Abstract
A 43-year-old woman of Mayan origin from Quintana Roo, Mexico, was diagnosed with diffuse lepromatous leprosy. The etiologic bacillus was determined to be Mycobacterium lepromatosis instead of Mycobacterium leprae. This case likely represents the first report of this leprosy form and its agent in the southeastern tip of Mexico.
CASE REPORT
A 43-year-old woman of Mayan ancestry sought dermatologic care in the town of Chetumal, Quintana Roo, Mexico. The patient complained of a 2-year history of burning skin sensation, loss of eyelashes and eyebrows, lower leg edema, and generalized weakness. She was a widowed housewife and had been previously healthy.
Physical examination revealed generalized skin infiltration, including areas of prominent “peau d′orange” (erythematous and turgid appearance) and shining skin on the central face, cheeks, and chin. There was extensive loss of hair on arms, legs, and the pubic area. There were no eye lashes or eyebrows. The cornea reflex was diminished. The earlobes were enlarged and indurated. The body skin was dry and did not sweat. There was a generalized loss of sensation to needle pricks over the body. Laboratory examinations showed a normal hemoglobin level (140g/liter), normal counts of blood leukocytes (6.0 × 109/liter) and platelets, a negative VDRL test, a negative human immunodeficiency virus test, and a normal urine test. No thickened nerves were noted. Smears of the nasal mucosa and an earlobe showed acid-fast bacilli with intensities of + and ++, respectively. These findings led to a working diagnosis of diffuse lepromatous leprosy (DLL) in view of the absence of skin nodules.
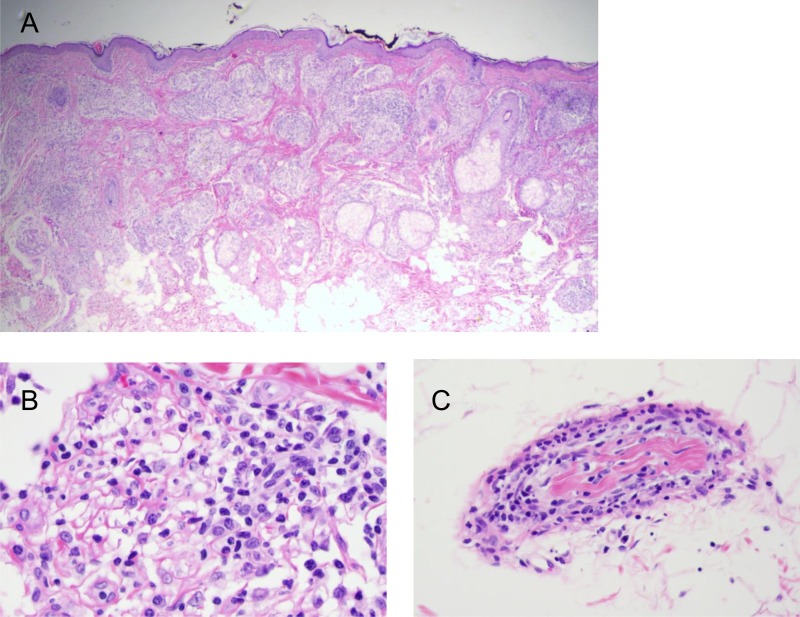
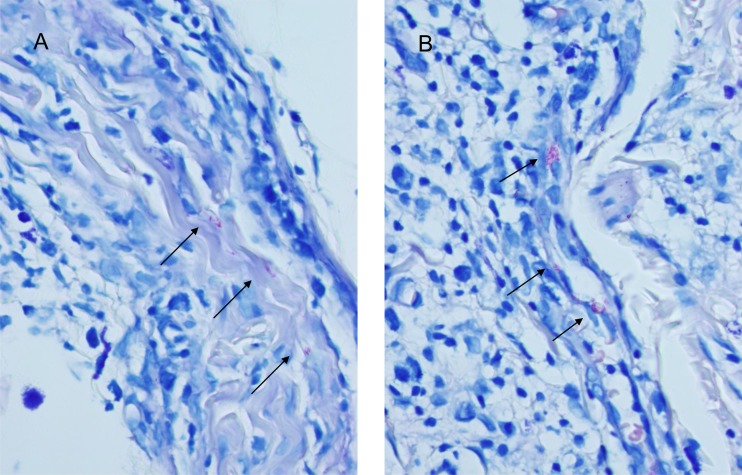
A skin biopsy of the chin was performed. Histopathology showed extensive infiltration of histiocytes that involved skin appendages and a small nerve (Fig. 1). A Fite stain revealed moderate number of acid-fast bacilli in the skin tissue, with additional locations in the endothelia and a nerve (Fig. 2). These results rendered the diagnosis of DLL.
FIG 1.
Histopathology of diffuse lepromatous leprosy in a 43-year-old woman. Shown are dense histiocytic infiltration in the dermis at low and high magnifications (A, 20×; B, 400×) and neuritis (C, 400×). All panels, hematoxylin and eosin.
FIG 2.
Acid-fast bacilli in the nerve (A) and endothelia (B). Fite stain, 1,000×.
The patient was treated with a multidrug regimen consisting of dapsone, rifampin, and clofazimine. In the third month of treatment, she developed weakness and red nodules on the face and arms, which was consistent with erythema nodosum leprosum. This mild reaction was controlled with 30 mg prednisone daily for 1 week with taper. Upon treatment for 12 months, nasal and earlobe smears remained positive for intact acid-fast bacilli, which led to continued treatment. The patient completed 18 months of treatment, resulting in negative smears and resolution of the skin infiltration and partial recovery of pain sensation, sweating, and hair (in the legs especially). At a 3-year follow-up, she showed nearly complete recovery of sensation and regrowth of hair on her eyelids and eyebrows. Thus, she was considered cured.
The diagnosis of DLL prompted a quest to determine the identity of the acid-fast bacillus. Thus, differential PCRs were used to test the DNA extracted from the formalin-fixed paraffin-embedded biopsy tissue. The PCRs targeted the 16S rRNA genes of the leprosy agents Mycobacterium leprae and Mycobacterium lepromatosis, as previously designed and used (1–4). In brief, heminested PCRs were used to maximize detection sensitivity: the first-round PCR used primers AFBFO (5′-GCGTGCTTAACACATGCAAGTC, common to all mycobacterial species) and MLER4 (5′-CCACAAGACATGCGCCTTGAAG, specific for the leprosy bacilli). The resulting amplicons, 171 bp in size and usually faint or undetectable, were diluted (100-fold) and further amplified by three separate second-round PCRs using MLER4 as the anchoring primer to pair with primer MLEFO (5′-GCAAGTCGAACGGAAAGGTCT, specific for the leprosy bacilli) for a common amplicon (159 bp), with primer LPMF2 (5′-GTCTCTTAATACTTAAACCTATTAA) for M. lepromatosis (142 bp), and with primer LERF2 (5′-CTAAAAAATCTTTTTTAGAGATAC) for M. leprae (135 bp). The thermocycles were performed as follows: activation of polymerase at 95°C for 2 min, 35 cycles of denaturation (95°C for 20 s), and primer annealing (58°C for 20 s for the first-round PCR or 48°C for 20 s for the second-round PCRs) and extension (72°C 40 s), and final extension for 5 min. A regular Taq polymerase was used. The target amplicons from the second-round PCRs were examined by agarose gel electrophoresis for the intended sizes.
As shown in Fig. 3, the common and M. lepromatosis-specific amplicons were detected, but not the M. leprae-specific amplicon. The common amplicon, visibly larger on proper electrophoretic separation, was also sequenced to resolve all 159 bp, which matched completely with M. lepromatosis. Therefore, this species was the etiologic agent of this infection.
FIG 3.
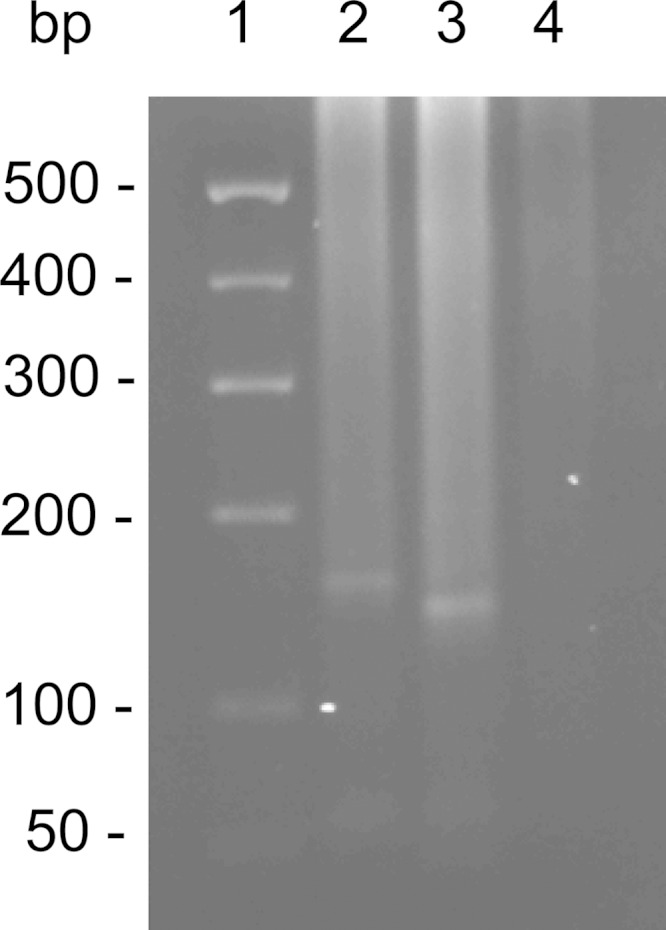
Detection of the 16S rRNA gene of Mycobacterium lepromatosis by heminested differential PCRs. Lane 1, 100-bp DNA size marker; lane 2, 159-bp amplicon from the common leprosy primers; lane 3, 142-bp specific amplicon for M. lepromatosis; lane 4, lack of the 135-bp amplicon for M. leprae.
How the patient contracted the infection was uncertain. She had no known contacts with other leprosy patients. She had lived all her life, with no travel more than 50 km away from home, in Quintana Roo, one of the three states in the Yucatan Peninsula, the southeastern tip of Mexico, where DLL had been unknown previously. Although she related that her villagers had armadillo in their diet, she had never eaten or cooked this animal. It is also noteworthy that there are no published or known studies of armadillo in the Yucatan Peninsula to show that this animal carries a leprosy agent. One study noted leprosy-like infection in an armadillo caught near Mexico City (5).
Leprosy (Hansen's disease) is likely the oldest human infection that can be traced to its African origin with humans (6–8) and possibly much earlier to the hominid era millions of years ago (9). M. leprae has been known to be the sole leprosy agent since its initial discovery in 1873 (10). In 2008, a novel Mycobacterium species named M. lepromatosis was recognized as the cause of death of two Mexican patients with DLL (11). Further phylogenetic studies of 20 genes and pseudogenes revealed a 9.1% genetic difference between the two leprosy bacilli (11). This large difference contrasts with the clonal nature of worldwide M. leprae strains (6, 8, 12). It also hints at the ancient divergence of the two bacilli, ∼10 million years ago, from their last common ancestor (9, 13). Most recently, genomes of two M. lepromatosis strains were sequenced, revealing an ∼13% genome-wide difference from M. leprae but with similar genome sizes and organizations between the species (14, 15). Analysis of the one of the draft genomes also refined the divergence time to 13.9 million years (14).
Independent studies have corroborated this new cause of leprosy. Vera-Cabrera et al. (16, 17) reported several cases of infection from Nuevo Leon, Mexico. Jessamine and colleagues (18) reported infection of a native Canadian man who manifested polyneuropathy and skin rashes but had no significant history of contact or travel to areas of endemicity.
DLL is a unique, severe form of leprosy initially recognized by Lucio and Alvarado in 1852 (19) and further described by Latapi and Chevez-Zamora in 1948 (20), both in Mexico. It is thus also called Lucio's leprosy. This form shows a diffuse cutaneous infiltration, with no nodule or plaque formation, and frequent skin ulceration in the late stage, known as Lucio's phenomenon (20–22). On histopathology, DLL shows the usual acid-fast bacillary infiltration of the skin and nerves, panniculitis, vasculitis, and in the late stage, unique endothelial proliferation and vascular occlusion (20, 21, 23). DLL has been endemic in western and central Mexico (20, 22) and Costa Rica (24) but rarely reported elsewhere.
Realization of two leprosy bacilli led us to conduct an etiologic analysis of 120 Mexican leprosy cases using archived biopsy tissue (1, 2). The study confirmed and differentiated the mycobacteria in 87 cases. Of these, M. lepromatosis alone caused 55 cases, M. leprae alone caused 18 cases, and both species together caused 14 cases. M. lepromatosis caused not only all 13 DLL cases specifically but also 42 cases of other clinical forms of leprosy. In the study, the M. lepromatosis cases came from nine western and central Mexico states, which matched the historical areas where areas of DLL is endemic (20, 25). Among other states in Mexico, studies (16, 17, 26) have noted M. lepromatosis infection in Tamaulipas, Nuevo Leon, and Coahuila in the northeast, which border Texas in the United States. The present case from a native of Quintana Roo further adds the far southeastern tip of Mexico to the list. Thus far, 13 of the 30 Mexico States were known to have M. lepromatosis leprosy. Recently, a likely family transmission of this agent was noted that involved a pair of Mexican siblings (A. F. Marsch and C. Hill, 2015 unpublished data).
In addition to Mexico and Canada, M. lepromatosis has been identified in Brazil (4), Singapore (3), and Myanmar (4). In these studies, the organism caused fatal DLL and other forms of leprosy, and dual infections with M. leprae were also seen. Thus, M. lepromatosis is a long-elusive second cause of leprosy with a wide trans-Pacific distribution. The long record of DLL and the likely dominance of M. lepromatosis in Mexico have led us to propose that the disease came with the first American settlers from Asia over 13,000 years ago (1). Finding M. lepromatosis in Myanmar (4) and in Singapore (3) supports this Asian origin. Finding it in Brazil (4) accords with further American spread from the North America to Central America, such as Costa Rica, where DLL has been endemic (24), and to South America, such as the Amazon region of Brazil. In the Brazilian Amazon, leprosy has been known for at least a century (27) and is still hyperendemic (28).
The present case also adds to our growing experience on the presentation, diagnosis, treatment, and follow-up of M. lepromatosis infection. The normal laboratory findings and the lack of skin ulcers—a usual feature of late-stage DLL—suggested mild infection. During treatment, the patient experienced mild erythema nodosum leprosum, a common reaction of leprosy that usually occurs during the early course of multidrug treatment. In two other featured cases of M. lepromatosis infection (26), erythema nodosum leprosum was a presenting sign along with high fever, lymphadenopathy, and skin rashes. The treatment success of the present case also echoes a similar recent report (26), earlier clinical experience of Rea and Jerskey with DLL (21), and the steady decline in leprosy incidence in Mexico in recent decades (25). Thus, the standard multidrug regimen for multibacillary leprosy likely works for M. lepromatosis infection.
Recently, leprosy-like dermatitides of animals have been described in cats in Australia (29), red squirrels in Scotland (30), and cows in France (31). Thus far, the etiologic acid-fast bacilli have not been cultivated, similar to the difficulty in cultivation of M. leprae and M. lepromatosis. Limited genetic studies of the organisms in cats and squirrels have indicated similarities to the leprosy bacilli (29, 30). The study of the cow agent analyzed portions of 6 genes totaling 3,231 nucleotides (31). Judged from the GenBank deposits (KJ095004 to KJ095009), the five protein-coding genes matched 88% to 93% those of M. leprae and/or M. lepromatosis, and the 16S rRNA gene—the most conserved bacterial gene—matched best with M. lepromatosis (98.4% [361 of 367 bp]). These results thus raise the likelihood of a new Mycobacterium species. Whether the cow agent contains pseudogenes—the hallmark of the leprosy bacilli—is yet to be seen.
(This work was presented at the 115th General Meeting of the American Society for Microbiology, New Orleans, LA, 2 June 2015.)
ACKNOWLEDGMENTS
The authors declare they have no conflicts of interest. There was no funding support for this work.
REFERENCES
- 1.Han XY, Sizer KC, Velarde-Felix JS, Frias-Castro LO, Vargas-Ocampo F. 2012. The leprosy agents Mycobacterium lepromatosis and Mycobacterium leprae in Mexico. Int J Dermatol 51:952–959. doi: 10.1111/j.1365-4632.2011.05414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han XY, Zhang JQ, Li L. 2015. Leprosy agents Mycobacterium lepromatosis and Mycobacterium leprae in Mexico: a clarification. J Clin Microbiol 53:3387–3388. doi: 10.1128/JCM.01588-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han XY, Sizer KC, Tan HH. 2012. Identification of the leprosy agent Mycobacterium lepromatosis in Singapore. J Drugs Dermatol 11:168–172. [PubMed] [Google Scholar]
- 4.Han XY, Aung FM, Choon S-E, Werner B. 2014. Analysis of the leprosy agents M. leprae and M. lepromatosis in four countries. Am J Clin Pathol 142:524–532. doi: 10.1309/AJCP1GLCBE5CDZRM. [DOI] [PubMed] [Google Scholar]
- 5.Amezcua ME, Escobar-Gutierrez A, Storrs EE, Dhople AM, Burchfiled HP. 1984. Wild Mexican armadillo with leprosy-like infection. Int J Leprosy 52:254–255. [PubMed] [Google Scholar]
- 6.Cole ST, Eiglmeier K, Parkhill J, James KD, Thomson NR, Wheeler PR, Honoré N, Garnier T, Churcher C, Harris D, Mungall K, Basham D, Brown D, Chillingworth T, Connor R, Davies RM, Devlin K, Duthoy S, Feltwell T, Fraser A, Hamlin N, Holroyd S, Hornsby T, Jagels K, Lacroix C, Maclean J, Moule S, Murphy L, Oliver K, Quail MA, Rajandream MA, Rutherford KM, Rutter S, Seeger K, Simon S, Simmonds M, Skelton J, Squares R, Squares S, Stevens K, Taylor K, Whitehead S, Woodward JR, Barrell BG. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007–1011. doi: 10.1038/35059006. [DOI] [PubMed] [Google Scholar]
- 7.Monot M, Honoré N, Garnier T, Araoz R, Coppée JY, Lacroix C, Sow S, Spencer JS, Truman RW, Williams DL, Gelber R, Virmond M, Flageul B, Cho SN, Ji B, Paniz-Mondolfi A, Convit J, Young S, Fine PE, Rasolofo V, Brennan PJ, Cole ST. 2005. On the origin of leprosy. Science 308:1040–1042. doi: 10.1126/science/1109759. [DOI] [PubMed] [Google Scholar]
- 8.Monot M, Honoré N, Garnier T, Zidane N, Sherafi D, Paniz-Mondolfi A, Matsuoka M, Taylor GM, Donoghue HD, Bouwman A, Mays S, Watson C, Lockwood D, Khamesipour A, Dowlati Y, Jianping S, Rea TH, Vera-Cabrera L, Stefani MM, Banu S, Macdonald M, Sapkota BR, Spencer JS, Thomas J, Harshman K, Singh P, Busso P, Gattiker A, Rougemont J, Brennan PJ, Cole ST. 2009. Comparative genomic and phylogeographic analysis of Mycobacterium leprae. Nat Genet 41:1282–1289. doi: 10.1038/ng.477. [DOI] [PubMed] [Google Scholar]
- 9.Han XY, Silva FJ. 2014. On the age of leprosy. PLoS Negl Trop Dis 8:e2544. doi: 10.1371/journal.pntd.0002544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen GHA. 1874. Undersøgelser Angående Spedalskhedens Årsager (on the etiology of leprosy). Norsk Mag Laegervidenskaben 4:1–88. [Google Scholar]
- 11.Han XY, Seo Y-H, Sizer KC, Taylor S, May GS, Spencer JS, Li W, Nair RG. 2008. A new Mycobacterium species causing diffuse lepromatous leprosy. Am J Clin Pathol 130:856–864. doi: 10.1309/AJCPP72FJZZRRVMM. [DOI] [PubMed] [Google Scholar]
- 12.Schuenemann VJ, Singh P, Mendum TA, Krause-Kyora B, Jäger G, Bos KI, Herbig A, Economou C, Benjak A, Busso P, Nebel A, Boldsen JL, Kjellström A, Wu H, Stewart GR, Taylor GM, Bauer P, Lee OY, Wu HH, Minnikin DE, Besra GS, Tucker K, Roffey S, Sow SO, Cole ST, Nieselt K, Krause J. 2013. Genome-wide comparison of medieval and modern Mycobacterium leprae. Science 341:179–183. doi: 10.1126/science.1238286. [DOI] [PubMed] [Google Scholar]
- 13.Han XY, Sizer KC, Thompson EJ, Kabanja J, Li J, Hu P, Gómez-Valero L, Silva FJ. 2009. Comparative sequence analysis of Mycobacterium leprae and the new leprosy-causing Mycobacterium lepromatosis. J Bacteriol 191:6067–6074. doi: 10.1128/JB.00762-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh P, Benjak A, Schuenemann VJ, Herbig A, Avanzi C, Busso P, Nieselt K, Krause J, Vera-Cabrera L, Cole ST. 2015. Insight into the evolution and origin of leprosy bacilli from the genome sequence of Mycobacterium lepromatosis. Proc Natl Acad Sci U S A 112:4459–4464. doi: 10.1073/pnas.1421504112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han XY, Mistry NA, Thompson EJ, Tang HL, Khanna K, Zhang L. 2015. Draft genome sequence of new leprosy agent Mycobacterium lepromatosis. Genome Announc 3:e00513–15. doi: 10.1128/genomeA.00513-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vera-Cabrera L, Escalante-Fuentes WG, Gomez-Flores M, Ocampo-Candiani J, Busso P, Singh P, Cole ST. 2011. A case of diffuse lepromatous leprosy associated with Mycobacterium lepromatosis. J Clin Microbiol 49:4366–4368. doi: 10.1128/JCM.05634-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vera-Cabrera L, Escalante-Fuentes W, Ocampo-Garza SS, Ocampo-Candiani J, Molina-Torres CA, Avanzi C, Benjak A, Busso P, Singh P, Cole ST. 2015. Mycobacterium lepromatosis infections in Nuevo Leon, Mexico. J Clin Microbiol 53:1945–1946. doi: 10.1128/JCM.03667-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jessamine PG, Desjardins M, Gillis T, Scollard D, Jamieson F, Broukhanski G, Chedore P, McCarthy A. 2012. Leprosy-like illness in a patient with Mycobacterium lepromatosis from Ontario, Canada. J Drugs Dermatol 11:229–233. [PubMed] [Google Scholar]
- 19.Lucio R, Alvarado Y. 1852. Opusculo sobre el mal de San Lazaro o elefanciasis de los Griegos. M. Murguia y Compania, Mexico. [Google Scholar]
- 20.Latapi F, Chevez-Zamora A. 1948. The “spotted” leprosy of Lucio: an introduction to its clinical and histological study. Int J Lepr 16:421–437. [Google Scholar]
- 21.Rea TH, Jerskey RS. 2005. Clinical and histologic variations among thirty patients with Lucio's phenomenon and pure and primitive diffuse lepromatosis (Latapi's lepromatosis). Int J Lepr Other Mycobact Dis 73:169–188. [PubMed] [Google Scholar]
- 22.Gelber RH. 2005. Leprosy (Hansen's disease), p 966–972. In Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL (ed), Harrison's principles of internal medicine, 16th ed McGraw-Hill, New York, NY. [Google Scholar]
- 23.Vargas-Ocampo F. 2007. Diffuse leprosy of Lucio and Latapí: a histologic study. Lepr Rev 78:248–260. [PubMed] [Google Scholar]
- 24.Romero A, Ibarra AB, Fallas M. 1949. Clinical study of lepromatous leprosy in Costa Rica. Int J Lepr 17:27–33. [PubMed] [Google Scholar]
- 25.Lopez Roa RI, Morris MF. 2006. Leprosy in Mexico. Jpn J Lepr 75:51–58. doi: 10.5025/hansen.75.51. [DOI] [PubMed] [Google Scholar]
- 26.Han XY, Jessurun J. 2013. Severe leprosy reactions due to Mycobacterium lepromatosis. Am J Med Sci 345:65–69. doi: 10.1097/MAJ.0b013e31826af5fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penna MLF, Wand-del-Rey de Oliveira ML, Penna F. 2009. Spatial distribution of leprosy in the Amazon region of Brazil. Emerging Infect Dis 15:650–652. doi: 10.3201/eid1504.081378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barreto JG, Bisanzio D, Guimaraes LdS, Spencer JS, Vazquez-Prokopec GM, Kitron U, Salgado CG. 2014. Spatial analysis spotlighting early childhood leprosy transmission in a hyperendemic municipality of the Brazilian Amazon region. PLoS Negl Trop Dis 8:e2665. doi: 10.1371/journal.pntd.0002665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes MS, James G, Taylor MJ, McCarroll J, Neill SD, Chen SC, Mitchell DH, Love DN, Malik R. 2004. PCR studies of feline leprosy cases. J Feline Med Surg 6:235–243. doi: 10.1016/j.jfms.2003.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meredith A, Del Pozo J, Smith S, Milne E. 2014. Leprosy in red squirrels in Scotland. Vet Record 175:285–286. doi: 10.1136/vr.g5680. [DOI] [PubMed] [Google Scholar]
- 31.Pin D, Guérin-Faublée V, Garreau V, Breysse F, Dumitrescu O, Flandrois JP, Lina G. 2014. Mycobacterium species related to M. leprae and M. lepromatosis from cows with bovine nodular thelitis. Emerg Infect Dis 20:2111–2114. doi: 10.3201/eid2012.140184. [DOI] [PMC free article] [PubMed] [Google Scholar]