Abstract
Trithorax group (TrxG) and Polycomb group (PcG) proteins are two mutually antagonistic chromatin modifying complexes, however, how they together mediate transcriptional counter-regulation remains unknown. Genome-wide analysis revealed that binding of Ezh2 and menin, central members of the PcG and TrxG complexes, respectively, were reciprocally correlated. Moreover, we identified a developmental change in the positioning of Ezh2 and menin in differentiated T lymphocytes compared to embryonic stem cells. Ezh2-binding upstream and menin-binding downstream of the transcription start site was frequently found at genes with higher transcriptional levels, and Ezh2-binding downstream and menin-binding upstream was found at genes with lower expression in T lymphocytes. Interestingly, of the Ezh2 and menin cooccupied genes, those exhibiting occupancy at the same position displayed greatly enhanced sensitivity to loss of Ezh2. Finally, we also found that different combinations of Ezh2 and menin occupancy were associated with expression of specific functional gene groups important for T cell development. Therefore, spatial cooperative gene regulation by the PcG and TrxG complexes may represent a novel mechanism regulating the transcriptional identity of differentiated cells.
INTRODUCTION
Trithorax group (TrxG) and Polycomb group (PcG) complexes exert opposing effects on the maintenance of transcriptional status and play a critical role in the expression of developmentally regulated transcription factors through methylation at histone H3-K4 (H3K4me3; a permissive mark) and H3-K27 (H3K27me3; a repressive mark), respectively (1–5). In embryonic stem (ES) cells, a set of genes encoding developmental regulators was found to be controlled by the PcG complex (4, 6), and the TrxG complex was found to be essential for self-renewal and reprogramming (7, 8). TrxG and PcG complexes have also been recognized as crucial factors regulating the terminal differentiation of some cell types, such as epidermal cells (9), germ cells (10), muscle cells (11), and T lymphocytes (3, 12–14), and mutations in these proteins are often associated with tumorigenic potential (15).
PcG proteins are subdivided into two major repressive complexes: Polycomb repressive complex 1 (PRC1) and PRC2. The PcG protein Enhancer of Zeste Homolog 2 (Ezh2) is a histone methyltransferase specific for H3K27 and is essential for repression of target gene transcription (16). In CD4+ T cells, Ezh2 has been shown to directly bind and facilitate correct expression the Gata3 gene during differentiation into effector cells (12, 14). In addition, Ezh2 has recently been found to play an essential role in regulating the germinal center (GC) response by facilitating normal activation-induced cytidine deaminase function and preventing terminal differentiation of GC B cells (17, 18).
Compared to PcG proteins, TrxG proteins show more diversity regarding the different molecules that they form complexes with. Menin is found in TrxG complexes containing MLL1 or MLL2, which are responsible for H3K4me3 (2, 19). The protein menin is encoded by the MEN1 gene, which is mutated in patients with multiple endocrine neoplasia type 1 (MEN1) syndrome (20, 21). Menin can act as a tumor suppressor and is required for binding of the complex to DNA (2). Menin also plays a crucial role in immune system since it has been shown to be important for Th2 cell function both in mice and humans (14, 22). Although a considerable number of studies have been carried out on the nature of PcG proteins or TrxG proteins individually, it has not been well defined how transcriptional counter-regulation is organized by the TrxG and PcG complexes. With the exception of recent pioneering work demonstrating dynamic transformations of histone modifications during T cell development (23), how the global signature of TrxG and PcG cooccupied genes is changed during developmental processes remains unclear.
In the present study, we address these unresolved but important biological questions by assessment of spatial interaction of chromatin regulators on a genome-wide scale in ES cells and mature B and T lymphocytes using chromatin immunoprecipitation (ChIP) coupled with high-throughput DNA sequencing (ChIP-Seq) (24). This study reveals a new cooperative regulation by the PcG/TrxG complex that controls the transcriptional identity of differentiated cells.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were purchased from CLEA (Tokyo, Japan). Mice with loxp sites flanking the SET domain of Ezh2 were generated as previously described (25) and backcrossed with C57BL/6 mice for 10 generations. These mice were then bred with mice expressing transgenic constructs for Cre recombinase under the control of the CD4 promoter, allowing for conditional knockout (KO) of Ezh2 function in CD4+ T cells (CD4-Cre). CD4-Cre mice were purchased from Taconic. All mice used in the present study were maintained under specific-pathogen-free conditions and ranged from 6 to 8 weeks of age. All experimental protocols using mice were approved by the Chiba University Animal Committee. All animal care was performed in accordance with the guidelines of Chiba University.
Antibodies.
The antibodies used for the ChIP assay were anti-Bmi1 (Santa Cruz, sc-10745), anti-Ezh2 (Diagenode, pAb-039-050), anti-menin (Bethyl, A300-105A), and anti-Ser5-P RNA polymerase II (RNAPII) (Abcam, ab5408).
Isolation of B220+ B cells and CD4+ T cells from mouse spleen.
B220+ B and CD4+ T cells were purified using magnetic beads and an AutoMACS sorter (Miltenyi Biotec) that yielded a purity of >98%.
Generation Th2 cells and TSA treatment.
Th2 cells were generated as previously described (13). In brief, splenic CD4+ T cells were stimulated with 3 μg of immobilized anti-TCR-β monoclonal antibody (MAb)/ml plus 1 μg of anti-CD28 MAb/ml under Th2-culture conditions for 5 days in vitro. For the Th2 conditions, we used interleukin-2 (IL-2) at 15 ng/ml and IL-4 at 10 ng/ml. These cells were used as Th2 cells. For trichostatin A (TSA) treatment, splenic CD4 cells were cultured under Th2 conditions and 10 nM TSA (Sigma, St. Louis, MO) was added in the culture on day 2 and, after another 3 days of culture, the CD4 T cells were collected for analysis.
ES cell culture.
B6N-22 ES cells (26) were established and characterized previously. ES cells were cultivated on mitomycin C-treated mouse embryonic fibroblast in Dulbecco modified Eagle medium containing 0.1 mM 2-mercaptoethanol, 1,000 U of leukemia inhibitory factor/ml, nonessential amino acids, sodium pyruvate, and 20% fetal bovine serum.
ChIP assay.
ChIP experiments for Bmi1, Ezh2, menin, RNAPII, and control antibody were carried out using Dynabeads (Invitrogen). In brief, 107 ES, B, and CD4+ T cells were fixed with 1% paraformaldehyde at 37°C for 10 min. The cells were sedimented, washed, lysed with sodium dodecyl sulfate (SDS) lysis buffer (50 mM Tris-HCl, 1% SDS, 10 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 μg of aprotinin/ml, 1 μg of leupeptin/ml). The lysates were sonicated to reduce DNA lengths to between 150 and 300 bp. The soluble fraction was diluted in ChIP dilution buffer and incubated with antibody conjugated with Dynabeads-protein A and G overnight at 4°C. The immune complexes were then captured by using a magnet and washed with low-salt, high-salt, LiCl, or Tris-EDTA wash buffer. Enriched chromatin fragments were eluted with elution buffer (0.1 M NaHCO3 containing 1% SDS). The eluted material was incubated at 65°C for 6 h to reverse the formaldehyde cross-links and then treated with RNase A (10 μg/ml) and proteinase K (40 μg/ml). DNA was extracted by using a QIAquick PCR purification kit (Qiagen). The total input DNA (cellular DNA without immunoprecipitation) was purified in parallel.
ChIP-Seq and Illumina sequencing.
Antibody-specific immunoprecipitates and total input DNA samples were prepared using a ChIP-Seq sample preparation kit (Illumina). Adaptor-ligated DNA of 170 to 250 bp was recovered by size fractionation on an acrylamide gel. This DNA was then amplified by 18 cycles of PCR and 1 ng was used for 36 cycles of sequencing reaction on an Illumina Genome Analyser IIx. Read sequences (36 bp) were then aligned to the mm9 mouse reference genome (University of California, Santa Cruz [UCSC], July 2007) using Eland software (Illumina). Only sequences with two or less mismatches were considered for alignment.
ChIP-Seq data analysis.
Each aligned read sequence was extended to 120 bp in order to efficiently detect duplicate reads aligned to identical locations. These 120-bp tags were used for further analysis (Bed file). For visualization of binding, data were converted to BedGraph file format using a 500-bp sliding window with step size of 100 bp and uploaded to the IGV genome browser (http://www.broadinstitute.org/igv/).
ChIP-Seq peak calling.
The numbers of tags at each base were calculated and normalized to the total tag number for both antibody-precipitated and total input DNA (cellular DNA without immunoprecipitation). Binding peaks were defined as a 10-fold increase in normalized tag count at all bases at any successive 121-bp window, compared to the normalized tag count obtained from input DNA samples at the same position. A cutoff of 10 ChIP tags at each base was used to exclude peaks with very low ChIP tag and low input DNA tag counts (27–29).
Definition of promoter regions and target genes.
We defined 21,035 mouse promoters, corresponding to each RefSeq gene, as the sequences between kb −5 and kb +3 with respect to the annotated transcription start site (TSS), using the mouse mm9 genome build from the RefSeq gene database (http://hgdownload.cse.ucsc.edu/goldenPath/mm9/database/). We first defined target promoters that contained at least one peak in the promoter region. We also used fold enrichment of ChIP tag counts compared to input DNA to calculate the binding level. A 2-fold increase in ChIP tag count compared to input DNA in the region from kb −5 to kb +3 was empirically shown to indicate enrichment of binding. In addition, we excluded promoter regions with very low normalized ChIP tag counts (<4 ppm) (see Fig. S7 in the supplemental material). Thus, promoter regions showing 2-fold-increased ChIP tag counts compared to input DNA and containing more than four normalized ChIP tag counts were defined as binding targets. We therefore took into account both defined peaks of binding for Bmi1, Ezh2, menin, and RNAPII and also extended the areas of increased binding (2-fold increase around TSS). Genes that passed either of these two criteria were selected for further analysis (i.e., target genes).
Compiled tag density profiles.
We compiled tag density profiles by dividing the promoter region into 100-bp bins and counted tag base numbers in each bin. Tag base counts were normalized by total tag counts for representation.
Definition of UD index.
At each gene promoter, the upstream/downstream (UD) index was calculated as follows: UD index = tag count in the 3-kb region downstream of the TSS/tag count in the 8-kb region across the TSS. For comparison purposes, we also used UD indices normalized by input DNA values or those calculated in the region from kb −5 to kb +5 relative to the TSS of each gene.
Model-based analysis of ChIP-Seq (MACS).
For comparison purposes, MACS 1.4.2 software (P value for peak calling set at 0.0001) was also used (30). We selected genes that contained at least one peak in the region between kb −5 and kb +3 of the annotated TSS. Total peak length of each protein (Ezh2 and menin) was calculated by the summation of length of all peaks found in the regions from kb −5 to kb +3 of these selected genes. Ezh2 and menin cooccupied genes were ranked based on total peak length with the ranking determined by the shorter peak of Ezh2 or menin. The correlation coefficient between Ezh2 UD indices and menin UD indices was calculated focusing on the top 50, 100, 150, 200, 250, 300, 350, and 400 cooccupied genes rank ordered as described above (see Fig. 3D).
FIG 3.
ChIP-Seq binding profiles reveal a novel feature of cooccupancy with Ezh2 and menin. (A) Compiled tag density profiles (upper) and heat map representation of binding profiles (lower) across the TSS from the kb −5 and kb +3 flanking regions with 100-bp resolution for Ezh2. The heat map is rank ordered from genes with the highest UD indices to the lowest UD indices. (B) Correlation matrix shows Pearson correlations of UD indices between indicated data sets. Dark and light pink, positive correlation; white, no correlation; light blue, negative correlation. (C) Comparison of UD indices of Ezh2 to those of menin at cooccupied genes. Scatter plots compare Ezh2 UD indices against menin UD indices in ES, B, and T cells. Sectors are demarcated by lines along which the subtraction of the menin UD index from the Ezh2 UD index yields values of ±0.25. (D) Comparison of UD indices of Ezh2 and menin at cooccupied genes defined by MACS peak calling. The cooccupied genes were rank ordered by MACS peak length, and rank-dependent changes in correlation coefficient between Ezh2 UD indices and menin UD indices were examined (see also Materials and Methods). The x axis indicates the number of analyzed genes (e.g., “x = 100” means that top 100 cooccupied genes are used for calculating correlation coefficient indicated in the y axis). P values were calculated by testing for no correlation (*, P < 0.05). (E) Scatter plots compare Ezh2 UD indices against menin UD indices in Th2 cells (left). Red dots indicate genes with increased expression (4-fold) in Ezh2-deficient Th2 cells, and blue dots indicate genes with decreased expression (4-fold) in Ezh2-deficient Th2 cells compared to wild-type Th2 cells. Sectors are demarcated as for panel C. The ratios of the number of genes in the central sector to that in the peripheral sectors are shown on the right.
Microarray data collection and analysis.
Total cellular RNA was extracted with TRIzol reagent (Invitrogen) according to the manufacturer's instructions. RNA was labeled using a 3′ IVT Express kit (Affymetrix) and hybridized to GeneChip Mouse Genome 430 2.0 arrays (Affymetrix) according to the manufacturer's protocols. Expression values were determined with Affymetrix GeneChip Command Console software (AGCC) and Console software (Expression Console). Upregulation or downregulation of Refseq gene mRNA level was defined if at least one Affymetrix-GeneChip probe corresponding to the RefSeq gene showed upregulation or downregulation (4-fold), respectively. The maximum Affymetrix-GeneChip probe data corresponding to each RefSeq gene were used for scatter plots in Fig. 4A and B and in Fig. 6B, C, and E.
FIG 4.
Comparison of mRNA levels with positions of Ezh2/menin binding. (A and B) The DNA microarray signal intensity in ES (A) and T (B) cells is plotted against values resulting from the subtraction of the menin UD index from the Ezh2 UD index. The maximum Affymetrix-GeneChip probe data corresponding to each RefSeq gene was used for scatter plots. All dots corresponding to the genes shown in Fig. 5A to F and Fig. S6J and K in the supplemental material are highlighted. (C and D) Comparison of mRNA levels with positions of Ezh2/menin binding at cooccupied genes defined by MACS peak calling. The cooccupied genes were rank ordered by MACS peak length, and rank-dependent changes in correlation coefficients between mRNA levels (after being log10 transformed) and the subtraction of the menin UD index from the Ezh2 UD index were examined. P values were calculated by testing for no correlation (*, P < 0.05).
FIG 6.
Changes in the binding states of Ezh2 and menin during T cell development from ES cells. (A) Circos visualization of comparison of Ezh2 and menin binding states between ES and T cells (43). The colors of the outer arch indicate Ezh2/menin binding states in ES and T cells. The colors of the inner arch on the right side indicate the original binding states of Ezh2 and menin in ES cells. Links of genes upregulated or downregulated during T cell development are indicated. The green rectangle indicates the region of enlarged view shown in the right panel. (B and C) Comparison of transcription levels (upper) and their binding positioning (lower) between ES and T cells. (B) Genes showing menin mono-occupancy in ES cells and Ezh2 mono-occupancy in T cells were analyzed, and genes downregulated in T cells compared to ES cells were used for the assessment of Ezh2 and menin binding. (C) Genes showing Ezh2 mono-occupancy in ES cells and menin mono-occupancy in T cells were analyzed, and genes upregulated in T cells compared to ES cells were used for the assessment of Ezh2 and menin binding. (D) Percentage of cooccupancy, Ezh2 mono-occupancy, menin mono-occupancy, or null occupancy-derived menin mono-occupied genes in T cells for the category shown on the left side of each bar. (E) Genes showing Ezh2 mono-occupancy in ES cells and Ezh2 and menin cooccupancy in T cells were analyzed, and genes upregulated in T cells compared to ES cells were used for the assessment of Ezh2 and menin binding. (B, C, and E) All dots corresponding to the genes shown in Fig. 5E and Fig. S6D to K in the supplemental material are highlighted.
RNA-Seq.
Total cellular RNA was extracted with TRIzol reagent (Invitrogen). For cDNA library construction, we used TruSeq RNA Sample Prep Kit v2 (Illumina) according to the manufacturer's protocol. Sequencing the library fragments was performed on the HiSeq 1500 or 2500 system. For data analysis, read sequences (50 bp) were aligned to the mm10 mouse reference genome (UCSC, December 2011) using Bowtie (version 0.12.8) and TopHat (version 1.3.2). Fragments per kilobase of exon per million mapped reads (FPKM) for each gene were calculated using Cufflinks (version 2.0.2). Genes with absolute an FPKM of >1 (mean from duplicate samples) were defined as expressed genes (see Fig. 2C to E). Differentially regulated genes were selected according to the following criteria: (i) an absolute FPKM of >1 in at least one condition (Th2 and TSA-treated Th2) and (ii) an expression change of >+2 or <−2 (see Fig. 7).
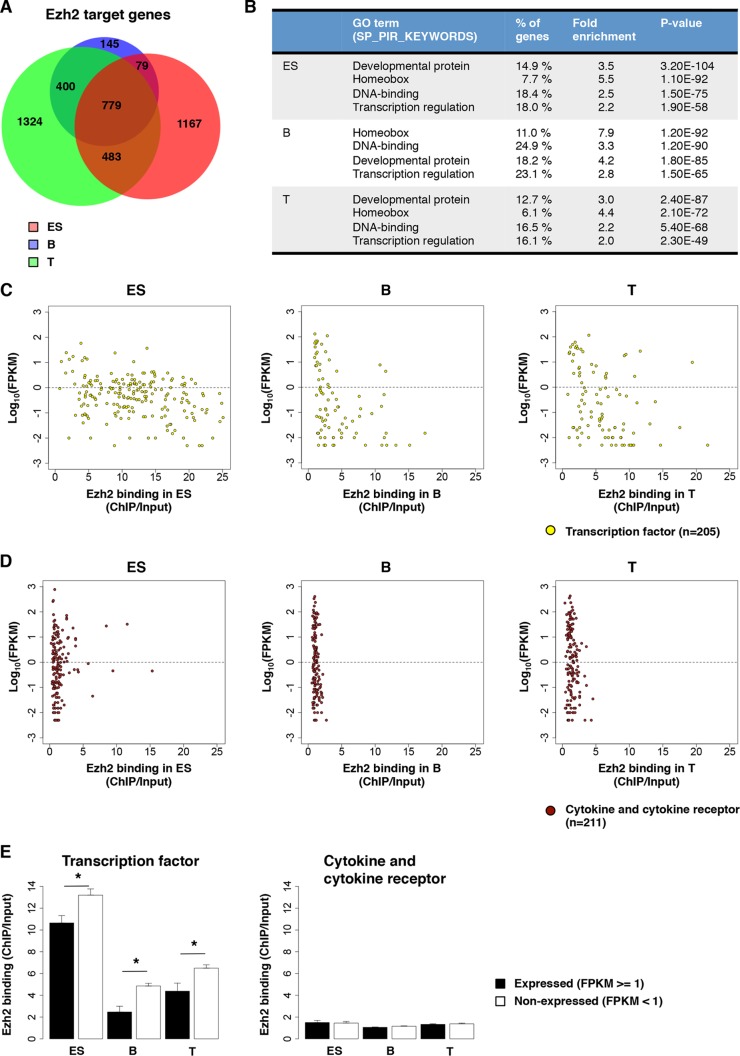
FIG 2.
Conserved signatures of PcG occupancy between ES cells and lymphocytes. (A) A Venn diagram shows the numbers of cell-type-specific and non-cell-type-specific Ezh2 target genes. (B) GO categories overrepresented in Ezh2-positive gene sets in ES, B, and T cells. (C and D) mRNA levels are plotted against levels of Ezh2 binding at 205 genes encoding transcription factors that are identified as PcG quadruple-positive genes in ES cells (6) (C) or at 211 cytokine and cytokine receptor genes (D). (E) The mean levels of Ezh2 binding at the expressed and nonexpressed genes are shown. Error bars indicate the standard errors of mean. P values were calculated by the Welch two-sample t test (*, P < 0.05).
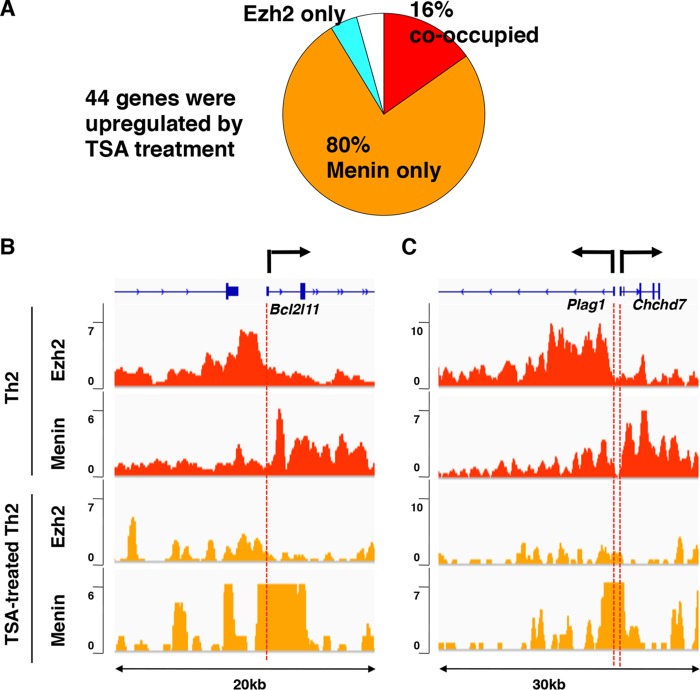
FIG 7.
Disruption of Ezh2/menin cooccupancy by trichostatin A (TSA). (A) TSA treatment upregulated 44 of 230 cooccupied genes in Th2 cells. A pie chart illustrates the frequency of Ezh2 and menin cooccupancy and mono-occupancy in these 44 genes. (B and C) Binding of Ezh2 and menin at representative loci in Th2 and TSA-treated Th2 cells. ChIP-Seq profiles are shown across two loci (chromosome 4, 3875000 to 3845000 [B]; chromosome 2, 127940000 to 127960000 [C]).
GO analysis.
Gene ontology (GO) functional annotation for Ezh2, Bmi1, and menin target genes was performed using the DAVID analysis tool (https://david.ncifcrf.gov).
Statistics.
Welch's s t test was used to compare Ezh2 binding levels at the expressed genes to those at the nonexpressed genes. The Pearson correlation coefficient was used to measure the correlation between two samples, and P values were calculated by test for no correlation. The Fisher exact test was used to analyze 2×2 contingency tables.
GEO database accession numbers.
The ChIP-Seq data sets of Bmi1, Ezh2, menin, and RNAPII, and the microarray data for the ES, B, and T cells are available in the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo) under accession number GSE72757. The ChIP-Seq data set for Ezh2 and the microarray data for WT Th2 and Ezh2 KO Th2 cells are available in the GEO database under accession numbers GSE51079 and GSE50729. For comparison purposes, Ezh2Ref (GSE23943), Dpy30 (GSE26136), and histone modification (GSE23943) data sets are available from the GEO database.
RESULTS
Genome-wide comparison of Ezh2 and menin binding between ES cells and B and T lymphocytes.
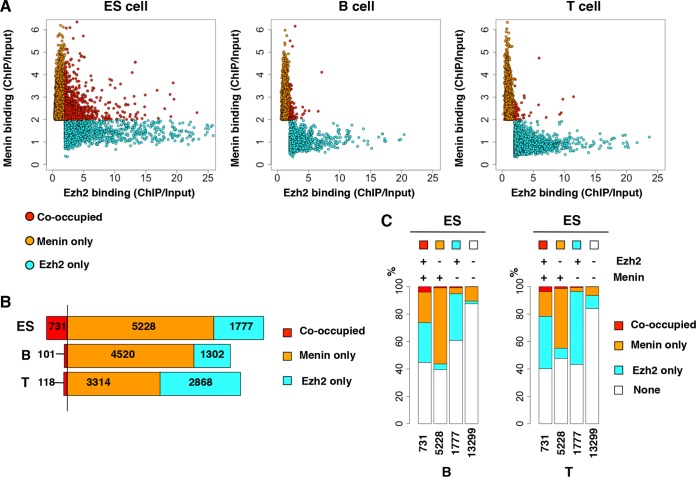
We first assessed the genome-wide binding pattern of Ezh2, a central member of PRC2 and menin, a critical component of the TrxG complex, using ES cells and B220+ B and CD4+ T lymphocytes by chromatin immunoprecipitation coupled with high-throughput DNA sequencing (ChIP-Seq) (24) (see Table S1 in the supplemental material). To identify target genes, we first called peaks by an established method whose validity is verified by using Poisson probabilities (27–29). We identified 8,148 and 2,352 peaks for Ezh2 and menin, respectively, in T cells. However, manual inspection of peaks using the IGV browser revealed a considerable number of genes that exhibited strong ChIP signals were classified as “peak-less” genes (e.g., Cd69, Cd28, Stat3, Nfkb1, Tox, etc., for menin). Based on these observations and the fact that this peak calling algorism is optimized for “sharp peaks,” a simple fold enrichment value (ChIP/input DNA) was taken into consideration (31, 32), and target genes were defined as described in Materials and Methods. We focused on the region from kb −5 to kb +3 relative to the TSS of each gene (23, 33, 34). In addition, our previous analysis of the Gata3 gene showed that this region contained both Ezh2 and menin peaks (14). As shown in Fig. 1A, this analysis defined a clear reciprocal pattern of binding between Ezh2 and menin in both ES cells and B and T lymphocytes. In addition, a considerable number of genes (n = 731) were cooccupied by both Ezh2 and menin in ES cells, and cooccupancy was less frequent (101 in B, 118 in T cells) in lymphocytes (Fig. 1B). In B and T lymphocytes, Ezh2 and menin cooccupancy was preserved at only a few percent (∼4%) of the genes that were cooccupied in ES cells (see leftmost bars in the B and T cell panels in Fig. 1C). Of the cooccupied genes in ES cells, approximately 40 and 20% of these genes showed Ezh2 or menin mono-occupancy in lymphocytes, respectively, and approximately half of the cooccupied genes lost both Ezh2 and menin in lymphocytes (45% in B cells and 40% in T cells). Mono-occupied genes in ES cells tended to either lose Ezh2 or menin binding or preserve their original binding characteristics in lymphocytes (the second and third bars in the B cell and T cell panels in Fig. 1C). Only about 10% of genes that were not bound by either Ezh2 or menin in ES cells acquired either Ezh2 or menin binding in lymphocytes. These genome-wide analyses revealed that (i) a reciprocal binding pattern of Ezh2 and menin is evident, (ii) cooccupancy occurs far more frequently in ES cells and tends to disappear during development into lymphocytes, and (iii) the exchange from Ezh2 single occupancy to menin single occupancy only rarely occurred and vice versa. Similar results were obtained in the analysis of Bmi1, a central member of PRC1 (see Fig. S1 in the supplemental material).
FIG 1.
Genome-wide comparison of Ezh2-menin cooccupancy between ES cells and lymphocytes. (A) Comparison of Ezh2 and menin binding in ES cells (left), B cells (middle), and T cells (right). Of all target genes shown in panel B, genes with >2-fold enrichment (ChIP/Input DNA) in Ezh2 and/or menin binding were used for the depiction. (B) Bar graph indicating the frequency of Ezh2 and menin cooccupancy and mono-occupancy. (C) Cooccupied, mono-occupied, and unbound gene groups in ES cells are compared for relative percentages of Ezh2 and menin occupancy in B cells (left) and T cells (right). Ezh2 and menin binding states at the ES cell stage are shown above the bars.
Conserved signatures of PcG occupancy between ES cells and B and T lymphocytes.
A considerable percentage of the Ezh2 target genes were shared by ES cells and lymphocytes (61% in B cells and 42% in T cells) (Fig. 2A). In addition, the intensity of Ezh2 binding appeared to be relatively higher for the genes that showed Ezh2 binding in ES cells and also in B and T cells (see Fig. S2A and B in the supplemental material). GO analyses in lymphocytes revealed that the Ezh2 targets contained a marked enrichment of genes encoding developmental proteins, including members of the Hox family (Fig. 2B). As expected, we found similar groups of genes enriched for targeting by Ezh2 in ES cells, an observation in agreement with previously reported findings (6, 35), and comparable results were obtained for the PRC1 protein Bmi1 (see Fig. S2C and D in the supplemental material). We next compared Ezh2 binding levels in ES cells and lymphocytes at the Polycomb targeted transcription factor (TF) genes (6) (Fig. 2C; see Table S2 in the supplemental material) with all known cytokine and cytokine receptor genes, whose expression is important for normal lymphocyte effector function (Fig. 2D; also see Table S2 in the supplemental material). In each cell type analyzed, TF genes showed much stronger Ezh2 binding compared to cytokine and cytokine receptor genes (Fig. 2C and D) (mean values = 12.309 versus 1.467 in ES cells, 4.552 versus 1.128 in B cells, and 6.175 versus 1.356 in T cells). In ES cells, 35% of the TF genes showed mRNA expression (FPKM values of ≥1). Ezh2 binding levels were lower for these expressed genes than for the nonexpressed genes (Fig. 2E). In B and T lymphocytes, comparatively fewer TF genes (13 and 15% for B and T cells, respectively) showed mRNA expression, and at these loci the Ezh2 binding levels were lower than for the nonexpressed genes, indicating that Ezh2-mediated repression was involved in these TF gene mRNA expression. In contrast, a considerable number of cytokine and cytokine receptor genes exhibited mRNA expression (36, 32, and 39% for ES, B, and T cells, respectively). Ezh2 binding levels were low for almost all cytokine and cytokine receptor genes, indicating that Ezh2 was not involved in the direct repression of these gene (Fig. 2E). Again, similar results were obtained for Bmi1 (see Fig. S2G and H in the supplemental material). These results indicate that the PcG complex favors genes encoding transcription factors and that this bias is conserved between ES cells and lymphocytes. In contrast, no functional bias was observed in the type of genes that menin targets (see Fig. S2E to H in the supplemental material).
ChIP-Seq binding profiles of Ezh2 and/or menin in ES cells and B and T lymphocytes.
To investigate the nature of Ezh2 binding in more detail, we performed assessment of the localization of Ezh2 binding at its target genes by generating tag density profiles relative the annotated TSS of each Ezh2 target gene in ES cells and B and T lymphocytes (Fig. 3A, upper row) (32, 36). Ezh2 bound broadly across the gene promoters with peaks either side of the TSS and an apparent exclusion of Ezh2 binding from the TSS itself. The clear definition of Ezh2 binding on either side of the TSS prompted us to measure the proportion of tags upstream and downstream of the TSS, termed an “UD index,” in which the proportion of tag counts downstream of TSS is displayed numerically (see Materials and Methods). Heat map displays (37) based on the UD index revealed large variations in the positioning of Ezh2 binding relative to the TSS (Fig. 3A, lower row). Some Ezh2 target genes showed strong binding at regions downstream of the TSS, whereas others displayed strong binding only at regions upstream of the TSS. Menin also showed similar large variation in positioning relative to the TSS (see Fig. S3A in the supplemental material). Next, we compared UD indices for cooccupied genes for at all combinations of Bmi1, Ezh2, and menin and also the RNA polymerase II complex (RNAPII) that composes the TrxG complex. The correlation coefficients for each pair of molecules in ES, B, and T cells are represented in the correlation matrix shown in Fig. 3B (36). This allowed us to assess similarities in the positioning of these regulators of chromatin and gene expression in ES, B, and T cells. The position of binding relative to the TSS was highly conserved between ES cells and B and T lymphocytes for any one particular molecule (Fig. 3B). Moreover, the positions of the PcG components, Bmi1 (PRC1) and Ezh2 (PRC2), were also highly conserved, indicating that these two PcG proteins possess similar positioning patterns in these three cell types (Fig. 3B, upper left). Likewise, the two molecules associated with gene activation, menin and RNAPII, also displayed comparatively strong conservation (Fig. 3B, lower right).
Developmental change in the positioning of Ezh2 and menin between ES and T cells.
Interestingly, when we compared the correlation coefficients of Ezh2 and menin between ES cells and T cells, we found that the positioning patterns of Ezh2 and menin were relatively similar in ES cells (0.351) but different in T cells (−0.263) (yellow-outlined boxes in Fig. 3B). To visualize this finding more precisely, we used scatter plots comparing Ezh2 UD indices versus menin UD indices at the cooccupied genes (731, 101, and 118 genes in ES, B, and T cells, respectively) (Fig. 3C). In ES cells, UD indices of Ezh2 and menin were positively correlated (R = 0.351, P < 2.2E–16), i.e., frequently found in a similar position relative to the annotated TSS (i.e., 88.9% of the genes showed subtraction values of −2.5 to +2.5) (Fig. 3C, left), whereas they were negatively correlated (R = −0.263, P value = 0.004), i.e., frequently found in discrete positions either side of the annotated TSS in T cells (20.3 + 11.9 = 32.2%) (Fig. 3C, right). The redistribution of Ezh2 and menin relative to the TSS of cooccupied genes in B cells was not as obvious at that observed for T cells (R = 0.056) (Fig. 3C, middle). We also confirmed these results by using another approach. We performed model-based analysis of ChIP-Seq (MACS) and detected 1,062, 139, and 390 cooccupied genes in ES, B, and T cells, respectively (30). Next, we rank ordered the cooccupied genes by MACS peak length to examine rank-dependent changes in correlation coefficient between Ezh2 UD indices and menin UD indices (38). In ES cells, a positive correlation between Ezh2 UD indices and menin UD indices was observed in a rank-independent manner (Fig. 3D). In T cells, the negative correlation become more evident when focusing on the top 50, 100, 150, and 200 cooccupied genes. When we used UD indices normalized by input DNA values or those calculated in the region from kb −5 to kb +5 relative to the TSS of each gene, similar results were obtained (see Fig. S3B in the supplemental material). Furthermore, these results were reproducible in another independent experiment (see Fig. S4 in the supplemental material). We also analyzed Ezh2 (GSE23943) and Dpy30 (GSE26136) ChIP-Seq data sets downloaded from GEO and found that the UD indices of Ezh2 and Dpy30, a component of TrxG complex, were positively correlated at 731 cooccupied genes in ES cells (R = 0.423, P < 2.2E–16). A clear negative correlation between Ezh2 and menin positioning (UD indices) was also observed in Th2 cells, a functional CD4+ T helper cell subset (R = −0.409, P = 1.1E–10) (Fig. 3E). Next, we compared the expression levels of each cooccupied gene in wild-type and Ezh2-deficient Th2 cells, and genes with increased mRNA levels in Ezh2-deficient Th2 cells are marked in the UD index scatter plots (Fig. 3E). Most of the Ezh2 and menin cooccupied genes (16 of 17 genes) that were upregulated in Ezh2-deficient cells were located in the central sector (depicted by red dots in Fig. 3E). These results indicate that cooccupied genes in which Ezh2 and menin bound at the same position in Th2 cells were highly sensitive to loss of Ezh2.
Connection between gene expression and the position of binding of Ezh2 and menin relative to the TSS.
Next, in order to identify a functional link between mRNA levels and Ezh2 and menin positioning, we counterplotted absolute gene expression levels against the subtraction of menin UD index from Ezh2 UD index in ES and T cells (see the scheme in Fig. S5A in the supplemental material). In ES cells, a weak inverse correlation was observed (R = −0.244, P = 4.1E–11) (Fig. 4A), indicating that the binding position of Ezh2 and menin had little effect on mRNA levels in ES cells. A list of cooccupied genes in ES cells (see Table S3 in the supplemental material) and actual binding patterns of Ezh2 and menin at some examples (Kcnc2, Fam184b, Gli2, and Tox) are shown in Fig. 5A to D. These genes had varied levels of expression and similar binding positioning for Ezh2 and menin, a finding typical of the cooccupied genes in ES cells (Fig. 4A). In contrast, in T cells, we found that mRNA levels displayed a strong negative correlation with the subtraction values of menin UD index from Ezh2 UD index (R = −0.490, P = 2.7E–8) (Fig. 4B). This was also true in B and Th2 cells (see Fig. S5B in the supplemental material). A strong negative correlation between mRNA levels and the subtraction values of menin UD index from Ezh2 UD index in T cells was confirmed in the cooccupied genes identified by MACS (Fig. 5C and D). These results indicate that the genes where Ezh2 binding tended to be upstream and menin binding tended to be downstream of the TSS (see Fig. S5A, left) had higher mRNA levels, and the genes with Ezh2-binding at the downstream region and menin binding at the upstream region in relation to the TSS had lower mRNA levels (see Fig. S5A, right, in the supplemental material). Two typical examples of cooccupied genes in T cells (Gata3 and Rab30) are shown in Fig. 5E and F. These results indicate that positioning of Ezh2 and menin at cooccupied genes appear to control both sensitivity to the presence of Ezh2 and the overall transcriptional state in T cells.
FIG 5.
Ezh2 and menin binding profiles at genes showing examples of cooccupancy in ES or T cells. Binding of Ezh2, Dpy30, and menin and modifications of histone H3K27me3 and H3K4me3 at representative loci in ES cells (pink) and T cells (green) are shown. ChIP-Seq profiles are shown across six loci (chromosome 10, 111650000 to 111750000 [A]; chromosome 5, 46000000 to 46050000 [B]; chromosome 1, 120900000 to 121000000 [C]; chromosome 4, 6873211 to 6950000 [D]; chromosome 2, 9750000 to 9850000 [E]; chromosome 7, 99850000 to 99950000 [F]). Ezh2Ref (GSE23943), Dpy30 (GSE26136), and histone modification (GSE23943) data sets were obtained from the GEO database. For the visualization of binding, data sets from GSE23943 underwent the same data processing as the data sets of the present study, as described in Materials and Methods. The data sets from GSE26136 were used without data processing. The Gata3 gene showed a low UD index for Ezh2 and a high UD index for menin in T cells (Ezh2, 0.109; menin, 0.501) and was highly transcribed (E) (Fig. 4B). The binding region of Ezh2 and menin around the Gata3 TSS region does not overlap, an observation consistent with our previous findings (14). The Rab30 gene was expressed at low levels, and Ezh2 bound mainly downstream of the TSS with menin binding mainly upstream of the TSS (F) (Fig. 4B).
Changes in the binding states of Ezh2 and menin during T cell development, and association with T cell function.
Finally, we analyzed the relationship between Ezh2 and menin binding states (cooccupancy or mono-occupancy) and up- or downregulation of mRNA levels between T lymphocytes and ES cells (CIRCOS visualization [Fig. 6A; see also Fig. S6A and Table S4 in the supplemental material]). This analysis allowed visualization of several important facets of Ezh2 and menin function in lymphocytes. In agreement with the role of Ezh2 as a positive regulator of genetic repression, the majority of genes bound only by Ezh2 in T cells showed lower levels of mRNA in T cells than ES cells (Fig. 6A, blue links; see also the third row in Fig. S6A in the supplemental material). In addition, these downregulated genes displayed a strong functional bias for genes associated with embryonic morphogenesis and development (see Fig. S6B in the supplemental material) that was largely independent of the Ezh2/menin binding states in ES cells. In T cells, mRNA levels of genes bound only by menin in ES cells and only by Ezh2 in T cells was most frequently decreased (170/387) (Fig. 6A, left, arrow B, and Fig. 6B, upper). The Ezh2 UD index in T cells was positively correlated with the menin UD index in ES cells (R = 0.341, P = 5.3E–6), indicating that relative to the TSS, Ezh2 binding in T cells was frequently found at a similar position as menin in ES cells (Fig. 6B, lower).
The majority of genes bound only by menin in T cells showed higher mRNA levels in T cells compared to ES cells (Fig. 6A, pink links; see also the second row in Fig. S6A in the supplemental material). Most of the genes bound only by Ezh2 in ES cells and only by menin in T cells were upregulated (40/52) (Fig. 6A, arrow C, and Fig. 6C, upper). The menin UD index in T cells was positively correlated with the Ezh2 UD index in ES cells (R = 0.313, P = 0.049), suggesting that the position of menin binding in T cells was similar to the Ezh2 binding position in ES cells relative to the TSS (Fig. 6C, lower).
Interestingly, in contrast to the case for Ezh2 binding in T cells, the functional biases found for menin bound genes in T cells were highly dependent on the Ezh2/menin binding states in ES cells (Fig. 6D; see Fig. S6C in the supplemental material). Genes that were bound by neither Ezh2 nor menin in ES cells contained a large number of genes broadly involved in regulation of immune responses (P = 5.9E–17), whereas genes with menin mono-occupancy (menin only) in both ES and T cells displayed a functional bias for genes encoding chromatin regulators (P = 3.7E–11). Cooccupancy-derived genes were enriched for transcription factors (P = 3.6E–7) that included a set of genes essential for T cell development, including Bcl11b, Bach2, Ikzf1, Ikzf2, Satb1, and Tox. In addition, although the overall number was relatively small, Ezh2 mono-occupancy-derived genes showed strong enrichment for genes associated with intracellular signaling (P = 4.3E–3).
Finally, we analyzed the genes that were cooccupied by Ezh2 and menin in T cells (see the first row in Fig. S6A in the supplemental material). Of the genes cooccupied in T cells and menin mono-occupied in ES cells, 9 were upregulated and 10 were downregulated (see Fig. S6A in the supplemental material). Genes cooccupied in both ES and T cells were more often upregulated (11/26) than downregulated (1/26) in T cells compared to ES cells (see Fig. S6A in the supplemental material). However, in these groups, no significant tendency was found regarding the positioning of Ezh2 or menin relative to the TSS. In contrast, of the 11 genes cooccupied in T cells, and Ezh2 mono-occupied in ES cells, 9 were expressed more strongly in T cells (Fig. 6A, right, arrow E, and Fig. 6E, upper), moreover, all of these upregulated genes displayed a lower Ezh2 UD index in T cells than ES cells, indicating that Ezh2 binding position had shifted upstream relative to the TSS during development from ES cells into T cells (Fig. 6E, lower). This group also includes essential T cell-related transcriptional regulators such as Gata3, Fli1, Nfatc1, Gfi1, and Bcl11a. The binding patterns of Ezh2 and menin at the genes indicated above are shown in Fig. 5E and F (also see Fig. S6D to K).
A genome-wide comparison between ES cells and T cells argued that physiological changes in the binding states of Ezh2 and menin during T cell development from ES cells were functionally associated with changes in transcriptional states. To explore the biological relevance of Ezh2/menin cooccupancy in a given cell type, we next examined whether experimental alternation of occupancy of Ezh2 may alter transcriptional at the cooccupied genes. We used trichostatin A (TSA) to alter Ezh2 binding because TSA treatment was reported to reduce PcG protein binding levels at several gene loci (14, 39). TSA treatment upregulated 44 of 230 cooccupied genes in Th2 cells. A total of 80% of these upregulated genes showed loss of Ezh2 binding, indicating that disruption of Ezh2/menin cooccupancy by TSA relives Ezh2-dependent gene silencing (Fig. 7). Thus, Ezh2/menin cooccupancy is fundamental for maintaining the transcriptional states at target genes.
Our analysis of co- and mono-occupancy characteristics of members of the PcG and TrxG chromatin regulator complexes identified differences in the functional biases of differentially regulated genes, depending on the combinations of Ezh2 and menin binding in ES cells and T cells. We propose that the positioning of Ezh2 and menin may control both sensitivity to the presence of chromatin regulators and also the overall transcriptional state.
DISCUSSION
We have here characterized the binding position of Ezh2 and menin at all annotated genes in ES cells and B and T lymphocytes. Our data define a clear reciprocal pattern of binding between Ezh2 and menin in these three cell types. We also demonstrate a dynamic developmental change in the positioning of Ezh2 and menin in differentiated T lymphocytes compared to ES cells at their cooccupied genes. Interestingly, different combinations of mono- or cooccupancy of Ezh2 and menin during development into T lymphocytes appear to regulate expression of different functional groupings of genes in T cells.
Our data indicate that the biological consequences of Ezh2-menin cooccupancy may be different between multipotent ES cells and differentiated T cells. In ES cells, Ezh2-menin cooccupancy is generally found at poised genes (40, 41). PcG proteins repress expression of these genes so that they are not activated without specific developmental cues (6), whereas TrxG proteins are required for immediate activation of these poised genes after receiving signals for differentiation (8). The present study identifies several previously unappreciated characteristics of these poised genes. In ES cells the positioning of Ezh2 and menin were relatively similar, and deficiency of PcG proteins in these cells often cause derepression of poised genes, including transcription factors important for normal tissue development (6). In contrast, in T cells, Ezh2-menin cooccupied genes exhibited variations in both the positioning of Ezh2 and menin and their transcriptional states. Among them, genes where Ezh2 and menin bound at the same position had similar characteristics as those in ES cells, i.e., sensitivity to loss of Ezh2. However, cooccupied genes where Ezh2 and menin were found at discrete positions on either side of TSS had defined characteristics in differentiated lymphocytes. Genes with Ezh2 binding upstream and menin binding downstream of the TSS showed higher mRNA levels (active cooccupied genes), and genes with Ezh2-binding downstream and menin binding upstream showed lower expression (silent cooccupied genes) in T lymphocytes. In active cooccupied genes, menin is likely acting as a positive regulator of transcription; however, the role of Ezh2 at these active cooccupied genes is currently unknown. We postulate that Ezh2 may serve as a regulator of expression at cooccupied genes where expression is essential while requiring tight control. A typical example of an active cooccupied gene is Gata3. Consistent with our previous report (14), the Gata3 gene exhibits Ezh2-binding upstream and menin binding downstream of the TSS. Since basal levels of Gata3 expression are required for CD4+ T cell development and survival (42), menin binding to the Gata3 gene may allow positive regulation of its transcription, while Ezh2 enables restriction of Gata3 expression. In differentiated CD4 T lymphocytes Ezh2 deficiency results in enhanced expression of Gata3 and hyperproduction of Th2 cytokines (12). Thus, Ezh2-menin cooccupancy at the Gata3 gene likely regulates both adequate expression for T cell development while maintaining multipotency of CD4+ T cells for differentiation into effector helper T cell subsets.
Our analysis also defined novel functional biases regarding Ezh2 and menin-mediated gene regulation. Menin was found at immune response genes that were active in T cells. In contrast, Ezh2 was not detected at genes encoding cytokines, cytokine receptors or other immune-related molecules that are silent in ES and B cells. For example, the Cd4, Cd28, and Cd247 (encoding CD3 zeta chain) genes were highly expressed and showed menin mono-occupancy in T cells. However, these genes showed low-level expression and low-level binding of Ezh2 in ES and B cells. Instead, Ezh2 binding was detected at many genes encoding transcription factors. These results indicate that Ezh2 indirectly regulates several genes, including immune-related genes via controlling expression of the upstream transcription factors. In T cells, Ezh2 binding was also found at many transcription factor genes that are important for nonimmune systems, and while Ezh2 appears indispensable for repression of these transcription factor genes during development, it was largely dispensable in differentiated cells. Our data indicate that in differentiated cells, the majority of Ezh2 target genes are insensitive to Ezh2-deficiency in the absence of specific activating signals.
In summary, we have identified a novel mechanism of gene regulation that is dependent on the spatial interplay between members of the PcG and TrxG complexes. We propose that the positioning of these chromatin regulators is an important determinant of their function at cooccupied genes during cellular development. We expect our data set to serve as a resource for the study of epigenetic regulatory mechanisms in ES cells and B and T lymphocytes. Further analysis of Ezh2 and/or menin target genes identified in the present study will provide important insight for understanding lymphocyte development and immune responses of B and T lymphocytes.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to John J. O'Shea, Yuka Kanno, and Golnaz Vahedi for their helpful comments and constructive criticisms in the preparation of the manuscript. We thank Atsushi Iwama and Satoru Miyagi for excellent experimental suggestions.
This study was supported by the Global COE Program (Global Center for Education and Research in Immune System Regulation and Treatment) and by grants from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT Japan; Grants-in-Aid for Scientific Research [S, 26221305; C, 24592083 and 15K08522], for Young Scientists [B, 23790523 and 25860351], and for Scientific Research on Innovative Areas [Genome Science, 221S0002]), by the Ministry of Health, Labor, and Welfare, by the Uehara Memorial Foundation, by the Princess Takamatsu Cancer Research Fund, and by the Takeda Science Foundation. D.J.T. was supported by a Japanese Society for the Promotion of Science postdoctoral fellowship (grant 2109747).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00677-15.
REFERENCES
- 1.Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. 2007. Genome regulation by polycomb and trithorax proteins. Cell 128:735–745. doi: 10.1016/j.cell.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Schuettengruber B, Martinez AM, Iovino N, Cavalli G. 2011. Trithorax group proteins: switching genes on and keeping them active. Nat Rev Mol Cell Biol 12:799–814. doi: 10.1038/nrm3230. [DOI] [PubMed] [Google Scholar]
- 3.Nakayama T, Yamashita M. 2009. Critical role of the Polycomb and Trithorax complexes in the maintenance of CD4 T cell memory. Semin Immunol 21:78–83. doi: 10.1016/j.smim.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Margueron R, Reinberg D. 2011. The Polycomb complex PRC2 and its mark in life. Nature 469:343–349. doi: 10.1038/nature09784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onodera A, Nakayama T. 2015. Epigenetics of T cells regulated by Polycomb/Trithorax molecules. Trends Mol Med 21:330–340. doi: 10.1016/j.molmed.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, Bell GW, Otte AP, Vidal M, Gifford DK, Young RA, Jaenisch R. 2006. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441:349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- 7.Ang YS, Tsai SY, Lee DF, Monk J, Su J, Ratnakumar K, Ding J, Ge Y, Darr H, Chang B, Wang J, Rendl M, Bernstein E, Schaniel C, Lemischka IR. 2011. Wdr5 mediates self-renewal and reprogramming via the embryonic stem cell core transcriptional network. Cell 145:183–197. doi: 10.1016/j.cell.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang H, Shukla A, Wang X, Chen WY, Bernstein BE, Roeder RG. 2011. Role for Dpy-30 in ES cell-fate specification by regulation of H3K4 methylation within bivalent domains. Cell 144:513–525. doi: 10.1016/j.cell.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hopkin AS, Gordon W, Klein RH, Espitia F, Daily K, Zeller M, Baldi P, Andersen B. 2012. GRHL3/GET1 and Trithorax group members collaborate to activate the epidermal progenitor differentiation program. PLoS Genet 8:e1002829. doi: 10.1371/journal.pgen.1002829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Hiller M, Sancak Y, Fuller MT. 2005. Tissue-specific TAFs counteract Polycomb to turn on terminal differentiation. Science 310:869–872. doi: 10.1126/science.1118101. [DOI] [PubMed] [Google Scholar]
- 11.Mousavi K, Zare H, Wang AH, Sartorelli V. 2012. Polycomb protein Ezh1 promotes RNA polymerase II elongation. Mol Cell 45:255–262. doi: 10.1016/j.molcel.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tumes DJ, Onodera A, Suzuki A, Shinoda K, Endo Y, Iwamura C, Hosokawa H, Koseki H, Tokoyoda K, Suzuki Y, Motohashi S, Nakayama T. 2013. The Polycomb protein Ezh2 regulates differentiation and plasticity of CD4+ T helper type 1 and type 2 cells. Immunity 39:819–832. doi: 10.1016/j.immuni.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita M, Hirahara K, Shinnakasu R, Hosokawa H, Norikane S, Kimura MY, Hasegawa A, Nakayama T. 2006. Crucial role of MLL for the maintenance of memory T helper type 2 cell responses. Immunity 24:611–622. doi: 10.1016/j.immuni.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Onodera A, Yamashita M, Endo Y, Kuwahara M, Tofukuji S, Hosokawa H, Kanai A, Suzuki Y, Nakayama T. 2010. STAT6-mediated displacement of polycomb by trithorax complex establishes long-term maintenance of GATA3 expression in T helper type 2 cells. J Exp Med 207:2493–2506. doi: 10.1084/jem.20100760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mills AA. 2010. Throwing the cancer switch: reciprocal roles of polycomb and trithorax proteins. Nat Rev Cancer 10:669–682. doi: 10.1038/nrc2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz YB, Pirrotta V. 2013. A new world of Polycombs: unexpected partnerships and emerging functions. Nat Rev Genet 14:853–864. doi: 10.1038/nrg3603. [DOI] [PubMed] [Google Scholar]
- 17.Caganova M, Carrisi C, Varano G, Mainoldi F, Zanardi F, Germain PL, George L, Alberghini F, Ferrarini L, Talukder AK, Ponzoni M, Testa G, Nojima T, Doglioni C, Kitamura D, Toellner KM, Su IH, Casola S. 2013. Germinal center dysregulation by histone methyltransferase EZH2 promotes lymphomagenesis. J Clin Invest 123:5009–5022. doi: 10.1172/JCI70626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beguelin W, Popovic R, Teater M, Jiang Y, Bunting KL, Rosen M, Shen H, Yang SN, Wang L, Ezponda T, Martinez-Garcia E, Zhang H, Zheng Y, Verma SK, McCabe MT, Ott HM, Van Aller GS, Kruger RG, Liu Y, McHugh CF, Scott DW, Chung YR, Kelleher N, Shaknovich R, Creasy CL, Gascoyne RD, Wong KK, Cerchietti L, Levine RL, Abdel-Wahab O, Licht JD, Elemento O, Melnick AM. 2013. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell 23:677–692. doi: 10.1016/j.ccr.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes CM, Rozenblatt-Rosen O, Milne TA, Copeland TD, Levine SS, Lee JC, Hayes DN, Shanmugam KS, Bhattacharjee A, Biondi CA, Kay GF, Hayward NK, Hess JL, Meyerson M. 2004. Menin associates with a trithorax family histone methyltransferase complex and with the hoxc8 locus. Mol Cell 13:587–597. doi: 10.1016/S1097-2765(04)00081-4. [DOI] [PubMed] [Google Scholar]
- 20.Matkar S, Thiel A, Hua X. 2013. Menin: a scaffold protein that controls gene expression and cell signaling. Trends Biochem Sci 38:394–402. doi: 10.1016/j.tibs.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balogh K, Racz K, Patocs A, Hunyady L. 2006. Menin and its interacting proteins: elucidation of menin function. Trends Endocrinol Metab 17:357–364. doi: 10.1016/j.tem.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Nakata Y, Brignier AC, Jin S, Shen Y, Rudnick SI, Sugita M, Gewirtz AM. 2010. c-Myb, Menin, GATA-3, and MLL form a dynamic transcription complex that plays a pivotal role in human T helper type 2 cell development. Blood 116:1280–1290. doi: 10.1182/blood-2009-05-223255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang JA, Mortazavi A, Williams BA, Wold BJ, Rothenberg EV. 2012. Dynamic transformations of genome-wide epigenetic marking and transcriptional control establish T cell identity. Cell 149:467–482. doi: 10.1016/j.cell.2012.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. 2007. High-resolution profiling of histone methylations in the human genome. Cell 129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Hirabayashi Y, Suzki N, Tsuboi M, Endo TA, Toyoda T, Shinga J, Koseki H, Vidal M, Gotoh Y. 2009. Polycomb limits the neurogenic competence of neural precursor cells to promote astrogenic fate transition. Neuron 63:600–613. doi: 10.1016/j.neuron.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 26.Tanimoto Y, Iijima S, Hasegawa Y, Suzuki Y, Daitoku Y, Mizuno S, Ishige T, Kudo T, Takahashi S, Kunita S, Sugiyama F, Yagami K. 2008. Embryonic stem cells derived from C57BL/6J and C57BL/6N mice. Comp Med 58:347–352. [PMC free article] [PubMed] [Google Scholar]
- 27.Tanimoto K, Tsuchihara K, Kanai A, Arauchi T, Esumi H, Suzuki Y, Sugano S. 2010. Genome-wide identification and annotation of HIF-1alpha binding sites in two cell lines using massively parallel sequencing. Hugo J 4:35–48. doi: 10.1007/s11568-011-9150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanai A, Suzuki K, Tanimoto K, Mizushima-Sugano J, Suzuki Y, Sugano S. 2011. Characterization of STAT6 target genes in human B cells and lung epithelial cells. DNA Res 18:379–392. doi: 10.1093/dnares/dsr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robertson G, Hirst M, Bainbridge M, Bilenky M, Zhao Y, Zeng T, Euskirchen G, Bernier B, Varhol R, Delaney A, Thiessen N, Griffith OL, He A, Marra M, Snyder M, Jones S. 2007. Genome-wide profiles of STAT1 DNA association using chromatin immunoprecipitation and massively parallel sequencing. Nat Methods 4:651–657. doi: 10.1038/nmeth1068. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, Liu XS. 2008. Model-based analysis of ChIP-Seq (MACS). Genome Biol 9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muto T, Sashida G, Oshima M, Wendt GR, Mochizuki-Kashio M, Nagata Y, Sanada M, Miyagi S, Saraya A, Kamio A, Nagae G, Nakaseko C, Yokote K, Shimoda K, Koseki H, Suzuki Y, Sugano S, Aburatani H, Ogawa S, Iwama A. 2013. Concurrent loss of Ezh2 and Tet2 cooperates in the pathogenesis of myelodysplastic disorders. J Exp Med 210:2627–2639. doi: 10.1084/jem.20131144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horiuchi S, Onodera A, Hosokawa H, Watanabe Y, Tanaka T, Sugano S, Suzuki Y, Nakayama T. 2011. Genome-wide analysis reveals unique regulation of transcription of Th2-specific genes by GATA3. J Immunol 186:6378–6389. doi: 10.4049/jimmunol.1100179. [DOI] [PubMed] [Google Scholar]
- 33.Endoh M, Endo TA, Endoh T, Fujimura Y, Ohara O, Toyoda T, Otte AP, Okano M, Brockdorff N, Vidal M, Koseki H. 2008. Polycomb group proteins Ring1A/B are functionally linked to the core transcriptional regulatory circuitry to maintain ES cell identity. Development 135:1513–1524. doi: 10.1242/dev.014340. [DOI] [PubMed] [Google Scholar]
- 34.Ferrari R, Pellegrini M, Horwitz GA, Xie W, Berk AJ, Kurdistani SK. 2008. Epigenetic reprogramming by adenovirus e1a. Science 321:1086–1088. doi: 10.1126/science.1155546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ku M, Koche RP, Rheinbay E, Mendenhall EM, Endoh M, Mikkelsen TS, Presser A, Nusbaum C, Xie X, Chi AS, Adli M, Kasif S, Ptaszek LM, Cowan CA, Lander ES, Koseki H, Bernstein BE. 2008. Genomewide analysis of PRC1 and PRC2 occupancy identifies two classes of bivalent domains. PLoS Genet 4:e1000242. doi: 10.1371/journal.pgen.1000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ram O, Goren A, Amit I, Shoresh N, Yosef N, Ernst J, Kellis M, Gymrek M, Issner R, Coyne M, Durham T, Zhang X, Donaghey J, Epstein CB, Regev A, Bernstein BE. 2011. Combinatorial patterning of chromatin regulators uncovered by genome-wide location analysis in human cells. Cell 147:1628–1639. doi: 10.1016/j.cell.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu H, D'Alessio AC, Ito S, Xia K, Wang Z, Cui K, Zhao K, Sun YE, Zhang Y. 2011. Dual functions of Tet1 in transcriptional regulation in mouse embryonic stem cells. Nature 473:389–393. doi: 10.1038/nature09934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benayoun BA, Pollina EA, Ucar D, Mahmoudi S, Karra K, Wong ED, Devarajan K, Daugherty AC, Kundaje AB, Mancini E, Hitz BC, Gupta R, Rando TA, Baker JC, Snyder MP, Cherry JM, Brunet A. 2014. H3K4me3 breadth is linked to cell identity and transcriptional consistency. Cell 158:673–688. doi: 10.1016/j.cell.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caslini C, Capo-chichi, Roland CDIH, Nicolas E, Yeung AT, Xu XX. 2006. Histone modifications silence the GATA transcription factor genes in ovarian cancer. Oncogene 25:5446–5461. doi: 10.1038/sj.onc.1209533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, Jaenisch R, Wagschal A, Feil R, Schreiber SL, Lander ES. 2006. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 41.Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, Chevalier B, Johnstone SE, Cole MF, Isono K, Koseki H, Fuchikami T, Abe K, Murray HL, Zucker JP, Yuan B, Bell GW, Herbolsheimer E, Hannett NM, Sun K, Odom DT, Otte AP, Volkert TL, Bartel DP, Melton DA, Gifford DK, Jaenisch R, Young RA. 2006. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell 125:301–313. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ho IC, Tai TS, Pai SY. 2009. GATA3 and the T-cell lineage: essential functions before and after T-helper-2-cell differentiation. Nat Rev Immunol 9:125–135. doi: 10.1038/nri2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. 2009. Circos: an information aesthetic for comparative genomics. Genome Res 19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.