Abstract
In 54/64 subjects with nosocomial diarrhea, fecal calprotectin levels correlated with the results of stool samples tested for Clostridium difficile toxin gene by PCR. Fecal calprotectin levels can be used as an adjunctive measure to PCR to support the diagnosis of C. difficile infection.
TEXT
Fecal calprotectin (fCPT), found predominantly in neutrophils, is a marker of inflammation in inflammatory bowel disease (IBD) (1) due to neutrophilic infiltration of the gut and shedding into the gut lumen and can therefore be quantified in feces. Serum procalcitonin (sPCT), a biomarker of bacterial infection, has been validated for use in monitoring responses to antibiotic therapy and as a prognosticator of sepsis in critically ill patients (2).
We performed a prospective exploratory observational study to assess whether fCPT and sPCT could aid in the diagnosis of Clostridium difficile infection (CDI) in the context of a positive PCR for C. difficile toxin gene (CD-PCR).
This study was undertaken at a single tertiary 637-bed university teaching hospital and was approved by the institutional research ethics committee. Inpatients whose stool samples were received for CD-PCR underwent testing for fCPT, as assessed by the two Quantum Blue calprotectin lateral flow rapid tests (Bühlmann, Basel, Switzerland), according to the manufacturer's instructions. Briefly, a 1:150 dilution of sample with the buffer was centrifuged, and 80 μl of the supernatant was loaded onto the test cartridge and placed in the dedicated reader. The assay selectively measures calprotectin by a sandwich immunoassay using monoclonal antibodies. The lower-range assay (LR-fCPT) (range, 30 to 300 μg/g) was developed as a screen for IBD, and the higher-range assay (HR-fCPT) (range, 100 to 1,800 μg/g) is used to follow IBD therapy and for predicting relapses. As per the manufacturer's recommendations for interpretation with IBD, HR-fCPT values of <100 μg/g represent a mild or noninflammatory state of the gut, whereas values of >200 μg/g indicate active organic disease with inflammation. sPCT levels were measured using the Vidas B.R.A.H.M.S PCT assay (bioMérieux, Marcy l'Etoile, France) on leftover serum from subject samples collected within 48 h prior to the date on which the stool sample was collected for CD-PCR. Written informed consent was obtained from all patients for serum and stool testing and for medical chart review.
Chi-square and Fisher exact tests were used to compare categorical variables, and a Student t test was used for continuous variables. Quantitative variables were compared by the nonparametric Mann-Whitney U test and Kruskal-Wallis test. P values of <0.05 were considered statistically significant. IBM SPSS software (version 22) was used for statistical analysis.
Between January and September 2013, 64 subjects (44 CD-PCR positive and 20 CD-PCR negative) were enrolled. There were no significant differences in gender (53% male) and age distribution between the 2 groups; 47 (73%) individuals were ≥60 years old. CD-PCR+ cases had a higher mean white cell count (11.86 × 109 ± 7.23 × 109 versus 8.15 × 109 ± 3.89 × 109 cells/liter; P < 0.01) but did not differ significantly from CD-PCR− cases with regard to serum albumin level, serum creatinine level, temperature, ATLAS score (3), presence of abdominal pain, presence of IBD, or concomitant antibiotic therapy for ≥1 day during CDI therapy. The presence of diarrhea, assessed by documentation in medical charts, was more frequently reported in CD-PCR+ patients (93.2% in CD-PCR+ versus 70% in CD-PCR− patients; P = 0.02). When clinically indicated, concurrent analyses for alternative infectious causes of diarrhea were negative. Among the 44 CD-PCR+ subjects, 2 (4.5%) suffered CDI complications, and 2 (4.5%) other patients died within 30 days from non-CDI-related causes.
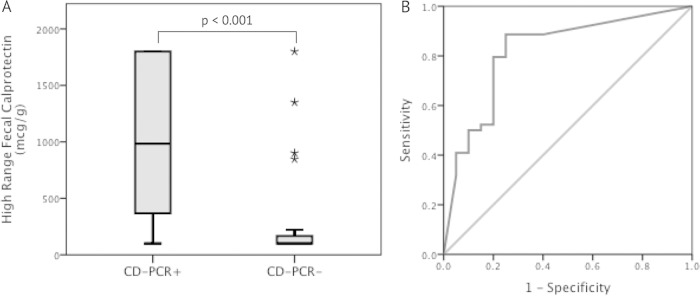
CD-PCR+ patients had higher (P < 0.001) median (interquartile range) HR-fCPT values than CD-PCR− patients, at 983 μg/g (351 to >1,800 μg/g) compared with <100 μg/g (<100 to 194 μg/g) (Fig. 1A). Similarly, the median (interquartile range) LR-fCPT values were significantly higher (P < 0.001) in CD-PCR+ samples (>300 μg/g [>300 to >300 μg/g]) than in CD-PCR− samples (77.5 μg/g [30 to 238 μg/g]). However, the median sPCT levels in CD-PCR+ subjects did not significantly differ (P = 0.08) from those in CD-PCR− subjects. The area under the curve (AUC) for HR-fCPT predicting CD-PCR positivity was 0.82 (95% confidence interval [CI], 0.70 to 0.94) with a sensitivity of 88.6% and a specificity of 75% at an HR-fCPT level of 135 μg/g (Fig. 1B), the threshold seen for an optimal AUC statistic. When using the manufacturer's suggested cutoff of 200 μg/g for IBD interpretation, the sensitivity decreased to 79.5% but maintained a 75% specificity. The LR-fCPT was less specific, with an AUC of 0.8 (95% CI, 0.67 to 0.92), sensitivity of 88.6%, and specificity of 55% at an LR-fCPT threshold of 81.5 μg/g. The AUC for sPCT was not significant (0.64; 95% CI, 0.5 to 0.78) for predicting a CD-PCR+ result.
FIG 1.
(A) Comparison of high-range fecal calprotectin levels correlated with the occurrence of C. difficile toxin gene detection by PCR in stool samples. (B) Receiver operating characteristic curve of high-range fecal calprotectin for predicting a positive C. difficile toxin gene assay by PCR (area under the curve, 0.82). CD-PCR, PCR assay for C. difficile toxin gene.
Within the CD-PCR+ group, the measured HR-fCPT level was greater than the cutoff of 135 μg/g in 39/44 (88.6%) subjects and above the IBD threshold (200 μg/g) in 35/44 (79.5%) subjects, compared to 5/20 (25%) subjects in the CD-PCR− group, regardless of the cutoff; statistical significance (P < 0.001) was maintained in both instances. Further analyses comparing the clinical and laboratory parameters of CD-PCR+ subjects with an HR-fCPT greater or less than either cutoff did not yield any significant parameters that could adjunctively support or refute a CDI diagnosis. The ATLAS score and its five components, analyzed independently, did not differ significantly between CD-PCR+ and CD-PCR− subjects and did not correlate with HR-fCPT and sPCT levels.
Our results are similar to those obtained in prior studies in which fCPT levels were found to correlate with the presence of C. difficile (4–6). In one study, fCPT concentrations were found to be higher in CDI cases diagnosed by a toxin assay than those detected by PCR for the toxin gene (4), supporting the hypothesis that some proportion of CD-PCR+ samples represent colonization only (7, 8).
We did not find that sPCT correlated with CD-PCR positivity. In a previous report (9), sPCT correlated with CDI severity, but the cases were not stratified according to the diagnostic methods used (i.e., toxin enzyme immunoassay [EIA] versus PCR) (7, 8, 10).
The principal limitations of our study are the small number of subjects enrolled, the inability to categorically discriminate between CDI and colonization, and the very small amount of CDI-related complications preventing a correlation of fCPT and sPCT with CDI severity. Ideally, a cell cytotoxicity assay and toxigenic culture of the stool samples might have assisted in determining the presence of CDI versus colonization.
Our data suggest that HR-fCPT levels are elevated in a large proportion of CD-PCR+ individuals with nosocomial diarrhea, with lower levels in CD-PCR− patients, suggesting a possible adjuvant role for these levels in diagnosing CDI. Further studies in a larger number of patients are required to determine the performance and reliability of using HR-fCPT levels as an adjunct for diagnosis of CDI.
ACKNOWLEDGMENTS
We thank Yves Longtin for support with the statistical analysis.
K.Y.P. reports no conflicts of interest relevant to this article; M.A.M. has been an employee of bioMérieux since October 2012.
REFERENCES
- 1.Dhaliwal A, Zeino Z, Tomkins C, Cheung M, Nwokolo C, Smith S, Harmston C, Arasaradnam RP. 2015. Utility of faecal calprotectin in inflammatory bowel disease (IBD): what cut-offs should we apply? Frontline Gastroenterol 6:14–19. doi: 10.1136/flgastro-2013-100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sridharan P, Chamberlain RS. 2013. The efficacy of procalcitonin as a biomarker in the management of sepsis: slaying dragons or tilting at windmills? Surg Infect (Larchmt) 14:489–511. doi: 10.1089/sur.2012.028. [DOI] [PubMed] [Google Scholar]
- 3.Miller MA, Louie T, Mullane K, Weiss K, Lentnek A, Golan Y, Kean Y, Sears P. 2013. Derivation and validation of a simple clinical bedside score (ATLAS) for Clostridium difficile infection which predicts response to therapy. BMC Infect Dis 13:148. doi: 10.1186/1471-2334-13-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitehead SJ, Shipman KE, Cooper M, Ford C, Gama R. 2014. Is there any value in measuring faecal calprotectin in Clostridium difficile positive faecal samples? J Med Microbiol 63:590–593. doi: 10.1099/jmm.0.067389-0. [DOI] [PubMed] [Google Scholar]
- 5.Shastri YM, Bergis D, Povse N, Schafer V, Shastri S, Weindel M, Ackermann H, Stein J. 2008. Prospective multicenter study evaluating fecal calprotectin in adult acute bacterial diarrhea. Am J Med 121:1099–1106. doi: 10.1016/j.amjmed.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 6.Swale A, Miyajima F, Roberts P, Hall A, Little M, Beadsworth MB, Beeching NJ, Kolamunnage-Dona R, Parry CM, Pirmohamed M. 2014. Calprotectin and lactoferrin faecal levels in patients with Clostridium difficile infection (CDI): a prospective cohort study. PLoS One 9:e106118. doi: 10.1371/journal.pone.0106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longtin Y, Trottier S, Brochu G, Paquet-Bolduc B, Garenc C, Loungnarath V, Beaulieu C, Goulet D, Longtin J. 2013. Impact of the type of diagnostic assay on Clostridium difficile infection and complication rates in a mandatory reporting program. Clin Infect Dis 56:67–73. doi: 10.1093/cid/cis840. [DOI] [PubMed] [Google Scholar]
- 8.Beaulieu C, Dionne LL, Julien AS, Longtin Y. 2014. Clinical characteristics and outcome of patients with Clostridium difficile infection diagnosed by PCR versus a three-step algorithm. Clin Microbiol Infect 20:1067–1073. doi: 10.1111/1469-0691.12676. [DOI] [PubMed] [Google Scholar]
- 9.Rao K, Walk ST, Micic D, Chenoweth E, Deng L, Galecki AT, Jain R, Trivedi I, Yu M, Santhosh K, Ring C, Young VB, Huffnagle GB, Aronoff DM. 2013. Procalcitonin levels associate with severity of Clostridium difficile infection. PLoS One 8:e58265. doi: 10.1371/journal.pone.0058265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Planche TD, Davies KA, Coen PG, Finney JM, Monahan IM, Morris KA, O'Connor L, Oakley SJ, Pope CF, Wren MW, Shetty NP, Crook DW, Wilcox MH. 2013. Differences in outcome according to Clostridium difficile testing method: a prospective multicentre diagnostic validation study of C. difficile infection. Lancet Infect Dis 13:936–945. doi: 10.1016/S1473-3099(13)70200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]