Abstract
Olanzapine (OLZ), an atypical antipsychotic, can be effective in treating patients with restricting type anorexia nervosa who exercise excessively. Clinical improvements include weight gain and reduced pathological hyperactivity. However the neuronal populations and mechanisms underlying OLZ actions are not known. We studied the effects of OLZ on hyperactivity using male mice lacking the hypothalamic neuropeptide melanin-concentrating hormone (MCHKO) that are lean and hyperactive. We compared the in vivo effects of systemic or intra-accumbens nucleus (Acb) OLZ administration on locomotor activity in WT and MCHKO littermates. Acute systemic OLZ treatment in WT mice significantly reduced locomotor activity, an effect that is substantially attenuated in MCHKO mice. Furthermore, OLZ infusion directly into the Acb of WT mice reduced locomotor activity, but not in MCHKO mice. To identify contributing neuronal mechanisms, we assessed the effect of OLZ treatment on Acb synaptic transmission ex vivo and in vitro. Intraperitoneal OLZ treatment reduced Acb GABAergic activity in WT but not MCHKO neurons. This effect was also seen in vitro by applying OLZ to acute brain slices. OLZ reduced the frequency and amplitude of GABAergic activity that was more robust in WT than MCHKO Acb. These findings indicate that OLZ reduced Acb GABAergic transmission and that MCH is necessary for the hypolocomotor effects of OLZ.
Keywords: anorexia, accumbens nucleus, mch, antipsychotic, locomotion, electrophysiology
Introduction
Anorexia nervosa is characterized by an inability to maintain a minimally normal body weight, starvation behaviors and intense fear of weight gain. In restricting type anorexia, individuals limit the amount of food they eat while exercising excessively. This hyperactivity reflects the underlying anxiety and compulsivity driving destructive body image and restrictive behaviors (Davis and Kaptein, 2006) and plays a critical role in disorder progression (Davis et al., 1997).
Most second generation, atypical antipsychotics used to treat schizophrenia and other psychiatric disorders are associated with weight gain (Almandil et al., 2013). This led to the evaluation of antipsychotics for treating anorexia (Brewerton, 2012). Olanzapine (OLZ), more commonly linked with weight gain than other antipsychotics, is among the most effective drugs for treating anorexia (Brewerton, 2012). Some studies do not find a therapeutic effect of OLZ (Kishi et al., 2012) but indeed, some anorexic patients treated with OLZ gained weight, showed improved attitudes towards eating and reduced their activity levels (Dennis et al., 2006; Dunican and DelDotto, 2007). In rodent models of activity-based anorexia, where food-restricted rodents housed with running wheels develop paradoxical hyperactivity and severe weight loss (Epling et al., 1983), both acute (Prinssen et al., 2000) and chronic OLZ treatment (Albaugh et al., 2011; Hillebrand et al., 2005; Klenotich et al., 2012) reduced locomotor activity and prolonged survival (Hillebrand et al., 2005; Klenotich et al., 2012).
Mice deficient in melanin-concentrating hormone (MCH), a hypothalamic neuropeptide that stimulates food intake (Tritos et al., 1998) and weight gain (Della-Zuana et al., 2002; Gomori et al., 2003), may also represent one model of anorexia (Siegfried et al., 2003). MCHKO mice are lean, hypophagic (Shimada et al., 1998) and hyperactive (Kokkotou et al., 2005; Zhou et al., 2005). They do not elicit homeostatic increases in food intake despite a steadfast demonstration of hyperactivity and higher energy expenditure (Alon and Friedman, 2006; Kokkotou et al., 2005; Whiddon and Palmiter, 2013; Zhou et al., 2005). Interestingly, the orexigenic actions of OLZ and MCH can act synergistically, such as in the accumbens nucleus (Acb) (Guesdon et al., 2010). The Acb is a critical region controlling motivated behavior and motor control (Graybiel et al., 1994) and integrates both feeding (Georgescu et al., 2005; Guesdon et al., 2009) and locomotor actions of MCH (Pissios et al., 2008).
OLZ may treat anorexia by reducing hyperactivity (Leggero et al., 2010) but its mechanisms of action are not known. Using the MCHKO as a mouse model of hyperactivity, we investigated the effect of OLZ to reduce locomotor activity. We demonstrated that the Acb partly mediates the hypolocomotor effects of OLZ, and then performed electrophysiological recordings from Acb medium spiny neurons (MSNs) to study the neuronal mechanisms underlying OLZ actions. OLZ treatment reduced GABAergic transmission at Acb MSNs. Both the behavioral and cellular actions of OLZ were less robust in MCHKO mice. These findings demonstrate that MCH is necessary for enabling the maximal effects of OLZ, which acts by suppressing local GABAergic transmission in the Acb and inhibit locomotor activity.
Experimental Procedures
Animals
Male WT and MCHKO littermates were backcrossed onto the C57BL/6 background for >20 generations (Kokkotou et al., 2005; Shimada et al., 1998). All mice were housed in a 12-hour light-dark cycle at 22 °C with ad libitum access to food (#5058, LabDiet) and water. All procedures were conducted following accepted guidelines of the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committees.
Wheel-running activity
Baseline wheel-running activity of male WT and MCHKO littermates (11–16 weeks old) was averaged over 5 days after acclimation (7 days) to running wheels (11.5 cm diameter; Mini Mitter). The number of wheel revolutions was monitored using a magnetic reed switch and recorded using VitalView (Mini Mitter) data acquisition hardware and software. The effect of acute OLZ administration on wheelrunning activity was assessed in WT and MCHKO mice injected intraperitoneally (ip) with vehicle (0.16% acetic acid (AcOH) in saline) or OLZ (2.5 mg/kg) 30 min before onset of the dark cycle.
Intra-Acb administration
WT and MCHKO mice (12–16 weeks old) were anesthetized with ketamine (100 µg/kg, ip; Butler Schein)-xylazine (10 µg/kg, ip; Butler Schein) and a bilateral 4.2 mm guide cannula (Plastics One) was stereotaxically placed using coordinates: anterioposterior +1.20 mm, mediolateral ±0.60 mm, dorsoventral −4.10 mm (Paxinos and Franklin, 2001). The tip of the cannula was dorsomedial to the anterior commissure but within the medial Acb. After surgery, mice were singly-housed in a homecage free of suspended obstructions (ie. food hoppers, wire mesh) to prevent the cannula from dislodging. Homecage locomotor activity was assessed over 24-hour periods by an Opto-M3 infrared beam break monitoring system (Columbus Instruments) to detect the number of sequential beam breaks along the x-axis. A Multi Device Interface (v1.3; Columbus Instruments) recorded the number of X-ambulations. After one week to recover from surgery, each mouse was habituated to handling for 6 consecutive days before intra-Acb treatment.
All intra-Acb infusions began 30 min before the dark cycle. Using a 33 G bilateral injector, we bilaterally infused WT and MCHKO mice with 1 µl of vehicle (0.7% DMSO, 0.01% AcOH in ACSF [technical information, Alzet], pH 7) on Day 1 and then 1 µl of 0.74 mM OLZ (0.23 µg per side) on Day 2. Solutions are sequentially delivered over 4 min (0.25 µl/min) to each side of the Acb, waiting 2 min after each infusion before pulling out the injector to allow the solution to be absorbed by the Acb and prevent backflow up the cannula. All mice were returned to their respective homecage and locomotor activity was monitored for 4 hours post-infusion.
At the end of the treatment period, bilateral cannula tips were coated with Dil stain (Life Technologies) to mark their placement in the Acb. The mice were deeply anesthetized with an overdose of ketamine-xylazine (ip) then transcardially perfused with chilled (4 °C) 0.9% NaCl saline followed with 10% buffered-formalin. The brains were removed, post-fixed, cryoprotected with 20% sucrose and 30 µm thick coronal sections were cut on a freezing microtome (SM20000R; Leica) into four equal series. We mounted one brain series to check the cannula placement. Results from mice where one or both cannula tips landed outside the medial Acb were excluded from analysis.
Electrophysiology
To study the effects of OLZ ex vivo, WT and MCHKO mice (10–12 weeks) were ip injected with vehicle (0.16% AcOH in saline) or OLZ (2.5 mg/kg) during the first half of the light cycle. Acute brain slices were prepared 2 hours after ip injection, as described below, and patch-clamp recordings were obtained from slices within 3 hours thereafter.
Mice were anesthetized with 0.7% chloral hydrate (Sigma-Aldrich) then decapitated. The brains were rapidly removed and submerged in an ice-cold, carbogenated (95% O2/5% CO2) sucrose-based artificial cerebrospinal fluid (aCSF) containing (in mM) 210 sucrose, 2.5 KCl, 1.24 NaH2PO4, 10 MgCl2•6H2O, 10 glucose, 26 NaHCO3, 0.5 CaCl2, 1 ascorbic acid (310 mOsm/L). Coronal sections (250 µm) between Bregma 1.0 – 1.9 mm (Paxinos and Franklin, 2001) were cut with a vibrating microtome (VT1000S; Leica) then incubated in aCSF containing (in mM) 124 NaCl, 3 KCl, 1.3 MgSO4, 1.4 NaH2PO4, 10 glucose, 26 NaHCO3, 2.5 CaCl2 (301 mOsm/L) at 35 °C for 20 minutes, then maintained at 22 °C.
MSN recordings (32 °C) were obtained with 7–8 MΩ patch pipettes when backfilled with an internal solution containing (in mM) 228 CsMS, 11 KCl, 10 HEPES, 0.1 CaCl2, 1 EGTA, 5 MgATP, 0.3 NaGTP (285 mOsm/L; pH 7.23). Data were acquired with a Multiclamp 700B amplifier (Molecular Devices) and digitized via a Digidata 1440A interface (Molecular Devices) running pCLAMP 10.3 software (Molecular Devices). All traces were sampled at 5 kHz, filtered at 1 kHz and analyzed off-line.
We patched MSNs in the medial Acb shell between Bregma 1.1 – 1.5 mm that were located beneath the tip of the lateral ventricle and horizontally aligned with the anterior commissure. MSNs comprise more than 90% of striatal neurons (Tepper and Bolam, 2004) and are distinguished from interneurons based on their size. Spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded at a holding potential of −5 mV and appear as outward currents. Miniature inhibitory postsynaptic currents (mIPSCs) were recorded in 500 nM tetrodotoxin (TTX) to block action potential-dependent transmitter release. OLZ was bath applied for 7–8 minutes.
Drugs
All chemical agents were purchased from Sigma-Aldrich, except OLZ (Toronto Research Chemicals, North York, Canada) and TTX (Alomone Labs, Jerusalem, Israel).
Statistical Analysis
Synaptic events were analyzed using MiniAnalysis (Synaptosoft). Statistical comparisons were performed using GraphPad Prism (v5.02; GraphPad), unless indicated otherwise. Data are shown as mean ± SEM and the number of mice or cells per group (n) is indicated in parentheses within the figures. Group means were analyzed by the unpaired Student’s t test and data distribution was tested with the Shapiro-Wilk normality test where applicable. Differences observed over time were analyzed using repeated measures two-way (RM)-ANOVA followed by post hoc Bonferroni test. To generate cumulative probability plots, 200 IPSC events from each cell (ex vivo experiments) or condition (in vitro experiments) were pooled into each group. Differences in cumulative probability were determined by the Kolmogorov-Smirnov two-sample statistical test (KS-test) using MiniAnalysis (Synaptosoft). All significant differences were determined at p < 0.05. The exponential dose-response curve was fitted to a standard sigmoidal dose-response curve equation: Y = [Bottom + (Top-Bottom)/(1+10(LogEC50-X)•HillSlope)]. Representative sample traces were plotted using Axum 5.0 (MathSoft).
Results
Acute systemic OLZ treatment reduced wheel-running activity of WT but not MCHKO mice
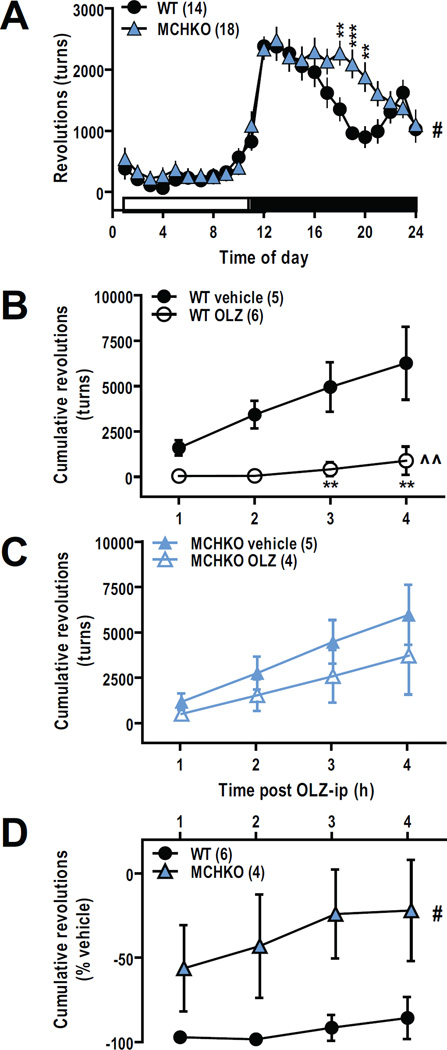
We assayed the MCHKO model of hyperactivity by comparing the baseline activity of MCHKO to WT littermates. Over a 24-hour period, MCHKO mice exhibited more wheel-running activity than WT littermates, a difference largely attributed to activity during the dark cycle (F(1,690) = 5.33, p < 0.05; Figure 1A).
Figure 1. Acute systemic OLZ treatment suppressed wheel-running activity of WT but not MCHKO mice.
A, Mean number of wheel revolutions averaged over 5 days show elevated baseline wheel-running activity of MCHKO mice. B–C, Comparison of cumulative wheel-running activity following intraperitoneal administration of vehicle (0.16% acetic acid) or OLZ (2.5 mg/kg) in WT (B) and MCHKO mice (C) show that OLZ reduced wheel-running activity of WT but not MCHKO mice. D, OLZ-mediated reduction in wheel-running was greater in WT than MCHKO mice. RM-ANOVA for WT vs MCHKO: #, p < 0.05; RM-ANOVA for WT vehicle vs WT OLZ: ^^, p < 0.01; Bonferroni post test: **, p < 0.01.
To determine the actions of OLZ on locomotor activity, we monitored the activity of WT and MCHKO mice following an acute ip injection of OLZ (2.5 mg/kg). OLZ-treated WT mice exhibited significantly less wheel-running activity than vehicle-treated counterparts (F(1,27) = 12.03, p < 0.005; Figure 1B). However even after 4 hours, OLZ had no significant effect on MCHKO mice (Figure 1C). Evidently, the reduction in wheel-running activity was greater in WT than MCHKO mice (F(1,24) = 7.07, p < 0.05; Figure 1D).
OLZ acts at the medial Acb to suppress locomotor activity
The hyperactivity of MCHKO mice is partly integrated at the Acb (Pissios et al., 2008), where synergistic OLZ and MCH actions were also reported (Guesdon et al., 2010). To determine if the Acb mediates inhibitory OLZ actions, we bilaterally infused vehicle (Day 1) or OLZ (Day 2) to each mouse (Figure 2A) and compared corresponding changes in locomotion. We removed the homecage running wheels after surgery to prevent the guide cannula from dislodging and monitored ambulatory beam break activity for up to 4 hours post treatment.
Figure 2. Bilateral infusion of OLZ into the medial accumbens reduced ambulatory activity of WT but not MCHKO mice.
A, Tip location of bilateral cannula placed in the medial Acb for all WT and MCHKO mice included in these experiments. Each cannula pair is color-matched. Ambulatory activity of each WT and MCHKO mouse after intra-Acb OLZ infusion (0.23 µg per side) was compared and normalized to vehicle (0.7% DMSO, 0.01% AcOH). B, Average baseline ambulation count along the x-axis (X-ambulations) over 5 days for MCHKO mice remained elevated following cannulation surgery. C, Comparison of percent decrease in ambulatory activity following intra-Acb vehicle versus OLZ infusion in individual WT and MCHKO mice. Unpaired t test: *, p < 0.05. D, Intra-Acb OLZ infusion reduced ambulatory activity of WT but not MCHKO mice. Inhibitory effects of OLZ in WT Acb were maximal within the first 2 hours but absent across all time points in MCHKO. RM-ANOVA for WT vs MCHKO: #, p < 0.05; Bonferroni post test: *, p < 0.05, **, p < 0.01.
Cannulation surgery did not affect locomotor activity of WT or MCHKO mice (F(1,299) = 6.34, p < 0.05; Figure 2B). After 2 hours, bilateral intra-Acb OLZ infusion reduced the ambulatory activity of WT mice by 50%; but had little effect in MCHKO mice (t(11) = 2.53, p < 0.05; Figure 2C). Over 4 hours, the effect of intra-Acb OLZ was more effective in WT than MCHKO mice (F(3,33) = 6.55, p < 0.05; Figure 2D).
OLZ treatment reduced GABAergic activity ex vivo in WT but not MCHKO Acb
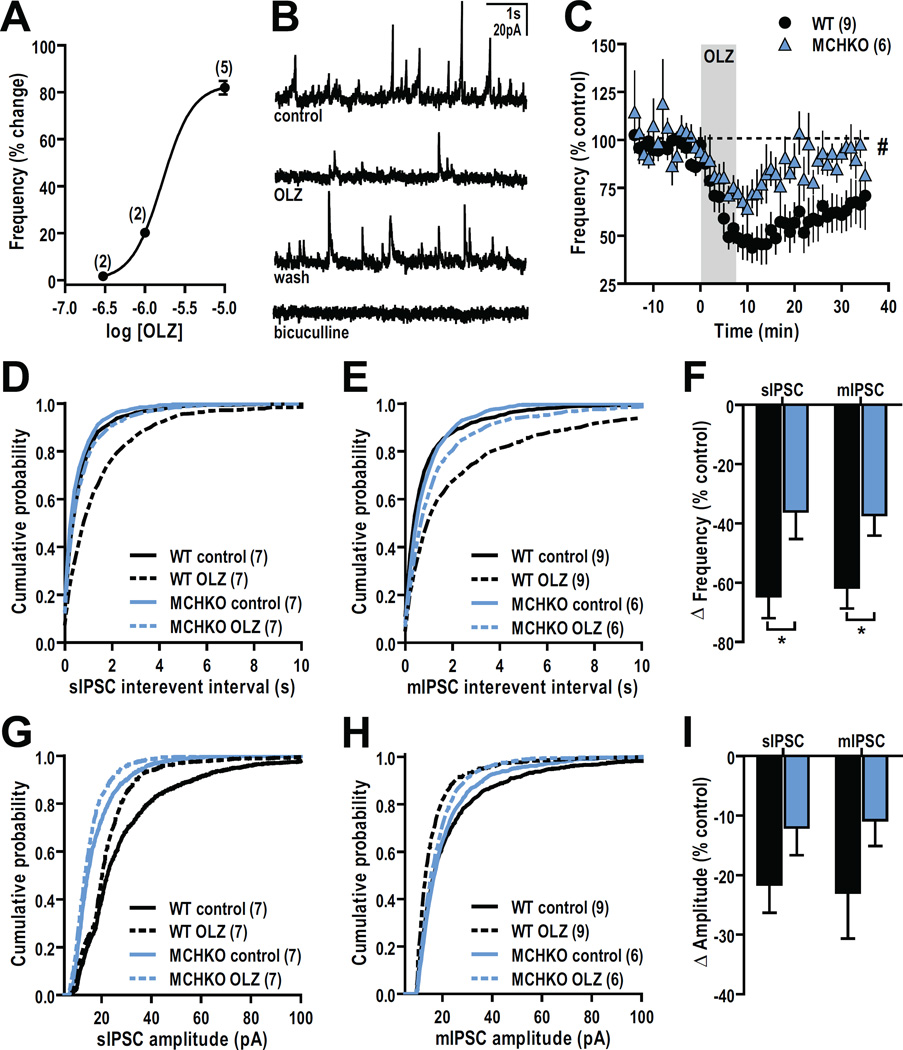
To determine the effect of OLZ in the Acb, we first determined if OLZ alters synaptic transmission between Acb MSNs. We tested whether OLZ alters intra-Acb GABAergic transmission in ex vivo MSN recordings within 2–5 hours of an acute ip injection of vehicle or OLZ (2.5 mg/kg).
Acb MSNs from OLZ-treated WT mice exhibited significantly lower sIPSC frequencies than MSNs from vehicle-treated WT mice (t(28) = 2.80, p < 0.01; Figure 3A). This reduction in WT GABAergic activity persisted in the presence of TTX to block activity-dependent transmission, thus suggesting an effect of OLZ at local MSNs (t(30) = 2.16, p < 0.05; Figure 3B). By contrast, MCHKO mice treated with vehicle or OLZ showed similar sIPSC (Figure 3A) and mIPSC frequencies (Figure 3B).
Figure 3. Systemic OLZ treatment reduced GABAergic activity in WT but not MCHKO MSNs ex vivo.
MSN recordings from WT and MCHKO Acb were obtained 2–5 hours after mice were injected intraperitoneally with vehicle (0.16% acetic acid; solid bars) or OLZ (2.5 mg/kg; hatched bars). A, Lower MSN sIPSC frequency from OLZ-treated WT but not MCHKO mice. B, MSNs pretreated and recorded in 500 nM TTX showed lower MSN mIPSC frequency from OLZ-treated WT but not MCHKO mice. Unpaired t test: *, p < 0.05, **, p < 0.01.
OLZ application in vitro reduced GABAergic synaptic transmission at Acb MSNs
We then identified the neuronal mechanisms underlying inhibitory OLZ actions by comparing the response of WT and MCHKO IPSCs to OLZ applied directly to naïve brain slices. Since MCHKO MSNs have an attenuated OLZ response, we conducted a dose-response curve using WT MSNs to select a maximal in vitro OLZ concentration so that even small OLZ effects in MCHKO MSNs may be detected. The change in sIPSC frequency with varied OLZ concentrations (300 nM – 10 µM) indicated that 10 µM OLZ is a near maximal dose (Figure 4A), which we applied in subsequent experiments. Pretreating our slices with bicuculline (10 µM) abolished all sIPSCs thus confirming that we are analyzing GABAA-mediated synaptic transmission (Figure 2B).
Figure 4. In vitro OLZ application produced a greater reduction in the frequency of GABAergic activity in WT than MCHKO MSNs.
A, Dose-response curve showing the reduction of IPSC frequency by different OLZ concentrations that reached a maximum at 10 µM OLZ. EC50 = 1.6 µM. B, Representative sIPSC sample traces before (control), during (10 µM OLZ), after OLZ washout (wash), and that were abolished by 10 µM bicuculline pretreatment. C, Time course of the change in GABAergic activity by bath application of OLZ showed a robust decrease in sIPSC frequency over time that was greater and longer-lasting in WT than MCHKO MSNs. RM-ANOVA for WT vs MCHKO: #, p < 0.05. D–E, OLZ bath application (dashed lines) to WT and MCHKO MSNs produced a right-shift in the cumulative probability plot of sIPSC (D) and mIPSC (E) interevent intervals compared to their respective controls (solid lines). K-S test for WT and MCHKO control vs OLZ, p < 0.0001. F, Decrease in sIPSC and mIPSC frequency was greater in WT than MCHKO MSNs. Unpaired t test: *, p < 0.05. G–H, Bath application of OLZ (dashed lines) to WT and MCHKO MSNs produced a left-shift in the cumulative probability plot of sIPSC (G) and mIPSC (H) amplitudes compared to their respective controls (solid lines). K-S test for WT and MCHKO control vs OLZ, p < 0.0001. I, OLZ reduced the amplitude of sIPSC and mIPSC amplitudes in WT and MCHKO MSNs.
Bath application of 10 µM OLZ reduced sIPSC frequency (Figure 4C) and produced a right-shift in the cumulative distribution plot of sIPSC interevent intervals from both WT (p < 0.0001) and MCHKO MSNs (p < 0.0001; Figure 4D). In the presence of TTX to block action potential-dependent transmission and isolate GABAergic activity originating within the brain slice, such as from local Acb MSNs, OLZ similarly right-shifted the cumulative distribution of mIPSC interevent intervals and reduced mIPSC frequency in both WT (p < 0.0001) and MCHKO MSNs (p < 0.0001) (Figure 4E). However the OLZmediated decrease in sIPSC (t(12) = 2.40, p < 0.05) and mIPSC frequency (t(13) = 2.34, p < 0.05) was nearly 2-fold greater in WT than MCHKO MSNs (Figure 4F). OLZ also affected the amplitude of GABAergic events, producing a leftward shift in the cumulative distribution of sIPSC (p < 0.0001; Figure 4G) and mIPSC amplitudes (p < 0.0001, Figure 4H). This reduction in amplitude was similar between WT and MCHKO MSNs (Figure 4I).
Discussion
MCH has well-documented orexigenic actions and can regulate locomotor activity. We show that MCH plays a key role in mediating the hypolocomotor effects of OLZ. We identified the Acb as one neural correlate mediating the hypolocomotor effects of OLZ, then determined its corresponding neuronal mechanism. OLZ administration peripherally or directly to the medial Acb suppressed the locomotor activity of WT mice. OLZ treatment reduced GABAergic activity at Acb MSNs. Interestingly, these effects were markedly attenuated in mice lacking MCH.
OLZ has been effective in treating restricting type anorexia nervosa, such as by reducing their pathological hyperactivity (Leggero et al., 2010). To explore its mechanisms that reduce hyperactivity, we tested its efficacy in MCHKO mice that have been proposed as a model of anorexia (Siegfried et al., 2003) because they are hyperactive but do not compensate by eating more. We did not measure food intake here since clear baseline hyperactivity can be distinguished. Surprisingly, we found that OLZ was substantially less effective in MCHKO mice, thus implicating MCH is necessary for OLZ actions. The Acb is a critical structure mediating the locomotor actions of MCH and OLZ. The Acb contains a high level of MCH receptor mRNA (Chee et al., 2013) and mediates the hyperactivity of MCHKO mice (Pissios et al., 2008). Furthermore, co-administration of OLZ with an MCH agonist has a synergistic effect to stimulate feeding when infused into the Acb (Guesdon et al., 2010). We show here that MCH is necessary for the actions of OLZ, especially those mediated by the Acb.
Systemic OLZ treatment nearly abolished all WT locomotor activity for at least 2 hours. Over the same time period, direct OLZ infusion into the medial Acb of WT mice reduced locomotor activity by more than 50% compared to vehicle-infused WT mice. This indicates that the Acb is one target of OLZ but concurrent effects in other brain regions are needed to fully inhibit locomotor activity. Potential candidate regions include the orexin neurons in the hypothalamus (Rasmussen et al., 2005), ventral tegmental area (Stockton and Rasmussen, 1996) and prefrontal cortex (Robertson and Fibiger, 1996; Sebens et al., 1998). These regions show an increase in c-fos immunoreactivity and/or neuronal firing with OLZ treatment and are implicated in locomotor activity, albeit via different mechanisms. For example, glutamatergic cortical efferents can regulate motor control (David, 2009), while locomotor actions of the ventral tegmental is mediated by cannabinoid signaling (Dubreucq et al., 2013) and/or innervation by orexin neurons (Rasmussen et al., 2007), which can also directly regulate locomotion (Nakamachi et al., 2006).
Additionally, we showed that OLZ actions in the Acb reduced MSN GABAergic activity. We observed a reduction of GABAergic activity ex vivo following systemic OLZ treatment as well as by applying OLZ to acute brain slices in vitro. Collateral projections between MSNs or from local interneurons that comprise the majority of GABAergic afferents in the Acb do not extend far from the soma (Koos et al., 2004; Tunstall et al., 2002). This suggests that the effect of OLZ on GABAergic synaptic transmission occur between proximal MSNs. The change in GABAergic frequency suggests a presynaptic site of action. By reducing the amplitude of GABAergic events, this further suggested mechanisms of OLZ action that can decrease GABAA receptor sensitivity (Skilbeck et al., 2008), induce GABAA receptor internalization (Chen et al., 2006) and reduce membrane density (Farnbach-Pralong et al., 1998). OLZ has no affinity for GABAA receptors and there is no consistent or direct correlation between GABAergic transmission and locomotor activity, as injection of GABA agonists may increase or decrease locomotion (Jones et al., 1981; Wachtel and Andén, 1978). However activation of direct (via dopamine D1 receptors) or indirect (via dopamine D2 receptors) striatal motor output pathways (Koos et al., 2004) can bias toward increased or decreased locomotion, respectively (Kravitz et al., 2010). OLZ increases dopamine D2 receptor binding (Vinish et al., 2013) and MSN D2 receptor activation decreases IPSC frequency (Watanabe et al., 2009) and amplitude (Kohnomi et al., 2012) similar to what we observe. Thus it is possible that OLZ preferentially activates D2-expressing MSNs to reduce locomotor activity. Moreover, D2 receptor availability may underlie OLZ insensitivity in MCHKO mice. Our results suggest that MCH-dependent Acb mechanisms are necessary for OLZ action. MCHKO hyperactivity is attributed to elevated dopamine release in the Acb (Pissios et al., 2008), which can lead to D2 receptor downregulation (Gomez-Sintes et al., 2014; Pissios et al., 2008). As OLZ preferentially increases D2 binding (Vinish et al., 2013) to inhibit locomotion, we speculate that in the absence of MCH, there is a reduced availability of D2 receptors, leading to the attenuation of OLZ action. Further work will define the precise mechanisms by which MCH changes the sensitivity to OLZ.
Despite the effectiveness of OLZ in the clinic, its mechanisms of actions are poorly understood. We showed here that the Acb is one brain region mediating the hypolocomotor actions of OLZ. At the synaptic level, OLZ reduced Acb GABAergic activity, which provided an effective readout for OLZ actions. It is possible that the efficacy of OLZ can be linked to MCH-dependent actions in the Acb. Although speculative, these findings implicate a potential role for MCH in anorexic patients that do not respond to OLZ.
Acknowledgements
We thank the NIH (Grant 5R01DK069983-02 to EMF; NRSA 5T32DK751627 to ND) and Davis Foundation (Postdoctoral fellowship to ACA) for their funding support.
Role of Funding Source
Funding for this study was provided by NIH Grant 5R01DK069983-02 and discretionary funds to EMF; Institutional Research Training Grant NRSA 5T32DK751627 to ND; Davis Foundation postdoctoral fellowship to ACA. None of these funding sources had a role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no competing financial interests.
Contributors
MJSC designed the study, performed experiments, analyzed data, wrote the manuscript. ND performed experiments and analyzed data. ABF, AM, SL, SEF performed experiments. ACA designed the study. EMF designed the study, wrote the manuscript. All authors have contributed to and approved the final manuscript.
References
- Albaugh VL, Judson JG, She P, Lang CH, Maresca KP, Joyal JL, Lynch CJ. Olanzapine promotes fat accumulation in male rats by decreasing physical activity, repartitioning energy and increasing adipose tissue lipogenesis while impairing lipolysis. Mol Psychiatry. 2011;16:569–581. doi: 10.1038/mp.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almandil NB, Liu Y, Murray ML, Besag FM, Aitchison KJ, Wong IC. Weight gain and other metabolic adverse effects associated with atypical antipsychotic treatment of children and adolescents: a systematic review and meta-analysis. Paediatr Drugs. 2013;15:139–150. doi: 10.1007/s40272-013-0016-6. [DOI] [PubMed] [Google Scholar]
- Alon T, Friedman JM. Late-onset leanness in mice with targeted ablation of melanin concentrating hormone neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:389–397. doi: 10.1523/JNEUROSCI.1203-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewerton TD. Antipsychotic agents in the treatment of anorexia nervosa: neuropsychopharmacologic rationale and evidence from controlled trials. Curr Psychiatry Rep. 2012;14:398–405. doi: 10.1007/s11920-012-0287-6. [DOI] [PubMed] [Google Scholar]
- Chee MJ, Pissios P, Maratos-Flier E. Neurochemical characterization of neurons expressing melanin-concentrating hormone receptor 1 in the mouse hypothalamus. The Journal of comparative neurology. 2013;521:2208–2234. doi: 10.1002/cne.23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Kittler JT, Moss SJ, Yan Z. Dopamine D3 receptors regulate GABAA receptor function through a phospho-dependent endocytosis mechanism in nucleus accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:2513–2521. doi: 10.1523/JNEUROSCI.4712-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David HN. Towards a reconceptualization of striatal interactions between glutamatergic and dopaminergic neurotransmission and their contribution to the production of movements. Curr Neuropharmacol. 2009;7:132–141. doi: 10.2174/157015909788848893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Kaptein S. Anorexia nervosa with excessive exercise: a phenotype with close links to obsessive-compulsive disorder. Psychiatry Res. 2006;142:209–217. doi: 10.1016/j.psychres.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Davis C, Katzman DK, Kaptein S, Kirsh C, Brewer H, Kalmbach K, Olmsted MP, Woodside DB, Kaplan AS. The prevalence of high-level exercise in the eating disorders: etiological implications. Compr Psychiatry. 1997;38:321–326. doi: 10.1016/s0010-440x(97)90927-5. [DOI] [PubMed] [Google Scholar]
- Della-Zuana O, Presse F, Ortola C, Duhault J, Nahon JL, Levens N. Acute and chronic administration of melanin-concentrating hormone enhances food intake and body weight in Wistar and Sprague-Dawley rats. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2002;26:1289–1295. doi: 10.1038/sj.ijo.0802079. [DOI] [PubMed] [Google Scholar]
- Dennis K, Le Grange D, Bremer J. Olanzapine use in adolescent anorexia nervosa. Eat Weight Disord. 2006;11:e53–e56. doi: 10.1007/BF03327760. [DOI] [PubMed] [Google Scholar]
- Dubreucq S, Durand A, Matias I, Bénard G, Richard E, Soria-Gomez E, Glangetas C, Groc L, Wadleigh A, Massa F, Bartsch D, Marsicano G, Georges F, Chaouloff F. Ventral tegmental area cannabinoid type-1 receptors control voluntary exercise performance. Biol Psychiatry. 2013;73:895–903. doi: 10.1016/j.biopsych.2012.10.025. [DOI] [PubMed] [Google Scholar]
- Dunican KC, DelDotto D. The role of olanzapine in the treatment of anorexia nervosa. Ann Pharmacother. 2007;41:111–115. doi: 10.1345/aph.1H297. [DOI] [PubMed] [Google Scholar]
- Epling W, Pierce W, Stefan L. A theory of activity-based anorexia. International Journal of Eating Disorders. 1983;3:27–49. [Google Scholar]
- Farnbach-Pralong D, Bradbury R, Copolov D, Dean B. Clozapine and olanzapine treatment decreases rat cortical and limbic GABA(A) receptors. Eur J Pharmacol. 1998;349:R7–R8. doi: 10.1016/s0014-2999(98)00285-4. [DOI] [PubMed] [Google Scholar]
- Georgescu D, Sears RM, Hommel JD, Barrot M, Bolaños CA, Marsh DJ, Bednarek MA, Bibb JA, Maratos-Flier E, Nestler EJ, DiLeone RJ. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:2933–2940. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Sintes R, Bortolozzi A, Artigas F, Lucas JJ. Reduced striatal dopamine DA D2 receptor function in dominant-negative GSK-3 transgenic mice. Eur Neuropsychopharmacol. 2014;24:1524–1533. doi: 10.1016/j.euroneuro.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Gomori A, Ishihara A, Ito M, Mashiko S, Matsushita H, Yumoto M, Tanaka T, Tokita S, Moriya M, Iwaasa H, Kanatani A. Chronic intracerebroventricular infusion of MCH causes obesity in mice. Melanin-concentrating hormone. American journal of physiology. Endocrinology and metabolism. 2003;284:E583–E588. doi: 10.1152/ajpendo.00350.2002. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- Guesdon B, Denis RG, Richard D. Additive effects of olanzapine and melanin-concentrating hormone agonism on energy balance. Behav Brain Res. 2010;207:14–20. doi: 10.1016/j.bbr.2009.09.032. [DOI] [PubMed] [Google Scholar]
- Guesdon B, Paradis E, Samson P, Richard D. Effects of intracerebroventricular and intra-accumbens melanin-concentrating hormone agonism on food intake and energy expenditure. Am J Physiol Regul Integr Comp Physiol. 2009;296:R469–R475. doi: 10.1152/ajpregu.90556.2008. [DOI] [PubMed] [Google Scholar]
- Hillebrand JJ, van Elburg AA, Kas MJ, van Engeland H, Adan RA. Olanzapine reduces physical activity in rats exposed to activity-based anorexia: possible implications for treatment of anorexia nervosa? Biol Psychiatry. 2005;58:651–657. doi: 10.1016/j.biopsych.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Jones DL, Mogenson GJ, Wu M. Injections of dopaminergic, cholinergic, serotoninergic and GABAergic drugs into the nucleus accumbens: effects on locomotor activity in the rat. Neuropharmacology. 1981;20:29–37. doi: 10.1016/0028-3908(81)90038-1. [DOI] [PubMed] [Google Scholar]
- Kishi T, Kafantaris V, Sunday S, Sheridan EM, Correll CU. Are antipsychotics effective for the treatment of anorexia nervosa? Results from a systematic review and meta-analysis. J Clin Psychiatry. 2012;73:e757–e766. doi: 10.4088/JCP.12r07691. [DOI] [PubMed] [Google Scholar]
- Klenotich SJ, Seiglie MP, McMurray MS, Roitman JD, Le Grange D, Dugad P, Dulawa SC. Olanzapine, but not fluoxetine, treatment increases survival in activity-based anorexia in mice. Neuropsychopharmacology. 2012;37:1620–1631. doi: 10.1038/npp.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohnomi S, Koshikawa N, Kobayashi M. D(2)-like dopamine receptors differentially regulate unitary IPSCs depending on presynaptic GABAergic neuron subtypes in rat nucleus accumbens shell. J Neurophysiol. 2012;107:692–703. doi: 10.1152/jn.00281.2011. [DOI] [PubMed] [Google Scholar]
- Kokkotou E, Jeon JY, Wang X, Marino FE, Carlson M, Trombly DJ, Maratos-Flier E. Mice with MCH ablation resist diet-induced obesity through strain-specific mechanisms. Am J Physiol Regul Integr Comp Physiol. 2005;289:R117–R124. doi: 10.1152/ajpregu.00861.2004. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM, Wilson CJ. Comparison of IPSCs evoked by spiny and fast-spiking neurons in the neostriatum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:7916–7922. doi: 10.1523/JNEUROSCI.2163-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggero C, Masi G, Brunori E, Calderoni S, Carissimo R, Maestro S, Muratori F. Low-dose olanzapine monotherapy in girls with anorexia nervosa, restricting subtype: focus on hyperactivity. J Child Adolesc Psychopharmacol. 2010;20:127–133. doi: 10.1089/cap.2009.0072. [DOI] [PubMed] [Google Scholar]
- Nakamachi T, Matsuda K, Maruyama K, Miura T, Uchiyama M, Funahashi H, Sakurai T, Shioda S. Regulation by orexin of feeding behaviour and locomotor activity in the goldfish. J Neuroendocrinol. 2006;18:290–297. doi: 10.1111/j.1365-2826.2006.01415.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KB. The mouse brain in stereotaxic coordinates. 2nd ed. San Diego, California: Academic; 2001. [Google Scholar]
- Pissios P, Frank L, Kennedy AR, Porter DR, Marino FE, Liu FF, Pothos EN, Maratos-Flier E. Dysregulation of the mesolimbic dopamine system and reward in MCH−/− mice. Biol Psychiatry. 2008;64:184–191. doi: 10.1016/j.biopsych.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Prinssen EP, Koek W, Kleven MS. The effects of antipsychotics with 5-HT(2C) receptor affinity in behavioral assays selective for 5-HT(2C) receptor antagonist properties of compounds. Eur J Pharmacol. 2000;388:57–67. doi: 10.1016/s0014-2999(99)00859-6. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Benvenga MJ, Bymaster FP, Calligaro DO, Cohen IR, Falcone JF, Hemrick-Luecke SK, Martin FM, Moore NA, Nisenbaum LK, Schaus JM, Sundquist SJ, Tupper DE, Wiernicki TR, Nelson DL. Preclinical pharmacology of FMPD [6-fluoro-10-[3-(2-methoxyethyl)-4-methyl-piperazin-1-yl]-2-methyl-4H-3-thia-4,9-diaza-benzo[f]azulene]: a potential novel antipsychotic with lower histamine H1 receptor affinity than olanzapine. J Pharmacol Exp Ther. 2005;315:1265–1277. doi: 10.1124/jpet.105.089326. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Hsu MA, Yang Y. The orexin-1 receptor antagonist SB-334867 blocks the effects of antipsychotics on the activity of A9 and A10 dopamine neurons: implications for antipsychotic therapy. Neuropsychopharmacology. 2007;32:786–792. doi: 10.1038/sj.npp.1301239. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Fibiger HC. Effects of olanzapine on regional C-Fos expression in rat forebrain. Neuropsychopharmacology. 1996;14:105–110. doi: 10.1016/0893-133X(95)00196-K. [DOI] [PubMed] [Google Scholar]
- Sebens JB, Koch T, Ter Horst GJ, Korf J. Olanzapine-induced Fos expression in the rat forebrain; cross-tolerance with haloperidol and clozapine. Eur J Pharmacol. 1998;353:13–21. doi: 10.1016/s0014-2999(98)00391-4. [DOI] [PubMed] [Google Scholar]
- Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- Siegfried Z, Berry EM, Hao S, Avraham Y. Animal models in the investigation of anorexia. Physiol Behav. 2003;79:39–45. doi: 10.1016/s0031-9384(03)00103-3. [DOI] [PubMed] [Google Scholar]
- Skilbeck KJ, O'Reilly JN, Johnston GA, Hinton T. Antipsychotic drug administration differentially affects [3H]muscimol and [3H]flunitrazepam GABA(A) receptor binding sites. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:492–498. doi: 10.1016/j.pnpbp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Stockton ME, Rasmussen K. Electrophysiological effects of olanzapine, a novel atypical antipsychotic, on A9 and A10 dopamine neurons. Neuropsychopharmacology. 1996;14:97–105. doi: 10.1016/0893-133X(94)00130-R. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Bolam JP. Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol. 2004;14:685–692. doi: 10.1016/j.conb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Tritos NA, Vicent D, Gillette J, Ludwig DS, Flier ES, Maratos-Flier E. Functional interactions between melanin-concentrating hormone, neuropeptide Y, and anorectic neuropeptides in the rat hypothalamus. Diabetes. 1998;47:1687–1692. doi: 10.2337/diabetes.47.11.1687. [DOI] [PubMed] [Google Scholar]
- Tunstall MJ, Oorschot DE, Kean A, Wickens JR. Inhibitory interactions between spiny projection neurons in the rat striatum. J Neurophysiol. 2002;88:1263–1269. doi: 10.1152/jn.2002.88.3.1263. [DOI] [PubMed] [Google Scholar]
- Vinish M, Elnabawi A, Milstein JA, Burke JS, Kallevang JK, Turek KC, Lansink CS, Merchenthaler I, Bailey AM, Kolb B, Cheer JF, Frost DO. Olanzapine treatment of adolescent rats alters adult reward behaviour and nucleus accumbens function. Int J Neuropsychopharmacol. 2013;16:1599–1609. doi: 10.1017/S1461145712001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtel H, Andén NE. Motor activity of rats following intracerebral injections of drugs influencing GABA mechanisms. Naunyn Schmiedebergs Arch Pharmacol. 1978;302:133–139. doi: 10.1007/BF00517980. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Kita T, Kita H. Presynaptic actions of D2-like receptors in the rat cortico-striatoglobus pallidus disynaptic connection in vitro. J Neurophysiol. 2009;101:665–671. doi: 10.1152/jn.90806.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiddon BB, Palmiter RD. Ablation of neurons expressing melanin-concentrating hormone (MCH) in adult mice improves glucose tolerance independent of MCH signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:2009–2016. doi: 10.1523/JNEUROSCI.3921-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Shen Z, Strack AM, Marsh DJ, Shearman LP. Enhanced running wheel activity of both Mch1r-and Pmch-deficient mice. Regul Pept. 2005;124:53–63. doi: 10.1016/j.regpep.2004.06.026. [DOI] [PubMed] [Google Scholar]