Abstract
Blastomyces spp. antigen testing was evaluated over a 10-year period in an area where blastomycosis is endemic. Antigen testing was less sensitive than previously reported, but serial urine testing was useful in monitoring disease resolution or progression. Culture and cytopathology remain the gold standard for diagnosis and exclusion of this infection.
TEXT
Blastomycosis is a serious and potentially fatal fungal infection. Blastomycosis may be asymptomatic or it can present as isolated pulmonary disease, disseminated disease (pulmonary and extrapulmonary infection), or extrapulmonary disease with manifestations in the central nervous system, bone, skin, or other locations (1–11).
Culture and cytopathology are the gold standard for the diagnosis of blastomycosis. However, a variety of other diagnostic tests, including antigen testing, antibody testing, and PCR, are commercially available (8, 12–22). Given that identification from culture may not be evident for 2 to 4 weeks and that it often requires invasive procedures to obtain specimens, there is great interest in using antigen enzyme immunoassays (EIA) for rapid, noninvasive diagnosis and monitoring of disease progression or resolution (2, 4, 8, 19).
We evaluated the use of Blastomyces urine, serum, and bronchoalveolar lavage (BAL) fluid antigen assays for the diagnosis of blastomycosis and the effects of treatment on the clearance of antigenuria at the Marshfield Clinic from 1995 to 2015. Marshfield Clinic is located in Wisconsin where blastomycosis is endemic (1, 3, 6, 9, 23–28). Research protocols were approved by the Marshfield Clinic Institutional Review Board. Waiver of informed consent was obtained.
Patients were included if blastomycosis was confirmed by culture or cytopathology and if urine, serum, or BAL fluid antigen EIA was completed at the time of diagnosis or within 30 days of starting antifungal medication. Blastomycosis was confirmed by culture or cytopathology at Marshfield Labs using conventional techniques. Commercially available blastomycosis antigen EIA was performed at the MiraVista Diagnostics reference laboratory at the time of specimen collection using qualitative EIA prior to March 2011 or quantitative EIA thereafter. EIA results are available within 24 h of sample submission (13, 14, 16). All specimens were obtained as part of routine clinical evaluations. Retrospective chart review was completed for all patients.
Data were abstracted into Excel 2010, and statistical analysis was completed using SAS 9.3. Categorical data were compared using the χ2 test or the Fisher exact test. Continuous variables were compared using Wilcoxon rank sum, Kruskal-Wallis, or analysis of variance (ANOVA). Correlation for serial urine antigen testing was measured using Spearman's coefficient. Significance was defined as a P of <0.05.
Patients with quantitative antigen EIA results were reported as negative, positive below the limit of quantification (<0.2 ng/ml), positive and quantifiable (0.2 to 14.7 ng/ml), or positive above the limit of quantification (>14.8 ng/ml). For the graphing of serial antigen studies, negative values were defined as 0 ng/ml, values below the limit of quantification were defined as 0.01 ng/ml, and values above the limit of quantification were defined as 14.8 ng/ml. Spearman correlations were calculated using quantified values only.
Sixty-seven patients met inclusion criteria. Serial urine antigen testing was completed in 19 patients. Urine antigen testing was completed in 59 patients, serum antigen testing was completed in 18 patients, and BAL fluid antigen testing was completed in 8 patients. A combination of urine, serum, and/or BAL fluid testing was completed in 14 patients. The mean age of patients tested was 37 ± 21 years. There was one death from blastomycosis in the cohort.
Blastomycosis antigen EIA had lower sensitivity than that which has been previously reported. Sensitivity data are shown in Table 1. Though urine sensitivity has been reported to be as high as 93% (12–14), and BAL fluid and serum sensitivity have been reported to be as high as 82% (12–14, 16, 17, 29), only 76.3% (45/59) of our patients had antigenuria, 55.6% (10/18) had antigenemia, and 62.5% (5/8) had positive BAL fluid results. Only 8/10 (80%) patients who had urine and serum antigen testing completed had at least one positive result; thus, the combination of urine and serum testing did not improve sensitivity significantly. This emphasizes the need to confirm negative antigen findings with culture and cytology. There were no significant differences between the sensitivities of urine, serum, or BAL fluid antigen testing (P = 0.203).
TABLE 1.
Differences in sensitivity by sample type and location of infection
| Sample source by location and type of infectiona | No. positive/no. tested (% sensitivity) for: |
||
|---|---|---|---|
| Urineb | Serumc | BAL fluid | |
| Isolated pulmonary | 43/52 (82.7) | 9/14 (64.3) | 5/8 (62.5) |
| Disseminatedd | 2/3 (66.7) | 1/3 (33.4) | 0/0 (NAe) |
| Extrapulmonary | 0/4 (0) | 0/1 (0) | 0/0 (NA) |
| Total | 45/59 (76.3) | 10/18 (55.6) | 5/8 (62.5) |
| Urine and serum (any positive) | 8/10 (80.0) | ||
No significant difference between sensitivity of urine, serum, or BAL fluid testing using Fischer's exact test (P = 0.203).
Significant difference in the sensitivity of urine antigen testing between patients with isolated pulmonary, disseminated, and extrapulmonary infection (P = 0.001).
No significant difference in serum sensitivity between isolated pulmonary, disseminated, or extrapulmonary infection (P = 0.08).
Disseminated includes patients with pulmonary and extrapulmonary infection.
NA, not applicable.
As has been previously described, antigen testing was most sensitive in patients with isolated pulmonary disease (12–14). Urine antigen testing was positive in 45/59 (76.3%) patients with isolated pulmonary infection, 2/3 (66.7%) patients with disseminated infection, and 0/4 (0%) patients with extrapulmonary infection (P < 0.01). Serum antigen testing was positive in 9/14 (64.3%) patients with isolated pulmonary disease, 1/3 (33.4%) patients with disseminated infection, and 0/1 (0%) patients with extrapulmonary infection (P = 0.08). Though prior studies have suggested that antigen testing can be useful in the diagnosis of extrapulmonary infection, in this study such testing was negative in all cases of extrapulmonary infections (12–14).
Qualitative testing was positive in 21/26 (80.1%) patients, and quantitative testing was positive in 25/33 (75.8%) patients (P = 0.383). For patients with quantitative testing performed, initial antigen concentration varied significantly between patients with BAL fluid, serum, or urine antigen testing completed. The mean BAL fluid concentration was 1.28 ± 1.54 ng/ml, it was 6.56 ± 5.31 ng/ml for serum, and it was 3.21 ± 1.68 ng/ml for urine (P = 0.03).
There were 14 patients with negative cytology at the time of diagnosis. Antigen testing was positive in 7 of those patients, which expedited the diagnosis.
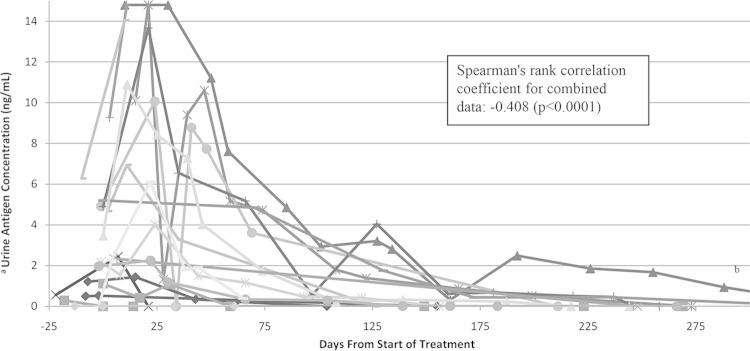
Serial urine antigen concentrations were measured in 19 patients (Fig. 1). Eighteen had isolated pulmonary disease, and one had disseminated infection. Of those with serial measurements, 8 were treated with oral itraconazole, 5 were treated with amphotericin B followed by itraconazole, and 6 were treated with amphotericin B and fluconazole, fluconazole alone, or voriconazole. Treatment variations were secondary to severity of illness, intolerance of medication, or insurance requirements. Itraconazole dosing was titrated to ensure therapeutic levels. Treatment method did not change the rate of clearance, though there were numerous confounders, including severity of illness that could not be addressed given the retrospective nature of the study. Thirteen patients had a rise in antigen concentration after antifungal treatment was started (median, 11 days; range, 0 to 70 days). Peak concentrations varied widely (0.01 ng/ml to above the limit of quantification). This may reflect the increased excretion of antigen after the death of fungal cells, the transient increase in fungal load from macrophage uptake after initiation of antifungal medication, or differences in location or severity of infection (30). Two patients converted to positive within 1 month of starting treatment, and 4 patients reverted to negative within 30 days of starting antifungal medications. The median number of days to the first negative result was 200 days (range, 0 to 672 days). One patient had detectable antigenuria 493 days after the start of treatment.
FIG 1.
Quantitative urine antigen concentration by days from the start of treatment for 19 patients with serial urine antigen testing. Each line represents a different patient. (a) Values were reported as negative (0 ng/ml), positive below the limit of quantification (assigned as 0.01 ng/ml), quantified (0.2 to 14.7 ng/dl), or positive above the limit of quantification (assigned as 14.8 ng/ml). (b) Patient had 5 additional tests completed, all at <1.0 ng/ml. The last positive test was 493 days after the start of treatment. The first negative test was 672 days after the start of treatment.
There is a moderate but significant negative correlation between length of treatment and clearance of antigenuria that is useful for monitoring blastomycosis patients in a noninvasive way during treatment. Spearman's correlation was −0.408 (P < 0.0001). There were three cases of treatment failure. In the one mortality, antigenuria increased from 9.27 ng/ml to above the limit of quantification prior to death. In one patient, antigenuria decreased from above the limit of quantification at initial discharge on itraconazole to 11.22 ng/ml at second admission. The patient had a prolonged 22-month symptomatic course with increases in urine antigen concentration correlating and improving with clinical findings. In the third case, antigenuria decreased from above the limit of quantification to 1.32 ng/ml after starting fluconazole but increased to 9.41 ng/ml during worsening clinical symptoms. It steadily declined to nondetectable after changing to amphotericin B. This validates previous case reports that have described the use of urine antigen testing in this way (18, 21).
Given the retrospective nature of this study, we are unable to determine the specificity of blastomycosis antigen EIA though previous studies have shown a high specificity when eliminating patients with histoplasmosis (12–14). The cross-reactivity of histoplasmosis and blastomycosis antigens can present a diagnostic dilemma for clinicians in areas where the two infections are endemic, but EIA is useful in making a preliminary diagnosis for initiation of treatment, particularly in regions where blastomycosis is most likely (12–14, 31).
In summary, the sensitivity of the blastomycosis antigen EIA was lower than what has been previously reported. In patients where there is a high degree of suspicion for blastomycosis, antigen testing alone should not be used to exclude the diagnosis. While antigen testing can be used to expedite the diagnosis of blastomycosis in some patients, culture and cytopathology remain the gold standard. Serial urine antigen concentrations are a useful means to determine resolution or relapse from infection.
ACKNOWLEDGMENTS
We have no conflicts of interest to disclose.
We have no funding source to disclose.
We thank the Marshfield Clinic Bioinfomatics Research Center for their help in identification of cases and Thomas Fritsche for his clinical expertise and review of the manuscript.
REFERENCES
- 1.Baumgardner DJ, Halsmer SE, Egan G. 2004. Symptoms of pulmonary blastomycosis: northern Wisconsin, United States. Wilderness Environ Med 15:250–256. [DOI] [PubMed] [Google Scholar]
- 2.Bush JW, Wuerz T, Embil JM, Del Bigio MR, McDonald PJ, Krawitz S. 2013. Outcomes of persons with blastomycosis involving the central nervous system. Diagn Microbiol Infect Dis 76:175–181. doi: 10.1016/j.diagmicrobio.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Crampton TL, Light RB, Berg GM, Meyers MP, Schroeder GC, Hershfield ES, Embil JM. 2002. Epidemiology and clinical spectrum of blastomycosis diagnosed at Manitoba hospitals. Clin Infect Dis 34:1310–1316. doi: 10.1086/340049. [DOI] [PubMed] [Google Scholar]
- 4.Fanella S, Skinner S, Trepman E, Embil JM. 2011. Blastomycosis in children and adolescents: a 30-year experience from Manitoba. Med Mycol 49:627–632. [DOI] [PubMed] [Google Scholar]
- 5.Laskey WK, Sarosi GA. 1980. Blastomycosis in children. Pediatrics 65:111–114. [PubMed] [Google Scholar]
- 6.Morris SK, Brophy J, Richardson SE, Summerbell R, Parkin PC, Jamieson F, Limerick B, Wiebe L, Ford-Jones EL. 2006. Blastomycosis in Ontario, 1994-2003. Emerg Infect Dis 12:274–279. doi: 10.3201/eid1202.050849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao GR, Narayan BL, Durga Prasad BK, Amareswar A, Sridevi M, Raju B. 2013. Disseminated blastomycosis in a child with a brief review of the Indian literature. Indian J Dermatol Venereol Leprol 79:92–96. doi: 10.4103/0378-6323.104676. [DOI] [PubMed] [Google Scholar]
- 8.Saccente M, Woods GL. 2010. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev 23:367–381. doi: 10.1128/CMR.00056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumgardner DJ, Buggy BP, Mattson BJ, Burdick JS, Ludwig D. 1992. Epidemiology of blastomycosis in a region of high endemicity in north central Wisconsin. Clin Infect Dis 15:629–635. doi: 10.1093/clind/15.4.629. [DOI] [PubMed] [Google Scholar]
- 10.Baumgardner DJ, Brockman K. 1998. Epidemiology of human blastomycosis in Vilas County, Wisconsin. II: 1991-1996. WMJ 97:44–47. [PubMed] [Google Scholar]
- 11.Meece JK, Anderson JL, Gruszka S, Sloss BL, Sullivan B, Reed KD. 2013. Variation in clinical phenotype of human infection among genetic groups of Blastomyces dermatitidis. J Infect Dis 207:814–822. doi: 10.1093/infdis/jis756. [DOI] [PubMed] [Google Scholar]
- 12.Bariola JR, Hage CA, Durkin M, Bensadoun E, Gubbins PO, Wheat LJ, Bradsher RW Jr. 2011. Detection of Blastomyces dermatitidis antigen in patients with newly diagnosed blastomycosis. Diagn Microbiol Infect Dis 69:187–191. doi: 10.1016/j.diagmicrobio.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Connolly P, Hage CA, Bariola JR, Bensadoun E, Rodgers M, Bradsher RW Jr, Wheat LJ. 2012. Blastomyces dermatitidis antigen detection by quantitative enzyme immunoassay. Clin Vaccine Immunol 19:53–56. doi: 10.1128/CVI.05248-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durkin M, Witt J, Lemonte A, Wheat B, Connolly P. 2004. Antigen assay with the potential to aid in diagnosis of blastomycosis. J Clin Microbiol 42:4873–4875. doi: 10.1128/JCM.42.10.4873-4875.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fanella S, Walkty A, Bridger N, Crockett M, Consunji-Araneta R, Embree J, Karlowsky J. 2010. Gastric lavage for the diagnosis of pulmonary blastomycosis in pediatric patients. Pediatr Infect Dis J 29:1146–1148. doi: 10.1097/INF.0b013e3181ecc94b. [DOI] [PubMed] [Google Scholar]
- 16.Hage CA, Davis TE, Egan L, Parker M, Fuller D, Lemonte AM, Durkin M, Connelly P, Joseph Wheat L, Blue-Hnidy D, Knox KS. 2007. Diagnosis of pulmonary histoplasmosis and blastomycosis by detection of antigen in bronchoalveolar lavage fluid using an improved second-generation enzyme-linked immunoassay. Respir Med 101:43–47. doi: 10.1016/j.rmed.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Hage CA, Knox KS, Davis TE, Wheat LJ. 2011. Antigen detection in bronchoalveolar lavage fluid for diagnosis of fungal pneumonia. Curr Opin Pulm Med 17:167–171. doi: 10.1097/MCP.0b013e3283447b60. [DOI] [PubMed] [Google Scholar]
- 18.Mongkolrattanothai K, Peev M, Wheat LJ, Marcinak J. 2006. Urine antigen detection of blastomycosis in pediatric patients. Pediatr Infect Dis J 25:1076–1078. doi: 10.1097/01.inf.0000241144.89426.2a. [DOI] [PubMed] [Google Scholar]
- 19.Patel AJ, Gattuso P, Reddy VB. 2010. Diagnosis of blastomycosis in surgical pathology and cytopathology: correlation with microbiologic culture. Am J Surg Pathol 34:256–261. doi: 10.1097/PAS.0b013e3181ca48a5. [DOI] [PubMed] [Google Scholar]
- 20.Sidamonidze K, Peck MK, Perez M, Baumgardner D, Smith G, Chaturvedi V, Chaturvedi S. 2012. Real-time PCR assay for identification of Blastomyces dermatitidis in culture and in tissue. J Clin Microbiol 50:1783–1786. doi: 10.1128/JCM.00310-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarr M, Marcinak J, Mongkolrattanothai K, Burns JL, Wheat LJ, Durkin M, Ismail M. 2007. Blastomyces antigen detection for monitoring progression of blastomycosis in a pregnant adolescent. Infect Dis Obstet Gynecol 2007:89059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meece JK, Anderson JL, Klein BS, Sullivan TD, Foley SL, Baumgardner DJ, Brummitt CF, Reed KD. 2010. Genetic diversity in Blastomyces dermatitidis: implications for PCR detection in clinical and environmental samples. Med Mycol 48:285–290. doi: 10.3109/13693780903103952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu JH, Feudtner C, Heydon K, Walsh TJ, Zaoutis TE. 2006. Hospitalizations for endemic mycoses: a population-based national study. Clin Infect Dis 42:822–825. doi: 10.1086/500405. [DOI] [PubMed] [Google Scholar]
- 24.Litvinov IV, St-Germain G, Pelletier R, Paradis M, Sheppard DC. 2013. Endemic human blastomycosis in Quebec, Canada, 1988-2011. Epidemiol Infect 141:1143–1147. doi: 10.1017/S0950268812001860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonough ES, Kuzma JF. 1980. Epidemiological studies on blastomycosis in the state of Wisconsin. Sabouraudia 18:173–183. doi: 10.1080/00362178085380321. [DOI] [PubMed] [Google Scholar]
- 26.Khuu D, Shafir S, Bristow B, Sorvillo F. 2014. Blastomycosis mortality rates, United States, 1990-2010. Emerg Infect Dis 20:1789–1794. doi: 10.3201/eid2011.131175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seitz AE, Adjemian J, Steiner CA, Prevots DR. 2015. Spatial epidemiology of blastomycosis hospitalizations: detecting clusters and identifying environmental risk factors. Med Mycol 53:447–454. doi: 10.1093/mmy/myv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seitz AE, Younes N, Steiner CA, Prevots DR. 2014. Incidence and trends of blastomycosis-associated hospitalizations in the United States. PLoS One 9:e105466. doi: 10.1371/journal.pone.0105466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martynowicz MA, Prakash UB. 2002. Pulmonary blastomycosis: an appraisal of diagnostic techniques. Chest 121:768–773. doi: 10.1378/chest.121.3.768. [DOI] [PubMed] [Google Scholar]
- 30.Sterkel AK, Mettelman R, Wuthrich M, Klein BS. 2015. The unappreciated intracellular lifestyle of Blastomyces dermatitidis. J Immunol 194:1796–1805. doi: 10.4049/jimmunol.1303089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kauffman CA. 2006. Endemic mycoses: blastomycosis, histoplasmosis, and sporotrichosis. Infect Dis Clin North Am 20:645–662. doi: 10.1016/j.idc.2006.07.002. [DOI] [PubMed] [Google Scholar]