pregnancy outcomes, including the study’s composite outcome (perinatal mortality, hypoglycemia, hyperbilirubinemia, neonatal hyperinsulinemia, and/or birth trauma), large-for-gestational age birth weight, small-for-gestational age birth weight, shoulder dystocia, neonatal hypoglycemia, gestational hypertension and preeclampsia.
RESULTS
Among 1360 eligible women, each timed OGTT value was linearly associated with increased odds of composite adverse outcome. We found evidence of a departure from linearity only for the association between fasting glucose and gestational hypertension/preeclampsia (gHTN), with a stronger association for values of 85-94 mg/dL (p=0.03). We found no evidence of departure from linearity for any other OGTT values and measured outcomes (all chi-square test p-values ≥0.05).
CONCLUSION
In a population of untreated women with mild gestational glucose intolerance and fasting OGTT < 95 mg/dL, we found an increasing risk of gestational hypertension with fasting glucose between 85 and 94 mg/dL.
Keywords: maternal glycemia, gestational diabetes, GDM
Introduction
Gestational diabetes (GDM) affects 2.2 to 8.8 percent of pregnant women. Treatment of impaired glucose tolerance during pregnancy, whether with diet, oral hypoglycemics, or insulin, can reduce perinatal morbidity and may also reduce the infant’s risk of obesity in later life 1, 2.
Since O’Sullivan first described gestational glucose intolerance3, progressively lower thresholds have been used to make the diagnosis of gestational diabetes. Recent data from a large, observational cohort study demonstrated that the risk of adverse outcomes increases with increasing glucose values, even among women with sub-threshold results in fasting and 2-hour post-load glucose screening4. These results have led to lower proposed thresholds for diagnosis and treatment of gestational diabetes5. It is not known, however, whether there is a glucose tolerance threshold above which there is an acceleration in risk of adverse outcomes. Such a glucose threshold could be used as a natural cut-point to identify women at elevated risk of adverse outcomes.
We therefore tested whether there is non-linear relationship between glucose tolerance measured on the 3-hour oral glucose tolerance test and adverse pregnancy outcomes in a cohort of women with untreated mild gestational glucose intolerance.
Methods
We performed a secondary analysis of women with untreated mild gestational glucose intolerance. Participants were derived from two groups: 1) women randomized to no treatment in the previously reported Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network multicenter randomized trial of treatment for mild gestational diabetes (GDM); and 2) women in the associated observational cohort, comprised of women with a 50-gram glucose screen ≥ 135 mg/dL who did not meet criteria for gestational diabetes. 1
To be eligible for participation in the primary study, women had to be between 24 weeks 0 days and 30 weeks 6 days gestation and have a 50-gram glucose loading test screen between 135 and 199 mg/dL. Eligible women underwent diagnostic testing with a 100-gram 3-hour OGTT. Women with normal fasting values (< 95 mg/dL) but at least 2 OGTT values exceeding established thresholds (1h 180 mg/dL, 2h 155 gm/dL, 3h 140 mg/dL) were randomized to treatment for mild GDM or usual care. In addition, women with normal OGTT results were followed in an observational cohort. In the primary study, participants in the observational cohort were frequency matched at each participating center to the GDM group by body mass index < or ≥ 27 kg/m2 and race/ethnicity (black, Hispanic, or non-black, non-Hispanic). Further details of the methodology of the study have been described elsewhere.1 Women were excluded from the primary study if they had any of the following conditions: preexisting diabetes, an abnormal result on a glucose screening test < 24 wks, or prior GDM; history of stillbirth, multifetal gestation, asthma, or chronic hypertension; active corticosteroids use; fetus with a known fetal anomaly, or were likely to have an imminent preterm delivery.
For the current analysis, we included women who were randomized to usual care or who were enrolled in the observational cohort (N=1360) in order to study the natural history of blood glucose concentration in the absence of treatment. For outcome vs. OGTT analyses, women with missing values for an outcome were excluded from the analysis for that outcome (primary outcome, N=57; gestational hypertension/preeclampsia, N=1; preeclampsia, N=1; LGA, N=1; SGA, N=1; shoulder dystocia, N=0; and neonatal hypoglycemia, N=288).
Measurement of exposures
Maternal glucose tolerance was measured using the 100-gram 3-hour Oral Glucose Tolerance Test (OGTT) after an overnight fast. Maternal age, self-reported race/ethnicity and parity were obtained by patient interview at the time of enrollment. Gestational age was confirmed by ultrasound prior to the OGTT
Measurement of outcomes
In this secondary analysis, we measured the association between maternal OGTT results and the primary outcome of the parent study, as well as gestational hypertension/preeclampsia, preeclampsia, LGA, SGA, shoulder dystocia, and neonatal hypoglycemia. The primary outcome of the parent study was a composite outcome of perinatal mortality, hypoglycemia, hyperbilirubinemia, neonatal hyperinsulinemia, or birth trauma1. Gestational hypertension was defined as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg or more on two occasions at least 4 hours apart, or one elevated blood-pressure value subsequently treated with medication. Preeclampsia was defined as elevation in blood pressure, as defined for gestational hypertension, with proteinuria (≥ 300 mg of protein / 24-hour collection or dipstick ≥2+ if 24-hour collection not available) or with AST ≥ 70 U/L or platelet count < 100,000/L. Large for gestational age (LGA) birth weight was defined as > 90% for gestational age, and small for gestational age (SGA) was defined as birth weight < 10% for gestational age6.
Analysis
Statistical analyses were performed using SAS statistical software (SAS Institute, Cary, NC) and R (www.r-project.org). We used the generalized additive models (GAM) with smoothing splines7 to explore non-linear associations between each 3-hour OGTT parameter and adverse outcome. Generalized additive models use non-parametric techniques to identify non-linear relationships between an exposure and an outcome. For example, both adolescent pregnancy and advanced maternal age are associated with adverse pregnancy outcomes, compared with pregnancies in women in their 20s and 30s. A simple logistic regression model measuring the association between maternal age and adverse pregnancy outcome would not detect this association, because it assumes a linear relationship between age and outcome, while a general additive model could detect a U-shaped association between these two variables. For the present analysis, we allowed for such departures from linearity, allowing for associations between OGTT values and outcome to increase, decrease or plateau across the range of values observed. If such an inflection point were identified, it might provide a natural cut-point for diagnosing pathological glucose intolerance. We set two as the target equivalent degrees of freedom (smoothing parameter). We considered a departure from linearity to be significant if the chi-square test for the non-linear splines showed a p-value < 0.05. If no non-linear association was found, we reduced the model to logistic regression with a linear term for each 3-hour OGTT parameter. We present figures with unadjusted predicted probabilities and 95% confidence intervals for each outcome for which we found a statistically significant association in order to illustrate their clinical utility for risk stratification. All models quantified the univariate association between an OGTT parameter and outcome, without adjustment for other factors.
Results
Of the 1360 participants eligible for inclusion in our study, 775 were Hispanic (57.0%), 362 were white (26.6%), 165 were black (12.1%) and 58 were of other race/ethnicity (4.3%). The mean maternal age was 27.9 (SD 5.6), and 32.9% were nulliparous (Table 1). About 1/3 of participants met criteria for composite adverse neonatal outcome (Table 2).
Table 1.
Characteristics of the study population (N=1360)
| N (%) | |
|---|---|
| Race/Ethnicity | |
| Black | 165 (12.1) |
| White | 362 (26.6) |
| Hispanic | 775 (57.0) |
| Other | 58 (4.3) |
| Nulliparous | 448 (32.9) |
| BMI | |
| Normal BMI (<25 kg/m2) | 656 (48.2) |
| Overweight BMI (25-<30 kg/m2) | 410 (30.2) |
| Obese BMI (≥30 kg/m2) | 294 (21.6) |
| Mean (SD) | |
| BMI (kg/m2) n=1250 | 26.6 (5.6) |
| Age, mean (SD) | 27.9 (5.6) |
| Fasting glucose | 85.3 (5.8) |
| 1h glucose, mg/dL | 166.9 (30.0) |
| 2h glucose | 144.2 (29.6) |
| 3h glucose | 116.9 (28.3) |
Table 2.
Prevalence of outcomes of interest (N=1360)
| Outcome | N (%) |
|---|---|
| N | 1360 |
| Composite outcome | 442 (33.9) |
| LGA | 161 (11.9) |
| SGA | 90 (6.6) |
| gHTN or preeclampsia | 145 (10.7) |
| Preeclampsia | 61 (4.5) |
| Shoulder dystocia | 35 (2.6) |
| Hypoglycemia | 175 (16.3) |
In our generalized additive models, we found no evidence of a non-linear association between any OGTT parameter and composite adverse neonatal outcome, preeclampsia, LGA, SGA, shoulder dystocia, or neonatal hypoglycemia (all chi-square test p-values ≥ 0.05). We did find evidence of non-linearity for the association between fasting glucose and gestational hypertension/preeclampsia, with an increase in the slope of the association for glucose values between 85 and 94 (chi-square test p-value=0.03). Within our study population, 59.4% had a glucose value between 85 and 94.
We found linear associations between 3-hour OGTT parameters and the primary outcome (all OGTT parameters), LGA (fasting, 1- and 2-hour), gestational hypertension/preeclampsia (1-, 2- and 3-hour ), and shoulder dystocia (1- and 2-hour), as illustrated in figure 1. We found no evidence of an association between OGTT parameters and preeclampsia, SGA or neonatal hypoglycemia.
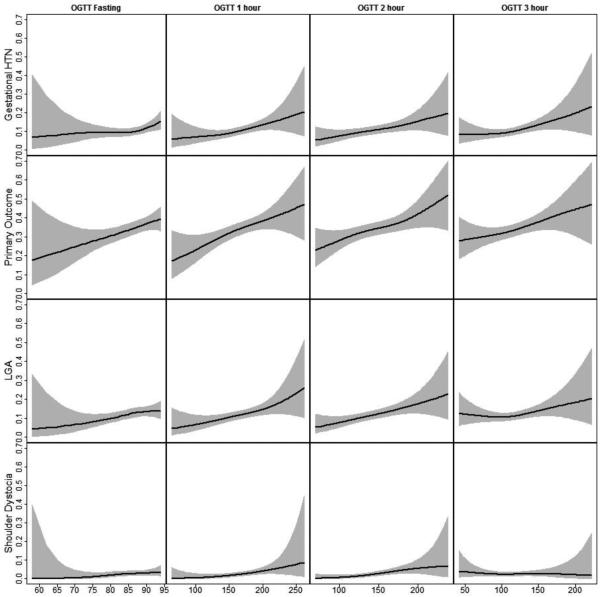
Figure.
Non-parametric associations between OGTT values and pregnancy outcomes. The black line indicates the predicted probability of an outcome, and the grey region indicates the 95% confidence interval around that predicted probability. We found a statistically significant departure from linearity for fasting glucose and gestational hypertension/preeclampsia (top left), with an increase in the slope of the association for glucose values between 85 and 94 mg/dL (top left). We found linear associations between 3-hour OGTT parameters and gestational hypertension/preeclampsia (1-, 2- and 3-hour ), the primary outcome (all OGTT parameters), LGA (fasting, 1- and 2-hour), and shoulder dystocia (1- and 2-hour).
Comment
In a prospective study of women with mild glucose intolerance and fasting glucose <95 mg/dL, we found evidence of a non-linear association between fasting glucose and gestational hypertension/preeclampsia, with an acceleration in risk for fasting glucose values from 85 through 94 mg/dL. We did not find evidence of a departure from linearity for 3-hour OGTT parameters and other outcomes.
Our findings confirm and extend earlier work linking mild glucose intolerance with adverse perinatal outcomes. In the HAPO study 4, authors reported a direct association between fasting glucose and adverse pregnancy outcomes among women with fasting glucose < 105 mg/dL and 2-hour, 75g OGTT < 200 mg/dL at 24 to 32 weeks’ gestation. Other authors have reported a linear increase in adverse outcomes with increasing 3-hour OGTT results among women without GDM by NDDG criteria8.
We found that almost all of the associations between blood glucose concentration and adverse pregnancy outcomes were linear. These results suggest that, within the range of concentrations we observed, there was no threshold below which the risk of adverse outcomes stopped decreasing, nor above which the risk remained stable. Rather, within this range, higher concentrations were always associated with increased risk of adverse outcome. Therefore, there is no "natural" cutoff to define gestational diabetes, and cutoff values should be based on the benefits versus the risks of treatment, as well as the burden to both the individual and the health care system of making a diagnosis of GDM.
Our finding that fasting glucose values of 85-94 mg/dL were more strongly associated with gHTN may reflect differences in the pathophysiology of fasting compared with post-load glucose intolerance. Fasting glucose reflects basal insulin secretion and hepatic insulin sensitivity. Post-load glucose regulation reflects increased insulin secretion and peripheral insulin sensitivity9, as well as diminished ability of insulin to suppress hepatic glucose production10. Our finding of a non-linear association between fasting glucose and gestational hypertension suggests that factors affecting basal insulin secretion and hepatic insulin sensitivity may also play a role in gestational hypertension. To the extent that impaired fasting glucose is correlated with body mass index, these results may also reflect greater adiposity among women with higher fasting glucose values. In a prior analysis of the same group of women, we found that pregravid BMI was associated with risk of gestational hypertension, independent of glucose tolerance11.
Strengths of our study include standardized, prospective assessment of glucose tolerance and obstetrical outcomes. Our multicenter design includes women from 14 centers in the United States, increasing generalizability. Nevertheless, our findings must be interpreted in the context of the study design. This is a secondary analysis of participants in a clinical trial, and enrollment was limited to women with glucose loading test results of 135 to 199 mg/dL and fasting glucose < 95mg/dL. Our results are therefore only generalizable to women with mild glucose intolerance. This analysis does not address glycemic control, but rather the prognostic implications of results of the 3-hour OGTT, Multiple testing is also a concern. We tested for non-linear associations for 4 different glucose parameters and seven outcomes, and, with a p value of 0.05, we would expect at least one statistically significant result by chance alone. Further studies will be needed to confirm our findings in other patient populations.
In conclusion, we found tentative evidence of a non-linear association between fasting glucose and gestational hypertension/preeclampsia among women with mild gestational glucose intolerance. We found no evidence of any other threshold values in the 3-hour oral glucose tolerance test above which there is a non-linear increase in adverse perinatal outcomes.
Acknowledgments
The project described was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) [HD27915, HD34116, HD40485, HD34208, HD27869, HD40500, HD40560, HD34136, HD40544, HD27860, HD40545, HD53097, HD21410, HD27917, HD40512, HD53118, HD36801], General Clinical Research Centers Grant [M01-RR00034] and the National Center for Research Resources [UL1-RR024989, M01-RR00080, UL1-RR025764, C06-RR11234 ]. Comments and views of the authors do not necessarily represent views of the NICHD.
The authors thank the following MFMU Network members who participated in protocol development and coordination between clinical research centers (Francee Johnson, R.N., M.S.N. and Jo-Ann Tillinghast, R.N., M.S.N.), protocol/data management and statistical analysis (Elizabeth Thom, Ph.D. and Lisa Mele, M.Sc.), and protocol development and oversight (Marshall W. Carpenter, M.D. and Catherine Y. Spong, M.D.).
Footnotes
In addition to the authors, other members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network are as follows:
University of North Carolina at Chapel Hill — J. Thorp, K. Dorman, S. Brody, S. Timlin, J. Bernhardt University of Texas Southwestern Medical Center — B. Casey, K. Leveno, L. Moseley, J. Gold, D. Bradford, L. Fay, M. Garcia, F. Capellan
Columbia University — M. Miodovnik, F. Malone, S. Bousleiman, H. Husami, V. Carmona, N. Fredericks, E. Gantioqui, B. Greenspan, M. Williams
University of Utah — K. Anderson (University of Utah Health Sciences Center), P. Ashby (University of Utah Health Sciences Center), S. McAllister (University of Utah Health Sciences Center), S. Quinn (LDS Hospital), A. Guzman (McKay-Dee Hospital), F. Castinella (LDS Hospital), J. Steiner (McKay-Dee Hospital), J. Parker (Utah Valley Regional Medical Center)
University of Alabama at Birmingham — J. Sheppard, J. Tisdale, A. Northen, W. Andrews
Brown University — M. Carpenter, D. Catlow, D. Allard, M. Seebeck, J. Tillinghast
The Ohio State University — J. Iams, F. Johnson, C. Latimer, E. Weinandy, B. Maselli
Drexel University — M. Hoffman, E. Guzman, M. Talucci, T. Grossman, C. Perez, L. Zeghibe, P. Tabangin
Case Western Reserve University-MetroHealth Medical Center — B. Mercer, B. Stetzer, C. Milluzzi, W. Dalton, S. Pichette
Wake Forest University Health Sciences — M. Harper, M. Swain, P. Meis, J. White
The University of Texas Health Science Center at Houston — L. Gilstrap, K. Cannon, J. Martinez, D. Dusek
University of Texas Medical Branch — J. Moss, J. Brandon, A. Jackson, G. Hankins, D. Sharp
University of Pittsburgh — S. Caritis, M. Bickus, H. Birkland, M. Cotroneo, N. Cuddy
Wayne State University — G. Norman, P. Lockhart, S. Blackwell, L. Quast
Northwestern University — P. Simon, G. Mallett
Oregon Health & Science University — J. Tolosa, L. Davis, E. Lairson, C. Cromett, C. Naze, M. Blaser
The George Washington University Biostatistics Center — E. Thom, L. Mele, J. Zachary, B. Getachew, C. Cobb, L. Leuchtenburg, S. Gilbert
Eunice Kennedy Shriver National Institute of Child Health and Human Development — C. Spong, S. Tolivaisa, K. Howell
MFMU Network Steering Committee Chair (University of Texas Medical Branch, Galveston, TX) — G.D. Anderson, M.D.
Preliminary results were presented at the Society for Maternal-Fetal Medicine, San Francisco, CA, February 11, 2011.
References
- 1.Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. The New England journal of medicine. 2009;361:1339–48. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. The New England journal of medicine. 2005;352:2477–86. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 3.O'Sullivan JB. Gestational diabetes. Unsuspected, asymptomatic diabetes in pregnancy. The New England journal of medicine. 1961;264:1082–5. doi: 10.1056/NEJM196105252642104. [DOI] [PubMed] [Google Scholar]
- 4.The Hapo Study Cooperative Research Group. Hyperglycemia and Adverse Pregnancy Outcomes. The New England journal of medicine. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 5.Metzger BE, Buchanan TA, Coustan DR, et al. Summary and Recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30:S251–60. doi: 10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]
- 6.Alexander GR, Kogan MD, Himes JH. 1994-1996 U.S. singleton birth weight percentiles for gestational age by race, Hispanic origin, and gender. Matern Child Health J. 1999;3:225–31. doi: 10.1023/a:1022381506823. [DOI] [PubMed] [Google Scholar]
- 7.Hastie T, Tibshirani R. Generalized additive models. Chapman and Hall; London: Number of pages. [Google Scholar]
- 8.Sermer M, Naylor CD, Gare DJ, et al. Impact of increasing carbohydrate intolerance on maternal-fetal outcomes in 3637 women without gestational diabetes. The Toronto Tri-Hospital Gestational Diabetes Project. American journal of obstetrics and gynecology. 1995;173:146–56. doi: 10.1016/0002-9378(95)90183-3. [DOI] [PubMed] [Google Scholar]
- 9.Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. 2002;19:708–23. doi: 10.1046/j.1464-5491.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 10.Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. American journal of obstetrics and gynecology. 1999;180:903–16. doi: 10.1016/s0002-9378(99)70662-9. [DOI] [PubMed] [Google Scholar]
- 11.Stuebe AM, Landon MB, Lai Y, et al. Maternal BMI, glucose tolerance, and adverse pregnancy outcomes. American journal of obstetrics and gynecology. 2012;207 doi: 10.1016/j.ajog.2012.04.035. 62.e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]