Abstract
Our case series showed that uncomplicated Yarrowia lipolytica fungemia might be treated with catheter removal alone. The Vitek 2 YST identification (ID) card system, matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS), and internal transcribed spacer and 25S nuclear ribosomal DNA (nrDNA) gene sequencing provided reliable identification. All isolates had low MICs to voriconazole, echinocandins, and amphotericin B.
TEXT
A growing number of non-albicans Candida spp. have emerged in recent years as important human pathogens among the growing, heterogeneous population of immunocompromised and critically ill patients (1–3). Yarrowia lipolytica (Candida lipolytica) is an ascomycetous yeast found ubiquitously in the environment and meat products, including sausages and dairy products, especially cheese (4). It may occasionally be found as a colonizer in the feces, oropharyngeal swabs, sputa, and skin swabs of asymptomatic persons (5). Its intense secretory activity is widely employed in the food, detergent, and pharmaceutical industries to produce aroma compounds, organic acids, polyalcohols, emulsifiers, and surfactants (4, 6, 7). Although Y. lipolytica was previously considered to be of low virulence, it has been increasingly recognized to cause sporadic cases and nosocomial clusters of human infections, especially catheter-related suppurative thrombophlebitis and fungemia associated with biofilm formation in immunocompromised or critically ill patients who experience prolonged hospitalization and who required long-dwelling intravascular catheterization and broad-spectrum antibacterial agents (5, 8–10). Other forms of clinical disease, including noncatheter-related fungemia, traumatic ocular infection, and acute exacerbation of chronic sinusitis, have also been reported (5, 8, 11). A major limitation of the literature on Y. lipolytica infection is that most studies were case reports or small case series focusing only on clinical characteristics (5, 8–17). Recently, Trabelsi and colleagues reported the epidemiological risk factors and clinical outcomes of 55 cases of Y. lipolytica fungemia in Tunisia and provided some data on the in vitro susceptibility test results of the isolates to a few antifungal drugs (6). However, details about the correlative microbiological characteristics, including phenotypic and genotypic identification and in vitro susceptibility test results for newer antifungal drugs such as the echinocandins and posaconazole, were lacking in this larger case series. In this multicenter, prospective surveillance study in China, we described the epidemiological and clinical characteristics of 14 cases of Y. lipolytica fungemia in correlation with the comparative performance of several commonly employed phenotypic and genotypic identification methods, and we also described the in vitro susceptibility of this emerging fungal pathogen to nine antifungal drugs, including the newer azoles and echinocandins.
This study was approved by the Institutional Review Boards of The University of Hong Kong/Hospital Authority Hong Kong West Cluster and the Peking Union Medical College Hospital. As described previously, the National China Hospital Invasive Fungal Surveillance Net (CHIF-NET) study was a prospective, laboratory-based, multicenter study of invasive yeast infections that started on 1 August 2009 (18). The present study included all of the Y. lipolytica blood culture isolates identified in the CHIF-NET study from 1 August 2009 to 31 July 2012. These isolates were identified as Y. lipolytica using the Vitek 2 YST identification (ID) card system (bioMérieux, France) as previously described (19). For isolates that had a single identification with a confidence value of <99.9%, multiple identifications, or no identification, internal transcribed spacer (ITS) region sequencing was used for confirmation (19). Additionally, we compared the performance of the API 20C Aux system (bioMérieux) and matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) for the identification of Y. lipolytica with that of the Vitek 2 YST ID Card system (see the supplemental material).
A total of 14 cases of Y. lipolytica fungemia were identified during the study period. The clinical characteristics of 13 patients with available information are listed in Table 1. One patient (case 9) had two Y. lipolytica isolates identified from the blood cultures obtained from the peripheral vein and central venous catheter simultaneously. Twelve (92.3%) were males. Their median age was 63 years (range, 1 to 82 years). Similar to previous reports, all had risk factors for Y. lipolytica fungemia, including prior use of broad-spectrum antibacterials (100.0%), prolonged hospitalization and admission to the intensive care unit (84.6%), and intravascular catheterization (100.0%). Seven (53.8%) had a central venous catheter. The time interval between the setup of the intravenous catheter and the onset of fungemia was longer in those with central venous catheters (mean, 38.9 days) than those with peripheral venous catheters (mean, 13.3 days). All patients had catheter removal after Y. lipolytica was isolated in blood culture, but the use of antifungal treatment was variable. Four (30.8%) did not receive any antifungal treatment, but they all survived after catheter removal. The remaining nine (69.2%) received systemic antifungal treatment, including fluconazole, itraconazole, and amphotericin B for a range of 5 to 40 days. Three (23.1%) died during hospitalization.
TABLE 1.
Clinical characteristics of 13 patients with Yarrowia lipolytica fungemiaa
| Case | Isolate | Sex/Age (yr) | Reason for admission (comorbidities) | i.v. catheters (days)b | i.v. antibiotics | ICU | Specimen(s) | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | PW3298 | M/65 | Road traffic accident with traumatic subdural hematoma, multiple fractures, and gastrectomy | PVC (9) | Yes | No | Blood (peripheral) | Catheter removal and i.v. fluconazole 200 mg daily | Discharged after 14 days of hospitalization |
| 2 | PW3299 | M/46 | Postoperative fever (CRHD with valvular replacement) | PVC (11) | Yes | Yes | Blood (peripheral) | Catheter removal | Discharged after 20 days of hospitalization |
| 3 | PW3300 | M/27 | Fever with hypotension (cervical spinal fracture with paraplegia) | PICC (44) | Yes | Yes | Blood (PICC) | Catheter removal | Transferred to another hospital after 55 days of hospitalization |
| 4 | PW3301 | M/67 | Ischemic stroke with hemorrhagic transformation (ESRF) | CVC (7) | Yes | Yes | Blood (peripheral and CVC) | Catheter removal and i.v. itraconazole 350 mg daily | Discharged after 30 days of hospitalization |
| 5 | PW3302 | M/73 | Recurrent peritonitis (carcinoma of pancreas) | CVC (40) | Yes | Yes | Blood (peripheral and CVC) | Catheter removal and i.v. amphotericin B 25 mg | Discharged against medical advice after 45 days of hospitalization |
| 6 | PW3303 | M/1 | Apnea of prematurity | PVC (15) | Yes | No | Blood (peripheral) | Catheter removal | Discharged after 16 days of hospitalization |
| 7 | PW3304 | M/3 | Respiratory distress (congenital heart disease) | CVC (75) | Yes | Yes | Blood (peripheral and CVC) | Catheter removal and oral fluconazole 150 mg daily for 12 days | Discharged after 4 mo of hospitalization |
| 8 | PW3305 | M/63 | Bronchiectactic exacerbation (bronchiectasis) | PVC (6) | Yes | Yes | Blood (peripheral) | Catheter removal and i.v. fluconazole 400 mg daily for 5 days | Died after 10 days of hospitalization |
| 9 | PW3306 and PW3307 | M/75 | Intracerebral hemorrhage | CVC (30) | Yes | Yes | Blood (peripheral and CVC, respectively) | Catheter removal and i.v. fluconazole 400 mg daily for 14 days | Died after 35 days of hospitalization |
| 10 | PW3309 | M/43 | Pneumonia (brainstem death) | PVC (8) | Yes | No | Blood (peripheral) | Catheter removal | Discharged after 10 days of hospitalization |
| 11 | PW3310 | M/45 | Intestinal obstruction | CVC (38) | Yes | Yes | Blood (peripheral and CVC) | Catheter removal and i.v. fluconazole 400 mg daily for 27 days | Discharged after 49 days of hospitalization |
| 12 | PW3311 | M/73 | Open skull fracture | PVC (31) | Yes | Yes | Blood (peripheral) | Catheter removal and i.v. fluconazole 200 mg daily for 37 days | Discharged after 2 mo of hospitalization |
| 13 | PW3312 | F/82 | Right hip fracture | CVC (38) | Yes | Yes | Blood (peripheral and CVC) | Catheter removal and i.v. fluconazole 400 mg daily for 40 days | Died after 45 days of hospitalization |
The clinical data of 1 of the 14 cases in this study was not available. CRHD, chronic rheumatic heart disease; CVC, central venous catheter; ESRF, end-stage renal failure; F, female; i.v., intravenous; ICU, intensive care unit; M, male; PVC, peripheral venous catheter; PICC, peripherally inserted central catheter.
Duration of intravenous catheter in situ before onset of fungemia.
A major knowledge gap in the literature on Y. lipolytica catheter-related fungemia is the optimal management strategy. While the clinical practice guideline for the management of candidiasis by the Infectious Diseases Society of America recommends the use of systemic antifungal treatment and catheter removal, some have suggested that catheter removal alone might be sufficient in treating Y. lipolytica catheter-related infection (5, 14, 20). Catheter removal without systemic antifungal treatment has rendered the blood culture negative in some cases of Y. lipolytica catheter-related fungemia, including those involving neutropenic patients (5, 14). Y. lipolytica-associated suppurative thrombophlebitis may also be successfully treated with catheter removal alone (5). Nearly one-third (cases 2, 3, 6, and 10) of our patients did not receive systemic antifungal treatment, but their fungemia resolved and their clinical conditions improved after catheter removal. Our data suggested that Y. lipolytica catheter-related infection may be treated with catheter removal without systemic antifungal therapy, provided that there is clinical improvement with resolution of systemic and/or local symptoms and signs, microbiological documentation of clearance of fungemia, and an absence of persistent infective foci such as endocarditis, abscess, and endophthalmitis.
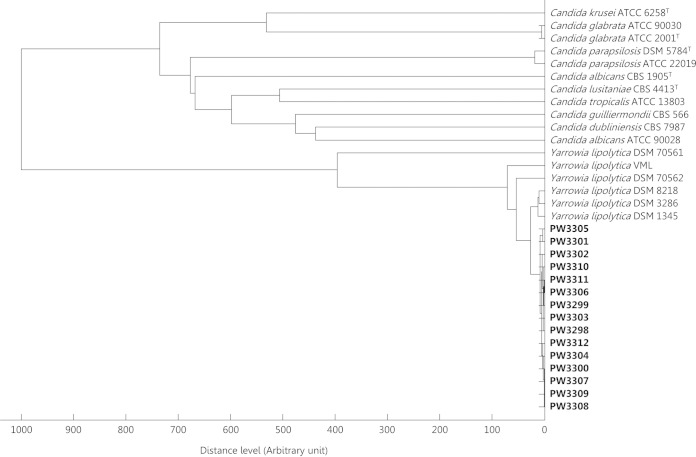
Rapid and accurate identification is especially important for Y. lipolytica, as the need and choice of systemic antifungal treatment may differ between infections caused by Y. lipolytica and other Candida spp. The Vitek 2 YST ID Card system identified all 15 (100.0%) isolates as Y. lipolytica with probabilities of 94.0% to 99.0% (see Table S1 in the supplemental material). None of the isolates were identified as Y. lipolytica by the API 20C Aux system. Nine (60.0%) were unidentifiable and the other six (40.0%) were misidentified as Candida magnoliae, Candida zeylanoides, Candida dubliniensis, or Sporobolomyces salmonicolor with probabilities of 42.9% to 99.5%. In contrast, MALDI-TOF MS accurately identified all (100.0%) isolates as Y. lipolytica with top match scores of 1.884 to 2.250. Hierarchical cluster analysis of the MALDI-TOF mass spectra of all 15 isolates also unambiguously showed that they were clustered with other Y. lipolytica isolates (Fig. 1).
FIG 1.
Dendrogram generated from the hierarchical cluster analysis (HCA) of the MALDI-TOF mass spectra of the case isolates, Y. lipolytica, and other Candida spp. showing their relative relatedness.
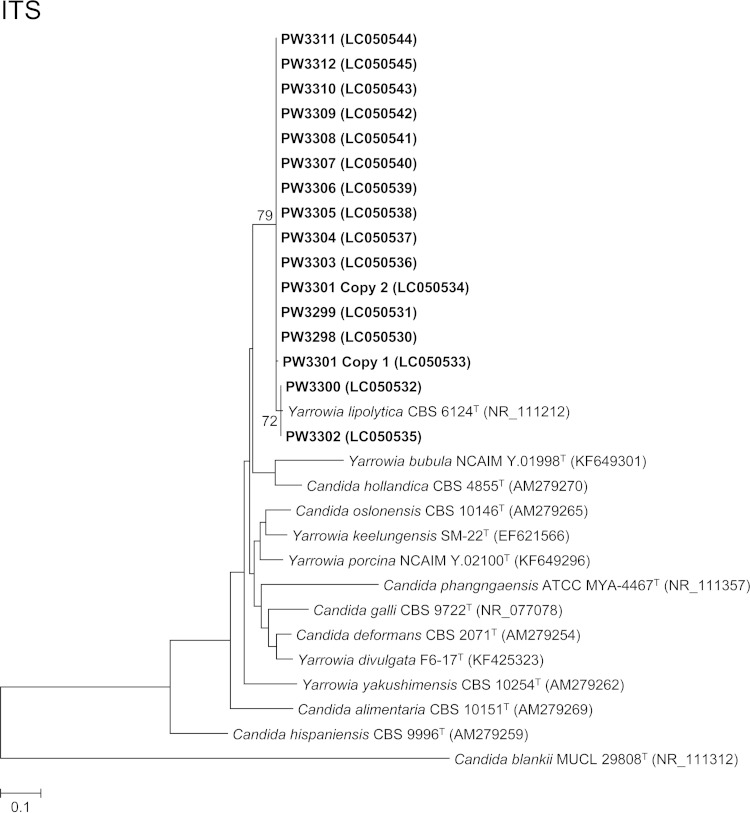
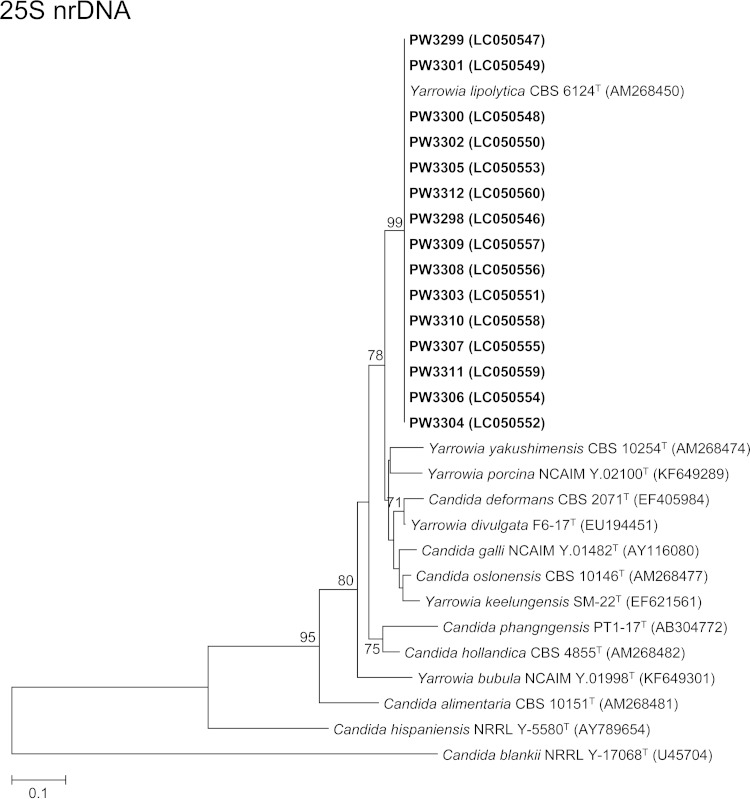
For phylogenetic analysis, DNA extraction, PCR amplification, and DNA sequencing of the ITS region for the isolates were performed according to our previous publications (21–28) using the primer pairs ITS1/ITS4 (29) for the ITS and NL1/NL4 (30) for the 25S nuclear ribosomal DNA (nrDNA) (see the supplemental material). ITS and partial 25S nrDNA gene sequencing identified 14 and all 15 isolates as Y. lipolytica, respectively, confirming the results of the Vitek 2 YST ID card system and MALDI-TOF MS. Since the electropherogram obtained from direct PCR product sequencing for the ITS of isolate PW3301 was uninterpretable where double or multiple peaks were observed frequently in the sequence trace, the PCR product of the ITS of isolate PW3301 was cloned into plasmids for another sequencing trial (see the supplemental material). Two of the four clones selected for sequencing showed one ITS copy while the other two clones showed another ITS copy, indicating the presence of intrastrain ITS heterogeneity in the isolate PW3301. Pairwise alignment of the two ITS copies of PW3301 showed that they differed by a single nucleotide insertion of an A residue at the ITS2 region. This is the first report of intrastrain ITS heterogeneity in Y. lipolytica. Pairwise alignment also showed that the ITS and 25S nrDNA sequences of the 15 isolates possessed 98.3% to 99.3% and 99.8% to 100% identities to that of Y. lipolytica strain CBS 6124T, respectively. Phylogenetic analyses based on the ITS and partial 25S nrDNA sequences showed that all 15 isolates were clustered with Y. lipolytica CBS 6124T (Fig. 2). The low intraspecies sequence variability for ITS and 25S nrDNA for Y. lipolytica makes these two gene loci good DNA markers for the identification of this fungus. In addition, ITS and 25S nrDNA sequencing confirmed that isolates PW3306 and PW3307 obtained from the same patient (case 9) were identical (100% sequence identities for both loci).
FIG 2.
Phylogenetic trees showing the relationship between the 15 fungal isolates and other members of the Yarrowia clade. The trees were inferred from ITS and 25S nrDNA sequence data by the maximum likelihood method with the substitution models T92 (Tamura 3-parameter model) + G (gamma-distributed rate variation) and K2 (Kimura 2-parameter model) + G, respectively. The numbers of nucleotide positions of the trimmed, aligned sequences included for phylogenetic analyses are 293 and 488, respectively. The trees were rooted using Candida blankii strains MUCL 29808T and NRRL Y-17068T, respectively. The scale bars indicate the estimated numbers of substitutions per base. The numbers at the nodes, expressed in percentages, indicate levels of bootstrap support calculated from 1,000 trees, and bootstrap values lower than 70 are not shown. All accession numbers (in parentheses) are given as cited in the DDBJ/ENA/GenBank databases. The case isolates reported in this study are highlighted in bold type.
The susceptibilities of the isolates to nine different antifungal drugs were tested by the broth microdilution method using Sensititre YeastOne plates (Trek Diagnostic Systems, United Kingdom) according to the manufacturer's instructions for Candida spp. and Pfaller et al. (31). The MIC values and ranges were calculated using WHONET 5.6 (World Health Organization Collaborating Center for Surveillance of Antibiotic Resistance, Boston, MA, USA). The susceptibilities of the isolates to fluconazole and voriconazole were also evaluated using Sensi-Disc antimicrobial susceptibility test disks (BBL; BD Diagnostic Systems, Sparks, MD, USA) by the disk diffusion method according to the Clinical and Laboratory Standards Institute M44-A2 standard (32). We performed antimicrobial susceptibility tests for only one of the two isolates obtained in case 9 because they were identified to be the same strain. All 14 (100.0%) Y. lipolytica isolates had MICs of ≤2.00 μg/ml to voriconazole by the broth microdilution method (Table 2). The MICs to the other azoles were less consistent, with many of them having MICs of ≥4.00 μg/ml to fluconazole, itraconazole, or posaconazole by the broth microdilution method. The broth microdilution and disk diffusion methods demonstrated that MICs and zone sizes were in general agreement with each other for voriconazole and fluconazole, with the isolates having lower MICs in the broth microdilution method also showing larger zone sizes in the disk diffusion method. As for the other antifungal agents, all 14 (100.0%) isolates had MICs of ≤2.00 μg/ml to caspofungin, micafungin, anidulafungin, and amphotericin B, whereas 10/14 (71.4%) isolates had MICs of ≥4.00 μg/ml to flucytosine.
TABLE 2.
In vitro antifungal susceptibilities of Yarrowia lipolytica isolates in this study
| Isolate | MIC (μg/ml)a for: |
Disk diffusion zone diameter (mm) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Azoles |
Echinocandins |
Others |
|||||||||
| FZ | IZ | VOR | PZ | CAS | MF | AND | AB | 5-FC | FZ | VOR | |
| PW3298 | 4 | 0.25 | 0.03 | 0.50 | 1 | 1 | 0.50 | 1 | 16 | 20 | 25 |
| PW3299 | 4 | 0.50 | 0.06 | 0.50 | 0.25 | 0.12 | 0.03 | 1 | 4 | 20 | 28 |
| PW3300 | 4 | 0.12 | 0.03 | 0.50 | 0.50 | 0.50 | 0.12 | 1 | 8 | 20 | 26 |
| PW3301 | 16 | 0.50 | 0.25 | 0.03 | 0.02 | 0.03 | 0.03 | 0.25 | 0.12 | 6 | 6 |
| PW3302 | 0.50 | 0.06 | 0.03 | 0.12 | 0.06 | 0.12 | 0.03 | 0.50 | 4 | 21 | 25 |
| PW3303 | 2 | 0.25 | 0.03 | 0.50 | 0.50 | 0.50 | 0.12 | 0.50 | 8 | 25 | 30 |
| PW3304 | 16 | 0.50 | 0.25 | 1 | 0.50 | 0.50 | 0.50 | 2 | 16 | 13 | 30 |
| PW3305 | >256 | 2 | 2 | 2 | 0.50 | 0.50 | 0.12 | 1 | 16 | 6 | 6 |
| PW3307 | 64 | 1 | 1 | 2 | 0.25 | 0.50 | 0.25 | 0.25 | 0.50 | 6 | 6 |
| PW3308 | 128 | >16 | 2 | >8 | 0.50 | 1 | 0.12 | 0.50 | 4 | 6 | 6 |
| PW3309 | 128 | >16 | 2 | >8 | 0.50 | 1 | 0.12 | 0.50 | 8 | 6 | 6 |
| PW3310 | 128 | >16 | 2 | >8 | 0.50 | 1 | 0.25 | 0.50 | 4 | 6 | 6 |
| PW3311 | 64 | 1 | 1 | 2 | 0.25 | 0.25 | 0.12 | 0.25 | 0.50 | 6 | 6 |
| PW3312 | 64 | 1 | 1 | 2 | 0.12 | 0.25 | 0.12 | 0.25 | 0.50 | 6 | 6 |
| MIC data | |||||||||||
| MIC range | 0.50–>256 | 0.06–>16 | 0.03–2 | 0.03–>8 | 0.02–1 | 0.03–1 | 0.03–0.50 | 0.25–2 | 0.12–16 | ||
| MIC50 | 16 | 0.50 | 0.25 | 1 | 0.50 | 0.50 | 0.12 | 0.50 | 4 | ||
| MIC90 | 128 | >16 | 2 | >8 | 0.50 | 1 | 0.50 | 1 | 16 | ||
5-FC, flucytosine; AB, amphotericin B; AND, anidulafungin; CAS, caspofungin; FZ, fluconazole; IZ, itraconazole; MF, micafungin; PZ, posaconazole; VOR, voriconazole.
Fluconazole was the most commonly used antifungal drug in the nine patients who received systemic antifungal treatment. This was based on previous observations that most Y. lipolytica isolates were susceptible to fluconazole in vitro (6, 8, 10). However, three of our patients (cases 8, 9, and 13) who received fluconazole died despite treatment and catheter removal. Their blood culture isolates had high MICs of 32 to >256 μg/ml to fluconazole. Importantly, all of these isolates also had MICs of ≥1 μg/ml to itraconazole and ≥2 μg/ml to posaconazole (Table 2). Thus, high MICs to fluconazole might help to predict high MICs to itraconazole and posaconazole, and infections caused by Y. lipolytica isolates with high MICs to fluconazole should not be treated with itraconazole and posaconazole. Our in vitro antifungal susceptibility data showed that voriconazole, caspofungin, micafungin, anidulafungin, and amphotericin B may be better treatment options.
Our study highlighted the need to establish standardized recommendations for determining in vitro antifungal susceptibility for Y. lipolytica using different testing methods. The in vitro susceptibility to fluconazole and voriconazole by the broth microdilution and disk diffusion methods differed markedly when the recommended MICs and zone sizes for other Candida spp. were used for interpretation. Y. lipolytica isolates with MICs of ≥16 μg/ml and ≥1 μg/ml against fluconazole and voriconazole, respectively, had zone sizes corresponding to the resistant category by the disk diffusion method. Furthermore, there are as yet no interpretative criteria for posaconazole and amphotericin B for Candida spp., although in general, an MIC of ≤1 μg/ml is considered to inhibit most clinical isolates (33). The in vitro antifungal susceptibility of more clinical isolates of Y. lipolytica with correlation to the patients' clinical response should be evaluated to determine the optimal breakpoints of this emerging fungal pathogen.
Nucleotide sequence accession numbers.
The ITS and partial 25S nrDNA sequences of the 15 Y. lipolytica isolates have been deposited in the DDBJ/ENA/GenBank databases with the accession numbers LC050530 to LC050560.
Supplementary Material
ACKNOWLEDGMENTS
This work is partly supported by the University Development Fund, the Committee for Research and Conference Grant, Cheng Yu Tung Fellowship, Mary Sun Medical Scholarship, Wong Ching Yee Medical Postgraduate Scholarship, and the University Postgraduate Scholarship, The University of Hong Kong, Hong Kong, the Croucher Senior Medical Research Fellowship, Croucher Foundation, Hong Kong, and by the donations of Larry Chi-Kin Yung and the Hui Hoy and Chow Sin Lan Charity Fund Limited. The CHIF-NET surveillance study is supported by an Investigator-Initiated Research Grant from Pfizer Corporation China. Jasper F. W. Chan has received travel grants from the Pfizer Corporation Hong Kong and Astellas Pharma Hong Kong Corporation Limited. The funding sources had no role in study design, data collection, analysis, interpretation, or writing of the report.
The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.01985-15.
REFERENCES
- 1.Hazen KC. 1995. New and emerging yeast pathogens. Clin Microbiol Rev 8:462–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papon N, Courdavault V, Clastre M, Bennett RJ. 2013. Emerging and emerged pathogenic Candida species: beyond the Candida albicans paradigm. PLoS Pathog 9:e1003550. doi: 10.1371/journal.ppat.1003550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miceli MH, Díaz JA, Lee SA. 2011. Emerging opportunistic yeast infections. Lancet Infect Dis 11:142–151. doi: 10.1016/S1473-3099(10)70218-8. [DOI] [PubMed] [Google Scholar]
- 4.Kurtzman CP. 2011. Yarrowia van der Walt & von Arx (1980), p 927–929. In Kurtzman CP, Fell JW, Boekhout T (ed), The yeasts: a taxonomic study, 5th ed Elsevier, London, UK. [Google Scholar]
- 5.Walsh TJ, Salkin IF, Dixon DM, Hurd NJ. 1989. Clinical, microbiological, and experimental animal studies of Candida lipolytica. J Clin Microbiol 27:927–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trabelsi H, Chtara K, Khemakhem N, Néji S, Cheikhrouhou F, Sellami H, Guidara R, Makni F, Bouaziz M, Ayadi A. 2015. Fungemia caused by Yarrowia lipolytica. Mycopathologia 179:437–445. doi: 10.1007/s11046-015-9859-4. [DOI] [PubMed] [Google Scholar]
- 7.Zinjarde SS. 2014. Food-related applications of Yarrowia lipolytica. Food Chem 152:1–10. doi: 10.1016/j.foodchem.2013.11.117. [DOI] [PubMed] [Google Scholar]
- 8.Liu WC, Chan MC, Lin TY, Hsu CH, Chiu SK. 2013. Candida lipolytica candidemia as a rare infectious complication of acute pancreatitis: a case report and literature review. J Microbiol Immunol Infect 46:393–396. doi: 10.1016/j.jmii.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Shin JH, Kook H, Shin DH, Hwang TJ, Kim M, Suh SP, Ryang DW. 2000. Nosocomial cluster of Candida lipolytica fungemia in pediatric patients. Eur J Clin Microbiol Infect Dis 19:344–349. doi: 10.1007/s100960050491. [DOI] [PubMed] [Google Scholar]
- 10.D'Antonio D, Romano F, Pontieri E, Fioritoni G, Caracciolo C, Bianchini S, Olioso P, Staniscia T, Sferra R, Boccia S, Vetuschi A, Federico G, Gaudio E, Carruba G. 2002. Catheter-related candidemia caused by Candida lipolytica in a patient receiving allogeneic bone marrow transplantation. J Clin Microbiol 40:1381–1386. doi: 10.1128/JCM.40.4.1381-1386.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nitzulescu V, Niculescu M. 1976. 3 cases of ocular candidiasis caused by Candida lipolytica. Arch Roum Pathol Exp Microbiol 35:269–272. (In French.) [PubMed] [Google Scholar]
- 12.Wehrspann P, Füllbrandt U. 1985. Report of a case of Yarrowia lipolytica (Wickerham et al.) van der Walt and von Arx isolated from a blood culture. Mycoses 28:217–222. (In German.) doi: 10.1111/j.1439-0507.1985.tb02119.x. [DOI] [PubMed] [Google Scholar]
- 13.García-Martos P, de la Rubia F, Palomo M, Alvarez M, Marín P, Mira J. 1993. Candida lipolytica, a new opportunistic pathogen. Enferm Infecc Microbiol Clin 11:163 (In Spanish.) [PubMed] [Google Scholar]
- 14.Chang CL, Park TH, Lee EY, Lim YT, Son HC. 2001. Recurrent self-limited fungemia caused by Yarrowia lipolytica in a patient with acute myelogenous leukemia. J Clin Microbiol 39:1200–1201. doi: 10.1128/JCM.39.3.1200-1201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal S, Thakur K, Kanga A, Singh G, Gupta P. 2008. Catheter-related candidemia caused by Candida lipolytica in a child with tubercular meningitis. Indian J Pathol Microbiol 51:298–300. doi: 10.4103/0377-4929.41709. [DOI] [PubMed] [Google Scholar]
- 16.Özdemir H, Karbuz A, Çiftçi E, Dinçaslan HU, Ince E, Aysev D, Yavuz G, Dogru U. 2011. Successful treatment of central venous catheter infection due to Candida lipolytica by caspofungin-lock therapy. Mycoses 54:e647–e649. doi: 10.1111/j.1439-0507.2010.01964.x. [DOI] [PubMed] [Google Scholar]
- 17.Lai CC, Lee MR, Hsiao CH, Tan CK, Lin SH, Liao CH, Huang YT, Hsueh PR. 2012. Infections caused by Candida lipolytica. J Infect 65:372–374. doi: 10.1016/j.jinf.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Xiao M, Chen SC, Kong F, Sun ZY, Liao K, Lu J, Shao HF, Yan Y, Fan H, Hu ZD, Chu YZ, Hu TS, Ni YX, Zou GL, Xu YC. 2012. In vitro susceptibilities of yeast species to fluconazole and voriconazole as determined by the 2010 National China Hospital Invasive Fungal Surveillance Net (CHIF-NET) study. J Clin Microbiol 50:3952–3959. doi: 10.1128/JCM.01130-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Xiao M, Wang H, Gao R, Fan X, Brown M, Gray TJ, Kong F, Xu Y-C. 2014. Yeast identification algorithm based on use of the Vitek MS system selectively supplemented with ribosomal DNA sequencing: proposal of a reference assay for invasive fungal surveillance programs in China. J Clin Microbiol 52:572–577. doi: 10.1128/JCM.02543-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pappas PG, Kauffman CA, Andes D, Benjamin DK Jr, Calandra TF, Edwards JE Jr, Filler SG, Fisher JF, Kullberg BJ, Zeichner LO, Reboli AC, Rex JH, Walsh TJ, Sobel JD. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.To KK, Lau SK, Wu AK, Lee RA, Ngan AH, Tsang CC, Ling IW, Yuen KY, Woo PC. 2012. Phaeoacremonium parasiticum invasive infections and airway colonization characterized by agar block smear and ITS and β-tubulin gene sequencing. Diagn Microbiol Infect Dis 74:190–197. doi: 10.1016/j.diagmicrobio.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Woo PC, Ngan AH, Tsang CC, Ling IW, Chan JF, Leung SY, Yuen KY, Lau SK. 2013. Clinical spectrum of Exophiala infections and a novel Exophiala species, Exophiala hongkongensis. J Clin Microbiol 51:260–267. doi: 10.1128/JCM.02336-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernando N, Hui SW, Tsang CC, Leung SY, Ngan AH, Leung RW, Groff JM, Lau SK, Woo PC. 2015. Fatal Fusarium solani species complex infections in elasmobranchs: the first case report for black spotted stingray (Taeniura melanopsila) and a literature review. Mycoses 58:422–431. doi: 10.1111/myc.12342. [DOI] [PubMed] [Google Scholar]
- 24.Tsang CC, Chan JF, Ip PP, Ngan AH, Chen JH, Lau SK, Woo PC. 2014. Subcutaneous phaeohyphomycotic nodule due to Phialemoniopsis hongkongensis sp. nov. J Clin Microbiol 52:3280–3289. doi: 10.1128/JCM.01592-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsang CC, Chan JF, Trendell-Smith NJ, Ngan AH, Ling IW, Lau SK, Woo PC. 2014. Subcutaneous phaeohyphomycosis in a patient with IgG4-related sclerosing disease caused by a novel ascomycete, Hongkongmyces pedis gen. et sp. nov.: first report of human infection associated with the family Lindgomycetaceae. Med Mycol 52:736–747. doi: 10.1093/mmy/myu043. [DOI] [PubMed] [Google Scholar]
- 26.Chan JF, Teng JL, Li IW, Wong SC, Leung SS, Ho PO, To KK, Lau SK, Woo PC, Yuen KY. 2014. Fatal empyema thoracis caused by Schizophyllum commune with cross-reactive cryptococcal antigenemia. J Clin Microbiol 52:683–687. doi: 10.1128/JCM.02770-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woo PC, Leung SY, To KK, Chan JF, Ngan AH, Cheng VC, Lau SK, Yuen KY. 2010. Internal transcribed spacer region sequence heterogeneity in Rhizopus microsporus: implications for molecular diagnosis in clinical microbiology laboratories. J Clin Microbiol 48:208–214. doi: 10.1128/JCM.01750-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng VC, Chan JF, Ngan AH, To KK, Leung SY, Tsoi HW, Yam WC, Tai JW, Wong SS, Tse H, Li IW, Lau SK, Woo PC, Leung AY, Lie AK, Liang RH, Que TL, Ho PL, Yuen KY. 2009. Outbreak of intestinal infection due to Rhizopus microsporus. J Clin Microbiol 47:2834–2843. doi: 10.1128/JCM.00908-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White T, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322. In Innis M, Gelfand D, Shinsky J, White T (ed), PCR protocols: a guide to methods and applications. Academic Press, San Diego, CA. [Google Scholar]
- 30.O'Donnell K. 1993. Fusarium and its near relatives, p 225–233. In Reynolds DR, Taylor JW (ed), The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. CAB International, Wallingford, UK. [Google Scholar]
- 31.Pfaller MA, Chaturvedi V, Diekema DJ, Ghannoum MA, Holliday NM, Killian SB, Knapp CC, Messer SA, Miskou A, Ramani R. Comparison of the Sensititre YeastOne colorimetric antifungal panel with CLSI microdilution for antifungal susceptibility testing of the echinocandins against Candida spp., using new clinical breakpoints and epidemiological cutoff values. Diagn Microbiol Infect Dis 73:365–368. [DOI] [PubMed] [Google Scholar]
- 32.Clinical and Laboratory Standards Institute. 2009. Method for antifungal disk diffusion susceptibility testing of yeasts; approved standard— 2nd ed CLSI document M44-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 33.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard— 3rd ed CLSI document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.