Abstract
The evolutionarily conserved Wnt signaling pathway plays essential roles during embryonic development and tissue homeostasis. Notably, comprehensive genetic studies in Drosophila and mice in the past decades have demonstrated the crucial role of Wnt signaling in intestinal stem cell maintenance by regulating proliferation, differentiation, and cell-fate decisions. Wnt signaling has also been implicated in a variety of cancers and other diseases. Loss of the Wnt pathway negative regulator adenomatous polyposis coli (APC) is the hallmark of human colorectal cancers (CRC). Recent advances in high-throughput sequencing further reveal many novel recurrent Wnt pathway mutations in addition to the well-characterized APC and β-catenin mutations in CRC. Despite attractive strategies to develop drugs for Wnt signaling, major hurdles in therapeutic intervention of the pathway persist. Here we discuss the Wnt-activating mechanisms in CRC and review the current advances and challenges in drug discovery.
Keywords: Wnt signaling pathway, colorectal cancer, adenomatous polyposis coli, β-catenin, drug targeting, small molecules
the gastrointestinal tract constitutes the key fundamental system for food digestion and nutrient absorption. The heavy task of food processing by the human intestine causes routine damage to the surface epithelium on a daily basis. Replenishment of cell loss caused by abrasion of the intestinal epithelium is constantly supported by the leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5)-expressing intestinal stem cells (ISC) located at the bottom of each crypt (116). These ISCs proliferate and generate transit-amplifying progenitor cells in the upper crypts, which will eventually differentiate into various cell types, including enterocytes, Paneth cells, goblet cells, enteroendocrine cells, tuft cells, and M cells. Such proliferation and differentiation processes are tightly regulated by various signaling pathways along the crypt-villus axis, including Wnt, BMP, EGF, and Notch pathways. Among these, the Wnt pathway constitutes the primary driving force for ISC proliferation and maintenance.
Wnt signaling is a highly conserved pathway that plays principal regulatory roles in many developmental and biological processes. Besides its crucial role in tissue homeostasis, Wnt signaling is also found to be activated aberrantly in many human diseases, including cancers and metabolic disorders. The Wnt signaling pathways are further subdivided into the canonical β-catenin-dependent pathway and the noncanonical β-catenin-independent (planar cell polarity or Wnt/calcium) pathway. In adult intestine, it is well established that the canonical Wnt signaling plays complementary roles in physiology and pathology: in health, it maintains crypt stem cell compartments, but, when activated by mutation, it is the cause of colon cancer. In this review, we discuss the canonical Wnt signaling pathway and its activating mechanisms in colorectal cancer (CRC). We will highlight the common Wnt pathway-associated mutations identified in CRC and will further review the current therapeutic agents targeting Wnt signaling regarding their perspectives and challenges.
Wnt Signaling Pathway
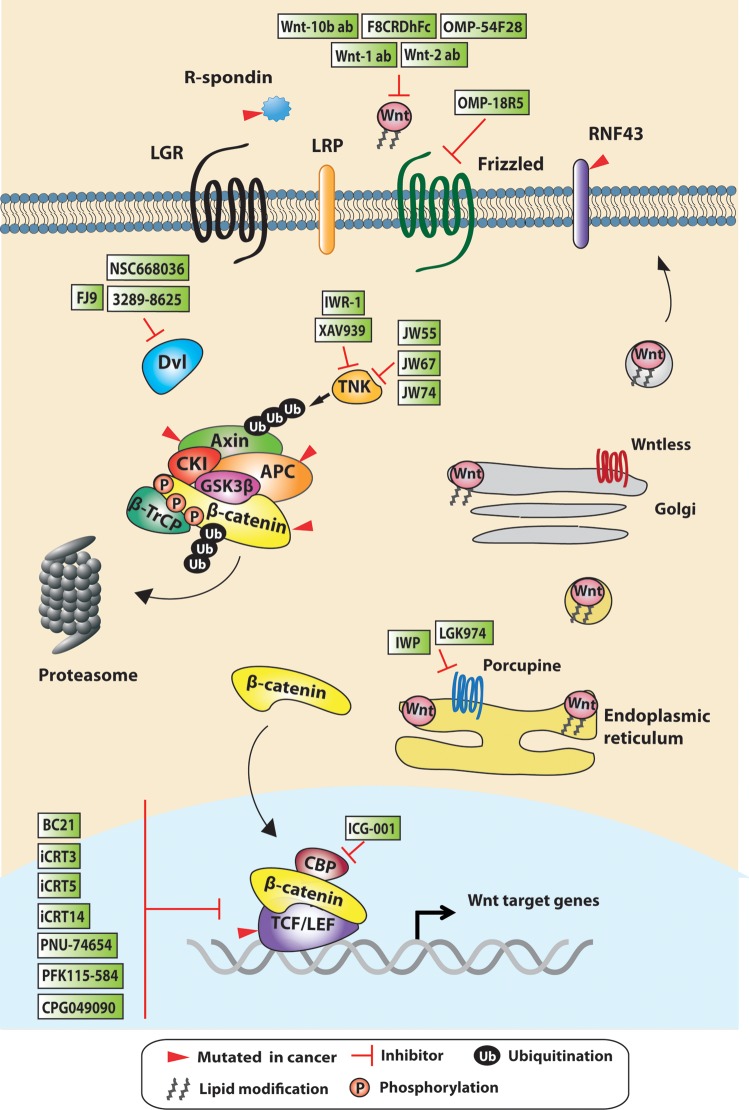
Wnt signaling controls the level of the key modulator β-catenin for signal transduction through processes involving phosphorylation and ubiquitin-mediated degradation. This is regulated by the cytoplasmic β-catenin destruction complex, which consists of the core proteins AXIN, adenomatous polyposis coli (APC), casein kinase 1 (CK1), and glycogen synthase kinase 3 (GSK3). In the absence of Wnt, β-catenin is phosphorylated by CK1 and GSK3 in the complex, which is followed by recruitment of the E3 ligase β-TrCP to the complex for ubiquitination and subsequent proteasomal degradation (Fig. 1) (1, 60, 72). Upon Wnt ligand engagement to the receptors Frizzled (FZD) and low-density lipoprotein-related protein 5/6 (LRP5/6), the β-catenin destruction complex is recruited to the membrane, whereas β-TrCP is dissociated from the complex (71). This is achieved by a scaffold protein Dishevelled (DVL) in the cytosol that transduces extracellular Wnt signals from receptors to downstream effectors (38). DVL has the ability to bind to FZD receptor and AXIN protein via its PDZ and DIX domain, respectively, thus facilitating the interaction between the AXIN complex and the Wnt receptors. The DVL-AXIN interaction has been reported to inhibit the destruction complex by interfering with AXIN oligomerization via the DIX domain, whereas DVL polymerization is also believed to enhance Wnt receptor signalosomes (31, 98). As a consequence, the complex-bound β-catenin is no longer ubiquitinated or degraded, leading to complex saturation and inhibition. This results in accumulation of free β-catenin in the cytoplasm and its subsequent nuclear translocation. In the nucleus, β-catenin displaces the repressor Groucho from T cell factor (TCF)/lymphoid enhancer-binding factor (LEF) transcription factors. β-Catenin/TCF/LEF, together with other coactivators, form an active transcriptional complex, leading to the expression of Wnt target genes (10, 81).
Fig. 1.
Schematic representation of the Wnt signaling pathway with selected inhibitors. Wnt ligands are palmitoylated by the acyltransferase porcupine in the endoplasmic reticulum and transported to the Golgi complex. The transmembrane protein Wntless facilitates the subsequent transfer of Wnt ligands to the plasma membrane for secretion. β-Catenin, the key effector of Wnt signaling, will be constantly degraded by the destruction complex in the absence of Wnt. Casein kinase 1 (CK1) and glycogen synthase kinase 3β (GSK3β) in the complex phosphorylate β-catenin, which is then recognized by the E3-ligase β-TrCP for ubiquitination and proteosomal degradation. Upon Wnt ligand engagement of the Frizzled and low-density lipoprotein-related protein (LRP) receptors, the destruction complex will be inhibited, and the free cytosolic β-catenin will be translocated to the nucleus. Within the nucleus, β-catenin binds to the T cell factor (TCF) transcription factor together with other coactivators such as cAMP-response element-binding protein-binding protein (CBP) and p300 to regulate Wnt target gene transcription. The membrane-bound E3 ligases RNF43/ZNRF3 are Wnt negative feedback targets that will suppress Wnt signaling by removing Frizzled receptors from the cell surface and neutralizing the Wnt agonist R-spondin/leucine-rich repeat-containing G protein-coupled receptor (LGR) complex formation. Mutations in different Wnt signaling components (represented with red arrows) are frequently observed in colorectal cancers and have been found to cause pathway deregulation and tumorigenesis. Inhibitors targeting Wnt signaling at different cellular levels of the cascade are summarized in green boxes. LEF, lymphoid enhancer-binding factor; IWP, inhibitor of Wnt production; APC, adenomatous polyposis coli; TNK, tankyrase; DVL, Dishevelled.
In 2007, the Clevers laboratory first identified the Lgr5 as an intestinal stem cell marker via transcriptomic analysis of Wnt/TCF targets (6). Subsequent studies demonstrated that LGR5 and its homologs mediate Wnt/β-catenin signaling via R-spondin (17, 42). Structural analysis of the receptor complex further revealed the interaction between LGR5, R-spondin, and the E3 ubiquitin ligases RING finger 43 (RNF43) and zinc and RING finger 3 (ZNRF3) (15, 90). RNF43 and ZNRF3 are two transmembrane E3 ligases that inhibit Wnt signaling through binding and removing the Wnt receptors from the cell surface (48, 62). It is believed that R-spondin potentiates Wnt signaling by forming a complex with LGR5 and RNF43/ZNRF3 to neutralize the Wnt negative feedback activity (18). This has added further complexity to the Wnt/β-catenin signaling regulation at the receptor level.
Wnt-Activating Mechanism in CRC
Wnt signaling is activated at the bottom of the intestinal crypts, which is crucial for stem cell maintenance and tissue homeostasis. Aberrant Wnt activation is frequently observed in human cancers, especially in CRC (12). Human CRCs can be broadly classified into two major categories based on their molecular profiles: nonhypermutated microsatellite stable (MSS) CRCs and hypermutated microsatellite instability (MSI) cancers. MSS CRCs constitute the vast majority, where loss of the tumor suppressor APC is the early event to initiate adenoma formation (85). Subsequent cancer progression requires stepwise accumulation of other mutations, such as in KRAS, PI3K, TGF-β, p53, and/or SMAD4, which is known as the multistep somatic evolution model, the “Vogelgram” (30). On the other hand, around 10–15% of CRCs are caused by defective DNA mismatch repair (MMR) machinery that is often associated with MLH1 hypermethylation (12, 54, 113, 119). These are characterized by the presence of insertions or deletions of nucleotides in microsatellite repeat regions widespread across the genome, hence their name as MSI tumors (54, 113).
The Wnt signaling pathway is aberrantly upregulated in both MSS and MSI CRCs (12). Inactivating mutations of the negative regulator APC constitute the principal mechanism by which the Wnt pathway is activated in MSS CRCs. In MSI tumors, MMR defects result in high mutation rates in the entire genome, which is known as the hypermutation phenotype. As a consequence, frequent mutations are observed in multiple oncogenes and tumor suppressor genes, including Wnt pathway components such as APC, β-catenin, and/or AXIN2 (12). In addition, epigenetic silencing of Wnt inhibitors by DNA hypermethylation has also been suggested as another common mechanism to activate the Wnt pathway. Indeed, several studies have reported epigenetic silencing of negative regulators of Wnt signaling, including the extracellular Wnt inhibitors SFRP1-5, WIF1, DKK1, and DKK3 (92, 93, 104, 107, 111, 120), as well as the destruction complex proteins APC and AXIN2 (27, 34, 61, 83, 84, 104). The high mutation rate of Wnt pathway components observed in human CRCs highlights the promise of Wnt signaling as a therapeutic target (12). We discuss below the commonly observed mutations and the mechanisms by which they act in human CRCs.
Loss-of-Function Mutations
APC is a multifunctional tumor suppressor. It is a large scaffold protein that consists of multiple binding domains for various cellular functions, including WNT signal regulation, cell adhesion, and chromosomal segregation during mitosis (32, 79). Mice with Apc mutations will develop adenomas in the intestine attributable to hyperactivation of Wnt (39). Functional loss of APC results in constitutive activation of Wnt signaling and in chromosomal instability in human colorectal adenocarcinomas (80). Germ-line mutations of the APC gene cause familial adenomatous polyposis (FAP) syndromes characterized by the presence of multiple polyps in the colon and predisposition to CRC development (29). Mutations in different regions of the gene result in different degrees of polyposis. Two mutation hotspots at codons 1,061 and 1,309 have been identified that result in high polyposis penetrance (36). In most cases of sporadic CRCs, APC mutations are frequently clustered between codons 1,309 and 1,450 (29). The majority of APC mutations are frameshift and nonsense mutations, which cause premature protein truncation. Biallelic loss of APC is achieved through second loss-of-heterozygosity mutations (which are somatic rather than germ line). APC protein truncation is believed to activate Wnt/β-catenin signaling through abrogation of destruction complex-mediated β-catenin ubiquitination (64).
AXIN1 and AXIN2 are negative Wnt regulators and tumor suppressors that are found mutated in sporadic CRCs and also some familial cancer syndromes (97). There are three germ-line mutations reported in AXIN2: two result in premature stop codons in exon 7 (65, 77), whereas the other is a missense mutation in exon 5 (94). These lead to genetic predisposition to CRC development and tooth agenesis. Several studies have described somatic mutations of AXIN2 in MSI CRCs, the majority of which are also localized in exon 7 (21, 74, 114). In addition to mutations, epigenetic silencing of AXIN2 has also been reported, mostly in MSI CRCs (61). Although no germ-line mutation of AXIN1 has been found in CRCs, several studies have reported AXIN1 somatic mutations (56, 103). AXIN is the scaffold protein in the destruction complex that mediates β-catenin ubiquitination and degradation. AXIN2 is also a well-known downstream Wnt target, which provides negative feedback upon Wnt activation. Loss of AXIN is believed to activate the Wnt pathway through disruption of the β-catenin destruction complex and the Wnt negative feedback mechanism. Further investigation is needed to define the functional and clinical difference between AXIN1 and AXIN2 mutations.
WTX (also known as FAM123B or adenomatous polyposis coli membrane recruitment 1, AMER1), is an X-linked gene mutated in Wilms tumors. In 2007, the Moon laboratory identified WTX as a component of the destruction complex that negatively regulates Wnt signaling by promoting β-catenin ubiquitination and degradation (76). However, a more recent study showed that WTX could be a Wnt activator by promoting Wnt-induced LRP6 phosphorylation (112), postulating a complex dual positive and negative role of WTX in Wnt signaling. In patients, truncating mutations of WTX are frequently observed in kidney and CRCs. More recent sequencing data show high mutation rates in CRCs, preferentially in MSI cases (12). Further characterization of the role of WTX in the Wnt signaling pathway and CRCs will aid understanding of its clinical significance.
RNF43 and its homolog ZNRF3 have been recently identified as negative Wnt regulators by promoting FZD receptor turnover (48, 62). In mouse intestine, depletion of both RNF43 and ZNRF3 results in expansion and proliferation of the stem cell/Paneth cell compartment (62). Recently, whole-exome sequencing of human CRCs identified a large number of somatic mutations in RNF43, with the majority associated with MSI cases (40). RNF43 and ZNRF3 are expressed at the bottom of intestinal crypts to negatively regulate Wnt signaling by controlling Wnt receptor levels (62). Loss of function mutations of RNF43 will lead to a failure of Wnt receptor removal in the crypt, thus activating Wnt signaling by increased sensitivity to the Wnt ligands from the surrounding niche. Such receptor-associated mutations are exogenous Wnt dependent, making it desirable for treatment with Wnt inhibitors targeting Wnt ligands/receptors.
Activating Mutations
The CTNNB1 gene encodes for β-catenin, the key transcription modulator of the Wnt signaling pathway. β-Catenin is also a subunit of cadherin protein complexes that play essential roles in coordinating cell-cell adhesion. Hotspot mutations within CTNNB1 that affect the regulatory NH2-terminal serine/threonine residues, such as S45F and T41A, are found in MSI CRCs (82). It is well characterized that β-catenin is first phosphorylated by CK1 at ser45, followed by GSK3β-mediated phosphorylation at ser33, ser37, and thr41 within the destruction complex (72). Activating point mutations at these amino acid residues will prevent β-catenin from being phosphorylated, thereby blocking the subsequent β-TrCP-mediated ubiquitination and degradation.
R-spondin proteins (RSPO1-4) are Wnt agonists that enhance Wnt signaling via engagement with the LGR and FZD receptors in the presence of Wnt ligands (17, 42). Overexpression of RSPO1 in mouse intestine results in hyperproliferation and expansion of the crypts (58). Exome sequencing data from a large cohort of patients have identified two recurrent gene fusions in CRCs, RSPO2-EIF3E (eukaryotic translation initiation factor 3) and RSPO3-PTPRK (receptor-type tyrosine-protein phosphatase κ) that can potentiate Wnt signaling (100). Interestingly, these fusions were found in non-APC-mutated CRCs, indicating an alternative Wnt-activating mechanism.
Uncharacterized Mutations
Several members of the TCF family are the downstream effectors of Wnt/β-catenin signaling, notably TCF7/TCF1, LEF, TCF7L1/TCF3, and TCF7L2/TCF4. Point mutations, deletions, and translocations of TCF7L1 and TCF7L2 have been reported in CRCs (9, 12, 16, 24, 105). However, the Wnt-activating potential of these mutations has not been fully characterized. Specific TCF4 frameshift mutations found in MSI-CRC cells have been shown to be activating mutations by selective loss of TCF4 isoforms with binding ability to the transcriptional repressor CtBP (16). On the other hand, genome-wide RNAi screening has identified TCF7L2 as a tumor suppressor in CRC cells (109). In mouse intestine, Tcf4 deletion results in crypt degeneration, whereas loss of Tcf1 and Tcf3 do not show any apparent defect (117). Together, these data suggest a functional discrepancy of different TCF members between mouse and human. Further investigation is required to explain the difference and to characterize transcriptional regulation mediated by TCF1, TCF3, and TCF4.
Targeting Wnt Signaling in CRC
APC mutations in CRCs were first discovered in 1991 (59, 87). Since the late 1990s, there has been growing interest in developing therapeutic agents against the Wnt signaling pathway. However, the development of Wnt inhibitors has only reached a promising start recently, and an effective clinically approved drug is still missing. A variety of therapeutic agents modulating the Wnt pathway have been reported, ranging from small-molecule compounds to antibodies and peptides (Fig. 1). Below, we summarize selected Wnt inhibitors into four classes according to their specific targets: generic, the Wnt-receptor complex, the β-catenin destruction complex, and nuclear/transcription factor complexes (Fig. 2).
Fig. 2.
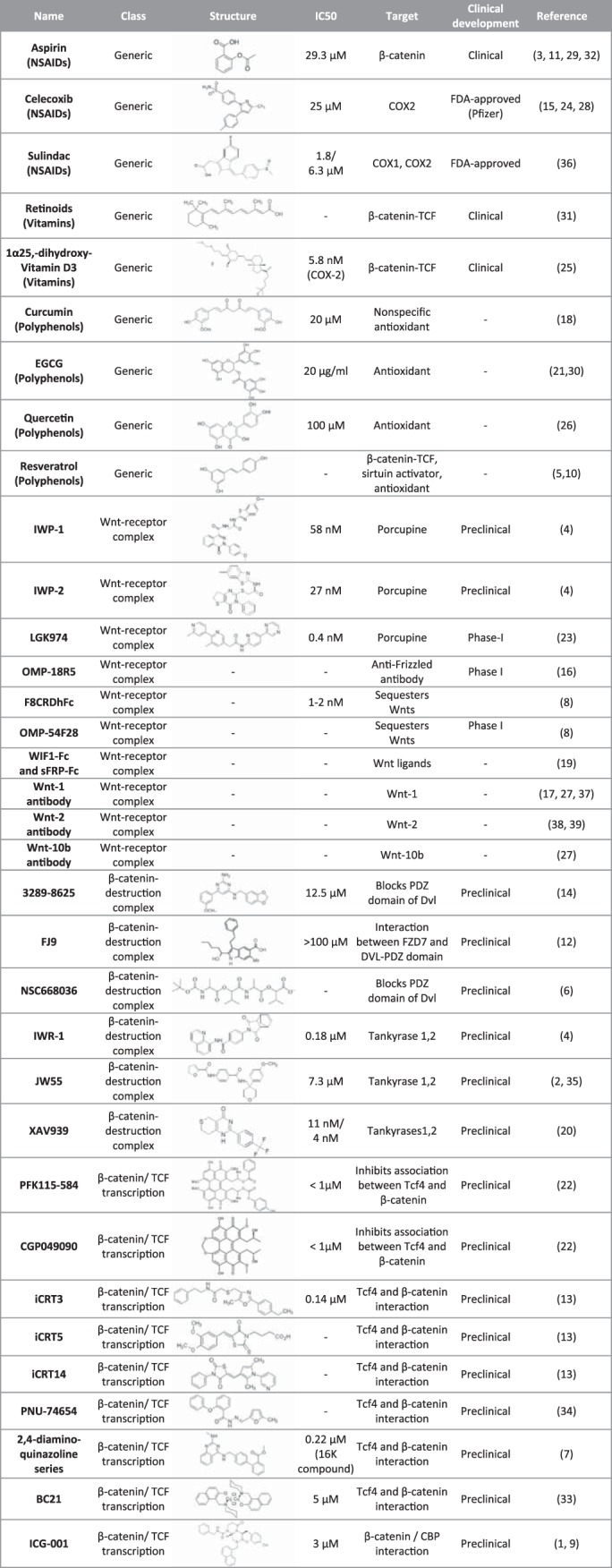
Negative modulators of the Wnt signaling pathway. COX, cyclooxygenase; EGCG, epigallocatechin gallate. See Supplemental Material for references (available online at the journal website).
Generic Wnt Inhibitors
There are in fact a few FDA-approved drugs that have been shown to modulate the Wnt pathway. However, these drugs belong to the generic category that target Wnt signaling nonspecifically. NSAIDs such as aspirin, indomethacin, and sulindac inhibit the activity of cyclooxygenase (COX), which is a key enzyme in the arachidonic acid cascade. Several studies have suggested that both NSAIDs and the COX2 inhibitor celecoxib can inhibit Wnt/β-catenin transcription in CRC cells and prevent polyp formation in patients with FAP and mouse models (7, 13, 23, 41, 46, 96). The data suggest that controlling COX2-dependent prostaglandin E2 (PGE2) production can potentially have chemopreventive effects in human colon cancer. Vitamins have also been reported to have the potential to reduce cancer risk. For example, Vitamin A (retinoid) has been shown to decrease β-catenin protein levels (20, 106), and Vitamin D (1α,25-dihydroxyvitamin D3) was also suggested to have therapeutic potential for cancer (3, 49). Several studies have suggested that vitamins can repress Wnt/β-catenin signaling at many levels, including competition for nuclear β-catenin binding with TCF upon activation of the vitamin nuclear receptors or activation of Wnt inhibitor (Dickkopf, DKK) transcription (88, 89, 101). Despite the potential benefit of these generic agents in Wnt suppression and cancer therapy, the detailed molecular mechanisms remain to be elucidated.
Wnt-Receptor Complex Inhibitors
Porcupine inhibitors.
Porcupine (PORCN) is a membrane-bound O-acyltransferase (MBOAT) residing in endoplasmic reticulum (ER), which adds the palmitoyl group to Wnt proteins, a critical lipid-modifying step for processing Wnt ligand secretion (2, 14, 53, 108, 121). In 2009, Chen and colleagues (14) identified a class of small molecules that inhibit Wnt protein production, inhibitors of Wnt production (IWPs) (14). IWP compounds are believed to act specifically on the Wnt pathway because they failed to suppress Notch-mediated signaling or to block general protein secretion, and they did not affect palmitoylation of Sonic hedgehog, a substrate of another MBOAT member, Hedgehog acyltransferase (14). A more recent study identified another potent porcupine inhibitor LGK974 that showed 63% tumor regression in mice with no adverse effect in normal Wnt-dependent tissues at the efficacious dose (73). The data indicate the therapeutic potential of PORCN inhibitors to treat cancers that are exogenous Wnt dependent, such as LKB1 and RNF43 mutations in CRCs (63). Loss-of-function mutations in the tumor suppressor kinase LKB1 fail to restrain the activity of FZD receptors (55, 75), whereas RNF43 loss fails to remove Wnt receptors from the cell surface (48, 62).
Receptor/ligand interference.
Another approach toward Wnt-receptor complex directed therapy is to target specific Wnt ligands or receptors that are found overexpressed in tumors. Therapeutic monoclonal antibodies against Wnt-1 and Wnt-2 have been shown to inhibit Wnt signaling and suppress tumor growth in vivo (110, 124, 126). These antibodies drive apoptosis in different tumor models, including melanoma, non-small cell lung carcinoma, mesothelioma, sarcoma, breast cancer, and CRC cells (50, 51, 78, 127). Another monoclonal antibody OMP-18R5 (OncoMed Pharmaceuticals/Bayer) has been reported to target the FZD receptors and inhibit tumor growth in patient-derived xenograft mouse models by blocking Wnt ligand engagement (47). This product is currently in phase I clinical trials. On the other hand, a soluble Fc-fusion protein with the cysteine-rich domain of FZD8 (FZD8-CRD-Fc) has also been shown to inhibit autocrine Wnt signaling in vitro and in xenograft models (19). Mice treated with this soluble receptor exhibited no signs of toxicity after several weeks of treatment, suggesting that pan-Wnt inhibition may be a safe approach for tumor-specific treatment. Finally, the therapeutic potential of secretory Wnt antagonists such as secreted FZD-related proteins (SFRPs) and Wnt inhibitory protein has been suggested by interrupting binding between Wnt ligands and receptors (11). SFRP1 protein or SFRP1-derived peptides suppress HCT116 xenograft tumor formation in mice (67). Similarly, another secretory Wnt inhibitor DKK antagonizes Wnt signaling by disrupting Wnt-induced FZD-LRP6 complex formation (25, 43, 99). Together, the data suggest that antibody or peptide treatment can be used to block Wnt signaling by targeting Wnt ligand-receptor complexes.
β-Catenin-Destruction Complex Inhibitors
Dishevelled inhibitors.
The ability of DVL to bind to both the Wnt receptors and AXIN indicates its unique role in Wnt signal transduction. DVL binds to the carboxyl terminal end of the FZD receptors through its PDZ domain, a common protein-interaction domain. Small molecule compounds targeting specifically the FZD and DVL-PDZ interaction (NSC668036, FJ9, and 3289–8625) have been identified to block the signal transduction cascade. These compounds have been shown to inhibit Wnt signaling and suppress tumor cell growth in prostate, colon, and lung cancer cells (35, 45, 102).
Tankyrase inhibitors.
Two small-molecule inhibitors targeting tankyrases [poly(ADP)-ribosylating enzymes] were identified in 2009 that show Wnt-antagonizing effects in colon cancer cells. Chen and colleagues (14) first discovered a group of compounds named inhibitors of Wnt response (IWR), which stabilize AXIN proteins and inhibit Wnt signaling (14). Treatment of IWR-1 in zebrafish inhibits tailfin regeneration and blocks normal gastrointestinal tissue homeostasis. Further analysis showed that IWR-1 inhibits Wnt/β-catenin transcriptional activity in DLD-1 human CRC cells, which harbor an inactivating APC mutation. Subsequent studies by Huang and collaborators (53) using a high-throughput screen identified another Wnt inhibitor, XAV939. This study further revealed the cellular targets of both XAV939 and IWR-1, suggesting that they regulate AXIN protein levels by inhibiting tankyrase 1 and tankyrase 2. XAV939 was also demonstrated to inhibit Wnt/β-catenin signaling in the APC-mutated CRC cell lines SW480 and DLD-1. It was later on reported that RNF146, a RING-domain E3 ubiquitin ligase, directly interacts with tankyrase and mediates tankyrase-dependent AXIN degradation (129). More recently, a number of new tankyrase inhibitors, JW55, JW67, and JW64, have been reported with the potential to inhibit Wnt signaling and suppress tumor growth in Apc mutant mice (121, 122). However, further detailed analysis of these inhibitor analogs demonstrated intestinal toxicity, which limits the full potential of the antitumor activity of these drugs (66). Together, these studies highlight the therapeutic potential of tankyrase inhibitors for APC-mutated cancers. However, safety concern remains to be addressed because of the observation of nontumor-specific Wnt inhibition in preclinical models.
β-Catenin/TCF Transcription Complex Inhibitors
β-Catenin/TCF antagonists.
Discovery of Wnt inhibitors targeting downstream transcription effectors is of great interest because of the diversity of Wnt pathway mutations identified at different subcellular levels. A high-throughput ELISA screen of ∼7,000 purified natural compounds has identified 8 compounds that disrupt β-catenin/TCF complex formation in a dose-dependent manner, among which PFK115-584 and CGP049090 were the two most effective compounds (70). However, these compounds were also found to target nonspecifically the β-catenin/APC interaction, indicating the unselective pharmacological interference with β-catenin complexes. Several other compounds have been reported in individual screens that inhibit Wnt target gene transcription and suppress CRC cell growth by targeting β-catenin/TCF interactions (44, 115, 123). Further investigation of these compounds will be important to validate how selective they are in β-catenin/TCF targeting and to evaluate their toxicity and whether they impact on normal homeostatic tissue functions at the effective dose.
Wnt coactivator antagonists.
Upon Wnt activation, β-catenin recruits the transcriptional coactivator cAMP-response element-binding protein-binding protein (CBP) or its homolog p300 to form a transcriptionally active complex together with other components. In 2004, a small-molecule compound ICG-001 was identified that antagonizes β-catenin/TCF transcription (26). ICG-001 binds specifically the coactivator CBP but not p300 and inhibits Wnt signaling by disrupting the interaction of CBP and β-catenin. Several studies have subsequently demonstrated the ability of ICG-001 to eliminate drug-resistant tumor cells by inducing apoptosis (37, 52, 68, 125). The promising tumor-suppressive effect and lack of toxicity highlight the therapeutic potential of ICG-001 for cancer treatment. More functional studies of β-catenin/CBP antagonists will be important to characterize the drug specificity regarding safety and its potential effect on other CBP-dependent signaling pathways.
Therapeutic Efficacy of Wnt Inhibitors in the Clinic
Considering the diversity of Wnt-targeting strategies and the Wnt pathway mutation spectrum, it is important to identify the appropriate agents to treat patients with cancer carrying specific mutations. Understanding where and how the Wnt modulator acts in the pathway will help determine the therapeutic efficacy of a particular agent. A general approach is to match the Wnt inhibitor with the mutation, such that it acts at the same target or equivalent subcellular level (i.e., membrane-receptor complex, cytoplasmic-destruction complex, or nuclear-β-catenin/TCF transcription complex). For instance, PORCN inhibitors function to block Wnt ligand secretion and thus are suitable for treating cancers that are exogenous Wnt dependent. These include tumors carrying mutations acting only at the receptor level, such as RNF43 and LKB1 that are both regulating FZD receptors. On the other hand, mutations acting downstream of the receptors, such as APC truncations, activate the Wnt pathway constitutively in the absence of Wnt ligand and are therefore resistant to PORCN inhibitors. Somatic mutations of RNF43 are reported in over 18% of CRCs, predominantly in MSI tumors, whereas LKB1 mutations are relatively rare, suggesting that PORCN inhibitors can potentially be efficacious in over 18% of CRCs (4, 40). However, the drug efficacy has to be reevaluated when mutations in more than one Wnt pathway component are detected in the same tumor. For example, hypermutated MSI CRCs harboring simultaneous inactivating mutations of RNF43 and APC will unlikely respond to PORCN inhibitors because of the dominant downstream mutations at APC. DVL inhibitors target FZD and DVL-PDZ interactions and therefore block signal transduction from receptor to cytoplasmic-destruction complex. This implies that most APC-mutated CRCs with mutations downstream of DVL will be resistant to DVL inhibitors. In contrast, tankyrase inhibitors are reported to target specifically APC-mutated tumors, which constitute >80% of CRCs. This would have a high therapeutic value if the toxicity concerns could be resolved. β-Catenin/TCF complex antagonists target the furthest downstream effectors, i.e., those involved in transcription, which ideally have the greatest therapeutic potential to treat most CRCs with various upstream mutations. Nevertheless, such targeting approaches may provide little if any selection between tumor and normal tissues, which may well restrain their ability to achieve both efficacy and safety in the clinic. Further investigation is needed to identify the safe therapeutic dosage. In a nutshell, identifying mutation-specific Wnt inhibitors as well as the safe efficacious dose are the two key elements to target the Wnt pathway safely in the clinic.
Challenges of Targeting the Wnt Pathway
Aberrant Wnt signaling has been associated with many types of cancer and other diseases and is therefore an attractive target for therapeutic intervention. Thus far, no approved drugs are available in the clinic for treatment via targeting of the Wnt signaling pathway despite the substantial effort put on therapeutic development of Wnt inhibitors in the past two decades. One major challenge of drugging the Wnt pathway is attributable to its crucial role in normal development and adult tissue homeostasis. Many high-turnover tissues such as gastrointestinal epithelium and hair follicles require Wnt signaling for stem cell maintenance and tissue regeneration. Interference of this pathway will target, not only Wnt-activated cancer cells, but also the normal stem cell populations of Wnt-dependent tissues, resulting in significant toxicity. Caution must be taken when utilizing Wnt inhibitors to identify the optimal safe and efficacious dose.
Another important point to consider when targeting Wnt signaling is the network crosstalk between pathways. Indeed, many studies have demonstrated the capability of Wnt signaling to transactivate other signaling pathways in different cellular contexts. For instance, suppression of Wnt signaling by Notch has been reported in keratinocytes and skin tissues (86, 91). In adult intestine, Wnt and Notch signaling are important in both intestinal homeostasis and tumorigenesis (33, 118). Wnt and Notch work synergistically together in the murine intestine to trigger intestinal tumorigenesis particularly in colon, which is unusual in Apc-deficient mouse models but typical in human CRCs (33). Recent studies show that activation of the mammalian target of rapamycin complex 1 (mTORC1) pathway is required for proliferation of APC-depleted cells (28). Treatment with the serine/threonine kinase mTOR inhibitor rapamycin will block Wnt-activated tumor growth, whereas normal intestinal homeostasis is unaffected. The data suggest that targeting the mTORC1 pathway would provide therapeutic benefit during the early development of CRC. Crosstalk between the Wnt and Hippo pathway has also been implicated in intestinal homeostasis. The downstream effector YAP was shown to restrict intestinal regeneration and suppress Wnt signaling by blocking Dvl nuclear localization (8), whereas potential regulation of the β-catenin destruction complex by YAP and TAZ has also been suggested (5). The recent discovery of tankyrase inhibitors as Wnt modulators has further unraveled the role of tankyrase dependent-poly(ADP-ribosyl)ation to regulate Axin degradation, whereas tankyrase has also been reported to regulate telomerase homeostasis, glucose metabolism, and mitotic spindle formation (69). Together, the data suggest a complex interaction of Wnt signaling with many other cellular pathways in both cancer and normal tissue homeostasis. Better understanding of the crosstalk will be key to design an effective therapeutic strategy and to explore the potential of combination therapy for targeting multiple pathways.
Although Wnt signaling has been extensively characterized in the past three decades, a number of novel Wnt regulators or mechanisms have only been reported recently. The identification of Lgr5 as an intestinal stem cell marker in 2007 has subsequently uncovered the role of R-spondin and LGR receptors in potentiating Wnt signal strength (6, 17, 42). Recent studies of Rnf43 and Znrf3 in mouse and zebrafish have further revealed the Wnt negative feedback mechanism via receptor regulation (48, 62). More recently, another Wnt inhibitor Notum has also been demonstrated to deacylate Wnt protein extracellularly, adding yet another level of regulation of the Wnt proteins (57, 128). At the cytosolic destruction complex level, it is well established that β-catenin is degraded via phosphorylation and ubiquitination. However, the fundamental mechanism of Wnt-induced Axin-destruction complex inactivation is still controversial. We have previously redefined the physiological Wnt-activating mechanism by showing that inactivation of β-catenin ubiquitination is the principal initial Wnt-activating mechanism, whereas phosphorylation is unaffected (71). Our data challenged the previous dogma that β-catenin phosphorylation is inhibited upon Wnt activation. It is important to note that the choices of cell lines, time points, and experimental strategy are all crucial parameters for mechanistic studies. Given that β-catenin is involved in multiple cellular processes including membrane adherens junctions, caution has to be taken when interpreting data generated from whole-cell lysates vs. specific complex pull down. Further investigation is needed to elucidate the complete picture of the Wnt signaling pathway in a time course- and context-dependent manner. Finally, it will be important to study the mechanistic differences involved in the destruction complex between wild-type and Wnt-activated tumor cells (such as APC mutants) for future development of tumor-specific Wnt inhibitors.
Future Prospects
Despite many exciting preclinical studies for drugging the Wnt signaling pathway in cancers and other disease models, only a limited number of agents have recently entered clinical trials. To develop more effective Wnt inhibitors, it is important to answer outstanding questions relating to the mechanisms by which the Wnt pathway functions. A better understanding of the mechanistic difference between physiological and pathological Wnt activation is crucial for identifying tumor-specific therapeutic targets with minimal toxicity to normal tissues. Recent advances in high-throughput sequencing of human cancers have uncovered more novel Wnt pathway mutations, which provide exciting avenues for drug targeting and personalized medicine. Given the wide variety of Wnt inhibitors targeting different subcellular levels, it is important to select the right agents for specific mutations. To date, most preclinical studies rely largely on nonphysiological cancer cell lines or complicated and time-consuming patient-derived xenografts. Recent development of cancer organoids allows us to establish efficient and long-term culture of primary human CRC tissues, which provide attractive physiological disease models for high-throughput drug screening (95). It is important to note that deregulation of the Wnt pathway is crucial but not sufficient for CRC tumorigenesis. Stepwise accumulation of other oncogene and/or tumor suppressor gene mutations such as KRAS and p53 is required for tumor progression. It remains unclear whether targeting Wnt signaling alone in advanced CRCs will be sufficient for tumor suppression. However, a recent study has demonstrated that Apc restoration can trigger differentiation and restore intestinal homeostasis in established tumors with Kras and p53 mutations using a transgenic mouse model (22). The data indicate that Wnt activation is absolutely required to sustain tumor growth in advanced CRC and provide compelling evidence that the Wnt signaling pathway can be an effective therapeutic target for treatment of CRC.
GRANTS
Work in the laboratory of V. S. W. Li has been funded by the UK Medical Research Council (MC_UP_1202/7) and now by the Francis Crick Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: L.N. and P.A. prepared figures; L.N., P.A., and V.S.L. drafted manuscript; L.N., P.A., and V.S.L. edited and revised manuscript; V.S.L. conception and design of research; V.S.L. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank R. Lovell-Badge for critical reading of the manuscript. This review is a snapshot of Wnt signaling pathway and drug targeting at the current status, and we apologize to many colleagues whose work could not be cited here due to space limitation.
REFERENCES
- 1.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J 16: 3797–3804, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrami L, Kunz B, Iacovache I, van der Goot FG. Palmitoylation and ubiquitination regulate exit of the Wnt signaling protein LRP6 from the endoplasmic reticulum. Proc Natl Acad Sci USA 105: 5384–5389, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akhter J, Chen X, Bowrey P, Bolton EJ, Morris DL. Vitamin D3 analog, EB1089, inhibits growth of subcutaneous xenografts of the human colon cancer cell line, LoVo, in a nude mouse model. Dis Colon Rectum 40: 317–321, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Avizienyte E, Roth S, Loukola A, Hemminki A, Lothe RA, Stenwig AE, Fossa SD, Salovaara R, Aaltonen LA. Somatic mutations in LKB1 are rare in sporadic colorectal and testicular tumors. Cancer Res 58: 2087–2090, 1998. [PubMed] [Google Scholar]
- 5.Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, Fassina A, Cordenonsi M, Piccolo S. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell 158: 157–170, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, McKeown-Eyssen G, Summers RW, Rothstein R, Burke CA, Snover DC, Church TR, Allen JI, Beach M, Beck GJ, Bond JH, Byers T, Greenberg ER, Mandel JS, Marcon N, Mott LA, Pearson L, Saibil F, van Stolk RU. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med 348: 891–899, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S, Kuo CJ, Camargo FD. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature 493: 106–110, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bass AJ, Lawrence MS, Brace LE, Ramos AH, Drier Y, Cibulskis K, Sougnez C, Voet D, Saksena G, Sivachenko A, Jing R, Parkin M, Pugh T, Verhaak RG, Stransky N, Boutin AT, Barretina J, Solit DB, Vakiani E, Shao W, Mishina Y, Warmuth M, Jimenez J, Chiang DY, Signoretti S, Kaelin WG, Spardy N, Hahn WC, Hoshida Y, Ogino S, Depinho RA, Chin L, Garraway LA, Fuchs CS, Baselga J, Tabernero J, Gabriel S, Lander ES, Getz G, Meyerson M. Genomic sequencing of colorectal adenocarcinomas identifies a recurrent VTI1A-TCF7L2 fusion. Nat Genet 43: 964–968, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 382: 638–642, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci 121: 737–746, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487: 330–337, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan TA. Nonsteroidal anti-inflammatory drugs, apoptosis, and colon-cancer chemoprevention. Lancet Oncol 3: 166–174, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, Roth MG, Amatruda JF, Chen C, Lum L. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol 5: 100–107, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen PH, Chen X, Lin Z, Fang D, He X. The structural basis of R-spondin recognition by LGR5 and RNF43. Genes Dev 27: 1345–1350, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuilliere-Dartigues P, El-Bchiri J, Krimi A, Buhard O, Fontanges P, Flejou JF, Hamelin R, Duval A. TCF-4 isoforms absent in TCF-4 mutated MSI-H colorectal cancer cells colocalize with nuclear CtBP and repress TCF-4-mediated transcription. Oncogene 25: 4441–4448, 2006. [DOI] [PubMed] [Google Scholar]
- 17.de Lau W, Barker N, Low TY, Koo BK, Li VS, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M, Stange DE, van Es JE, Guardavaccaro D, Schasfoort RB, Mohri Y, Nishimori K, Mohammed S, Heck AJ, Clevers H. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476: 293–297, 2011. [DOI] [PubMed] [Google Scholar]
- 18.de Lau W, Peng WC, Gros P, Clevers H. The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes Dev 28: 305–316, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeAlmeida VI, Miao L, Ernst JA, Koeppen H, Polakis P, Rubinfeld B. The soluble Wnt receptor Frizzled8CRD-hFc inhibits the growth of teratocarcinomas in vivo. Cancer Res 67: 5371–5379, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Dillard AC, Lane MA. Retinol decreases beta-catenin protein levels in retinoic acid-resistant colon cancer cell lines. Mol Carcinog 46: 315–329, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Domingo E, Espin E, Armengol M, Oliveira C, Pinto M, Duval A, Brennetot C, Seruca R, Hamelin R, Yamamoto H, Schwartz S Jr. Activated BRAF targets proximal colon tumors with mismatch repair deficiency and MLH1 inactivation. Genes Chromosomes Cancer 39: 138–142, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Dow LE, O'Rourke KP, Simon J, Tschaharganeh DF, van Es JH, Clevers H, Lowe SW. APC restoration promotes cellular differentiation and reestablishes crypt homeostasis in colorectal cancer. Cell 161: 1539–1552, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DuBois RN, Giardiello FM, Smalley WE. Nonsteroidal anti-inflammatory drugs, eicosanoids, and colorectal cancer prevention. Gastroenterol Clin North Am 25: 773–791, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Duval A, Gayet J, Zhou XP, Iacopetta B, Thomas G, Hamelin R. Frequent frameshift mutations of the TCF-4 gene in colorectal cancers with microsatellite instability. Cancer Res 59: 4213–4215, 1999. [PubMed] [Google Scholar]
- 25.Ellwanger K, Saito H, Clement-Lacroix P, Maltry N, Niedermeyer J, Lee WK, Baron R, Rawadi G, Westphal H, Niehrs C. Targeted disruption of the Wnt regulator Kremen induces limb defects and high bone density. Mol Cell Biol 28: 4875–4882, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, Moon RT, Teo JL, Kim HY, Moon SH, Ha JR, Kahn M. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected]. Proc Natl Acad Sci USA 101: 12682–12687, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esteller M, Sparks A, Toyota M, Sanchez-Cespedes M, Capella G, Peinado MA, Gonzalez S, Tarafa G, Sidransky D, Meltzer SJ, Baylin SB, Herman JG. Analysis of adenomatous polyposis coli promoter hypermethylation in human cancer. Cancer Res 60: 4366–4371, 2000. [PubMed] [Google Scholar]
- 28.Faller WJ, Jackson TJ, Knight JR, Ridgway RA, Jamieson T, Karim SA, Jones C, Radulescu S, Huels DJ, Myant KB, Dudek KM, Casey HA, Scopelliti A, Cordero JB, Vidal M, Pende M, Ryazanov AG, Sonenberg N, Meyuhas O, Hall MN, Bushell M, Willis AE, Sansom OJ. mTORC1-mediated translational elongation limits intestinal tumour initiation and growth. Nature 517: 497–500, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol 6: 479–507, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 61: 759–767, 1990. [DOI] [PubMed] [Google Scholar]
- 31.Fiedler M, Mendoza-Topaz C, Rutherford TJ, Mieszczanek J, Bienz M. Dishevelled interacts with the DIX domain polymerization interface of Axin to interfere with its function in down-regulating beta-catenin. Proc Natl Acad Sci USA 108: 1937–1942, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fodde R, Smits R, Clevers H. APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer 1: 55–67, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Fre S, Pallavi SK, Huyghe M, Lae M, Janssen KP, Robine S, Artavanis-Tsakonas S, Louvard D. Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine. Proc Natl Acad Sci USA 106: 6309–6314, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu X, Li L, Peng Y. Wnt signalling pathway in the serrated neoplastic pathway of the colorectum: possible roles and epigenetic regulatory mechanisms. J Clin Pathol 65: 675–679, 2012. [DOI] [PubMed] [Google Scholar]
- 35.Fujii N, You L, Xu Z, Uematsu K, Shan J, He B, Mikami I, Edmondson LR, Neale G, Zheng J, Guy RK, Jablons DM. An antagonist of dishevelled protein-protein interaction suppresses beta-catenin-dependent tumor cell growth. Cancer Res 67: 573–579, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Galiatsatos P, Foulkes WD. Familial adenomatous polyposis. Am J Gastroenterol 101: 385–398, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Gang EJ, Hsieh YT, Pham J, Zhao Y, Nguyen C, Huantes S, Park E, Naing K, Klemm L, Swaminathan S, Conway EM, Pelus LM, Crispino J, Mullighan CG, McMillan M, Muschen M, Kahn M, Kim YM. Small-molecule inhibition of CBP/catenin interactions eliminates drug-resistant clones in acute lymphoblastic leukemia. Oncogene 33: 2169–2178, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao C, Chen YG. Dishevelled: The hub of Wnt signaling. Cell Signal 22: 717–727, 2010. [DOI] [PubMed] [Google Scholar]
- 39.Gaspar C, Fodde R. APC dosage effects in tumorigenesis and stem cell differentiation. Int J Dev Biol 48: 377–386, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Giannakis M, Hodis E, Jasmine Mu X, Yamauchi M, Rosenbluh J, Cibulskis K, Saksena G, Lawrence MS, Qian ZR, Nishihara R, Van Allen EM, Hahn WC, Gabriel SB, Lander ES, Getz G, Ogino S, Fuchs CS, Garraway LA. RNF43 is frequently mutated in colorectal and endometrial cancers. Nat Genet 46: 1264–1266, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJ. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med 328: 1313–1316, 1993. [DOI] [PubMed] [Google Scholar]
- 42.Glinka A, Dolde C, Kirsch N, Huang YL, Kazanskaya O, Ingelfinger D, Boutros M, Cruciat CM, Niehrs C. LGR4 and LGR5 are R-spondin receptors mediating Wnt/beta-catenin and Wnt/PCP signalling. EMBO Rep 12: 1055–1061, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391: 357–362, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Gonsalves FC, Klein K, Carson BB, Katz S, Ekas LA, Evans S, Nagourney R, Cardozo T, Brown AM, DasGupta R. An RNAi-based chemical genetic screen identifies three small-molecule inhibitors of the Wnt/wingless signaling pathway. Proc Natl Acad Sci USA 108: 5954–5963, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grandy D, Shan J, Zhang X, Rao S, Akunuru S, Li H, Zhang Y, Alpatov I, Zhang XA, Lang RA, Shi DL, Zheng JJ. Discovery and characterization of a small molecule inhibitor of the PDZ domain of dishevelled. J Biol Chem 284: 16256–16263, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grosch S, Tegeder I, Niederberger E, Brautigam L, Geisslinger G. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J 15: 2742–2744, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, Lam A, Lazetic S, Ma S, Mitra S, Park IK, Pickell K, Sato A, Satyal S, Stroud M, Tran H, Yen WC, Lewicki J, Hoey T. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci USA 109: 11717–11722, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hao HX, Xie Y, Zhang Y, Charlat O, Oster E, Avello M, Lei H, Mickanin C, Liu D, Ruffner H, Mao X, Ma Q, Zamponi R, Bouwmeester T, Finan PM, Kirschner MW, Porter JA, Serluca FC, Cong F. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 485: 195–200, 2012. [DOI] [PubMed] [Google Scholar]
- 49.Harris DM, Go VL. Vitamin D and colon carcinogenesis. J Nutr 134: 3463S–3471S, 2004. [DOI] [PubMed] [Google Scholar]
- 50.He B, Reguart N, You L, Mazieres J, Xu Z, Lee AY, Mikami I, McCormick F, Jablons DM. Blockade of Wnt-1 signaling induces apoptosis in human colorectal cancer cells containing downstream mutations. Oncogene 24: 3054–3058, 2005. [DOI] [PubMed] [Google Scholar]
- 51.He B, You L, Uematsu K, Xu Z, Lee AY, Matsangou M, McCormick F, Jablons DM. A monoclonal antibody against Wnt-1 induces apoptosis in human cancer cells. Neoplasia 6: 7–14, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He K, Xu T, Xu Y, Ring A, Kahn M, Goldkorn A. Cancer cells acquire a drug resistant, highly tumorigenic, cancer stem-like phenotype through modulation of the PI3K/Akt/beta-catenin/CBP pathway. Int J Cancer 134: 43–54, 2014. [DOI] [PubMed] [Google Scholar]
- 53.Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, Hild M, Shi X, Wilson CJ, Mickanin C, Myer V, Fazal A, Tomlinson R, Serluca F, Shao W, Cheng H, Shultz M, Rau C, Schirle M, Schlegl J, Ghidelli S, Fawell S, Lu C, Curtis D, Kirschner MW, Lengauer C, Finan PM, Tallarico JA, Bouwmeester T, Porter JA, Bauer A, Cong F. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461: 614–620, 2009. [DOI] [PubMed] [Google Scholar]
- 54.Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 363: 558–561, 1993. [DOI] [PubMed] [Google Scholar]
- 55.Jacob LS, Wu X, Dodge ME, Fan CW, Kulak O, Chen B, Tang W, Wang B, Amatruda JF, Lum L. Genome-wide RNAi screen reveals disease-associated genes that are common to Hedgehog and Wnt signaling. Sci Signal 4: ra4, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jin LH, Shao QJ, Luo W, Ye ZY, Li Q, Lin SC. Detection of point mutations of the Axin1 gene in colorectal cancers. Int J Cancer 107: 696–699, 2003. [DOI] [PubMed] [Google Scholar]
- 57.Kakugawa S, Langton PF, Zebisch M, Howell SA, Chang TH, Liu Y, Feizi T, Bineva G, O'Reilly N, Snijders AP, Jones EY, Vincent JP. Notum deacylates Wnt proteins to suppress signalling activity. Nature 519: 187–192, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, Liu Y, Boyle B, Park E, Emtage P, Funk WD, Tomizuka K. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science 309: 1256–1259, 2005. [DOI] [PubMed] [Google Scholar]
- 59.Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D, Finnear R, Markham A, Groffen J, Boguski M, Altschul S, Horii A, Ando H, Miyoshi Y, Miki Y, Nishisho I, Nakamura Y. Identification of FAP locus genes from chromosome 5q21. Science 253: 661–665, 1991. [DOI] [PubMed] [Google Scholar]
- 60.Kitagawa M, Hatakeyama S, Shirane M, Matsumoto M, Ishida N, Hattori K, Nakamichi I, Kikuchi A, Nakayama K, Nakayama K. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of beta-catenin. EMBO J 18: 2401–2410, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koinuma K, Yamashita Y, Liu W, Hatanaka H, Kurashina K, Wada T, Takada S, Kaneda R, Choi YL, Fujiwara SI, Miyakura Y, Nagai H, Mano H. Epigenetic silencing of AXIN2 in colorectal carcinoma with microsatellite instability. Oncogene 25: 139–146, 2006. [DOI] [PubMed] [Google Scholar]
- 62.Koo BK, Spit M, Jordens I, Low TY, Stange DE, van de Wetering M, van Es JH, Mohammed S, Heck AJ, Maurice MM, Clevers H. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature 488: 665–669, 2012. [DOI] [PubMed] [Google Scholar]
- 63.Koo BK, van Es JH, van den Born M, Clevers H. Porcupine inhibitor suppresses paracrine Wnt-driven growth of Rnf43;Znrf3-mutant neoplasia. Proc Natl Acad Sci USA 112: 7548–7550, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science 275: 1784–1787, 1997. [DOI] [PubMed] [Google Scholar]
- 65.Lammi L, Arte S, Somer M, Jarvinen H, Lahermo P, Thesleff I, Pirinen S, Nieminen P. Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal cancer. Am J Hum Genet 74: 1043–1050, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lau T, Chan E, Callow M, Waaler J, Boggs J, Blake RA, Magnuson S, Sambrone A, Schutten M, Firestein R, Machon O, Korinek V, Choo E, Diaz D, Merchant M, Polakis P, Holsworth DD, Krauss S, Costa M. A novel tankyrase small-molecule inhibitor suppresses APC mutation-driven colorectal tumor growth. Cancer Res 73: 3132–3144, 2013. [DOI] [PubMed] [Google Scholar]
- 67.Lavergne E, Hendaoui I, Coulouarn C, Ribault C, Leseur J, Eliat PA, Mebarki S, Corlu A, Clement B, Musso O. Blocking Wnt signaling by SFRP-like molecules inhibits in vivo cell proliferation and tumor growth in cells carrying active beta-catenin. Oncogene 30: 423–433, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lazarova DL, Chiaro C, Wong T, Drago E, Rainey A, O'Malley S, Bordonaro M. CBP activity mediates effects of the histone deacetylase inhibitor butyrate on Wnt activity and apoptosis in colon cancer cells. J Cancer 4: 481–490, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lehtio L, Chi NW, Krauss S. Tankyrases as drug targets. FEBS J 280: 3576–3593, 2013. [DOI] [PubMed] [Google Scholar]
- 70.Lepourcelet M, Chen YN, France DS, Wang H, Crews P, Petersen F, Bruseo C, Wood AW, Shivdasani RA. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell 5: 91–102, 2004. [DOI] [PubMed] [Google Scholar]
- 71.Li VS, Ng SS, Boersema PJ, Low TY, Karthaus WR, Gerlach JP, Mohammed S, Heck AJ, Maurice MM, Mahmoudi T, Clevers H. Wnt signaling through inhibition of beta-catenin degradation in an intact Axin1 complex. Cell 149: 1245–1256, 2012. [DOI] [PubMed] [Google Scholar]
- 72.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108: 837–847, 2002. [DOI] [PubMed] [Google Scholar]
- 73.Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T, Kasibhatla S, Schuller AG, Li AG, Cheng D, Li J, Tompkins C, Pferdekamper A, Steffy A, Cheng J, Kowal C, Phung V, Guo G, Wang Y, Graham MP, Flynn S, Brenner JC, Li C, Villarroel MC, Schultz PG, Wu X, McNamara P, Sellers WR, Petruzzelli L, Boral AL, Seidel HM, McLaughlin ME, Che J, Carey TE, Vanasse G, Harris JL. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci USA 110: 20224–20229, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu W, Dong X, Mai M, Seelan RS, Taniguchi K, Krishnadath KK, Halling KC, Cunningham JM, Boardman LA, Qian C, Christensen E, Schmidt SS, Roche PC, Smith DI, Thibodeau SN. Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating beta-catenin/TCF signalling. Nat Genet 26: 146–147, 2000. [DOI] [PubMed] [Google Scholar]
- 75.Lum L, Clevers Cell biology H. The unusual case of Porcupine. Science 337: 922–923, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Major MB, Camp ND, Berndt JD, Yi X, Goldenberg SJ, Hubbert C, Biechele TL, Gingras AC, Zheng N, Maccoss MJ, Angers S, Moon RT. Wilms tumor suppressor WTX negatively regulates WNT/beta-catenin signaling. Science 316: 1043–1046, 2007. [DOI] [PubMed] [Google Scholar]
- 77.Marvin ML, Mazzoni SM, Herron CM, Edwards S, Gruber SB, Petty EM. AXIN2-associated autosomal dominant ectodermal dysplasia and neoplastic syndrome. Am J Med Genet A 155A: 898–902, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mikami I, You L, He B, Xu Z, Batra S, Lee AY, Mazieres J, Reguart N, Uematsu K, Koizumi K, Jablons DM. Efficacy of Wnt-1 monoclonal antibody in sarcoma cells. BMC Cancer 5: 53, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mimori-Kiyosue Y, Shiina N, Tsukita S. Adenomatous polyposis coli (APC) protein moves along microtubules and concentrates at their growing ends in epithelial cells. J Cell Biol 148: 505–518, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mimori-Kiyosue Y, Shiina N, Tsukita S. The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules. Curr Biol 10: 865–868, 2000. [DOI] [PubMed] [Google Scholar]
- 81.Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell 86: 391–399, 1996. [DOI] [PubMed] [Google Scholar]
- 82.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 275: 1787–1790, 1997. [DOI] [PubMed] [Google Scholar]
- 83.Murakami T, Mitomi H, Saito T, Takahashi M, Sakamoto N, Fukui N, Yao T, Watanabe S. Distinct WNT/beta-catenin signaling activation in the serrated neoplasia pathway and the adenoma-carcinoma sequence of the colorectum. Mod Pathol 28: 146–158, 2015. [DOI] [PubMed] [Google Scholar]
- 84.Muto Y, Maeda T, Suzuki K, Kato T, Watanabe F, Kamiyama H, Saito M, Koizumi K, Miyaki Y, Konishi F, Alonso S, Perucho M, Rikiyama T. DNA methylation alterations of AXIN2 in serrated adenomas and colon carcinomas with microsatellite instability. BMC Cancer 14: 466, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nagase H, Nakamura Y. Mutations of the APC (adenomatous polyposis coli) gene. Hum Mutat 2: 425–434, 1993. [DOI] [PubMed] [Google Scholar]
- 86.Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, van Noort M, Hui CC, Clevers H, Dotto GP, Radtke F. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet 33: 416–421, 2003. [DOI] [PubMed] [Google Scholar]
- 87.Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, Koyama K, Utsunomiya J, Baba S, Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science 253: 665–669, 1991. [DOI] [PubMed] [Google Scholar]
- 88.Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M, Munoz A. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol 154: 369–387, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pendas-Franco N, Aguilera O, Pereira F, Gonzalez-Sancho JM, Munoz A. Vitamin D and Wnt/beta-catenin pathway in colon cancer: role and regulation of DICKKOPF genes. Anticancer Res 28: 2613–2623, 2008. [PubMed] [Google Scholar]
- 90.Peng WC, de Lau W, Forneris F, Granneman JC, Huch M, Clevers H, Gros P. Structure of stem cell growth factor R-spondin 1 in complex with the ectodomain of its receptor LGR5. Cell Rep 3: 1885–1892, 2013. [DOI] [PubMed] [Google Scholar]
- 91.Proweller A, Tu L, Lepore JJ, Cheng L, Lu MM, Seykora J, Millar SE, Pear WS, Parmacek MS. Impaired Notch signaling promotes de novo squamous cell carcinoma formation. Cancer Res 66: 7438–7444, 2006. [DOI] [PubMed] [Google Scholar]
- 92.Qi J, Zhu YQ, Luo J, Tao WH. Hypermethylation and expression regulation of secreted frizzled-related protein genes in colorectal tumor. World J Gastroenterol 12: 7113–7117, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rawson JB, Manno M, Mrkonjic M, Daftary D, Dicks E, Buchanan DD, Younghusband HB, Parfrey PS, Young JP, Pollett A, Green RC, Gallinger S, McLaughlin JR, Knight JA, Bapat B. Promoter methylation of Wnt antagonists DKK1 and SFRP1 is associated with opposing tumor subtypes in two large populations of colorectal cancer patients. Carcinogenesis 32: 741–747, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rivera B, Perea J, Sanchez E, Villapun M, Sanchez-Tome E, Mercadillo F, Robledo M, Benitez J, Urioste M. A novel AXIN2 germline variant associated with attenuated FAP without signs of oligondontia or ectodermal dysplasia. Eur J Hum Genet 22: 423–426, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sachs N, Clevers H. Organoid cultures for the analysis of cancer phenotypes. Curr Opin Genet Devel 24: 68–73, 2014. [DOI] [PubMed] [Google Scholar]
- 96.Sakoguchi-Okada N, Takahashi-Yanaga F, Fukada K, Shiraishi F, Taba Y, Miwa Y, Morimoto S, Iida M, Sasaguri T. Celecoxib inhibits the expression of survivin via the suppression of promoter activity in human colon cancer cells. Biochem Pharmacol 73: 1318–1329, 2007. [DOI] [PubMed] [Google Scholar]
- 97.Salahshor S, Woodgett JR. The links between axin and carcinogenesis. J Clin Pathol 58: 225–236, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schwarz-Romond T, Fiedler M, Shibata N, Butler PJ, Kikuchi A, Higuchi Y, Bienz M. The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat Struct Mol Biol 14: 484–492, 2007. [DOI] [PubMed] [Google Scholar]
- 99.Semenov MV, Zhang X, He X. DKK1 antagonizes Wnt signaling without promotion of LRP6 internalization and degradation. J Biol Chem 283: 21427–21432, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Seshagiri S, Stawiski EW, Durinck S, Modrusan Z, Storm EE, Conboy CB, Chaudhuri S, Guan Y, Janakiraman V, Jaiswal BS, Guillory J, Ha C, Dijkgraaf GJ, Stinson J, Gnad F, Huntley MA, Degenhardt JD, Haverty PM, Bourgon R, Wang W, Koeppen H, Gentleman R, Starr TK, Zhang Z, Largaespada DA, Wu TD, de Sauvage FJ. Recurrent R-spondin fusions in colon cancer. Nature 488: 660–664, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shah S, Hecht A, Pestell R, Byers SW. Trans-repression of beta-catenin activity by nuclear receptors. J Biol Chem 278: 48137–48145, 2003. [DOI] [PubMed] [Google Scholar]
- 102.Shan J, Shi DL, Wang J, Zheng J. Identification of a specific inhibitor of the dishevelled PDZ domain. Biochemistry 44: 15495–15503, 2005. [DOI] [PubMed] [Google Scholar]
- 103.Shimizu Y, Ikeda S, Fujimori M, Kodama S, Nakahara M, Okajima M, Asahara T. Frequent alterations in the Wnt signaling pathway in colorectal cancer with microsatellite instability. Genes Chromosomes Cancer 33: 73–81, 2002. [DOI] [PubMed] [Google Scholar]
- 104.Silva AL, Dawson SN, Arends MJ, Guttula K, Hall N, Cameron EA, Huang TH, Brenton JD, Tavare S, Bienz M, Ibrahim AE. Boosting Wnt activity during colorectal cancer progression through selective hypermethylation of Wnt signaling antagonists. BMC Cancer 14: 891, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, Szabo S, Buckhaults P, Farrell C, Meeh P, Markowitz SD, Willis J, Dawson D, Willson JK, Gazdar AF, Hartigan J, Wu L, Liu C, Parmigiani G, Park BH, Bachman KE, Papadopoulos N, Vogelstein B, Kinzler KW, Velculescu VE. The consensus coding sequences of human breast and colorectal cancers. Science 314: 268–274, 2006. [DOI] [PubMed] [Google Scholar]
- 106.Soprano DR, Qin P, Soprano KJ. Retinoic acid receptors and cancers. Annu Rev Nutr 24: 201–221, 2004. [DOI] [PubMed] [Google Scholar]
- 107.Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, Pretlow TP, Yang B, Akiyama Y, Van Engeland M, Toyota M, Tokino T, Hinoda Y, Imai K, Herman JG, Baylin SB. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet 36: 417–422, 2004. [DOI] [PubMed] [Google Scholar]
- 108.Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell 11: 791–801, 2006. [DOI] [PubMed] [Google Scholar]
- 109.Tang W, Dodge M, Gundapaneni D, Michnoff C, Roth M, Lum L. A genome-wide RNAi screen for Wnt/beta-catenin pathway components identifies unexpected roles for TCF transcription factors in cancer. Proc Natl Acad Sci USA 105: 9697–9702, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tang Y, Simoneau AR, Liao WX, Yi G, Hope C, Liu F, Li S, Xie J, Holcombe RF, Jurnak FA, Mercola D, Hoang BH, Zi X. WIF1, a Wnt pathway inhibitor, regulates SKP2 and c-myc expression leading to G1 arrest and growth inhibition of human invasive urinary bladder cancer cells. Mol Cancer Ther 8: 458–468, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Taniguchi H, Yamamoto H, Hirata T, Miyamoto N, Oki M, Nosho K, Adachi Y, Endo T, Imai K, Shinomura Y. Frequent epigenetic inactivation of Wnt inhibitory factor-1 in human gastrointestinal cancers. Oncogene 24: 7946–7952, 2005. [DOI] [PubMed] [Google Scholar]
- 112.Tanneberger K, Pfister AS, Brauburger K, Schneikert J, Hadjihannas MV, Kriz V, Schulte G, Bryja V, Behrens J. Amer1/WTX couples Wnt-induced formation of PtdIns(4,5)P2 to LRP6 phosphorylation. EMBO J 30: 1433–1443, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science 260: 816–819, 1993. [DOI] [PubMed] [Google Scholar]
- 114.Thorstensen L, Lind GE, Lovig T, Diep CB, Meling GI, Rognum TO, Lothe RA. Genetic and epigenetic changes of components affecting the WNT pathway in colorectal carcinomas stratified by microsatellite instability. Neoplasia 7: 99–108, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tian W, Han X, Yan M, Xu Y, Duggineni S, Lin N, Luo G, Li YM, Han X, Huang Z, An J. Structure-based discovery of a novel inhibitor targeting the beta-catenin/Tcf4 interaction. Biochemistry 51: 724–731, 2012. [DOI] [PubMed] [Google Scholar]
- 116.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 71: 241–260, 2009. [DOI] [PubMed] [Google Scholar]
- 117.van Es JH, Haegebarth A, Kujala P, Itzkovitz S, Koo BK, Boj SF, Korving J, van den Born M, van Oudenaarden A, Robine S, Clevers H. A critical role for the Wnt effector Tcf4 in adult intestinal homeostatic self-renewal. Mol Cell Biol 32: 1918–1927, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435: 959–963, 2005. [DOI] [PubMed] [Google Scholar]
- 119.Vilar E, Tabernero J. Molecular dissection of microsatellite instable colorectal cancer. Cancer Discov 3: 502–511, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Voorham QJ, Janssen J, Tijssen M, Snellenberg S, Mongera S, van Grieken NC, Grabsch H, Kliment M, Rembacken BJ, Mulder CJ, van Engeland M, Meijer GA, Steenbergen RD, Carvalho B. Promoter methylation of Wnt-antagonists in polypoid and nonpolypoid colorectal adenomas. BMC Cancer 13: 603, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Waaler J, Machon O, Tumova L, Dinh H, Korinek V, Wilson SR, Paulsen JE, Pedersen NM, Eide TJ, Machonova O, Gradl D, Voronkov A, von Kries JP, Krauss S. A novel tankyrase inhibitor decreases canonical Wnt signaling in colon carcinoma cells and reduces tumor growth in conditional APC mutant mice. Cancer Res 72: 2822–2832, 2012. [DOI] [PubMed] [Google Scholar]
- 122.Waaler J, Machon O, von Kries JP, Wilson SR, Lundenes E, Wedlich D, Gradl D, Paulsen JE, Machonova O, Dembinski JL, Dinh H, Krauss S. Novel synthetic antagonists of canonical Wnt signaling inhibit colorectal cancer cell growth. Cancer Res 71: 197–205, 2011. [DOI] [PubMed] [Google Scholar]
- 123.Wang W, Liu H, Wang S, Hao X, Li L. A diterpenoid derivative 15-oxospiramilactone inhibits Wnt/beta-catenin signaling and colon cancer cell tumorigenesis. Cell Res 21: 730–740, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wei W, Chua MS, Grepper S, So SK. Blockade of Wnt-1 signaling leads to anti-tumor effects in hepatocellular carcinoma cells. Mol Cancer 8: 76, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wend P, Fang L, Zhu Q, Schipper JH, Loddenkemper C, Kosel F, Brinkmann V, Eckert K, Hindersin S, Holland JD, Lehr S, Kahn M, Ziebold U, Birchmeier W. Wnt/beta-catenin signalling induces MLL to create epigenetic changes in salivary gland tumours. EMBO J 32: 1977–1989, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.You L, He B, Uematsu K, Xu Z, Mazieres J, Lee A, McCormick F, Jablons DM. Inhibition of Wnt-1 signaling induces apoptosis in beta-catenin-deficient mesothelioma cells. Cancer Res 64: 3474–3478, 2004. [DOI] [PubMed] [Google Scholar]
- 127.You L, He B, Xu Z, Uematsu K, Mazieres J, Fujii N, Mikami I, Reguart N, McIntosh JK, Kashani-Sabet M, McCormick F, Jablons DM. An anti-Wnt-2 monoclonal antibody induces apoptosis in malignant melanoma cells and inhibits tumor growth. Cancer Res 64: 5385–5389, 2004. [DOI] [PubMed] [Google Scholar]
- 128.Zhang X, Cheong SM, Amado NG, Reis AH, MacDonald BT, Zebisch M, Jones EY, Abreu JG, He X. Notum is required for neural and head induction via Wnt deacylation, oxidation, and inactivation. Dev Cell 32: 719–730, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang Y, Liu S, Mickanin C, Feng Y, Charlat O, Michaud GA, Schirle M, Shi X, Hild M, Bauer A, Myer VE, Finan PM, Porter JA, Huang SM, Cong F. RNF146 is a poly(ADP-ribose)-directed E3 ligase that regulates axin degradation and Wnt signalling. Nat Cell Biol 13: 623–629, 2011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.