Abstract
Understanding the factors that predict and guide variation in behavioral change can lend insight into mechanisms of motor plasticity and individual differences in behavior. The performance of adult birdsong changes with age in a manner that is similar to rapid context-dependent changes to song. To reveal mechanisms of vocal plasticity, we analyzed the degree to which variation in the direction and magnitude of age-dependent changes to Bengalese finch song could be predicted by variation in context-dependent changes. Using a repeated-measures design, we found that variation in age-dependent changes to the timing, sequencing, and structure of vocal elements (“syllables”) was significantly predicted by variation in context-dependent changes. In particular, the degree to which the duration of intersyllable gaps, syllable sequencing at branch points, and fundamental frequency of syllables within spontaneous [undirected (UD)] songs changed over time was correlated with the degree to which these features changed from UD song to female-directed (FD) song in young-adult finches (FDyoung). As such, the structure of some temporal features of UD songs converged over time onto the structure of FDyoung songs. This convergence suggested that the FDyoung song could serve as a stable target for vocal motor plasticity. Consequently, we analyzed the stability of FD song and found that the temporal structure of FD song changed significantly over time in a manner similar to UD song. Because FD song is considered a state of heightened performance, these data suggest that age-dependent changes could reflect practice-related improvements in vocal motor performance.
Keywords: Bengalese finch, birdsong, sequencing, tempo, social context
motor plasticity and learning are characterized by increases in the speed, consistency, and accuracy of motor performance and are driven by reinforcement, practice, and feedback-dependent mechanisms (Adams 1987; Doya 2000; Drake and Palmer 2000; Ericsson et al. 1993; Hickok and Poeppel 2007; Sanes and Donoghue 2000; Schmidt and Lee 1988; Shadmehr and Krakauer 2008; Ungerleider et al. 2002; Willingham 1998; Wolpert et al. 2003). Empirical and computational models of motor plasticity indicate that reinforcement and error signals based on sensory targets shape the performance of a variety of behaviors (Dayan and Cohen 2011; Magill 2004; Willingham 1998) and that as performance improves, the neural coding of these behaviors can become more efficient [e.g., Karni et al. (1995, 1998); Petersen et al. (1998); Poldrack (2000); Zatorre et al. (2007)]. These mechanisms continue operating to maintain or improve motor performance, even after individuals acquire expertise. For example, baseline and peak performances of trained musicians and singers continue to improve as they engage in practice (Drake and Palmer 1999; Ericsson 2008; Keith and Ericsson 2007). However, despite our understanding of fundamental mechanisms of motor learning and plasticity, relatively little is known about the factors that predict and regulate individual variation in motor plasticity [e.g., Golestani et al. (2002, 2007); Herholz and Zatorre (2012); Landi et al. (2011); Tomassini et al. (2011)]. Furthermore, our understanding of neural mechanisms of motor plasticity is based primarily on the performance of relatively simple behaviors, and little is known about mechanisms underlying plasticity in the performance of more complex and natural behaviors.
Birdsong is an important model system for understanding mechanisms that guide the plasticity of evolutionarily important behaviors, as well as individual variation in motor plasticity. Like other forms of motor learning and plasticity, the learning and development of birdsong involve the acquisition of a sensory target, motor practice, and changes in the speed and consistency of motor performance (Brainard and Doupe 2000, 2013; Doupe and Kuhl 1999; Mooney 2009). During a sensitive period in development, juvenile songbirds memorize the songs of adult tutors, and these memorized songs serve as sensory templates or targets that guide song development. Thereafter, juveniles engage in extensive vocal motor practice to refine their initially “noisy” and variable vocalizations into structured and stereotyped vocal elements (“syllables”) that resemble the sensory targets [e.g., Kelly and Sober (2014); Ölveczky et al. (2005); Tchernichovski et al. (2001)]. In addition to changes to syllable structure, song becomes faster, and syllable sequencing becomes more consistent over the course of juvenile song development (Doupe and Kuhl 1999; Glaze and Troyer 2006, 2013; Okanoya 1997; Troyer and Doupe 2000). By the time individual songbirds reach sexual maturity, their songs are relatively stable and similar to the songs memorized during development. Whereas such developmental changes are generally thought to be mediated by target-based plasticity mechanisms, changes to the speed and consistency of song are also observed in birds deprived of the opportunity to acquire a sensory target (Kojima and Doupe 2007; Livingston et al. 2000; Morrison and Nottebohm 2003). As such, there could be additional mechanisms that act in concert with target-based mechanisms to sculpt the development of song.
Despite the overall convergence of juvenile songs onto the structure of tutor songs, there exists substantial individual variation in song development (Catchpole and Slater 2008; Doupe and Kuhl 1999). The structure, timing, and sequencing of song elements can vary significantly among pupils that share a tutor, as well as between tutors and pupils, highlighting the importance of understanding individual variation in vocal motor plasticity. It has recently been found that the developmental trajectory of a juvenile's song could be predicted by analyzing acute context-dependent changes to song performance (Kojima and Doupe 2011). In particular, it was found that juvenile songbirds significantly change the structure of their songs when singing to females [female-directed (FD) songs] compared with when producing spontaneous songs in isolation [undirected (UD) songs] and moreover, that the structure of the juvenile's FD song was highly similar to the UD song that the individual ultimately produced as an adult. These data indicate that social context not only reveals a heightened level of vocal performance but also provides predictive insight into individual variation in vocal plasticity. Additionally, these data suggest that the understanding of mechanisms of social influences on vocal performance could reveal mechanisms of vocal plasticity.
Despite the relative stability of adult song structure, adult song continues to change in a manner that resembles vocal motor plasticity during development. Specifically, the spontaneous UD songs of adult Bengalese finches continue to become faster and more stereotyped in sequencing over time (James and Sakata 2014). The similarity in the nature of developmental and adult vocal motor plasticity suggests that similar mechanisms could guide vocal motor change in juvenile and adult songbirds (James and Sakata 2014; Kao and Brainard 2006; Kojima and Doupe 2011; Pytte et al. 2007; Sakata and Vehrencamp 2012). For example, just as social context predicts developmental song plasticity, context-dependent changes to adult song could similarly predict adult vocal plasticity. Indeed, just as UD song becomes faster and more stereotyped in sequencing over time, song acutely becomes faster and more stereotyped in sequencing when adult Bengalese finches produce FD song, indicating broad similarity in context- and age-dependent changes to song (Dunning et al. 2014; Hampton et al. 2009; Heinig et al. 2014; Sakata et al. 2008).
Here, we conducted a series of analyses to reveal potential mechanisms underlying adult vocal plasticity. We first used a repeated-measures design to analyze the degree to which variation in long-term age-dependent changes to adult Bengalese finch song (i.e., changes to UD song over time) could be predicted by understanding variation in acute context-dependent changes to song when birds were young adults (i.e., changes from UDyoung to FDyoung song). Thereafter, we proposed two models to explain the nature of age-dependent changes to UD song and tested these models by independently analyzing how age affected the structure of FD song.
MATERIALS AND METHODS
Animals and data collection.
Bengalese finches (2–3 mo old) were purchased from vendors (Exotic Wings & Pet Things, Ontario, Canada) and shipped to McGill University. All birds were housed on a 14-h light:10-h dark cycle with other birds and provided food and water ad libitum. All procedures were approved by the McGill University Animal Care and Use Committee in accordance with the guidelines of the Canadian Council on Animal Care.
For all song recordings, birds were housed individually in sound-attenuating chambers (TRA Acoustics, Ontario, Canada). Song was recorded using an omnidirectional microphone (Countryman Associates, Menlo Park, CA) positioned above the male's cage. Computerized, song-activated recording systems were used to detect and digitize song (Sound Analysis Pro, v 1.04, digitized at 44.1 kHz; http://ofer.sci.ccny.cuny.edu/sound_analysis_pro). Recorded songs were digitally filtered (0.3–8 kHz) for offline analysis using software custom written in the Matlab programming language (MathWorks, Natick, MA).
To assess context-dependent changes to song, we collected renditions of UD and FD song of adult Bengalese finches following a protocol similar to Sakata et al. (2008). UD songs are spontaneously produced when birds are alone, whereas FD songs are produced during courtship interactions with females. Males were moved into a sound-attenuating chamber at least one night before collecting UD and FD song. To collect FD songs, we placed a cage with a female next to the experimental male's cage and monitored his behavior via video. FD songs are readily distinguishable from UD songs because they are produced after a male approaches or faces a female, accompanied by a courtship dance (e.g., pivoting body from side to side) and associated with the fluffing of the male's plumage (Morris 1954; Zann 1996). Only songs that were accompanied by at least two of the above behaviors were categorized as FD songs. FD songs were almost always produced soon after the introduction of a female, and we removed females after 30–60 s to ensure that FD songs were produced at a short latency following exposure to a female. The modal interval between exposures to females was 4–5 min, which allowed for the collection of UD song between female exposures. However, not all birds produced renditions of UD song between female presentations; therefore, UD songs were also recorded for 30 min before and after the testing session. The number of UD [28.8 ± 1.7 (mean ± SE)] and FD (12.6 ± 0.8) songs collected was comparable with previous studies (Hampton et al. 2009; Heinig et al. 2014; James and Sakata 2014; Matheson et al. 2015; Sakata and Brainard 2009; Sakata et al. 2008).
We collected the UD and FD songs of young-adult Bengalese finches (UDyoung and FDyoung songs; n = 14 birds; 4–7 mo). We then returned males to their group cage (n = 10) or housed them with females to serve as breeders (n = 4) for 10–39 mo (21.0 ± 2.7 mo). Thereafter, we recorded the UD songs of these males as older adults (UDolder songs). This design allowed us to assess the degree to which context-dependent changes to song in young-adult Bengalese finches (i.e., from UDyoung to FDyoung) predicted age-dependent changes to UD song (from UDyoung to UDolder) (James and Sakata 2014). Some of the older-adult recordings (n = 7) were included in a previous study (James and Sakata 2014). The magnitudes of context- and age-dependent changes to song were not significantly different between group-housed and breeding males; therefore, both were combined in the analysis.
Results from the analysis of context- and age-dependent changes suggested two models of vocal motor change, and the differentiation of these models required the analysis of the FD songs of older birds (FDolder). We were able to record the FD songs of a subset (n = 7) of Bengalese finches when they were older adults, allowing us to analyze subsequently how social context and age interacted to affect song performance using a factorial design.
Song analysis.
Bengalese finch song consists of distinct acoustic elements arranged in both stereotyped and variable sequences. An example of a Bengalese finch song is provided in Fig. 1. For purposes of description and analysis, we use the term “syllable” to refer to individual acoustic elements that are separated from each other by >5 ms of silence (James and Sakata 2014; Okanoya and Yamaguchi 1997; Warren et al. 2012). Identical to previous studies, we manually labeled syllables based on visual inspection of spectrograms following amplitude-based syllable segmentation in Matlab [e.g., Heinig et al. (2014); James and Sakata (2014); Long and Fee (2008); Matheson et al. (2015); Sakata et al. (2008); Stepanek and Doupe (2010); Warren et al. (2012)].
Fig. 1.
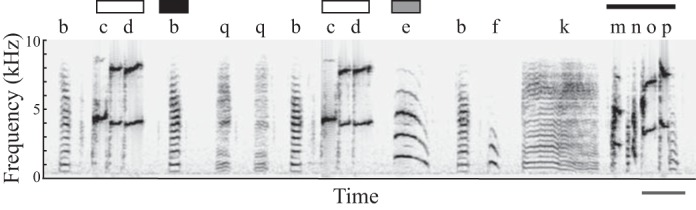
Adult Bengalese finch song. A spectrogram (time on the x-axis, frequency on the y-axis, darkness as amplitude) of a rendition of Bengalese finch song. Labels for syllables are located above the spectrogram. As with other Bengalese finches, this bird produces both variable (branch point) and stereotyped sequences within his song. The sequence “cd” (white bars) is an example of a branch point because it can be followed by a “b” (black bar), “e” (gray bar), or “q” (transition to q is not observed in this rendition). In contrast, the sequence “mnop” (black line) is a stereotyped sequence in which syllables are always produced in this order. Scale bar, 200 ms.
Nodes in song with variable sequencing are called “branch points,” and we analyzed context- and age-dependent changes to syllable sequencing at such branch points (n = 46 branch points in 14 males). For example, in Fig. 1, the branch point “cd” can be seen transitioning to both “b” and “e.” Sequence variability at branch points is not simply biological noise but reflects a controlled aspect of song that is stable over days and weeks and modulated by social context (Hampton et al. 2009; Heinig et al. 2014; James and Sakata 2014; Matheson and Sakata 2015; Okanoya and Yamaguchi 1997; Sakata et al. 2008; Warren et al. 2012). Stereotyped and branch points were identified during manual labeling and confirmed using bigram plots [e.g., Fujimoto et al. (2011); Heinig et al. (2014); Kakishita et al. (2009); Matheson et al. (2015); Okanoya and Yamaguchi (1997)]. We analyzed the probability of different syllable transitions (typically two to five distinct transitions per branch point) immediately following a specific sequence of syllables, paying close attention to longer-range statistics in sequencing (Fujimoto et al. 2011; James and Sakata 2014; Matheson et al. 2015; Warren et al. 2012). Sequences were considered to be branch points if transition probabilities for all transitions were <95% under any experimental condition. We computed the transition entropy, a measure of variability, of each branch point using the following formula
where the sum is over all transitions produced at the branch point, and pi is the probability of the ith transition across all renditions of the branch point. For our intents and purposes, the transition entropy indicates the number of bits of information required to summarize the extent of variation in syllable transitions. Branch points with transitions that are more variable (i.e., closer to uniform probability) have higher transition entropy scores. Entropy scores in our dataset ranged from 0 to 1.83 bits (sequences with entropy scores of 0 are included in the analysis because some branch points become completely stereotyped over time or across contexts). Only branch points that occurred at least 15 times during each recording session were analyzed (mean ± SE: 73.0 ± 4.4 renditions; range: 15–342). Instances in which song was terminated immediately following the branch point were not included in the calculation of entropy.
Changes to song tempo were analyzed using methods similar to previous studies (Cooper et al. 2012; James and Sakata 2014). Specifically, we identified a single, commonly produced sequence in an individual's songs (e.g., “mnop” in Fig. 1) and measured the duration of all syllables and intersyllable gaps within the sequence. Because the songs of young- and older-adult birds could have been acquired under different recording conditions, we compared normalized acoustic envelopes of syllable sequences to assess changes to song tempo. For this, we extracted the waveform of the sequence and then rectified, smoothed (5 ms square window), and resampled (1 kHz) the waveform. Thereafter, we normalized the envelope between zero and one using the minimum and peak values. After this normalization, the amplitude traces across ages and contexts were comparable, and we applied a common threshold on these normalized traces to find syllable onsets and offsets for each recording. This normalization allowed us to analyze age- and context-dependent changes to syllable and intersyllable gap durations, which can independently contribute to changes in sequence durations. We computed the mean duration of each syllable (range: 10–189 ms) and of each intersyllable gap within the sequence (range: 16–146 ms). We examined context- and age-dependent changes to song tempo by analyzing the mean duration of each individual syllable and gap, as well as the sum of all syllables and of all gaps within a sequence (i.e., total duration of syllables or total duration of gaps within a sequence). We also summed all mean syllable and gap durations together to compute sequence durations (i.e., interval from the onset of the first syllable to the onset of the last syllable of the sequence; range: 188–549 ms). Because sequence durations increase as the song progresses (Chi and Margoliash 2001; Cooper and Goller 2006; Glaze and Troyer 2006) and because song durations can change as a function of social context and age (James and Sakata 2014; Kao and Brainard 2006), we restricted our analysis to the first occurrence of the sequence in each song. Given the range of syllable, gap, and sequence durations, we also calculated the percent change in durations across social contexts and ages for data visualization and analysis.
To analyze changes to syllable structure, we analyzed the fundamental frequency (FF) of syllables that had distinct and stable harmonic structure (n = 33 syllables from 14 males; e.g., syllables “c” and “d” in Fig. 1). The FF of such syllables is tightly regulated by the nervous system and represents an important metric for song development and performance (Brainard and Doupe 2013; Kao and Brainard 2006; Sakata et al. 2008; Sakata and Vehrencamp 2012). To compute the FF, we calculated the autocorrelation of a segment of the sound waveform and defined the FF as the distance, in Hz, between the zero-offset peak and the highest peak in the autocorrelation function. To improve the resolution and accuracy of frequency estimates, we performed a parabolic interpolation of the peak of the autocorrelation function (de Cheveigné and Kawahara 2002). Each rendition of a syllable was visually screened to ensure that we analyzed only examples devoid of sound artifacts that could affect FF calculations [e.g., sound of movement, female calls in background; 105.1 ± 6.7 renditions (range: 18–317)]. For each syllable, we computed the mean and variability of FF across renditions, two aspects of song that change over development and across social contexts [e.g., Hampton et al. (2009); Kelly and Sober (2014); Sakata et al. (2008)]. The mean FF of syllables that we measured ranged from 0.4 to 4.5 kHz. Because of this wide range of values, we also calculated the percent change in mean FF across social contexts and ages for data visualization and analysis. We characterized the variability of FF across renditions using the interquartile region (IQR; distance between the 25th and 75th percentiles) divided by the median (50th percentile). This normalized IQR (normIQR; range: 0.0059–0.0784) is analogous to the coefficient of variation (SD/mean) in that it normalizes for differences in central tendencies (i.e., median or mean) but is more robust to outliers than other measures of variability (Samuels and Witmer 2002). Results were comparable regardless of whether we used the normIQR or coefficient of variation to characterize variability.
Statistical analyses.
A central objective of this study was to assess the relationship between acute context-dependent changes (from UDyoung to FDyoung song) (Dunning et al. 2014; Hampton et al. 2009; Heinig et al. 2014; Sakata et al. 2008) and longer-term plasticity in the structure of UD song (UDyoung to UDolder) (James and Sakata 2014). Therefore, we first analyzed variation in song structure among the UDyoung, FDyoung, and UDolder songs of individual birds. Because we measured multiple distinct examples of branch points and syllables (e.g., transition entropies of multiple branch points, the duration of multiple gaps within a sequence, the duration and FF of multiple syllables) within the songs of individual Bengalese finches across different social contexts and ages, we used mixed-effects models to analyze changes to transition entropy, syllable and gap durations, and FF. For these models, independent variables were GROUP (UDyoung, FDyoung, and UDolder songs); BIRD ID; and BRANCH POINT ID, GAP ID, or SYLLABLE ID, nested within BIRD ID. BIRD ID, SYLLABLE ID, GAP ID, and BRANCH POINT ID were random variables. Because only one sequence was measured per bird, we used a repeated-measures ANOVA to analyze context- and age-dependent changes to sequence durations. Tukey's honestly significant difference was used for post hoc comparisons. We used mixed-effects models (transition entropy, transition probabilities, syllable and gap durations, normIQR of FF) and Pearson's product moment correlation (sequence durations) to analyze the relationship between the magnitudes of context- and age-dependent changes to song. To analyze how social context and age interacted to influence song control for the subset of males in which UD and FD songs were collected when birds were young and older adults, we used a factorial design with CONTEXT, AGE, and CONTEXT × AGE as independent variables and BIRD ID and BRANCH POINT ID, GAP ID, or SYLLABLE ID, nested within BIRD ID, as random variables. Analyses were conducted using JMP 10 (SAS Institute, Cary, NC) and Matlab, and α = 0.05 for all tests.
RESULTS
Relationship between context- and age-dependent changes to syllable sequencing.
The variability with which syllables are sequenced within spontaneously produced UD song generally decreases over time (James and Sakata 2014). However, there is considerable individual variation in the direction and magnitude of age-dependent changes to syllable sequencing. Age-dependent changes to syllable sequencing generally resemble context-dependent changes: sequence variability decreases significantly when adult Bengalese finches produce FD song relative to when they produce UD song (Hampton et al. 2009; Heinig et al. 2014; Matheson et al. 2015; Sakata et al. 2008). This suggests the possibility that context-dependent changes (UDyoung to FDyoung) could predict the direction and magnitude in which the sequencing of syllables within UD song changes over time (UDyoung to UDolder). As such, we compared the UDyoung, FDyoung, and UDolder songs of individual Bengalese finches using a repeated-measures design.
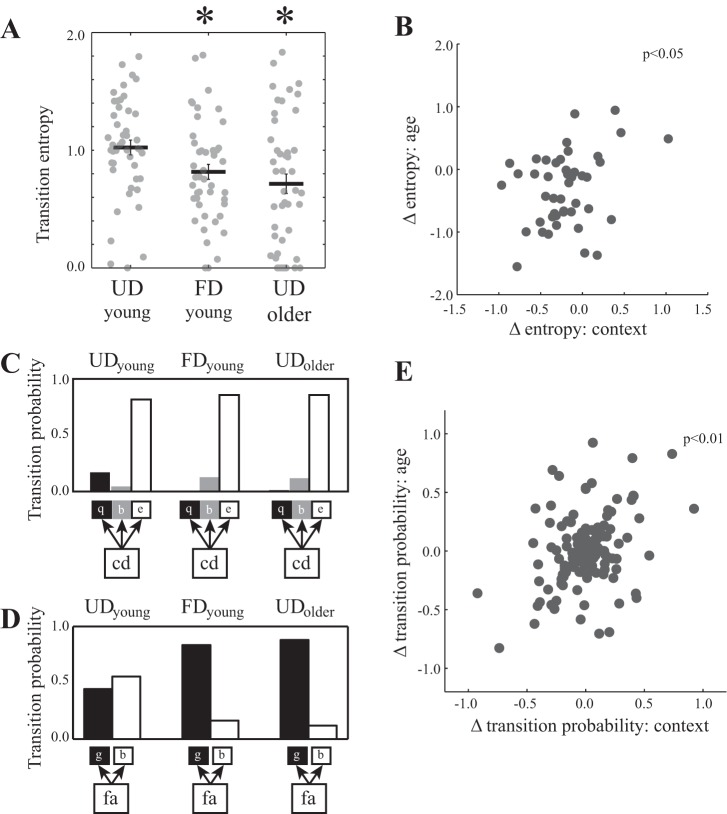
We first compared the magnitude of age- and context-dependent changes to the transition entropy of branch points (n = 46 branch points from 14 birds). Transition entropy was significantly different among the UDyoung, FDyoung, and UDolder songs of individual birds (per branch point; F2,90 = 8.4, P = 0.0005). In particular, transition entropy was significantly lower for FDyoung and UDolder songs than for UDyoung song (P < 0.025 for both) and not significantly different between FDyoung and UDolder songs (Fig. 2A; P = 0.3896). Consequently, the magnitude of context-dependent changes to the transition entropy of individual branch points (i.e., differences between UDyoung and FDyoung songs) was not significantly different than the magnitude of age-dependent changes (i.e., differences between UDyoung and UDolder songs; per branch point; F1,45 = 1.4, P = 0.2487).
Fig. 2.
Context-dependent changes to syllable sequencing predicted age-dependent changes to syllable sequencing. A: transition entropy, a measure of sequence variability, decreased significantly across social context and age. Plotted is the mean (±SE) transition entropy of branch points for the undirected and female-directed young-adult (UDyoung and FDyoung, respectively) and UD older-adult (UDolder) songs of individual birds (n = 46 branch points), with raw data values plotted in gray. Transition entropy is significantly lower for FDyoung and UDolder song than for UDyoung song and not different between FDyoung and UDolder song. *P < 0.05, significantly different than UDyoung song. B: there is a significant, positive relationship between the magnitude of change (Δ) in entropy caused by social context and by age (P = 0.0430). Plotted is the difference in transition entropy across context (FDyoung − UDyoung) and the difference across age (UDolder − UDyoung). C: an example of context- and age-dependent changes to syllable sequencing at branch points. The bird can produce the syllables q (black bar), b (gray bars), and e (white bars), following the branch-point sequence cd (same bird depicted in Fig. 1). Social context and age led to a virtual elimination of the transition to q and increases in transitions to b and e. D: another example of context- and age-dependent changes to syllable sequencing at branch points. The bird produced the syllables “g” (black bars) and b (white bars) following the branch point “fa,” and the changes to both transitions across context and age were comparable. E: context-dependent changes to transition probabilities for all branch points were significantly correlated with age-dependent changes to transition probabilities (P < 0.0001). Plotted are the differences in transition probabilities across context (FDyoung − UDyoung) and across age (UDolder − UDyoung).
Similarities in the extent of context- and age-dependent changes to transition entropy suggest the possibility that individual variation in context-dependent changes to syllable sequencing could predict individual variation in age-dependent changes to syllable sequencing. To this end, we first analyzed the relationship between context- and age-dependent changes to transition entropy and found that the magnitude of acute context-dependent changes was significantly and positively correlated with the magnitude of long-term age-dependent changes (Fig. 2B; per branch point; F1,43.2 = 4.3, P = 0.0430). Branch points in which transition entropy changed more across social contexts also changed more over time.
The correlation between context- and age-dependent changes to transition entropy suggested the possibility that individual transition probabilities could change in similar manners across social context and age. However, because distinct types of changes to syllable sequencing could drive similar changes to entropy, it remained possible that context- and age-dependent changes to specific sequences could be independent. Therefore, we assessed the relationship between context- and age-dependent changes to the transition probabilities of all transitions across all branch points. At one branch point (Fig. 2C), social context and age had similar effects on transition probabilities: the probability of transitions from cd to “q” was virtually eliminated across context (from UDyoung to FDyoung) and age (from UDyoung to UDolder), and the transition probabilities from cd to b and e increased across both context and age. For another branch point (Fig. 2D), the transition probabilities from “fa” to “g” and b were approximately equal when the bird produced UDyoung song, and the transition probability to g increased across both social context and age. Such examples were common, and consequently, across all transitions in all branch points, there was a significant and positive relationship between the changes in transition probabilities caused by social context and by age (Fig. 2E; per transition; F1,134.1 = 119.7, P < 0.0001). These analyses highlight that the direction and magnitude of context-dependent changes to syllable sequencing significantly predicted the direction and magnitude of age-dependent changes.
Relationship between context- and age-dependent changes to syllable timing.
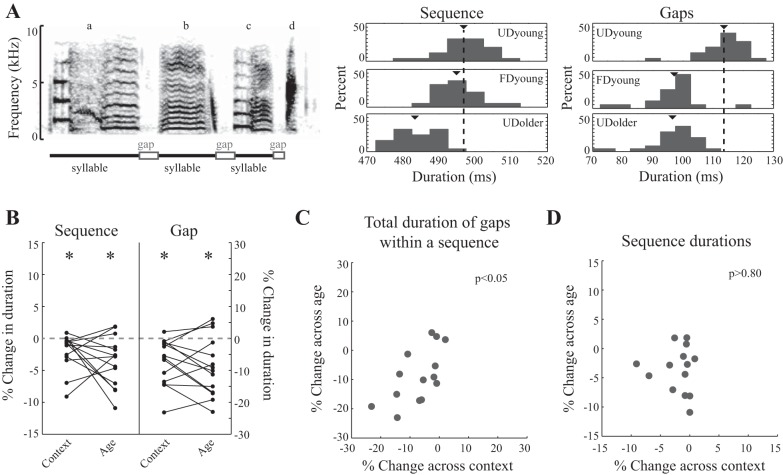
Previous experiments have found that the FD songs of adult Bengalese finches are faster than their UD songs and that UD songs become faster with age (Dunning et al. 2014; Hampton et al. 2009; James and Sakata 2014; Matheson et al. 2015; Sakata and Brainard 2009; Sakata et al. 2008). We found similar patterns of change to song tempo, as depicted in the example in Fig. 3A. The average duration of the sequence “abcd” for an individual bird decreased from UDyoung song [497.0 ± 1.2 (mean ± SE) ms] to FDyoung song (495.1 ± 1.4 ms) and to UDolder song (483.6 ± 1.0 ms). Such changes were prevalent across birds, and consequently, we observed significant differences in sequence durations among UDyoung, FDyoung, and UDolder songs (per bird; F2,26 = 5.3, P = 0.0114). Sequence durations were shorter for UDolder than for UDyoung songs (P = 0.0083) but not significantly different between UDyoung and FDyoung songs (P = 0.2672) or between FDyoung and UDolder songs (P = 0.2330). The lack of significant difference between UDyoung and FDyoung songs observed in the post hoc contrasts was not consistent with previous studies. Therefore, we also computed the percent changes in sequence durations from UDyoung to FDyoung song for each bird and assessed whether the average change across social contexts was significantly different than zero (t-test; H0: mean = 0). In contrast to the above analysis but consistent with previous studies (Dunning et al. 2014; Hampton et al. 2009; Matheson et al. 2015; Sakata and Brainard 2009; Sakata et al. 2008), we observed that the average change in sequence durations from UDyoung to FDyoung song was significantly less than zero, confirming that young-adult Bengalese finches produce faster songs when singing to females (−2.0 ± 0.8% change; per bird; t13 = 2.6, P = 0.0201). Analyses of the percent changes from UDyoung to UDolder song and from FDyoung to UDolder song were consistent with the previous analysis: the percent change in sequence durations from UDyoung to UDolder was significantly less than zero (per bird; P = 0.0044), indicating that UD song became faster over time, and the percent change from FDyoung to UDolder was not significantly different from zero (per bird; P = 0.2883), supporting the previous analysis that song tempo was similar between FDyoung and UDolder songs. Given these patterns, it is not surprising that the magnitudes of context- and age-dependent changes to sequence durations were not statistically different (Fig. 3B; per bird; F1,13 = 1.3, P = 0.2790).
Fig. 3.
Context-dependent changes to syllable timing predicted age-dependent changes to syllable timing. A: spectrogram of a stereotyped sequence used to analyze context- and age-dependent changes to song tempo. Labels for syllables are located above the spectrogram, and syllables (lines) and intersyllable gaps (empty boxes) are highlighted under the spectrogram. Histograms display sequence durations (middle) and the total duration of gaps within the sequence (right) across renditions, and triangles indicate the mean duration for each condition. Dashed lines correspond to the mean duration for UDyoung song. In this example, both sequence and gap durations were shorter for both FDyoung and UDolder song than for UDyoung song. B: sequence (left) and gap (right) durations change significantly across social context and age, and the magnitudes of context- and age-dependent changes to sequence and gap durations were not significantly different (n = 14 sequences). Plotted are the percent changes to sequence and gap durations across context (UDyoung to FDyoung) and age (UDyoung to UDolder). *P < 0.05 indicates that the mean percent change was significantly different than 0 (t-test). C: context-dependent changes to gap durations (percent change) are correlated with age-dependent changes (P = 0.0174). Plotted are the percent changes to gap durations across context (UDyoung to FDyoung) and across age (UDyoung to UDolder). D: context-dependent changes to sequence durations (percent change) were not correlated with age-dependent changes (P = 0.8141). This lack of correlation at the sequence level (despite the correlation at the gap level) is likely due to the lack of correlation in syllable durations (P = 0.5492) as well as the fact that syllables contribute more to sequence durations than gaps.
The duration of a sequence consists of the durations of multiple syllables and of multiple intersyllable gaps, and syllables and gaps could be differentially affected by social context and age [e.g., Cooper and Goller (2006); Glaze and Troyer (2006); James and Sakata (2014); Thompson et al. (2011)]. In Fig. 3A, we plot an example from an individual bird, and the total duration of gaps within this sequence decreased from UDyoung song (113.6 ± 1.4 ms) to FDyoung song (97.1 ± 2.1 ms) and to UDolder song (96.5 ± 1.6 ms). This pattern was consistent across birds, and consequently, we found that the total duration of gaps within individual sequences was significantly different among UDyoung, FDyoung, and UDolder songs (per bird; F2,26 = 10.8, P = 0.0004). Total gap durations were shorter for FDyoung and UDolder songs than for UDyoung song (P < 0.0070 for both), indicating a significant effect of age and context on gap durations, and not significantly different between UDolder and FDyoung songs (P = 0.5131). Similarly, when we analyzed changes to each individual intersyllable gap, we found a significant difference among UDyoung, FDyoung, and UDolder songs (per gap; F2,70 = 10.2, P < 0.0001) that was driven by a significant decrease in FDyoung and UDolder songs relative to UDyoung song (P < 0.01 for both contrasts). As such, the magnitudes of context- and age-dependent changes to gap durations were not statistically different (Fig. 3B; per bird; F1,13 = 0.5, P = 0.4907).
In contrast to gap durations, the total duration of syllables within a sequence was not significantly different among UDyoung, FDyoung, and UDolder songs (per bird; F2,26 = 1.7, P = 0.2075). The variation in syllable duration was also not significant when each syllable was examined individually (per syllable; F2,71 = 1.15, P = 0.3205). Taken together, these analyses indicate that changes to sequence durations are driven primarily by changes to gap durations, not syllable durations.
We then analyzed the degree to which variation in context-dependent changes to gap, syllable, and sequence durations predicted variation in age-dependent changes. The magnitudes of context- and age-dependent changes to the total duration of gaps within a sequence were significantly and positively correlated (Fig. 3C; per bird; r = 0.62, n = 14, P = 0.0174): young adults that demonstrated larger context-dependent decreases in the total duration of intersyllable gaps within a sequence also demonstrated larger decreases in gap durations with age. This positive relationship was also observed when we analyzed each individual gap within a sequence, but the relationship was not statistically significant (per gap; F1,20.4 = 2.04, P = 0.1686). The magnitudes of context- and age-dependent changes to the total duration of syllables within a sequence (per bird; r = 0.18, n = 14, P = 0.5492), the duration of each syllable within a sequence (per syllable; F1,31.7, P = 0.2661), and sequence durations were not significantly correlated (Fig. 3D; per bird; r = −0.07, n = 14, P = 0.8141). The lack of correlation at the sequence level, despite the correlation at the gap level, is likely due to the lack of correlation between context and age effects on syllable durations and the fact that syllables contribute more to sequence durations than gaps.
Taken together, these analyses highlight the importance of changes to gap durations for rapid context-dependent and longer-term age-dependent changes to song tempo. Gap durations (but not syllable durations) were affected by social context and by age, and the magnitude of context-dependent changes to gap durations (but not to syllable durations) was correlated with the magnitude of age-dependent changes.
Relationship between context- and age-dependent changes to syllable structure.
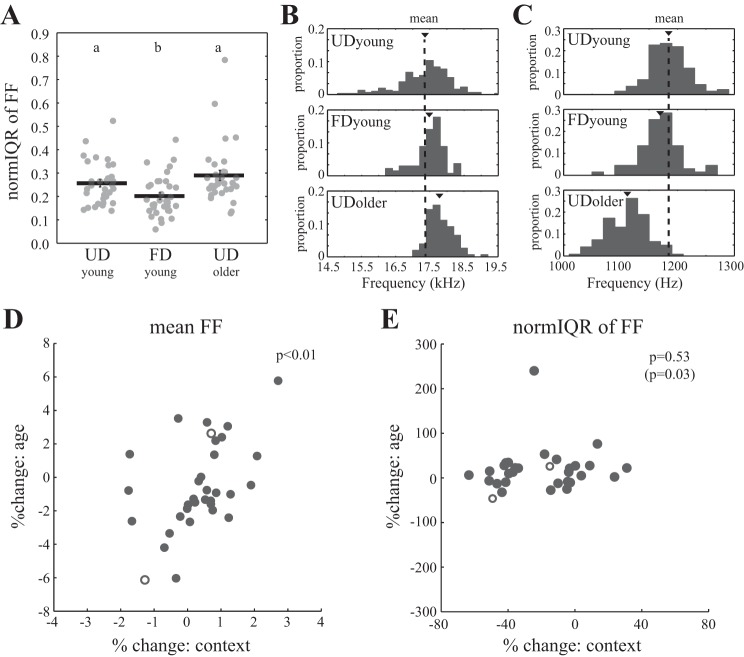
The FF of syllables with flat, harmonic structure is higher and less variable when male Bengalese finches produce FD song than when they produce UD song (Hampton et al. 2009; Matheson et al. 2015; Sakata et al. 2008). In contrast, the mean and variability of the FF of syllables for UD song do not change significantly with age (James and Sakata 2014). We observed similar context- but not age-dependent changes to syllable structure (n = 33 syllables in 14 males). The mean FF of syllables with flat, harmonic structure was significantly different among the UDyoung, FDyoung, and UDolder songs of individual birds (per syllable; F2,64 = 3.7, P = 0.0304). Mean FF was higher during FDyoung song than during both UDyoung and UDolder song, but the difference was only significant between FDyoung and UDolder song (P = 0.0228). The variability of FF (normIQR) was also significantly different among UDyoung, FDyoung, and UDolder songs (Fig. 4A; per syllable; F2,64 = 11.9, P < 0.0001). Variability was significantly lower during FDyoung song than during UDyoung or UDolder song (P < 0.011 for both) but not different between UDyoung and UDolder songs (P = 0.1711).
Fig. 4.
Context-dependent changes to fundamental frequency (FF) predicted age-related variation in FF (n = 33 syllables in 14 males). A: social context but not age affected the variability of FF. Plotted are normalized interquartile region (normIQR) values for each individual syllable, with lines indicating the mean ± SE for each condition. Groups with different letters indicate groups that are significantly different. B: distributions of FF across renditions for an individual syllable with flat, harmonic structure. Triangles indicate the mean FF for each condition, and the dashed line corresponds to the mean FF for UDyoung song to help visualize context- and age-dependent changes. The mean FF of this syllable increased across both social contexts (from UDyoung to FDyoung) and age (from UDyoung to UDolder). The variability of FF for this syllable decreased across both social contexts and age. C: distributions of FF across renditions for another syllable. The mean FF of this syllable decreased across both context and age. The variability of FF for this syllable decreased slightly across context and increased with age. D: variation in the magnitude of change in the mean FF across social context (percent change from UDyoung to FDyoung) predicts variation in the magnitude of change across age (percent change from UDyoung to UDolder; P = 0.0049). Open circles represent example syllables depicted in B and C. E: overall, there was no significant relationship between the magnitude of context-dependent changes (percent change from UDyoung to FDyoung) to the variability (normIQR) of FF and the magnitude of age-dependent changes (percent change from UDyoung to UDolder; P = 0.5308). However, after the removal of the value for a single outlying syllable, the relationship between context- and age-dependent changes to normIQR was significant (P = 0.0342). Open circles represent syllables summarized in B and C.
Despite that social context and age differentially affected the mean and variability of FF, we found that variation in context-dependent changes to syllable structure tended to predict variation in age-dependent changes. For example, for one syllable, mean FF increased from UDyoung song (1,736 ± 5 Hz) to FDyoung song (1,748 ± 5 Hz) and to UDolder song (1,782 ± 3 Hz), which amounted to a 0.7% increase across social contexts and a 2.6% increase with age (Fig. 4B). For another syllable, mean FF decreased from UDyoung song (1,185 ± 3 Hz) to FDyoung song (1,169 ± 4 Hz) and to UDolder song (1,112 ± 3 Hz), amounting to a 1.4% decrease across social contexts and a 6.2% decrease with age (Fig. 4C). For both examples, the direction in which mean FF changed was similar across social contexts and age. As such, there was a significant and positive relationship between the magnitudes of context- and age-dependent changes to mean FF (Fig. 4D; per syllable; F1,27.0 = 9.4, P = 0.0049).
There was generally a linear relationship between context- and age-dependent changes to the variability of FF. For example, for the syllable summarized in Fig. 4B, the variability of FF decreased from UDyoung song (0.052) to FDyoung song (0.027) and to UDolder song (0.028), which corresponded to a 48% and 46% decrease in the variability of FF, respectively, across social contexts and age. For the syllable summarized in Fig. 4C, the variability of FF decreased from UDyoung song (0.036) to FDyoung song (0.031) but increased from UDyoung song to UDolder song (0.045), corresponding to a 15% decrease and 26% increase across social context and age, respectively. When examining all syllables, the magnitudes of context- and age-dependent changes to the variability of FF were not related significantly (Fig. 4E; per syllable; F1,30.9 = 0.4, P = 0.5308). However, there was a single outlying value, and this relationship was significant and positive when this outlier was removed from the analysis (F1,22.5 = 5.1, P = 0.0342). Generally speaking, large context-dependent decreases in the variability of FF were related to decreases in the variability of FF with age, whereas smaller context-dependent decreases or context-dependent increases were related to increases in the variability of FF with age.
Testing models of adult vocal motor change.
The preceding analyses confirm our hypothesis that context-dependent vocal motor changes in young-adult Bengalese finches (UDyoung to FDyoung) lend predictive insight into age-dependent vocal motor changes to UD song (UDyoung to UDolder). In addition, we observed that some temporal features of adult UD song converge over time onto the structure of FDyoung song. Specifically, whereas UDyoung song differed in many ways from FDyoung song, there was no significant difference in sequence variability and syllable timing between UDolder and FDyoung songs that these birds produced. We propose two models—a “target model” and a “performance model”—that could account for this convergence of UD song onto the FD song of young adults. The target model postulates that the FDyoung song represents a stable “target” for age-dependent changes to UD song. The performance model proposes that context- and age-dependent changes reflect changes in vocal performance. It has been hypothesized that FD song represents a state of heightened performance, possibly indicative of an individual's current “best” performance (Byers et al. 2010; Dunning et al. 2014; Kao and Brainard 2006; Podos et al. 2009; Sakata et al. 2008; Sakata and Vehrencamp 2012; Woolley and Doupe 2008). Because the UDyoung songs change toward the structure of FDyoung songs over time, the performance model interprets age-dependent changes to song as increases in vocal performance, possibly due to practice. Whereas the two models are consistent in their predictions about age-dependent changes to UD song, the models differ in their predictions regarding age-dependent changes to FD song. The performance model predicts that the age-dependent changes to song are the result of an overall increase in vocal performance. Thus the age-dependent changes observed for UD songs should similarly be observed for FD songs: gap durations and sequence variability of FD songs should decrease over time (Fig. 5A). In contrast, the target model predicts that the FDyoung song represents a stable target for age-dependent change, and thus FD song should not change across time (Fig. 5A). As such, the target but not performance model predicts a significant interaction between age and social context on transition entropy and gap durations, whereas the performance but not target model predicts significant and independent effects of age and context.
Fig. 5.
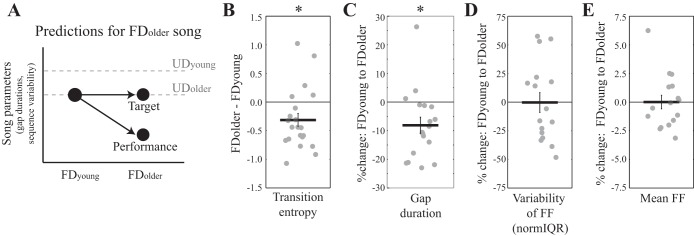
The testing of the “target” and “performance” models of vocal motor change. A: predictions of the target and performance models. The performance but not the target model predicts that features that changed significantly over time for UD song (syllable sequencing and tempo) should similarly change for FD song. As such, the performance model predicts that gap durations and sequence variability of FD song (black circles) should decrease over time. The target model predicts that these features of FD song should remain the same over time. Dashed gray lines represent UDyoung and UDolder songs and highlight the decrease for UD song over time. B: the transition entropy of branch points decreased from FDyoung to FDolder songs [Tukey's honestly significant difference (HSD); P = 0.0241], indicating age-dependent changes to syllable sequencing in FD song. Whereas analyses were performed using a factorial design (see results), depicted in this panel (as well as subsequent panels of this figure) are changes to song features for FD song with age. We depict the FDyoung-FDolder contrast because this contrast is central for the comparison of target and performance models and plot changes instead of mean values for each condition, as differences in mean values are difficult to see for some features because of the large range of values (see materials and methods). C: gap durations decreased from FDyoung to FDolder song (Tukey's HSD; P = 0.0085), demonstrating an age-dependent increase to the tempo of FD song. D: the variability (normIQR) of FF does not change significantly from FDyoung to FDolder song (Tukey's HSD; P = 0.7680). E: mean FF does not change significantly from FDyoung to FDolder song (Tukey's HSD; P = 0.9833). *P < 0.05 indicates features of FD song that change significantly over time.
We were able to collect renditions of UD and FD songs from seven Bengalese finches when they were both young and older adults to test these models of vocal motor change. When simultaneously examining context- and age-dependent changes to sequence variability (n = 21 branch points in seven birds), we found a significant effect of age (per branch point; F1,60 = 24.3, P < 0.0001), a trend for social context (F1,60 = 3.3, P = 0.0751), and no significant interaction between age and social context on the transition entropy of individual branch points (F1,60 = 0.6, P = 0.4372). Importantly, post hoc contrasts revealed that the transition entropy of FD songs was significantly lower in older birds than in younger birds (P = 0.0241; Fig. 5B).
Similar effects of age and social context were observed for gap durations. Age and social context independently affected the duration of individual gaps within a sequence (per gap; age: F1,51 = 34.4, P < 0.0001; context: F1,51 = 10.8, P = 0.0018; age × context: F1,18 = 1.3, P = 0.2564) and the total duration of gaps within a sequence (per bird; age: F1,18 = 13.8, P = 0.0016; context: F1,18 = 4.3, P = 0.0519; age × context: F1,18 = 0.5, P = 0.4765). Importantly, post hoc contrasts revealed that the duration of individual gaps during FD song were significantly shorter in older adults than in younger adults (per gap; P = 0.0085; Fig. 5C).
Social context but not age or the interaction between age and context significantly affected the variability of FF (normIQR) of individual syllables (per syllable; age: F1,45 = 0.3, P = 0.5793; context: F1,45 = 25.9, P < 0.0001; age × context: F1,45 = 0.7, P = 0.4216). Within this subset of birds, the mean FF of individual syllables tended to increase from UD to FD song, but the effects of context, age, or the interaction between age and context were not significant (per syllable; age: F1,45 = 0.2, P = 0.6448; context: F1,45 = 2.0, P = 0.1689; age × context: F1,45 = 0.0, P = 0.9597). Consistent with the lack of age-dependent changes to the mean and variability of FF for UD songs (Fig. 4), post hoc contrasts reveal no difference in the variability of FF (per syllable; P = 0.7680; Fig. 5D) or mean FF (per syllable; P = 0.9833; Fig. 5E) between FDyoung and FDolder songs.
Taken together, these data indicate that age and social context did not significantly interact to affect any song feature measured but that age and context independently affected sequence variability and gap durations. Of particular importance was that syllable sequencing and timing were significantly different between FDyoung and FDolder songs. As such, temporal features changed in the same manner over time for FD song as for UD song, lending greater support for the performance than the target model of vocal motor change.
DISCUSSION
The structure of evolutionarily important behaviors is not static but can change considerably over time (Catchpole and Slater 2008; Colonnese et al. 1996; Doupe and Kuhl 1999; Fentress 1992; Glaze and Troyer 2013; James and Sakata 2014; Sakata and Vehrencamp 2012). The understanding of the processes that guide and predict such behavioral changes can lend insight into mechanisms of motor plasticity and control as well as processes mediating individual differences in behavior [e.g., Kojima and Doupe (2011); Sakata and Crews (2003)]. Here, we demonstrate that understanding individual variation in the acute effects of social context on syllable sequencing, song tempo, and syllable structure lends predictive insight into variation in the direction and magnitude of age-dependent changes to syllable sequencing, timing, and structure. For example, when comparing the effect of social context (UDyoung to FDyoung) with the effect of age on UD song in these same individuals (UDyoung to UDolder songs), we found that the direction and magnitude of context-dependent changes to the duration of silent gaps between syllables predicted the direction and magnitude of age-dependent changes to gap durations. Consequently, by examining individual variation in social context-dependent changes to adult Bengalese finch song, we can predict individual trajectories and magnitudes of age-dependent changes to some important features of song.
The correlations in the magnitude of age- and context-dependent changes to song suggest that neurophysiological changes that drive context-dependent changes to syllable sequencing, timing, and structure could resemble the neurophysiological changes that underlie age-dependent variation in syllable sequencing, timing, and structure. Birdsong is controlled primarily by two forebrain circuits. Neurons in the vocal motor pathway (VMP), which includes the HVC (used as proper name) and robust nucleus of the arcopallium (RA), encode the motor commands for song and are functionally analogous to neurons in the mammalian premotor, supplementary, and primary motor cortical areas (Doupe and Kuhl 1999; Fee and Scharff 2010). The activity of neurons in HVC affects and encodes information about syllable sequencing and timing (Andalman et al. 2011; Ashmore et al. 2005; Basista et al. 2014; Fujimoto et al. 2011; Hahnloser et al. 2002; Kosche et al. 2015; Long and Fee 2008; Long et al. 2010; Prather et al. 2008; Sakata and Brainard 2008; Schmidt 2003; Vu et al. 1994; Wang et al. 2008; Yu and Margoliash 1996). Therefore, we propose that context- and age-dependent changes to syllable sequencing at branch points and to gap durations are mediated by neurophysiological changes in HVC.
The control and plasticity of song are also regulated by activity in the anterior forebrain pathway (AFP), a circuit that is homologous to cortical-basal ganglia-thalamic loops in mammals and includes the basal ganglia nucleus Area X, the dorsal lateral nucleus of the anterior thalamus, and the lateral magnocellular nucleus of the anterior nidopallium (LMAN) (Brainard and Doupe 2013; Doupe et al. 2005; Reiner et al. 2004; Woolley and Kao 2015). For example, lesions of LMAN, the primary interface between the AFP and VMP, consistently affect the variability of syllable structure [reviewed in Woolley and Kao (2015)]. As such, social context and age could affect LMAN activity in similar ways to shape syllable structure. Manipulations of LMAN activity have also been found to affect song tempo persistently and to affect syllable sequencing transiently and persistently [e.g., Brainard and Doupe (2001); Hamaguchi and Mooney (2012); Kao and Brainard (2006); Kobayashi et al. (2001); Kubikova et al. (2014); Thompson et al. (2011); Williams and Mehta (1999)]. However, a number of studies do not observe significant AFP contributions to the sequencing and timing of song elements [e.g., Ali et al. (2013); Hampton et al. (2009); Leblois and Perkel (2012); Stepanek and Doupe (2010)]. Of particular relevance is that lesions of LMAN do not affect the social modulation of syllable sequencing and song tempo in Bengalese finches (Hampton et al. 2009). Consequently, we hypothesize that the AFP could mediate the covariation in age- and context-dependent changes to syllable structure but not the correlated changes to syllable sequence and timing for adult Bengalese finch song.
Given the similarity in context- and age-dependent changes to syllable timing and sequencing, we hypothesize that the pattern of neural activity across song control nuclei should be similar when older Bengalese finches produce UD song and when young-adult Bengalese finches produce FD song. Fewer neurons in both the VMP and AFP express the immediate early gene early growth response protein 1 (EGR-1) when adult Bengalese finches produce the faster and less variable FD song than when they produce the slower and more variable UD song (Matheson et al. 2015). As such, we propose that fewer neurons in song control circuitry, in particular, the VMP, will express EGR-1 when older-adult Bengalese finches produce song than when young adults produce song. Such neural changes would be consistent with models linking motor learning and performance to increased neural efficiency (Poldrack 2000). For example, the supplementary motor area and premotor cortex are less activated by finger tapping in expert piano players than in nonmusicians, and such reductions have been hypothesized to reflect more efficient neural processing for motor performance (Hund-Georgiadis and von Cramon 1999; Jäncke et al. 2000; Krings et al. 2000; Lotze et al. 2003). Similar decreases have also been observed in premotor cortex as individuals learn how to play melodies (Chen et al. 2012), and in language-related areas as individuals become more proficient with languages [e.g., Briellmann et al. (2004); Perani et al. (2003); Vingerhoets et al. (2003)]. In songbirds, developmental changes to EGR-1 mRNA expression that are consistent with this hypothesis have been observed in RA: singing causes a smaller increase in EGR-1 mRNA expression in RA when adult zebra finches produce their stereotyped songs than when juvenile zebra finches produce their variable songs (Jarvis et al. 1998; Jin and Clayton 1997; Whitney et al. 2000).
Regardless of the nature of social context and age effects on neural activity, these data provide some insight into the process of adult vocal motor change. Our data demonstrate that the temporal patterning of UD songs converge over time onto the temporal structure of FDyoung songs (Figs. 2 and 3). By examining how both the UD and FD songs of individual birds changed over time, we tested the hypotheses that FDyoung songs served as stable targets for song plasticity (target model) or that age-dependent changes simply reflect a change in vocal motor performance (performance model), focusing on how FD song changed over time to distinguish between these models. We found greater support for the performance model of vocal motor change because the stereotypy of syllable sequencing and tempo of an individual's FD song increased significantly over time (Fig. 5). It has been proposed that FD song reveals the physiological limit of a bird's performance (e.g., FD song is the bird's fastest possible song) (Podos et al. 2009), and our results indicate that the best (FD) rendition of song can change as baseline (UD) vocal performance changes.
Despite the evidence against the notion that the FD song of young birds represents a target for adult song plasticity, it remains possible that sensory targets could guide adult vocal plasticity. In particular, it is possible that tutor songs memorized during the first month of development serve as the target for adult vocal motor change. Indeed, some studies suggest that neurons in the adult songbird brain continue to represent the structure of the tutor's song [e.g., Gobes and Bolhuis (2007); Phan et al. (2006); van der Kant et al. (2013)], although the representation of tutor song seems to diminish over development (Achiro and Bottjer 2013; Nick and Konishi 2005; Solis and Doupe 2000). We were unable to test this hypothesis here because of the lack of tutor song recordings for the birds in this study (all birds were purchased from outside vendors), but this study motivates targeted analyses of similarities in the temporal patterning of tutor and pupil songs in Bengalese finches.
Variation in context-dependent changes to syllable structure were also significantly related to age-dependent variation, although the relationship was more complicated than that for temporal features of song. Social context and age affected syllable sequencing and timing to similar degrees, and individual variation in the direction and magnitude of context-dependent changes predicted variation in age-dependent changes. In contrast, social context but not age significantly affected the mean and variability of FF. Despite the lack of significant changes to the mean and variability of FF over time, variation in age-dependent changes to these spectral features of song were correlated with variation in context-dependent changes. For example, syllables that increased more in FF from UDyoung to FDyoung song tended to increase in FF from UDyoung to UDolder song, whereas syllables that increased less or decreased in FF across social context tended to decrease in FF with age (Fig. 4). Despite the lack of overall change in FF over time, these data highlight the potential for context-dependent changes to predict variation in the nature of changes to the spectral structure over time.
In summary, these data demonstrate that by examining variation in the degree to which social context affects the control of an evolutionarily important behavior, one can gain insight into individual differences in the trajectory of behavioral change over time. Our data are consistent with the notion that individual variation in motor ability predicts variation in the magnitude of motor learning [e.g., Adams (1987); Magill (2004)] and suggest that age-dependent changes could reflect improvements to vocal performance (e.g., shorter gap durations). Furthermore, these data suggest the possibility that mechanisms that acutely regulate vocal performance could shape vocal motor plasticity.
GRANTS
Support for this research was provided by funding from the National Science and Engineering Research Council, McGill University, and a group infrastructure grant from the Fonds de Recherche du Québec-Santé (Center for Studies in Behavioral Neurobiology, Concordia University).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: L.S.J. and J.T.S. conception and design of research; L.S.J. and J.T.S. performed experiments; L.S.J. and J.T.S. analyzed data; L.S.J. and J.T.S. interpreted results of experiments; L.S.J. and J.T.S. prepared figures; L.S.J. and J.T.S. drafted manuscript; L.S.J. and J.T.S. edited and revised manuscript; L.S.J. and J.T.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank S. C. Woolley, L. E. Matheson, D. M. Toccalino, A. Bernard, and R. Krahe for important contributions to data collection, analysis, interpretation, and presentation.
REFERENCES
- Achiro JM, Bottjer SW. Neural representation of a target auditory memory in a cortico-basal ganglia pathway. J Neurosci 33: 14475–14488, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JA. Historical review and appraisal of research on the learning, retention, and transfer of human motor skills. Psychol Bull 101: 41–71, 1987. [Google Scholar]
- Ali F, Otchy TM, Pehlevan C, Fantana AL, Burak Y, Ölveczky BP. The basal ganglia is necessary for learning spectral, but not temporal, features of birdsong. Neuron 80: 494–506, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andalman AS, Foerster JN, Fee MS. Control of vocal and respiratory patterns in birdsong: dissection of forebrain and brainstem mechanisms using temperature. PLoS One 6: e25461, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore RC, Wild JM, Schmidt MF. Brainstem and forebrain contributions to the generation of learned motor behaviors for song. J Neurosci 25: 8543–8554, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basista MJ, Elliott KC, Wu W, Hyson RL, Bertram R, Johnson F. Independent premotor encoding of the sequence and structure of birdsong in avian cortex. J Neurosci 34: 16821–16834, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Auditory feedback in learning and maintenance of vocal behaviour. Nat Rev Neurosci 1: 31–40, 2000. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Postlearning consolidation of birdsong: stabilizing effects of age and anterior forebrain lesions. J Neurosci 21: 2501–2517, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Translating birdsong: songbirds as a model for basic and applied medical research. Annu Rev Neurosci 36: 489–517, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briellmann RS, Saling MM, Connell AB, Waites AB, Abbott DF, Jackson GD. A high-field functional MRI study of quadri-lingual subjects. Brain Lang 89: 531–542, 2004. [DOI] [PubMed] [Google Scholar]
- Byers J, Hebets E, Podos J. Female mate choice based upon male motor performance. Anim Behav 79: 771–778, 2010. [Google Scholar]
- Catchpole CK, Slater PJ. Bird Song Biological Themes and Variations (2nd ed). Cambridge, UK: Cambridge University Press, 2008. [Google Scholar]
- Chen JL, Rae C, Watkins KE. Learning to play a melody: an fMRI study examining the formation of auditory-motor associations. Neuroimage 59: 1200–1208, 2012. [DOI] [PubMed] [Google Scholar]
- Chi Z, Margoliash D. Temporal precision and temporal drift in brain and behavior of zebra finch song. Neuron 32: 899–910, 2001. [DOI] [PubMed] [Google Scholar]
- Colonnese MT, Stallman EL, Berridge KC. Ontogeny of action syntax in altricial and precocial rodents: grooming sequences of rat and guinea pig pups. Behaviour 133: 1165–1195, 1996. [Google Scholar]
- Cooper BG, Goller F. Physiological insights into the social-context-dependent changes in the rhythm of the song motor program. J Neurophysiol 95: 3798–3809, 2006. [DOI] [PubMed] [Google Scholar]
- Cooper BG, Méndez JM, Saar S, Whetstone AG, Meyers R, Goller F. Age-related changes in the Bengalese finch song motor program. Neurobiol Aging 33: 564–568, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron 72: 443–454, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cheveigné A, Kawahara H. YIN, a fundamental frequency estimator for speech and music. J Acoust Soc Am 111: 1917–1930, 2002. [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci 22: 567–631, 1999. [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Perkel DJ, Reiner A, Stern EA. Birdbrains could teach basal ganglia research a new song. Trends Neurosci 28: 353–363, 2005. [DOI] [PubMed] [Google Scholar]
- Doya K. Complementary roles of basal ganglia and cerebellum in learning and motor control. Curr Opin Neurobiol 10: 732–739, 2000. [DOI] [PubMed] [Google Scholar]
- Drake C, Palmer C. Skill acquisition in music performance: relations between planning and temporal control. Cognition 74: 1–32, 2000. [DOI] [PubMed] [Google Scholar]
- Dunning JL, Pant S, Bass A, Coburn Z, Prather JF. Mate choice in adult female Bengalese finches: females express consistent preferences for individual males and prefer female-directed song performances. PLoS One 9: e89438, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson KA. Deliberate practice and acquisition of expert performance: a general overview. Acad Emerg Med 15: 988–994, 2008. [DOI] [PubMed] [Google Scholar]
- Ericsson KA, Ralf TK, Tesch-Römer C. The role of deliberate practice in the acquisition of expert performance. Psychol Rev 100: 363, 1993. [Google Scholar]
- Fee MS, Scharff C. The songbird as a model for the generation and learning of complex sequential behaviors. ILAR J 51: 362–377, 2010. [DOI] [PubMed] [Google Scholar]
- Fentress JC. Emergence of pattern in the development of mammalian movement sequences. J Neurobiol 23: 1529–1556, 1992. [DOI] [PubMed] [Google Scholar]
- Fujimoto H, Hasegawa T, Watanabe D. Neural coding of syntactic structure in learned vocalizations in the songbird. J Neurosci 31: 10023–10033, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaze CM, Troyer TW. Development of temporal structure in zebra finch song. J Neurophysiol 109: 1025–1035, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaze CM, Troyer TW. Temporal structure in zebra finch song: implications for motor coding. J Neurosci 26: 991–1005, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobes SM, Bolhuis JJ. Birdsong memory: a neural dissociation between song recognition and production. Curr Biol 17: 789–793, 2007. [DOI] [PubMed] [Google Scholar]
- Golestani N, Molko N, Dehaene S, LeBihan D, Pallier C. Brain structure predicts the learning of foreign speech sounds. Cereb Cortex 17: 575–582, 2007. [DOI] [PubMed] [Google Scholar]
- Golestani N, Paus T, Zatorre RJ. Anatomical correlates of learning novel speech sounds. Neuron 35: 997–1010, 2002. [DOI] [PubMed] [Google Scholar]
- Hahnloser RH, Kozhevnikov AA, Fee MS. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature 419: 65–70, 2002. [DOI] [PubMed] [Google Scholar]
- Hamaguchi K, Mooney R. Recurrent interactions between the input and output of a songbird cortico-basal ganglia pathway are implicated in vocal sequence variability. J Neurosci 32: 11671–11687, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton CM, Sakata JT, Brainard MS. An avian basal ganglia-forebrain circuit contributes differentially to syllable versus sequence variability of adult Bengalese finch song. J Neurophysiol 101: 3235–3245, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinig A, Pant S, Dunning J, Bass A, Coburn Z, Prather JF. Male mate preferences in mutual mate choice: finches modulate their songs across and within male-female interactions. Anim Behav 97: 1–12, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herholz SC, Zatorre RJ. Musical training as a framework for brain plasticity: behavior, function, and structure. Neuron 76: 486–502, 2012. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci 8: 393–402, 2007. [DOI] [PubMed] [Google Scholar]
- Hund-Georgiadis M, von Cramon DY. Motor-learning-related changes in piano players and non-musicians revealed by functional magnetic-resonance signals. Exp Brain Res 125: 417–425, 1999. [DOI] [PubMed] [Google Scholar]
- James LS, Sakata JT. Vocal motor changes beyond the sensitive period for song plasticity. J Neurophysiol 112: 2040–2052, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäncke L, Shah NJ, Peters M. Cortical activations in primary and secondary motor areas for complex bimanual movements in professional pianists. Brain Res Cogn Brain Res 10: 177–183, 2000. [DOI] [PubMed] [Google Scholar]
- Jarvis ED, Scharff C, Grossman MR, Ramos JA, Nottebohm F. For whom the bird sings: context-dependent gene expression. Neuron 21: 775–788, 1998. [DOI] [PubMed] [Google Scholar]
- Jin H, Clayton DF. Localized changes in immediate-early gene regulation during sensory and motor learning in zebra finches. Neuron 19: 1049–1059, 1997. [DOI] [PubMed] [Google Scholar]
- Kakishita Y, Sasahara K, Nishino T, Takahasi M, Okanoya K. Ethological data mining: an automata-based approach to extract behavioral units and rules. Data Min Knowl Disc 18: 446–471, 2009. [Google Scholar]
- Kao MH, Brainard MS. Lesions of an avian basal ganglia circuit prevent context-dependent changes to song variability. J Neurophysiol 96: 1441–1455, 2006. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 377: 155–158, 1995. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci USA 95: 861–868, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith N, Ericsson KA. A deliberate practice account of typing proficiency in everyday typists. J Exp Psychol Appl 13: 135–145, 2007. [DOI] [PubMed] [Google Scholar]
- Kelly CW, Sober SJ. A simple computational principle predicts vocal adaptation dynamics across age and error size. Front Integr Neurosci 8: 75, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Uno H, Okanoya K. Partial lesions in the anterior forebrain pathway affect song production in adult Bengalese finches. Neuroreport 12: 353–358, 2001. [DOI] [PubMed] [Google Scholar]
- Kojima S, Doupe AJ. Social performance reveals unexpected vocal competency in young songbirds. Proc Natl Acad Sci USA 108: 1687–1692, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Doupe AJ. Song selectivity in the pallial-basal ganglia song circuit of zebra finches raised without tutor song exposure. J Neurophysiol 98: 2099–2109, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosche G, Vallentin D, Long MA. Interplay of inhibition and excitation shapes a premotor neural sequence. J Neurosci 35: 1217–1227, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krings T, Töpper R, Foltys H, Erberich S, Sparing R, Willmes K, Thron A. Cortical activation patterns during complex motor tasks in piano players and control subjects. A functional magnetic resonance imaging study. Neurosci Lett 278: 189–193, 2000. [DOI] [PubMed] [Google Scholar]
- Kubikova L, Bosikova E, Cvikova M, Lukacova K, Scharff C, Jarvis ED. Basal ganglia function, stuttering, sequencing, and repair in adult songbirds. Sci Rep 4: 6590, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi SM, Baguear F, Della-Maggiore V. One week of motor adaptation induces structural changes in primary motor cortex that predict long-term memory one year later. J Neurosci 31: 11808–11813, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblois A, Perkel DJ. Striatal dopamine modulates song spectral but not temporal features through D1 receptors. Eur J Neurosci 35: 1771–1781, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston FS, White SA, Mooney R. Slow NMDA-EPSCs at synapses critical for song development are not required for song learning in zebra finches. Nat Neurosci 3: 482–488, 2000. [DOI] [PubMed] [Google Scholar]
- Long MA, Fee MS. Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature 456: 189–194, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, Jin DZ, Fee MS. Support for a synaptic chain model of neuronal sequence generation. Nature 468: 394–399, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M, Scheler G, Tan HR, Braun C, Birbaumer N. The musician's brain: functional imaging of amateurs and professionals during performance and imagery. Neuroimage 20: 1817–1829, 2003. [DOI] [PubMed] [Google Scholar]
- Magill RA. Motor Learning and Control: Concepts and Applications (7th ed) New York: McGraw-Hill, 2004. [Google Scholar]
- Matheson LE, Sakata JT. Catecholaminergic contributions to vocal communication signals. Eur J Neurosci 14: 1180–1194, 2015. [DOI] [PubMed] [Google Scholar]
- Matheson LE, Sun H, Sakata JT. Forebrain circuits underlying the social modulation of vocal communication signals. Dev Neurobiol. First published June 11, 2015; doi: 10.1002/dneu.22298. [DOI] [PubMed] [Google Scholar]
- Mooney R. Neurobiology of song learning. Curr Opin Neurobiol 19: 654–660, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. The reproductive behaviour of the zebra finch, with special reference to pseudofemale behaviour and displacement activities. Behaviour 6: 271–322, 1954. [Google Scholar]
- Morrison RG, Nottebohm F. Role of a telencephalic nucleus in the delayed song learning of socially isolated zebra finches. J Neurobiol 24: 1045–1064, 1993. [DOI] [PubMed] [Google Scholar]
- Nick TA, Konishi M. Neural song preference during vocal learning in the zebra finch depends on age and state. J Neurobiol 62: 231–242, 2005. [DOI] [PubMed] [Google Scholar]
- Okanoya K. Voco-auditory behavior in the Bengalese finch: a comparison with the zebra finch. Biomed Res 18: 53–70, 1997. [Google Scholar]
- Okanoya K, Yamaguchi A. Adult Bengalese finches (Lonchura striata var. domestica) require real-time auditory feedback to produce normal song syntax. J Neurobiol 33: 343–356, 1997. [PubMed] [Google Scholar]
- Ölveczky BP, Andalman AS, Fee MS. Vocal experimentation in the juvenile songbird requires a basal ganglia circuit. PLoS Biol 3: e153, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani D, Abutalebi J, Paulesu E, Brambati S, Scifo P, Cappa SF, Fazio F. The role of age of acquisition and language usage in early, high-proficient bilinguals: an fMRI study during verbal fluency. Hum Brain Mapp 19: 170–182, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, van Mier H, Fiez JA, Raichle ME. The effects of practice on the functional anatomy of task performance. Proc Natl Acad Sci USA 95: 853–860, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan ML, Pytte CL, Vicario DS. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proc Natl Acad Sci USA 103: 1088–1093, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podos J, Lahti DC, Moseley DL. Vocal performance and sensorimotor learning in songbirds. In: Advances in the Study of Behavior. Atlanta, GA: Elsevier, 2009, vol. 40, chapt. 5, p. 159–195. [Google Scholar]
- Poldrack RA. Imaging brain plasticity: conceptual and methodological issues—a theoretical review. Neuroimage 12: 1–13, 2000. [DOI] [PubMed] [Google Scholar]
- Prather JF, Peters S, Nowicki S, Mooney R. Precise auditory-vocal mirroring in neurons for learned vocal communication. Nature 451: 305–310, 2008. [DOI] [PubMed] [Google Scholar]
- Pytte CL, Gerson M, Miller J, Kirn JR. Increasing stereotypy in adult zebra finch song correlates with a declining rate of adult neurogenesis. Dev Neurobiol 67: 1699–1720, 2007. [DOI] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Güntürkün O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol 473: 377–414, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata JT, Brainard MS. Online contributions of auditory feedback to neural activity in avian song control circuitry. J Neurosci 28: 11378–11390, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata JT, Brainard MS. Social context rapidly modulates the influence of auditory feedback on avian vocal motor control. J Neurophysiol 102: 2485–2497, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata JT, Crews D. Embryonic temperature shapes behavioural change following social experience in male leopard geckos, Eublepharis macularius. Anim Behav 66: 839–846, 2003. [Google Scholar]
- Sakata JT, Hampton CM, Brainard MS. Social modulation of sequence and syllable variability in adult birdsong. J Neurophysiol 99: 1700–1711, 2008. [DOI] [PubMed] [Google Scholar]
- Sakata JT, Vehrencamp SL. Integrating perspectives on vocal performance and consistency. J Exp Biol 215: 201–209, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels M, Witmer J. Statistics for the Life Sciences (3rd ed). Upper Saddle River, NJ: Prentice Hall, 2002. [Google Scholar]
- Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci 23: 393–415, 2000. [DOI] [PubMed] [Google Scholar]
- Schmidt MF. Pattern of interhemispheric synchronization in HVc during singing correlates with key transitions in the song pattern. J Neurophysiol 90: 3931–3949, 2003. [DOI] [PubMed] [Google Scholar]
- Schmidt RA, Lee T. Motor Control and Learning. Champaign, IL: Human Kinetics, 1998. [Google Scholar]
- Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res 185: 359–381, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis MM, Doupe AJ. Compromised neural selectivity for song in birds with impaired sensorimotor learning. Neuron 25: 109–121, 2000. [DOI] [PubMed] [Google Scholar]
- Stepanek L, Doupe AJ. Activity in a cortical-basal ganglia circuit for song is required for social context-dependent vocal variability. J Neurophysiol 104: 2474–2486, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernichovski O, Mitra PP, Lints T, Nottebohm F. Dynamics of the vocal imitation process: how a zebra finch learns its song. Science 291: 2564–2569, 2001. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Basista MJ, Wu W, Bertram R, Johnson F. Dual pre-motor contribution to songbird syllable variation. J Neurosci 31: 322–330, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassini V, Jbabdi S, Kincses ZT, Bosnell R, Douaud G, Pozzilli C, Matthews PM, Johansen-Berg H. Structural and functional bases for individual differences in motor learning. Hum Brain Mapp 32: 494–508, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer TW, Doupe AJ. An associational model of birdsong sensorimotor learning II. Temporal hierarchies and the learning of song sequence. J Neurophysiol 84: 1224–1239, 2000. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Doyon J, Karni A. Imaging brain plasticity during motor skill learning. Neurobiol Learn Mem 78: 553–564, 2002. [DOI] [PubMed] [Google Scholar]
- van der Kant A, Derégnaucourt S, Gahr M, Van der Linden A, Poirier C. Representation of early sensory experience in the adult auditory midbrain: implications for vocal learning. PLoS One 8: e61764, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingerhoets G, Van Borsel J, Tesink C, van den Noort M, Deblaere K, Seurinck R, Vandemaele P, Achten E. Multilingualism: an fMRI study. Neuroimage 20: 2181–2196, 2003. [DOI] [PubMed] [Google Scholar]
- Vu ET, Mazurek ME, Kuo YC. Identification of a forebrain motor programming network for the learned song of zebra finches. J Neurosci 14: 6924–6934, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Herbst JA, Keller GB, Hahnloser RH. Rapid interhemispheric switching during vocal production in a songbird. PLoS Biol 14: e250, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren TL, Charlesworth JD, Tumer EC, Brainard MS. Variable sequencing is actively maintained in a well learned motor skill. J Neurosci 32: 15414–15425, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney O, Soderstrom K, Johnson F. Post-transcriptional regulation of zenk expression associated with zebra finch vocal development. Brain Res Mol Brain Res 80: 279–290, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams H, Mehta N. Changes in adult zebra finch song require a forebrain nucleus that is not necessary for song production. J Neurobiol 39: 14–28, 1999. [PubMed] [Google Scholar]
- Willingham DB. A neuropsychological theory of motor skill learning. Psychol Rev 105: 558–584, 1998. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Doya K, Kawato M. A unifying computational framework for motor control and social interaction. Philos Trans R Soc Lond B Biol Sci 358: 593–602, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SC, Doupe AJ. Social context-induced song variation affects female behavior and gene expression. PLoS Biol 6: e62, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SC, Kao MH. Variability in action: contributions of a songbird cortical-basal ganglia circuit to vocal motor learning and control. Neuroscience 296: 39–47, 2015. [DOI] [PubMed] [Google Scholar]
- Yu AC, Margoliash D. Temporal hierarchical control of singing in birds. Science 273: 1871–1875, 1996. [DOI] [PubMed] [Google Scholar]
- Zann RA. The Zebra Finch: A Synthesis of Field and Laboratory Studies. New York: Oxford University Press, 1996. [Google Scholar]
- Zatorre RJ, Chen JL, Penhune VB. When the brain plays music: auditory-motor interactions in music perception and production. Nat Rev Neurosci 8: 547–558, 2007. [DOI] [PubMed] [Google Scholar]